Abstract
A Thermus thermophilus selector strain for production of thermostable and thermoactive α-galactosidase was constructed. For this purpose, the native α-galactosidase gene (agaT) of T. thermophilus TH125 was inactivated to prevent background activity. In our first attempt, insertional mutagenesis of agaT by using a cassette carrying a kanamycin resistance gene led to bacterial inability to utilize melibiose (α-galactoside) and galactose as sole carbohydrate sources due to a polar effect of the insertional inactivation. A Gal+ phenotype was assumed to be essential for growth on melibiose. In a Gal− background, accumulation of galactose or its metabolite derivatives produced from melibiose hydrolysis could interfere with the growth of the host strain harboring recombinant α-galactosidase. Moreover, the AgaT− strain had to be Kms for establishment of the plasmids containing α-galactosidase genes and the kanamycin resistance marker. Therefore, a suitable selector strain (AgaT− Gal+ Kms) was generated by applying integration mutagenesis in combination with phenotypic selection. To produce heterologous α-galactosidase in T. thermophilus, the isogenes agaA and agaB of Bacillus stearothermophilus KVE36 were cloned into an Escherichia coli-Thermus shuttle vector. The region containing the E. coli plasmid sequence (pUC-derived vector) was deleted before transformation of T. thermophilus with the recombinant plasmids. As a result, transformation efficiency and plasmid stability were improved. However, growth on minimal agar medium containing melibiose was achieved only following random selection of the clones carrying a plasmid-based mutation that had promoted a higher copy number and greater stability of the plasmid.
α-Galactosidases catalyze the hydrolysis of α-1,6-linked α-galactose residues from oligosaccharides and polymeric galactomannans (9, 25, 26, 42). They have considerable potential in various industrial applications, e.g., in the sugar industry for the elimination of d-raffinose from sugar beet syrup. Due to the elevated temperatures used during the sugar manufacturing process, as well as in other industrial applications, stability and activity at high temperatures are important properties of α-galactosidases.
We have been studying α-galactosidases from various bacteria with regard to their application as oligosaccharide-hydrolyzing enzymes (9, 11, 14). Our intention was to subject α-galactosidase to thermoadaptation by introducing genes encoding enzymes inactive at high temperatures into a thermophilic bacterium for subsequent selection of enzyme variants active at high temperatures. We chose Thermus thermophilus as a host due to its high transformation ability (17) and ability to use melibiose (α-galactoside) as a sole carbohydrate source (10). Thermus species have been used for expression of heterologous genes and selection of thermostable enzyme variants (16, 19, 34, 36). They possess a natural transformation system (17) and are competent regardless of their growth phase (12). Genetic systems based on the application of autonomously replicating plasmids, as well as integration vectors or vectors containing cassettes, for chromosomal integration have been established (13, 18, 22, 23, 35, 40). So far, the only antibiotic selection markers described for Thermus bacteria are thermostabilized variants of the kanamycin nucleotidyltransferase gene derived from a thermophilic Bacillus gene (28) or from the kan gene of Staphylococcus aureus (24).
Expression of heterologous genes requires the inactivation of analogous genes in the host strain. In our previous work (10), we cloned the α-galactosidase gene (agaT) from T. thermophilus TH125 into Escherichia coli and subsequently disrupted the gene by site-specific integration of the kanamycin resistance marker into the agaT locus in the T. thermophilus chromosome. Sequence analysis of agaT along with flanking sequences revealed an open reading frame (ORF) downstream of and overlapping the agaT gene. The predicted translation product displayed similarity to galactose-1-phosphate uridylyltransferases (GalT) of E. coli (2) and Streptomyces lividans (1). The 3′ region of agaT and the 5′ region of galT were left intact in the site-specific integration due to the overlapping coding regions. However, characterization of the integration mutants revealed their inability to use melibiose as well as galactose. This indicated a polar transcriptional effect on the downstream galT gene. The polar effect was considered an obstruction for our purpose due to a potential growth inhibition effect of the accumulated galactose (or its metabolite derivatives) produced from melibiose hydrolysis in Gal− strains harboring recombinant α-galactosidases. Interference with growth by galactose has been described for Gal− mutants of, e.g., E. coli (30) and Bacillus subtilis (20).
In this paper, we describe the establishment of a Thermus strain suitable for production of heterologous α-galactosidases. Thereby, two problems were circumvented which restricted the use of the previously constructed agaT deletion strains (10): the galactose-negative phenotype and their kanamycin resistance, which otherwise prevented plasmid selection with the kanamycin marker. Further, we demonstrate the practical value of the strain established in this work for the production of recombinant α-galactosidases and as a potential selection system for α-galactosidases active at high temperatures.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
T. thermophilus TH125 (trpB5) (12) was generously provided by T. Hoshino. The Thermus strains were grown under strong aeration in mineral medium 162 (8) with 0.25% tryptone and 0.25% yeast extract at pH 7.5 (T162). Growth under nonselective conditions was carried out at 65 to 70°C. Growth was carried out at 60°C when the cultures contained kanamycin (20 μg ml−1) for selection of plasmid-containing cells. Growth of T. thermophilus TH125 on sole carbon sources was tested at 65 to 70°C on agar plates with minimal medium 162 (8) with a slight modification. Instead of titriplex I, EGTA was used as a chelating agent (15 mg liter−1). The medium contained galactose (0.3%) or melibiose (0.1, 0.2, or 0.4%) and 0.05% NH4Cl as carbon and nitrogen sources, respectively, biotin (50 μg liter−1), thiamine (1 mg liter−1), and tryptophan (50 μg ml−1) when needed. The pH was adjusted to 7.8. All of the E. coli plasmids constructed were brought into strain JM109 [supE44 Δ(lac-proAB) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 (F′ traD36 proAB laclqZΔM15)] (38) by transformation (6). Transformants were selected on agar plates either for ampicillin resistance (100 μg ml−1) or for kanamycin resistance (25 μg ml−1).
