Abstract
Purpose
Exosomes are extracellular membrane vesicles. Their content directly reflects the metabolic state of the cells from which they originate and play an important role in cellular functions and pathological states, for example, cancer. The aim was to establish the effect of exosomes from patients diagnosed with CIN1 (grade one cervical intraepithelial neoplasia) on the viability of HeLa cells in culture. It had not been documented, nor had the vesicles obtained by cervicovaginal samples taken by the patients themselves (self-taken vaginal).
Patients and Methods
Exosomes were obtained from self-taken vaginal by patients diagnosed with CIN1 and healthy. The exosomes were characterized by determining the AChE (acetylcholinesterase) activity, obtaining a protein profile, and obtaining images of these by STEM. The effect on cell viability was made in HeLa and HaCaT cells in culture.
Results
Vesicles between 185 nm and 415 nm were observed by STEM. Exosomes show a “protective” effect when those patients without injury are confronted with HeLa cells. On the other hand, exosomes promote viability when they come from injured patients in the presence of the same cells.
Conclusion
Exosomes can be used to identify ideal biomarkers for the early diagnosis of CC (cervical cancer), follow-up of patients, and even treatment given the effects observed on cell cultures.
Keywords: papillomavirus infections, exosomes, uterine cervical dysplasia, patients
Introduction
Exosomes are extracellular membrane vesicles with a diameter ranging from 30 to 150 nm which are secreted by living cells.1,2 They carry several molecules such as DNA, mRNA, microRNA, functional proteins (from the cytosol and some components of the nucleus, mitochondria, endoplasmic reticulum, etc.), and lipids that include cholesterol, sphingomyelin, among others.3–5
The link between cervical cancer (CC) and HPV was demonstrated in the 1980s by Dr. Harald zur Hausen. Persistent high-risk HPV infection is a requirement for the development of this disease,6 as well as microenvironmental factors that can influence tumor transformation and progression, such as immune, endothelial, vascular, and other cells in the tissues, although cytokines and microvesicles7 that sprout from the cell membrane and exosomes may also highlight.8
The macromolecular components of exosomes play an important role in cellular functions and pathological states such as inflammation, immune responses, angiogenesis, cell death, neurodegenerative diseases, and cancer,9 reasons why they have been proposed as biomarkers together with some of their contents,8 hence the importance of their study and how to obtain them easily and non-invasively, specifically in patients with CC and its precursor lesions.
As is known, science is not typical of a profession, the vision of a multidisciplinary group allows the development of higher quality work that opens paths toward solving health problems, which is the case in the present work. The effect of exosomes on cell cultures has not been tested before.
Due to the above, the objective of this work was to evaluate the effect of exosomes of patients diagnosed with grade one Cervical Intraepithelial Neoplasia (CIN1) and of healthy patients (negative HPV) on the viability of HeLa and HaCaT cells in culture. The samples from which the exosomes were obtained were collected by vaginal self-sampling as a new method for this. The usefulness of self-taken vaginal has been demonstrated in the detection of HPV for the prevention of CC and is a method widely accepted by women.10,11
Materials and Methods
Donor Patients
Three patients with CIN1 diagnosed by histopathology donated a sample for the present study. The 3 control samples were donated by women with a negative HPV test for 13 oncogenic types (molecular PCR test). Donors were screened for cervical cancer early detection at a state health center. None of the 6 women reported having symptoms of any other sexually transmitted infection (pain during intercourse, itching, burning when urinating, lesions in the vulvovaginal region, bad smell, discharge, among others). The written informed consent form was signed by each patient. All procedures were done by the Declaration of Helsinki. The protocol was approved by the Ethics and Research Committee of the Faculty of Nursing and Nutrition of the UASLP with folio: CEIFE-2019-286.
Samples for Obtaining Exosomes
The samples were obtained by self-taken vaginal.11 The transport tube contained 2 mL of PBS pH 7.4. Each patient donated 2 samples on the same day, 3.5 mL were used for obtaining exosomes and 0.5 mL for DNA extraction.
