Ventricular–arterial coupling (VAC) is a very interesting variable in the field of heart failure (HF), enabling a more in‐depth evaluation of patient profile. Indeed, ‘vascular’ scenarios have been mentioned for years as an important entity within the scope of HF, yet without any practical approach to efficiently identify them. The assessment of VAC can fill in this gap.
A consensus document was published in the European Journal of Heart Failure in April 2019 presenting the assessment, clinical implications and therapeutic perspectives related to VAC in a clinical HF setting. 1 To show the clinical usefulness of VAC, this viewpoint presents the assessment of VAC in various clinical scenarios such as systemic hypertension and HF with reduced (HFrEF) and preserved ejection fraction (HFpEF), respectively. In order to facilitate the utilization of VAC into everyday clinical practice, a simplifying Excel sheet for the VAC calculation is provided (online supplementary Appendix S1).
The interplay between the heart and the arterial system has recently gained much attention since interventions that improve both myocardial and vascular functions may delay the progression to HF, valvular heart disease and possibly even improve prognosis. 1 , 2 Today, the assessment of VAC in clinical practice is being facilitated by advances in non‐invasive assessment of cardiac imaging. Traditionally, VAC has been defined as the combined marker of arterial and myocardial function, expressed as Ea/Ees ratio, where Ea reflects arterial elastance (an index of arterial load on the left ventricle) and Ees ventricular elastance (an index of the contractility of the left ventricle). 3 The Ea/Ees ratio has shown to be a key determinant of HF and increased arterial stiffness, both independently associated with impaired microcirculation causing damage to the end organs such as the kidneys.
Arterial elastance (Ea) is defined as the ratio of end‐systolic pressure and stroke volume (ESP/SV) 4 which is influenced by the vascular resistance, pulsatile load and heart rate. In contrast, Ees is a load‐independent measure of left ventricular (LV) contractility and reflects the slope of the end‐systolic pressure–volume relationship, originated from the principles of pressure–volume curve as the ratio of ESP and end‐systolic volume (ESP/ESV). 5 Subsequently, Ea/Ees (ESP/SV)/(ESP/ESVi) can be further simplified as ESV/SV, after removing ESP in the equation. Ees is affected by LV chamber stiffness and geometry and has an inverse correlation with LV mass.
In order to calculate Ees, invasive multi‐beat intraventricular catheterization has been regarded as the gold standard method. However, the non‐invasive method by Chen et al. 3 is commonly used where Ees can be calculated by the formula: Ees = [DBP – (End(est) × SBP × 0.9)]/End(est) × SV where DBP and SBP are diastolic and systolic arm‐cuff blood pressures, End(est) is the estimated normalized ventricular elastance at the onset of ejection, and SV is Doppler‐derived SV (Figure 1 ).
Figure 1.
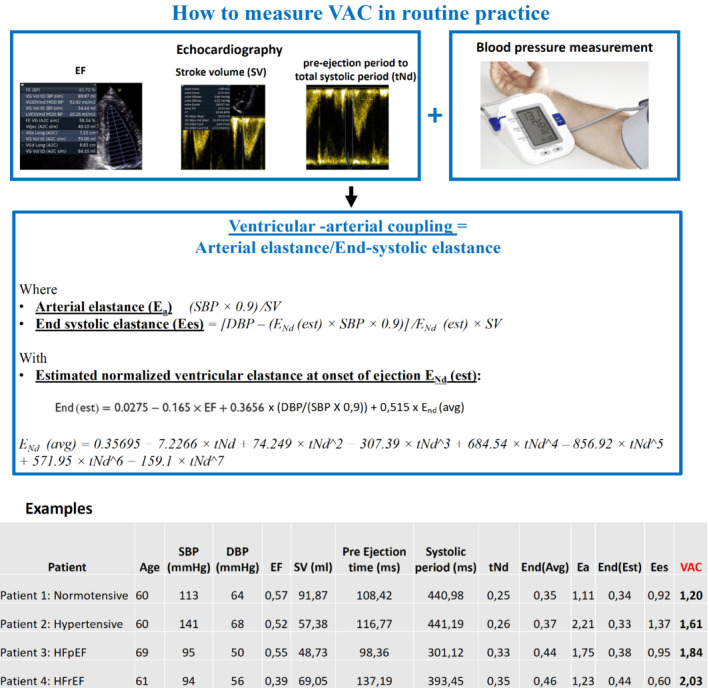
How to measure ventricular–arterial coupling (VAC) in routine practice. DBP, diastolic blood pressure; Ea, arterial elastance; Ees, ventricular elastance; EF, ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; SBP, systolic blood pressure; SV, stroke volume from four cavities pulsed Doppler; tNd, ratio of the pre‐ejection period to the total systolic period measured on the aortic pulse Doppler.
To elicit VAC results in routine practice, this viewpoint presents the assessment of VAC in various archetypal clinical scenarios such as systemic hypertension and HFrEF and HFpEF. We hope these examples will promote the use of this formula among physicians managing patients with HF. In addition, an Excel sheet providing embedded calculations is provided in online supplementary Appendix S1. Within routine care, physicians will only have to enter key variables from their echocardiographic exams (namely SBP, DBP, LV ejection fraction, stroke volume, pre‐ejection time and ejection time) and the sheet will provide correct calculations of Ees, Ea and VAC. This simplified sheet is more easily usable that the previous iOS‐based VAC calculators (iElastance); it reaches a wider audience as it is not tied to a platform/operating system and also allows decimals for the included variables. 6
Previous studies have shown that the optimal value of VAC derived from the Ea/Ees ratio should range from 0.5 to 1 reflecting the state when the stroke work of left ventricle is ideal. 7 , 8 Patient 1 (Figure 1) consequently corresponds to a ‘normal’ situation, as both blood pressure, ejection fraction, SV, and their interplay are within normal range.
