Abstract
Irrigation plays an essential role in root canal treatment. The purpose of this narrative review was to critically appraise the experimental methods and models used to study irrigants and irrigation systems and to provide directions for future research. Studies on the antimicrobial effect of irrigants should use mature multispecies biofilms grown on dentine or inside root canals and should combine at least two complementary evaluation methods. Dissolution of pulp tissue remnants should be examined in the presence of dentine and, preferably, inside human root canals. Micro‐computed tomography is currently the method of choice for the assessment of accumulated dentine debris and their removal. A combination of experiments in transparent root canals and numerical modeling is needed to address irrigant penetration. Finally, models to evaluate irrigant extrusion through the apical foramen should simulate the periapical tissues and provide quantitative data on the amount of extruded irrigant. Mimicking the in vivo conditions as close as possible and standardization of the specimens and experimental protocols are universal requirements irrespective of the surrogate endpoint studied. Obsolete and unrealistic models must be abandoned in favour of more appropriate and valid ones that have more direct application and translation to clinical Endodontics.
Keywords: biofilm, debris, extrusion, irrigant, penetration, pulp tissue, smear layer
INTRODUCTION
Irrigation plays an essential role during root canal treatment (Zehnder, 2006). The currently accepted paradigm suggests that irrigants accomplish the major part of cleaning and disinfection of the root canal system, whereas instrumentation is primarily a means to obtain access to the apical anatomy (Gulabivala et al., 2005), as instruments are unable to reach many areas (Peters, 2004). Anatomical complexities and the presence of bacteria as surface‐adherent biofilm structures are the foremost challenges for irrigation (Chávez de Paz & Ordinola Zapata, 2019) and motivate a continuous research interest. As a result, a plethora of studies have been published on this topic and new studies are continuously undertaken. Evidently, not all of them are accepted for publication. A recent report suggested that approximately 85% of all manuscripts (including those on irrigation) that are submitted to a leading Endodontic journal are rejected (Ahmad et al., 2019), often because of major experimental design and methodological flaws (Nagendrababu et al., 2021). This is an important problem because of the time and resources invested in these studies.
Efforts to improve the quality of the submitted manuscripts have led to the development of reporting guidelines for various study types in Endodontology (Nagendrababu et al., 2020), but these guidelines focus on manuscript preparation rather than study design and methodology. The methodology of studies on root canal irrigation was critically appraised a decade ago (Shen et al., 2012), so an update is warranted to cover the latest developments in this field. Such information could assist researchers in the selection of the most suitable methods and models. Knowledge of their strengths and weaknesses may also assist the authors of future systematic reviews and readers to interpret the findings of published studies and distinguish between reliable and unreliable research.
Research on root canal irrigation entails a wide variety of methods and models, ranging from basic and translational (or applied) research to clinical research. Basic research aims to generate knowledge on fundamental mechanisms related, for instance, to the biofilm, chemical reactions and physical action of irrigants, without attempting to extrapolate the findings directly to clinical practice. Translational research, on the other hand, aims to refine this knowledge and translate it into improved patient treatments using laboratory‐based or animal‐based models that mimic closely the in vivo conditions in humans (Fang & Casadevall, 2010). Clinical studies are the ultimate test for these treatments where they are evaluated under real‐life conditions.
The primary outcome of interest in clinical Endodontology is the prevention or healing of apical periodontitis (Azarpazhooh et al., 2022; Ørstavik, 2019), but very few publications on root canal irrigation have actually reported it (Liang et al., 2013). To facilitate laboratory‐based basic and translational research, surrogate end‐points, such as the antimicrobial effect, the dissolution of pulp tissue remnants, the removal of dentine debris and the smear layer, and the penetration of the irrigant in the root canal system, have been used instead. Likewise, inadvertent irrigant extrusion through the apical foramen has been used as a surrogate end‐point for extrusion accidents, which are an important, yet rare, side effect of irrigation (Boutsioukis et al., 2013). These end‐points are easier to quantify than the corresponding primary outcomes and they require shorter observation periods, so they have dominated the literature in this field, even though they often lack the necessary validation and their correlation with the primary outcomes is based on assumptions. Some examples of basic and translational research experiments per surrogate end‐point can be found in Table 1.
Table 1.
Examples of basic and translational research experiments for various surrogate endpoints used in root canal irrigation studies
| Surrogate endpoint | Basic research | Translational research |
|---|---|---|
| Antimicrobial effect | Measurement of the minimum inhibitory concentration of an irrigant against planktonic bacteria | Killing/removal of a multispecies biofilm grown inside a human root canal ex vivo by an irrigant |
| Dissolution of pulp tissue remnants | Dissolution of bovine pulp tissue by an irrigant in a test tube | Histological evaluation of pulp tissue remnants in human root canals irrigated ex vivo |
| Removal of dentine debris | Dissolution of dentine debris by an irrigant in a test tube | Micro‐CT evaluation of the accumulated dentine debris in root canals of extracted human teeth following irrigation. |
The purpose of this narrative review was to critically appraise the current experimental methods and models that are used to study irrigants and irrigation systems and to provide directions for future research. The review is organized according to the various end‐points of interest.
ANTIMICROBIAL EFFECT
Due to the key role of bacteria in the development of pulpal and periapical disease (Chávez de Paz, 2007; Kakehashi et al., 1965; Möller et al., 1981), the reduction of the intracanal microbial load is undoubtedly the most relevant surrogate end‐point when studying root canal irrigation. There is evidence that this end‐point is correlated to the healing of apical periodontitis, at least in single‐rooted teeth assessed by two‐dimensional imaging (Sjögren et al., 1997), but there is a need to confirm these findings in posterior teeth using three‐dimensional imaging.
Direct contact tests on planktonic cultures (in vitro)
Planktonic cultures have been widely used to determine the antimicrobial effect of root canal irrigants (Generali et al., 2020; Li et al., 2020; Nudera et al., 2007; Tong et al., 2011; Torabinejad et al., 2003). These simple tests on bacteria in a liquid phase measure the minimal inhibitory concentration and minimal bactericidal concentration of an irrigant (Andrews, 2011). As planktonic bacteria rarely occur in real‐life conditions in the root canal, the clinical relevance of these tests is very limited.
Another method that was used extensively in the past is the agar diffusion test (Sassone et al., 2008; Siqueira et al., 2000). Although simple and easy to perform, this test has important limitations that compromise the validity of the results. The measured antimicrobial effect is strongly affected by the ability of the irrigants to diffuse through agar, which depends on their molecular weight. Therefore, comparisons between different irrigants are unreliable. Moreover, the bacteria are in a planktonic state and the test cannot distinguish between bacteriostatic and bactericidal effects (Tobias, 1988). Contrary to its use for determining the effectiveness of systemic antibiotics against specific bacteria (Bonev et al., 2008), there are no accepted standards for its use to compare irrigants (Shen et al., 2012). Thus, this test should not be used to compare the antimicrobial activity of root canal irrigants, not even as a preliminary screening step (Editorial Board of the Journal of Endodontics, 2007).
Biofilm models in vitro and ex vivo
Root canal infections are caused by multispecies microbial biofilms organized in heterogeneous communities attached to dentinal surfaces (Svensäter & Bergenholtz, 2004). The biofilm mode of growth offers several advantages over their planktonic counterparts, for example an increased resistance to antimicrobial agents (Costerton et al., 1999). Several factors contribute to the antimicrobial resistance; the extracellular polymeric substance (EPS) acts as a diffusion barrier and hinders the penetration of antimicrobials into the biofilm; the different oxygen and nutrient availability force the cells to enter slow‐growing or starved metabolic states in the inner layers of the biofilm, rendering them less susceptible to antimicrobials; existing ‘persister cells’ express a highly persistent phenotype when exposed to antimicrobials (Costerton et al., 1999; Folkesson et al., 2008; Hall‐Stoodley et al., 2004; Lewis, 2007); the large number and high diversity of the microorganisms within the biofilm facilitate gene transfer that can confer antibiotic and antimicrobial resistance (Lerminiaux & Cameron, 2019). This greater resistance to antimicrobials has been observed in bacteria isolated from infected roots canals (Chávez de Paz et al., 2007). Overall, biofilms are the primary target of irrigants and, therefore, a variety of methods are used to determine their antimicrobial effect against biofilm grown in vitro or ex vivo.
Structure and composition of the biofilm
Most of the in vitro biofilm models used to study the effect of root canal irrigation have been composed of a single bacterial species (Du et al., 2014; Morago et al., 2019; Rodrigues et al., 2018; Zeng et al., 2020), predominantly Enterococcus faecalis. Single‐species biofilms are easy to grow and allow a high experimental throughput (Swimberghe et al., 2021). In the past, this species was thought to survive treatment procedures and persist even as a mono‐infection, leading to treatment failure (Siren et al., 1997; Sundqvist et al., 1998). However, more recent studies have questioned its role (Zehnder & Guggenheim, 2009; Zehnder & Paqué, 2011). E. faecalis is not present in many failed cases, and, when found, it is hardly ever among the most prevalent species (Bouillaguet et al., 2018; Siqueira et al., 2016; Zandi et al., 2018). Perhaps the most attractive feature of E. faecalis is its ability to tolerate a wide range of growth conditions, which greatly facilitates laboratory handling. The unwarranted attention paid to a single species is likely to have misled our understanding of the antimicrobial effect of various irrigants. For example, E. faecalis is particularly susceptible to chlorhexidine and early laboratory studies using only this species came to the erroneous conclusion that chlorhexidine is a very strong antimicrobial agent that could potentially replace NaOCl (Menezes et al., 2004). More recent work using multispecies biofilms has overturned this conclusion (Ruiz‐Linares et al., 2017).
Although single‐species biofilm models represent a clear improvement over the planktonic bacteria used in the past, they still do not resemble real‐life conditions, as these have a multispecies nature (Bouillaguet et al., 2018; Gomes et al., 2015; Siqueira & Rôças, 2014; Zandi et al., 2018). Complex interspecies interactions (Tan et al., 2017) result in larger biofilm production, increased virulence, as well as higher resistance to several antimicrobials (Jiang et al., 2011a; Stojicic et al., 2012). Thus, multispecies biofilm models formed from laboratory strains or clinical isolates of root canal bacteria have been established (Busanello et al., 2019; Chávez de Paz, 2012; Marinković et al., 2020; Swimberghe et al., 2021). These models usually include a small number of species selected based on their availability and interspecies compatibility. Nevertheless, even multispecies models may not be able to replicate natural root canal communities due to the differences in the environmental conditions and the difficulty to include microorganisms with restricted culture requirements (Chávez de Paz & Marsh, 2015). Increasing the biodiversity of multispecies biofilm models may compromise their standardization and reproducibility. Additionally, laboratory strains used in multispecies models have been shown to express different phenotypic characteristics than their clinical counterparts (Chávez de Paz et al., 2015).
As an alternative, natural biofilms can be grown directly from infected root canal samples (Clegg et al., 2006; Du et al., 2013; Ruiz‐Linares et al., 2017; Shen et al., 2011; Figure 1) or dental plaque (Shen et al., 2009, 2011; Stojicic et al., 2013). For example, naturally formed dental plaque biofilms have been grown on special intra‐oral orthodontic devices (del Carpio‐Perochena et al., 2015; Ordinola‐Zapata et al., 2013). These naturally formed multispecies biofilms, especially those composed of root canal bacteria, resemble more closely the composition, interspecies interactions and metabolic cooperation of root canal biofilms in vivo, leading to an increased resistance to antimicrobials (Chávez de Paz, 2007; Chávez de Paz & Marsh, 2015; Tan et al., 2017). However, selection of the most suitable incubation time, growth media and atmospheric conditions is challenging and some species, such as nonculturable ones, may be lost along the laboratory workflow (Rudney et al., 2012). Furthermore, the exact microbial composition is initially unknown; the biodiversity within each sample can only be explored afterwards using molecular techniques such as 16S rRNA gene sequencing (see section ‘Molecular methods’). Hence, natural multispecies biofilms cannot be reproduced in different laboratories or at different times. Despite these limitations, multispecies biofilm models are the models of choice for future studies to investigate the antimicrobial effect of irrigants. Single‐species biofilms are not recommended anymore for this purpose (Swimberghe et al., 2021).
FIGURE 1.