Viral Typing
DNA was extracted using the Wizard® Genomic DNA Purification Kit (Promega). Viral typing was done for the high-risk genotypes of HPV-16 (direct oligonucleotide E6-HPV-16: 5´-AATGTTTCAGGACCCACAGG-3´ and reverse oligonucleotide E6-HPV-16: 5´- GTTGCTTGCAGTACACACATTC-3´) and HPV −18 (direct oligonucleotide E6-HPV-18: 5´- ACCCTACAAGCTACCTGATCT-3´ and reverse oligonucleotide E6-HPV-18: 5´- ACCTCTGTAAGTTCCAATACTGTC-3´). The real-time polymerase chain reaction (qPCR) assay was carried out on the StepOne Real-Time PCR System (Thermo Fisher Scientific) to detect HPV DNA using the following protocol: 7 pmol/µL of the direct oligonucleotide, 5 pmol/µL of the reverse oligonucleotide, 5 µL of SYBR Green (Real-time PCR Master Mix, Thermo Fisher Scientific), 50 ng/µL of DNA extracted from the samples were used and adjusted to a final volume of 10 µL with sterile H2O. The amplification reaction was started at 95 ° C for 15 min followed by 40 cycles of amplification (denaturation at 95 ° C for 5 sec, alignment at 60 ° C for 30 sec and extension at 72 ° C for 15 sec). The reaction mixture without DNA was used as a negative control. Oligonucleotides of the human β-globin gene (PCO4: 5’-CAACTTCATCCACGTTCACC −3 “and GH20: 5”-GAAGAGCCAAGGACAGGTAC-3’) were used to guarantee the quality of the DNA of the samples.
Exosome Isolation
It was made using the Total Exosome Isolation Reagent from the cell culture media Kit (Invitrogen™). Briefly: 750 µL of self-collected cervicovaginal fluid were centrifuged at 2000 × g for 30 minutes in a refrigerated micro centrifuge (Z 216 MK, HERMLE) for the removal of cells and cell debris. The supernatant was transferred to a sterile 2mL Eppendorf tube and 500 µL of the exosome isolation reagent kit was added to the mix by strong inversion and allowed to refrigerate at 4 ° C for 12 hours. The mixture was centrifuged again at 10,000 × g for 1h obtaining a pellet that was resuspended in 1600 μL of sterile 1 X PBS pH 7.4. In this mix are exosomes.
Characterization of Exosomes
Determination of Acetylcholinesterase (AChE) Activity
The determination was carried out with the Amplex® Red Acetylcholine/Acetylcholinesterase Assay Kit, using 10-acetyl 3,7-dihydroxyphenoxazine (Amplex Red Reagent) and a fluorogenic probe for hydrogen peroxide (H2O2). The final product generated is resorufin, which has high fluorescence. A 100 mM acetylcholine stock was prepared, and a standard curve was made. Samples were read on a Thermo Scientific ™ Multiskan ™ microplate fluorometer at 530 nm excitation and 560 nm emission.
Determination of Protein Concentration
The determination was made with a commercial reagent based on the Bradford spectrophotometric method (Bio-Rad Protein Assay; Bio-Rad Lab., Germany) in microassay, which has linearity for protein concentrations between 1 µg/mL to 25 µg/mL.
Electrophoresis in Polyacrylamide Gels Under Denaturing Conditions (SDS-PAGE)
Once the amount of proteins in the exosome sample had been quantified, a 12% SDS-PAGE was performed. Previously, 30 µL of exosomes were placed in the presence of 20 µL of Proteinase K and incubated at 56 ° C/24h. Subsequently, the proteins were denatured by immersing the sample in an Eppendorf tube in boiling water for 3 min and then applying it to the wells of the gel; the run was developed at a constant voltage of 80 V for approximately 2 h or until the bromophenol blue reached the base of the gel. The gel was stained with Coomassie blue G-250 (Bio-Rad).
