Abstract
Raw milk was artificially contaminated with declumped cells of Mycobacterium avium subsp. paratuberculosis at a concentration of 104 to 105 CFU/ml and was used to manufacture model hard (Swiss Emmentaler) and semihard (Swiss Tisliter) cheese. Two different strains of M. avium subsp. paratuberculosis were tested, and for each strain, two model hard and semihard cheeses were produced. The survival of M. avium subsp. paratuberculosis cells was monitored over a ripening period of 120 days by plating out homogenized cheese samples onto 7H10-PANTA agar. In both the hard and the semihard cheeses, counts decreased steadily but slowly during cheese ripening. Nevertheless, viable cells could still be detected in 120-day cheese. D values were calculated at 27.8 days for hard and 45.5 days for semihard cheese. The most important factors responsible for the death of M. avium subsp. paratuberculosis in cheese were the temperatures applied during cheese manufacture and the low pH at the early stages of cheese ripening. Since the ripening period for these raw milk cheeses lasts at least 90 to 120 days, the D values found indicate that 103 to 104 cells of M. avium subsp. paratuberculosis per g will be inactivated.
Mycobacterium avium subsp. paratuberculosis is the etiologic agent of Paratuberculosis, a chronic granulomatous enteritis in ruminants, also known as Johne's disease (4, 5, 7). Most often, the infection is acquired by calves. After a prolonged incubation phase, lasting 2 to 3 years in cattle, the infection results in disease. Typical clinical symptoms of this disease are rapid weight loss, chronic diarrhea, and failure to respond to treatment. Paratuberculosis was first recognized in Europe in 1895 and was detected in an increasing number of countries during the twentieth century. It occurs worldwide in several other domesticated species, including goats, sheep, deer, and South American camelids. It has also been recorded in a wide range of other domesticated, wild, and zoo animals.
Crohn's disease is a chronic inflammatory bowel disease that primarily affects the ileum in humans and bears considerable similarity to the histopathology of cattle with Johne's disease (2). Recent studies have shown that a high percentage of people with Crohn's disease are infected with M. avium subsp. paratuberculosis. Whether the association of M. avium subsp. paratuberculosis and Crohn's disease is causal or coincidental is not known (7). However, the similarities between Johne's and Crohn's disease have raised the question of whether milk, among other factors, could be a vector to transmit M. avium subsp. paratuberculosis from cattle to humans.
The possible link to Crohn's disease is not the only disturbing feature of M. avium subsp. paratuberculosis. Together with Mycobacterium lepraemurium, the three subspecies of M. avium, i.e., M. avium subsp. avium, M. avium subsp. paratuberculosis, and M. avium subsp. silvaticum form the M. avium complex (MAC) (37). MAC infections have become increasingly common in North America and Europe, and MAC is the most common cause of nontuberculous mycobacteria human disease. The greatest increase in MAC infections during the last decade has been in patients with AIDS (24). In a study on environmental risk factors for MAC acquisition in persons with immunodeficiency virus infection, consumption of hard cheeses was found to be a risk factor (17). In this study, hard cheese samples were examined for mycobacteria, but MAC organisms could not be recovered.
M. avium subsp. paratuberculosis is a small gram-positive, acid-fast, slow-growing bacterium with fastidious growth requirements. It has a tendency to occur in large clumps. Its growth in vitro is dependent on the presence of mycobactin, an iron-chelating agent produced by other mycobacteria (21). As a consequence, multiplication outside a host cell is not possible. The bacterium is more resistant to adverse conditions, such as low pH, high temperature, and salt, than most other pathogenic bacteria (34). These properties allow M. avium subsp. paratuberculosis to survive for long periods in the environment, as was demonstrated for soil, feces, and liquid manure (9, 19, 23), as well as surface water and drinking water (6, 8, 38).
It has been documented that cows with clinical Johne's disease or asymptomatically infected cows in the latter stages of infection shed viable M. avium subsp. paratuberculosis cells into their milk, albeit at low concentrations, i.e., 2 to 8 CFU/50 ml of milk (32, 35, 36). Since fecal material from clinically infected cows may contain as much as 109 CFU of M. avium subsp. paratuberculosis cells per g of feces, fecal contamination of raw milk may provide a much larger contribution of this microorganism rather than the actual shedding of the bacteria directly into milk by clinically infected cows (28).
Chiodini and Hermon-Taylor reported laboratory studies demonstrating that M. avium subsp. paratuberculosis was more heat resistant than Mycobacterium bovis, one of the bacterial pathogens used to define pasteurization time and temperature standards (3). Their findings led several research groups to investigate the death of M. avium subsp. paratuberculosis cells under different pasteurization conditions (10, 11, 12, 15, 16, 21, 26, 31, 33; J. R. Stabel, Letter, Appl. Environ. Microbiol. 64:2760–2761, 1998). The high-temperature, short-time (HTST) pasteurization method (71.7°C for 15 s) resulted in a high death rate, but according to some reports, survivors may occur. Since the effect of pasteurization on M. avium subsp. paratuberculosis seems to be limited, it is obvious that any treatment of raw milk below the minimal HTST pasteurization requirements will not be effective in eliminating this potentially human-pathogenic mycobacterium. As a consequence, cheese and other milk products made from either raw or pasteurized milk may harbor survivors which could pose a risk to consumers.
