Abstract
Introduction
Paravalvular leak (PVL) is a well‐recognized complication after mitral valve replacement (MVR). However, there are only a few studies analyzing leak occurrence and postoperative results after surgical MVR. The aim of this study was to assess the rate and determinants of early mitral PVL and to evaluate the impact on survival.
Methods
We performed a retrospective analysis involving patients who underwent MVR from January 2012 to December 2019 at our Institution. Postoperative transthoracic echocardiography evaluation was done for all subjects before hospital discharge. Multivariable analysis was carried out by constructing a logistic regression model to identify predictors for PVL occurrence.
Results
Four hundred ninety‐four patients were enrolled. Operative mortality was 4.9%. Early mitral PVL was found in 16 patients (3.2%); the majority were mild (75%). Leaks occurred more frequently along the posterior segment of the mitral valve annulus (62.5%). Only one individual with moderate‐to‐severe PVL underwent reoperation during the same hospital admission. Multivariable analysis revealed that preoperative diagnosis of infective endocarditis was the only factor associated with early leak after MVR (odds ratio: 4.96; 95% confidence interval: 1.45–16.99; p = .011). Overall mortality at follow‐up (mean follow‐up time: 4.7 [SD: 2.5] years) was 19.6% and favored patients without early mitral PVL.
Conclusion
The incidence of early PVL after MVR is low. PVL is usually mild and develop more frequently along the posterior segment of the mitral valve annulus. Preoperative diagnosis of infective endocarditis increases the risk of PVL formation.
Keywords: mitral regurgitation, mitral valve replacement, paravalvular leak
Abbreviations
- CPB
cardiopulmonary bypass
- IABP
intra‐aortic balloon pump
- MV
mitral valve
- MVA
mitral valve annulus
- MVR
mitral valve replacement
- PVL
paravalvular leak (or leaks)
- SD
standard deviation
- TEE
transesophageal echocardiography
- TMVR
transcatheter mitral valve replacement
- TTE
transthoracic echocardiography
1. INTRODUCTION
Paravalvular regurgitation or leak (PVL) is a known complication of surgical valve replacement characterized by eccentric jets originating from the outside of the sewing ring. The PVL can result from technical failure, which can be identified intraoperatively or by early postoperative echocardiographic assessment. It may also develop as a late complication of valvular operation as a consequence of suture dehiscence caused by prosthetic infection or the gradual resorption of incompletely debrided annular calcification. 1 Surgical mitral valve replacement (MVR) has a reported incidence of early PVL between 1.9% and 8%. 2 , 3 Although most PVL are trivial‐to‐mild and have a benign course, moderate‐to‐severe PVL may cause hemolysis, heart failure through MV regurgitation, or both. To our knowledge, only a few papers have analyzed mitral PVL incidence and pattern profiles, and little attention has been paid to the potential relationship between anatomical and surgical factors and PVL occurrence. Our retrospective study, therefore, attempted to assess the incidence and risk factors of early PVL after conventional MVR, and to evaluate the impact on survival. Further, we separated the PVL incidence for infectious endocarditis versus noninfective endocarditis patients as a benchmark for emerging approaches such as transcatheter‐MVR (TMVR).
2. MATERIALS AND METHODS
2.1. Ethical statement
The institutional review board approved submission and publication of the retrospective study and waived the need for informed patient consent.
2.2. Study design and population
This was an observational retrospective single‐center analysis in which we collected data from patients who underwent isolated or combined surgical MVR at Circolo Hospital between January 2012 and December 2019. Inclusion and exclusion criteria are described in Table 1. Demographic parameters (e.g., age, sex, and comorbidities) were collected. The etiology of mitral valve disease, including active infective endocarditis, degenerative regurgitation, ischemic/functional tethering, and rheumatic disease was ascertained. The type of prosthesis implanted, associated surgery, and duration of operation were recorded. In‐hospital mortality and main postoperative complications were also collected. Most of the subjects had a complete transesophageal echocardiographic (TEE) evaluation intraoperatively after weaning from cardiopulmonary bypass (CPB). In our institution, transthoracic echocardiogram (TTE) is performed systematically after MVR, before discharge, as a part of the clinical follow‐up. Patients who died in the hospital were excluded from the analysis if a postoperative TTE was not performed. Operative mortality was defined as any death within 30 days after surgery or during the same hospital admission. Information about long‐term survival was obtained from the Regional Institutional Health Database System. The study protocol was approved by the Institutional Ethics Committee (No. 6/2020).