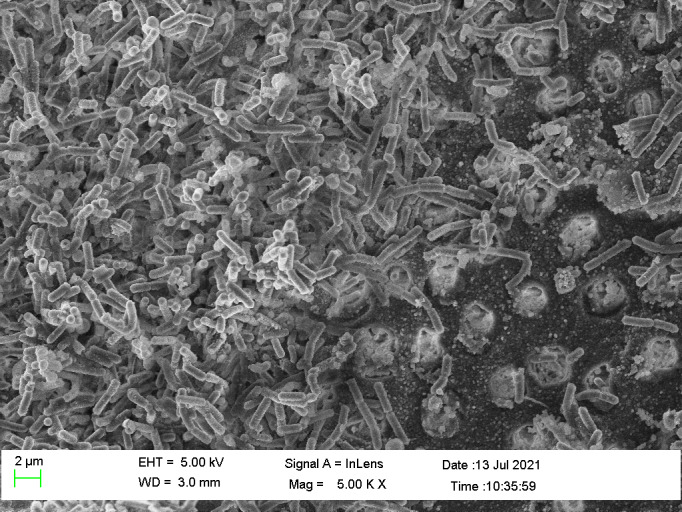
SEM photomicrograph of a natural multispecies biofilm grown from a necrotic root canal sample on dentine for 3 weeks
Substrate and surface coating
Both synthetic and natural surfaces have been used to grow biofilms in vitro. Synthetic surfaces, such as glass, polystyrene, polydimethylsiloxane, epoxy resin and hydroxyapatite (Layton et al., 2015; Liu et al., 2010; Pereira et al., 2021b; Petridis et al., 2019; Shen et al., 2010a; Townsend & Maki, 2009), allow better standardization of the specimens in terms of size, shape, composition and surface characteristics. However, they may alter the initial stages of biofilm formation because bacteria adhere to organic receptors on dentine, such as collagen fibrils, which influence the biofilm structure and composition (Kishen et al., 2008; Love & Jenkinson, 2002). In addition, synthetic substrates cannot reproduce the fine details of dentine microanatomy and the chemical interactions between irrigants and dentine, for example the consumption of the free available chlorine of NaOCl solutions (Macedo et al., 2010; Shen et al., 2012) or the detachment of biofilm bacteria due to chelation by EDTA (Banin et al., 2006). When a synthetic surface is needed for the growth of a multispecies biofilm, hydroxyapatite seems to have the most advantages (Shen et al., 2010a).
Natural surfaces, for example human dentine blocks and complete roots, have also been used to investigate the antimicrobial effect of irrigants ex vivo (Haapasalo & Ørstavik, 1987; Ma et al., 2011; Morago et al., 2019). However, age‐related changes such as the continuous deposition of peritubular dentine (Carrigan et al., 1984; Eldarrat et al., 2010), the reduced permeability (Thaler et al., 2008) and the reduced number of infected dentinal tubules (Kakoli et al., 2009), along with variability in the configuration and mineralization of dentine, are difficult to control and may confound the results (Thaler et al., 2008). Still, human dentine is the substrate of choice for biofilm growth. Using pairs of extracted teeth from the same patient may reduce these differences (Baumgartner et al., 2007; Kho & Baumgartner, 2006; Miller & Baumgartner, 2010) but only two groups can be compared at a time and it may be difficult to obtain enough sound specimens.
To overcome some of the limitations of human dentine, bovine dentine is often used as an alternative. Bovine teeth are easier to obtain and their age can be standardized along with various other environmental conditions. Nevertheless, bovine dentine has slightly different morphology, chemical composition and properties (Yassen et al., 2011), most notably a lower mineral content and collagen crosslinking (Enrich‐Essvein et al., 2021), as well as a significantly larger number of dentinal tubules compared to human dentine (Camargo et al., 2007). Its use as a substitute for human dentine when growing biofilm remains to be validated.
Sterilization of the specimens before biofilm growth is necessary to standardize the initial conditions. The choice of the sterilization method depends on the tolerance of the material/tissue to heat, humidity and chemicals. Sterilization must ensure complete elimination of all microorganisms without affecting its physical, chemical and biological properties. Steam autoclaving is the most widely used method for dentine sterilization (Nawrocka & Łukomska‐Szymańska, 2019), and it has only a minimal effect on its structure and mineral content (Parsell et al., 1998; Pashley et al., 1993). However, when dentine is pre‐treated with a chelating agent, autoclaving denatures and disintegrates the exposed collagen fibrils, so it affects dentine permeability (Jiang et al., 2019; Soares et al., 2011) and bacterial adhesion (Chivatxaranukul et al., 2008), which are important during the initial steps of biofilm formation. Gamma radiation has given promising results without notable adverse effects on dentine (White et al., 1994), but it requires costly equipment and careful adjustment of its operating parameters (Nawrocka & Łukomska‐Szymańska, 2019). Ethylene oxide and immersion in ethanol, hydrogen peroxide, glutaraldehyde, quaternary ammonium compounds or sodium hypochlorite are not sufficiently effective (Dominici et al., 2001; Pashley et al., 1993; Sandhu et al., 2012) and they may also interfere with bacterial adhesion (Sandhu et al., 2012).
Once the samples have been sterilized, they are often coated with saliva, mucin, bovine serum albumin or collagen to facilitate bacterial adhesion (Busanello et al., 2019; Kayaoglu et al., 2005; Layton et al., 2015; Li & Bowden, 1994; Lundstrom et al., 2010). Coating mimics the conditioning film that is naturally adsorbed on dentine and acts as a receptor for bacterial adhesins, so it is useful for all types of substrates. It can influence the formation and structural organization of a biofilm (Shen et al., 2010a; Stepanović et al., 2004) and its resistance to antimicrobials (Chávez de Paz et al., 2010; Violante et al., 2013). Nevertheless, it remains unclear which coating protocol is the most suitable for biofilm formation.
Biofilm growth
Biofilm can be grown under static conditions, which may lead to the nutrient supply becoming scarce at some stage (Kishen & Haapasalo, 2012; Merritt et al., 2005). Alternatively, it can be grown under dynamic conditions in flow chambers (Chávez de Paz et al., 2010) or fermenting devices (Pereira et al., 2020; Petridis et al., 2019) where a controlled continuous flow of medium provides fresh nutrients and drains the old medium and the waste products (Busanello et al., 2019; Chin et al., 2006; Pavarina et al., 2011; Shen et al., 2012; Tolker‐Nielsen & Sternberg, 2011). It is likely that the exudate penetrating an infected root canal in vivo flows very slowly and the applied shear force is not strong enough to affect biofilm formation. Consequently, static biofilm models seem to resemble more closely the root canal biofilms in vivo than dynamic ones (Shen et al., 2012), although neither type is able to fully replicate an in vivo infection.
The ‘dentine block’ model has been widely used to grow biofilm under static or dynamic conditions and evaluate the effect of root canal irrigants on infected dentine (Haapasalo & Ørstavik, 1987). The standardized geometry and infection of the specimens enhance the reproducibility. Owing to their small size, multiple blocks can be obtained from the same tooth (particularly from bovine teeth or from the coronal third of human teeth), which enables matching of the specimens prior to randomization (Baca et al., 2011). Biofilm growth on the surface of the blocks needs to be confirmed (see section ‘Microscopy’) before the experiment (Shen et al., 2012). It should be noted that the size of the dentine blocks varies between studies, which hinders direct comparisons, and that the effect of root canal geometry is excluded, so the results should be interpreted with some caution. Nevertheless, this model can be used for the initial screening of the antimicrobial effect of irrigants. In another similar model, bacteria are forced into the dentinal tubules by centrifugation to create a more standardized deep infection (Ma et al., 2011). This contamination method is markedly different from the way root canals are infected in vivo. Centrifugation may have a negative impact on the bacteria (Ma et al., 2011), so obligate anaerobes are not suitable as they may not survive the process. Another important limitation of these models is that the blocks are typically obtained from the coronal third of young teeth to avoid sclerotic dentine (Paqué et al., 2006; Vasiliadis et al., 1983a); therefore, the findings cannot be directly extrapolated to the apical third.
Biofilms have also been grown inside root canals ex vivo (Gazzaneo et al., 2019). This model resembles real‐life conditions more closely than dentine blocks, but the anatomy of the root canal may compromise the effectiveness of the infection process. Thus, it is important to include root canals with similar anatomy. Pre‐instrumentation is usually required to facilitate the entrance of bacteria in the root canal following the immersion in the bacterial suspension (Gazzaneo et al., 2019) or inoculation (Villalta‐Briones et al., 2021). Either way, successful biofilm formation on the root canal wall should be confirmed (Bhuva et al., 2010).
Biofilm age
Biofilm formation is initiated by the attachment of planktonic bacteria to a pre‐coated surface. Subsequently, microcolonies lead to total colonization and growth on the surface and form a complex biofilm community (Sauer et al., 2002). As the biofilm matures, its resistance to various antimicrobials increases (Lim et al., 2009; Shen et al., 2011; Stojicic et al., 2013; Swimberghe et al., 2021; Wang et al., 2012; Yang et al., 2016). In view of these findings and given that most in vivo biofilms are likely to be quite old (weeks‐, months‐ or even years‐old) at the time of root canal treatment, mature biofilms are a more realistic choice when evaluating the antimicrobial activity of irrigants in vitro or ex vivo. Unfortunately, the majority of the available studies on this topic used very young biofilms (<7 days; Swimberghe et al., 2019a), so they may have overestimated the effect of the irrigants. An important milestone seems to be reached after 3 weeks of biofilm maturation irrespective of the initial composition of the biofilm (Stojicic et al., 2013). However, this period may be intrinsic to that particular model and environmental conditions, so examining the biofilm growth kinetics in each particular model is recommended before initiating a study to identify and understand its maturation stage (Swimberghe et al., 2019a).
During biofilm growth, the media is usually refreshed once per week (Shen et al., 2011; Stojicic et al., 2013; Wang et al., 2012). The constant nutrient supply maintains the bacteria in an exponential growth phase and favours the removal of nonadhered bacteria and metabolic by‐products. This condition is clearly different from the clinical situation, particularly regarding secondary or persistent infections. Nutrients are very scarce in a previously treated root canal, and starvation makes the bacteria more resistant to adverse environmental conditions, including antimicrobials. Therefore, the type of biofilm used (starved/stressed or metabolically active) should be decided based on the particular focus of each study. Growing starved/stressed biofilms seems more reasonable when simulating a persistent infection.
Evaluation of the antimicrobial effect of irrigants on in vitro and ex vivo biofilm
So far, there is no gold standard method for the assessment of the antimicrobial effect of irrigants on biofilm, so it is recommended to combine two or more complementary methods that together can provide a more thorough view (Camilleri et al., 2020).
Direct contact test on biofilm
A direct contact test can be performed on biofilm grown in vitro to determine the minimal biofilm eradication concentration (MBEC; Figure 2). This test is quick, easy to perform and reproducible (Arias‐Moliz et al., 2010; Ceri et al., 1999). However, the biofilm is brought in contact with an excess of irrigant in the absence of dentine, so MBEC is a very poor predictor of the antimicrobial effect of the irrigant ex vivo and in vivo. Hence, this test is only suitable for initial screening and should always be complemented by more accurate methods (Arias‐Moliz et al., 2010; Ferrer‐Luque et al., 2010; Giardino et al., 2020a, 2020b).
FIGURE 2.

MBEC™‐high‐throughput device (Innovotech) that can be used to grow biofilms on 96 polystyrene pegs (top half). A flute trough (bottom half) guides the inoculated growth medium across the pegs when the device is placed on a rocker
Sampling
Effective sampling of the bacteria in a biofilm depends on the location. When the biofilm resides on a dentine block or an inert surface, it can be recovered in a liquid (e.g., broth or saline) by vortexing, sonication, or centrifugation (Baca et al., 2011). Fine‐tuning of the recovery protocol is required for each particular biofilm/substrate combination to ensure that the applied shear force is enough to completely detach the biofilm from the surface without damaging the bacteria.
When the biofilm is grown inside a root canal, samples can be obtained by a combination of paper points, files and/or burs (Ercan et al., 2004; Ferrer‐Luque et al., 2014; Gomes et al., 2003; Möller, 1966; Peters et al., 2011), a difficult and technique‐sensitive procedure (Sathorn et al., 2007). Paper points alone can only sample planktonic bacteria from the main root canal lumen and bacteria loosely adhered to the wall. The procedure can be improved by introducing a solution into the root canal and scraping the wall with files or burs in an effort to loosen the biofilm and adjacent dentine before inserting the paper points (Möller, 1966). Vortexing or sonication of the paper points and the files/burs is then used to recover the sampled bacteria. The information on the precise location of the bacteria in the root canal is lost during sampling. In addition, the microbial load in the sampled areas may not be representative of the remaining bacteria in isthmuses, lateral canals and other anatomic irregularities that are difficult to reach with instruments and irrigants (Sathorn et al., 2007).
These sampling limitations can be partially overcome in ex vivo experiments by cryo‐pulverization of the roots, which facilitates the recovery of bacteria from difficult‐to‐reach areas (Alves et al., 2009). However, this is a destructive method, so repeated testing of the same specimens is not possible, and care must be taken to disinfect the external root surface before pulverization.
Culturing
The number of viable and cultivable bacteria in a sample can be determined by plating on agar plates (Hannig et al., 2007) and counting the colonies formed (colony forming units—CFUs), a method that has been used extensively (Figure 3). It should be emphasized that only viable bacteria that are able to divide and form colonies in the supplied culture medium are quantified by this method (Azeredo et al., 2017). A high proportion of the bacteria present in root canal infections are viable but nonculturable (VBNC), which means that they are metabolically active and virulent bacteria that can initiate biofilm formation, albeit to a lesser degree than viable bacteria, but they lack the ability to grow in culture media (Li et al., 2014). As a result, culturing of samples from a natural multispecies biofilm model that includes VBNC bacteria will underestimate the diversity and the total number of bacteria. In addition, culture methods are laborious and time‐consuming; a large range of culture media have to be prepared and samples must be incubated for extended periods of time in order to detect slow‐growing microorganisms.
