Abstract
Background
Underrepresentation of females in randomized controlled trials (RCTs) limits generalizability and quality of the evidence guiding treatment of females. This study aimed to measure the sex disparities in participants' recruitment in RCTs of atrial fibrillation (AF) and determine associated factors, and to describe the frequency of outcomes reported by sex.
Methods
MEDLINE was searched to identify RCTs of AF published between January 1, 2011, and November 20, 2021, in 12 top‐tier journals. We measured the enrollment of females using the enrollment disparity difference (EDD) which is the difference between the proportion of females in the trial and the proportion of females with AF in the underlying general population (obtained from the Global Burden of Disease). Random‐effects meta‐analyses of the EDD were performed, and multivariable meta‐regression was used to explore factors associated with disparity estimates. We also determined the proportion of trials that included sex‐stratified results.
Results
Out of 1133 records screened, 142 trials were included, reporting on a total of 133 532 participants. The random‐effects summary EDD was −0.125 (95% confidence interval [CI] = −0.143 to −0.108), indicating that females were under‐enrolled by 12.5 percentage points. Female enrollment was higher in trials with higher sample size (<250 vs. >750, adjusted odds ratio [aOR] 1.065, 95% CI: 1.008–1.125), higher mean participants' age (aOR: 1.006, 95% CI: 1.002–1.009), and lower in trials conducted in North America compared to Europe (aOR: 0.945, 95% CI: 0.898–0.995). Only 36 trials (25.4%) reported outcomes by sex, and of these 29 (80.6%) performed statistical testing of the sex‐by‐treatment interaction.
Conclusion
Females remain substantially less represented in RCTs of AF, and sex‐stratified reporting of primary outcomes is infrequent. These findings call for urgent action to improve sex equity in enrollment and sex‐stratified outcomes' reporting in RCTs of AF.
Keywords: atrial fibrillation, enrollment, reporting, sex
1. INTRODUCTION
Atrial fibrillation (AF), the most common sustained arrhythmia, is an increasingly important threat to global health owing to its rapidly growing prevalence 1 and associated devastating complications including stroke, heart failure, and death. 2 , 3 There are significant sex differences in the epidemiology of AF. 4 Women have a lower prevalence of AF compared to men: of the 37.6 million individuals with AF globally in 2017, 17.8 million (47.3%) were women. 1 However, AF has been shown to be a stronger risk factor for stroke, cardiovascular mortality, and all‐cause mortality in women compared with men. 5
Sex differences in disease pathophysiology, clinical patterns, and response to treatment should be considered in the design, analysis, and reporting of research studies. 6 , 7 Recent analyses have shown inadequate enrollment of women in randomized controlled trials (RCTs) of various cardiovascular diseases. 8 , 9 , 10 Given the well‐established importance of sex in the epidemiology of AF, 4 the underrepresentation of women in RCTs of therapies for patients with AF might undermine their generalizability and consequently the validity of the evidence guiding treatment of women. Furthermore, reporting of trial results in women and men separately is essential to determine when sex‐specific therapeutic strategies should be applied. Despite the call for reporting of trial results by sex made by several initiatives over more than a decade, 11 , 12 a number of previous studies have observed that results of most trials are not reported according to sex. 9 , 13 , 14 , 15 The current study, which focuses on recent RCTs of AF, aims to measure the sex disparities in participants' recruitment, determine associated factors, and describe the frequency of reporting of primary endpoint results by sex.
2. METHODS
This project was registered with PROSPERO (CRD42021291258).
2.1. Literature search
PubMed/MEDLINE was searched to identify all RCTs of AF published between January 1, 2011, and November 20, 2021, in top journals (based on Clarivate Analytics impact factor 2020) of general medicine (New England Journal of Medicine [NEJM], The Lancet, Journal of the American Medical Association [JAMA], JAMA Internal Medicine and British Medical Journal [BMJ]), of general cardiology (European Heart Journal [EHJ], Circulation, Journal of the American College of Cardiology [JACC], and JAMA Cardiology), and of cardiac electrophysiology (JACC Clinical Electrophysiology [JACC CE], Circulation Arrhythmia and Electrophysiology [Circ AE], Hearth Rhythm, Europace, and Journal of Cardiac Electrophysiology [JCE]). The search strategy was built based on the combination of relevant terms related to AF, clinical trials, and the names of the targeted journals (Table S1). One investigator (Jean Jacques Noubiap) conducted the bibliographic searches. The reference lists of eligible articles were also scrutinized and ClinicalTrial.gov to identify additional relevant RCTs.
