Abstract
Significant gaps remain in understanding the response of plant reproduction to environmental change. This is partly because measuring reproduction in long‐lived plants requires direct observation over many years and such datasets have rarely been made publicly available. Here we introduce MASTREE+, a data set that collates reproductive time‐series data from across the globe and makes these data freely available to the community. MASTREE+ includes 73,828 georeferenced observations of annual reproduction (e.g. seed and fruit counts) in perennial plant populations worldwide. These observations consist of 5971 population‐level time‐series from 974 species in 66 countries. The mean and median time‐series length is 12.4 and 10 years respectively, and the data set includes 1122 series that extend over at least two decades (≥20 years of observations). For a subset of well‐studied species, MASTREE+ includes extensive replication of time‐series across geographical and climatic gradients. Here we describe the open‐access data set, available as a.csv file, and we introduce an associated web‐based app for data exploration. MASTREE+ will provide the basis for improved understanding of the response of long‐lived plant reproduction to environmental change. Additionally, MASTREE+ will enable investigation of the ecology and evolution of reproductive strategies in perennial plants, and the role of plant reproduction as a driver of ecosystem dynamics.
Keywords: demography, flowering, general flowering, masting, plant reproduction, recruitment, regeneration
MASTREE+ is an open‐access data set that collates reproductive time‐series data from across the globe. It includes 73,828 georeferenced observations of annual reproduction (e.g. seed and fruit counts) in perennial plant populations, consisting of 5,971 population‐level time‐series from 974 species. MASTREE+ will provide the basis for improved understanding of the response of plant reproduction to environmental change and can be used to investigate the ecology and evolution of plant reproductive strategies and the role of plant reproduction as a driver of ecosystem dynamics.

Resumen
Aún existen importantes vacíos en la comprensión de la respuesta reproductiva de las plantas al cambio medioambiental, en parte, porque su monitoreo en especies de plantas longevas requiere una observación directa durante muchos años, y estos conjuntos de datos rara vez han estado disponibles. Aquí presentamos a MASTREE +, una base de datos que recopila series de tiempo de la reproducción de las plantas de todo el planeta, poniendo a disposición estos datos de libre acceso para la comunidad científica. MASTREE + incluye 73.828 puntos de observación de la reproducción anual georreferenciados (ej. conteos de semillas y frutos) en poblaciones de plantas perennes en todo el mundo. Estas observaciones consisten en 5971 series temporales a nivel de población provenientes de 974 especies en 66 países. La mediana de la duración de las series de tiempo es de 10 años (media = 12.4 años) y el conjunto de datos incluye 1.122 series de al menos dos décadas (≥20 años de observaciones). Para un subconjunto de especies bien estudiadas, MASTREE +incluye un amplio conjunto de series temporales replicadas en gradientes geográficos y climáticos. Describimos el conjunto de datos de acceso abierto disponible como un archivo.csv y presentamos una aplicación web asociada para la exploración de datos. MASTREE+ proporcionará la base para mejorar la comprensión sobre la respuesta reproductiva de plantas longevas al cambio medioambiental. Además, MASTREE+ facilitará los avances en la investigación de la ecología y la evolución de las estrategias reproductivas en plantas perennes y el papel de la reproducción vegetal como determinante de la dinámica de ecosistemas.
1. INTRODUCTION
Climate change and other anthropogenic drivers are altering plant demographics, with reported changes in plant mortality, growth and reproduction (Allen et al., 2010; McDowell et al., 2020; Pearse et al., 2017; Senf et al., 2018). These demographic shifts are changing the composition and structure of vegetation, with far‐reaching effects on ecosystem functioning and services, including complex effects on biodiversity and terrestrial carbon sinks (Carnicer et al., 2011; Chen et al., 2019; Clark et al., 2016; Ruiz‐Benito et al., 2017). In most plant species, seed production is a key process limiting sexual reproduction. However, our understanding of climate‐driven changes in seed production lags behind other key demographic processes such as growth and mortality (Clark et al., 2021), where inventory data, tree‐ring networks and remote sensing have transformed understanding of responses to environmental change (Buras et al., 2020; Changenet et al., 2021; Klesse et al., 2020). Reproduction and other processes associated with plant recruitment require direct and intensive field‐based observation over many years. However, there have been few previous attempts to collate, archive and make available original data from long‐term monitoring studies across taxa and wide geographic areas (Ascoli, Maringer, et al., 2017; Koenig & Knops, 2000; Pearse et al., 2020). Consequently, the response of plant reproduction to ongoing environmental change remains poorly understood, and paucity of data compromises the parameterisation of reproduction in models used to predict future vegetation dynamics (Fisher et al., 2018; Vacchiano et al., 2018).
Recent analysis of long‐term data sets indicates that seed production may be sensitive to climate change. Where increases in temperature favour reproduction, warming is linked to increased seed production (Bogdziewicz et al., 2020; Buechling et al., 2016; Caignard et al., 2017), whereas in drought‐limited populations seed production has declined in association with warming (Redmond et al., 2012). Additionally, environmental change may alter the interannual variability and spatial synchrony of reproduction (Hacket‐Pain & Bogdziewicz, 2021; Pearse et al., 2017). These shifts in reproduction have consequences for recruitment and wider ecosystem dynamics (Pau et al., 2018; Redmond et al., 2012; Schupp et al., 2019). For example, long‐term reductions in tropical rainforest fruit production have been linked with declining vitality of herbivorous megafauna (Bush et al., 2020), and low seed availability can limit forest recovery after large‐scale mortality events (Redmond et al., 2018). Beyond changes in mean seed and fruit production, shifts in the spatiotemporal variability of flowering and fruiting (i.e. masting) will also have impacts on key ecosystem services and habitat management (Pearse et al., 2021) including commercial and subsistence food crops (Calama et al., 2011; Ladio & Lozada, 2004; Shelef et al., 2017), seed‐eating animal population dynamics (Touzot et al., 2020), and human health through the trophic interactions that drive vector‐borne zoonotic disease dynamics (Bennett et al., 2010; Bregnard et al., 2020). However, the direction and magnitude of reported changes in masting are inconsistent, and this variability in response remains poorly understood (Hacket‐Pain & Bogdziewicz, 2021).
As the magnitude of plant reproduction is highly variable across time and space (Figure 1), multi‐decadal time‐series of plant reproductive effort with high replication and sampling across environmental gradients are needed to derive meaningful inferences and predictions from modelling efforts (Clark et al., 2021; Pearse et al., 2021; Pennekamp et al., 2019; Vacchiano et al., 2018). The availability of such data will enable robust estimates of the response of plant reproduction to recent environmental change, and through identification of the underlying drivers, prediction of future trends. MASTREE+ provides these time‐series of plant reproductive effort, and will enable testing of changes in masting patterns associated with recent environmental change across multiple species and geographical regions (Hacket‐Pain & Bogdziewicz, 2021; LaMontagne et al., 2021; Pearse et al., 2017). Such data sets will also enable new insights into the ecology and evolution of perennial plant reproduction (Dale et al., 2021), and the role of plant reproduction as a driver of other ecological processes including plant recruitment and animal population dynamics (Brumme et al., 2021; Connell & Green, 2000; Curran & Leighton, 2000; Schupp et al., 2019).
FIGURE 1.
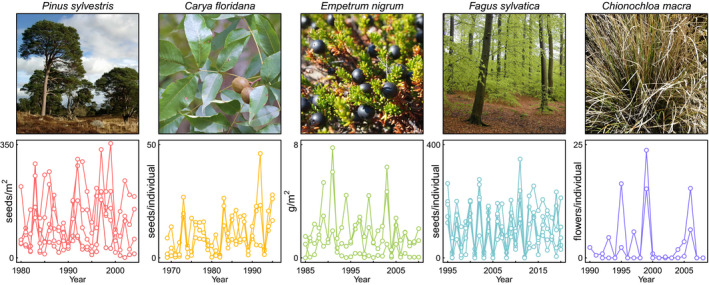
Examples of population‐level time‐series of reproductive effort from MASTREE+. For five diverse plant species, data from several local populations are plotted to illustrate the range of spatiotemporal variation in reproduction that is typical in long‐lived plants. Note that axis scales and units vary between plots
2. MASTREE+
Here we introduce a project to collate data of perennial plant reproductive time‐series. Time‐series originate from diverse sources, including 17th century European forestry records of seed production (‘mast years’) (Ascoli, Vacchiano, et al., 2017), data from ongoing plant reproductive biology and phenology monitoring programmes (e.g. RENECOFOR, LTER, California Acorn Survey), and projects studying the dynamics of ecosystems including the relationships between seed production and animal demographics (Boutin et al., 2006). Many of these data sets record the number or mass of flowers, seeds, fruits or cones per individual or unit area on a continuous scale. We also include ordinal time‐series, which record annual reproduction output according to an ordered categorical scale (e.g. failure/partial/full crop) which can be successfully used to investigate the variability and synchrony of plant reproduction (Bogdziewicz et al., 2021).
The current version of MASTREE+ currently includes 5971 species‐specific and georeferenced time‐series representing 73,828 annual observations of reproductive effort in perennial plant populations, and the project is designed to continue to assemble and update records (see Sections 4 and 5). Mean and median time‐series length are 12.6 and 10 years respectively. 2846 series are based on continuous measures of reproductive effort, and 3125 are ordinal series. Ordinal series originate mainly from Europe. Importantly, MASTREE+ contains 1122 time‐series ⋝20 years, of which 187 time‐series exceed 40 years of observations. Such records will enable quantification of recent changes in plant reproduction, including mean reproductive effort and spatiotemporal variability, and the identification of key drivers of change.