Table 1.
Study inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Adult patients (≥18 years) underwent conventional MVR | Pediatric patients (<18 years old) |
| Isolated or combined MVR | MVR for congenital valve disease or anomalies |
| MVR for native or prosthetic mitral valve disease (redo) | Minimally invasive mitral valve surgery |
| MVR in elective, urgent, or emergent clinical status | Transcatheter MVR |
| Postoperative TTE evaluation before hospital discharge | Patients dead in‐hospital in which at least one postoperative TTE was not available |
Abbreviations: MVR, mitral valve replacement; TTE, transthoracic echocardiography.
2.3. Surgical procedure
All operations were performed under aorto‐bicaval cannulation, moderate systemic hypothermia, and cold cardioplegic arrest through median sternotomy. MVR was performed using an everting or noneverting pledgeted horizontal mattress suture technique. The use of continuous suture technique was avoided. If planned, concomitant cardiac procedures were performed in the standard fashion. The following parameters were documented by surgeon at the time of operation: the presence of annular calcification, the type of anatomopathological lesion, the size of the prosthetic valve, and the type of suture technique used for the implantation of the mitral valve prosthesis.
2.4. Echocardiography evaluation
Early mitral PVL was defined as PVL detected by postoperative TTE performed before hospital discharge. Echocardiography was performed with patients in the left lateral decubitus position. Color Doppler was used to assess the competence of prosthetic valves. A flow within the orifice of the prosthesis was considered transvalvular or physiologic regurgitation. Deviations from this pattern (i.e., asymmetric, or eccentric jet originating from outside of the sewing ring) were considered PVL. The severity of PVL was assessed semiquantitatively using visual estimation and graded from mild to severe, as recommended by the American Society of Echocardiography. 4 The location of the leakage was established by analyzing the mitral annulus in a clock‐wise format from a surgeon's view, as suggested by the De Cicco et al. 5
2.5. Statistical analysis
Values were expressed as mean (standard deviation) or counts and percentages. Differences between patients with and without early mitral PVL in baseline characteristics and intraoperative data were analyzed using χ 2 test or Fisher's exact test for categorical variables and Student's t test or Mann–Whitney U test for continuous variables, as appropriate. For continuous variables, the normality assumptions were verified with the Shapiro–Wilk tests. Multiple imputations was performed for missing values, including in the analysis only variables presenting up to 10% of missing data. Variables that had >10% missing values were nonessential to the purpose of the study and were not included in the analysis. Backward stepwise logistic regression was used to identify independent risk factors for early PVL, with the variables showing statistical significance (p < .2) in univariate analysis as covariates. Survival rates were estimated using Kaplan–Meier method and groups (patients with vs. patients without early mitral PVL) were compared on the basis of the log‐rank test. Statistical analyses were performed using the SPSS v.24.0 (Statistical Package for Social Science, SPSS Inc.). A p < .05 was considered statistically significant.