2.2. Selection of studies to include in the review
The inclusion criteria were: (1) studies reporting final primary results of an RCT; (2) published in one of the 12 abovementioned target journals between January 1, 2011, and November 20, 2021; (3) trial population consisting of patients with AF. The exclusion criteria were: (1) sample size of less than 100 participants; (2) articles not reporting primary results such as subsequent analyses of trial data; (3) RCTs of AF screening or detection in at risk populations such as elderly or patients with stroke. Two investigators (Jean Jacques Noubiap and Ulrich Flore Nyaga) independently selected records from bibliographic searches based on title and abstract screening. Full texts of articles deemed potentially eligible were retrieved and screened independently by the same investigators for final inclusion. Selection discrepancies were solved through discussion and consensus.
2.3. Data extraction and management
Data were extracted using a standardized data abstraction form and included: name of the first author, year of publication, period of participants' recruitment, whether it was a single or multicenter trial, total trial population size, whether participants were recruited in one or multiple countries, trial name, trial registration number, trial phase (as reported by the investigators), masking (unmasked, single‐blinded, or double‐blinded trial), type of intervention, primary outcome, involvement of industry, involvement of 1 or more females represented in trial leadership, total sample size, number of enrolled females, whether trial results were reported by sex, and whether there was a sex difference in the trial results. Detailed variable definitions are provided in Table S2. Data were extracted by one investigator (Jean Jacques Noubiap). This study did not aim to assess treatment efficacy in the included RCTs. Therefore, we did not assess the risk of bias.
2.4. Assessment of sex enrollment disparities
To measure enrollment of females, we used a modified version of the enrollment disparity difference (EDD), a metric which accounts for sex prevalence inequalities in the general population, initially developed to characterize enrollment disparities in lung cancer treatment RCTs, 16 and also used in a recent study of sex enrollment disparities in RCTs of acute stroke therapies. 8 For each RCT, we calculated the proportion of females enrolled in the trial (PFT), and estimated the proportion of females with AF among the general population (PFG) using data from the Global Burden of Disease (GBD) database (https://gbd2017.healthdata.org/gbd-search/). Each trial was matched to GBD prevalence data on the basis of the approximate median year of the trial recruitment period and geographic area. Because data in the GBD database were available up to year 2017, data for 2017 were attributed to all trials in which participants were recruited from 2017 onward. For trials conducted in one country, we considered the GBD estimates of that country. For trials conducted in two or more countries, if they were in the same GBD region, we included estimates for the corresponding region; otherwise, we considered the global estimates. We abstracted the number of people with AF and associated a 95% uncertainty interval for females, males, and both.
The EDD was calculated as the difference between the PFT and PFG. We also calculated the standard errors (SE) of the PFT, PFG, and EDD. The following calculations were performed:
To calculate the standard error of the PFG, we accounted for the uncertainty of the estimates from the GBD. For each trial, we used the matched number of males and females with AF and the associated 95% uncertainty intervals from the GBD to fit γ distributions and drew 100 000 samples from each of the γ distributions to compute the corresponding PFG and its standard error.
2.5. Statistical analysis
Sex disparities in enrollment across RCTs were summarized using a random‐effects meta‐analysis of the EDDs. We conducted subgroup analyses according to trial characteristics including journal categories (general medicine, general cardiology, and cardiac electrophysiology), period of publication, sample size category, geographical region, type of trial intervention, presence of females in a leadership position, and industry involvement. Heterogeneity was assessed by the χ² test on Cochrane's Q statistic, 17 which was quantified by I² values, assuming I² values of 25%, 50%, and 75%, respectively, representing low, medium, and high heterogeneity. 18 We performed univariable and multivariable meta‐regression to explore factors associated with the enrollment of females. Analyses were conducted using the R statistical software (version 3.5.03, The R Foundation for Statistical Computing) and IBM SPSS Statistics version 27.0.