DNA manipulation and general plasmid construction.
Recombinant DNA techniques, i.e., plasmid preparation, subcloning, agarose gel electrophoresis, and Southern blotting, were performed by the conventional protocol (31). Structures of the plasmids were confirmed by restriction mapping, and the inserts of pOF5712, pOF5713, and pOF1172 were also confirmed by sequencing. Sequencing reactions of double-stranded DNA were carried out in accordance with the dideoxy-chain termination method with universal and internal primers (32). The plasmids used in this study are listed in Table 1.
TABLE 1.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pOF1154 | pUC18 with the NdeI restriction site deleted | This study |
| pOF1155 | kan as an NdeI-BamHI fragment between the 5′-flanking sequence and the 3′ region of agaT along with the 5′ sequence of galT in pOF1154 | This study |
| pOF1053 | Identical to pOF1155 except for an NdeI restriction site in the vector | This study |
| pOF1271 | galT along with an ∼1.3-kb upstream sequence from OF1053GD in pOF1154 | This study |
| pJOE930 | Positive selection vector | 3 |
| pIC20R | Apr, pBR322 ori, lacZ, EcoRI restriction sites on each side of a polylinker | 27 |
| pCG1 | agaA in pUC12 | 11 |
| pCG3 | agaB in pUC12 | 14 |
| pMY1 | Apr, kan downstream of PslpA, pUC, RepA | 23 |
| pOF1056 | kan downstream of PslpA in pJOE930 | This study |
| pBTac1 | Apr, pBR322 ori | 4 |
| pOF665 | kan downstream of Ptac in pBTac1 | This study |
| pOF477 | EcoRI site in pOF655 deleted | This study |
| pOF962 | 3′ region of agaA downstream of PslpA in pOF1154 | This study |
| pOF963 | 3′ region of agaB downstream of PslpA in pOF1154 | This study |
| pOF964 | agaA downstream of PslpA in pOF1154 | This study |
| pOF965 | agaB downstream of PslpA in pOF1154 | This study |
| pOF576 | repA from pMY1 on a ∼4.7-kb PstI fragment in pIC20R | This study |
| pOF578 | Ptac-kan amplified from pOF477 in pOF576 | This study |
| pOF5712 | agaA downstream of PslpA from pOF964 in pOF578 | This study |
| pOF5713 | agaB downstream of PslpA from pOF965 in pOF578 | This study |
| pOF5714 | pIC portion of pOF5712 deleted | This study |
| pOF5715 | pIC portion of pOF5713 deleted | This study |
| pOF5714M | Stable mutant plasmid of pOF5714 (plasmid stability in T. thermophilus) | This study |
| pOF10726 | pOF5714M linearized with EcoRI in pOF1154 | This study |
| pOF1172 | NdeI fragment from pOF10726 (agaA) replaced with NdeI fragment from pOF5713 (agaB) | This study |
| pOF1176 | pUC portion of pOF1172 deleted | This study |
Transformation of T. thermophilus.
The method of Koyama et al. (17), with a slight modification, was used for the transformation of T. thermophilus as previously described (9). Transformants were selected on T162-agar plates containing 20 μg of kanamycin ml−1 incubated at 60°C for 48 h (selection of agaT deletion strains) or on minimal 162-agar plates containing 0.3% galactose incubated for at 70°C for 4 to 7 days (selection of Gal+ strain OF1271).
Construction of Thermus expression plasmids.