In total, 974 species are represented, drawn from 136 families across the plant Tree of Life. This increases species representation by 168% compared with the largest previously available compilation (Pearse et al., 2020), which is incorporated into MASTREE+. This expands the potential to quantify reproductive traits that describe the spatiotemporal variability of reproduction (i.e. masting) with other life‐history traits to better understand the evolution of plant reproductive strategies (Dale et al., 2021; Fernandez‐Martinez et al., 2019; Pesendorfer et al., 2021). For example, we have 67 species overlap with the plant demographic database COMPADRE (Salguero‐Gomez et al., 2015), 442 species overlap with seed mass data from the Kew Seed Information Database (Royal Botanic Gardens Kew, 2021) and 82 species overlap with the seed germination database SylvanSeeds (Fernandez‐Pascual, 2021). Reflecting a bias in sampling to temperate forests, woody species from the genera Quercus (60 species), Nothofagus (10), Pinus (25), Abies (13), Acer (13) and Eucalyptus (15) are highly represented, but other well‐represented genera include Acacia (11), Shorea (9) and Chionochloa (11). We include data from 66 countries, six continents (Figure 2), and from all the major vegetated biomes (Figure 3). Importantly, we increase data representation from regions that have been unrepresented in previous data sets (Ascoli, Maringer, et al., 2017; Pearse et al., 2020), including south and central America, Africa, and Asia, although these regions remain strongly under‐represented.
FIGURE 2.
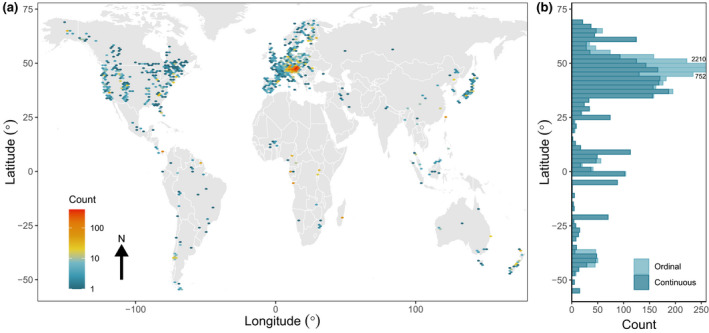
The geographical distribution of time‐series within MASTREE+. The (a) spatial and (b) latitudinal distribution of species‐specific time‐series. For (b), series are stacked and coloured according to the variable type (Continuous, Ordinal). Plotting of counts for ordinal data in the northern mid‐latitudes are truncated due to high sampling intensity in central Europe. Unprojected map, datum = WGS84
FIGURE 3.
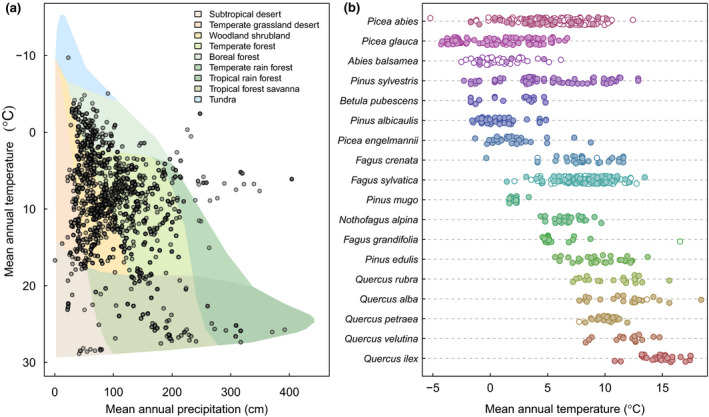
Distribution of time‐series in MASTREE+ according to local climate (Worldclim v2.1, 30 arcsecond resolution, Mosier et al., 2014). Only time‐series representing reproduction at the stand or patch scale are plotted (regional records are excluded, as local climate data based on coordinates may not be representative). (a) Series plotted according to Whittaker biomes (Whittaker, 1970) and (b) Species with high replication (≥20 species‐specific time‐series), plotted according to local mean annual temperature. Species are labelled according to the first three characters of the genus followed by the first three characters of the species name, and species are ordered according to the sample site with the lowest mean annual temperature. Unfilled points represent ordinal time‐series and filled points represent continuous time‐series
Sampling intensity varies between species. For example, 71% of species are represented by a single time‐series, but other species have high replication, often covering large parts of their geographical distribution. 51 species are represented by at least 10 location‐specific time‐series. The most replicated species are Fagus sylvatica (913 site‐specific time‐series), Picea abies (844), Pinus sylvestris (419), Larix decidua (395), Abies alba (393), Quercus robur (188), Quercus petraea (161), Pinus cembra (135) and Picea glauca (108). These and other well‐replicated species include data spanning large climatic gradients (Figure 3). These records will enable investigation of intraspecific variation in plant reproduction across climate, space, and time, including trends in the spatiotemporal variability of reproduction. It will also enable comprehensive assessments of intraspecific variability of masting characteristics (i.e. interannual variability, autocorrelation), including variation with environmental conditions that are predicted by theory but have rarely been tested (Pearse et al., 2020; Pesendorfer et al., 2021), and analysis of interspecific variation in spatial synchrony of reproduction (Dale et al., 2021), in functionally diverse plant species.
3. APPLICATIONS OF MASTREE+
MASTREE+ provides the data sets to establish how fecundity, and specifically seed masting, responds to environmental change. It includes the high replication of long time‐series required to isolate climate change effects on plant reproductive effort (Hacket‐Pain & Bogdziewicz, 2021; Mundo et al., 2021) (Figure 4), while high spatial replication across environmental gradients (e.g. Figure 3b) provides the opportunity for a complementary space‐for‐time substitution approach (Wion et al., 2020). The expected response of masting to climate change remains unresolved, and MASTREE+ will enable testing of contrasting predictions that masting will be unresponsive to trends in mean temperature (Kelly et al., 2013), or will shift predictably based on climate‐driven changes in resource limitation (Bogdziewicz, 2021). Resolving this uncertainty is a priority because changes in seed masting will impact plant reproductive success, and more widely affect ecosystem services and habitat management (Ida, 2021; Pearse et al., 2021; Touzot et al., 2020).
FIGURE 4.
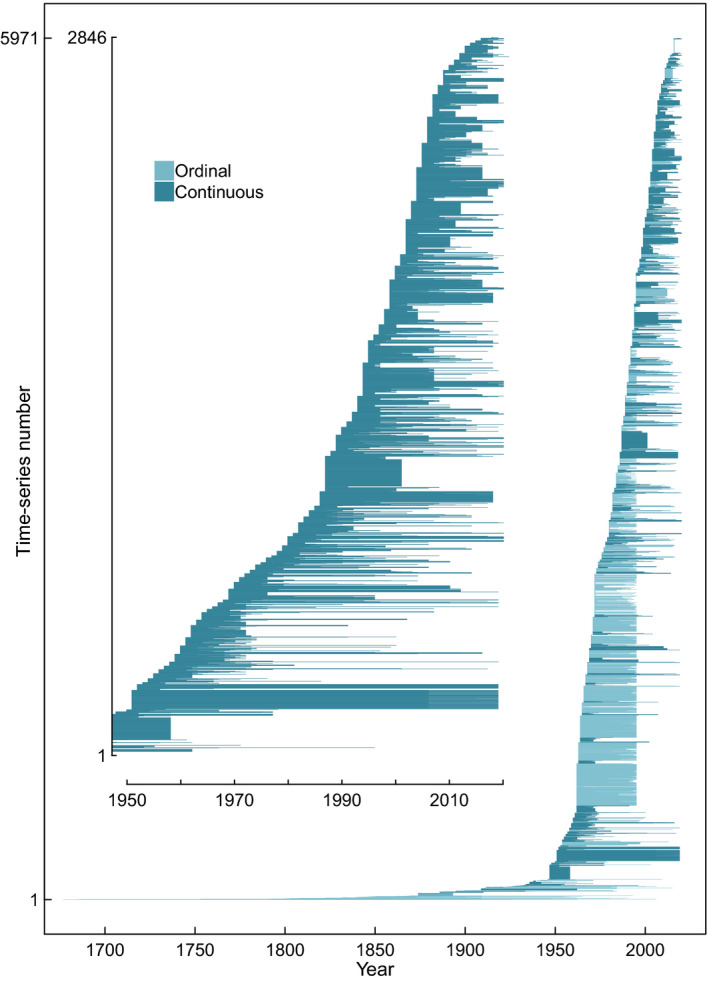
Timespans covered by species‐specific time‐series in MASTREE+, coloured by data class. Inset plot shows continuous data since 1950 when time‐series replication is highest
In systems where seed production limits recruitment, MASTREE+ can be utilised to understand the drivers of plant reproduction and regeneration (Abraham et al., 2018; Manríquez et al., 2016; Oliva et al., 2013). Intraspecific differences in fecundity and masting influence regeneration success, determining species composition and vegetation structure, including during the colonisation of new habitats (Joubert et al., 2013), and after natural and anthropogenic disturbance (Martin‐DeMoor et al., 2010; Mokake et al., 2018; Peters et al., 2005). Masting characteristics of hundreds of species can be investigated using MASTREE+, and integration with plant trait and demographic databases will enable deeper integration of masting and reproductive strategies within life history theory (Salguero‐Gomez et al., 2016). Many ecologically and economically important species show highly variable investment in reproduction between years, and the ability to accurately forecast occasional years of high seed production is a priority for habitat management, with wide ranging applications (Chiavetta & Marzini, 2021; Pearse et al., 2021; Pukkala et al., 2010). Predictive models of masting developed and tested using MASTREE+ data may enable more effective seed collection for afforestation and restoration schemes (Fargione et al., 2021; Kettle et al., 2010), inform wildlife and conservation management (Choquenot & Ruscoe, 2000; Fujiki, 2018; Ida, 2021; O'Donnell & Hoare, 2012), and enable forecasting of periods of elevated infection risk from tick‐borne disease, which predictably follow years of high seed production in many forest ecosystems (Brugger et al., 2018; Cunze et al., 2018; Heyman et al., 2012; Ostfeld et al., 1996).