3. RESULTS
3.1. Baseline characteristics and surgical details
During the 8‐year study period, 494 patients (mean age 68.4 [SD: 11.3] years; 44.5% men) underwent MVR procedures at our Institution. Pre‐existing hypertension was the most common comorbidity (64.8%), followed by dyslipidemia (32.2%) and diabetes mellitus (14.6%). Clinical state at surgery was elective in 88.5% of cases. One hundred and sixty‐one patients (32.6%) underwent isolated MVR, while combined procedures were performed in 333 subjects (67.4%). A redo operation was undertaken in 75 individuals (15.2%). The most common underlying etiologies for MVR were degenerative (48.6%) and rheumatic valve disease (18.4%); active infective endocarditis accounted for 12.8% of the cases (Figure 1). Almost 20% of patients presented with calcification of the mitral valve annulus (MVA). In two‐thirds of the cases, the mitral valve was replaced with a bioprosthesis; 167 subjects (33.8%) received a mechanical valve. Noneverting pledgeted horizontal mattress sutures were used in 276 (55.9%) MVR procedures, while everting suture was applied in 218 (44.1%), according to surgeon's preference. Average CPB and aortic cross‐clamp time were 154.4 (SD: 55.5) minutes and 121.1 (SD: 45.3) minutes, respectively. TEE was performed intraoperatively in approximately 95% of patients, and no significant mitral PVL requiring immediate reintervention was detected. The demographic and operative data of the enrolled subjects are shown in Table 2.
Figure 1.
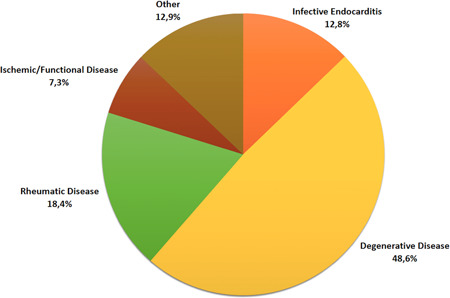
Proportion of patients undergoing mitral valve replacement for each underlying etiology of mitral valve disease
Table 2.
Baseline characteristics, operative data, and early outcome of the study patients
| Clinical characteristics | Total MVR (n = 494) | PVL absent (n = 478) | PVL present (n = 16) | p Value |
|---|---|---|---|---|
| Mean age (years) | 68.4 (11.3) | 68.3 (11.4) | 71.5 (5.6) | .27 |
| Age > 65 years | 331 (67) | 317 (66.3) | 14 (87.5) | .133 |
| Female | 275 (55.7) | 264 (55.2) | 11 (68.7) | .41 |
| Male | 219 (44.3) | 214 (44.8) | 5 (31.2) | |
| Mean BSA | 25 (4.8) | 25.1 (4.7) | 24.6 (8.3) | .73 |
| Surgical priority | ||||
| Elective | 437 (88.5) | 424 (88.7) | 13 (81.2) | .59 |
| Urgent/emergency | 57 (11.5) | 54 (11.3) | 3 (18.7) | .60 |
| Previous cardiac surgery | 75 (15.2) | 72 (15.1) | 3 (18.7) | .96 |
| Indication for MVR | ||||
| Stenosis | 139 (28.1) | 135 (28.2) | 4 (25) | .99 |
| Regurgitation | 292 (59.1) | 285 (59.6) | 7 (43.7) | .31 |
| Endocarditis | 63 (12.7) | 58 (12.1) | 5 (31.2) | .061 |
| Annular calcification | 99 (20) | 93 (19.5) | 6 (37.5) | .145 |
| Isolated MVR | ||||
| Yes | 161 (32.6) | 158 (33) | 3 (18.7) | .35 |
| No | 333 (67.4) | 320 (66.9) | 13 (81.2) | |
| Valve type | ||||
| Bioprosthetic | 327 (66.2) | 317 (66.3) | 10 (62.5) | .96 |
| Mechanical | 167 (33.8) | 161 (33.7) | 6 (37.5) | |
| Median valve size (mm) | 28.3 (2.3) | 28.3 (2.3) | 27.7 (2.3) | .32 |
| Type of interrupted suture | ||||
| Everting | 218 (44.1) | 212 (44.3) | 6 (37.5) | .77 |
| Noneverting | 276 (55.9) | 266 (55.7) | 10 (62.5) | |
| Type of atriotomy | ||||
| Single | 478 (96.8) | 463 (96.9) | 15 (93.7) | .98 |
| Biatrial | 16 (3.2) | 15 (3.1) | 1 (6.2) | |
| Mean CPB time (min) | 154.3 (55.5) | 154.3 (55.8) | 155.5 (47.3) | .93 |
| Mean cross‐clamp time (min) | 121.1 (45.3) | 120 (45.4) | 124.4 (42) | .70 |
| Operative mortality | 24 (4.9) | 21 (4.4) | 3 (18.7) | .037 |
Note: Data are shown as number (%) or mean (SD), as appropriate.