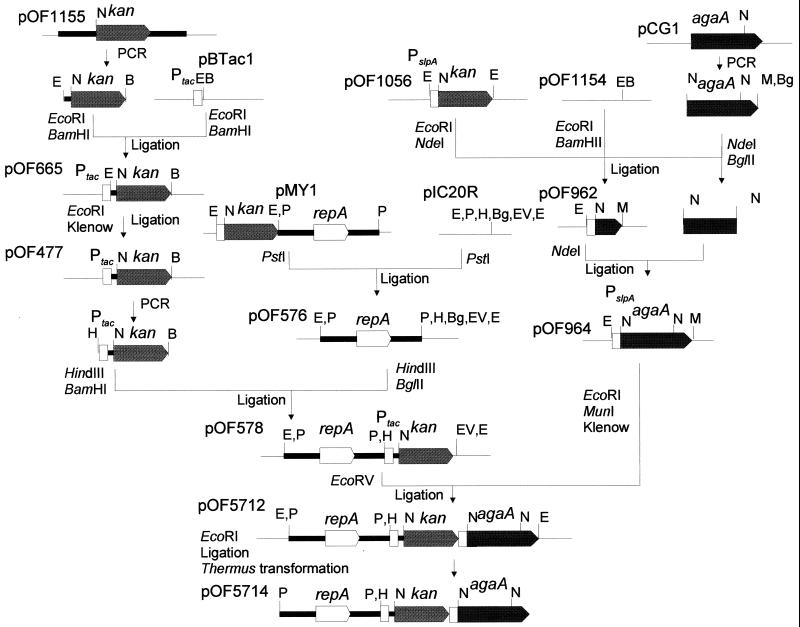
Thermus expression plasmids were constructed by cloning the α-galactosidase isogenes from Bacillus stearothermophilus KVE39 (11) downstream of the slpA promoter from T. thermophilus HB8 (22, 23) into a plasmid which contained the origin of replication from pMY1 (7) and a thermostable kanamycin marker (kan) (28). The construction of plasmid pOF5714, carrying an AgaA-encoding gene, agaA, is summarized in Fig. 1 and explained below. A BamHI-EcoRI fragment containing the kan gene downstream of the slpA promotor in pMY1 was made blunt ended and cloned into the EcoRV site of pJOE930 (3) to produce pOF1056. agaA from pCG1 (14) was amplified by PCR with primer S950 (GGAATTCCATATGTCAGTTGCATACAA), containing an NdeI site (underlined), and S952 (GAAGATCTCAATTGTCTTATTGTTGAACAG), with BglII and MunI sites (underlined). The gene was cloned into pOF1154 (a pUC18 derivative with the NdeI restriction site deleted) along with the slpA promotor from pOF1056. This was done in two steps. First, the 3′ region of agaA, an NdeI-BglII fragment, and the slpA promotor as an EcoRI-NdeI fragment was ligated into pOF1154 to produce pOF962. The 5′ region of agaA (NdeI fragment) was then ligated into the NdeI site of pOF962 to produce pOF964. pIC20R is a pUC-derived plasmid (38) with EcoRI restriction sites on each side of a polylinker (27). The pTSP1 portion of pMY1 (23) containing repA (minimal replication unit) (7) was cloned in pIC20R as a PstI fragment to produce pOF576. pOF1155 contains the kan gene (28) between the 5′-flanking sequence and the 3′ region of agaT along with the 5′ sequence of galT in pOF1154. The kanamycin marker with a preceding Thermus SD sequence in pOF1155 was amplified with primers S1065 (CGGAATTCTACCTGGGCGGCAAGGA), with an EcoRI site (underlined), and S718 (CGGGATCCGTCATCGTTCAAAATGG), with a BamHI site (underlined), and cloned, following restriction, between the EcoRI and BamHI restriction sites of pBTac1 (4) to produce pOF665. The EcoRI restriction site of pOF665 was deleted by performing EcoRI digestion, Klenow filling, and ligation to produce pOF477. The kanamycin resistance gene, along with the tac promoter (Ptac) in pOF477, was amplified in a PCR with primers S1318 (CCCCAAGCTTATCGACTGCACGGTG), with a HindIII site (underlined), and S718. The amplified Ptac-kan fragment, following HindIII-BamHI digestion, was ligated between the HindIII and BglII restriction sites of pOF576 to produce pOF578. The agaA gene downstream of the slpA promotor was cut from pOF964 with EcoRI and MunI, made blunt ended, and ligated into the EcoRV site of pOF578 to produce pOF5712. The pIC region of pOF5712 was deleted by EcoRI digestion, and the remaining plasmid was self-ligated before transformation of T. thermophilus. The corresponding agaB plasmid, pOF5715, was generated in the same way, except that plasmid pCG3 (14) was the initial source of agaB, which was amplified with primers S951 (GGAATTCCATATGGCGGTTACATACAA [the NdeI site is underlined]) and S952 (GAAGATCTCAATTGTCTTATTGTTGAACAG [the BglII and MunI sites are underlined]). pOF1176 carrying agaB2 is based on the stable plasmid mutant of pOF5714 (designated pOF5714M), which was brought back to E. coli by transformation following linearization with EcoRI and ligation into the pUC18 derivative pOF1154. The NdeI fragment from the resulting plasmid, pOF10726 (with an agaA sequence), was replaced with an NdeI fragment from pOF5713 (with an agaB sequence) to produce pOF1172. The pUC sequence of pOF1172 was deleted by digestion with EcoRI. The remaining plasmid, pOF1176, was recircularized by ligation and brought into T. thermophilus by transformation.
FIG. 1.
Construction of Thermus replication vector pOF5714. The procedure used is explained in Materials and Methods. Thin lines represent an E. coli cloning vector. Thick lines represent sequences from T. thermophilus, and genes are represented with pointed boxes. Restriction and modifying enzymes used for the plasmid constructions are indicated. Abbreviation for restriction enzyme sites: B, BamHI; Bg, BglII; E, EcoRI; H, HindIII; M, MunI; N, NdeI; P, PstI; EV, EcoRV.
Cloning of galT and the upstream sequence region from Gal+ strain OF1053GD.
The intact galT gene, along with an about 1.3-kb upstream sequence, in OF1053GD was amplified with primers S944 (CGGAATTCCGCCGCCATGGGAATT), with a EcoRI site (underlined), and S1167 (CCCAAGCTTGGCCGTCACGGCAAC), with a HindIII site (underlined), and cloned into a pUC18 derivative (pOF1154) to produce pOF1271.