Of all mycobacteria possibly present in raw milk M. bovis (sometimes referred to as Mycobacterium tuberculosis typus bovinus) has invited the most attention regarding its behavior in cheese. Cheese contaminated with M. bovis has been shown to be infective for long periods of time: more than 180 days in Camembert, 220 days in Cheddar (14), and 2 months in Camembert and Edam (13). Virulent tubercle bacilli were found in Bulgarian white cheese made from artificially infected milk after 120 days of storage (18). Kästli and Binz produced different cheeses from raw milk naturally infected with M. bovis cells (estimated 1 to 10 CFU/ml). In Swiss Emmentaler, infective M. bovis cells were found after 5 but not 22 days, and in Gruyère, cells survived for 22 but not 31 days. In Swiss Tilsiter, cells still remained virulent after 305 days. Camembert harbored infective M. bovis cells for 47 days (20). In six emmental cheeses made from artificially contaminated milk, one was found to produce tuberculosis when injected into guinea pigs after 3 months of maturation (13). During ripening of blue cheese made from raw milk with bovine tubercle bacilli (104/ml), a decrease in numbers was observed during the first and second weeks, but they were still present after 3 to 4 months (22).
To our knowledge, the only previous work with M. avium subsp. paratuberculosis in cheese was carried out by Sung and Collins (34), who prepared a soft Hispanic-style cheese under laboratory conditions from milk spiked with M. avium subsp. paratuberculosis (final concentration, 104 CFU/ml of milk). They observed a decimal reduction time (D value) of 59.9 days. In cheeses made with sublethally injured (by heat treatment at 62°C for 240 s) cells, M. avium subsp. paratuberculosis numbers declined more rapidly (D value of 36.5 days).
The aim of this work was to study the length of time that M. avium subsp. paratuberculosis cells may survive in two different Swiss types of hard and semihard cheeses made from raw milk under conditions similar to traditional cheese manufacture. The findings will form a basis to estimate the possible risk that such cheeses may pose to consumers.
MATERIALS AND METHODS
M. avium subsp. paratuberculosis strains.
Two strains of M. avium subsp. paratuberculosis were tested: ATCC 19698 (obtained from DSMZ-German Collection of Microorganisms and Cell Cultures GmbH, Braunschweig, Germany) and strain Niebüll, which was originally isolated from raw milk (kindly provided by P. Hammer, Federal Dairy Research Centre, Institute for Hygiene and Food Safety, Kiel, Germany).
Cultures were maintained on Middlebrook and Cohn 7H10 agar slants containing 10% (vol/vol) Middlebrook OADC (oleic acid-dextrose-catalase) enrichment (Becton Dickinson Microbiology Systems, Sparks, Md), 0.5% glycerol (Fluka, Buchs, Switzerland), and 0.0002% (wt/vol) mycobactin J (Synbiotics Europe SAS, Lyon Cedex, France). The slants were incubated at 37°C for 4 weeks and then held at 5°C.
Fluid cultures were grown similar to the procedures used by Sung and Collins (33) and Keswani and Frank (21) using Middlebrook 7H9 broth medium containing 10% (vol/vol) Middlebrook ADC enrichment (albumine-dextrose-catalase; Becton Dickinson Microbiology Systems), 0.2% (vol/vol) glycerol (Fluka), and 0.0002% (wt/vol) mycobactin J (Synbiotics Europe SAS). The M. avium subsp. paratuberculosis strains were inoculated in 50 ml of medium in 100-ml glass bottles and incubated at 37°C for 3 months with constant gentle shaking.
Preparation of cell suspensions for cheese making.
Aliquots of 25 ml of M. avium subsp. paratuberculosis fluid cultures were centrifuged at 4,000 × g for 20 min and resuspended in 2.5 ml of Middlebrook 7H9 broth. To break up clumps of M. avium subsp. paratuberculosis cells, the suspensions were syringed 20 times as was described by O'Connor et al. (29): the suspensions were drawn up by the syringe and expelled in less than 2 s through a 0.2-by-40-mm needle (Becton Dickinson Microbiology Systems). Sterile glycerol was added to a final concentration of 10% (vol/vol). The cell suspensions were stored at −40°C before being used as inoculum in the cheese making. Seven to 10 days prior to cheese making, the cell suspensions were thawed at room temperature and 0.5 ml was transferred to 9.5 ml of sterile reconstituted skim milk to achieve an inoculation culture of the desired concentration which was stored at 5°C.
Manufacture, ripening, and sampling of cheese.
Cheeses were manufactured according to traditional procedures as previously published (1) and described below. With each strain, two model hard (Swiss Emmentaler) and two model semihard (Swiss Tilsiter) cheeses were manufactured in a special cheese-making system that was self-contained. Rigorous safety precautions were taken to prevent any infection of persons and contamination of the environment as required by Swiss legislation regarding the use of microorganisms of risk class 2. One model cheese was produced each day. Raw bovine milk made up of a mixture of evening and morning milk was used: 90 liters for hard cheese and 70 liters for semihard cheese. For the semihard cheese, the raw milk was heat treated prior to cheese manufacture by heating to 60°C within 20 min. After holding this temperature for 15 s, the milk was immediately cooled to 31°C. The milk was inoculated with lactic starter culture and at the same time with the M. avium subsp. paratuberculosis test strain to obtain 104 to 105 CFU/ml of vat milk. In addition, a culture of propionic acid bacteria was added for hard cheese. Rennet was added to induce coagulation within 35 min. Curds were cut into cubes with dimensions of 2 to 8 mm per side for hard cheese and 5 to 10 mm per side for semihard cheese and maintained at 53°C for 45 min and 44°C for 10 min, respectively. The whey was removed, and cheeses were pressed (22 h) in a 30-cm-diameter form. The temperature in the pressing station was controlled in order to achieve uniform cooling rates in the outer and inner zones of the model cheeses. After salting in 20% (wt/vol) brine for 24 h, the cheeses were individually vacuum sealed in plastic foil and stored at temperatures usually applied in traditional cheese ripening, i.e., for hard cheese, 10 days at 12°C, then 60 days at 22°C, and 50 days at 12°C. Semihard cheese was ripened for 120 days at 14 to 15°C.