FIGURE 3.
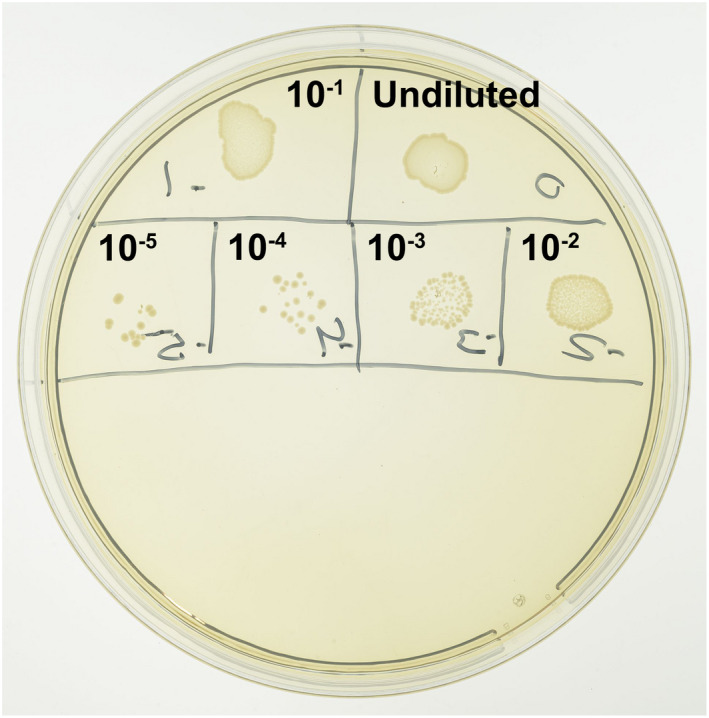
CFUs of an E. faecalis suspension grown on a Brain Heart Infusion (BHI; Scharlau Chemie SA) agar plate (bottom side shown). A 10‐μl drop of the suspension, either undiluted or diluted over the range 10−1 to 10−5, was plated at each location
Molecular methods
Molecular methods are based on the detection of nucleic acids from microorganisms. Among them, the polymerase chain reaction (PCR) is the one that revolutionized the field of molecular biology (Mullis et al., 1994). Early implementations lacked the ability to quantify the amount of DNA in the sample. The real‐time/quantitative polymerase chain reaction (qPCR) overcame this problem by monitoring the number of thermal cycles until a certain amount of DNA is produced. This number is correlated with the initial amount of DNA, thus with the number of bacteria. qPCR is very sensitive and as few as 10 bacterial cells can be detected in a sample (Siqueira & Rôças, 2017). The bacteria can be identified using either species‐specific primers or universal primers that detect a broad spectrum of bacteria. The latter are very useful when analyzing natural multispecies biofilms, but they are not as sensitive and even they cannot target all the species in a sample (Döring et al., 2008; Horz et al., 2005).
qPCR has been proposed as an alternative to culturing when studying the effect of root canal irrigation (Blome et al., 2008; Rodrigues et al., 2017; Zandi et al., 2016, 2019). This method is more sensitive than culturing and can detect microorganisms independently of their growth phase. Although a positive correlation of these findings to CFU counts has been reported (Aul et al., 1998; Malawista et al., 1994), qPCR can also detect free extracellular DNA and DNA from dead cells (Brundin et al., 2014, 2015; Klein et al., 2012; Siqueira & Rôças, 2005a, 2005b; Young et al., 2007). Therefore, the effect of antimicrobials on biofilm may be underestimated. Some studies reported that the half‐life of free DNA in an infected root canal seems to be very short because of the action of DNases and, therefore, it has only a minor effect on bacteria quantification by qPCR (Siqueira, 2008) but others have come to the conclusion that this DNA can be preserved for months, so it can be an important source of error (Brundin et al., 2014, 2015; Young et al., 2007). To overcome this problem, pre‐processing to degrade the free DNA prior to qPCR has been proposed (İriboz et al., 2018). This can be achieved by DNase treatment during sample preparation (İriboz et al., 2018). Alternatively, the samples can be treated with a photo‐reactive viability dye which binds selectively to the DNA of membrane‐compromised cells and leads to its degradation upon light exposure before DNA extraction and amplification, a technique named viability‐PCR (Codony et al., 2020; Nkuipou‐Kenfack et al., 2013). However, some DNA from diseased cells may persist (Codony et al., 2020). Recently, the disinfection procedure of the operative field and the sterility controls that had been used in a number of studies employing PCR and qPCR were also criticized because they were based on the requirements of culture‐based methods rather than molecular ones (Figdor & Brundin, 2016). To serve their purpose, sterility controls need to be analyzed in the same manner as the experimental samples in a study. It should also be emphasized that molecular methods are generally subject to the same sampling limitations as culture‐based methods.
Reverse transcriptase PCR is another molecular method that has been used to quantify the remaining bacteria following different irrigation protocols. This method is based on the detection of bacterial RNA and it provides more reliable information about the viability of the microorganisms in the sample compared to qPCR because RNA has a shorter half‐life than DNA and it is degraded rapidly after cell death (Kempsell et al., 2000; Miskin et al., 1999; Teske et al., 1996). However, RNA is also more difficult to isolate and preserve (Kawane et al., 2014).
A more recent development in molecular methods is next‐generation sequencing (NGS), also known as high‐throughput sequencing (İriboz et al., 2018; Zandi et al., 2018). NGS is based on PCR amplification and sequencing of the 16S rRNA gene, which allows the analysis of the taxonomic composition of microbiological ecosystems. It is a very sensitive method but the DNA extraction process may not be equally effective for all the taxa (Manoil et al., 2020). In addition, NGS is also unable to determine the viability of the bacteria and can detect free DNA and DNA originating from dead cells (Siqueira, 2008), like other molecular methods. Finally, the cost of this method is still high and the necessary equipment is not widely available. Even though NGS has been mainly employed to characterize the composition of the microbiota present in root canal infections (Keskin et al., 2017; Sánchez‐Sanhueza et al., 2018; Zahran et al., 2021), it has also been used to study the effect of chemomechanical preparation on the microbial diversity (Gomes et al., 2015; İriboz et al., 2018; Zandi et al., 2018). Moreover, the rRNA/DNA ratio calculated from NGS data has been used to estimate the proportion of active bacteria in a sample before and after instrumentation (Nardello et al., 2020). Thus, NGS can be a valuable tool for analyzing the effect of irrigants on both the composition and the viability of multispecies biofilms.
Microscopy
Light microscopy combined with histological staining allows for visualization of microorganisms in a specimen (Vera et al., 2012b). Although the resolution is not very high, it can provide valuable qualitative information about the biofilm, its location within the root canal system and its relation with pulp tissue remnants (Nair et al., 2005; Peters et al., 2011; Ricucci & Siqueira, 2008). Thanks to its low magnification, larger parts of a specimen can be imaged compared to other methods. However, it requires laborious sample preparation that includes fixation, decalcification, sectioning and staining. Moreover, it only provides two‐dimensional information and findings gathered from 2–3 sections may not be representative of the entire root canal. In addition, light microscopy is not a very sensitive method to detect bacteria and it does not provide information about their viability. Therefore, it is not suitable for the quantification of the antimicrobial effect of root canal irrigants but it could be used as a supplement to quantitative methods.
Scanning electron microscopy (SEM) provides high‐resolution and high‐magnification images of surface structures that allow morphological characterization of the biofilm on a specimen. Irregular surfaces can be easily imaged due to its larger depth of field compared to light microscopy (Azeredo et al., 2017; Morago et al., 2016; Figure 1). It has been widely used to confirm the presence or growth of a biofilm on a specimen and for qualitative evaluation of the effect of root canal irrigants on biofilm (Arias‐Moliz et al., 2021; Marinković et al., 2020; Shen et al., 2011). However, the specimens must undergo laborious preparation before imaging, including fixation, freeze‐ or critical‐point‐drying and coating with a conductive material. These processes may alter the cell morphology and introduce artefacts (Hannig et al., 2010). Drying also leads to the collapse of biofilm matrix polymers (Kachlany et al., 2001; Little et al., 1991) and conductive coating may obscure some structures (Bergmans et al., 2005; Little et al., 1991). Similar to light microscopy, SEM does not provide information about bacteria viability and it does not allow their identification or quantification. Moreover, it does not provide any data about the biofilm layers below the surface. Some additional limitations of SEM are discussed in the section on the removal of debris and smear layer.
Environmental scanning electron microscopy (ESEM) is based on the same principles as SEM, but the specimen can be imaged in low vacuum following minimal or no preparation, so biological specimens, including delicate biofilm structures such as the EPS matrix, can be imaged without prior dehydration or coating (Bergmans et al., 2005; Collins et al., 1993; Priester et al., 2007). This reduces the artefacts (McKinlay et al., 2004) and enables longitudinal evaluation of the same area at different times, for instance before and after irrigation (Bergmans et al., 2008; Reis et al., 2008), although exact repositioning of the specimen in the microscope chamber is not straightforward (Reis et al., 2008). Its resolution is lower than SEM, so less topographical details can be obtained (Bergmans et al., 2005) and, similarly to SEM, only the surface of the specimen can be examined and no information is provided about the viability of the bacteria.
Confocal laser scanning microscopy (CLSM) is currently among the most valuable techniques for in situ visualization and quantification of a biofilm (Lawrence et al., 1991; Neu & Lawrence, 2014). Its resolution allows for visualization of single cells (Daddi Oubekka et al., 2012). Capturing of multiple images along varying focal planes and computer‐based processing enable the three‐dimensional reconstruction of the biofilm and measurement of parameters such as the biofilm volume, thickness and surface coverage (Chávez de Paz, 2009). Unlike SEM, fixation, drying and coating are not required, so the biofilm remains hydrated and without alterations. To visualize the various components of the biofilm, the specimen can be stained with fluorochromes. Dual staining with SYTO 9 and Propidium Iodide (PI; Live/Dead Baclight bacterial viability kit; Invitrogen) is probably the most widely used method to discriminate between intact (stained by SYTO 9) and damaged cells (stained by PI) based on the integrity of their membrane. SYTO 9 labels both viable and VBNC cells (Netuschil et al., 2014). Therefore, this viability kit can reveal the three‐dimensional cell distribution in a biofilm (Hope et al., 2002) and the effect of antimicrobials on them, both on a surface and inside dentinal tubules (Ma et al., 2011; Villalta‐Briones et al., 2021). It is considered good practice to adjust and validate the staining protocol before each study (Stocks, 2004) to cope with interspecies differences, particularly when examining a multispecies biofilm (Zotta et al., 2012). CLSM has a small depth of field, so to image biofilm grown on dentine, its surface needs to be flattened in advance, a process that inevitably alters its morphology (Figure 4). CLSM also works at very high magnification, so it is not feasible to scan the entire specimen and evaluation is limited to a few selected spots. In some cases, dentine debris and the smear layer may also retain the fluorochromes and lead to errors. Finally, a common pitfall in the interpretation of CLSM findings is that, following Live/Dead staining, green‐stained and red‐stained cells are incorrectly regarded as live and dead cells, respectively. However, cells with intact membranes (green‐stained) can be metabolically inactive, thus dead, and cells with a damaged membrane (red‐stained) may still be alive (Netuschil et al., 2014).
FIGURE 4.
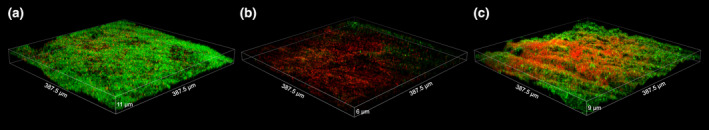
Three‐dimensional reconstruction of CLSM scans of natural multispecies biofilms grown from an infected root canal sample on dentine for 3 weeks: (a) untreated control, (b) after treatment with 2.5% NaOCl for 1 min, (c) after treatment with 0.2% cetrimide for 1 min. Green‐coloured bacteria are cells with intact membranes and red‐coloured bacteria are cells with damaged membranes following Live/Dead staining (BacLight; Invitrogen)
In fluorescence in situ hybridization (FISH), fluorescent probes bind to specific 16S rRNA sequences in permeabilized bacteria during incubation under controlled conditions. FISH can assist microscopic identification of bacteria and it also provides detailed information on the spatial organization of mixed microbial communities (Figure 5; Chávez de Paz et al., 2015; Lukic et al., 2020; Sunde et al., 2003). Furthermore, it is very sensitive and can detect microorganisms independent of their growth, although limited data are available concerning the detection of VBNC bacteria (Gao et al., 2021). However, it requires an extensive preparation of the specimens, only a limited number of probes are available and hybridization may not be equally efficient in all cases (Azeredo et al., 2017). The number of different microorganisms that can be detected simultaneously is also limited. Finally, similarly to other molecular techniques, FISH can also detect the free extracellular DNA and the DNA derived from dead cells.
FIGURE 5.