Enzyme assays.
Cells of Thermus cultures were harvested by centrifugation, washed, and resuspended in 10 mM potassium phosphate buffer (pH 6.5). Crude extracts were prepared by sonication, and debris was removed by centrifugation. The protein concentration of crude extracts was estimated by the method of Bradford (5) using bovine serum albumin as the standard. α-Galactosidase activity was determined by measuring the rate of hydrolysis of para-nitrophenyl-α-d-galactoside (4 mg ml−1) in 0.1 M potassium buffer (pH 6.5) as previously described (11). One unit of activity is defined as the amount of enzyme that liberates 1 μmol of p-nitrophenol per min under given assay conditions. Colonies that displayed α-galactosidase activity were detected by histochemical staining. Single colonies were immobilized on a nylon membrane (Qiagen, Hilden, Germany). The membrane was placed on filter papers, saturated with phosphate buffer containing 6-bromo-2-naphthyl-α-d-galactopyranoside (0.5 mg ml−1) in a petri plate, and incubated at 50°C for 30 min in a water bath. Following the incubation, the membrane was again placed on a filter paper saturated with phosphate buffer containing Fast Blue RR (1.3 mg ml−1). Positive colonies became intensely purple within a few seconds. Production of the recombinant α-galactosidases in T. thermophilus was estimated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (21) of crude extracts.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the nucleotide sequences of agaA and agaB are AY013286 and AY013287, respectively.
RESULTS AND DISCUSSION
Selection of a Gal+ revertant from a Gal− strain.
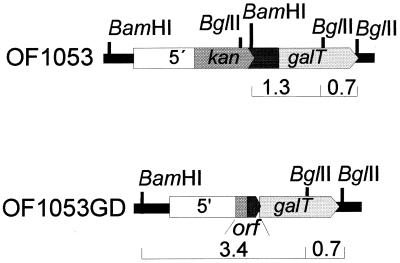
Deletion strain OF1053 (ΔagaT::kan) was constructed by applying site-specific integration mutagenesis as previously described (10), using an integration cassette with the kan marker located between the flanking sequences of agaT. The strain was unable to utilize galactose, which was interpreted as a polar transcriptional effect of the integration mutagenesis on the expression of the galT gene. The importance of the Gal+ phenotype for growth on melibiose minimal agar medium of AgaT− strains carrying recombinant α-galactosidases was revealed in our preliminary work by using Gal+ mutants that displayed amplification of the galT locus in the genome (data not shown). Consequently, the Gal+ phenotype was unstable. Gal+ mutants harboring recombinant α-galactosidase grew on minimal melibiose, whereas strains that had lost the ability to utilize galactose following deletion of the amplified galT locus were concurrently unable to grow on minimal melibiose medium. Subsequently, it was demonstrated that addition of galactose to the rich culture medium (T162) of Gal− strains promoted growth interference, whereas no growth interference was observed for Gal+ strains following the addition of galactose (results not shown). A Gal+ revertant of OF1053, designated OF1053GD, was isolated following incubation on galactose minimal medium at 70°C for 6 days. Southern hybridization of the mutant chromosomal DNA revealed a deletion of BglII-BamHI restriction sites upstream of the intact galT gene in strain OF1053 (data not shown). The intact galT gene, along with upstream sequences, was cloned as explained in Materials and Methods. The resulting plasmid was designated pOF1271. Sequence analysis of the cloned fragment revealed a deletion of a 1,257-bp fragment in the kan::agaT fusion gene, beginning 119 bp downstream of the start codon of the kan gene to 154 nucleotides upstream of the putative start codon of galT. The deletion resulted in the formation of a new ORF, from the start codon of the kan gene to a stop codon about 2 bp upstream the putative ribosome binding site of galT. Figure 2 shows a map of AgaT− strain OF1053 and deletion strain OF1053GD according to the restriction and sequence analysis. To demonstrate that the Gal+ phenotype of strain OF1053GD was dependent on galT and its upstream sequences, pOF1271 was used to transform Kmr Gal− strain OF1053 to strain OF1271 with a Kms Gal+ phenotype. The site-specific insertion of the integration module in pOF1271 into the chromosome of OF1053 was verified by Southern blotting (results not shown).
FIG. 2.
Map of strains OF1053 and OF1053GD. The 1-kb upstream region of agaT is represented by a open box, and corresponding genes by pointed boxes. Restriction fragments detected by Southern hybridization, referred to in Results, by using a galT fragment as a probe are indicated below the maps, along with their sizes in kilobases.
