Abstract
Objective
Previous studies identified essential user preferences for seizure detection devices (SDDs), without addressing their relative strength. We performed a discrete choice experiment (DCE) to quantify attributes' strength, and to identify the determinants of user SDD preferences.
Methods
We designed an online questionnaire targeting parents of children with epilepsy to define the optimal balance between SDD sensitivity and positive predictive value (PPV) while accounting for individual seizure frequency. We selected five DCE attributes from a recent study. Using a Bayesian design, we constructed 11 unique choice tasks and analyzed these using a mixed multinomial logit model.
Results
One hundred parents responded to the online questionnaire link; 49 completed all tasks, whereas 28 completed the questions, but not the DCE. Most parents preferred a relatively high sensitivity (80%–90%) over a high PPV (>50%). The preferred sensitivity‐to‐PPV ratio correlated with seizure frequency (r = −.32), with a preference for relative high sensitivity and low PPV among those with relative low seizure frequency (p = .04). All DCE attributes significantly impacted parental choices. Parents expressed preferences for consulting a neurologist before device use, personally training the device's algorithm, interaction with their child via audio and video, alarms for all seizure types, and an interface detailing measurements during an alarm. Preferences varied between subgroups (learning disability or not, SDD experience, relative low vs. high seizure frequency based on the population median).
Significance
Various attributes impact parental SDD preferences and may explain why preferences vary among users. Tailored approaches may help to meet the contrasting needs among SDD users.
Keywords: caregivers, epilepsy, SUDEP, users, wearables
Key Points.
We examined the strength of attributes influencing parental preferences for SDDs
Five different DCE attributes of the SDD all impacted choices
Most caregivers preferred a relatively high sensitivity over low false alarm rate
User preferences contrasted among subgroups
Contrasting needs among SDD users demand user‐centered and tailored approaches
1. INTRODUCTION
Seizure detection has rapidly advanced in epilepsy care as various new devices have been launched. 1 , 2 , 3 , 4 , 5 Meaningful implementation of these devices requires a good fit with the end users. Seizure detection devices (SDDs) are used mainly by people caring for an individual with epilepsy in an institution or at home. Caregivers' rapid response to SDD alarms might help prevent dangerous complications of seizures, including injury, status epilepticus, and sudden unexpected death in epilepsy (SUDEP). 6 , 7 , 8 , 9 SDDs may also help reduce the burden of seizure monitoring and promote independence. 4 These beneficial effects, however, can only be gained when the device meets the user's needs and is successfully implemented in the care setting. 5 Most SDD studies have focused on technological aspects and placed less emphasis on the user's role in coshaping SDDs. 10 People with epilepsy and caregivers have expressed the importance of an accurate device, 5 , 11 , 12 but little is known about how they evaluate the balance between sensitivity and positive predictive value (PPV) while accounting for individual seizure frequency. Previous research among potential users showed that design aspects also matter. 5 , 11 , 12 Several studies stressed the importance of attractive, nonintrusive, nonstigmatizing, comfortable devices, preferably wearable and removable, but securely fitted. 13 , 14 , 15 , 16 , 17 , 18 A recent qualitative context mapping study 19 explored caregivers' dreams and fears, and identified several key attributes influencing their trust in a device (e.g., ability to view all parameters overnight, personal adjustment of the algorithm, recommendation by a neurologist, and a setup period). 19
Previous studies did not examine the relative strength of the attributes determining the user's choice of an SDD. A discrete choice experiment (DCE) is a method to quantify the strength of different aspects influencing users' preferences. 20 The scope of DCE applications is expanding, including the design of complex interventions. 21 Few DCE studies have evaluated preferences for diagnostic and treatment options in epilepsy care. 22 , 23 , 24
This study builds on our context mapping study 19 by extracting the most important themes regarding SDD needs as attributes. We aimed to examine to what extent these attributes affect users' preferences for an SDD, using a DCE, and assess whether user characteristics influence SDD preferences. We also explored the optimal balance between sensitivity and PPV, while accounting for the seizure frequency of the individual.
2. MATERIALS AND METHODS
We designed an online questionnaire to explore the preferences of parents of children with epilepsy. The questionnaire consisted of three components: (1) background information about the parents and the child with epilepsy; (2) questions on motives for using an SDD, and the optimal balance between SDD sensitivity and PPV; and (3) a DCE.
2.1. Background information
We recorded family composition, parental educational level, the child's age and presence or absence of learning and/or physical disability, seizure frequency and types, and parental experience with SDD use. In the DCE, for the sake of ease, we referred to seizure types as "major" or "minor." We requested parents to describe the seizures of their child in the questionnaire and to indicate how they would label them (i.e., major or minor).
2.2. Questioning motives for using an SDD and the optimal SDD performance
Parents were asked to indicate their agreement on a 5‐point Likert scale with the following motives for using an SDD: (1) to enable timely intervention in potentially dangerous seizures, (2) to be alerted for every seizure type of my child, and (3) to get a better overview of my child's epilepsy. The scale varied from 1 point (totally disagree) to 5 points (totally agree). We calculated the mean total score for each motive. The higher the score, the more parents agreed with the motive.