The availability of seed and fruit production data sets in MASTREE+ will be broadly relevant when paired with existing animal population data sets. The pulses of resources associated with large reproductive events are key drivers of the population dynamics of seed‐eating insects, mammals and birds, with cascading impacts through ecosystems (Bouchard et al., 2018; Kanamori et al., 2017; Selonen et al., 2016). Time‐series in MASTREE+ can be combined with existing long time‐series of animal populations and behaviour to identify the drivers of population dynamics, both in seed‐dependent species and further down the trophic cascade (Kleef & Wijsman, 2015; Lithner & Jönsson, 2002). Where species are well replicated in MASTREE+, the spatial synchrony of masting can also be quantified, allowing researchers to determine where regional estimates of masting can be appropriately used as indicators of local variability in seed or fruit availability. The scale of spatial synchrony of masting appears to be highly variable between species (Bogdziewicz et al., 2019), but this has only been quantified of a handful of species so far (Koenig & Knops, 2013; LaMontagne et al., 2020).
In masting species, highly variable allocation to reproduction has wider effects on plant resource allocation, and carbon and nutrient cycling through ecosystems, but this remains poorly explored (Brumme et al., 2021; Khanna et al., 2009; Muller‐Haubold et al., 2015). Data in MASTREE+ can be combined with existing field and remote‐sensing data sets of plant growth or productivity, and with data sets of whole‐ecosystem or soil carbon and nutrient fluxes to understand how variable allocation to reproduction influences carbon sequestration above and belowground, and how this varies between species and across environmental gradients (Bajocco et al., 2021; Nussbaumer et al., 2021; Oddou‐Muratorio et al., 2021; Zhang et al., 2022). Related work can use MASTREE+ data combined with existing or retrospective sampling (e.g. tree‐rings) to address outstanding question regarding resource allocation between growth, reproduction, and defence, particularly how this varies interspecifically and with environmental stress, and how this may shape species and community responses to environmental change (Lauder et al., 2019; Redmond et al., 2019).
4. DATA SOURCES, ACQUISITION AND COMPILATION
We collected species‐specific time‐series of annual reproductive effort for terrestrial perennial plant populations, including trees, shrubs, herbs and grasses. We included data from unmanaged and managed populations, but excluded agricultural crop species subject to selective breeding. Where reproduction was monitored under experimentally manipulated conditions (e.g. fertilisation, warming, rainfall exclusion), we only included data from control plots.
Data were collected for reproductive effort at different stages of the reproductive cycle (e.g. flowers or inflorescences, pollen abundance, number of fruits, cones or seeds), but 90% of data were mature seed, fruit, or cone production. We did not set a minimum time‐series length but prioritised compiling effort on time‐series ≥4 years. All time‐series represent reproductive effort at the population level, ranging from local populations with <10 individuals to regional estimates of reproduction, and we recorded information on the number of monitored individuals in each population and the spatial scale represented by the time‐series (Table 1). We also included information on the original data collection methods, which included litter traps (19.3% of all records), seed, cone and fruit counts (18.3%), other methods including estimates of cone production using cone or fruit scars and categorical classification of seed and fruit crops by wildlife managers or foresters.
TABLE 1.
Overview of the data variables in the MASTREE+ data set. A more detailed description of the variables is included in Appendix 5
| Variable | Description |
|---|---|
| Alpha_Number | Unique code associated with each original source of data, that is, the publication, report or thesis containing extracted data, or the previously unpublished data set included in MASTREE+ |
| Segment | Temporal segment of a time‐series containing gaps (note that years with no observations are not recorded). Individual time‐series can consist of multiple segments |
| Site_number | Code to differentiate multiple sites from the same original source (Alpha_Number/Study_ID) |
| Variable_number | Code to differentiate multiple measures of reproductive output from the same species‐site combination (e.g. where seeds and cones were recorded separately) |
| Year | Year of observation |
| Species | Species identifier, standardised to The Plant List nomenclature. ‘spp.’ is used to indicate a record identified to the genus level only. ‘MIXED’ indicates a non‐species‐specific community‐level estimate of annual reproductive effort |
| Species_code | Six‐character species identifier |
| Mono_Poly | Monocarpic (semelparous) or Polycarpic (iteroparous) species |
| Value | The measured value of annual reproductive output |
| VarType | Continuous or ordinal data. Continuous time‐series are recorded on a continuous scale. Ordinal series are recorded on an ordered categorical scale. All ordinal series are rescaled to start at 1 (lowest reproductive effort) and to contain only integer values |
| Unit | The unit of measurement, where VarType is continuous |
| Max_Value | The maximum value in a time‐series |
| Variable | Categorical classification of the measured variable. Options limited to: cone, flower, fruit, seed, pollen, total reproduction organs |
| Collection_method | Classification of the method used to measure reproductive effort. Options are limited to: cone count, cone scar count, flower count, fruit count, fruit scar sound, seed count, seed trap, pollen count, lake sediment pollen count, harvest record, visual crop assessment, other quantification, dendrochronological reconstruction |
| Latitude | Latitude of the record, in decimal degrees |
| Longitude | Longitude of the record, in decimal degrees |
| Coordinate_flag |
A flag to indicate the precision of the latitude and longitude. A = coordinates provided in the original source B = coordinates estimated by the compiler based on a map or other location information provided in the original source C = coordinates estimated by the compiler as the approximate centre point of the smallest clearly defined geographical unit provided in the original source (e.g. county, state, island), and potentially of low precision |
| Site | A site name or description, based on information in the original source |
| Country | The country where the observation was recorded |
| Elevation | The elevation of the sample site in metres above sea level, where provided in the original source |
| Spatial_unit |
Categorical classification of spatial scale represented by the record, estimated by the compiler based on information provided in the original source. stand = <100 ha patch = 100–10,000 ha region = 10,000–1,000,000 ha super‐region = >1,000,000 ha |
| No_individuals | Either the number of monitored individual plants, or the number of litter traps. NA indicates no information in the original source, and 9999 indicates that while the number of monitored individuals was not specified, the source indicated to the compiler that the sample size was likely ≥10 individuals or litter traps |
| Start | The first year of observations for the complete time‐series, including all segments |
| End | The final year of observations for the complete time‐series, including all segments |
| Length | The number of years of observations. Note that may not be equal to the number of years between the Start and End of the time‐series, due to gaps in the time‐series. |
| Reference | Identification for the original source of the data, see Appendix 4 for the complete list of references |
| Record_type |
Categorisation of the original source. Peer‐reviewed = extracted from peer reviewed literature Grey = extracted from grey literature Unpublished = unpublished data |
| ID_enterer |
Identification of the original compiler of the data. AHP, Andrew Hacket‐Pain; ES, Eliane Schermer; JVM, Jose Moris; XTT, Tingting Xue; TC, Thomas Caignard; DV, Davide Vecchio; DA, Davide Ascoli; IP, Ian Pearse; JL, Jalene LaMontagne; JVD, Joep van Dormolen |
| Date_entry | Date of data entry into MASTREE+ in the format yyyy‐mm‐dd |
| Note on data location | Notes on the location of the data within the original source, such as page or figure number |
| Comments | Additional comments |
| Study_ID | Unique code associated with each source of data. M_ = series extracted from published literature; A_ = series incorporated from Ascoli et al. (2020), Ascoli, Maringer, et al. (2017) and Ascoli, Vacchiano, et al. (2017); PLK_ = series incorporated from Pearse et al. (2017); D_ = unpublished data sets |
Data were collected from several sources. We harmonised data from previously published compilations of plant reproductive effort displaying differences in data architecture (Ascoli et al., 2020; Ascoli, Maringer, et al., 2017; Pearse et al., 2020). To identify other time‐series, we searched Google Scholar and Scopus with multiple combinations of search terms (see Appendix 2). Spanish‐ and French‐language searches was used to increase data representation from South America and Africa. An initial screen was based on the title and abstract to exclude irrelevant sources. Then, potential sources were classified based on the inclusion of useful time‐series data of reproductive effort, available as either data tables, figures, descriptions in the text or in supplementary data files or in online data repositories. Finally, we solicited contributions of previously unpublished data sets from our research networks. Time‐series were extracted from the original sources. In the case of values published in tables, in the text, or in online data repositories or supplementary data files, we extracted values directly from the source. In cases where data were contained in figures, we used the WebPlotDigitizer tool (Rohatgi, 2020). Metadata associated with each time‐series was also extracted from the sources, or directly from data set contributors, and copies of original sources were archived.
4.1. Data set variables
For each monitored population we recorded annual observations of reproductive effort, the units of measurement, the method used to assess reproductive output and the number of monitored individuals (Table 1). Where multiple measures of reproductive output were recorded for the same population (e.g. where seeds and cones were recorded separately), this was recorded to enable filtering of the data set for pseudoreplicates (Table 1). For ordinal series, we maintained the original number of classes, but we rescaled to integer scales starting at 1 (lowest reproductive output). For continuous series, where possible we converted data into a common unit (e.g. we converted ‘seeds/ha’ to ‘seeds/m2’). Years with missing observations are not recorded, and time‐series that would otherwise have gaps consist of a set of segments. The Start and End year corresponds to the first and last observation year for each time‐series, respectively, including all segments. Length is the number of observations within each time‐series, and can therefore be lower than the number of years between the Start and End. The location (decimal degrees), site name, elevation and country of each time‐series were recorded. The spatial scale represented by the time‐series was estimated on a four‐point scale, from individual stand to region, based on information contained in the original source. Information on the nature of the source, and reference information was also recorded. Full details of data variables are listed in Table 1. Each time‐series can be uniquely defined by combining Alpha_Number, Site_number, Variable_number and Species_code.