Abbreviations: BSA, body surface area; CPB, cardiopulmonary bypass; MVR, mitral valve replacement; PVL, paravalvular leak.
3.2. Outcomes and determinants of early PVL
Operative mortality rate was 4.9% (24 of 494 patients), with three early deaths occurring in the PVL group. The main postoperative complications included: acute kidney injury requiring hemofiltration (24; 4.9%), respiratory complications (72; 14.6%), new‐onset atrial fibrillation (206; 41.7%), bleeding requiring reoperation (30; 6.1%), stroke (6; 1.2%), and intra‐aortic balloon pump (IABP) support for low cardiac output syndrome (11; 2.2%). Early mitral PVL was detected in 16 subjects (3.2%); after excluding patients with preoperative endocarditis, PVL occurred in only 2.5% of MVR (11 patients). Twelve patients had mild PVL, one had mild‐to‐moderate and two had moderate PVL. One subject presented with moderate‐to‐severe regurgitation and underwent reintervention during the same hospital admission; the patient died from multiorgan failure on the first postoperative day. PVL occurred more frequently along the posterior segment of the MV annulus (10; 62.5%). PVL locations and severity are shown in Figure 2. Early mitral PVL was more frequent in older (>65 years old) patients (4.2% vs. 1.2%; p = .133), in subjects with infective endocarditis (7.9% vs. 2.5%; p = .061), and in those with mitral annular calcification (6% vs. 2.5%; p = .145). However, multivariable logistic regression analysis revealed that preoperative diagnosis of infective endocarditis was the only factor associated with the development of early mitral PVL (odds ratio: 4.96; 95% confidence interval: 1.45–16.99; p = .011; Table 3). During the follow‐up (mean follow‐up time: 4.7 [SD: 2.5] years) late deaths occurred in 73 patients (7.9%), with two deaths in patients with mitral PVL. No patients required reoperation for the progression of the PVL. Overall cumulative survival was compared based on the presence, or not, of early PVL after MVR. Analysis revealed that patients with PVL had poorer survival when compared to subjects with no PVL, but this trend did not reach statistical significance (p = .114; Figure 3).
Figure 2.
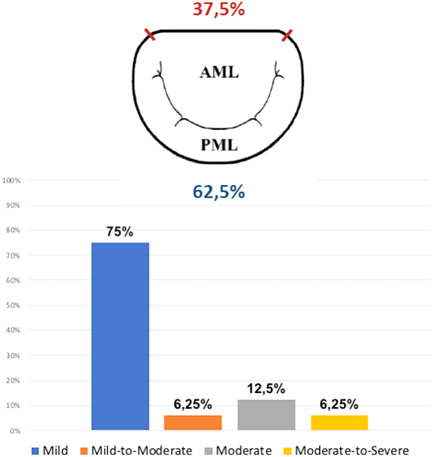
Location (above) and severity (below) of early paravalvular leak (PVL) after conventional mitral valve replacement
Table 3.
Multivariable logistic regression analysis to assess risk factors for early mitral PVL
| Variable | Univariate analysis | Multivariable analysis | |
|---|---|---|---|
| p Value | Odds ratio (95% CI) | p Value | |
| Age > 65 years | .133 | 0.26 (0.06–1.18) | .082 |
| Infective endocarditis | .061 | 4.96 (1.45–16.99) | .011 |
| Annular calcification | .145 | 2.86 (0.96–8.51) | .059 |
Abbreviations: CI, confidence interval; PVL, paravalvular leak.
Figure 3.