The fact that agaT overlaps galT in the progenitor strain T. thermophilus TH125 indicates translational coupling of those genes, which may explain the polar effect of the agaT insertional inactivation. Ribosome progression along the mRNA from agaT may be required to open the mRNA at the initiation site of galT, which is otherwise trapped in a secondary structure. Indeed, regions of dyad symmetry are observed in the 3′ region of agaT (Fig. 3). Following translation of kan in the integration mutants (OF1053), ribosomes are released about 750 nucleotides upstream of the ribosome binding site of galT. Thereby, the translational initiation site of galT may remain enclosed in a secondary structure, which blocks the access of a ribosome and eventually protein synthesis. The ORF preceding galT in OF1053GD (and OF1271) possibly contributes to the translational initiation of the galT gene by translation coupling. Translational coupling is known to occur at an intercistronic boundary of the E. coli galactose operon; i.e., the galK gene is translationally coupled to galT immediately preceding galK (33). Gene clusters containing closely linked or overlapping genes are a common feature of Thermus bacteria. This is generally true of organisms with small genomes (15, 29, 39). Due to the high GC content of Thermus RNA molecules, formation of stable folding structures at high temperatures is possible. Translational coupling with upstream ORFs may thus be an important factor in the expression of closely linked genes in a polycistronic message. Such a mechanism has been proposed to play a role in the regulated expression of the Thermus strain ZO5 pyr gene cluster (37).
FIG. 3.
Overlapping coding regions of agaT and galT in T. thermophilus TH125. The divergent broken arrows indicate regions of dyad symmetry. The ORF, of agaT and galT are shown as shaded boxes. The putative ribosome binding site (RBS) of galT is underlined, as well as a stop codon corresponding to the translation termination codon of the ORF preceding galT in strain OF1053GD. The GenBank accession number of the nucleotide sequence containing the α-galactosidase gene and flanking sequences in T. thermophilus TH125 is AF135399 (10).
Production of recombinant α-galactosidases in T. thermophilus OF1053GD.
agaA and agaB from B. stearothermophilus KVE36 were used as test genes for the expression of heterologous α-galactosidase genes in T. thermophilus. The respective gene products are designated AgaA and AgaB. Although the enzymes share 97% amino acid sequence identity, their properties are different (14). AgaA displays maximal hydrolyzing activity at 65 to 67°C under given assay conditions (14) and a high affinity for melibiose and raffinose. On the other hand, AgaB displays very low activity at 65 to 67°C (maximal hydrolyzing activity at 45 to 50°C) and a low affinity for melibiose and raffinose. The aim was to use AgaA as a positive control enzyme during the development of a selection system based on the coupling of cell growth with enzyme activity. A future approach will be the thermoadaptation of AgaB, i.e., selection for enhanced activity at elevated temperatures in the thermophilic bacterium T. thermophilus.
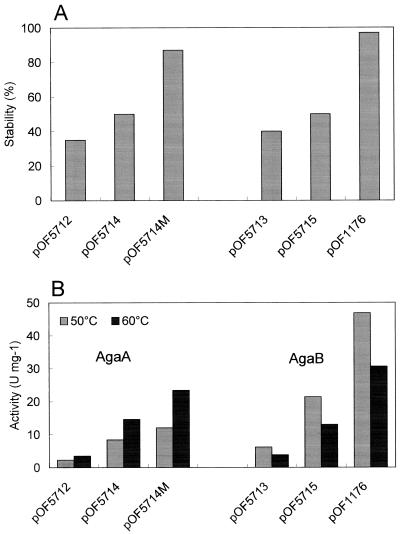
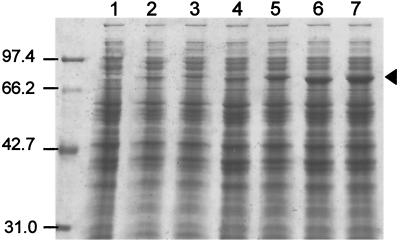
Plasmids containing the α-galactosidase genes downstream of the slpA promoter from plasmid pMY1 (23) were constructed as described in Materials and Methods and Fig. 1. The kan gene (28) with a Thermus ribosome binding site downstream of a the E. coli tac hybrid promoter (4) was used as a plasmid selection marker. The origin of replication came from a pTSP1 portion of pMY1 (7). The pIC region (E. coli plasmid sequence) of pOF5712 and pOF5713 was deleted by EcoRI digestion, and the remaining plasmid segments were recircularized by ligation (pOF5714 and pOF5715) before the transformation of T. thermophilus. In this way, the transformation efficiency was 1 order of magnitude higher than that of plasmids without the deletion (pOF5712 and pOF5713; ∼103 versus 102 transformants per μg of DNA, respectively). Furthermore, the E. coli plasmid sequences contributed to the instability of the shuttle vector in strain OF1053GD (Fig. 4A). How they affected the shuttle vector's stability remains unclear. However, such an effect is known to occur in other shuttle vector systems, e.g., for E. coli-Streptomyces (41). Strain OF1053GD harboring pOF5714 (agaA with the pIC sequence deleted), however, exhibited poor growth on agar medium containing melibiose, even though further selection with kanamycin was applied (data not shown). A mutant was isolated that grew significantly faster than the wild type on minimal melibiose agar medium. Colonies appeared following 4 days of incubation, compared to 7 to 8 days for the wild type. Higher α-galactosidase activity, observed in crude extracts of the mutant strain compared to the progenitor (Fig. 4B), correlated with a higher concentration of the recombinant enzyme in the crude extract, consistent with the intensity of a band detected by SDS-polyacrylamide gel electrophoresis (Fig. 5). The plasmid was isolated from this strain and introduced into plasmid-free strain OF1053GD. The resulting strain grew on minimal melibiose agar plates and displayed α-galactosidase activity identical to that of the original mutant strain. Moreover, the stability of the mutant plasmid was significantly higher than that of the progenitor plasmid (Fig. 4A). The copy number of the plasmids in Thermus cells in the exponential growth phase was determined. The number was about threefold higher in cells carrying the mutant plasmid than in cells carrying the progenitor (15 to 16 versus 5 to 6 for pOF5714M and pOF5714, respectively). More than 90% of the T. thermophilus OF1053GD cells transformed with pOF5714M contained the plasmid following overnight cultivation (14 h) in a nonselective medium. Further, the plasmid supported the growth of T. thermophilus OF1053GD on a minimal agar medium containing melibiose. Analysis of this plasmid mutation will be the subject of another study.