These procedures resulted in cheese wheels weighing 8 kg for hard cheese, which is approximately 7 to 10% of a normal Swiss Emmentaler cheese wheel. The water content of the cheese after 24 h was 375 to 385 g/kg of cheese, and the salt (NaCl) content after 120 days was (mean ± standard deviation [SD]) 4 ± 1 g/kg. Semihard cheese yielded wheels weighing 6.5 kg, which corresponds to the actual size of marketed cheese of this type. The water content after 24 h was 400 to 410 g/kg, and the salt content after 120 days was 16 ± 2 g/kg. The final pH of the 120-day cheese ranged from 5.7 to 5.8 in hard and semihard cheese.
Ten milliliters of raw milk after contamination was used to determine the contamination level of raw milk in the vat. Also, 10 ml was taken before adding the M. avium subsp. paratuberculosis inoculum to check for natural contamination of the raw milk with M. avium subsp. paratuberculosis. Cheese samples were taken after 1, 7, 30, 60, 90, and 120 days of ripening. The vacuum-sealed cheeses were unpacked, and samples were taken with a cheese borer with a 20-mm diameter. Then, cheeses were vacuum sealed again in a new plastic foil.
Enumeration method.
Fluid samples were diluted 1:10 in 0.8% (wt/vol) sodium cloride containing 0.1% (wt/vol) caseine peptone. Two grams of cheese plugs was aseptically transferred into a sterile stomacher bag. Eighteen milliliters of prewarmed (40°C) sterile peptone water consisting of 0.5% (wt/vol) sodium cloride, 1.0% (wt/vol) caseine peptone, and 2.0% (wt/vol) sodium citrate was added. The mixture was homogenized in a Stomacher peristaltic lab-blender 400 (Seward Medical, London, United Kingdom) for 3 min. The resulting suspension was diluted 1:10 and plated on 7H10-PANTA agar. This agar was made up of Middlebrook and Cohn 7H10 agar base supplemented with 10% (vol/vol) Middlebrook OADC enrichment, 5.0% (vol/vol) PANTA PLUS antibiotic supplement (polymyxin B-amphotericin B-nalidixic acid-trimethoprim-azlocillin; Becton Dickinson Microbiology Systems), 0.5% glycerol, and 0.0002% (wt/vol) mycobactin J (Synbiotics Europe SAS).
To monitor the CFU of M. avium subsp. paratuberculosis on the cheese surface, contact samples were taken with 7H10-PANTA agar in RODAC plates (Becton Dickinson Microbiology Systems) with a contact surface of 25 cm2. Samples were taken after 30, 60, and 90 days of ripening.
All agar plates were packed in sterile plastic bags and incubated at 37°C. After 21 days of incubation, colonies typical of M. avium subsp. paratuberculosis were counted. A representative number of typical colonies and all colonies of uncertain appearance were confirmed by Ziehl-Neelsen acid-fast staining (Difco, Detroit, Mich.).
Decontamination of cheese samples.
To decontaminate the cheese samples from bacteria originating from the raw milk flora that might grow on 7H10-PANTA agar, the N-acetyl-l-cysteine (NALC)-NaOH method as described in the Manual of Clinical Microbiology (24) was applied. In a 50-ml centrifuge tube, 10 ml of the homogenized sample was mixed with 10 ml of digestant consisting of sterile 0.1 M trisodium-citrate, 4% (wt/vol) sodium hydroxide, and 0.5% NALC (Fluka) and vigorously shaken for 20 s. The mixture was allowed to stand at room temperature for 15 min, and then 30 ml of 0.067 M phosphate buffer (pH 6.8) was added. After centrifugation at 4,000 × g for 15 min, the pellet was resuspended in 5 ml of phosphate buffer.
Qualitative detection.
Samples suspected of having CFU levels below the detection limit of the enumeration method were submitted to the Institute for Infectious Diseases, Division of Clinical Microbiology, University of Bern, Bern, Switzerland, to test for the presence of M. avium subsp. paratuberculosis cells with the BACTEC radiometric culture method (27). Two 0.5-ml aliquots of the homogenized suspension were injected into BACTEC 12B vials containing 4 ml of 7H12 medium supplemented with 0.1 ml of PANTA PLUS (Becton Dickinson Microbiology Systems) and 0.1 ml of mycobactin J (Synbiotics Europe SAS). The vials were incubated at 35°C, and growth index readings were recorded, according to the instructions of the manufacturer, using the BACTEC 460 TB instrument (Johnston Laboratories, Inc., Towson, Md.) for a maximum of 8 weeks.
D value.
The D value was calculated from the slope of the linear regression line generated with the SYSTAT program (release 9.0; SPSS Inc., Chicago, IIl.) by plotting the log10 values of M. avium subsp. paratuberculosis survivors per gram of cheese versus the ripening time.
RESULTS
The unusual safety layout of the experimental environment did not affect the manufacture of the model cheese since the pH value of the 24-h cheeses and the appearance and firmness of the cheese wheels were normal. Also, cheese ripening in plastic foil, the only nontraditional feature in our cheese manufacture, did not affect the cheese quality. However, eye formation in the hard cheeses was poor, possibly caused by unexpected turbulence during the pumping of the curd into the forms; nevertheless, cheese odor confirmed that propionic acid was present.