Optimal SDD performance was presented on a 6‐point scale, varying from an optimal PPV with relatively low sensitivity, to an optimal sensitivity with relatively low PPV. The questionnaire included the following sensitivity (%)/PPV (%) balances: 50/100, 60/83, 70/67, 80/50, 90/33, 100/17. The chosen values reflect the overall discriminative power of current SDDs, 3 , 8 with different set points for the tradeoff between sensitivity and specificity. SDD performance was expressed as numbers of missed seizures and false alarms while considering the individual seizure frequency. The data were presented as number of events per day, week, month, or year depending on the child's seizure frequency. For example, if a child experienced one seizure per day, one of the answer options would include four missed seizures per week and no false alarms (ratio sensitivity vs. PPV: 50%/100%), whereas the 60%/83% ratio would be presented as three missed seizures and one false alarm per week.
2.3. Discrete choice experiment
A DCE is often applied in health economics to evaluate preferences for health care products or programs. 25 , 26 The product, in our case an SDD, is described by several attributes, and the assumption is made that variation within these attributes (levels) affects SDD preferences. 25 Each exercise presents two hypothetical scenarios constructed by assembling random levels for each attribute. Respondents were asked to indicate their preference for one of the two scenarios. Next, the exercises were repeated with different scenarios, thus helping to identify the relative importance of each attribute and corresponding levels.
2.4. Identifying attributes and levels
We extracted the key themes regarding SDD needs from the context mapping study, 19 and converted them into five attributes to minimize study burden. Attribute levels were based on different preferences that emerged from the group discussion in this study. The list was finalized in a consensus meeting with clinicians, experts, a parent, and a patient representative, and included (1) introduction to use (three levels), (2) alert (three levels), (3) interface (three levels), (4) interaction (four levels), and (5) personalization (three levels). The attribute "interface" refers to a display of the device's measurements. All attributes and their different levels are shown in Figure 1A.
FIGURE 1.
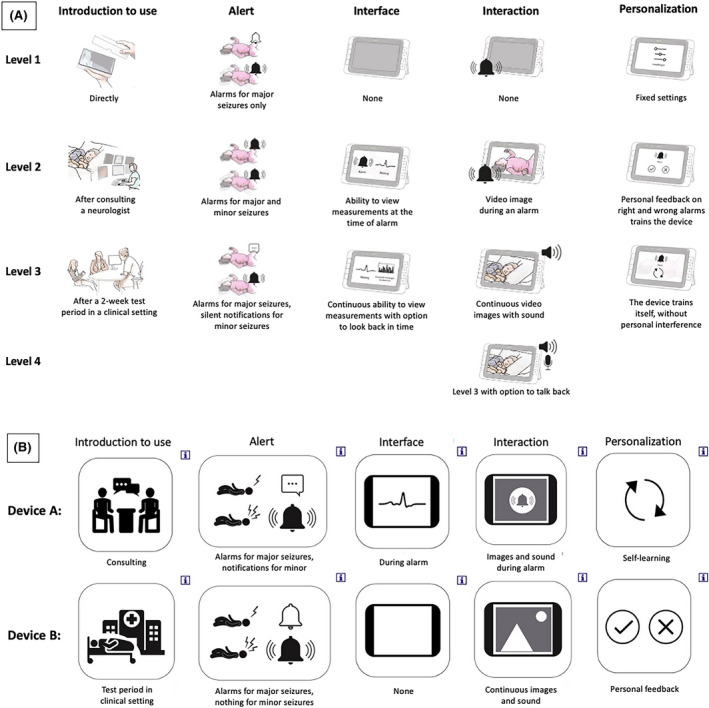
Illustrations and pictograms used in the questionnaire. (A) Illustrations for all attributes’ levels. (B) Example of discrete choice task with pictograms of different levels. Parents could click on the “i” displayed in the upper right corner of each pictogram to open additional illustrations and textual explanation of each level, as shown in A
2.5. Designing choice sets
The four attributes with three levels and one attribute with four levels used in this study could create 34 × 41 = 324 hypothetical scenarios. We used a subset of these scenarios for practical reasons, applying an algorithm to generate a Bayesian optimal design. 27 This method allows for a statistically efficient design that maximizes D‐efficiency (i.e., the precision of estimated parameters). The choice set was constructed using Stata version 16 (module DCREATE). 28 The Bayesian design assumes a prior distribution of likely parameter values (e.g., the beta coefficients in the regression analysis) for some or all parameters. We assumed that all coefficients had a positive sign (i.e., higher levels were assumed to be more preferred). To minimize participant burden, the number of choice tasks was limited to 11.
The final version consisted of 11 unique choice tasks and one repeated task to examine the test–retest reliability. There was no opt‐out option, so respondents were forced to choose between two hypothetical, unlabeled scenarios. A designer specializing in health care was asked to provide illustrations for each level, which were presented at the start of the DCE, together with an explanation of the five attributes and their levels (Figure 1A). To simplify the exercise, we provided the choice tasks with pictograms (Figure 1B). Parents could click on the pictogram for additional textual explanation.
2.6. Testing the full questionnaire
Before distributing the questionnaire, we performed a pilot study with five parents of children with epilepsy admitted to our epilepsy center, to optimize question format, pictograms, and language. The full Dutch version of the questionnaire is available from the authors on request.