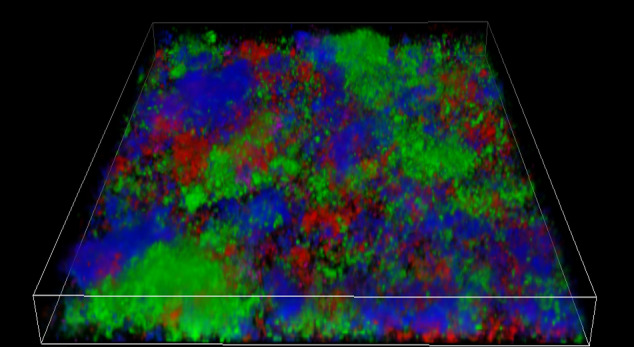
Three‐dimensional reconstruction of CLSM scans of 16S rRNA FISH‐labelled biofilm cells in cocultures of 3 species isolated from infected root canals. Streptococcus gordonii cells bind to probe STR405 (green fluorescence) and were detected by Ar‐laser excitation (480 nm). Lactobacillus salivarius (red) and Actinomyces naeslundii (blue) were simultaneously detected using lasers G‐HeNe (543 nm) and UV (390 nm). [for further information see Chávez de Paz (2012)]
Optical coherence tomography (OCT) is an imaging method based on low‐coherence interferometry. Light scattered by the biofilm is recorded and processed to obtain a cross‐sectional or fully three‐dimensional image of the biofilm (Wagner & Horn, 2017). This method is non‐invasive and requires no sample preparation, so repeated evaluation of the biofilm in its native state is possible. Changes in the biofilm structure (volume, thickness, porosity and roughness) after the application of an irrigant can be visualized and quantified through image analysis (Busanello et al., 2019; Wagner & Horn, 2017; Figure 6). It also provides a superior view of the substrate–biofilm and fluid–biofilm interfaces (Busanello et al., 2019; Pereira et al., 2020, 2021a). Its spatial resolution is lower than other microscopy techniques such as SEM and CLSM but the field of view is larger, so it is possible to scan the entire specimen in a very short time. Direct optical access to the biofilm is required at least from one direction. Another disadvantage is that OCT provides no information on the composition of the biofilm and the viability of the bacteria (Wagner & Horn, 2017).
FIGURE 6.
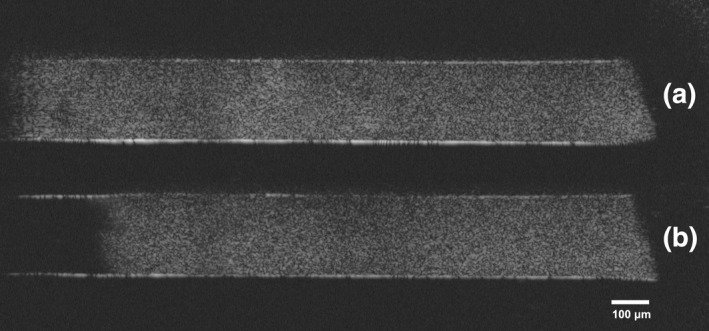
OCT scans of a dual‐species biofilm (S treptococcus oralis and Actinomyces naeslundii) grown inside artificial lateral canals made of Polydimethylsiloxane using a constant depth film fermenter (a) before and (b) after ultrasonic activation of 2% NaOCl in the adjacent main root canal (to the left)
Atomic force microscopy (AFM) is a high‐resolution scanning probe microscopy method that can measure the cohesive strength of the biofilm and the adhesion force between the biofilm and the substrate (James et al., 2017). No special preparation of the biofilm specimen is required (Müller et al., 2009) but the scanned surface has to be flat, so the natural morphology of dentine must be altered in most cases. AFM has been used to study the effect of irrigants on the short‐term adhesion force between bacteria and root canal dentine or filling materials (Kishen et al., 2008; Xu et al., 2019). However, it is not possible to functionalize the AFM tips with the same number of bacteria each time, which may affect the magnitude of the measured force (Kishen et al., 2008).
Chemical methods
The crystal violet assay is a colorimetric assay that has been used for rapid approximate quantification of the biofilm mass after exposure to irrigants (Christensen et al., 1985; Li et al., 2020; Mohmmed et al., 2016). This assay has been applied to biofilms grown on microtiter plates (Alves et al., 2013; Li et al., 2020; Mohmmed et al., 2016; Wilson et al., 2015) and also inside artificial root canals created in acrylic blocks (Layton et al., 2015; Townsend & Maki, 2009). It is easy to perform, inexpensive, and it can be applied to different bacterial species. However, it cannot differentiate between living and dead cells (Peeters et al., 2008; Pitts et al., 2003), so it can only quantify biofilm removal (Peeters et al., 2008). Moreover, reproducibility is a problem (Arnold, 2008; Peeters et al., 2008) and there is no standard protocol, so comparisons between different studies are hindered. Due to these limitations, its use should be limited to screening of potential antibiofilm agents before using more laborious and accurate quantification methods (Alves et al., 2013).
The adenosine triphosphate (ATP) assay measures the ATP production of the bacteria and reflects the metabolic activity of viable and VBNC cells (Beumer et al., 1992; Sánchez et al., 2013). Measurements can be taken using a variety of enzymatic assays, for example the one based on luciferase (Braissant et al., 2020). This assay is easy to perform, the results are obtained within a few minutes and it can detect as few as 10 bacterial cells (Tan et al., 2015). The results have been correlated with the CFU counts over a wide range of bacterial species (Choi et al., 2018; Solana et al., 2017; Tan et al., 2015). However, the reaction is non‐specific, so it is not possible to identify the microorganisms, and the amount of ATP produced may vary depending on the species, so it is difficult to calculate the number of microbial cells in a multispecies biofilm (Stewart, 1990). Therefore, the ATP assay can be used mainly as a complement to other methods.
The XTT assay is based on the reduction of the XTT dye to a formazan (Roehm et al., 1991). The amount of the formazan is proportional to the number of metabolically active microbial cells. This assay has been used to quantify the effect of irrigants on biofilm (Rana et al., 2019; Wright et al., 2021; Ye et al., 2019). However, problems regarding intra‐ and interspecies variability have been reported (Peeters et al., 2008). It is also expensive, more time‐consuming and less sensitive (>106 CFU/ml) than other chemical methods (Honraet & Nelis, 2006; Peeters et al., 2008). Consequently, its use is not recommended.
Resazurin is a stable redox indicator that is reduced to resorufin by metabolically active bacteria (O'Brien et al., 2000; Pettit et al., 2005). Similarly to the XTT assay, it can quantify both viable and VBNC bacteria (Gao et al., 2021) and its results correlate well with CFU counts (Jiang et al., 2011a; Pettit et al., 2005; Sandberg et al., 2009). It is a rapid, inexpensive and less time‐consuming method compared to the XTT assay (O'Brien et al., 2000; Peeters et al., 2008) and it has been used as an initial screening method to explore the effect of different irrigant concentrations on biofilm (Jiang et al., 2011a). Unfortunately, its sensitivity is relatively low (>105–107 CFU/ml; Jiang et al., 2011a; Sandberg et al., 2009) and microorganisms metabolize resazurin at a varying rate, so different incubation times are required for multispecies biofilms (Peeters et al., 2008). Similarly to the ATP assay, resazurin can be used to complement other methods.
Ex vivo models to evaluate irrigant substantivity
Some irrigants can bind on dentine and exert an antimicrobial effect over time (substantivity) which may prevent bacterial (re)colonization after root canal treatment (Komorowski et al., 2000; Rosenthal et al., 2004). However, published ex vivo studies often evaluated this property under unrealistic conditions. The dentine blocks that served as test specimens were totally immersed in the irrigant (Baca et al., 2012; Barrios et al., 2013; Komorowski et al., 2000; Parsons et al., 1980; Rosenthal et al., 2004), in some cases for up to 40 min (Parsons et al., 1980), which exaggerated the effect, and they were subsequently exposed to high concentrations of bacteria (Baca et al., 2012; Barrios et al., 2013), which also differs from the conditions in a treated root canal. The use of a single species, namely E. faecalis, as a test microorganism introduced further bias, as already explained. Some studies assessed the antimicrobial effect rather than substantivity (Khademi et al., 2006). The root canal was rarely filled (Rosenthal et al., 2004), so the potential adverse effect of the filling materials on substantivity was mostly ignored. Finally, irrigants that demonstrate substantivity (such as chlorhexidine) have a strong affinity for dentine, so they can be transferred together with dentine into the test assays and lead to false‐negative results (Rosenthal et al., 2004). Therefore, careful neutralization of the irrigants prior to evaluation is essential but it was rarely done in published studies.
Considerations for animal studies
Animal studies can reproduce more closely the in vivo conditions in humans than in vitro and ex vivo studies (Haapasalo, 2016). Many of the methods described already can be also applied in animal studies but would be unethical to apply in clinical studies (Garcia de Paula‐Silva et al., 2009; Holland, 1992; López et al., 2015; Silva et al., 2004; Sperandio et al., 2008; Tanomaru Filho et al., 2002). However, ethical guidelines for animal studies are also strict and the studies can be very costly. Contamination is a concern when sampling animal root canals in vivo and, due to the polymicrobial nature of real‐life biofilms, it is recommended not to rely exclusively on culture‐dependent quantification methods (Cohenca et al., 2010). Moreover, the control of confounding factors is not as effective as in in vitro and ex vivo studies, so a larger sample size may be required (Shen et al., 2012). There may also be differences in the root canal anatomy, host response and tissues between animals and humans, so the findings should not be directly extrapolated to the clinical situation, although they are usually more clinically relevant than those of laboratory‐based studies (Haapasalo, 2016).
Considerations for clinical studies
Clinical studies are a higher level of evidence as they allow testing of irrigants and irrigation techniques under real‐life conditions (Haapasalo, 2016). Root canal anatomy, temperature, nutrients, dentine, host response and the biofilm are all present (Shen et al., 2012). Nevertheless, some of these parameters cannot be controlled, so they act as confounders. For instance, it is not possible (or ethical) to create standardized root canal infections. A larger sample size is usually recommended to circumvent this problem (Haapasalo, 2016) but recruiting enough patients may be difficult. Obtaining a representative sample from root canals in vivo is also notoriously challenging (Ruksakiet et al., 2020) and special protocols must be followed to disinfect the operating field (Figdor & Brundin, 2016; Möller, 1966). Similarly to animal studies, it is preferable not to rely only on culture‐dependent methods when studying natural biofilms in vivo (Vianna et al., 2006). Clinical studies also need to follow very strict ethical guidelines (Shen et al., 2012). Depending on the design of each study, it may be possible to treat the teeth in vivo and evaluate them ex vivo (Nair et al., 2005; Vera et al., 2012b). However, the root canals can be easily contaminated even during tooth extraction (Kapalas et al., 2011).
DISSOLUTION OF PULP TISSUE REMNANTS
Pulp tissue remnants are considered a potential source of nutrients for bacteria surviving in the root canal (Love, 2012), and they may also interact with the irrigants and limit their antimicrobial action (Haapasalo et al., 2007). Therefore, their dissolution and removal from the root canal system is one of the goals of irrigation (Zehnder, 2006), even though there is still no evidence that it has any effect on the healing of apical periodontitis.
Human pulp tissue remains the first choice for in vitro and ex vivo experiments focusing on this surrogate end‐point. However, the difficulty to obtain it in sufficient quantity (Cullen et al., 2015; Slutzky‐Goldberg et al., 2013) has motivated the use of other tissues, such as bovine pulp tissue (Al‐Jadaa et al., 2009a, 2009b; Camps et al., 2009; Guneser et al., 2015) or meat (Haapasalo et al., 2014; Stojicic et al., 2010; Tartari et al., 2015, 2017; Tejada et al., 2019), pig pulp tissue (Clarkson et al., 2012) or palatal mucosa (Conde et al., 2017; Naenni et al., 2004), rat tissue (Hand et al., 1978), and shrimp meat (Ballal et al., 2021). These tissues are easily available and can be cut into standardized specimens (Stojicic et al., 2010). It is strongly recommended that the use of any substitute tissue is sufficiently justified concerning its similarity to human pulp tissue.
In vitro studies have often immersed a standardized tissue specimen in abundant irrigant inside a test tube or other similar container and measured its dissolution rate (Cullen et al., 2015; Guneser et al., 2015; Haapasalo et al., 2014; Hand et al., 1978; Stojicic et al., 2010). Such basic‐science experiments examine the direct chemical effect of the irrigant on tissue under optimum well‐controlled conditions, so they are useful for initial screening but the results should not be directly extrapolated to clinical practice. The absence of dentine leads to an overestimation of the dissolution capacity (Tejada et al., 2019). Moreover, test tubes cannot reproduce the fluid dynamics of a human root canal when the irrigant is delivered or agitated (see section ‘General points’), so experiments of this kind should not be used to compare irrigation methods. Apart from their weight, the tissue specimens also need to be standardized in terms of size and shape (Haapasalo et al., 2014; Stojicic et al., 2010) because the exposed surface area of the tissue is one of the parameters that affect their dissolution rate (Guneser et al., 2015). When quantification is based on the time until complete dissolution (Cullen et al., 2015), the end of the reaction may be difficult to determine because of the large number of bubbles produced (Shen et al., 2012). Hence, the weight loss of the specimen after contact with the irrigant for a fixed time has been used instead (Hand et al., 1978; Naenni et al., 2004; Stojicic et al., 2010). However, in this case, the measurements can be affected by the hydration state of the specimen, which needs to be standardized before weighing (Hand et al., 1978; Stojicic et al., 2010; Tartari et al., 2017). In addition, hypertonic irrigants will draw water out of the specimen and reduce its weight, whereas hypotonic irrigants will have the opposite effect. Tissue dissolution has also been evaluated indirectly through measurement of the available chlorine in the NaOCl solution before and after interaction with the tissue (Moorer & Wesselink, 1982) or measurement of the amount of the amino acid hydroxyproline in the remaining tissue (Koskinen et al., 1980). Evidently, these methods are more complicated and time‐consuming than weighing.