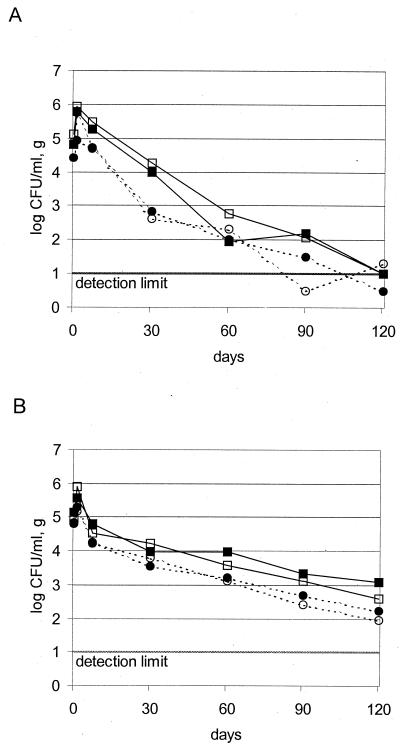
Figure 1 shows the survival of M. avium subsp. paratuberculosis in all eight individual model cheeses as determined by enumeration of nondecontaminated samples. For both hard cheeses and semi-hard cheeses, a slow but constant decrease in CFU over the whole ripening period was observed. Within the first 30 days of ripening, the decrease was most rapid. The levels decreased by a factor of approximately 100 in both types of cheese and then decreased more slowly in semihard cheese than in hard cheese. After 90 and 120 days, when ripening was complete, M. avium subsp. paratuberculosis counts in two hard cheeses fell below the detection limit of 10 CFU/g. This was confirmed by the BACTEC radiometric culture method (Table 1). In semihard cheeses, the CFU numbers were well above the detection limit. The increase in the number of M. avium subsp. paratuberculosis in the 1-day cheeses over those of the original inoculated level can be attributed to the physical concentration of bacteria due to syneresis of the curd.
FIG. 1.
Development of M. avium subsp. paratuberculosis in hard cheese (Swiss Emmentaler) (A) and semihard cheese (Swiss Tilsiter) (B). Shown are log CFU counts for each single model cheese. Unbroken lines indicate strain Niebüll and dotted lines indicate strain ATCC 19698. Open symbols, cheese 1; solid symbols, cheese 2.
TABLE 1.
Qualitative detection of M. avium subsp. paratuberculosis in hard cheese by using BACTEC radiometric culture
| Days of ripening | Presence of M. avium subsp. paratuberculosis
|
|||
|---|---|---|---|---|
| Cheese 1 (ATCC 19698) | Cheese 2 (ATCC 19698) | Cheese 1 (Niebüll) | Cheese 2 (Niebüll) | |
| 60 | + | + | + | + |
| 90 | − | + | + | + |
| 120 | + | − | + | + |
The two strains used did not differ in their behavior during cheese ripening. The lower counts for the reference strain ATCC 19698 compared to the wild strain Niebüll are due to a somewhat lower initial contamination level of the raw milk. The curves are parallel over the whole ripening period; thus, we do not believe that this difference reflects a strain property.
The relatively short time of incubation of 21 days was sufficient to produce countable colonies. Incubation for an additional 5 weeks did not result in more colonies but increased the risk of growth of contaminating flora. The detection limit of the enumeration method with plating out on 7H10-PANTA agar was estimated to be at 10 CFU per ml or g. An experimental comparison with a most probable number approach with the BACTEC radiometric culture method confirmed this estimation (results not shown). The decontamination of cheese samples with NALC-NaOH was shown not to be necessary. The raw milk flora did not affect the enumeration of M. avium subsp. paratuberculosis. Only a few colonies on 7H10-PANTA agar originated from the raw milk flora but could easily be distinguished from mycobacterium colonies, either by their macroscopic aspect or by acid-fast staining. All bacterial species used as starter cultures did not grow on 7H10-PANTA agar. Furthermore, we observed a loss of about 60% in the CFU of M. avium subsp. paratuberculosis in decontaminated samples compared to nondecontaminated samples. As a consequence, we discontinued sample decontamination after 30 days of ripening and did not use the enumeration results of decontaminated samples for Fig. 1.
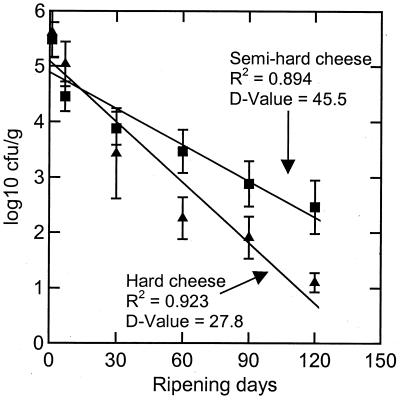
Figure 2 shows the inactivation curves for M. avium subsp. paratuberculosis over the whole ripening period. The mean of the log CFU per gram of the four cheeses for each of the hard and semihard cheese was calculated and plotted versus the ripening time starting with the values for the 24-h cheese. The regression lines show a good correlation. The D values for M. avium subsp. paratuberculosis were 27.8 days in hard cheese and 45.5 days in semihard cheese.
FIG. 2.
Inactivation curves for M. avium subsp. paratuberculosis in hard cheese (Swiss Emmentaler) and semihard cheese (Swiss Tilsiter) during 120 days of ripening.
M. avium subsp. paratuberculosis did not survive the cheese ripening process on the cheese surface, as can be seen in Table 2. All cheeses were tested systematically for the presence of M. avium subsp. paratuberculosis on their surface only after 90 days of ripening. In earlier stages of ripening, samples were taken only sporadically but CFU counts indicate clearly that M. avium subsp. paratuberculosis cells died rapidly on the cheese surface during the first period of cheese ripening.
TABLE 2.