Protein Band Density
Using the ChemiDoc XRS system (Bio-Rad) using Image Lab software, the density analysis of the bands was made. An image was obtained from SDS-PAGE at 12% density of the protein bands, providing a quantitative result. The system takes molecular weight markers as a standard to establish concentration.
Preparation of Samples for Microscopy (STEM) and Image Acquisition
Images of the exosomes were obtained by scanning transmission electron microscopy (STEM). The negative staining method “Side Blotting” was used to prepare the sample.12 Briefly, the grid was taken with negative forceps and 3–5 µL of the sample was placed on the grid (Holey carbon grid, Ted Pella Inc., USA) and allowed to absorb at room temperature for one minute. Then, the edge of the grid was touched with a filter paper to allow capillary action to remove the liquid. Subsequently, a 50 µL drop of ultra-pure water (Milli-Q) was placed on a parafilm paper, the drop was touched with the grid and again with the filter paper (this process was repeated twice). Subsequently, a drop of the staining agent (2% uranyl acetate) was placed on the grid and left for 30 seconds at room temperature to remove the agent with filter paper (three times). Finally, the samples were allowed to air dry at room temperature. Images were obtained with the FEI Helios G4 CX dual-beam workstation microscope, Hillsboro, Oregon, USA, at the National Laboratory of Science and Technology in Terahertz (CIACYT, San Luis Potosí, Mexico). Brightfield images were obtained with a voltage of 30 kV.
Effect of Exosomes on the Proliferation and Viability of HeLa and HaCaT Cells
HeLa and HaCaT cell lines were purchased from the ATCC (American Type Culture Collection). 2.5×104 cells were cultured in 96-well plates in DMEM medium with 10% fetal bovine serum (FBS) and 1% antibiotic (penicillin) at 37 °C and 5% CO2 flux, in the presence of different volumes (20, 40, 60 and 80 µL) of exosomes for 24 h. Cells without exosomes (0 µL) were also quantified and triplicates of each cell line were made. The determination of cell viability was carried out using an MTT (3- (4,5-dimethylthiazol-2-yl) −2,5-diphenyltetrazole) assay to measure cell survival.
Statistical Analysis
The GraphPad Prism 8.0.2 program was used. An ANOVA analysis of variances was performed, to know if the data were within normality, the Shapiro–Wilk test, Kolmogorov–Smirnov test, and qqPlot were performed. With the normalized data, the Tukey’s test (p = < 0.05) was used.
Results
Exosome Characterization
Once the exosomes had been purified, the activity of the AChE enzyme was determined as part of its characterization. This enzyme is a marker for exosomes. All the samples presented absorbance within the ranges established through the calibration (data not shown).
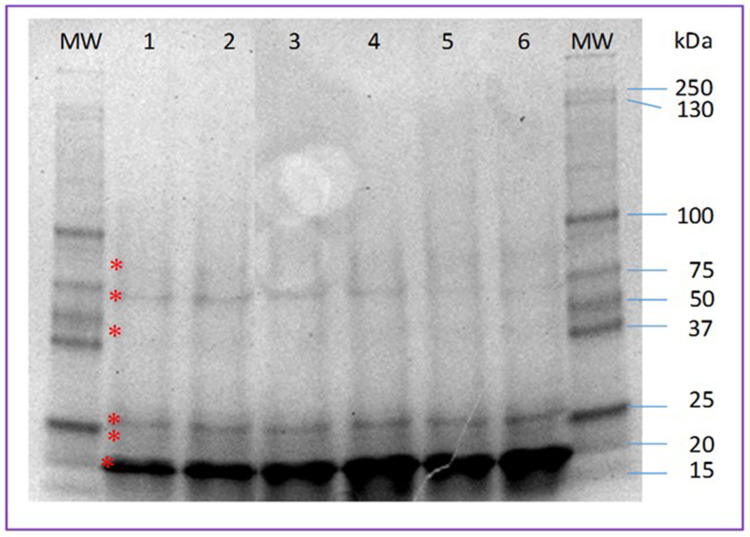
Figure 1 shows the protein profile of the exosomes of women with injury and without injury. The upper band of the doublet at the 25 kDa marker level as well as the majority band at the 20 kDa marker level are more intense in samples from patients with lesions.