2.7. Data collection
A link to the online questionnaire was distributed via multiple social media used by three large epilepsy centers in the Netherlands (Epilepsy Institutes of the Netherlands Foundation, Academic Center for Epileptology Kempenhaeghe, and University Medical Center Utrecht), EpilepsieNL, the Dutch Epilepsy Foundation, and Facebook groups of representatives of people with epilepsy in the Netherlands and Belgium. We aimed to include a population that was as diverse as possible to represent a wide range of preferences. Any Dutch‐speaking parent of a child with epilepsy, with or without SDD experience, was invited to participate. The questionnaire completion time was about 45 min. The study was evaluated by the Medical Research Ethics Committee Utrecht. An official approval was not required under the Medical Research Involving Human Subjects Act. All parents participated voluntarily and anonymously. Data were collected between March 2020 and March 2021.
2.8. Statistics and data analysis
Data on background information, motivation for using an SDD, and the optimal sensitivity/PPV balance are presented using descriptive statistics. We used χ2 statistics to analyze differences between groups for categorical data. To analyze the correlation between seizure frequency and preferences for SDD sensitivity‐to‐PPV ratio, we performed a 10 log transformation to create a normally distributed dataset and then used an analysis of variance test to estimate differences. Categories with a small number of responders (n < 5) were clustered together.
DCE data were analyzed using the statistics software package R (v4.0.4). We used a mixed multinomial logit (MMNL) model to determine the relative strength for each attribute on parents' preferences, using the following steps:
Defining the regression model: The regression function was constructed with the attributes as independent variable and the choice of the parents (i.e., either a “0” or “1” depending on which of the two alternatives was chosen for each question) as dependent variable. No constant term was included in the final model, as this was deemed irrelevant (i.e., it would be the mean of the unobserved effects for each of the alternatives). All attributes consisted of categorical variables and were included in the model as dummies using effect coding. We normalized the first level of each attribute to zero, and calculated preference weights relative to the effect of this first attribute's level.
Assigning distributions to each independent variable: All parameters included in the MMNL model were treated as random parameters (assuming a normal distribution), estimated using 2000 Halton draws.
Performing primary analysis: Data from all parents who completed the DCE were used to perform the primary analysis to test the attributes for significance.
Performing subgroup analyses: We tested interactions between responders' characteristics and attributes for three subgroups: learning disability of the child with epilepsy (yes/no), experience with SDD use (yes/no), and seizure frequency (relatively low/high). Seizure frequency was categorized as either relatively high or low using the median seizure frequency of all participants as a cutoff. A p‐value < .05 was considered to be statistically significant.
MMNL was chosen to allow for possible preference heterogeneity across respondents and to account for the panel nature of the data (i.e., repeated measures within individuals and hence correlated observations). 29 A positive output for a level illustrates a positive effect on parental preferences with the first attribute's level as a reference.
The resulting regression coefficients show the relative importance of the attribute. Relative importance weights to ease interpretation were calculated using the method described by Malhotra et al. 30
3. RESULTS
3.1. Respondent characteristics
In total, 100 parents responded to the link to the online questionnaire, and 49 responders completed the full questionnaire, including all DCE choice tasks, whereas 28 responders completed part of the questionnaires but did not start the DCE. Everyone who started the DCE, completed it.
Table 1 shows characteristics of the participants per subgroup; those who completed all tasks including DCE, and the subgroup who answered only some of the questions. A slightly higher parental educational level and a lower frequency of learning disabilities in the child were found among those who completed the DCE, but no other differences were noted between groups. Most responders lived as a family of two parents/caregivers with one or more children and had finished secondary vocational education or higher. The median age of the child with epilepsy was 11.5 years. Approximately half had a learning disability, and one quarter of the children experienced physical disabilities. Seizure frequency varied from one per year to several per day (median seizure frequency = one per week). Most parents reported major seizures (with or without minor seizures). Their descriptions of major seizures included "tonic–clonic," "loss of consciousness with intense jerks and salivation," "stiffen/overstretching and turning blue," "lots of movements and screaming," and "status epilepticus." Minor seizures were described as "absences/staring/freezing," "small jerks/myoclonias," "vibrations/jerks on one side of the face or body," and "loss of muscle tone or falls." Approximately 40% of responders had ever used an SDD.
TABLE 1.