Enumeration of M. avium subsp. paratuberculosis on cheese surface
| Cheese type | Days of ripening | Counts (CFU/25 cm2) of M. avium subsp. paratuberculosis on cheese surface
|
|||
|---|---|---|---|---|---|
| Cheese 1 (ATCC 19698) | Cheese 2 (ATCC 19698) | Cheese 1 (Niebüll) | Cheese 2 (Niebüll) | ||
| Hard | 30 | NDa | ND | ND | ND |
| 60 | ND | ND | 0 | 1 | |
| 90 | 0 | 0 | 0 | 0 | |
| Semihard | 30 | ND | ND | 2 | 4 |
| 60 | ND | ND | 0 | 0 | |
| 90 | 0 | 0 | 0 | 0 | |
ND, not determined.
DISCUSSION
Given the fastidious growth requirements of M. avium subsp. paratuberculosis, this organism will not multiply in cheese. This fact could be demonstrated very clearly with both the reference strain ATCC 19698 and the wild strain Niebüll, which was originally isolated from raw milk. In both cheese types tested, M. avium subsp. paratuberculosis counts decreased slowly over the ripening period of 120 days. The decrease was slower in semihard cheese than in hard cheese. The survival curve of M. avium subsp. paratuberculosis in semihard cheese was very similar to that found in previous experiments with Listeria monocytogenes, which survived 90 days in Swiss Tilsiter-type cheese, but no increase was observed (1). Both of those microorganisms appear to be the most tenacious microorganisms in semihard cheese. In hard cheese, the long survival of M. avium subsp. paratuberculosis is rather surprising. Compared to other pathogenic bacteria possibly present in raw milk, M. avium subsp. paratuberculosis differs greatly in its capacity to survive cheese manufacture and ripening. Recent studies of this hard cheese variety showed the rapid death of several species of pathogenic bacteria (1). The gram-negative species (Yersinia enterocolitica, Salmonella enterica serovar Typhimurium, Aeromonas hydrophila, Pseudomonas aeruginosa, and Escherichia coli) were detectable only in the curd after cooking but not in the 24-h cheese; Campylobacter jejuni did not even survive the cooking process. Of the gram-positive species tested, L. monocytogenes died between curd cooking and the 24-h ripening. The species which survived longest in Swiss Emmentaler-type cheese was Staphylococcus aureus. It was detectable in the 24-h cheese but not after 7 days of ripening.
The long survival of M. avium subsp. paratuberculosis in our model cheeses corresponds with the findings of Kästli and Binz (20), who determined survival times for M. bovis of 305 days in Swiss Tilsiter and between 7 and 22 days in Swiss Emmentaler. In their experiments with naturally contaminated raw milk, the initial level of contamination was estimated to be 1 to 10 CFU/ml, compared to the 104 to 105 CFU/ml in our study, which would explain the shorter survival time of M. bovis in Swiss Emmentaler. In the above-mentioned study, no quantitative analyses were performed; instead, virulent M. bovis cells were detected in guinea pigs, and the authors were not able to calculate or estimate D values. This makes it difficult to compare the behavior of M. bovis and M. avium subsp. paratuberculosis in cheese.
Sung and Collins (34) previously published D values for M. avium subsp. paratuberculosis ATCC 19698 in cheese made of spiked milk. They produced a Hispanic-style soft white cheese (Queso fresco) under laboratory conditions with characteristics (2% NaCl, pH 6.15; maximum temperature during manufacture was 37.7°C) that differed greatly from our hard and semihard cheeses. The D value found in Hispanic-style soft cheese was 59.9 days. When the tested strain was heat treated prior to the cheese making (62°C for 240 s), a D value of 36.5 days was determined. The D values observed in our hard and semihard cheeses are much lower, indicating a faster rate of death of M. avium subsp. paratuberculosis than was observed in the soft cheese. This is not surprising since the survival of pathogenic bacteria in cheese is correlated with its composition. The higher the water content (water activity) in cheese, the longer the survival time that can be expected. Other reasons for the more rapid disappearance in semihard and hard cheeses are the much lower pH values (5.7 to 5.8) compared to that of the Mexican-style soft cheese (6.15). Also, at least for hard cheese, the cooking process at 53°C for 45 min may lead to sublethal injuries to M. avium subsp. paratuberculosis cells that affect their survival during the cheese ripening period.
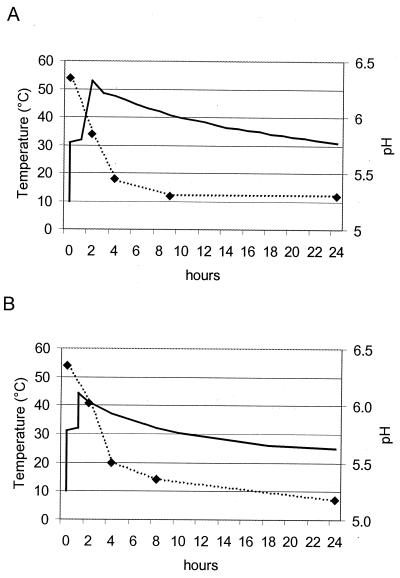
Of all the different factors responsible for the decrease in numbers of M. avium subsp. paratuberculosis in cheese, the most important are temperature, pH, and salt. Also, water content, lactic acid, and propionic acid may contribute to some extent. Mycobacteria are rather temperature-resistant microorganisms. Most of the published data on heat sensitivity deal with pasteurization temperatures. Data on temperatures applied in cheese manufacture are limited. In a recent study, Keswani and Frank (21) demonstrated only minimal thermal inactivation of M. avium subsp. paratuberculosis at a temperature of 55°C for 12 min. At 58°C for 12 min, a reduction of only 0.3 to 0.7 logs was found for clumped and declumped cells. Figure 3 shows the temperatures occurring in model cheese over the first 24 h. In our cheese manufacture, the curd-cooking temperature for hard cheese was 53°C (applied for 45 min), suggesting a limited contribution to the reduction of CFU. In semihard cheese, the cooking temperature reached only 44°C, a temperature far below a value that might have had an influence on the survival of M. avium subsp. paratuberculosis. After curd cooking, the temperature profiles in Fig. 3 represent the temperatures occurring in the outer zones of normal cheeses. In normal-cheese manufacture, the cooling rates differ significantly in various zones of the cheese wheel. The temperature in the center of the cheese decreases much more slowly and may still reach 40°C after 24 h in hard cheese. However, these temperature conditions during the first 24 h of a cheese will not affect the behavior of M. avium subsp. paratuberculosis greatly.