Figure 1.
Protein profile of exosomes obtained from samples of patients with and without cervical intraepithelial lesion. Lanes 1–3, samples from patients without injury; lanes 4–6, samples from patients with injury. 12% SDS-PAGE. The asterisk indicates the 6 main bands found.
Abbreviations: MW, molecular weight markers; kDa, molecular weight in kilodaltons.
Tables 1 and 2 show the molecular weight and percentage of intensity of the protein bands found in the exosomes of the samples from patients with and without injury, respectively. Most are similar both in weight and intensity, however, those that are different between control samples and problem samples stand out.
Table 1.
Molecular Weight and Intensity Percentage of Each Protein Bands Analyzed in Patient with CIN1 Diagnosis
| MW (KDa) | Int (%) | Samp. 1 (kDa) | Int (%) | Samp. 2 (kDa) | Int (%) | Samp. 3 (kDa) | Int (%) |
|---|---|---|---|---|---|---|---|
| 250 | 1.6 | —– | —– | —– | —– | —– | —– |
| 150 | 1.6 | —– | —– | —– | —– | —– | —– |
| 100 | 8.7 | —– | —– | —– | —– | —– | —– |
| 75 | 5.3 | 75.8 | 1.3 | —– | —– | —– | —– |
| 50 | 10.3 | 60.1 | 6.1 | 58.5 | 4.7 | 57 | 3.3 |
| 37 | 13.5 | —– | —– | —– | —– | —- | —– |
| 25 | 30.3 | 24.6 | 9.6 | 23.8 | 21.0 | 22.9 | 18.8 |
| 20 | 14.4 | —– | —– | —– | —– | —– | —– |
| 15 | 14.3 | 16.5 | 82.9 | 15.9 | 74.3 | 14.8 | 77.9 |
Notes: The additional bands found are in bold.
Abbreviations: MW, molecular weight in kilodaltons; kDa, kilodaltons; Samp, sample; Int, intensity of the bands.
Table 2.
Molecular Weight and Intensity Percentage of Each of the Protein Bands Analyzed in Samples from Patients Without Lesion
| MW (KDa) | Int. (%) | Samp. 1 (kDa) | Int. (%) | Samp. 2 (kDa) | Int. (%) | Samp. 3 (kDa) | Int. (%) |
|---|---|---|---|---|---|---|---|
| 250 | 1.6 | —– | —– | —– | —– | —– | —– |
| 150 | 1.6 | —– | —– | —– | —– | —– | —– |
| 100 | 8.7 | —– | —– | 101.8 | 0.2 | 106.4 | 0.2 |
| 75 | 5.3 | 62.9 | 0.8 | —– | —– | 75.8 | 0.1 |
| 50 | 10.3 | —– | —– | 59.5 | 0.4 | —– | —– |
| 37 | 13.5 | —– | —– | —– | —– | 36.1 | 0.2 |
| 25 | 30.3 | 23.6 | 18.1 | 23.9 | 18.9 | 24.3 | 18.2 |
| 20 | 14.4 | —– | —– | —– | —– | —– | —– |
| 15 | 14.3 | 15.8 | 81.1 | 16.5 | 80.5 | 17.4 | 81.4 |
Notes: The additional bands found are in bold.
Abbreviations: MW, molecular weight in kilodaltons; kDa, kilodaltons; Samp, sample; Int, intensity of the bands.