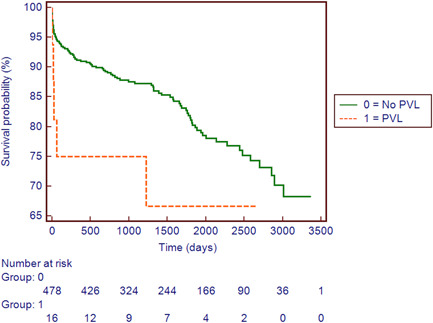
Kaplan–Meier estimates of survival stratified by presence or absence of early paravalvular leak (PVL) after standard mitral valve replacement
4. DISCUSSION
MVR is a common procedure in cardiac surgery with a low in‐hospital mortality rate and considerable benefits in terms of quality of life and long‐term survival. 6 Despite the refinement of surgical technique and recent advances in prosthesis design and tissue preservation, leading to more efficient and durable artificial heart valves, the postoperative course after MVR is not yet completely free of complications. One of the most frequent determinants of reoperation is PVL. 5 However, only a few reports in the available literature evaluated the incidence and predictors of early PVL after conventional MVR in contemporary series. 2 , 3 , 7 The main findings of the current study were as follows: (i) early PVL occurs in approximately 3% of MVR operations; (ii) most PVL (93.7%) after MVR are mild or moderate; (iii) the main risk factor for postoperative PVL development is preoperative infective endocarditis; (iv) such a complication develops more frequently (62.5%) in the posterior segment of the MV annulus.
PVL, a regurgitant flow between the sewing ring and the native valve annulus, can occur early or late, even several years, after valve replacement. The real incidence of mitral PVL is unclear and differs widely among registries. Indeed, the reported incidence ranges from 1.9% to 8% in contemporary series. 2 , 3 In the current analysis, mitral PVL in the early postoperative period was detected in 3.2% of the patients. Previous articles suggested that body surface area (BSA), suture technique, the presence of MVA calcification, and the type of the prosthesis were associated with mitral PVL. 2 , 8 , 9 However, we did not find such factors to reflect the previous findings. Indeed, the only independent predictor for PVL, in the present analysis, was preoperative diagnosis of infective endocarditis, which is in accordance with the study of Hwang and colleagues. 3 In the context of infective endocarditis, the presence of abscesses and fragile inflammatory tissue may cause leakage that progress with time. To avoid this complication, particularly in cases with locally uncontrolled infection, excision of infected and devitalized tissue should be followed by repair of associated defects to secure valve fixation. Besides, the development of a PVL in the early postoperative period, in a patient with preoperative diagnosis of infective endocarditis, may indicate a failure to control the infection. In patients with endocarditis, therefore, appropriate antibiotic therapy should be promptly instituted and surgery, whenever possible, postpone to allow 1 or 2 weeks of antibiotic treatment.
Although we observed a higher rate of early PVL in patients with mitral annular calcification, this correlation did not reach statistical significance in multivariable analysis. Notwithstanding, as also advocated by Yanartas et al., 10 we suggest that MV annulus calcifications should be completely removed, whenever feasible, at the time of surgery otherwise the residual calcium can cause a reason for the development of early PVL after MVR. Dhasmana and colleagues revealed the suture technique to be associated with a high incidence of mitral PVL. 8 Our study results were in disagreement with the latter study. This might be owing to the fact that we avoided the continuous suture technique, which has demonstrated to be related with PVL occurrence following valve replacement in the previous series. 8 , 11 Early PVL, in fact, is usually related to disruption of the annular tissue during the sewing ring suture. In an attempt to avoid such a serious complication, it's our practice to performed MVR using a pledgetted horizontal mattress suture technique. The findings of the present study seem to indicate that the pledgetted mattress suture, protecting the tissue from being cut through during tying, may be effective in preventing PVL.
Location of PVL around MV has been poorly investigated. De Cicco et al. 5 demonstrated that PVL occurs more frequently at the anterolateral and posteromedial segments of MVA. In our study, the most common leakage locations were around the posterior segment of the MVA. The collagen fibers distribution in the MVA is not homogeneous, either quantitatively or qualitatively. 12 In fact, a well‐formed chord‐like fibrous annulus is not present all around the MVA, particularly along the posterior sector of the annulus 13 ; the intrinsic anatomic weakness of the posterior segment of the annulus could explain the highest rate of PVL encountered in this area, as well as the proximity of the circumflex artery and the risk of damaging this vessel with sutures.