4.2. Technical validation and quality control
A two‐stage approach was adopted to validate time‐series data. Initially, we standardised attribute data and checked for errors and inconsistencies within time‐series. Species names were checked and standardised to The Plant List nomenclature, using the ‘Taxonstand’ package for R (v. 2.3) (Cayuela et al., 2021). Country names were converted to the English short name (ISO3166‐1) using the ‘countrycode’ package for R (v. 1.2.0) (Arel‐Bundock et al., 2018). Automatic checks were performed to ensure that each time‐series was uniquely identified by the identification variables and that time‐series' observations were uniquely identified by Year. Species_code was assigned by automatically combining the first three characters from the TPL‐standardised genus and species names. Where separate species shared a Species_code, a unique combination was manually created. The final character of Species_code for populations of a hybrid origin was changed to ‘X’. We ran various automatic checks to ensure all observations in a time‐series had uniform attribute data where such uniformity was expected (i.e. within a time‐series, there was only a single value for variables such as Unit). Interrelated variables were checked to ensure consistency, for example that time‐series spatial data (Latitude, Longitude) fell within the boundaries of the indicated Country. Time‐series duration variables (i.e. Segment, Start, End, Length) were directly calculated from time‐series.
The second stage involved the detection and removal of duplication problems between time‐series, that is, series added multiple times, including with partial overlap, usually when data were published in more than one source. First, we created ‘potential duplication groups’ that contained sets of time‐series that shared the same study species and approximate location (using a ±0.1 decimal degree buffer between pairs of time‐series). PDGs containing time‐series from multiple sources (Alpha_Number) were then inspected further. Suspect pairs of time‐series within PDGs were initially identified based on a correlation test (Spearman's ρ > 0.97), and we then inspected manually for duplication using information including location, units, and collection methods to identify possible duplication. To supplement the semi‐automated detection of duplicates, we performed a further manual check, examining groups of time‐series that shared the same country and species. Suspect pairs of series might, for example, share matching spatial references, matching site descriptions and/or matching author names.
Where duplicated series were identified, or where independence could not be confirmed, we selected a single time‐series for inclusion in MASTREE+. Generally, the longest time‐series was prioritised, unless there were clear signs that a shorter time‐series was of higher quality (e.g. the data were directly shared by the author and not extracted from a graph).
5. DATA SET AVAILABILITY AND MASTREE+ DATA EXPLORER
The data set is provided as a csv file in the online supporting information (Appendix 1) and is distributed under a CC‐BY‐4.0 licence so that it can be freely used, shared and modified so long as appropriate credit is given. The data set will be expanded and updated over time, so users are encouraged to check for the latest version of the data set on GitHub (https://github.com/JJFoest/MASTREEplus) and via associated updates to the MASTREE+ Data Explorer. The MASTREE+ Data Explorer allows users to explore the MASTREE+ data set and provides an alternative for downloading the data set, including user‐defined subsets thereof. The MASTREE+ Data Explorer was created using the shiny package in R (Chang et al., 2021) and can be accessed at https://mastreeplus.shinyapps.io/mastreeplus/. Time‐series are plotted on a zoomable world map, with updating summary plots showing the time‐series lengths and species/genera for the selected region, as well as scaled time‐series for initial visualisation of the data within the selected region of interest (Figure 5). Individual time‐series can be selected on the map to reveal associated meta‐data, including the location, species and original source. Various filter options allow the user to subset the full data set. An R script is provided in Appendix 6 that illustrates how to load, manipulate and visualise the data set.
FIGURE 5.
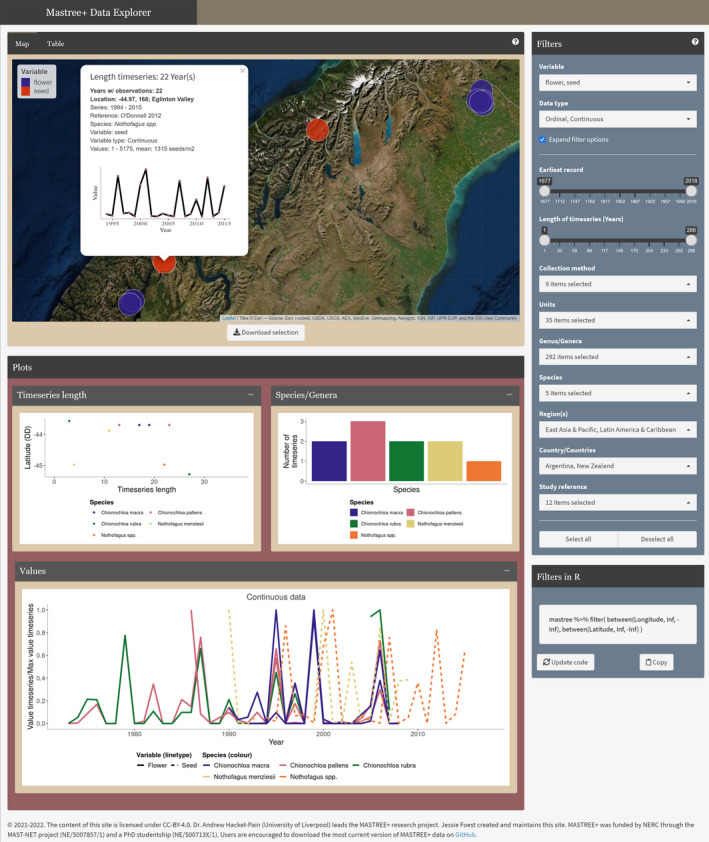
Example of the MASTREE+ Shiny Data Explorer, showing data from the South Island of New Zealand. The Data Explorer allows the user to explore data availability within MASTREE+, and download the full or user‐defined subsets of the data set
6. CALL FOR DATA
We have increased taxonomic and geographic representation in MASTREE+, but many gaps remain in the coverage of our data set. Our goal is to provide a global platform for sharing data on long‐lived plant reproduction, and we encourage scientists to submit time‐series of annual reproductive effort in perennial plant populations for inclusion in MASTREE+ (Table 2). We will consider all species‐specific time‐series of four or more years, including continuous and ordinal observations. We include time‐series data on flower, seed, fruit and cone production, which are associated with geographical coordinates. We can include data that represent small local populations through to large regional‐scale assessments of reproductive effort. Note that we only record annual reproductive effort. Where data are collected at sub‐annual timesteps, this means that reproduction must be aggregated to annual units (e.g. April–March).
TABLE 2.
Minimum data requirements for submissions to MASTREE+. For further details see Table 1
| Minimum data requirements and metadata |
|---|
| Minimum of four consecutive measurements of annual reproductive output |
| Measurement at the population level (local population through regional scale estimates acceptable) |
| Species name according to The Plant List. Records identified to the genus level are acceptable, and measurements of non‐species‐specific community reproductive effort may be included. |
| Spatial coordinates of the monitored population |
| Details of the method used to measure reproductive effort (e.g. litter traps, seed counts, visual crop estimate, see Table 1) |
Potential contributors of data are encouraged to search the latest version of the data set to check whether the data are already included in MASTREE+, either by downloading the latest version from GitHub, Dryad (Section 5) or via the MASTREE+ Data Explorer. If the data are not already included, potential contributors are encouraged to contact the corresponding author to discuss arrangements for sharing data. The minimum data requirements are included in Table 2.