3. RESULTS
3.1. Study selection and characteristics
From 1133 records identified from bibliographic search and additional sources, we finally included 142 articles reporting trial data from a total of 133 532 participants (Figure 1). The list of included trials and their individual characteristics are presented in Tables S3–S5. These articles were most frequently published in Circ AE (14.8%), EHJ (14.1%), Europace (12.7%), NEJM (11.3%), and Heart Rhythm (10.6%). Most RCTs were conducted in multiple centers (71.8%), or in one country (59.2%). About a third of RCTs were conducted in several regions, whereas the single most represented region was Europe (31.0%). Most RCTs did not have a woman in a leadership role (86.6%), and industry (for‐profit entity) was involved in 46.5% of them. The number of participants enrolled in the included trials ranged from 100 to 21 105, with a median of 222 (interquartile range [IQR] = 150–501.5). The most common interventions evaluated in the RCTs were catheter ablation (49.3%), any adjuvant therapy to catheter ablation or electrical cardioversion (12.7%), periprocedural oral anticoagulation (9.9%), long‐term oral anticoagulation (7.7%), and risk factor management or integrated care (7.7%; Table 1).
Figure 1.
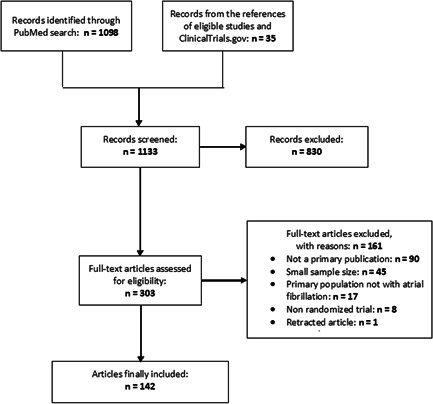
Study selection
Table 1.
Characteristics of included trials
| Characteristics | Number | Percentage |
|---|---|---|
| Journal | ||
|
16 | 11.3 |
|
6 | 4.2 |
|
8 | 5.6 |
|
1 | 0.7 |
|
20 | 14.1 |
|
14 | 9.9 |
|
9 | 6.3 |
|
1 | 0.7 |
|
7 | 4.9 |
|
21 | 14.8 |
|
15 | 10.6 |
|
18 | 12.7 |
|
6 | 4.2 |
| Journal category | ||
|
31 | 21.8 |
|
44 | 40.0 |
|
67 | 47.2 |
| Year of publication | ||
|
27 | 19.0 |
|
34 | 23.9 |
|
24 | 16.9 |
|
27 | 19.0 |
|
30 | 21.1 |
| Sample size | ||
|
81 | 57.0 |
|
37 | 26.1 |
|
24 | 16.9 |
| Centers | ||
|
102 | 71.8 |
|
40 | 28.2 |
| Multinational | ||
|
58 | 40.8 |
|
84 | 59.2 |
| Region | ||
|
26 | 18.3 |
|
44 | 31.0 |
|
25 | 17.6 |
|
1 | 0.7 |
|
46 | 32.4 |
| Intervention | ||
|
70 | 49.3 |
|
3 | 2.1 |
|
2 | 1.4 |
|
18 | 12.7 |
|
14 | 9.9 |
|
11 | 7.7 |
|
4 | 2.8 |
|
4 | 2.8 |
|
11 | 7.7 |
|
5 | 3.5 |
| Industry involvement | ||
|
66 | 46.5 |
|
76 | 53.5 |
| Female(s) in trial leadership role | ||
|
19 | 13.4 |
|
123 | 86.6 |
Abbreviation: RFM, risk factor management.
3.2. Enrollment disparity differences
There were wide variations in the PFT and PFG across studies (Figure 2). The PFT ranged from 10.0% to 72.8% (median 30.9%, IQR = 24.6%–38.1%), whereas the PFG ranged from 34.7% to 55.9% (median 45.3%, IQR = 40.6%–47.2%). The random‐effects pooled EDD of the 142 trials was −0.125 (95% CI = −0.143 to −0.108), representing an under‐enrollment of females by an absolute difference of 12.5 percentage points relative to their representation in the general populations of people with AF. Female enrollment varied significantly across trials (I² 88%). The results of subgroup analysis according to journals, publication period, sample size, region, type of intervention tested in the trial, industry involvement, and whether female(s) had a leadership role in the trial are presented in Figure 3 and Table S6.
Figure 2.
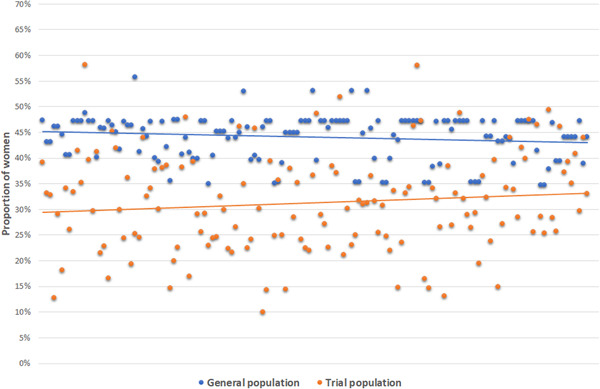
Proportions of females in trials and matched Global Burden of Disease populations with atrial fibrillation
Figure 3.