Respondents' characteristics
| Characteristic | Subgroup with full data, including DCE, n = 49 | Subgroup with incomplete data, n = 51 | |
|---|---|---|---|
| Family | |||
| Family composition | Parents/caregivers | 41 (84%) | 25 (81%) |
| Single parent/caregiver | 3 (6%) | 6 (19%) | |
| Composed family | 5 (10%) | 0 (0%) | |
| Missing | 20 (not calculated) | ||
| Parental educational level | No school finished | 0 (0%) | 1 (3%) |
| Primary education | 0 (0%) | 1 (3%) | |
| Secondary education | 5 (10%) | 11 (36%) | |
| Secondary vocational education | 36 (74%) | 16 (52%) | |
| Higher education | 8 (16%) | 2 (6%) | |
| Missing | 20 (not calculated) | ||
| Child | |||
| Age of child | Median (range) | 10 years (2–39) | 15 years (1–43) |
| Learning disability | Yes | 19 (39%) | 20 (65%) |
| No | 30 (61%) | 11 (35%) | |
| Missing | 20 (not calculated) | ||
| Physical disability | Yes | 11 (22%) | 8 (26%) |
| No | 38 (78%) | 23 (74%) | |
| Missing | 20 (not calculated) | ||
| Seizure frequency | Daily | 12 (25%) | 8 (29%) |
| Weekly | 15 (31%) | 4 (14%) | |
| Monthly | 11 (22%) | 6 (21%) | |
| Yearly | 11 (22%) | 10 (36%) | |
| Missing | 23 (not calculated) | ||
| Type of seizures a | Mainly major | 19 (39%) | 11 (38%) |
| Mainly minor | 9 (18%) | 5 (17%) | |
| Major and minor | 21 (43%) | 13 (45%) | |
| Missing | 22 (not calculated) | ||
| SDD usage | Yes | 21 (43%) | 9 (32%) |
| No | 28 (57%) | 19 (68%) | |
| Missing | 23 (not calculated) | ||
| Type of SDD used | NightWatch | 15 | 4 |
| Pulse oximeter | 4 | 1 | |
| Empatica Embrace | 1 | 2 | |
| Epi‐Care Free | 1 | 1 | |
| Emfit | 2 | ||
| Seizure alert dog | 1 | ||
Abbreviations: DCE, discrete choice experiment; SDD, seizure detection device.
Parents were asked to indicate whether their child suffered from major or minor seizures and to detail the seizure types they were referring to (see Results section).
3.2. Motives for using an SDD and the optimal SDD performance
The parents strongly agreed with all three motives for using an SDD: "to enable timely intervention in potentially dangerous seizures" (4.74), "to be alerted for every seizure type" (4.35), and "to get a better overview of their child's epilepsy" (4.18; Figure 2A).
FIGURE 2.
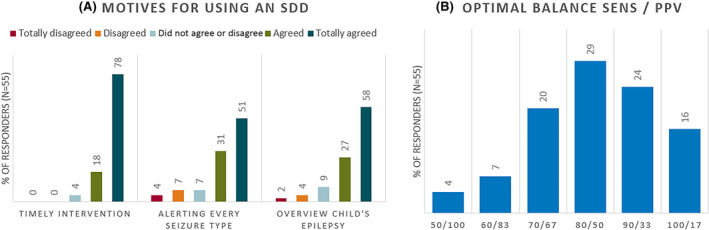
Responders’ preferred motives for using a seizure detection device (SDD) and balance between sensitivity and positive predictive value (PPV). (A) Parental motives for using an SDD: (1) to enable timely intervention in potentially dangerous seizures (timely intervention: 4.74), (2) to be alerted for every seizure type of my child (alerting every seizure type: 4.18), and (3) to get a better overview of my child's epilepsy (overview child's epilepsy: 4.35). (B) Parental choices for the optimal balance between the sensitivity (SENS) and positive predictive value of an SDD. The bars show the percentage of parents (n = 55) who chose the corresponding answer
The most frequently chosen category of SSD performance included 80% sensitivity and 50% PPV (29% of responders), followed by 90%/33% (24% of responders; Figure 2B). The SDD preference depended on the individual seizure frequency: the higher the seizure frequency, the lower the sensitivity to PPV ratio (r = −.32; p = .04). Whether the parent had used an SDD before did not impact parental tradeoff choice.
3.3. Discrete choice experiment
Forty‐five of 49 responders (92%) successfully completed the test–retest exercise (by providing the same answer), indicating a high reliability of the DCE. All attributes of the DCE were statistically significant, showing that they all had an important influence on parental preferences for an SDD. The relative importance of each attribute was expressed as a percentage, illustrating which attributes had the largest influence on parental choices (Figure 3). The relative effects of the attributes' levels by representing the output from the MMNL model expressed in log‐odds are shown in Table 2.
FIGURE 3.
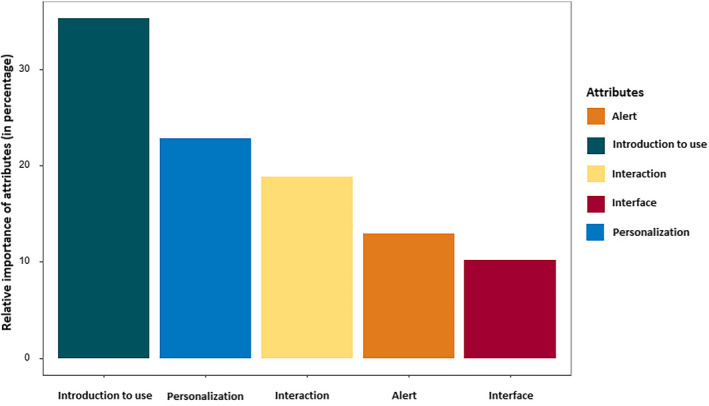
Relative importance of the five attributes used in the discrete choice experiment expressed as a percentage per attribute
TABLE 2.