FIG. 4.
(A) Plasmid stability in strain OF1053GD. Stability was defined as the titer of cells with α-galactosidase activity against the total cell titer following overnight growth in T162 nonselective medium. OF1053GD strains harboring the different α-galactosidase plasmids were cultivated in T162 kanamycin medium. Mid-exponential-phase cells were diluted in nonselective T162 medium (to 5 × 106 cells ml−1). Following cultivation at 67°C for 14 h, the cells were diluted and plated on nonselective T162-agar medium (triplicates). The plates were incubated at 67°C for 2 days for growth of single colonies. Colonies that displayed α-galactosidase activity were identified by histochemical staining as explained in Materials and Methods. The mean values are indicated by column height. Maximum variation was less than 5%. (B) α-Galactosidase activity in crude extracts of OF1053GD cells harboring different plasmids containing α-galactosidase genes and cultivated as explained above. Activity tests were done in triplicate. The maximum variation from the mean values (shown) was less than 5%. pOF5712, shuttle vector; pOF5714, vector following deletion of E. coli pIC sequences by EcoRI digestion and self-ligation; pOF5714M, a stable mutant plasmid. pOF5713, pOF5715, and pOF1176 are the corresponding AgaB-type plasmids.
FIG. 5.
SDS–10% polyacrylamide gel with crude extracts from OF1053GD. Strains harboring different α-galactosidase plasmids were cultivated in T162 nonselective medium as explained in the legend to Fig. 4 for the stability experiments. Crude extracts were prepared, and 10 μg of protein was loaded in each lane of the SDS-gel. Lanes: 1, strain without plasmid; 2, strain harboring pOF5712 (with the E. coli pIC plasmid sequence); 3, pOF5713 (with pIC sequences); 4, pOF5714 (pIC sequence deleted); 5, pOF5715 (pIC sequence deleted); 6, pOF5714M (stable plasmid mutant containing agaA); 7, pOF1176 (stable plasmid containing agaB2). Molecular mass markers are on the left of lane 1, and the sizes (in kilodaltons) of the marker proteins are indicated. Bands attributed to the ∼80 kDa AgaA and AgaB2 proteins are indicated by the arrowhead.
The corresponding stable AgaB-type plasmid (pOF1176 [agaB2]) was constructed from pOF5714M. The structure of the inserted α-galactosidase gene in the corresponding shuttle vector, pOF1172, was verified by sequence analysis. Although the 3′ region of the gene was derived from agaA, the gene product, designated AgaB2, exhibited the characteristic properties of AgaB, such as optimum activity at a temperature of 50°C and a low affinity for melibiose and raffinose. Growth on minimal melibiose agar medium of strains harboring α-galactosidases AgaA and AgaB2 was tested under different conditions (temperature and melibiose concentration). The results are summarized in Table 2. The host strains harboring pOF5714M grew well at 67°C on agar medium with all of the concentrations of melibiose tested. The strains harboring pOF1176 did not grow at 67°C on 0.1 and 0.2% melibiose minimal agar medium.
TABLE 2.
Growth of T. thermophilus OF1053GD with pOF5714M or pOF1176 on 162 minimal agar medium containing various melibiose concentrationsa
| Plasmid | Growth at 60°C at a [melibiose] of:
|
Growth at 67°C at a [melibiose] of:
|
||||
|---|---|---|---|---|---|---|
| 0.4% | 0.2% | 0.1% | 0.4% | 0.2% | 0.1% | |
| pOF5714M | +++ | +++ | +++ | +++ | +++ | +++ |
| pOF1176 | +++ | ++ | + | + | − | − |
+++, growth following 3 to 4 days of incubation; ++, growth following 5 to 6 days of incubation; +, growth following 7 days of incubation; −, no growth.
Concluding remarks.
We succeeded in establishing a strain suitable for the expression of heterologous α-galactosidase genes, which enables selection based on the coupling of growth with enzyme activity, i.e., an AgaT− strain that is capable of metabolizing galactose and permits the application of the kan marker for plasmid selection. The results presented in this paper demonstrate that T. thermophilus can be used for the expression of heterologous α-galactosidase genes. Recombinant α-galactosidases can support the growth of agaT deletion-containing strains on minimal agar medium containing melibiose as a sole carbohydrate source. Although T. thermophilus OF1053GD/pOF5714M (agaA) grows slowly on such a medium, selection of thermostable enzyme mutants, e.g., from AgaB2, should be possible. By varying the temperature and melibiose concentration, we established growth conditions suitable for the thermoadaptation of AgaB2. Work dealing with the selection of thermostable enzyme variants by using this thermophile is in progress.