To include the chemical interactions with dentine in the experiments, some studies have added dentine powder (Guneser et al., 2015; Tejada et al., 2019) or bars to the solution (Cullen et al., 2015) or the experiments have been performed inside artificial dentine cavities instead of inert containers (Slutzky‐Goldberg et al., 2013). Dentine powder has an exaggerated surface to volume ratio compared to the root canal wall, which probably leads to overestimation of its chemical effect on the irrigant. In addition, preparation of dentine specimens, whether in the form of powder, bars, or cavities, leads to inevitable structural modifications of dentine, which may also affect the chemical reactions (Shen et al., 2012).
Artificial root canal systems created in transparent plastic blocks have also been employed to mimic the flow conditions and irrigant‐tissue contact inside a real root canal (Al‐Jadaa et al., 2009a, 2009b; Malentacca et al., 2012), albeit without including the chemical effects of dentine. These models contain accessory canals that are filled with minced tissue and its dissolution is quantified by digital photography. It may be difficult to ensure complete and homogeneous filling of these accessory canals and minced tissue may be easier to dissolve than intact pulp tissue in vivo. Moreover two‐dimensional evaluation may not be able to describe a three‐dimensional effect in full.
Experiments have also been conducted in human teeth with artificial grooves (Conde et al., 2017) or resorption cavities (Ballal et al., 2021; Ulusoy et al., 2018). The roots are split to create these irregularities and fill them with a pre‐weighed amount of soft tissue and then they are reassembled. The tissue remaining after irrigation is weighed again. Although standardization of the tissue may not be as accurate as in the in vitro experiments in test tubes, these models combine more realistic flow conditions with the chemical effect of dentine and allow an improved understanding of tissue dissolution inside the root canal. Nonetheless, the artificial grooves are often much wider than real fins and isthmuses and the cavities resemble advanced cases of internal resorption, so tissue dissolution may be overestimated due to the exaggerated contact surface.
Instead of artificially placed tissue specimens, a few studies have used human teeth with a vital pulp that had already been scheduled for extraction. In some cases, the teeth were treated in vivo and subsequently extracted, fixed and processed for histological examination (Burleson et al., 2007; Gutarts et al., 2005), whereas other studies used freshly extracted teeth with a vital pulp that were immediately fixed and then treated ex vivo (De‐Deus et al., 2013; Varela et al., 2019). An in vivo study design requires ethical approval and it may be difficult to recruit enough patients for such a procedure and control potential confounders (Shen et al., 2012). The ex vivo design, on the other hand, may allow better post‐extraction standardization of the anatomy, but it should be emphasized that fixed pulp tissue is more difficult to dissolve than unfixed tissue (Thé, 1979), so the effect of the irrigants may be underestimated. Regardless of the design, the presence of intact pulp tissue must be confirmed to ensure a standardized initial condition.
Histological examination of the specimens after irrigation requires time‐consuming and complex preparation which may introduce artefacts because of tissue shrinkage. Two‐dimensional evaluation of the slices can give quantitative information about the surface covered by pulp tissue remnants in the main root canal, uninstrumented fins and isthmuses but the amount of histological detail provided exceeds what is necessary for this purpose. Evaluation is usually limited to a few slices that may not be representative of the entire root canal. Finally, this is a destructive method that does not allow longitudinal evaluation of the same specimens before and after irrigation.
Recently, the removal of pulp tissue remnants stained with a radiopaque solution was examined ex vivo by contrast‐enhanced micro‐computed tomography (De‐Deus et al., 2021). This new method is nondestructive, so repeated imaging of the specimens before and after irrigation is feasible. Extracted teeth with an intact pulp can be used instead of split roots with artificially placed tissue and there are no restrictions on the anatomy. This method is also easier and less time‐consuming than histological evaluation while at the same time providing three‐dimensional quantitative data. A similar approach using nano‐computed tomography has also been described (Hildebrand et al., 2021). At the moment, the contrast achieved is not very high, but with some further improvement, these methods could become the first choice for ex vivo studies in the future.
Artificial collagen films (Bryce et al., 2018; Huang et al., 2008; McGill et al., 2008) or hydrogels (Macedo et al., 2014a; Robinson et al., 2018; Swimberghe et al., 2019b) have also been used as targets for root canal irrigants. These materials were originally proposed as biofilm substitutes; however, they resemble pulp tissue remnants more than biofilm, so they are described here. A standardized amount of the material is easily applied to the main root canal wall following splitting of the root (Bryce et al., 2018; Huang et al., 2008; McGill et al., 2008) or inserted in transparent artificial isthmuses and lateral canals (Macedo et al., 2014a; Robinson et al., 2018; Swimberghe et al., 2019b). The specimens can be evaluated before and after irrigation and transparent models even allow for real‐time visualization of the removal. However, the interaction between the irrigant and dentine is missing in these cases. At this point, it should be emphasized that even cleared teeth cannot reproduce these chemical interactions in full because the composition of dentine is altered during clearing (Huang et al., 2012; Marshall et al., 1997; Rosales et al., 1999). The removal of collagen films and hydrogels may also differ to some extent from the removal of pulp tissue remnants, so the most promising irrigants and irrigation methods should be further tested against actual pulp tissue.
It is worthwhile mentioning that a potentially unrealistic initial condition may be created in the laboratory when an already prepared root canal (be it real or artificial) is completely filled or covered with pulp tissue or any substitute material. Clinically, preparation with instruments would remove the bulk of the pulp tissue from the main root canal, it would debride a large part of its wall and it would also create a pathway for the irrigant. However, this step is often omitted in laboratory experiments and the specimens are directly exposed to irrigants. Under these conditions, agitation techniques employing oscillating metal or plastic files/tips are favoured compared to other irrigation methods because of their direct physical action on the pulp tissue/substitute material in addition to their indirect action due to irrigant agitation. This additional direct action may seem desirable in the laboratory setting but clinically the same result would have already been produced by root canal preparation without the need for oscillating files/tips. Therefore, this laboratory model is not suitable for the evaluation of such agitation techniques. The problem can be circumvented if the tissue or substitute material is placed in a fin, groove, isthmus or lateral canal where it cannot be contacted physically by the oscillating files/tips, so its removal can only be achieved by the agitated irrigant.
REMOVAL OF DENTINE DEBRIS AND THE SMEAR LAYER
The removal of dentine debris and the smear layer, by‐products of instrumentation, is of interest because it is believed that they can harbour bacteria or hinder the access of irrigants to them (Gulabivala et al., 2005; Paqué et al., 2009). Similarly to other surrogate end‐points used in root canal irrigation studies, so far, there is no evidence that the removal of dentine debris or the smear layer increases the likelihood of healing of apical periodontitis.
Scanning Electron Microscopy
The debris and smear layer on the root canal wall were assessed for decades at very high magnification using the widely available SEM (Figure 7). Numerous ex vivo studies focused almost exclusively on single‐rooted teeth that were split longitudinally to allow evaluation (Baumgartner & Cuenin, 1992; Baumgartner & Mader, 1987; McComb & Smith, 1975; Yamada et al., 1983). Nevertheless, several key questions about the removal of dentine debris and the smear layer have not been answered and this has been largely attributed to the methodological limitations and lack of reproducibility of most SEM studies (De‐Deus et al., 2011; Hülsmann et al., 2005).
FIGURE 7.
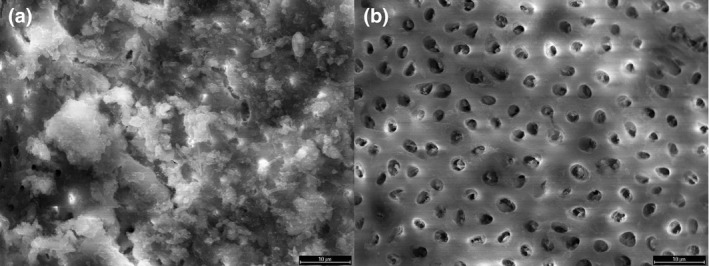
SEM photomicrographs of dentine (a) covered with smear layer, and (b) after the removal of the smear layer with 2.5% NaOCl followed by 17% EDTA
Examination under SEM requires dehydration of the specimens and coating with a conductive material. This procedure can introduce artefacts that may interfere with the assessment (De‐Deus et al., 2011) and it is destructive, so the specimens can only be examined once, after irrigation. The prior status of the root canal is unknown; therefore, it is impossible to conclude beyond doubt that a certain area was initially covered with dentine debris or smear layer and these were removed by irrigation (Gulabivala et al., 2005). A large portion of the root canal wall is left untouched by instruments (Peters, 2004), and no smear layer is formed on those areas (Sen et al., 1995). Moreover, additional dentine debris and smear layer may be produced by irrigant agitation devices that are used to remove them (Boutsioukis & Tzimpoulas, 2016; Kanaan et al., 2020; Retsas et al., 2016; Rodrigues et al., 2021), which also confounds the results of cross‐sectional examinations. A nonirrigated control group is not enough proof of the pre‐irrigation condition of the root canal because the area of interest is very large and diverse compared to the few selected spots that are actually examined (De‐Deus et al., 2011). A genuinely random selection of these spots is also rare. Instead, operators tend to select relatively clean areas and often there is additional bias due to the lack of blinding (Gulabivala et al., 2005; Hülsmann et al., 2005). Furthermore, the specimens are examined at varying magnifications (De‐Deus et al., 2011; Hülsmann et al., 2005).
Assessment of the remaining dentine debris and smear layer on SEM images is also problematic. The evaluation is inevitably limited to two dimensions and it is qualitative or semiquantitative. Subjective scoring systems are often used but the observers are not calibrated and the reproducibility of the findings is rarely checked (Gulabivala et al., 2005; Hülsmann et al., 2005). The difference between dentine debris and smear layer is not well‐defined. The scoring of the residual smear layer is often based on the number of open tubules (Lottanti et al., 2009), which is inevitably confounded by the amount of sclerotic dentine in each specimen (Vasiliadis et al., 1983a, 1983b), but this is very rarely taken into account (Lottanti et al., 2009). The age of the specimens is hardly ever reported even though the amount of sclerotic dentine increases with age (Vasiliadis et al., 1983a). It should also be emphasized that the clinical relevance of residual debris and smear layer on the wall of the main root canal or their removal as viewed on SEM images remains unclear (Gulabivala et al., 2005; Zehnder, 2012). Given the abundance of published studies, the widely recognized methodological limitations and the uncertain clinical relevance of the findings, further SEM evaluation of dentine debris and smear layer removal is discouraged, which is in line with the policy of the International Endodontic Journal (Zehnder, 2012).
Alternative methods to study the removal of the smear layer
ESEM is a version of SEM adapted for the examination of hydrated specimens (further details have been provided in the section ‘Antimicrobial effect/Microscopy’) and it has been proposed as an alternative for the study of the smear layer. The specimens can be examined repeatedly before and after irrigation (Kanaan et al., 2020), so the problem of the small field of view is partially ameliorated, but the assessment is still two‐dimensional and it is limited by the same problems as SEM. Another option is AFM which provides high‐resolution data on the three‐dimensional surface topography of the specimens following minimal sample preparation. However, image acquisition is slow, so repeated imaging of rapidly progressing phenomena at short intervals is not possible. Moreover, there are limitations in the surface height variation of the specimen, so polishing of the specimens is usually required (De‐Deus et al., 2006). Finally, Co‐site Optical Microscopy is another nondestructive method that allows almost real‐time evaluation of smear‐layer removal from a specimen through software‐based analysis. Polishing of the specimens before the experiment is again required due to the limited depth of field of the microscope at the required magnification (De‐deus et al., 2007; Reis et al., 2008).
Alternative methods to study the removal of dentine debris
In principle, ESEM and AFM could also be used to examine dentine debris on the wall of the main root canal following splitting of the root. However, currently, the research interest is focused on the large amounts of dentine debris that accumulate in uninstrumented areas of the root canal system, such as fins, isthmuses and accessory canals, during instrumentation. In infected cases, such accumulated debris could hinder the access of irrigants to intact biofilm (Gulabivala et al., 2005; Paqué et al., 2009; Siqueira et al., 2018) and this is arguably a more important problem than scattered dentine particles or a thin smear layer covering instrumented areas (Haapasalo et al., 2012).