Results from the mixed multinominal logit regression model illustrating the strength of different attributes on parental preferences for SDDs
| Attribute | Level | SDD preference | ||
|---|---|---|---|---|
| Log‐odds | CI | p | ||
| Introduction to use | Directly | Reference | NA | NA |
| After consulting a neurologist | 1.75 | 1.38 to 2.12 | <.001 a | |
| After a 2‐week test period in a clinical setting | −1.80 | −2.17 to −1.43 | <.001 a | |
| Alert | Alarms for major seizures only | Reference | NA | NA |
| Alarms for major and minor seizures | 1.31 | .97 to 1.65 | <.001 a | |
| Alarms for major seizures, silent notifications for minor seizures | .86 | .49 to 1.23 | <.001 a | |
| Interface | None | Reference | NA | NA |
| Ability to view measurements at the time of alarm | 1.03 | .68 to 1.37 | <.001 a | |
| Continuous ability to view measurements with option to look back in time | .81 | .52 to 1.10 | <.001 a | |
| Interaction | None | Reference | NA | NA |
| Video image during an alarm | .75 | .40 to 1.10 | <.001 a | |
| Continuous video images with sound | 1.90 | 1.43 to 2.36 | <.001 a | |
| Continuous video images with sound and the option to talk back via the device | 1.97 | 1.56 to 2.39 | <.001 a | |
| Personalization | Fixed settings | Reference | NA | NA |
| Personal feedback on right and wrong alarms to adjust the algorithm | .80 | .46 to 1.14 | <.001 a | |
| The device trains itself, without personal interference | .32 | .02 to .62 | .037 a | |
The table shows the output from the mixed multinominal logit regression model. The log‐odds represent the effect of the attributes’ levels relative to the mean effect of the different levels of the attribute in the respondent sample. A positive output for a level illustrates a positive effect on parental preferences, compared to the first attribute's level. The p‐value represents the statistical significance of the attribute's level effect (either positive or negative) relative to the reference level. To obtain the relative likelihood of choosing for a hypothetical scenario, one needs to sum the log‐odds of the levels of interest and take the exponential (elog odds = odds ratio).
Abbreviations: CI, confidence interval; NA, not applicable; SDD, seizure detection device.
Statistically significant.
The attribute "introduction to use" had the largest impact on parental preferences. Parents expressed a high preference for consulting a neurologist before putting the SDD into use, whereas a 2‐week test period in a clinical setting had a strong negative effect on parental preferences. Personalization was the second most important attribute; parents preferred the option of personalizing the device's algorithm, favoring giving personal feedback on right or wrong alarms over automatic personalization. For the attribute "interaction," parental response was: the more interaction, the better. Parents preferred to be alerted for major and minor seizures, and an alarm for both types was mostly favored. The attribute "interface" appeared to be less important; parents indicated a preference for an interface option, with no large differences in whether this option was given during an alarm or continuously with the ability to look back in time.
3.4. DCE subgroup analyses
Users' preferences differed among subgroups (Table 3). Parents of a child with a learning disability, compared to those without, were more likely to prefer consultation with a neurologist before SDD use, device interface options during an alarm, and the option to adjust the device's algorithm by giving personal feedback (Table 3). Parents who already used an SDD had a stronger preference to be alerted for both major and minor seizures and a device that could tailor its algorithms for the individual to personalize, compared to the ones without any SDD experience. Parents with SDD experience and those of a child with a relatively high seizure frequency expressed a higher preference for continuous video and audio, and the option to talk back through the device, whereas they were less likely to choose the ability to view alarms and measurements at the time of an alarm, compared to parents without SDD experience and parents of a child with relatively low seizure frequency.
TABLE 3.
Contrasts between parental preferences for seizure detection devices among three subgroups of respondents: parents of a child with learning disability (n = 19), parents with previous SDD use (n = 21), parents of a child with a relative high seizure frequency (n = 25) a
| Attributes | Levels | Learning disability | SDD usage | High seizure frequency a |
|---|---|---|---|---|
| Introduction to use | After consulting a neurologist | ++ | ++ | = |
| After a 2‐week test period in a clinical setting | −− | = | ||
| Alert | Alarms for major and minor seizures | − | ++ | = |
| Alarms for major seizures, silent notifications for minor seizures | = | = | = | |
| Interface | Ability to view measurements at the time of alarm | ++ | −− | −− |
| Continuous ability to view measurements with option to look back in time | = | = | = | |
| Interaction | Video image during an alarm | = | = | = |
| Continuous video images with sound | = | + | − | |
| Continuous video images with sound and the option to talk back via the device | = | ++ | ++ | |
| Personalization | Personal feedback on right and wrong alarms to adjust the algorithm | ++ | = | = |
| The device trains itself, without personal interference | = | ++ | = |
Abbreviations: −/−−, negative effect on parental preferences with p < .05/p < .01; +/++, positive effect on parental preferences with p < .05/p < .01; =, no effect on parental preferences; SDD, seizure detection device.
Seizure frequency was labeled as high if the frequency exceeded the median seizure frequency among participants (one seizure/week).
4. DISCUSSION
We explored parental preferences regarding usage motives, the tradeoff between sensitivity and PPV, and the attributes influencing SDD choice. We found that parents would rather have more false alarms than missed seizures. All DCE attributes had a high impact on parental choices, in the following order of importance: “introduction to use,” “personalization,” “interaction,” “alert,” and “interface.” Users' preferences varied between subgroups (learning disability or not, SDD experience, low vs. high seizure frequency based on the population median).