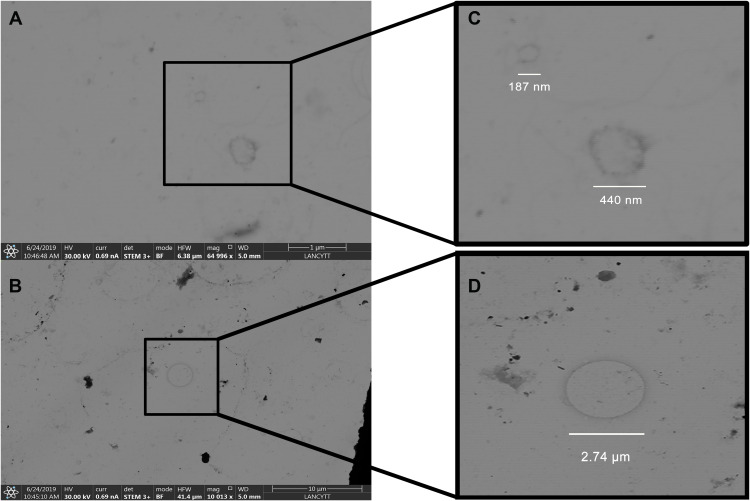
As a crucial part of the characterization, vesicles whose sizes vary between 185 nm and 415 nm were observed through STEM (Figure 2A and B). On the other hand, Figure 2C shows classic-shaped vesicles up to 274 nm with a defined membrane profile. A magnification of Figure 2C is shown in Figure 2D.
Figure 2.
Images of exosomes. Samples were obtained by vaginal self-sampling. The images were obtained by STEM. (A and B) shows vesicles of different sizes corresponding to exosomes. (C) corresponds to magnification of figure (A), where it can be observed two exosomes of different diameters. (D) is the magnification of the exosome found in figure (B), which was six times bigger than the others one. The images were taken by Dr. John Eder Sánchez and Dr. Alejandra Loyola Leyva at the National Laboratory of Science and Technology in Terahertz (LANCYTT) located in the Coordination for Innovation and Application of Science and Technology (CIACYT), San Luis Potosí, S.L.P., Mexico.
Viral Typing of Samples
There was no amplification for HPV 16 and HPV 18 in any of the cervicovaginal swab samples, both from women with and without a lesion (data not shown).
Effect of Exosomes on the Viability of HeLa and HaCaT Cells
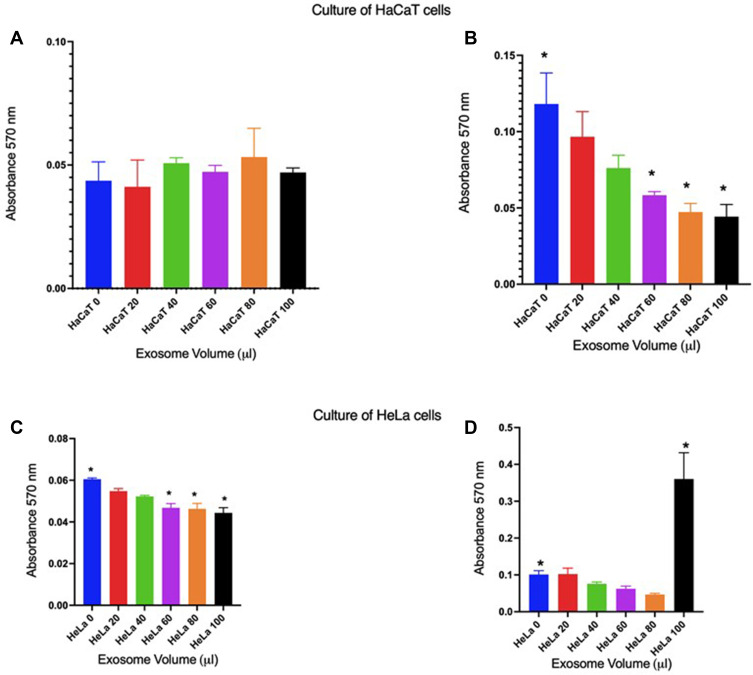
Figure 3 shows the cell viability of the HaCaT line (non-cancerous) when placed in the presence of different volumes of exosomes. In the case of those obtained from women without injury (Figure 3A), it is observed that no significant change in viability occurs compared to the culture without the presence of exosomes, except when 80µL is added, where it seems that viability increases, however, there is no significance compared to the control. On the contrary, when HaCaT cells are cultured in the presence of different volumes of exosomes from patients with lesions (Figure 3B), it is observed that the effect on cell viability is inversely proportional to the decrease in cell viability about the volumes of exosomes, that is, when a larger volume of exosomes is used, cell viability is lower. It should be noted that the effect of 60, 80 and 100 µL are significant (p = <0.05) compared to the control without exosomes. When doing the same experiment but in HeLa (CC) cell cultures, it is observed that in the presence of exosomes from patients without injury, viability is minimal compared to the control, however, there is significance when 60, 80, and 100 µL are added (p = <0.05), which may be due to the “content” of the exosomes of these patients, which generates an effect on cancer cells at least in this case. On the other hand, when exosomes from a patient with HeLa cell injury are confronted, it is observed that there is no effect with volumes between 20 and 80 µL, but when 100 µL are added there is an effect of a significant increase in proliferation (p = <0.05).
Figure 3.
Effect of exosomes on the viability of HaCaT and HeLa cells in culture. (A and B), HaCaT cells; in (C and D), HeLa cells. (A and C), exosomes from patients without injury; (B and D), exosomes from patients with injury. * = cell viability with significance compared to the control without exosomes (p = <0.05).
Discussion