FIG. 3.
Temperature (solid line) and pH (broken line) during first 24 h of hard cheese (Swiss Emmentaler) (A) and semihard cheese (Swiss Tilsiter) (B) manufacture.
M. avium subsp. paratuberculosis tolerates very low pH values for several hours. Sung and Collins (34) reported the effect of low pH on survival when suspended in acetate buffer (50 mM) containing 1% (vol/vol) lactic acid (50 mM). The average D values for two strains tested at pH 4.0, 5.0, and 6.0 in acetate buffer supplemented with 1% (vol/vol) lactic acid were (mean ± SD) 10.0 ± 2.5, 19.0 ± 3.9, and 33.3 ± 4.4 days, respectively. Our model cheeses reached their lowest pH values within the first 24 h after cheese manufacture, when pH was 5.3 in hard cheese and 5.2 in semihard cheese (Fig. 3). But as cheese ripening progressed, the pH value rose again, reaching 5.7 in hard cheese and 5.8 in semihard cheese after 120 days. A pH value of around 5.2 to 5.4 with an estimated D value of about 15 days lasted only for a relatively short time, 10 days for hard cheese and 25 days for semihard cheese. This would suggest that the low pH in the cheese may have accounted for a reduction of 1 to 2 logs within the first month of ripening. With the pH rising towards 6.0 during the later ripening, the bacteriocidal contribution of the pH would be reduced, resulting in an estimated D value of approximately 30 days, which corresponds well with previously published data (33.3 days observed at pH 6.0 under in vitro conditions) (34). The calculated D values over the whole ripening period of 120 days was 27.8 days for hard cheese and 45.5 days for semihard cheese. This indicates that the contribution of pH to the reduction of M. avium subsp. paratuberculosis counts is in fact lower. The influence of pH may be limited, but its contribution to the destruction of M. avium subsp. paratuberculosis seems to be more important than that of the temperature.
The pH in cheese is predominantly determined by the content of lactic acid. Lactic acid may have a bacteriostatic but not necessarily a bacteriocidal effect on acid-sensitive bacteria in cheese (30). Swiss Emmentaler and Swiss Tilsiter contain approximately 1.2% lactic acid 24 h after manufacture. But in the subsequent ripening of hard cheese, lactic acid serves as a source of energy for ripening organisms and its concentration decreases again. There is no information available on the effect of lactic acid on M. avium subsp. paratuberculosis or other mycobacteria. In their experiments on the effect of pH on M. avium subsp. paratuberculosis, Sung and Collins (34) used a test medium based on acetate buffer supplemented with 1% (vol/vol) lactic acid in order to more closely mimic conditions in cheese. The possible bactericidal action of lactic acid on M. avium subsp. paratuberculosis was not investigated by the authors.
Salting of cheese affects the survival rates of microorganisms only in cheese types with a very high salt content (30). Under in vitro conditions, NaCl concentrations from 2 to 6% had little or no effect on M. avium subsp. paratuberculosis survival rates at pH 4 to 6.8 (34). Since the salt content in our model cheese was much lower (0.4% for Swiss Emmentaler and 1.6% for Swiss Tilsiter), we conclude that no effect or only a negligible detrimental effect can be attributed to salt.
In hard cheese of the Swiss Emmentaler type, propionic acid bacteria play an important role in the ripening process by contributing to the typical flavor of this cheese. Meyer et al. (25) suggested that rapidly growing propionic acid bacteria might be a factor responsible for the death of mycobacteria. Unfortunately, there is no information available on the effect of propionic acid on M. avium subsp. paratuberculosis or other mycobacteria, leaving this suggestion open to further investigations.
The possible contribution of the ripening organisms (lactic acid and propionic acid bacteria) and their enzymes to the reduction of M. avium subsp. paratuberculosis can only be speculated on. There is no information available on the antagonistic behavior of cheese-ripening flora towards M. avium subsp. paratuberculosis.
In conclusion, the decrease of M. avium subsp. paratuberculosis in cheese cannot be attributed to one distinct factor. Of all the factors discussed above, temperature and pH seem to be the most important. Since these factors are not constant but behave dynamically during the manufacture and ripening of cheese, it will be very difficult to elucidate their individual contribution to the destruction of M. avium subsp. paratuberculosis. However, in cheese manufacture and ripening, these factors act together in an ideal and unique combination, resulting in a combined bacteriocidal effect that is stronger than could be expected for each single factor determined on the basis of in vitro results.