4.1. Strengths and limitations
Our study has its limitations. First, despite our efforts to draw attention to our online questionnaire among parents of children with epilepsy, we received a limited response. Additionally, only about half of the responders completed the DCE. The limited response might be explained by the complexity and length of the questionnaire. We tried to minimize the DCE complexity by providing pictograms and illustrations, and limiting the number of choice tasks, but the question format remains challenging. A recent review on DCEs in health economics indicated that the majority of DCEs have more than five attributes (our study uses five) and 54% use 9–16 choice tasks (we used 11). 31 A simpler, less onerous questionnaire would therefore need another question format. These studies have been performed previously, but lack information on the relative strength of different attributes that determine the user's choice of an SDD.
Estimates regarding the minimal required sample size for DCEs vary. For example, previous literature suggested various “rules of thumb,” ranging from equations such as n = 500c / (t × a) (in which c = equal to the largest product of levels of any two attributes, t = number of choice tasks, and a = number of alternatives, resulting in 273 participants for this study), to studies stating that 20 respondents per questionnaire version is sufficient to estimate reliable models, based on empirical experience. 32 Other studies have mentioned a minimal sample size of 30 for an adequate level of accuracy, based on econometric criteria. 33 The sample size of our study is on the lower end of this range and thus underpowered our subgroup analyses. These results should therefore be interpreted with caution. Despite our small sample size, we found large DCE effects. Hence, we believe that the sample size was sufficient to indicate the direction (i.e., which level has a positive or negative impact) and the importance (i.e., which attribute matters most) of participants' preferences. We found a slightly higher parental educational level in the subgroup that completed the DCE, which may have caused selection bias and thereby impacted the generalizability of our results. We had no signs to suggest that the task itself was too complex, as all responders who started the DCE also completed it. We speculate that the lower response rate relates to the required time to complete the study, which may have been too long for those parents with a high burden of care. The DCE design of this study is also one of its strengths; the method allows for a better understanding of how parents make choices for an SDD, and quantifies the strength of their preferences. We carefully selected DCE attributes from a context mapping study, 19 and the results show that all selected attributes had a significant impact. Another strength is the way we investigated the preferred tradeoff between sensitivity and specificity. Previous survey studies including people with epilepsy and caregivers examined the preferred sensitivity and false alarm rate (FAR) independently, thus reflecting an unrealistic scenario. 12 , 14 One study found that “detecting all seizures” was the most important device feature, but an accompanying FAR was not mentioned. 14 Most responders from another study required 100% sensitivity and allowed one false alarm per seizure, and one false alarm per week in those with seizure freedom. 12 We expressed the performance by calculating the absolute number of missed seizures and false alarms, taking into account the individual seizure frequency, to represent a realistic and recognizable scenario for the parents. Our results also showed a preferred FAR of one per seizure (PPV = 50%). We complement these findings with a preferred balanced sensitivity of 80%, and a tendency to favor more false alarms over a lower sensitivity. Finally, we included both parents who had experience with SDDs as well as those who did not, to include different perspectives. The question on SDD experience did not allow us to distinguish between current SDD users and parents who had used an SDD in the past. We therefore cannot examine whether the current or past use of a specific SDD influenced parental preferences. This might be an interesting topic for further research.
4.2. Main findings and related research
Previous surveys stressed the importance of design (attractive appearance, low visibility, low intrusiveness), comfort of use, confidentiality of recorded data, and timely support from both technical and clinical ends. 5 The attribute "introduction to use" had the most influence on parental preferences in our DCE, which might be explained by the strong positive (consulting a neurologist) and strong negative (clinical test period) effect of the different attribute's levels. A value‐sensitive design study among different stakeholders, including parents, highlighted that the values "health," "reliability," and "trust" were most relevant for SDD design. 11 We assume that a neurologist's advice helps to build trust in a device and optimizes implementation. Although a 2‐week test period in a clinical setting could provide meaningful information on device accuracy, it is presumably outweighed by the time and effort it costs.
Parental descriptions of major and minor seizures matched our earlier criteria quite accurately, 34 where we labeled seizures as "major" due to risk of SUDEP (tonic–clonic seizures), respiratory distress (generalized tonic seizures of >30 s), injury (hyperkinetic seizures), or status epilepticus (cluster of minor seizures). Most available SDDs offer high sensitivity/PPV ratios, meeting parental preferences, but predominantly target focal to bilateral (FBTCS) or generalized tonic–clonic seizures (GTCS). In accordance with previous surveys, we found that caregivers prefer to be alerted for a broader range of seizure types. 3 , 5 Incorporating a broader range of seizures will likely result in a lower sensitivity/PPV ratio, as minor seizures are often more subtle. The recent International League Against Epilepsy (ILAE) and International Federation of Clinical Neurophysiology (IFCN) guidelines on automated seizure detection recommend the clinical use of wearable devices for automated detection of GTCS and FBTCS, especially in unsupervised people with epilepsy, where alarms may promote rapid intervention. 34 Our survey confirms the expressed need for the detection of seizures other than GTCS or FBTCS. The ILAE/IFCN working group does not recommend the clinical use of the currently available devices for these seizure types in view of the low‐quality evidence and the lower sensitivities. Our framework provides guidance on how to evaluate the tradeoff between sensitivity and FARs. It also highlights the need to take individual seizure frequencies into account. In this respect, it is important to stress that the SDD studies so far 3 , 35 are skewed toward populations with a high seizure burden, thus impacting user evaluations.