6.1. Data licence
MASTREE+ is published under a CC‐BY‐4.0 licence, which enables users to copy and redistribute, adapt and modify the data set in any medium or format and for any purpose, including commercial. You must give appropriate credit by citing this publication, provide a link to the license and indicate if changes were made (see https://creativecommons.org/licenses/by/4.0/ for further details). Data can be accessed via Github: https://github.com/JJFoest/MASTREEplus, Dryad: https://doi.org/10.5061/dryad.18931zd02, or via the MASTREE+ Siny App. Publications using the RENECOFOR data (Reference = RENECOFOR_2020) are requested to acknowledge the RENECOFOR network, and send copies of publications to manuel.nicolas@onf.fr. Publications using the Lopé data (Reference = Bush_2021) are requested to cite the original data set (http://hdl.handle.net/11667/152), acknowledge The National Parks Agency of Gabon (ANPN) and the University of Stirling, and send copies of any resulting publications to science@parcsgabon.ga and k.a.abernethy@stir.ac.uk.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualisation: Andrew Hacket‐Pain, Ian S. Pearse, Walter D. Koenig, Giorgio Vacchiano, Michał Bogdziewicz, Mario Pesendorfer, Akiko Satake, Andrew J. Tanentzap, Peter A. Thomas, Thomas Wohlgemuth, Davide Ascoli. Methodology, including literature search, source classification, data extraction and compilation: Andrew Hacket‐Pain, Jessie J. Foest, Ian S. Pearse, Jalene M. LaMontagne, Walter D. Koenig, Giorgio Vacchiano, Michał Bogdziewicz, Thomas Caignard, Paulina Celebias, Joep van Dormolen, Marcos Fernández‐Martínez, Jose V. Moris, Ciprian Palaghianu, Mario Pesendorfer, Akiko Satake, Eliane Schermer, Andrew J. Tanentzap, Peter A. Thomas, Davide Vecchio, Andreas P. Wion, Thomas Wohlgemuth, Tingting Xue, Davide Ascoli. Methodology, including data validation: Andrew Hacket‐Pain, Jessie F. Foest. Data Explorer: Jessie J. Foest. Data contribution: Andrew Hacket‐Pain, Ian S. Pearse, Jalene M. LaMontagne, Walter D. Koenig, Michał Bogdziewicz, Peter A. Thomas, Katharine Abernethy, Marcelo Daniel Barrera, Jessica H. Barton, Stan Boutin, Emma R. Bush, Sergio Donoso Calderón, Felipe S. Carevic, Carolina Volkmer de Castilho, Juan Manuel Cellini, Hazel Chapman, Colin A. Chapman, Francesco Chianucci, Patricia da Costa, Luc Croisé, Andrea Cutini, Ben Dantzer, R. Justin DeRose, Jean‐Thoussaint Dikangadissi, Edmond Dimoto, Fernanda Lopes da Fonseca, Leonardo Gallo, Georg Gratzer, David F. Greene, Martín A. Hadad, Alejandro Huertas Herrera, Kathryn J. Jeffery, Jill F. Johnstone, Urs Kalbitzer, Władysław Kantorowicz, Christie A. Klimas, Jonathan G.A. Lageard, Jeffrey Lane, Katharina Lapin, Mateusz Ledwoń, Abigail Leeper, Maria Vanessa Lencinas, Ana Cláudia Lira‐Guedes, Michael C. Lordon, Paula Marchelli, Shealyn Marino, Harald Schmidt Van Marle, Andrew G. McAdam, Ludovic R.W. Momont, Manuel Nicolas, Lúcia Helena de Oliveira Wadt, Parisa Panahi, Guillermo Martínez Pastur, Thomas Patterson, Pablo Luis Peri, Łukasz Piechnik, Mehdi Pourhashemi, Claudia Espinoza Quezada, Fidel A. Roig, Karen Peña Rojas, Yamina Micaela Rosas, Silvio Schüler, Barbara Seget, Rosina Soler, Michael A. Steele, Mónica Toro‐Manríquez, Caroline E.G. Tutin, Tharcisse Ukizintambara, Lee White, Biplang Yadok, John L. Willis, Anita Zolles, Magdalena Żywiec, Davide Ascoli. Writing—original draft: Andrew Hacket‐Pain. Writing—Review and editing: All authors. Supervision and Project administration: Andrew Hacket‐Pain. Funding acquisition: Andrew Hacket‐Pain, Andrew J. Tanentzap, Peter A. Thomas.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
ACKNOWLEDGEMENTS
We thank Mark Green and Esther Dale. This study was funded by the UK Natural Environment Research Council grant no. NE/S007857/1 to AHP, AJT and PAT. JJF was supported a PhD studentship under Natural Environment Research Council grant number NE/S00713X/1. Original data collection was supported by many funders, including the Natural Sciences and Engineering Research Council of Canada (SB, AM, BD, JL) the National Science Foundation (AM, BD), the Austrian Climate Research Program (ACRP) of the ‘Klima‐ und Energiefonds’ (9th Call, project: MoreSeedsAdapt—KR16AC0K13339), the Brazilian Agricultural Research Corporation (EMBRAPA) through the Kamukaia III Project ‘Appreciation of non‐timber forest products in the Amazon’ (grant number SEG 12.13.07.007.00.00), the W. Szafer Institute of Botany of the Polish Academy of Sciences and the Polish National Science Foundation (2019/33/B/NZ8/01345), the French ministry of Agriculture and Food, the French ministry and the French agency for Ecological Transition, the French national forest office, and the European Commission (under successive regulations from No 1091/94 until No 2152/2003). As the French part of the ICP Forests intensive (Level II) monitoring programme, the RENECOFOR network has benefited from its scientific framework and shared expertise. Data from Bonanza Creek LTER come from a partnership between the University of Alaska Fairbanks and the U.S. Forest Service, with funding from the National Science Foundation Long‐Term Ecological Research program (NSF Grant numbers DEB‐1636476, DEB‐1026415, DEB‐0620579, DEB‐0423442, DEB‐0080609, DEB‐9810217, DEB‐9211769, DEB‐8702629) and by the USDA Forest Service, Pacific Northwest Research Station (Agreement # RJVA‐PNW‐01‐JV‐11261952‐231). The contribution of TX was supported by the National Natural Science Foundation (Grant No. 32001310) and Chuzhou University Start‐up Foundation for Research (2021qd05). Data from Italian Central Apennines were available, thanks to the support of the research project ‘Monitoraggio della produzione di seme di specie forestali, rinnovazione naturale e relazioni con la fauna selvatica (Pasciona)’ funded by the Foreste Casentinesi, Monte Falterona e Campigna National Park. The contribution from RJD would not have been possible without the effort of Dr. Theodore W. (‘Doc’) Daniel. Doc Daniel had the long‐term vision to commence and oversee the annual cone counts for hundreds of trees from 1947‐1981 on the Utah State Agricultural College (now Utah State University) School Forest, later named in his honour as the T.W. Daniel Experimental Forest. The Rothwald data were collected as part of the research project ‘Sporadic seed production in mast seeding trees’, P30381 of the Austrian Science Fund (FWF). We thank Charley Krebs, Rudy Boonstra, Dennis Murray for sharing unpublished data, and we gratefully acknowledge the scientists responsible for the following open‐access data sets that were incorporated into MASTREE+: https://doi.org/10.5061/dryad.4qrfj6q9m, https://doi.org/10.5061/dryad.1s625, https://doi.org/10.5061/dryad.v6wwpzgrb, https://doi.org/10.5061/dryad.772g3, https://doi.org/10.5061/dryad.pv608, https://doi.org/10.5061/dryad.stqjq2c0c, https://doi.org/10.5061/dryad.61m318c, https://doi.org/10.5061/dryad.75v7c. We thank the many collaborators, students, friends and family who have helped to support long‐term data collection. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions of this publication are those of the author(s) and should not be construed to represent an official USDA, Forest Service, or United States Government determination or policy.
Hacket‐Pain, A. , Foest, J. J. , Pearse, I. S. , LaMontagne, J. M. , Koenig, W. D. , Vacchiano, G. , Bogdziewicz, M. , Caignard, T. , Celebias, P. , van Dormolen, J. , Fernández‐Martínez, M. , Moris, J. V. , Palaghianu, C. , Pesendorfer, M. , Satake, A. , Schermer, E. , Tanentzap, A. J. , Thomas, P. A. , Vecchio, D. , … Ascoli, D. (2022). MASTREE+: Time‐series of plant reproductive effort from six continents. Global Change Biology, 28, 3066–3082. 10.1111/gcb.16130
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article, and are openly available via Github: https://github.com/JJFoest/MASTREEplus, Dryad: https://doi.org/10.5061/dryad.18931zd02, or via the MASTREE+ Data Explorer: https://mastreeplus.shinyapps.io/mastreeplus
REFERENCES
- Abraham, E. M. , Sklavou, P. , Loufi, A. , Parissi, Z. M. , & Kyriazopoulos, A. P. (2018). The effect of combined herbivory by wild boar and small ruminants on the regeneration of a deciduous oak forest. Forests, 9(9), 580. 10.3390/f9090580 [DOI] [Google Scholar]
- Allen, C. D. , Macalady, A. K. , Chenchouni, H. , Bachelet, D. , McDowell, N. , Vennetier, M. , Kitzberger, T. , Rigling, A. , Breshears, D. D. , Hogg, E. H. (Ted) , Gonzalez, P. , Fensham, R. , Zhang, Z. , Castro, J. , Demidova, N. , Lim, J.‐H. , Allard, G. , Running, S. W. , Semerci, A. , & Cobb, N. (2010). A global overview of drought and heat‐induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management, 259(4), 660–684. 10.1016/j.foreco.2009.09.001 [DOI] [Google Scholar]
- Arel‐Bundock, V. , Enevoldsen, N. , & Yetman, C. J. (2018). countrycode: An R package to convert country names and country codes. Journal of Open Source Software, 3(28), 848. 10.21105/joss.00848 [DOI] [Google Scholar]