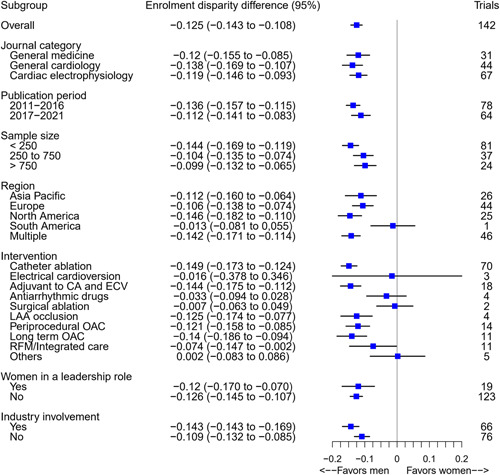
Random‐effects pooled enrollment disparity difference in trials of atrial fibrillation
In univariable meta‐regression analysis, an age limit for participants' inclusion in the trial, the mean age of participants, and the period of publication of the trial's results were associated with female enrollment, whereas a trend toward an association was noted for sample size, and industry involvement (Table 2). In multivariable analysis (Model 1), larger sample sizes were associated with an increased likelihood of female enrollment (categories < 250 vs. >750: adjusted odds ratio [aOR] = 1.065, 95% CI = 1.008–1.125). Compared to trials conducted in Europe, those in North America (USA or Canada) were associated with lower female enrollment (aOR = 0.945, 95% CI = 0.898–0.995). Because the periods of recruitment of trials were very heterogeneous and difficult to categorize, we used the period of publication of the trial results as a surrogate of the period of recruitment. The participation of females was higher in trials published in 2020 or 2021 compared to those published between 2011 and 2013 (aOR = 1.058, 95% CI = 1.004–1.115). Higher mean age of the trial population was also significantly associated with a greater likelihood of female enrollment (aOR = 1.006, 95% CI = 1.002–1.009; Model 2).
Table 2.
Random‐effects multivariable meta‐regression analysis of the enrollment disparity difference in trials of atrial fibrillation
| Univariable | Multivariable Model 1 | Multivariable Model 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | ||||
| LCL | UCL | LCL | UCL | LCL | UCL | |||||||
| Upper age limit | ||||||||||||
|
Ref | |||||||||||
|
1.045 | 1.007 | 1.086 | .021 | 1.036 | 0.995 | 1.078 | .083 | ||||
| Mean age (per 1 year increase) | 1.007 | 1.004 | 1.010 | <.001 | 1.006 | 1.002 | 1.009 | .002 | ||||
| Sample size | ||||||||||||
|
Ref | |||||||||||
|
1.041 | 1.000 | 1.084 | .052 | 1.054 | 1.012 | 1.097 | .011 | 1.041 | 1.000 | 1.084 | .050 |
|
1.047 | 0.999 | 1.097 | .054 | 1.065 | 1.008 | 1.125 | .025 | 1.042 | 0.986 | 1.102 | .144 |
| Female(s) in a leadership role | ||||||||||||
|
||||||||||||
|
1.006 | 0.956 | 1.059 | .814 | 0.983 | 0.933 | 1.034 | .502 | 0.976 | 0.928 | 1.026 | .340 |
| Industry involvement | ||||||||||||
|
Ref | |||||||||||
|
0.966 | 0.933 | 1.000 | .050 | 0.968 | 0.928 | 1.010 | .138 | 0.960 | 0.922 | 1.000 | .053 |
| Intervention | ||||||||||||
|
Ref | |||||||||||
|
0.961 | 0.925 | 0.999 | .043 | 0.971 | 0.927 | 1.017 | .211 | 0.986 | 0.941 | 1.032 | .542 |
| Region | ||||||||||||
|
Ref | |||||||||||
|
0.994 | 0.944 | 1.046 | .813 | 0.981 | 0.932 | 1.032 | .448 | 0.990 | 0.942 | 1.040 | .681 |
|
0.961 | 0.912 | 1.013 | .138 | 0.945 | 0.898 | 0.995 | .033 | 0.958 | 0.912 | 1.007 | .091 |
|
0.964 | 0.923 | 1.007 | .100 | 0.939 | 0.890 | 0.991 | .023 | 0.951 | 0.903 | 1.001 | .055 |
| Period of publication | ||||||||||||
|
Ref | |||||||||||
|
0.997 | 0.946 | 1.050 | .901 | 1.017 | 0.965 | 1.071 | .537 | 1.018 | 0.968 | 1.071 | .483 |
|
0.975 | 0.921 | 1.032 | .39 | 0.994 | 0.939 | 1.051 | .829 | 0.993 | 0.940 | 1.049 | .793 |
|
0.987 | 0.934 | 1.043 | .644 | 0.999 | 0.946 | 1.056 | .982 | 1.003 | 0.951 | 1.057 | .923 |
|
1.057 | 1.001 | 1.115 | .046 | 1.058 | 1.004 | 1.115 | .035 | 1.047 | 0.994 | 1.103 | .081 |
Note: Due to collinearity, the variables “highest permitted age of participants” and “mean age” could not be analyzed together. Therefore, two separate multivariable models including each of these variables were conducted. Model 1 and Model 2 are fully adjusted, with Model 1 including “highest permitted age of participants” whereas Model 2 includes “mean age.”