Other important features to consider with SDD development are the parental preference for an interface allowing them to interact with their child through the device and to view the device's measurements.
Our study population favored personalization of the algorithms of their device over fixed settings. This requires considerable interaction with the device, which contrasts with previous results that showed preference for a limited number of interactions. 5 , 16 , 17 The same studies emphasize that device design, especially its appearance, visibility, and intrusiveness, is an important factor influencing user acceptance and that users desired a minimal number of alerts. 5 , 16 , 17 Following a previous survey of people with epilepsy and caregivers, 14 most parents in our study choose to be alerted for every seizure type (e.g., major and minor). This contrasts with the findings of other studies addressing only people with epilepsy predominantly expressing their preference for detecting major seizures, thus underscoring heterogeneity among user groups.
Our results also show varying needs between different user groups. We found that preferences for a higher sensitivity and lower PPV (more false alarms) were associated with lower seizure frequencies. We speculate that sensitivity is critically important for those with low seizure frequencies, and a higher FAR, even at lower PPV, is still acceptable. This may differ for parents of children with relatively high seizure frequency, as even with relatively high PPV the alarm rate may still be a substantial burden.
5. CONCLUSIONS
We identified variation in SDD preferences between different user groups, both within our study and compared to other studies. People with epilepsy who live independently might consider the device's appearance and visibility more important, whereas parents caring for a child with epilepsy and severe learning disabilities might prefer to provide personal feedback on alarms, because they know their child best. We therefore expect that a generic device will not meet all users' needs and thus encourage the development of user‐centered and tailored approaches to foster SDD implementation.
CONFLICT OF INTEREST
R.D.T. has received research support from Medtronic and New Life Wearables and has received consultancy fees from Theravance Biopharma and Arvelle and fees for lectures from Medtronic, UCB, Zogenix, and Novartis. Neither of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
This work was supported by the Netherlands Organization for Health Research and Development (ZonMW; project number 446001009), the Dutch National Epilepsy Fund and Health Holland (project number 40‐41200‐98‐9335), and the “Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie”. We would like to thank Tessa Souhoka from Productzaken for her contribution in creating all the illustrations for the discrete choice experiment, and we are grateful to Dr F. S. S. Leijten, Dr R. H. C. Lazeron, Dr G. S. Bell, and Prof. J. W. Sander for critically reviewing the manuscript.
van Westrhenen A, Wijnen BFM, Thijs RD. Parental preferences for seizure detection devices: A discrete choice experiment. Epilepsia. 2022;63:1152–1163. 10.1111/epi.17202
REFERENCES
- 1. van Westrhenen A, De Cooman T, Lazeron RHC, Van Huffel S, Thijs RD. Ictal autonomic changes as a tool for seizure detection: a systematic review. Clin Auton Res. 2019;29(2):161–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van de Vel A, Cuppens K, Bonroyb B, Milosevic M, Jansen K, Van Huffel S, et al. Non‐EEG seizure detection systems and potential SUDEP prevention: review and update. Seizure. 2016;41:141–53. [DOI] [PubMed] [Google Scholar]
- 3. Beniczky S, Jeppesen J. Non‐electroencephalography‐based seizure detection. Curr Opin Neurol. 2019;32(2):198–204. [DOI] [PubMed] [Google Scholar]
- 4. van Andel J, Thijs RD, de Weerd A, Arends J, Leijen FS. Non‐EEG based ambulatory seizure detection designed for home use: what is available and how will it influence epilepsy care? Epilepsy Behav. 2016;57(Pt A):82–9. [DOI] [PubMed] [Google Scholar]
- 5. Bruno E, Viana PF, Sperling MR, Richardson MP. Seizure detection at home: do devices on the market match the needs of people living with epilepsy and their caregivers? Epilepsia. 2020;61(S1):S11–24. [DOI] [PubMed] [Google Scholar]
- 6. van der Lende M, Hesdorffer DC, Sander JW, Thijs RD. Nocturnal supervision and SUDEP risk at different epilepsy care settings. Neurology. 2018;91(16):e1508–18. [DOI] [PubMed] [Google Scholar]
- 7. Maguire MJ, Jackson CF, Marson AG, Nevitt SJ. Treatments for the prevention of sudden unexpected death in epilepsy (SUDEP). Cochrane Database Syst Rev. 2020;4(4):CD011792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rugg‐Gunn F, Duncan J, Hjalgrim H, Seyal M, Bateman S. From unwitnessed fatality to witnessed rescue: nonpharmacologic interventions in sudden unexpected death in epilepsy. Epilepsia. 2016;57(suppl 1):26–34. [DOI] [PubMed] [Google Scholar]
- 9. Ryvlin P, Ciumas C, Wisniewski I, Beniczky S. Wearable devices for sudden unexpected death in epilepsy prevention. Epilepsia. 2018;59:61–6. [DOI] [PubMed] [Google Scholar]