- Ascoli, D. , Hacket‐Pain, A. , LaMontagne, J. M. , Cardil, A. , Conedera, M. , Maringer, J. , Motta, R. , Pearse, I. S. , & Vacchiano, G. (2020). Climate teleconnections synchronize Picea glauca masting and fire disturbance: Evidence for a fire‐related form of environmental prediction. Journal of Ecology, 108(3), 1186–1198. 10.1111/1365-2745.13308 [DOI] [Google Scholar]
- Ascoli, D. , Maringer, J. , Hacket‐Pain, A. , Conedera, M. , Drobyshev, I. , Motta, R. , Cirolli, M. , Kantorowicz, W. , Zang, C. , Schueler, S. , Croisé, L. , Piussi, P. , Berretti, R. , Palaghianu, C. , Westergren, M. , Lageard, J. G. A. , Burkart, A. , Gehrig Bichsel, R. , Thomas, P. A. , … Vacchiano, G. (2017). Two centuries of masting data for European beech and Norway spruce across the European continent. Ecology, 98(5), 1473. 10.1002/ecy.1785 [DOI] [PubMed] [Google Scholar]
- Ascoli, D. , Vacchiano, G. , Turco, M. , Conedera, M. , Drobyshev, I. , Maringer, J. , Motta, R. , & Hacket‐Pain, A. (2017). Inter‐annual and decadal changes in teleconnections drive continental‐scale synchronization of tree reproduction. Nature Communications, 8. 10.1038/s41467-017-02348-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajocco, S. , Ferrara, C. , Bascietto, M. , Alivernini, A. , Chirichella, R. , Cutini, A. , & Chianucci, F. (2021). Characterizing the climatic niche of mast seeding in beech: Evidences of trade‐offs between vegetation growth and seed production. Ecological Indicators, 121, 9. 10.1016/j.ecolind.2020.107139 [DOI] [Google Scholar]
- Bennett, E. , Clement, J. , Sansom, P. , Hall, I. , Leach, S. , & Medlock, J. M. (2010). Environmental and ecological potential for enzootic cycles of Puumala hantavirus in Great Britain. Epidemiology and Infection, 138(1), 91–98. 10.1017/s095026880999029x [DOI] [PubMed] [Google Scholar]
- Bogdziewicz, M. (2021). How will global change affect plant reproduction? A framework for mast seeding trends. New Phytologist, Early View. 10.1111/nph.17682 [DOI] [PubMed] [Google Scholar]
- Bogdziewicz, M. , Hacket‐Pain, A. , Ascoli, D. , & Szymkowiak, J. (2021). Environmental variation drives continental‐scale synchrony of European beech reproduction. Ecology, 102(7), 10. 10.1002/ecy.3384 [DOI] [PubMed] [Google Scholar]
- Bogdziewicz, M. , Kelly, D. , Thomas, P. A. , Lageard, J. G. A. , & Hacket‐Pain, A. (2020). Climate warming disrupts mast seeding and its fitness benefits in European beech. Nature Plants, 6(2), 88–94. 10.1038/s41477-020-0592-8 [DOI] [PubMed] [Google Scholar]
- Bogdziewicz, M. , Szymkowiak, J. , Fernández‐Martínez, M. , Peñuelas, J. , & Espelta, J. M. (2019). The effects of local climate on the correlation between weather and seed production differ in two species with contrasting masting habit. Agricultural and Forest Meteorology, 268, 109–115. 10.1016/j.agrformet.2019.01.016 [DOI] [Google Scholar]
- Bouchard, M. , Regniere, J. , & Therrien, P. (2018). Bottom‐up factors contribute to large‐scale synchrony in spruce budworm populations. Canadian Journal of Forest Research, 48(3), 277–284. 10.1139/cjfr-2017-0051 [DOI] [Google Scholar]
- Boutin, S. , Wauters, L. A. , McAdam, A. G. , Humphries, M. M. , Tosi, G. , & Dhondt, A. A. (2006). Anticipatory reproduction and population growth in seed predators. Science, 314(5807), 1928–1930. 10.1126/science.1135520 [DOI] [PubMed] [Google Scholar]
- Bregnard, C. , Rais, O. , & Voordouw, M. J. (2020). Climate and tree seed production predict the abundance of the European Lyme disease vector over a 15‐year period. Parasites & Vectors, 13(1). 10.1186/s13071-020-04291-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger, K. , Walter, M. , Chitimia‐Dobler, L. , Dobler, G. , & Rubel, F. (2018). Forecasting next season's Ixodes ricinus nymphal density: The example of southern Germany 2018. Experimental and Applied Acarology, 75(3), 281–288. 10.1007/s10493-018-0267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumme, R. , Ahrends, B. , Block, J. , Schulz, C. , Meesenburg, H. , Klinck, U. , Wagner, M. , & Khanna, P. K. (2021). Cycling and retention of nitrogen in European beech (Fagus sylvatica L.) ecosystems under elevated fructification frequency. Biogeosciences, 18(12), 3763–3779. 10.5194/bg-18-3763-2021 [DOI] [Google Scholar]
- Buechling, A. , Martin, P. H. , Canham, C. D. , Shepperd, W. D. , & Battaglia, M. A. (2016). Climate drivers of seed production in Picea engelmannii and response to warming temperatures in the southern Rocky Mountains. Journal of Ecology, 104(4), 1051–1062. 10.1111/1365-2745.12572 [DOI] [Google Scholar]
- Buras, A. , Rammig, A. , & Zang, C. S. (2020). Quantifying impacts of the 2018 drought on European ecosystems in comparison to 2003. Biogeosciences, 17(6), 1655–1672. 10.5194/bg-17-1655-2020 [DOI] [Google Scholar]
- Bush, E. R. , Whytock, R. C. , Bahaa‐el‐din, L. , Bourgeois, S. , Bunnefeld, N. , Cardoso, A. W. , Dikangadissi, J. T. , Dimbonda, P. , Dimoto, E. , Edzang Ndong, J. , Jeffery, K. J. , Lehmann, D. , Makaga, L. , Momboua, B. , Momont, L. R. W. , Tutin, C. E. G. , White, L. J. T. , Whittaker, A. , & Abernethy, K. (2020). Long‐term collapse in fruit availability threatens Central African forest megafauna. Science, 370(6521), 1219–1221. 10.1126/science.abc7791 [DOI] [PubMed] [Google Scholar]
- Caignard, T. , Kremer, A. , Firmat, C. , Nicolas, M. , Venner, S. , & Delzon, S. (2017). Increasing spring temperatures favor oak seed production in temperate areas. Scientific Reports, 7, 8. 10.1038/s41598-017-09172-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calama, R. , Mutke, S. , Tome, J. , Gordo, J. , Montero, G. , & Tome, M. (2011). Modelling spatial and temporal variability in a zero‐inflated variable: The case of stone pine (Pinus pinea L.) cone production. Ecological Modelling, 222(3), 606–618. 10.1016/j.ecolmodel.2010.09.020 [DOI] [Google Scholar]
- Carnicer, J. , Coll, M. , Ninyerola, M. , Pons, X. , Sanchez, G. , & Penuelas, J. (2011). Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change‐type drought. Proceedings of the National Academy of Sciences of the United States of America, 108(4), 1474–1478. 10.1073/pnas.1010070108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayuela, L. , Macarro, I. , Stein, A. , & Oksanen, J. (2021). Taxonstand: Taxonomic standardization of plant species names. Retrieved From https://CRAN.R‐project.org/package=Taxonstand [Google Scholar]
- Chang, W. , Cheng, J. , Allaire, J. J. , Sievert, C. , Schloerke, B. , Xie, Y. , … Borges, B. (2021). shiny: Web application framework for R (Version 1.6.0). Retrieved from https://CRAN.R‐project.org/package=shiny [Google Scholar]
- Changenet, A. , Ruiz‐Benito, P. , Ratcliffe, S. , Fréjaville, T. , Archambeau, J. , Porte, A. J. , Zavala, M. A. , Dahlgren, J. , Lehtonen, A. , & Benito Garzón, M. (2021). Occurrence but not intensity of mortality rises towards the climatic trailing edge of tree species ranges in European forests. Global Ecology and Biogeography, 30(7), 1356–1374. 10.1111/geb.13301 [DOI] [Google Scholar]
- Chen, J. M. , Ju, W. M. , Ciais, P. , Viovy, N. , Liu, R. G. , Liu, Y. , & Lu, X. H. (2019). Vegetation structural change since 1981 significantly enhanced the terrestrial carbon sink. Nature Communications, 10. 10.1038/s41467-019-12257-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavetta, U. , & Marzini, S. (2021). foreMast: an R package for predicting beech (Fagus sylvatica L.) masting events in European countries. Annals of Forest Science, 78(4), 10. 10.1007/s13595-021-01109-5 [DOI] [Google Scholar]
- Choquenot, D. , & Ruscoe, W. A. (2000). Mouse population eruptions in New Zealand forests: the role of population density and seedfall. Journal of Animal Ecology, 69(6), 1058–1070. 10.1046/j.1365-2656.2000.00462.x [DOI] [Google Scholar]
- Clark, J. S. , Andrus, R. , Aubry‐Kientz, M. , Bergeron, Y. , Bogdziewicz, M. , Bragg, D. C. , Brockway, D. , Cleavitt, N. L. , Cohen, S. , Courbaud, B. , Daley, R. , Das, A. J. , Dietze, M. Fahey, T. J. , Fer, I. , Franklin, J. F. , Gehring, C. A. , Gilbert, G. S. , Greenberg, C. H. , … Zlotin, R. (2021). Continent‐wide tree fecundity driven by indirect climate effects. Nature Communications, 12(1), 1242. 10.1038/s41467-021-22025-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, J. S. , Iverson, L. , Woodall, C. W. , Allen, C. D. , Bell, D. M. , Bragg, D. C. , D'Amato, A. W. , Davis, F. W. , Hersh, M. H. , Ibanez, I. , Jackson, S. T. , Matthews, S. , Pederson, N. , Peters, M. , Schwartz, M. W. , Waring, K. M. , & Zimmermann, N. E. (2016). The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Global Change Biology, 22(7), 2329–2352. 10.1111/gcb.13160 [DOI] [PubMed] [Google Scholar]
- Connell, J. H. , & Green, P. T. (2000). Seedling dynamics over thirty‐two years in a tropical rain forest tree. Ecology, 81(2), 568–584. 10.1890/0012-9658(2000)081%5b0568:sdotty%5d2.0.co;2 [DOI] [Google Scholar]