When arterial load (Ea) increases to the point when Ea/Ees >1, a VAC mismatch appears with subsequent lower LV contractile efficiency. 9 This mismatch in VAC is often seen as the effect of increasing age and development of hypertension. Yet, the increase of Ea is met by a simultaneous increase of Ees (i.e. LV contractility) which preserves the VAC despite the presence of hypertension observed in patient 2. It should be acknowledged that Ea/Ees ratio has some limitations, i.e. it does not characterize the LV loading sequence. 10 Also, in HFpEF, it may be normal because both Ea and Ees are increased (patient 3). In the example of patient 4 with HFrEF, Ees is decreased as expected whilst Ea is slightly increased resulting in Ea/Ees ≥2. We would consequently like to emphasize that the use of the pulse wave velocity/global longitudinal strain (PWV/GLS) ratio may be more appropriate in a number of settings to characterize VAC since it incorporates the gold standard methods to assess arterial load (PWV) and LV contractility (GLS). Importantly, PWV/GLS has been shown to be better correlated with subclinical target organ damage compared with the traditional echocardiographic method (Ea/Ees). 11 Further, PWV/GLS might also help predicting response to cardiac resynchronization therapy and the benefit from sodium–glucose cotransporter 1 inhibitor, glucagon‐like peptide‐1 receptor agonists and anti‐inflammatory treatment in patients with rheumatoid arthritis. 12 , 13 , 14 Yet, even if PWV/GLS is likely more appropriate in a research setting, we can already use VAC in routine practice, only using simple echocardiographic measurements.
We hope that the figure presented herein (along with the provided online calculator, https://cic‐p‐nancy.fr/vac‐calculation‐tool‐sharing/) will promote the adequate calculation of the Ea/Ees ratio and prompt the use of VAC in patients with HF.
Conflict of interest: none declared.
Supporting information
Appendix S1. VAC_Calculation_sheet.
References
- 1. Ikonomidis I, Aboyans V, Blacher J, Brodmann M, Brutsaert DL, Chirinos JA, et al. The role of ventricular‐arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail. 2019;21:402–24. [DOI] [PubMed] [Google Scholar]
- 2. Fortuni F, Butcher SC, Dietz MF, van der Bijl P, Prihadi EA, de Ferrari GM, et al. Right ventricular‐pulmonary arterial coupling in secondary tricuspid regurgitation. Am J Cardiol. 2021;148:138–45. [DOI] [PubMed] [Google Scholar]
- 3. Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PYA, et al. Noninvasive single‐beat determination of left ventricular end‐systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–34. [DOI] [PubMed] [Google Scholar]
- 4. Chirinos JA, Rietzschel ER, Shiva‐Kumar P, de Buyzere ML, Zamani P, Claessens T, et al. Effective arterial elastance is insensitive to pulsatile arterial load. Hypertension. 2014;64:1022–31. [DOI] [PubMed] [Google Scholar]
- 5. Chang MC, Mondy JS 3rd, Meredith JW, Miller PR, Owings JT, Holcroft JW. Clinical application of ventricular end‐systolic elastance and the ventricular pressure‐volume diagram. Shock. 1997;7:413–9. [DOI] [PubMed] [Google Scholar]
- 6. Trambaiolo P, Figliuzzi I, Salvati M, Bertini P, Brizzi G, Tocci G, et al. Ventriculo‐arterial coupling in the intensive cardiac care unit: a non‐invasive prognostic parameter. Int J Cardiol. 2021;348:85–9. [DOI] [PubMed] [Google Scholar]
- 7. Burkhoff D, Sagawa K. Ventricular efficiency predicted by an analytical model. Am J Physiol. 1986;250:R1021–7. [DOI] [PubMed] [Google Scholar]
- 8. de Tombe PP, Jones S, Burkhoff D, Hunter WC, Kass DA. Ventricular stroke work and efficiency both remain nearly optimal despite altered vascular loading. Am J Physiol. 1993;264:H1817–24. [DOI] [PubMed] [Google Scholar]
- 9. Monge Garcia MI, Santos A. Understanding ventriculo‐arterial coupling. Ann Transl Med. 2020;8:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chirinos JA. Ventricular‐arterial coupling: invasive and non‐invasive assessment. Artery Res. 2013;7. 10.1016/j.artres.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ikonomidis I, Katsanos S, Triantafyllidi H, Parissis J, Tzortzis S, Pavlidis G, et al. Pulse wave velocity to global longitudinal strain ratio in hypertension. Eur J Clin Invest. 2019;49:e13049. [DOI] [PubMed] [Google Scholar]
- 12. Ikonomidis I, Pavlidis G, Katsimbri P, Lambadiari V, Parissis J, Andreadou I, et al. Tocilizumab improves oxidative stress and endothelial glycocalyx: a mechanism that may explain the effects of biological treatment on COVID‐19. Food Chem Toxicol. 2020;145:111694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, Kousathana F, et al. Effects of glucagon‐like peptide‐1 receptor agonists, sodium‐glucose cotransporter‐2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12‐month treatment. J Am Heart Assoc. 2020;9:e015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karamichalakis N, Ikonomidis I, Parissis J, Simitsis P, Filippatos G. Association of ventricular‐arterial interaction with the response to cardiac resynchronization therapy. Eur J Heart Fail. 2021;23:1238–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. VAC_Calculation_sheet.