Abbreviations: CI, confidence interval; LCL, lower confidence limit; OR, odds ratio; UCL, upper confidence limit.
3.3. Reporting of results by sex
Out of 142 trials, 36 (25.4%) reported primary endpoint results by sex (Table 3). Reporting by sex was higher (p < .001) in general medicine journals (64.5%), compared to general cardiology journals (27.3%) and cardiac electrophysiology journals (7.5%). The NEJM (87.5%) and Lancet (50.0%) had the highest reporting rates. Reporting also differed by period of publication, geographic region, sample size, intervention, and industry involvement. Trials published in 2020 and 2021 were more commonly reported by sex compared to those published between 2011 and 2019 (40.0% vs. 21.4%, p = .038). Reporting rates were higher in trials conducted in more than one country or more than one region compared to those conducted in a single country (36.2% vs. 17.9%, p = .013) or a single region (43.5% vs. 16.7, p < .001). Increased sample size was associated with more frequent reporting, with 66.6% of trials with more than 750 participants reporting compared to only 32.4% of trials with less than 250 participants (p < .001). Trials evaluating long‐term oral anticoagulation (81.8%), left atrial appendage occlusion (75.0%), and risk factor management or integrated care interventions (63.6%) had significantly higher reporting rates compared to trials on other interventions (p < .001). Reporting was higher in trials with industry involvement compared to those with no industry involvement (34.5% vs. 15.8%, p = .007). Twenty‐nine of the 36 trials (80.6%) that reported results by sex performed statistical testing of the sex‐by‐treatment interaction (Table S4).
Table 3.
Study‐level characteristics associated with reporting of sex‐specific trial results
| Characteristics | Number of trials (N) | Results reported by sex, n (%) | No results reported by sex, n (%) | p |
|---|---|---|---|---|
| Total | 142 | 36 (25.4) | 106 (74.6) | |
| Journal | <.001 | |||
|
16 | 14 (87.5) | 2 (12.5) | |
|
6 | 3 (50.0) | 3 (50.0) | |
|
8 | 3 (37.5) | 5 (62.5) | |
|
1 | 0 (0.0) | 1 (100.0) | |
|
20 | 5 (25.0) | 15 (75.0) | |
|
14 | 5 (28.6) | 10 (71.4) | |
|
9 | 2 (22.2) | 7 (77.8) | |
|
1 | 0 (0.0) | 1 (100.0) | |
|
7 | 0 (0.0) | 7 (100.0) | |
|
21 | 2 (9.5) | 19 (90.5) | |
|
15 | 0 (0.0) | 15 (100.0) | |
|
18 | 3 (16.7) | 15 (83.3) | |
|
6 | 0 (0.0) | 6 (100.0) | |
| Journal category | <.001 | |||
|
31 | 20 (64.5) | 11 (35.5) | |
|
44 | 12 (27.3) | 32 (72.7) | |
|
67 | 5 (7.5) | 62 (92.5) | |
| Year of publication | .133 | |||
|
27 | 6 (22.2) | 21 (77.8) | |
|
34 | 4 (11.8) | 30 (88.2) | |
|
24 | 7 (29.3) | 17 (70.8) | |
|
27 | 7 (25.9) | 20 (74.1) | |
|
30 | 12 (40.0) | 18 (60.0) | |
| Year of publication (Binary) | .038 | |||
|
112 | 24 (21.4) | 88 (78.6) | |
|
30 | 12 (40.0) | 18 (60.0) | |
| Sample size | ||||
|
81 | 12 (32.4) | 25 (67.6) | <.001 |
|
37 | 8 (9.9) | 73 (90.1) | |
|
24 | 16 (66.6) | 8 (33.3) | |
| Centers | ||||
|
102 | 30 (29.4) | 72 (70.6) | .076 |
|
40 | 6 (15.0) | 34 (85.0) | |
| Multinational | .013 | |||
|
58 (40.8) | 21 (36.2) | 37 (63.8) | |
|
84 (59.2) | 15 (17.9) | 69 (82.1) | |
| Region | .003 | |||
|
26 (18.3) | 4 (15.4) | 22 (84.6) | |
|
44 (31.0) | 6 (13.6) | 38 (86.4) | |
|
25 (17.6) | 5 (20.0) | 20 (80.0) | |
|
1 (0.7) | 1 (100.0) | 0 (0.0) | |
|
46 (32.4) | 20 (43.5) | 26 (56.5) | |
| Region (Binary) | <.001 | |||
|
96 | 16 (16.7) | 80 (83.3) | |
|
46 (32.4) | 20 (43.5) | 26 (56.5) | |
| Intervention | <.001 | |||
|
70 (49.3) | 9 (12.9) | 61 (87.1) | |
|
3 (2.1) | 0 (0.0) | 3 (100.0) | |
|
2 (1.4) | 0 (0.0) | 2 (100.0) | |
|
18 (12.7) | 3 (16.7) | 15 (83.3) | |