Different studies mention the presence of proteins in exosomes as possible biomarkers in different types of cancer, for example, the protein profile of exosomes derived from colorectal cancer cells allowed us to observe several proteins that are differentially expressed. Therefore, these vesicles have been considered potential sources of tumor markers for different types of cancer.13 Exosomes have been found in a variety of biological fluids such as urine, saliva, milk, and cervicovaginal discharge. These structures are of great interest because of their relationship with diseases such as cancer and because of their potential for diagnosis.14 Therefore, it was decided to carry out the present work in which the exosomes were isolated from samples taken by the participants. The usefulness of self-taken vaginal has been previously reported. The greatest advantage is that it does not need to be acquired by a health professional and that the patient can take the sample in the comfort of a bathroom or at home.10,11 This implied demonstrating that the exosomes were isolated with this technique, for which characterization was made.
The protein profiles in SDS-PAGE vary between the sample from which the exosomes were obtained, thus, in the present work the profile was similar between the samples of patients with and without injury, although by density it was possible to observe some differences between the molecular weights of the bands. In previous work, the protein profile of exosomes isolated from the plasma of healthy individuals and patients with retinoblastoma was reported; practically no differences were observed between some samples and others (14 bands).15 In another study, the protein profile of human plasma exosomes was observed, 22 bands were identified in a 4% −12% SDS-PAGE.16 Another part included in the characterization of the exosomes obtained in the present study was their observation using STEM, whose sizes varied between 200 and 400 nm. The presence of exosomes in cervicovaginal lavage samples using the same type of microscopy has been reported and spherical vesicles with sizes between 40–100 nm have been described, although the isolation was done by differential centrifugation.17 In another work, the structure of exosomes purified from plasma with sizes from 30 −100 nm was observed.15 In a work where exosomes were obtained from samples taken directly from the endocervix, it was observed that they measured between 100 nm and 280 nm.18 The effect of exosome contents has been studied and differential expression proteins have been identified,19 but to date, the effect of exosomes from patients with precancerous lesions compared with exosomes from patients without lesions (negative HPV) on cells in culture, in this case, HaCaT (non-cancer) and HeLa (CC) cells. The findings found in the present work indicate that the content of these vesicles has a “protective” effect when exosomes of patients without injury are confronted with cancer cells and that at a certain moment they could behave as control of tumor growth. On the other hand, exosomes promote viability when they come from patients with lesions in the presence of the same cells, which indicates that they contain some molecule (s) that influence the viability of non-cancer cells, but in turn, they favor the proliferation of cancer cells, it is understood that the “content” of these exosomes has some molecule (s) that favors cell viability and, therefore, could favor tumor growth in patients.