A number of in vitro and ex vivo studies have examined the removal of dentine debris from artificial depressions or grooves created along straight root canals in split roots after instrumentation. Both artificial and real root canals have been used for this purpose (Jiang et al., 2011b; Lee et al., 2004; Rödig et al., 2010; van der Sluis et al., 2006). The ‘split‐tooth’ model allows for standardization of the root canal anatomy and the pre‐operative amount of debris, and repeated examination under a regular stereoscopic microscope can take place without any dehydration or coating. However the fabrication of the specimens is time‐consuming, the model is mostly limited to straight root canals, and the grooves and depressions are relatively large compared to real uninstrumented fins, isthmuses and accessory canals. In addition, the dentine debris is manually packed instead of gradually accumulating during instrumentation and the assessment of its removal is based on the scoring of a two‐dimensional image. One additional concern is that assembly and disassembly of the models may move the debris.
Another approach is to pre‐section the root at a few levels perpendicularly to the root canal before instrumentation and reassemble it (Howard et al., 2011; Klyn et al., 2010; Thomas et al., 2014). This model is not limited to straight root canals, dentine debris accumulates naturally during instrumentation, and repeated evaluation at various stages of the chemomechanical preparation is possible. The amount of dentine debris is measured in two‐dimensions at the pre‐selected levels under a stereoscopic microscope following disassembly of the specimens, but the findings from these levels may not be representative of the entire root canal. Fabrication of the specimens is also time‐consuming and the location of the debris may also be altered during handling of the specimens.
The evaluation of dentine debris removal from root canals was greatly improved by the introduction of micro‐computed tomography (micro‐CT; Paqué et al., 2009, 2011). This method provides high‐resolution three‐dimensional images of the root canal system ex vivo (Figure 8) without damaging the specimens (Peters et al., 2000; Stock, 2008), so quantitative longitudinal evaluation before and after irrigation is possible even for teeth with complex anatomy. Dentine debris is gradually accumulated during instrumentation (Paqué et al., 2009) but the amount of debris cannot be standardized, so a larger sample size may be required. It is not recommended to alter the chemomechanical preparation protocols to favour debris accumulation (Leoni et al., 2017; Paqué et al., 2009) because it could create an unrealistic challenge for the irrigants. At high resolution, the scanning time of an entire root is still in the order of hours, although this is likely to decrease in the future. Scanning parameters can be easily standardized, but there are several critical steps during data processing that require attention (Moinzadeh et al., 2015). Filtering of the scans is necessary to reduce the noise and avoid spurious findings. Consecutive scans should also be coregistered automatically through digital image correlation analysis, rather than being aligned manually, to allow more accurate image subtraction in three dimensions. Automated observer‐independent segmentation of the scans to distinguish dentine from air is preferable to visual determination of the threshold, the latter being highly subjective (Moinzadeh et al., 2015). Finally, it should be kept in mind that quantitative data extracted from micro‐CT scans are strongly affected by the voxel size, so findings from studies that used different voxel sizes are not comparable (Paqué & Peters, 2011). It is noteworthy that such three‐dimensional analysis cannot be carried out using the currently available cone‐beam computerized tomography scanners because their spatial resolution (Talwar et al., 2016) is still not enough for accurate detection of accumulated dentine debris.
FIGURE 8.
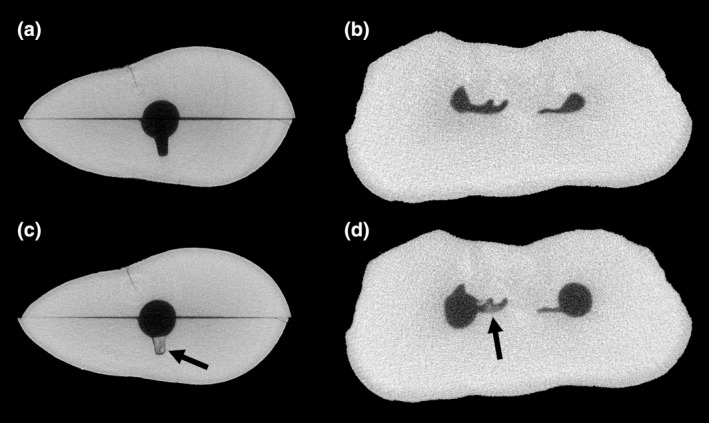
Micro‐CT cross‐sections of extracted human teeth: a split and reassembled maxillary canine (a) before, and (c) after manual packing of dentine debris in an artificially‐created depression (arrow), and the mesial root of a mandibular molar (b) before, and (d) after preparation with rotary Ni‐Ti instruments. Note the dentine debris that accumulated in the uninstrumented fin of the molar during preparation (arrow)
IRRIGANT FLOW AND PENETRATION
To exert any physical or chemical effect on biofilm, pulp tissue remnants, dentine debris and the smear layer, irrigants must first reach these targets. Therefore, information on their penetration in the root canal system can be a useful guide to select the irrigation methods that have the best chance of reaching the areas of interest for further testing in ex vivo and in vivo studies. Additionally, the irrigant velocity in artificial isthmuses and lateral canals has been correlated to biofilm removal from those areas (Pereira et al., 2021a). However, a direct link between irrigant penetration and the healing of apical periodontitis has not been demonstrated so far.
Experiments using radiopaque solutions
Tracing of radiopaque solutions delivered in root canals in vitro or ex vivo with the help of periapical radiographs was one of the earliest proposed methods to study irrigant penetration (de Gregorio et al., 2009; Munoz & Camacho‐Cuadra, 2012; Peeters & Gutknecht, 2014; Ram, 1977; Teplitsky et al., 1987). More recently, this method was also combined with micro‐CT to obtain a three‐dimensional view of the penetration pattern (Tay et al., 2010; Versiani et al., 2015). Unfortunately, radiopaque solutions (contrast agents or their mixtures with commonly used irrigants) have a much higher density and viscosity than NaOCl and other irrigants. Depending on the type of the experiment, other properties such as the solution's surface tension and its contact angle on dentine may also be relevant (Boutsioukis et al., 2014) and they are usually quite different as well. These physical properties have an impact on irrigant penetration, particularly in the narrower parts of the root canal system (Teplitsky et al., 1987), so radiopaque solutions are not reliable substitutes for irrigants in such experiments.
Even if a hypothetical radiopaque solution could mimic the flow of an irrigant perfectly, its penetration inside the root canal system is a dynamic process that should be examined in real time. Radiographs and micro‐CT scans can only capture a static image of the root canal a few seconds to a few hours after irrigation. Pressure changes, buoyancy, vibration of the specimen or the inevitable increase in temperature during micro‐CT scanning could alter the irrigant distribution and the size and location of any bubbles (Boutsioukis et al., 2014). Moreover, the detection limit of radiopaque solutions inside the root canal by periapical radiographs or micro‐CT is unknown (de Gregorio et al., 2009).
When radiographic tracing of these solutions takes place in clinical studies (Munoz & Camacho‐Cuadra, 2012; Vera et al., 2012a), there are additional ethical concerns. NaOCl is a very reactive solution and its effects depend primarily on the amount of free available chlorine (Zehnder et al., 2002). Thus, when mixing NaOCl with contrast agents, it is imperative to verify that the available chlorine is not reduced. Furthermore, repeated exposure of patients to radiation that is neither beneficial to their treatment nor provides any reliable data on irrigant penetration violates the ALARA principle (As Low As Reasonably Achievable). In view of the above‐mentioned limitations, tracing of radiopaque solutions in order to study irrigant penetration ex vivo or in vivo is discouraged.
Experiments using dyes
Other studies have opted for the visual tracing of dyes or mixtures of dyes and irrigants in transparent resin blocks or cleared teeth (de Gregorio et al., 2009; Park et al., 2013; Vera et al., 2012a). Both dye penetration and clearance of a pre‐injected dye from the root canal have been evaluated. Even though dyes resemble root canal irrigants more closely than radiopaque solutions in terms of physical properties, their flow patterns may still differ to some extent (Boutsioukis et al., 2014). There are no validated standards regarding the type of dye that should be used in place of the irrigant or its optimum concentration. Thus, the physical properties of the dye and its resemblance to commonly used irrigants should be examined in advance. As NaOCl may bleach the dye, distilled or tap water is often used instead as an irrigant in dye‐clearance experiments. Irrigation should be continued for a clinically relevant period of time or at least until a quasi‐steady‐state is reached but even then interpretation may be difficult because there may not be any sharp interface to mark the maximum penetration/clearance level. Transition may take place over an area occupied by diluted dye (Bronnec et al., 2010). Dye molecules will continue to diffuse across this area overtime at a rate that may not match the diffusion rate of irrigant molecules/ions, so evaluation immediately after the end of irrigation is critical.
Substitution of dentine by transparent acrylic blocks or 3D‐printed teeth may also affect irrigant penetration because the surface properties of polymethyl methacrylate and other hydrophobic resins are different from those of hydrophilic dentine (Boutsioukis et al., 2014). The same applies to cleared teeth (Robertson et al., 1980; Venturi et al., 2003) whose surface is also rendered less hydrophilic than intact dentine during processing (Huang et al., 2012; Marshall et al., 1997; Rosales et al., 1999). These problems are particularly important when examining dye penetration in empty root canals where a two‐phase flow is developed (dye and air). The hydrophobic environment may favour the entrapment of air bubbles in these cases (Boutsioukis et al., 2014).
Infrared imaging
Another way to study irrigant penetration is to deliver warm irrigant (50°C) in cooled root canals (10°C) ex vivo and monitor the temperature gradients with an infrared camera (Hsieh et al., 2007). This method has the advantage of using human teeth and real irrigants but increasing the temperature may decrease the viscosity of the irrigant slightly and lead to a more favourable penetration pattern. Real‐time imaging is feasible but the camera records heat transfer rather than irrigant flow, which may differ in some cases. Its spatial and temporal resolution may also be insufficient for a detailed analysis of the flow.
High‐speed imaging
To visualize and quantify the flow in detail, high‐speed imaging of the flowing irrigant seeded with microscopic neutrally buoyant tracer particles and analysis with Particle Tracking Velocimetry or Particle Image Velocimetry algorithms is currently considered the state‐of‐the‐art method (Boutsioukis et al., 2010a; Koch et al., 2014; Verhaagen et al., 2014a). This method provides high‐resolution time‐resolved data on the irrigant velocity inside the root canal (Figure 9). Being an optical method, it requires direct optical access to the irrigant at least from two sides, so artificial root canals made of transparent resin or polydimethylsiloxane have to be used. The concentration of the tracer particles must be balanced so that enough details of the flow can be captured without altering the flow itself (Westerweel, 1993). Imaging needs to be performed with a high‐speed camera that is fast enough to capture the important characteristics of the flow at the relevant time‐scale. The camera should be able to record several consecutive frames at frame rates at least 2× faster (and preferably 5–10× faster) than the highest temporal frequency of the high‐speed event of interest (Versluis, 2013). For example, to capture the oscillatory flow around an ultrasonic file during irrigant activation (f ≈ 30 kHz), the camera should be capable of recording at least one full oscillation cycle at a rate of 150 000–300 000 frames per second to capture the unsteady streaming; even higher frame rates may be required to capture the dynamics of transient cavitation bubbles (Macedo et al., 2014b). Otherwise, the analysis will reveal only a coarse time‐averaged view of the flow and important transient phenomena may be missed (Koch et al., 2016; Layton et al., 2015).
FIGURE 9.