ACKNOWLEDGMENTS
We thank Gisela Kwiatkowski for technical assistance and Josef Altenbuchner and Joachim Klein for critical reading of the manuscript. Also, we thank T. Hoshino for T. thermophilus strain TH125 and J. Berenguer for plasmid pMY1.
This work was supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie.
REFERENCES
- 1.Adams C W, Fornwald J A, Schmidt F J, Rosenberg M, Brawner M E. Gene organization and structure of the Streptomyces lividans gal operon. J Bacteriol. 1988;170:203–212. doi: 10.1128/jb.170.1.203-212.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhya S. The galactose operon. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 1503–1512. [Google Scholar]
- 3.Altenbuchner J, Viell P, Pelletier I. Positive selection vectors based on palindromic DNA sequences. Methods Enzymol. 1992;216:457–466. doi: 10.1016/0076-6879(92)16042-i. [DOI] [PubMed] [Google Scholar]
- 4.Amann E, Brosius J, Ptashne M. Vectors bearing a hybrid trp-lac-promoter useful for regulated expression of cloned genes in Eshcerichia coli. Gene. 1983;25:167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Chung C T, Niemela S L, Miller R H. One-step preparation of competent Escherischia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Grado M, Lasa I, Berenguer J. Characterization of a plasmid replicative origin from an extreme thermophile. FEMS Microbiol Lett. 1998;165:51–57. doi: 10.1111/j.1574-6968.1998.tb13126.x. [DOI] [PubMed] [Google Scholar]
- 8.Degryse E, Glansdorff N, Piérard A. A comparative analysis of extreme thermophilic bacteria belonging to the genus Thermus. Arch Microbiol. 1978;117:189–196. doi: 10.1007/BF00402307. [DOI] [PubMed] [Google Scholar]
- 9.Fridjonsson O, Watzlawick H, Gehweiler A, Rohrhirsch T, Mattes R. Cloning of the gene encoding a novel thermostable α-galactosidase from Thermus brockianus ITI360. Appl Environ Microbiol. 1999;65:3955–3963. doi: 10.1128/aem.65.9.3955-3963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridjonsson O, Watzlawick H, Mattes R. The structure of the α-galactosidase gene loci in Thermus brockianus ITI360 and Thermus thermophilus TH125. Extremophiles. 2000;4:23–33. doi: 10.1007/s007920050004. [DOI] [PubMed] [Google Scholar]
- 11.Ganter C, Böck A, Buckel P, Mattes R. Production of thermostable recombinant α-galactosidase suitable for raffinose elimination from sugar beet syrup. J Biotechnol. 1988;8:301–310. [Google Scholar]
- 12.Hidaka Y, Hasegawa M, Nakahara T, Hoshino T. The entire population of Thermus thermophilus cells is always competent at any growth phase. Biosci Biotech Biochem. 1994;58:1338–1339. doi: 10.1271/bbb.58.1338. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino T, Maseda H, Nakahara T. Plasmid marker rescue transformation in Thermus thermophilus. J Ferment Bioeng. 1993;76:276–279. [Google Scholar]
- 14.Janz L, Ganter C, Stezowski J, Mattes R. Elucidation of functional domains in thermostable isoenzymes of α-galactosidase in Bacillus stearothermophilus. Enzymatic properties are encoded in a genetically exchangeable domain. In: Reuss M, Chmiel H, Gilles E-D, Knackmuss H-J, editors. Biochemical engineering—Stuttgart. Stuttgart, Germany: Gustav Fischer; 1991. pp. 170–173. [Google Scholar]
- 15.Kosuge T, Tabata K, Hoshino T. Molecular cloning and sequence analysis of the proBA operon from an extremely thermophilic eubacterium Thermus thermophilus. FEMS Microbiol Lett. 1994;123:55–62. doi: 10.1111/j.1574-6968.1994.tb07201.x. [DOI] [PubMed] [Google Scholar]
- 16.Kotsuka T, Akanuma S, Tomuro M, Yamagishi A, Oshima T. Further stabilization of 3-isopropylmalate dehydrogenase of an extreme thermophile, Thermus thermophilus, by a suppressor mutation method. J Bacteriol. 1996;178:723–727. doi: 10.1128/jb.178.3.723-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama Y, Hoshino T, Tomizuka N, Furukawa K. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J Bacteriol. 1986;166:338–340. doi: 10.1128/jb.166.1.338-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama Y, Arikawa Y, Furukawa K. A plasmid vector for an extreme thermophile, Thermus thermophilus. FEMS Microbiol Lett. 1990;72:97–102. doi: 10.1016/0378-1097(90)90352-q. [DOI] [PubMed] [Google Scholar]
- 19.Koyama Y, Okamoto S, Furukawa K. Cloning of α- and β-galactosidase genes from an extreme thermophile, Thermus strain T2, and their expression in Thermus thermophilus HB27. Appl Environ Microbiol. 1990;56:2251–2254. doi: 10.1128/aem.56.7.2251-2254.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krispin O, Allmansberger R. The Bacillus subtilis galE gene is essential in the presence of glucose and galactose. J Bacteriol. 1998;180:2265–2270. doi: 10.1128/jb.180.8.2265-2270.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Lasa I, Caston J R, Fernandez-Herrero L A, de Pedro M A, Berenguer J. Insertional mutagenesis in the extreme thermophilic eubacteria Thermus thermophilus HB8: expression of a heterologous, plasmid-borne kanamycin nucleotidyl-transferase gene. Appl Environ Microbiol. 1992;58:421–425. [Google Scholar]