|
14 (9.9) | 3 (21.4) | 11 (78.6) | |
|
11 (7.7) | 9 (81.8) | 2 (18.2) | |
|
4 (2.8) | 3 (75.0) | 1 (25.0) | |
|
4 (2.8) | 1 (25.0) | 3 (75.0) | |
|
11 (7.7) | 7 (63.6) | 4 (36.4) | |
|
5 (3.5) | 1 (20.0) | 4 (80.0) | |
| Industry involvement | .007 | |||
|
66 (46.5) | 24 (36.4) | 42 (63.6) | |
|
76 (53.5) | 12 (15.8) | 64 (84.2) | |
| Female(s) in a trial leadership role | .258 | |||
|
19 (13.4) | 7 (36.8) | 12 (63.2) | |
|
123 (86.6) | 29 (23.6) | 94 (76.4) |
Abbreviation: RFM, risk factor management.
4. DISCUSSION
This study aimed to describe sex disparities in participants' recruitment and associated factors, and to describe the frequency of primary endpoint results reported by sex in contemporaneous RCTs of AF. Our major findings are: (i) females were substantially less represented, with an absolute difference of 12.5 percentage points relative to their representation in the general populations of people with AF; (ii) more recently published trials, those conducted in Europe, with larger sample sizes, and with a higher mean age of participants were associated with a greater likelihood of female enrollment; and (iii) only one‐quarter of trials reported sex‐specific primary endpoint results; reporting by sex was more common in general medicine journals (mainly NEJM and Lancet), in multinational trials, those with higher sample sizes, those with industry involvement, those evaluating long‐term oral anticoagulation, left atrial appendage occlusion, and risk factor management or integrated care interventions, and in the most recently published trials (2020 and 2021).
The marked underrepresentation of females in RCTs of AF is a major issue considering that females have poor AF‐related outcomes including more frequent strokes, cardiovascular and all‐cause deaths compared with men. 5 This underrepresentation of females also raises concerns about the applicability and generalizability of trial results to females. For instance, the enrollment of females was lowest in RCTs of catheter ablation. A recent analysis of the Get With The Guidelines‐AF registry in the United States found sex differences in AF ablation strategies that are not supported by current evidence, 19 highlighting the need for data to inform optimal ablation strategies by sex. This requires an appropriate representation of females in clinical trials.
We observed that trials with an upper age limit of 80 years or less had lower female representation in their study population. Furthermore, a younger mean age was associated with a reduced likelihood of female enrollment. Because females with AF are generally older than their male counterparts and diagnosed later in life, 20 trials that include younger patients by chance or by design are likely to recruit fewer females. Therefore, an upper age limit as a selection criterion in trials of AF should be considered only if medically indicated. If age is used as a surrogate of age‐related health condition such as frailty, it would be desirable to exclude participants based on a formal assessment of the condition rather than age. 8 , 21 We also observed that smaller trials enrolled fewer females. Special attention is required to ensure appropriate female representation in such trials. Overall, trials published in 2020 and 2021 had enrolled significantly more females compared to those published in 2011–2013. Although the recruitment in these trials might have occurred several years before publication of their results, it is likely that the enrollment of females in RCTs of AF has increased in recent years. More efforts are needed to further increase the enrollment of females in future trials.