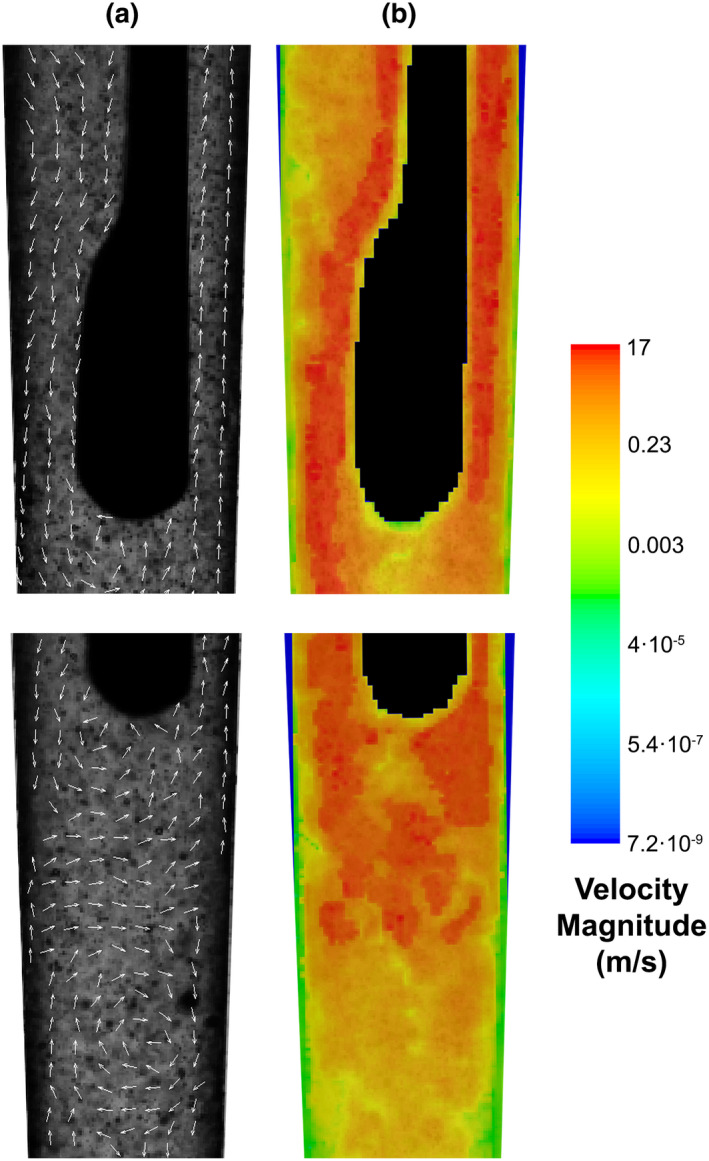
Experimental measurement of irrigant velocity by high‐speed imaging and Particle Image Velocimetry analysis: (a) velocity vectors and (b) velocity magnitude in the apical third of an artificial root canal during syringe irrigation with a 30G closed‐ended needle. The needle is coloured in black. [Reprinted and modified with permission from Wiley (Boutsioukis et al., 2010a)]
Penetration in dentinal tubules
Assessment of irrigant penetration in dentinal tubules, a very slow diffusion‐dominated process (Verhaagen et al., 2014b), requires a different approach. Direct penetration tests using dyes or mixtures of dyes and irrigants are of limited value because of their properties, as already explained. Moreover, lack of penetration may also be attributed to dentinal sclerosis (Vasiliadis et al., 1983a). Therefore, a two‐step approach is recommended: first, a dye should penetrate all patent tubules (positive control) and then, the irrigant (mostly NaOCl) should be allowed to penetrate these tubules and bleach the dye (Zou et al., 2010). The penetration pattern is then determined based on the extent of the bleached zone. Parameters known to affect diffusion, such as the temperature, the concentration of the irrigant and the exposure time (Verhaagen et al., 2014b) should be carefully controlled to avoid bias. Still, it must be underscored that a chlorine concentration gradient will be developed along the tubules (Verhaagen et al., 2014b) and it remains unclear whether the concentration that is sufficient to bleach the dye is also enough to kill bacteria or disrupt biofilm. One additional requirement is that the specimens must be kept fully hydrated, as close as possible to their natural in vivo condition (Jameson et al., 1994; Papa et al., 1994). It only takes a few minutes in a dry environment for dentine to lose a significant amount of free water due to dehydration (Jameson et al., 1994). Dry dentine is far more hydrophobic than wet dentine (Rosales et al., 1999), and under these conditions, the surface tension of the irrigant may limit penetration ex vivo, something very unlikely to happen in wet dentine in vivo.
Computational fluid dynamics
Numerical models have also been used to obtain additional information on the flow of irrigants inside the root canal system (Boutsioukis et al., 2009, 2010a; Chen et al., 2014; Shen et al., 2010b; Verhaagen et al., 2014b). These versatile models supplement experiments and provide information on irrigant velocity and pressure as well as their derivatives, such as the wall shear stress, in areas of the root canal system where experimental measurements are difficult or even impossible (Figure 10). However, they are based on a large number of assumptions and settings and even small modifications in these may produce very different results. Therefore, confirmation that the model predictions are a sufficient approximation of reality (validation) is a universal requirement for all new models before they are used to simulate any actual case. This can be done by comparison to properly designed validation experiments (Oberkampf & Trucano, 2002) that reproduce the essential elements of the model and provide quantitative data on the relevant physical quantities (usually irrigant velocity and pressure). A perfect match between experiments and numerical simulations is hardly ever possible because of unavoidable experimental and numerical errors, so the level of agreement should be taken into account when interpreting the predictions of the model. The simplified root canal geometries that were modelled in early studies (Boutsioukis et al., 2009, 2014; Chen et al., 2014; Shen et al., 2010b) have been gradually replaced by more realistic ones based on micro‐CT scans of human teeth (Boutsioukis & Gutierrez Nova, 2021; Loroño et al., 2020; Snjaric et al., 2012; Wang et al., 2015) but the benefits of the additional complexity are yet to be proven. Geometrical simplification is only one of many potential sources of error in a numerical model, so switching to more realistic root canal geometries does not guarantee the accuracy of the results though it does increase the workload and the computational time and resources required. In the process of model optimization, it is more reasonable to reduce all types of error to the same order of magnitude, so that none of them has a disproportionately large effect on the results, than to eliminate only one type of error (Oberkampf & Trucano, 2002).
FIGURE 10.
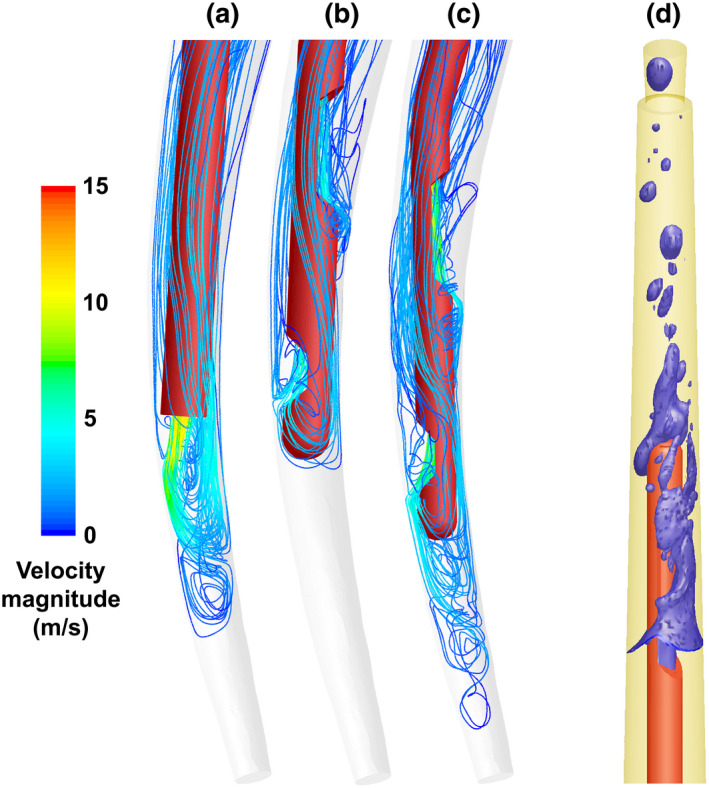
Irrigant flow calculated by different Computational Fluid Dynamics models: time‐averaged streamlines indicating the main irrigant flow created by (a) a 30G open‐ended, (b) a 30G closed‐ended, and (c) a 31G closed‐ended needle in a mesial root canal of a mandibular molar. [Reprinted and modified with permission from Elsevier (Boutsioukis & Gutierrez Nova, 2021)]. (d). Two‐phase flow (irrigant and air) in the apical part of a simplified straight root canal during the initial phase of irrigant delivery by a 30G closed‐ended needle. The air‐irrigant interface is depicted as a blue surface. Note the air bubbles apically to the needle (upper part of the image). The needles are coloured in red
IRRIGANT EXTRUSION THROUGH THE APICAL FORAMEN
Inadvertent extrusion of irrigant through the apical foramen may result in tissue damage and pronounced symptomatology (Guivarc'h et al., 2017; Hülsmann & Hahn, 2000), so it is considered an important side effect of root canal irrigation. A variety of methods have been employed to investigate the parameters involved in these accidents.
Measurement of the extruded irrigant ex vivo
One approach is to use the amount of irrigant that is extruded through the apical foramen ex vivo as a surrogate end‐point. Even though the hypothesis that this amount is correlated with the risk or the severity of an accident seems plausible, it has not been confirmed in any clinical study (Boutsioukis et al., 2013).
The model proposed by Fairbourn et al., (1987) and later modified by Myers and Montgomery (1991) has been used extensively to quantify the extruded irrigant ex vivo under various conditions and it still remains in use (Dos Reis et al., 2020; Vidas et al., 2020). In this model, the root of the specimen is attached to an empty vial where the extruded irrigant is collected during irrigation, so the apical foramen is entirely surrounded by ambient air. The periapical tissues are not simulated at all, even though they may act as a natural barrier in vivo (Salzgeber & Brilliant, 1977), and this leads to considerable overestimation of irrigant extrusion (Psimma et al., 2013a). Thus, this model has very limited clinical relevance. Another model based on the quantification of irrigant droplets ejected through the apical foramen (George & Walsh, 2008) shares the same limitations. It should be emphasized that air, whether free‐flowing or confined inside a vial (Araquam et al., 2009; Mangalam et al., 2002), is not enough to simulate the resistance of the periapical tissues to irrigant extrusion.
In an effort to mimic the effect of the periapical tissues and develop more realistic models, the root apices have been immersed in water (Psimma et al., 2013a, 2013b), various types of gels (Fukumoto et al., 2006; Hauser et al., 2007; Mitchell et al., 2011), silicone putty (Azim et al., 2018; Rodríguez‐Figueroa et al., 2014), or floral foam (Altundasar et al., 2011; Genc Sen & Kaya, 2018). Water may still exert less resistance to extrusion compared to a periapical lesion but it facilitates real‐time quantification of the extruded irrigant by electrochemical methods (Psimma et al., 2013a). Gels and silicone putty may resemble the tissue inside a periapical lesion more closely but they hinder the detection of the extruded irrigant. Chemical methods that rely on colour change of the gel are often used (Fukumoto et al., 2006; Mitchell et al., 2011; Yost et al., 2015) and quantification is based on the two‐dimensional discoloured area which may not be representative of the three‐dimensional effect. Conversely, floral foam is a porous material with little clinical relevance that may absorb or lose moisture over time. This may interfere with the measurements of the extruded irrigant, so its use is not recommended. Apart from these materials, an adjustable electronic valve that allows irrigant extrusion only when the apical irrigant pressure exceeds a certain threshold (Charara et al., 2016) and an apparatus that applies a pre‐defined opposing pressure at the apical foramen to resist irrigant extrusion (Cai et al., 2018) have also been proposed. It is very unlikely that the periapical tissues in vivo behave like a valve and there is no validated pressure threshold for irrigant extrusion (see further details in the section ‘Apical irrigant pressure’), so the clinical relevance of these models is questionable.
In general, the selection of the material or method to simulate the periapical tissues and its relevance to the in vivo conditions should be justified. Moreover, it is recommended that extrusion is evaluated quantitatively rather than qualitatively and all measurements are converted to volume of extruded irrigant, which can be easily compared across studies. Binary evaluation (yes/no) assigns the same importance to minor and major extrusion, which could be misleading. Passive extrusion of irrigant through the apical foramen seems to occur continuously at a very low rate (~1 μl/s) during root canal treatment ex vivo and in vivo for as long as the canal contains irrigant (Chu, 2010; Psimma et al., 2013a), but there is no evidence of consequent clinical manifestations in the vast majority of the treated clinical cases. Thus, there may be no difference between no extrusion and extrusion of small amounts of irrigant.
Apical irrigant pressure
Instead of evaluating the amount of extruded irrigant, a number of studies measured the irrigant pressure at or near the apical foramen during irrigation (Conard, 2012; Khan et al., 2013; Magni et al., 2021; Park et al., 2013; Verhaagen et al., 2012a). This pressure is easier to measure than irrigant extrusion and it has been regarded as a suitable surrogate end‐point, despite the lack of appropriate validation. Miniature pressure transducers fitted at the apical foramen can reduce the experimental error during in vitro and ex vivo measurements (Conard, 2012; Verhaagen et al., 2012a), so they should be preferred. Large transducers connected to the apex through long tubes and containers (Khan et al., 2013; Park et al., 2013) may allow the irrigant to flow through the apical foramen. In such a case, the location where the pressure is measured becomes uncertain because a pressure gradient develops along the path from the apical foramen to the transducer. It is also important to measure the apical pressure at each root independently even if irrigation takes place simultaneously in all canals (Ordinola‐Zapata et al., 2021). Otherwise, an unrealistic flow may develop through the surrounding container due to differences in the apical pressure at each foramen and this can introduce additional error in the measurements.
Several arbitrary pressure thresholds have been used to translate apical pressure measurements to risk of extrusion (Charara et al., 2016; Khan et al., 2013; Park et al., 2013), often based on the hypothesis that there is a constant pressure in the periapical area that opposes irrigant extrusion (Cai et al., 2018; Zhu et al., 2013). So far, none of these thresholds has been validated, so their use to formulate clinical safety recommendations is strongly discouraged. Additionally, the hypothesis of a constant opposing pressure in the periapical area is contradicted by the available in vivo evidence (Mohorn et al., 1971a, 1971b) and also leads to a number of paradoxes (Psimma & Boutsioukis, 2019). Apical pressure values are mainly useful to compare the relative risk of different irrigation methods or protocols when a direct measurement of the amount of extruded irrigant is not feasible, for example during numerical simulations (Boutsioukis et al., 2010b; Shen et al., 2010b) but they should not be overinterpreted. Moreover, the comparison of the apical pressure created by positive‐ and negative‐pressure irrigation methods (Chen et al., 2021; Haapasalo et al., 2016; Zhu et al., 2013) may be misleading, as the clinical significance and potential risks of high negative pressure at the apical foramen are not well understood yet.