It should be mentioned that it is reported that exosomes derived from cancer cells inhibit cell proliferation, have cytotoxic effects on natural killer (NK) cells, and induce apoptosis in T cells by containing the Fas ligand. However, in these studies, the effect of exosomes was observed by determinations of the presence of proteins on the surface that act as ligands or by Western blot analysis where the expression of typical exosome markers such as class I HCM and TSG101 was demonstrated (protein from tumor susceptibility gene 101).20,21
In various studies, it is mentioned that exosomes have a bidirectional exchange of content, for which they collaborate in maintaining cell homeostasis, but there is also exchange within a tumor environment influencing tumor progression, metastasis, and a response to treatment.1,2
In the work reported by Wei W-F, Zhou C-F, Wu X-G et al cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. It was found that exosomal miR-221-3p secreted in cervical squamous cell carcinoma could be a potential therapeutic target as well as a diagnostic biomarker for the development of this type of cancer.22 On the other hand, exosomal TUG1 (taurine up-regulated 1) affected HUVEC (human umbilical vein endothelial cells) growth by inhibiting the activity of caspase-3 and modifying apoptotic proteins. Collectively, exosomal TUG1 could be a new molecular marker for the initial diagnosis of CC.23
Finally, it is necessary to specify that the samples from women with CIN1 had a negative result when typified for HPV types 16 and 18. This could be because the lesion is generated by some other viral type of high oncogenic risk, or which is caused by some low-risk viral type such as 6 or 11. In this regard, it is known that low-risk viruses can cause low-grade lesions (CIN1 and CIN2). In a population-based research work, they analyzed the 3-year risks for women to develop CIN2 + or CIN3 + associated with HPV 6, 11, and 42 infections. The authors found that a small number of women with infection by the viral types mentioned low risk or with multiple infections with these were diagnosed with CIN2 + but not with CIN3 +.24
In conclusion, the standardization of the isolation and characterization of exosomes by the method proposed here is essential to obtain reproducible results of the analyzes for their possible clinical and therapeutic application, since the identification of protein markers in exosomes derived from tumors could be used in the development of diagnostic tests, and specifically in the case of CC, using a minimally invasive method would be important.
Exosomes can be used to identify ideal biomarkers for the early diagnosis of CC, follow-up of patients, and even treatment given the effects observed on cell cultures. It would be important to repeat the effect of exosomes on cell cultures using samples from patients with CIN2 or CIN3 as well as neoplastic lesions and to identify their content.
Acknowledgments
This work was supported by the National Laboratory of Science and Technology of Terahertz (LANCYTT) [project 278291 (SRE-CONACYT) and project P105 of CEMIE-Solar (Secretariat of Energy-CONACYT)] and FAI project, number 29010009, UASLP.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhang M, Zhang CG, Ding W. Exosome in cancer diagnosis and treatment. Sheng Li Ke Xue Jin Zhan. 2014;5:372–378. Chinese. PMID: 25764798. [PubMed] [Google Scholar]
- 2.Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells. 2019;4:E307. doi: 10.3390/cells8040307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobius W, van Donselaar E, Ohno-Iwashita Y, et al. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x [DOI] [PubMed] [Google Scholar]
- 4.Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb15 [DOI] [PubMed] [Google Scholar]