- 10. Papoutsi C, Collins CDE, Christopher A, Shaw SE, Greenhalgh T. Interrogating the promise of technology in epilepsy care: systematic, hermeneutic review. Sociol Health Illn. 2021;43(4):928–47. https://doi.org/ 10.1111/1467-9566.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Andel J, Leijten F, van Delden H, van Thiel G. What makes a good home‐based nocturnal seizure detector? A value sensitive design. PLoS One. 2015;10(4):e0121446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van de Vel A, Smets K, Wouters K, Ceulemans B. Automated non‐EEG based seizure detection: do users have a say? Epilepsy Behav. 2016;62:121–8. [DOI] [PubMed] [Google Scholar]
- 13. Hoppe C, Feldmann M, Blachut B, et al. Novel techniques for automated seizure registration: patients’ wants and needs. Epilepsy Behav. 2015;52:1–7. [DOI] [PubMed] [Google Scholar]
- 14. Patel AD, Moss R, Rust SW, et al. Patient‐centered design criteria for wearable seizure detection devices. Epilepsy Behav. 2016;64:116–21. [DOI] [PubMed] [Google Scholar]
- 15. Tovar Quiroga DF, Britton JW, Wirrell EC. Patient and caregiver view on seizure detection devices: a survey study. Seizure. 2016;64:179–81. [DOI] [PubMed] [Google Scholar]
- 16. Bruno E, Simblett S, Lang A, Biondi A, Odoi C, Schulze‐Bonhage A, et al. Wearable technology in epilepsy: the views of patients, caregivers, and healthcare professionals. Epilepsy Behav. 2018;85:141–9. [DOI] [PubMed] [Google Scholar]
- 17. Simblett SK, Biondi A, Bruno E, Ballard D, Stoneman A, Lees S, et al. Patients’ experience of wearing multimodal sensor devices intended to detect epileptic seizures: a qualitative analysis. Epilepsy Behav. 2020;102:106717. [DOI] [PubMed] [Google Scholar]
- 18. Bruno E, Biondi A, Böttcher S, Lees S, Schulze‐Bonhage A, Richardson MP, et al. Day and night comfort and stability on the body of four wearable devices for seizure detection: a direct user‐experience. Epilepsy Behav. 2020;112:107478. [DOI] [PubMed] [Google Scholar]
- 19. van Westrhenen A, Souhoka T, Ballieux ME, Thijs RD. Seizure detection devices: exploring caregivers’ needs and wishes. Epilepsy Behav. 2021;116:107723. [DOI] [PubMed] [Google Scholar]
- 20. Clark MD, Determann D, Petrou S, Moro D, de Bekker‐Grob EW. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics. 2014;32(9):883–902. [DOI] [PubMed] [Google Scholar]
- 21. Terris‐Prestholt F, Neke N, Grund JM, Plotkin M, Kuringe E, Osaki H, et al. Using discrete choice experiments to inform the design of complex interventions. Trials. 2019;20(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atkinson‐Clark E, Charokopou M, Van Osselaer N, Hiligsmann M. A discrete‐choice experiment to elicit preferences of patients with epilepsy for self‐management programs. Epilepsy Behav. 2018;79:58–67. [DOI] [PubMed] [Google Scholar]
- 23. Wijnen BFM, de Kinderen RJA, Colon AJ, Dirksen CD, Essers BAB, Hiligsmann M, et al. Eliciting patients’ preferences for epilepsy diagnostics: a discrete choice experiment. Epilepsy Behav. 2014;31:102–9. [DOI] [PubMed] [Google Scholar]
- 24. Ettinger AB, Carter JA, Rajagopalan K. Patient versus neurologist preferences: a discrete choice experiment for antiepileptic drug therapies. Epilepsy Behav. 2018;80:247–53. [DOI] [PubMed] [Google Scholar]
- 25. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user's guide. Pharmacoeconomics. 2008;26:661–77. [DOI] [PubMed] [Google Scholar]
- 26. Ryan M, Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy. 2003;2:55–64. [PubMed] [Google Scholar]
- 27. Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Muhlbacher A, Regier DA, et al. Constructing experimental designs for discrete‐choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16:3–13. [DOI] [PubMed] [Google Scholar]
- 28. Hole AR. DCREATE: Stata module to create efficient designs for discrete choice experiments. Statistical Software Component S458059, Boston College Department of Economics, revised 25 Aug 2017. Available from: https://ideas.repec.org/c/boc/bocode/s458059.html
- 29. Hensher DA, Rose JM, Greene WH. Applied choice analysis: a primer. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- 30. Malhotra NK, Nunan D, Birks DF. Marketing research: an applied approach. London, UK: Pearson Education; 2017. [Google Scholar]
- 31. Soekhai V, de Bekker‐Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Bekker‐Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete‐choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryan M, Kolstad JR, Rockers PC, Dolea C. How to conduct a discrete choice experiment for health workforce recruitment and retention in remote and rural areas: a user guide with case studies. Geneva, Switzerland: World Health Organization; 2012.
- 34. Arends J, Thijs RD, Gutter T, Ungureanu C, Cluitmans P, Van Dijk J, et al. Multimodal nocturnal seizure detection in a residential care setting: a long‐term prospective trial. Neurology. 2018;91(21):e2010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beniczky S, Wiebe S, Jeppesen J, Tatum WO, Brazdil M, Wang Y, et al. Automated seizure detection using wearable devices: a clinical practice guideline of the International League Against Epilepsy and the International Federation of Clinical Neurophysiology. Epilepsia. 2021;62(3):632–46. [DOI] [PubMed] [Google Scholar]