Managing patients with PVL is often challenging since there is no effective medical therapy, thus making reoperative cardiac surgery or percutaneous PVL closure the only two reasonable options. 5 , 14 Despite there is widespread agreement among cardiologists and surgeons alike that moderate‐to‐severe PVL should be corrected immediately, the management of subjects with mild‐to‐moderate and moderate leaks has not been elucidated yet. Furthermore, there is a paucity of data regarding the incidence of PVL and the time course in the available literature. Previous reports have shown that the majority of PVL after surgical valve replacement are clinically not significant and seem to have a benign course in the absence of endocarditis. 1 , 15 Early mild PVL, in fact, may spontaneously resolve with the endothelialization of the sewing ring. 15 In this study, patients with mild or moderate PVL at the time of hospital discharge did not require reintervention during follow‐up. However, the current analysis also demonstrated that individuals with mitral PVL had a trend, albeit not statistically significant, toward worse survival when compared to those without leakage. Our results mirror those of another study by Cho et al. who reported poor prognosis over time in patients with PVL after MVR, even with mild and moderate leaks. 16 Thus, in patients who develop > mild‐to‐moderate PVL early after MVR surgery, an aggressive therapeutic approach can be considered. Anyhow, due to the small sample size and the relatively short follow‐up period, further and dedicated studies are required to provide evidence and confirm such an assumption.
The present report confirms the direct link between MV annulus calcification and PVL. Mitral annular calcification is a well‐known challenge for cardiac surgeons. Interestingly, in recent years transcatheter MVR (TMVR) has been raised as an alternative approach to surgery for inoperable patients with severe mitral valve disease and extensive annular calcification. The initial data on follow‐up are encouraging, however, potential concerns may relate to the risks of the prothesis atrial displacement and PVL. Besides, because of the complexity of the mitral valve apparatus, TMVR poses unique and potentially fatal complications, such as left ventricular outflow tract obstruction. 17 This study provides important references with which TMVR can be compared. Indeed, we report the PVL rate for nonendocarditis patients, as a benchmark for alternative approaches.
The present study has certain limitations which deserve additional comment. First, it was a single‐center study in an academic medical center with inherent bias, thus the current results may not be generalized. Second, we only focused on the incidence of PVL in the immediate postoperative period, the late occurrence of leakage was not evaluated. Third, echocardiographic data were not evaluated by a centralized core echo laboratory; this may have caused some variability in the identification and localization of PVL, and grading of its severity. Even though 494 subjects were operated during the study period, the group of interest comprised of 16 individuals only; larger sample sizes are required to better establish the natural course of early PVL, and establish the impact on survival. Finally, only all‐cause mortality was assessed, specific causes of mortality were not investigated.
5. CONCLUSIONS
Early PVL represents a rare complication after conventional MVR. PVL occurs more frequently along the posterior segment of the MVA, and in the majority of the cases is mild. Preoperative diagnosis of infective endocarditis significantly increases the risk of PVL development.
CONFLICTS OF INTEREST
Roberto Lorusso consultant for Medtronic and LivaNova, and member of the Advisory Board of Eurosets and PulseCath. The remaining authors declare no conflicts of interest.
6. ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi dell'Insubria within the CRUI‐CARE Agreement.