Reporting of sex‐specific endpoint results has increased in recent years. This may be due to growing awareness of sex disparities in the epidemiology of disease in general, and particularly AF. Indeed, the number of publications on sex differences in diseases has substantially increased in the last decade (trends data from PubMed). Similar increasing temporal trends were observed in two recent analyses of reporting of results by sex in clinical trials of acute stroke therapies and Alzheimer's disease. 13 , 22 However, the reporting of outcomes by sex is still largely suboptimal as only 40% of RCTs of AF published in 2020 and 2021 reported sex‐specific outcomes. We observe higher rates of reporting in NEJM (87.5%) and Lancet (50.0%), in keeping with a 2016 review on sex‐related reporting in RCTs in the NEJM and Lancet journals in which 48% of trials reported results by sex, 15 and with a more recent review on trials of acute stroke therapies which showed 61% and 40% sex‐specific reporting in trials published in the NEJM and Lancet, respectively. 13 This likely reflects editorial policies in these journals that strongly recommend reporting sex‐specific outcomes. Consideration of such reporting recommendations should be strongly encouraged among authors and journals, especially those with lower rates of sex‐specific reporting. This can be achieved by including reporting by sex as a requirement in journals' reporting guidelines as recommended by the International Committee of Medical Journal Editors. 23 We also observed that increased sample size was associated with more frequent sex‐specific reporting. This suggests that perhaps trials that are more statistically powered are more likely to have sex‐stratified data reported. Indeed, potential sex differences in endpoints should be considered early in the design of trials.
The findings of this study should be interpreted with caution. Because we focused on trials published in selected high‐impact journals, some important RCTs could have been missed. However, RCTs with the greatest impact of AF management are more likely to be published in journals we selected. Furthermore, the precision of the measurement of enrollment disparities might have been influenced by the source of data for the representation of females in the general population. Indeed, data from the GBD are estimates derived from modeling analysis and therefore have some degree of imprecision. Moreover, because data in the GBD database were available up to year 2017, data for 2017 were attributed to all trials in which participants were recruited from 2017 onward. This could have introduced additional uncertainty to our estimates. Nevertheless, the GBD data were the most suitable for this study considering their disaggregation by year and geographic areas. Our study is an important contribution as it is the first description of the frequency of reporting by sex in RCTs of AF. Furthermore, we used a strong metric to measure sex enrollment disparities, accounting for the sex distribution of people with AF in the general population. We also provide important information on factors associated with female enrollment in these trials using adjusted meta‐regression analysis.
5. CONCLUSION
Despite recent progress, females remain substantially less represented in RCTs of AF. This calls into question the generalizability of these trials and the validity of the evidence guiding the treatment of females. More efforts are needed to increase female enrollment, with a special attention in trials conducted in Northern America and those with lower sample size. Avoiding the exclusion of older individuals may also improve female representation. Furthermore, sex‐stratified reporting of primary outcomes infrequently occurs in RCTs of AF, with the exception of top‐tier general medical journals. Reporting by sex should become a requirement in journals' reporting guidelines in a bid to reduce the sex disparity observed in enrollment and reporting of major trials in AF.
AUTHOR CONTRIBUTIONS
Conception and Design, Data interpretation, Manuscript drafting, Guarantor of the study, and Search strategy: Jean Jacques Noubiap. Study selection and Data extraction: Jean Jacques Noubiap and Ulrich Flore Nyaga. Data synthesis: Jean Jacques Noubiap and Gijo Thomas. Manuscript revision: Jean Jacques Noubiap, Ulrich Flore Nyaga, John L. Fitzgerald, Melissa E. Middeldorp, Gijo Thomas, Celine Gallagher, and Prashanthan Sanders. Approval of the final manuscript: Jean Jacques Noubiap, Ulrich Flore Nyaga, John L. Fitzgerald, Melissa E. Middeldorp, Gijo Thomas, Celine Gallagher, and Prashanthan Sanders.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Drs Jean Jacques Noubiap and John L. Fitzgerald are supported by a Postgraduate Scholarship from the University of Adelaide. Drs Celine Gallagher and Melissa E. Middeldorp are supported by Postdoctoral Fellowships from the University of Adelaide. Dr Prashanthan Sanders is supported by a Practitioner Fellowships from the National Health and Medical Research Council of Australia and by the National Heart Foundation of Australia. Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
Noubiap JJ, Thomas G, Nyaga UF, et al. Sex disparities in enrolment and reporting of outcomes by sex in contemporary clinical trials of atrial fibrillation. J Cardiovasc Electrophysiol. 2022;33:845‐854. 10.1111/jce.15421
Disclosures: Dr Prashanthan Sanders reports having served on the advisory board of Medtronic, Abbott Medical, Boston Scientific, CathRx, and PaceMate. He reports that the University of Adelaide has received on his behalf research funding, lecture and/or consulting fees from Medtronic, Abbott Medical, Boston Scientific, and Microport. Other authors: No disclosures.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the Supporting Information of this article.