- 5.Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a Web-Based Compendium of Exosomal Cargo. J Mol Biol. 2016;428:688–692. doi: 10.1016/j.jmb.2015.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walboomers JMM, Jacobs MV, Manos MM. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. Pathol J. 1999;189:12–19. doi::: [DOI] [PubMed] [Google Scholar]
- 7.Becker A, Thakur BK, Weiss JM, et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guenat D, Hermetet F, Pretet JL, Mougin C. Exosomes and other extracellular vesicles in HPV transmission and carcinogenesis. Viruses. 2017;9:e211. doi: 10.3390/v9080211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howitt J, Hill AF. Exosomes in the Pathology of Neurodegenerative Diseases. J Biol Chem. 2016;291:26589–26597. doi: 10.1074/jbc.R116.757955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;26:1868–1873. doi: 10.1016/S0140-6736(11)61522-5 [DOI] [PubMed] [Google Scholar]
- 11.Terán-Figueroa Y, Muñiz-Carreón P, Gallegos-Arévalo YG, et al. Acceptability of self-taken vaginal for early detection of HPV DNA in women with limited access to health services: an alternative to increase the coverage in a state of the Mexican Republic. Health. 2013;12:2162–2168. doi: 10.4236/health.2013.512295 [DOI] [Google Scholar]
- 12.Scarff CA, Fuller MJ, Thompson RF, Ladaza MG. Variations on Negative Stain Electron Microscopy Methods: tools for Tackling Challenging Systems. J Vis Exp. 2018;132:e57199. doi: 10.3791/57199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji H, Greening DW, Barnes TW, et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics. 2013;13:1672–1686. doi: 10.1002/pmic.201200562 [DOI] [PubMed] [Google Scholar]
- 14.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orozco-Romero MJ, Borja-Urby R, Ponce-Castañeda MV, Durán-Figueroa NV. Isolation and cellular characterization of exosomes from plasma for their use as diagnostic biomarkers. Acta Bioquím Clín Latinoam. 2016;4:783–790. [Google Scholar]
- 16.Looze C, Yui D, Leung L, et al. Proteomic Profiling of Human Plasma Exosomes Identifies PPARγ as an Exosome-associated Protein. Biochem Biophys Res Commun. 2009;3:433–438. doi: 10.1016/j.bbrc.2008.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Sun H, Wang X, et al. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int J Mol Sci. 2014;1:758–773. doi: 10.3390/ijms15010758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mata-Rocha M, Rodríguez-Hernández RM, Chávez-Olmos P, et al. Presence of HPV DNA in extracellular vesicles from HeLa cells and cervical samples. Enferm Infecc Microbiol Clin. 2020;4:159–165. doi: 10.1016/j.eimc.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 19.Xiao Y, Zhong J, Zhong B, et al. Exosomes as potential sources of biomarkers in colorectal cancer. Cancer Lett. 2020;476:13–22. doi: 10.1016/j.canlet.2020.01.033 [DOI] [PubMed] [Google Scholar]
- 20.Clayton A, Mitchell JP, Court J, et al. Human Tumor-Derived Exosomes Down-Modulate NKG2D Expression. Journal Immunol. 2008;11:7249–7258. doi: 10.4049/jimmunol.180.11.7249 [DOI] [PubMed] [Google Scholar]
- 21.Andreola G, Rivoltini L, Castelli C, et al. Induction of Lymphocyte Apoptosis by Tumor Cell Secretion of FasL-bearing Microvesicles. J Exp Med. 2002;10:1303–1316. doi: 10.1084/jem.20011624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu XG, Zhou CF, Zhang YM, et al. Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis. 2019;22:397–410. doi: 10.1007/s10456-019-09665-1 [DOI] [PubMed] [Google Scholar]
- 23.Lei L, Mou Q. Exosomal taurine up-regulated 1 promotes angiogenesis and endothelial cell proliferation in cervical cancer. Cancer Biol Ther. 2020;21:717–725. doi: 10.1080/15384047.2020.1764318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castle PE, Hunt WC, Langsfeld E, Wheeler CM; New Mexico HPV Pap Registry Steering Committee. Three-year risk of cervical precancer and cancer after the detection of low-risk human papillomavirus genotypes targeted by a commercial test. Obstet Gynecol. 2014;1:49–56. doi: 10.1097/AOG.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]