Matteucci M, Ferrarese S, Cantore C, et al. Early paravalvular leak after conventional mitral valve replacement: a single‐center analysis. J Card Surg. 2022;37:1559‐1566. 10.1111/jocs.16422
REFERENCES
- 1. Duncan BF, McCarthy PM, Kruse J, et al. Paravalvular regurgitation after conventional aortic and mitral valve replacement: a benchmark for alternative approaches. J Thorac Cardiovasc Surg. 2015;150(4):860‐868. [DOI] [PubMed] [Google Scholar]
- 2. Verdonk C, Cimadevilla C, Lepage L, et al. Systematic transoesophageal echocardiography after mitral valve replacement: rates and determinants of paravalvular regurgitation. Arch Cardiovasc Dis. 2018;111(8‐9):528‐533. [DOI] [PubMed] [Google Scholar]
- 3. Hwang HY, Choi JW, Kim HK, Kim KH, Kim KB, Ahn H. Paravalvular leak after mitral valve replacement: 20‐year follow‐up. Ann Thorac Surg. 2015;100(4):1347‐1352. [DOI] [PubMed] [Google Scholar]
- 4. Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22(9):975‐1014. [DOI] [PubMed] [Google Scholar]
- 5. De Cicco G, Russo C, Moreo A, et al. Mitral valve periprosthetic leakage: Anatomical observations in 135 patients from a multicentre study. Eur J Cardiothorac Surg. 2006;30(6):887‐891. [DOI] [PubMed] [Google Scholar]
- 6. Schnittman SR, Itagaki S, Toyoda N, Adams DH, Egorova NN, Chikwe J. Survival and long‐term outcomes after mitral valve replacement in patients aged 18 to 50 years. J Thorac Cardiovasc Surg. 2018;155(1):96‐102. 10.1016/j.jtcvs.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 7. Hassanin N, Sharaf Y, Ammar W, Sayed AYH. Early postoperative paravalvular leak among Egyptian population: an observational study. J Saudi Heart Assoc. 2017;29(3):160‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhasmana JP, Blackstone EH, Kirklin JW, Kouchoukos NT. Factors associated with periprosthetic leakage following primary mitral valve replacement: with special consideration of the suture technique. Ann Thorac Surg. 1983;35(2):170‐178. [DOI] [PubMed] [Google Scholar]
- 9. O'Rourke DJ, Palac RT, Malenka DJ, Marrin CA, Arbuckle BE, Plehn JF. Outcome of mild periprosthetic regurgitation detected by intraoperative transesophageal echocardiography. J Am Coll Cardiol. 2001;38(1):163‐166. [DOI] [PubMed] [Google Scholar]
- 10. Yanartaş M, Demir S, Baysal A, et al. The relation between location of paravalvular leakage and time to reoperation after mitral valve replacement. Anadolu Kardiyol Derg. 2014;14(1):61‐67. [DOI] [PubMed] [Google Scholar]
- 11. Englberger L, Schaff HV, Jamieson WR, et al. AVERT Investigators. Importance of implant technique on risk of major paravalvular leak (PVL) after St. Jude mechanical heart valve replacement: a report from the Artificial Valve Endocarditis Reduction Trial (AVERT). Eur J Cardiothorac Surg. 2005;28(6):838‐843. [DOI] [PubMed] [Google Scholar]
- 12. Kunzelman KS, Cochran RP, Murphree SS, Ring WS, Verrier ED, Eberhart RC. Differential collagen distribution in the mitral valve and its influence on biomechanical behaviour. J Heart Valve Dis. 1993;2(2):236‐244. [PubMed] [Google Scholar]
- 13. Angelini A, Yen HoS, Thiene G, Anderson RH. Anatomy of the mitral valve. In: Boudoulas H, Wooley CF, eds. Mitral Valve: Floppy Mitral Valve, Mitral Valve Prolapse, Mitral Valvular Regurgitation. 2nd revised ed. Futura Publishing Company; 2000:14. [Google Scholar]
- 14. Perl L, Cohen A, Dadashev A, et al. Long‐term outcomes of catheter‐based intervention for clinically significant paravalvular leak. EuroIntervention. 2021;98:736‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matteucci M, Ferrarese S, Cantore C, et al. Early aortic paravalvular leak after conventional cardiac valve surgery: a single‐center experience. Ann Thorac Surg. 2020;109(2):517‐525. [DOI] [PubMed] [Google Scholar]
- 16. Cho IJ, Moon J, Shim CY, et al. Different clinical outcome of paravalvular leakage after aortic or mitral valve replacement. Am J Cardiol. 2011;107(2):280‐284. [DOI] [PubMed] [Google Scholar]
- 17. Yoon SH, Bleiziffer S, Latib A, et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2019;12(2):182‐193. [DOI] [PubMed] [Google Scholar]