The numbers of M. avium subsp. paratuberculosis in raw milk has not been well investigated in detail. Sweeney et al. (35) found 2 to 8 CFU per 50 ml of milk in nine samples of milk obtained from asymptomatic cows infected with M. avium subsp. paratuberculosis. But, estimations suggest that contamination rates may reach as high as 104 CFU/ml of milk due to fecal contamination (12). For example, let us presume that the raw milk in a local cheese factory delivered from several farms harbors 100 CFU of M. avium subsp. paratuberculosis per liter. During the manufacture of cheese made from this raw milk, approximately 90% of this load will be entrapped in the milk coagulum after addition of the rennet. Following the syneresis of the curd, this 90 CFU/liter of coagulum will be concentrated to 90 CFU/100 g of cheese after 24 h. During the subsequent ripening periods of 4 months for Swiss Emmentaler and 3 months for Swiss Tilsiter, the numbers of M. avium subsp. paratuberculosis cells will be reduced by 4.3 logs and 2.0 logs respectively, as calculated from our D values. Thus, in the cheese ready for consumption, we do not expect more than 0.005 CFU/100 g in Swiss Emmentaler and 0.9 CFU/100 g in Swiss Tilsiter. By applying the above considerations, we can calculate that in Swiss Emmentaler, an initial load of 2 × 104 cells of M. avium subsp. paratuberculosis per liter of raw milk would be reduced to 0.9 CFU/100 g of cheese. Swiss Emmentaler is normally consumed only after 6 months of ripening, and this provides an additional safety margin.
In their experiments with similar contamination levels, Sung and Collins (34) concluded that cheese production using pasteurized milk and a 60-day curing period will largely eliminate the predicted level of M. avium subsp. paratuberculosis contamination. Based on our results, this conclusion is also true for hard cheese and to some extent for semihard cheese made of raw milk. There is strong evidence that the manufacturing and ripening conditions of hard cheese from raw milk are, in effect, equivalent to pasteurization as regards M. avium subsp. paratuberculosis.
Regarding the possible link to Crohn's disease, a risk assessment for human exposure of M. avium subsp. paratuberculosis in raw milk cheese is very difficult to evaluate because of the lack of data on the frequency and the level of raw milk contamination and the impossibility of determining an infective dose for M. avium subsp. paratuberculosis in humans. More accurate data will be necessary for a proper risk assessment.
ACKNOWLEDGMENTS
We express our thanks to Philipp Hammer, Federal Dairy Research Centre, Institute for Hygiene and Food Safety, in Kiel, Germany, for providing the Mycobacterium avium subsp. paratuberculosis strain Niebüll. We thank Thomas Bodmer, Institute for Infectious Diseases, Division of Clinical Microbiology, University of Bern, Bern, Switzerland, for his advice and support in the qualitative detection of mycobacteria in our samples. We thank Michael Casey, Federal Dairy Research Station Liebefeld, for his assistance in writing the manuscript.
REFERENCES
- 1.Bachmann H P, Spahr U. The fate of potentially pathogenic bacteria in swiss hard and semihard cheeses made from raw milk. J Dairy Sci. 1995;78:476–483. doi: 10.3168/jds.S0022-0302(95)76657-7. [DOI] [PubMed] [Google Scholar]
- 2.Chiodini R J. Crohn's disease and the mycobacterioses: a review and comparison of two disease entities. Clin Microbiol Rev. 1989;2:90–117. doi: 10.1128/cmr.2.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiodini R J, Hermon-Taylor J. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurization. J Vet Diagn Investig. 1993;5:629–631. doi: 10.1177/104063879300500424. [DOI] [PubMed] [Google Scholar]
- 4.Chiodini R J, van Kruiningen H J, Merkal R S. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 5.Cocito C, Gillot P, Coene M, De Kesel M, Poupart P, Vannuffel P. Paratuberculosis. Clin Microbiol Rev. 1994;7:328–345. doi: 10.1128/cmr.7.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins C H, Grange J M, Yates M D. Mycobacteria in water. J Appl Bacteriol. 1984;57:193–211. doi: 10.1111/j.1365-2672.1984.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 7.Collins M T. Mycobacterium paratuberculosis: a potential food-borne pathogen? J Dairy Sci. 1997;80:3445–3448. doi: 10.3168/jds.S0022-0302(97)76321-5. [DOI] [PubMed] [Google Scholar]
- 8.Covert T C, Rodgers M R, Reyes A L, Stelma G J. Occurrence of nontuberculous mycobacteria in environmental samples. Appl Environ Microbiol. 1999;65:2492–2496. doi: 10.1128/aem.65.6.2492-2496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle T M. Johne's disease. Vet Rec. 1956;68:869–878. [Google Scholar]
- 10.Grant I R, Ball H J, Rowe M T. Heat sensitivity of Mycobacterium paratuberculosis in cows' milk at pasteurization temperatures. In: Chiodini R J, Collins M T, Bassey E O E, editors. Proceedings of the Fourth International Colloquium on Paratuberculosis. Rehoboth, Mass: International Association of Paratuberculosis; 1994. pp. 313–319. [Google Scholar]
- 11.Grant I R, Ball H J, Rowe M T. High temperature, short time (HTST) pasteurization of milk containing low levels of Mycobacterium paratuberculosis. In: Chiodini R J, Hines II M E, Collins M T, editors. Proceedings of the Fifth International Colloquium on Paratuberculosis. Rehoboth, Mass: International Association of Paratuberculosis; 1996. pp. 333–338. [Google Scholar]