- Cunze, S. , Kochmann, J. , Kuhn, T. , Frank, R. , Dorge, D. D. , & Klimpel, S. (2018). Spatial and temporal patterns of human Puumala virus (PUUV) infections in Germany. Peerj, 6, 20. 10.7717/peerj.4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran, L. M. , & Leighton, M. (2000). Vertebrate responses to spatiotemporal variation in seed production of mast‐fruiting Dipterocarpaceae. Ecological Monographs, 70(1), 101–128. 10.1890/0012-9615(2000)070%5b0101:VRTSVI%5d2.0.CO;2 [DOI] [Google Scholar]
- Dale, E. E. , Foest, J. J. , Hacket‐Pain, A. , Bogdziewicz, M. , & Tanentzap, A. J. (2021). Macroevolutionary consequences of mast seeding. Philosophical Transactions of the Royal Society, 376, 2020372. 10.1098/rstb.2020.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargione, J. , Haase, D. L. , Burney, O. T. , Kildisheva, O. A. , Edge, G. , Cook‐Patton, S. C. , Chapman, T. , Rempel, A. , Hurteau, M. D. , Davis, K. T. , Dobrowski, S. , Enebak, S. , De La Torre, R. , Bhuta, A. A. R. , Cubbage, F. , Kittler, B. , Zhang, D. , & Guldin, R. W. (2021). Challenges to the reforestation pipeline in the United States. Frontiers in Forests and Global Change, 4, 18. 10.3389/ffgc.2021.629198 [DOI] [Google Scholar]
- Fernández‐Martínez, M. , Pearse, I. , Sardans, J. , Sayol, F. , Koenig, W. D. , LaMontagne, J. M. , Bogdziewicz, M. , Collalti, A. , Hacket‐Pain, A. , Vacchiano, G. , Espelta, J. M. , Peñuelas, J. , & Janssens, I. A. (2019). Nutrient scarcity as a selective pressure for mast seeding. Nature Plants, 5(12), 1222. 10.1038/s41477-019-0549-y [DOI] [PubMed] [Google Scholar]
- Fernandez‐Pascual, E. (2021). SylvanSeeds, a seed germination database for temperate deciduous forests. Journal of Vegetation Science, 32, e12960. 10.1111/jvs.12960 [DOI] [Google Scholar]
- Fisher, R. A. , Koven, C. D. , Anderegg, W. R. L. , Christoffersen, B. O. , Dietze, M. C. , Farrior, C. E. , Holm, J. A. , Hurtt, G. C. , Knox, R. G. , Lawrence, P. J. , Lichstein, J. W. , Longo, M. , Matheny, A. M. , Medvigy, D. , Muller‐Landau, H. C. , Powell, T. L. , Serbin, S. P. , Sato, H. , Shuman, J. K. , … Moorcroft, P. R. (2018). Vegetation demographics in Earth System Models: A review of progress and priorities. Global Change Biology, 24(1), 35–54. 10.1111/gcb.13910 [DOI] [PubMed] [Google Scholar]
- Fujiki, D. (2018). Can frequent occurrence of Asiatic black bears around residential areas be predicted by a model‐based mast production in multiple Fagaceae species? Journal of Forest Research, 23(5), 260–269. 10.1080/13416979.2018.1488653 [DOI] [Google Scholar]
- Hacket‐Pain, A. , & Bogdziewicz, M. (2021). Climate change and plant reproduction: trends and drivers of mast seeding change. Philosophical Transactions of the Royal Society B, 376, 20200379. 10.1098/rstb.2020.0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman, P. , Thoma, B. R. , Marié, J.‐L. , Cochez, C. , & Essbauer, S. S. (2012). In search for factors that drive hantavirus epidemics. Frontiers in Physiology, 3, 237. 10.3389/fphys.2012.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida, H. (2021). A 15‐year study on the relationship between beech (Fagus crenata) reproductive‐organ production and the numbers of nuisance Japanese black bears (Ursus thibetanus japonicus) killed in a snowy rural region in central Japan. Landscape and Ecological Engineering, 17(4), 507–514. 10.1007/s11355-021-00472-9 [DOI] [Google Scholar]
- Joubert, D. F. , Smit, G. N. , & Hoffman, M. T. (2013). The influence of rainfall, competition and predation on seed production, germination and establishment of an encroaching Acacia in an arid Namibian savanna. Journal of Arid Environments, 91, 7–13. 10.1016/j.jaridenv.2012.11.001 [DOI] [Google Scholar]
- Kanamori, T. , Kuze, N. , Bernard, H. , Malim, T. P. , & Kohshima, S. (2017). Fluctuations of population density in Bornean orangutans (Pongo pygmaeus morio) related to fruit availability in the Danum Valley, Sabah, Malaysia: A 10‐year record including two mast fruitings and three other peak fruitings. Primates, 58(1), 225–235. 10.1007/s10329-016-0584-5 [DOI] [PubMed] [Google Scholar]
- Kelly, D. , Geldenhuis, A. , James, A. , Penelope Holland, E. , Plank, M. J. , Brockie, R. E. , Cowan, P. E. , Harper, G. A. , Lee, W. G. , Maitland, M. J. , Mark, A. F. , Mills, J. A. , Wilson, P. R. , & Byrom, A. E. (2013). Of mast and mean: Differential‐temperature cue makes mast seeding insensitive to climate change. Ecology Letters, 16(1), 90–98. 10.1111/ele.12020 [DOI] [PubMed] [Google Scholar]
- Kettle, C. J. , Ghazoul, J. , Ashton, P. S. , Cannon, C. H. , Chong, L. , Diway, B. , … Hollingsworth, P. (2010). Mass fruiting in Borneo: A missed opportunity. Science, 330(6004), 584. [DOI] [PubMed] [Google Scholar]
- Khanna, P. K. , Fortmann, H. , Meesenburg, H. , Eichhorn, J. , & Meiwes, K. J. (2009). Biomass and element content of foliage and aboveground litterfall on the three long‐term experimental beech sites: Dynamics and significance. In Brumme R. & Khanna P. K. (Eds.), Functioning and management of European beech ecosystems (Vol. 208, pp. 183–205). Springer. [Google Scholar]
- Kleef, H. L. , & Wijsman, H. (2015). Mast, mice and pine marten (Martes martes): the pine marten's reproductive response to wood mouse (Apodemus sylvaticus) fluctuations in the Netherlands. Lutra, 58, 23–33. [Google Scholar]
- Klesse, S. , DeRose, R. J. , Babst, F. , Black, B. A. , Anderegg, L. D. L. , Axelson, J. , Ettinger, A. , Griesbauer, H. , Guiterman, C. H. , Harley, G. , Harvey, J. E. , Lo, Y.‐H. , Lynch, A. M. , O'Connor, C. , Restaino, C. , Sauchyn, D. , Shaw, J. D. , Smith, D. J. , Wood, L. , … Evans, M. E. K. (2020). Continental‐scale tree‐ring‐based projection of Douglas‐fir growth: Testing the limits of space‐for‐time substitution. Global Change Biology, 26(9), 5146–5163. 10.1111/gcb.15170 [DOI] [PubMed] [Google Scholar]
- Koenig, W. D. , & Knops, J. M. H. (2000). Patterns of annual seed production by northern hemisphere trees: A global perspective. The American Naturalist, 155(1), 59–69. 10.1086/303302 [DOI] [PubMed] [Google Scholar]
- Koenig, W. D. , & Knops, J. M. H. (2013). Large‐scale spatial synchrony and cross‐synchrony in acorn production by two California oaks. Ecology, 94(1), 83–93. 10.1890/12-0940.1 [DOI] [PubMed] [Google Scholar]
- Ladio, A. H. , & Lozada, M. (2004). Patterns of use and knowledge of wild edible plants in distinct ecological environments: A case study of a Mapuche community from northwestern Patagonia. Biodiversity and Conservation, 13(6), 1153–1173. 10.1023/b:bioc.0000018150.79156.50 [DOI] [Google Scholar]
- LaMontagne, J. M. , Pearse, I. S. , Greene, D. F. , & Koenig, W. D. (2020). Mast seeding patterns are asynchronous at a continental scale. Nature Plants, 6(5), 460. 10.1038/s41477-020-0647-x [DOI] [PubMed] [Google Scholar]
- LaMontagne, J. M. , Redmond, M. , Greene, D. , & Koenig, W. D. (2021). An assessment of temporal variability in mast seeding of North American Pinaceae. Philosophical Transactions of the Royal Society B, 376, 20200373. 10.1098/rstb.2020.0373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder, J. D. , Moran, E. V. , & Hart, S. C. (2019). Fight or flight? Potential tradeoffs between drought defense and reproduction in conifers. Tree Physiology, 39(7), 1071–1085. 10.1093/treephys/tpz031 [DOI] [PubMed] [Google Scholar]
- Lithner, S. , & Jönsson, I. (2002). Abundance of owls and Bramblings Fringilla montifringilla in relation to mast seeding in south‐eastern Sweden. Ornis Svecica, 12(1), 35–45. [Google Scholar]
- Manríquez, M. T. , Mestre, L. , Lencinas, M. V. , Promis, Á. , Pastur, G. M. , & Soler, R. (2016). Flowering and seeding patterns in pure and mixed Nothofagus forests in Southern Patagonia. Ecological Processes, 5(1), 21. 10.1186/s13717-016-0065-1 [DOI] [Google Scholar]
- Martin‐DeMoor, J. , Lieffers, V. J. , & Macdonald, S. E. (2010). Natural regeneration of white spruce in aspen‐dominated boreal mixedwoods following harvesting. Canadian Journal of Forest Research‐Revue Canadienne De Recherche Forestiere, 40(3), 585–594. 10.1139/x10-016 [DOI] [Google Scholar]
- McDowell, N. G. , Allen, C. D. , Anderson‐Teixeira, K. , Aukema, B. H. , Bond‐Lamberty, B. , Chini, L. , Clark, J. S. , Dietze, M. , Grossiord, C. , Hanbury‐Brown, A. , Hurtt, G. C. , Jackson, R. B. , Johnson, D. J. , Kueppers, L. , Lichstein, J. W. , Ogle, K. , Poulter, B. , Pugh, T. A. M. , Seidl, R. , … Xu, C. (2020). Pervasive shifts in forest dynamics in a changing world. Science, 368(6494), 964. 10.1126/science.aaz9463 [DOI] [PubMed] [Google Scholar]
- Mokake, S. E. , Chuyong, G. B. , Egbe, A. E. , Tabot, P. T. , Jumbam, B. , Biyon, B. J. N. , & Dibong, S. D. (2018). Plant reproductive phenology following selective logging in a semideciduous tropical forest in the East Region of Cameroon. Journal of Applied Biosciences, 128, 12901–12919. 10.4314/jab.v128i1.4 [DOI] [Google Scholar]
- Mosier, T. M. , Hill, D. F. , & Sharp, K. V. (2014). 30‐Arcsecond monthly climate surfaces with global land coverage. International Journal of Climatology, 34(7), 2175–2188. 10.1002/joc.3829 [DOI] [Google Scholar]
- Muller‐Haubold, H. , Hertel, D. , & Leuschner, C. (2015). Climatic drivers of mast fruiting in European beech and resulting C and N allocation shifts. Ecosystems, 18(6), 1083–1100. 10.1007/s10021-015-9885-6 [DOI] [Google Scholar]