- 23.Lasa I, de Grado M, de Pedro M A, Berenguer J. Development of Thermus-Escherichia shuttle vectors and their use for expression of the Clostridium thermopcellum celA gene in Thermus thermophilus. J Bacteriol. 1992;174:6424–6431. doi: 10.1128/jb.174.20.6424-6431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao H, McKenzie T, Hageman R. Isolation of a thermostable enzyme variant by cloning and selection in a thermophile. Proc Natl Acad Sci USA. 1986;83:576–580. doi: 10.1073/pnas.83.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luonteri E, Tenkanen M, Viikari L. Substrate specificities of Penicillium simplicissimum α-galactosidases. Enzyme Microb Technol. 1998;22:192–198. doi: 10.1016/s0141-0229(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 26.Margolles-Clark E, Tenkanen M, Luonteri E, Penttilä M. Three α-galactosidase genes of Thrichoderma reesei cloned by expression in yeast. Eur J Biochem. 1996;240:104–111. doi: 10.1111/j.1432-1033.1996.0104h.x. [DOI] [PubMed] [Google Scholar]
- 27.Marsh J L, Erfle M, Weykes E J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984;32:481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura M, Katakura Y, Imanaka T, Shuichi A. Enzymatic and nucleotide sequence studies of a kanamycin-inactivation enzyme encoded by a plasmid from thermophilic bacilli in comparison with that encoded by plasmid pUB110. J Bacteriol. 1984;160:413–420. doi: 10.1128/jb.160.1.413-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeiffer T, Jorcke D, Feltens R, Hartmann R K. Direct linkage of str-, S10- and spc-related gene clusters in Thermus thermophilus HB8, and sequences of ribosomal proteins L4 and S10. Gene. 1995;167:141–145. doi: 10.1016/0378-1119(95)00698-2. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg D, Keston A S. Interference with growth of galactokinaseless Escherichia coli mutants by galactose. Arch Biochem Biophys. 1967;120:239–241. doi: 10.1016/0003-9861(67)90627-3. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;7:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schumperli D, McKenney K, Sobieski D A, Rosenberg M. Translational coupling at an intercistronic boundary of the Escherichia coli galactose operon. Cell. 1982;30:865–871. doi: 10.1016/0092-8674(82)90291-4. [DOI] [PubMed] [Google Scholar]
- 34.Tamakoshi M, Yamagishi A, Oshima T. Screening of stable proteins in an extreme thermophile Thermus thermophilus. Mol Microbiol. 1995;16:1031–1036. doi: 10.1111/j.1365-2958.1995.tb02328.x. [DOI] [PubMed] [Google Scholar]
- 35.Tamakoshi M, Uchida M, Tanabe K, Fukuyama S, Yamagishi A, Oshima T. A new Thermus-Escherichia coli shuttle integration vector system. J Bacteriol. 1997;179:4811–4814. doi: 10.1128/jb.179.15.4811-4814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Touhara N, Taguchi H, Koyama Y, Ohta T, Matsuzawa H. Production and extracellular secretion of aqualysin 1 (a thermophilic subtilisin type protease) in a host-vector system for Thermus thermophilus. Appl Environ Microbiol. 1991;57:3385–3387. doi: 10.1128/aem.57.11.3385-3387.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van de Casteele M, Chen P, Roovers M, Legrain C, Glansdorff N. Structure and expression of a pyrimidine gene cluster from the extreme thermophile Thermus strain ZO5. J Bacteriol. 1997;179:3470–3481. doi: 10.1128/jb.179.11.3470-3481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vieira J, Messing J. The pUC plasmids and M13mp7 derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 39.Vornlocher H P, Kreutzer R, Sprinzl M. Organization of the Thermus thermophilus nusA/infB operon and overexpression of the infB gene in Escherichia coli. Biochimie. 1997;79:195–203. doi: 10.1016/s0300-9084(97)83506-7. [DOI] [PubMed] [Google Scholar]
- 40.Weber J M, Johnson S P, Vonstein V, Casadabanand M J, Demirjian D C. A chromosome integration system for stable gene transfer into Thermus flavus. Bio/Technology. 1995;13:271–275. doi: 10.1038/nbt0395-271. [DOI] [PubMed] [Google Scholar]
- 41.Wohlleben W, Pühler A. The Streptomyces ghanaensis low copy plasmid pSG2 and its use for vector construction. Arch Microbiol. 1987;148:298–304. doi: 10.1007/BF00456708. [DOI] [PubMed] [Google Scholar]
- 42.Zeilinger S, Kristufek D, Arisan-Atac I, Hodits R, Kubicek C P. Conditions of formation, purification, and characterization of an α-galactosidase of Trichoderma reesei RUT C-30. Appl Environ Microbiol. 1993;59:1347–1353. doi: 10.1128/aem.59.5.1347-1353.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]