- 12.Grant I R, Ball H J, Neill S D, Rowe M T. Inactivation of Mycobacterium paratuberculosis in cow's milk at pasteurization temperatures. Appl Environ Microbiol. 1996;62:631–636. doi: 10.1128/aem.62.2.631-636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn H. Is manufacture of Emmental cheese from raw milk safe from the viewpoint of public health? Tierärztl Umsch. 1959;14:254–256. [Google Scholar]
- 14.Hammer B W, Babel F J. Dairy bacteriology. 4th ed. New York, N.Y: John Wiley & Sons Inc; 1957. [Google Scholar]
- 15.Hammer P, Knappstein K. Mycobacterium paratuberculosis als Zoonoseerreger. Kiel Milchwirtsch Forschungsber. 1998;50:235–247. [Google Scholar]
- 16.Hope A F, Tulk P A, Condron R J. Pasteurization of Mycobacterium paratuberculosis in whole milk. In: Chiodini R J, Hines II M E, Collins M T, editors. Proceedings of the Fifth International Colloquium on Paratuberculosis. Rehoboth, Mass: International Association of Paratuberculosis; 1997. pp. 377–382. [Google Scholar]
- 17.Horsburgh C R, Chin D P, Yajko D M, Hopewell P C, Nassos P S, Elkin E P, Hadley W K, Stone E N, Simon E M, Gonzales P, Ostroff S, Reingold A L. Environmental risk factors for acquisition of Mycobacterium avium complex in persons with human immunodeficiency virus infection. J Infect Dis. 1994;170:362–367. doi: 10.1093/infdis/170.2.362. [DOI] [PubMed] [Google Scholar]
- 18.Iotov I, Todorov D. Study of survival of Mycobacterium tuberculosis in Bulgarian white cheese during ripening and storage. Nauchni Trud. 1959;1:33–38. [Google Scholar]
- 19.Johnson-Ifearulundu Y J, Kaneene J B. Relationship between soil type and Mycobacterium paratuberculosis. J Am Vet Med Assoc. 1997;210:1735–1740. [PubMed] [Google Scholar]
- 20.Kästli P, Binz M. Die Lebensfähigkeit von Mycobacterium tuberculosis in verschiedenen Käsesorten. Milchwissenschaft. 1949;4:391–394. [Google Scholar]
- 21.Keswani J, Frank J F. Thermal inactivation of Mycobacterium paratuberculosis in milk. J Food Prot. 1998;61:974–978. doi: 10.4315/0362-028x-61.8.974. [DOI] [PubMed] [Google Scholar]
- 22.Lafont J, Lafont P. Some modifications in Koch's bacillus during ripening of Blue cheese. Bull Acad Vet Fr. 1981;53:457–461. [Google Scholar]
- 23.Lovell R, Levi M, Francis J. Studies on the survival of Johne's bacilli. J Comp Pathol. 1944;54:120–129. [Google Scholar]
- 24.Metchok B G, Nolte F S, Wallace R J., Jr . Mycobacterium. In P. R. Murray (ed.), Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. [Google Scholar]
- 25.Meyer J, Touillier J, Malgras J. Les facteurs bactériolytiques antituberculeux des fromages. Le Lait. 1952;32:512–515. [Google Scholar]
- 26.Meylan M, Rings D M, Shulaw W P, Kowalski J J, Bechnielsen S, Hoffsis G F. Survival of Mycobacterium paratuberculosis and preservation of immunoglobulin G in bovine colostrum under experimental conditions simulating pasteurization. Am J Vet Res. 1996;57:1580–1585. [PubMed] [Google Scholar]
- 27.Middlebrook G, Reggiardo Z, Tigerit W D. Automatable radiometric detection of growth of Mycobacterium tuberculosis in selective media. Am Rev Respir Dis. 1977;115:1066–1069. doi: 10.1164/arrd.1977.115.6.1066. [DOI] [PubMed] [Google Scholar]
- 28.Nauta M J, van der Giessen J W. Human exposure to Mycobacterium paratuberculosis via pasteurised milk: a modelling approach. Vet Rec. 1998;143:293–296. doi: 10.1136/vr.143.11.293. [DOI] [PubMed] [Google Scholar]
- 29.O'Connor R E, Ewings K N, Hollywood N W. Effect of agitation on bacterial aggregates in pure cultures and raw milk. J Food Prot. 1983;46:681–685. doi: 10.4315/0362-028X-46.8.681. [DOI] [PubMed] [Google Scholar]
- 30.Spahr U, Url B. Behaviour of pathogenic bacteria in cheese—a synopsis of experimental data. Bull Intl Dairy Federation. 1994;298:2–16. [Google Scholar]
- 31.Stabel J R, Steadham E M, Bolin C A. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Appl Environ Microbiol. 1997;63:4975–4977. doi: 10.1128/aem.63.12.4975-4977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streeter R N, Hoffsis G F, Bech-Nielson S, Shulaw W P, Rings D M. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am J Vet Res. 1995;56:1322–1324. [PubMed] [Google Scholar]
- 33.Sung N, Collins M T. Thermal tolerance of Mycobacterium paratuberculosis. Appl Environ Microbiol. 1998;64:999–1005. doi: 10.1128/aem.64.3.999-1005.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung N, Collins M T. Effect of three factors in cheese production (pH, salt, and heat) on Mycobacterium avium subsp. paratuberculosis viability. Appl Environ Microbiol. 2000;66:1334–1339. doi: 10.1128/aem.66.4.1334-1339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweeney R W, Whitlock R H, Rosenberger A E. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J Clin Microbiol. 1992;30:166–171. doi: 10.1128/jcm.30.1.166-171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor T K, Wilks C R, McQueen D S. Isolation of Mycobacterium paratuberculosis from the milk of a cow with Johne's disease. Vet Rec. 1981;109:532–533. [PubMed] [Google Scholar]
- 37.Thorel M F, Krichevsky M, Lévy-Frébault V V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int J Syst Bacteriol. 1990;40:254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- 38.von Reyn C F, Maslow J N, Barber T W, Falkingham III J O, Arbeit R D. Persistent colonization of potable water as a source of Mycobacterium avium infection in AIDS. Lancet. 1994;343:1137–1141. doi: 10.1016/s0140-6736(94)90239-9. [DOI] [PubMed] [Google Scholar]