REFERENCES
- 1. Lippi G, Sanchis‐Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2020;19:1747493019897870. [DOI] [PubMed] [Google Scholar]
- 2. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920‐2925. [DOI] [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983‐988. [DOI] [PubMed] [Google Scholar]
- 4. Odening KE, Deiß S, Dilling‐Boer D, et al. Mechanisms of sex differences in atrial fibrillation: role of hormones and differences in electrophysiology, structure, function, and remodelling. Europace. 2019;21(3):366‐376. [DOI] [PubMed] [Google Scholar]
- 5. Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta‐analysis of cohort studies. BMJ. 2016;532:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mauvais‐Jarvis F, Bairey Merz N, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clayton JA. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol Behav. 2018;187:2‐5. [DOI] [PubMed] [Google Scholar]
- 8. Strong B, Pudar J, Thrift AG, et al. Sex disparities in enrollment in recent randomized clinical trials of acute stroke: a meta‐analysis. JAMA Neurol. 2021;78(6):666‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carcel C, Woodward M, Balicki G, et al. Trends in recruitment of women and reporting of sex differences in large‐scale published randomized controlled trials in stroke. Int J Stroke Off J Int Stroke Soc. 2019;14(9):931‐938. [DOI] [PubMed] [Google Scholar]
- 10. Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL. Women's participation in cardiovascular clinical trials from 2010 to 2017. Circulation. 2020;141(7):540‐548. [DOI] [PubMed] [Google Scholar]
- 11.Women's Health Research: Progress, Pitfalls, and Promise. Washington DC: 2010 by the National Academy of Sciences; 2010.
- 12. Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 2016;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pudar J, Strong B, Howard VJ, Reeves MJ. Reporting of results by sex in randomized controlled trials of acute stroke therapies (2010‐2020). Stroke. 2021;52(11):e702‐e705. [DOI] [PubMed] [Google Scholar]
- 14. Phillips SP, Hamberg K. Doubly blind: a systematic review of gender in randomised controlled trials. Glob Health Action. 2016;9:29597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avery E, Clark J. Sex‐related reporting in randomised controlled trials in medical journals. Lancet. 2016;388(10062):2839‐2840. [DOI] [PubMed] [Google Scholar]
- 16. Pang HH, Wang X, Stinchcombe TE, et al. Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(33):3992‐3999. www.jco.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101‐129. [Google Scholar]
- 18. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 19. Yunus FN, Perino AC, Holmes DN, et al. Sex differences in Ablation Strategy, Lesion Sets, and complications of Catheter Ablation for atrial fibrillation: an analysis from the GWTG‐AFIB registry. Circ Arrhythm Electrophysiol. 2021;14(11):e009790. [DOI] [PubMed] [Google Scholar]
- 20. Camm AJ, Accetta G, Al Mahmeed W, et al. Impact of gender on event rates at 1 year in patients with newly diagnosed non‐valvular atrial fibrillation: contemporary perspective from the GARFIELD‐AF registry. BMJ Open. 2017;7(3):e014579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carcel C, Reeves M. Under‐enrollment of women in stroke clinical trials: what are the causes and what should be done about it? Stroke. 2021;52(2):452‐457. [DOI] [PubMed] [Google Scholar]
- 22. Martinkova J, Quevenco FC, Karcher H, et al. Proportion of women and reporting of outcomes by sex in clinical trials for Alzheimer disease: a systematic review and meta‐analysis. JAMA Netw Open. 2021;4(9):e2124124. [DOI] [PubMed] [Google Scholar]
- 23.International Committee of Medical Journal Editors. Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. 2019. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information of this article.