- Mundo, I. A. , Sanguinetti, J. , & Kitzberger, T. (2021). Multi‐centennial phase‐locking between reproduction of a South American conifer and large‐scale drivers of climate. Nature Plants, 7(12), 1560. 10.1038/s41477-021-01038-1 [DOI] [PubMed] [Google Scholar]
- Nussbaumer, A. , Gessler, A. , Benham, S. , de Cinti, B. , Etzold, S. , Ingerslev, M. , Jacob, F. , Lebourgeois, F. , Levanic, T. , Marjanović, H. , Nicolas, M. , Ostrogović Sever, M. Z. , Priwitzer, T. , Rautio, P. , Roskams, P. , Sanders, T. G. M. , Schmitt, M. , Šrámek, V. , Thimonier, A. , … Rigling, A. (2021). Contrasting resource dynamics in mast years for European beech and oak—A continental scale analysis. Frontiers in Forests and Global Change, 4, 17. 10.3389/ffgc.2021.689836 [DOI] [Google Scholar]
- Oddou‐Muratorio, S. , Petit‐Cailleux, C. , Journé, V. , Lingrand, M. , Magdalou, J.‐A. , Hurson, C. , Garrigue, J. , Davi, H. , & Magnanou, E. (2021). Crown defoliation decreases reproduction and wood growth in a marginal European beech population. Annals of Botany, 128(2), 193–204. 10.1093/aob/mcab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, C. F. J. , & Hoare, J. M. (2012). Quantifying the benefits of long‐term integrated pest control for forest bird populations in a New Zealand temperate rainforest. New Zealand Journal of Ecology, 36(2), 131–140. [Google Scholar]
- Oliva, G. , Collantes, M. , & Humano, G. (2013). Reproductive effort and seed establishment in grazed tussock grass populations of Patagonia. Rangeland Ecology & Management, 66(2), 164–173. 10.2111/REM-D-11-00121.1 [DOI] [Google Scholar]
- Ostfeld, R. S. , Jones, C. G. , & Wolff, J. O. (1996). Of mice and mast. BioScience, 46(5), 323–330. 10.2307/1312946 [DOI] [Google Scholar]
- Pau, S. , Okamoto, D. K. , Calderon, O. , & Wright, S. J. (2018). Long‐term increases in tropical flowering activity across growth forms in response to rising CO2 and climate change. Global Change Biology, 24(5), 2105–2116. 10.1111/gcb.14004 [DOI] [PubMed] [Google Scholar]
- Pearse, I. S. , LaMontagne, J. M. , & Koenig, W. D. (2017). Inter‐annual variation in seed production has increased over time (1900–2014). Proceedings of the Royal Society B‐Biological Sciences, 284(1868), 1900–2014. 10.1098/rspb.2017.1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse, I. S. , LaMontagne, J. M. , Lordon, M. , Hipp, A. L. , & Koenig, W. D. (2020). Biogeography and phylogeny of masting: Do global patterns fit functional hypotheses? New Phytologist, 227(5), 1557–1567. 10.1111/nph.16617 [DOI] [PubMed] [Google Scholar]
- Pearse, I. S. , Wion, A. , Gonzalez, A. , & Pesendorfer, M. B. (2021). Understanding masting for conservation and land management. Philosophical Transactions of the Royal Society B, 376, 20200383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennekamp, F. , Iles, A. C. , Garland, J. , Brennan, G. , Brose, U. , Gaedke, U. , Jacob, U. , Kratina, P. , Matthews, B. , Munch, S. , Novak, M. , Palamara, G. M. , Rall, B. C. , Rosenbaum, B. , Tabi, A. , Ward, C. , Williams, R. , Ye, H. , & Petchey, O. L. (2019). The intrinsic predictability of ecological time series and its potential to guide forecasting. Ecological Monographs, 89(2). 10.1002/ecm.1359 [DOI] [Google Scholar]
- Pesendorfer, M. B. , Ascoli, D. , Bogdziewicz, M. , Hacket‐Pain, A. , Pearse, I. S. , & Vacchiano, G. (2021). The ecology and evolution of synchronized reproduction in long‐lived plants. Philosophical Transactions of the Royal Society B, 376, 20200369. 10.1098/rstb.2020.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, V. S. , MacDonald, S. E. , & Dale, M. R. T. (2005). The interaction between masting and fire is key to white spruce regeneration. Ecology, 86(7), 1744–1750. 10.1890/03-0656 [DOI] [Google Scholar]
- Pukkala, T. , Hokkanen, T. , & Nikkanen, T. (2010). Prediction models for the annual seed crop of Norway spruce and scots pine in Finland. Silva Fennica, 44(4), 629–642. 10.14214/sf.131 [DOI] [Google Scholar]
- Redmond, M. D. , Davis, T. S. , Ferrenberg, S. , & Wion, A. P. (2019). Resource allocation trade‐offs in a mast‐seeding conifer: Pinon pine prioritizes reproduction over defence. Aob Plants, 11(6), plz070. 10.1093/aobpla/plz070 [DOI] [Google Scholar]
- Redmond, M. D. , Forcella, F. , & Barger, N. N. (2012). Declines in pinyon pine cone production associated with regional warming. Ecosphere, 3(12), 14. 10.1890/es12-00306.1 [DOI] [Google Scholar]
- Redmond, M. D. , Weisberg, P. J. , Cobb, N. S. , & Clifford, M. J. (2018). Woodland resilience to regional drought: Dominant controls on tree regeneration following overstorey mortality. Journal of Ecology, 106(2), 625–639. 10.1111/1365-2745.12880 [DOI] [Google Scholar]
- Rohatgi, A. (2020). WebPlotDigitizer (Version 4.4). Retrieved from http://arohatgi.info/WebPlotDigitizer [Google Scholar]
- Royal Botanic Gardens Kew . (2021). Seed Information Database (SID) . [Google Scholar]
- Ruiz‐Benito, P. , Ratcliffe, S. , Zavala, M. A. , Martínez‐Vilalta, J. , Vilà‐Cabrera, A. , Lloret, F. , Madrigal‐González, J. , Wirth, C. , Greenwood, S. , Kändler, G. , Lehtonen, A. , Kattge, J. , Dahlgren, J. , & Jump, A. S. (2017). Climate‐ and successional‐related changes in functional composition of European forests are strongly driven by tree mortality. Global Change Biology, 23(10), 4162–4176. 10.1111/gcb.13728 [DOI] [PubMed] [Google Scholar]
- Salguero‐Gomez, R. , Jones, O. R. , Archer, C. R. , Buckley, Y. M. , Che‐Castaldo, J. , Caswell, H. , Hodgson, D. , Scheuerlein, A. , Conde, D. A. , Brinks, E. , de Buhr, H. , Farack, C. , Gottschalk, F. Hartmann, A. , Henning, A. , Hoppe, G. , Römer, G. , Runge, J. , Ruoff, T. , … Vaupel, J. W. (2015). The COMPADRE Plant Matrix Database: An open online repository for plant demography. Journal of Ecology, 103(1), 202–218. 10.1111/1365-2745.12334 [DOI] [Google Scholar]
- Salguero‐Gómez, R. , Jones, O. R. , Jongejans, E. , Blomberg, S. P. , Hodgson, D. J. , Mbeau‐Ache, C. , Zuidema, P. A. , de Kroon, H. , & Buckley, Y. M. (2016). Fast‐slow continuum and reproductive strategies structure plant life‐history variation worldwide. Proceedings of the National Academy of Sciences of the United States of America, 113(1), 230–235. 10.1073/pnas.1506215112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp, E. W. , Zwolak, R. , Jones, L. R. , Snell, R. S. , Beckman, N. G. , Aslan, C. , Cavazos, B. R. , Effiom, E. , Fricke, E. C. , Montaño‐Centellas, F. , Poulsen, J. , Razafindratsima, O. H. , Sandor, M. E. , & Shea, K. (2019). Intrinsic and extrinsic drivers of intraspecific variation in seed dispersal are diverse and pervasive. Aob Plants, 11(6), 20. 10.1093/aobpla/plz067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selonen, V. , Wistbacka, R. , & Korpimaki, E. (2016). Food abundance and weather modify reproduction of two arboreal squirrel species. Journal of Mammalogy, 97(5), 1376–1384. 10.1093/jmammal/gyw096 [DOI] [Google Scholar]
- Senf, C. , Pflugmacher, D. , Zhiqiang, Y. , Sebald, J. , Knorn, J. , Neumann, M. , Hostert, P. , & Seidl, R. (2018). Canopy mortality has doubled in Europe's temperate forests over the last three decades. Nature Communications, 9. 10.1038/s41467-018-07539-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelef, O. , Weisberg, P. J. , & Provenza, F. D. (2017). The value of native plants and local production in an era of global agriculture. Frontiers in Plant Science, 8. 10.3389/fpls.2017.02069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzot, L. , Schermer, É. , Venner, S. , Delzon, S. , Rousset, C. , Baubet, É. , Gaillard, J.‐M. , & Gamelon, M. (2020). How does increasing mast seeding frequency affect population dynamics of seed consumers? Wild boar as a case study. Ecological Applications, 30(6). 10.1002/eap.2134 [DOI] [PubMed] [Google Scholar]
- Vacchiano, G. , Ascoli, D. , Berzaghi, F. , Lucas‐Borja, M. E. , Caignard, T. , Collalti, A. , Mairota, P. , Palaghianu, C. , Reyer, C. P. O. , Sanders, T. G. M. , Schermer, E. , Wohlgemuth, T. , & Hacket‐Pain, A. (2018). Reproducing reproduction: How to simulate mast seeding in forest models. Ecological Modelling, 376, 40–53. 10.1016/j.ecolmodel.2018.03.004 [DOI] [Google Scholar]
- Whittaker, R. (1970). Communities and ecosystems. Macmillan. [Google Scholar]
- Wion, A. P. , Weisberg, P. J. , Pearse, I. S. , & Redmond, M. D. (2020). Aridity drives spatiotemporal patterns of masting across the latitudinal range of a dryland conifer. Ecography, 43(4), 569–580. 10.1111/ecog.04856 [DOI] [Google Scholar]
- Zhang, W. Y. , Wang, Y. , Xiao, J. Y. , & Lyu, L. X. (2022). Species‐specific coupling of tree‐ring width and litter production in a temperate mixed forest. Forest Ecology and Management, 504, 9. 10.1016/j.foreco.2021.119831 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article, and are openly available via Github: https://github.com/JJFoest/MASTREEplus, Dryad: https://doi.org/10.5061/dryad.18931zd02, or via the MASTREE+ Data Explorer: https://mastreeplus.shinyapps.io/mastreeplus