Clinical studies
Randomized clinical trials on inadvertent irrigant extrusion cannot be conducted due to ethical restrictions but clinical studies examining radiographically the penetration of radiopaque solutions beyond the apical foramen have been published (Salzgeber & Brilliant, 1977; Souza et al., 2021). It should be noted that radiopaque solutions have different physical properties than irrigants (see section ‘Irrigant flow and penetration’), so these experiments mainly demonstrate the presence of a continuous space in the periapical area adjacent to the apical foramen and not irrigant extrusion per se. Moreover, even if a small amount of irrigant leaks towards the periapical tissues, this alone may not be sufficient to trigger the signs and symptoms collectively described as ‘extrusion accident’, as already explained. Repeated exposure of the patients to radiation is another concern.
GENERAL POINTS
Basic and translational (or applied) research are complementary areas of science that interact in a bidirectional way. Basic research provides the fundamental knowledge to be translated into clinical applications (bottom‐up approach) but it is not uncommon for observations in translational research to generate new questions that must be addressed at the basic science level (top‐down approach; Fang & Casadevall, 2010). The boundary between these two areas is not well‐defined, with most studies falling somewhere on the spectrum between pure basic science and pure translational research.
The translation of ideas generated by basic science experiments into improved treatments requires the use of validated models that mimic the in vivo conditions in humans as closely as possible. One important consideration with regard to irrigation is that, in most cases, the root canal in vivo is an apically closed system (Tay et al., 2010), which is a very challenging domain for irrigants to penetrate. The same applies to lateral canals, isthmuses and dentinal tubules (Boutsioukis, 2019). Thus, in laboratory studies, the surface of the root and particularly the apical foramen should be sealed with enough layers of cyanoacrylate or composite before irrigation, taking care not to block the root canal. This is a critical requirement for all types of studies on irrigation with the exception of studies on the inadvertent irrigant extrusion through the apical foramen. It should be noted that wax, nail polish and silicone putty may not be enough to create a fluid‐ and air‐tight apical seal (Tay et al., 2010). In addition, creating an apically closed system is more difficult when the roots are split/sectioned and reassembled (Bhuva et al., 2010; Villalta‐Briones et al., 2021). The clinical relevance of laboratory models that do not ensure an apically closed root canal system during irrigation is very limited (Parente et al., 2010; Tay et al., 2010).
The geometry of the root canal is also of paramount importance. Experiments in glass tubes, beakers or large bovine root canals are unable to reproduce the fluid dynamics that occur inside the confined space of a human root canal (Verhaagen et al., 2012a) and may therefore over‐ or underestimate the performance of various irrigation systems. For instance, irrigant penetration and removal of entrapped air bubbles is much easier under these conditions (Boutsioukis et al., 2014). Likewise, sonic and ultrasonic files/tips oscillating at large amplitude (Jiang et al., 2010; Neuhaus et al., 2016; Verhaagen et al., 2012b) probably perform much better when oscillating unconstrained than inside a human root canal.
Proper standardization of the experimental protocols is imperative to increase the internal validity of the study and reduce the confounders. The apical preparation size is known to affect both the penetration of irrigants in the apical third (Boutsioukis et al., 2010b; Hsieh et al., 2007) and its debridement (Huang et al., 2008), so it should be standardized. A common misconception is that standardization can be based on the size of the first file that ‘binds’ at the canal terminus (Saini et al., 2012; Topçuoğlu et al., 2018a, 2018b). There is ample evidence that the size of this file does not correspond to the initial diameter of the root canal (Paqué et al., 2010; Weiger et al., 2006; Wu et al., 2002). Instead of relying on subjective tactile feedback, it is recommended to select root canals of similar shape and prepare all of them to the same apical size and taper.
Another critical parameter that needs to be standardized is the insertion depth of needles, cannulas and agitation files/tips (Adorno et al., 2016; Boutsioukis et al., 2010c; Malki et al., 2012; Perez et al., 2017). The insertion depth has been defined in some studies based on the binding point of these components inside the root canal (Desai & Himel, 2009; Hauser et al., 2007). This point is very subjective and may vary even in root canals prepared to the same apical size and taper. Therefore, it is preferable to define the insertion depth using the apical end of instrumentation as a reference point. It should be noted that the constant in‐and‐out movement of these components along the root canal, which may be applied by some clinicians, is difficult to standardize in laboratory studies without resorting to robotic arms.
The chemical effect of irrigation is sensitive to differences in the irrigant concentration, volume, contact surface, temperature and time (Chau et al., 2015; Moorer & Wesselink, 1982), whereas the mechanical effect is sensitive to differences in the flow rate and the intensity of agitation (Boutsioukis & Gutierrez Nova, 2021; Jiang et al., 2011b). Thus, depending on the type of the experiment and the particular irrigants or irrigation methods used, the relevant parameters should also be standardized. Battery‐powered irrigation devices should be connected to an external power supply to ensure their stable performance. Finally, if any parameters known to affect irrigation cannot be standardized, then the sample size should be increased and these parameters should be included in the analysis of the results as covariates.
To assist the interpretation of the findings, it is essential to compare new irrigants and irrigation methods also to the current clinical standards (Dutner et al., 2012; Willershausen et al., 2015). A comparison between two irrigation methods that are rarely used or between these methods and no irrigation at all provides very little useful information for the vast majority of clinicians because it lacks a common point of reference. The clinical standards should be applied according to an optimum clinically relevant protocol, otherwise the relative effectiveness of new irrigants and irrigation methods may be overestimated. Additionally, the value of a simple ranking of irrigants or irrigation methods according to a surrogate end‐point is limited to the materials or devices compared. Once these are replaced or withdrawn from the market, this information is no longer useful. Therefore, apart from ranking, it is worthwhile also to understand the fundamental mechanisms responsible for the observed performance, which often requires a basic‐science approach. This knowledge may help to design new irrigants and irrigation methods or to predict their performance.
As already mentioned, with the exception of the antimicrobial effect, commonly used surrogate end‐points, such as the removal of pulp tissue remnants, hard‐tissue debris or the smear layer, have not been directly correlated to the healing of apical periodontitis. Instead, their use has been based on a number of hypotheses and assumptions that link them to the reduction of the microbial load. However, a plausible hypothesis is not enough to justify the use of a surrogate end‐point. The examples of leakage (De‐Deus, 2012; Editorial Board of the Journal of Endodontics, 2007; Wu & Wesselink, 1993), which was used extensively in the past to rank root canal filling materials, and, more recently, apical extrusion of debris (Pappen et al., 2019), which is still reported in root canal preparation studies, serve as reminders that surrogate end‐points need to be validated. The purpose of validation is not only to confirm that they are actually correlated with the primary clinical outcomes but also to ascertain the minimum effect size that leads to a clinically relevant change in the primary outcome. It is strongly recommended that these limitations are acknowledged in the Discussion section of future studies on root canal irrigation.
Even though a detailed description of statistical tests is beyond the scope of this review, two important points that are frequently overlooked are worth mentioning. Contrary to common belief (Leoni et al., 2017; Liang et al., 2013; Thomas et al., 2014; Versiani et al., 2016), statistical testing of pre‐operative parameters or anatomical indices derived from specimens randomly allocated to two or more groups and failure to demonstrate a significant difference does not prove that these groups are equivalent at baseline. It only shows that there is not enough evidence to reject the null hypothesis (no difference). The difference between the groups has to be quite substantial to lead to a p‐value below .05 (the commonly used alpha level). Depending on the variability within each group and the sample size, which is rarely selected to ensure sufficient power when comparing these pre‐operative parameters, large between‐group differences may easily pass undetected. When multiple parameters are examined, spurious significant results (type I errors) are to be expected as well (Altman, 1985; Altman & Bland, 1995; de Boer et al., 2015). Therefore, such tests are considered illogical and misleading and their use is strongly discouraged (Harvey, 2018). Creating balanced groups with regard to any important pre‐operative parameters should be primarily based on clearly defined inclusion criteria and proper randomization (stratified if needed) along with a sufficiently large sample size.
Another problem often encountered in research is unaccounted data clustering. Dentine blocks originating from the same tooth, teeth originating from the same patient, and measurements on various areas of the same biofilm specimen are examples of cases where the obtained data are related at various levels. Thus, the fundamental assumption of independent samples that underlies many common statistical tests (Altman, 1991) is violated. Clustered data require special statistical methods for their analysis (Masood et al., 2015). Alternatively, the experiments can be designed to prevent data clustering, for example by including truly independent specimens in each group.
CONCLUDING REMARKS
Irrigation is one of the key elements of root canal treatment. Despite decades‐long efforts, there are still a lot of gaps in our understanding of the penetration of irrigants in the root canal system, their interaction with bacterial biofilm, pulp tissue remnants, and dentine debris, and their side effects, so additional research is needed in these areas. A wide variety of experimental methods and models have been used for this purpose. Unreliable or unrealistic ones are not uncommon and this may have contributed to the emergence of conflicting findings in the literature. When a method or model is not sufficiently reliable or realistic (criteria that may be tightening over time as new evidence comes to light), sound scientific judgement dictates that it should be replaced by a better one that has more direct application and translation to clinical Endodontics. Prolonging its use simply because ‘there is no alternative’ encourages stagnation and overreliance on flawed methodology. Research methods should be evolving continuously. Models also need to be validated and surrogate end‐points should be correlated with real clinical outcomes instead of being based solely on assumptions. Finally, in most cases, there are no ideal methods and models that work perfectly irrespective of the conditions while providing all the answers. Therefore, it may be necessary to combine two or more complementary ones and take into account their strengths and weaknesses when interpreting the results.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interests in connection with this article.
Boutsioukis, C. , Arias‐Moliz, M.T. & Chávez de Paz, L.E. (2022) A critical analysis of research methods and experimental models to study irrigants and irrigation systems. International Endodontic Journal, 55(Suppl. 2), 295–329. Available from: 10.1111/iej.13710
REFERENCES
- Adorno, C.G. , Fretes, V.R. , Ortiz, C.P. , Mereles, R. , Sosa, V. , Yubero, M.F. et al. (2016) Comparison of two negative pressure systems and syringe irrigation for root canal irrigation: an ex vivo study. International Endodontic Journal, 49, 174–183. [DOI] [PubMed] [Google Scholar]
- Ahmad, P. , Dummer, P.M.H. , Noorani, T.Y. & Asif, J.A. (2019) The top 50 most‐cited articles published in the International Endodontic Journal. International Endodontic Journal, 52, 803–818. [DOI] [PubMed] [Google Scholar]
- Al‐Jadaa, A. , Paqué, F. , Attin, T. & Zehnder, M. (2009a) Acoustic hypochlorite activation in simulated curved canals. Journal of Endodontics, 35, 1408–1411. [DOI] [PubMed] [Google Scholar]
- Al‐Jadaa, A. , Paqué, F. , Attin, T. & Zehnder, M. (2009b) Necrotic pulp tissue dissolution by passive ultrasonic irrigation in simulated accessory canals: impact of canal location and angulation. International Endodontic Journal, 42, 59–65. [DOI] [PubMed] [Google Scholar]
- Altman, D.G. (1985) Comparability of randomised groups. The Statistician, 34, 125–136. [Google Scholar]
- Altman, D.G. (1991) Practical statistics for medical research, 1st edition. London, UK: Chapman & Hall, pp. 1–611. [Google Scholar]
- Altman, D.G. & Bland, M.J. (1995) Statistics notes: absence of evidence is not evidence of absence. British Medical Journal, 311, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altundasar, E. , Nagas, E. , Uyanik, O. & Serper, A. (2011) Debris and irrigant extrusion potential of 2 rotary systems and irrigation needles. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 112, e31–e35. [DOI] [PubMed] [Google Scholar]
- Alves, F.R. , Silva, M.G. , Rôças, I.N. & Siqueira, J.F. Jr. (2013) Biofilm biomass disruption by natural substances with potential for endodontic use. Brazilian Oral Research, 27, 20–25. [DOI] [PubMed] [Google Scholar]
- Alves, F.R.F. , Siqueira, J.F. , Carmo, F.L. , Santos, A.L. , Peixoto, R.S. , Rôças, I.N. et al. (2009) Bacterial community profiling of cryogenically ground samples from the apical and coronal root segments of teeth with apical periodontitis. Journal of Endodontics, 35, 486–492. [DOI] [PubMed] [Google Scholar]
- Andrews, J.M. (2011) Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy, 48(Suppl. 1), 5–16. [DOI] [PubMed] [Google Scholar]
- Araquam, K.R. , Britto, M.L.B. & Nabeshima, C.K. (2009) Evaluation of apical extrusion of debris during ultrasonic versus rotary instrumentation. Revista Odonto Ciência, 24, 32–35. [Google Scholar]
- Arias‐Moliz, M.T. , Baca, P. , Solana, C. , Toledano, M. , Medina‐Castillo, A.L. , Toledano‐Osorio, M. et al. (2021) Doxycycline‐functionalized polymeric nanoparticles inhibit Enterococcus faecalis biofilm formation on dentine. International Endodontic Journal, 54, 413–426. [DOI] [PubMed] [Google Scholar]
- Arias‐Moliz, M.T. , Ferrer‐Luque, C.M. , González‐Rodríguez, M.P. , Valderrama, M.J. & Baca, P. (2010) Eradication of Enterococcus faecalis biofilms by cetrimide and chlorhexidine. Journal of Endodontics, 36, 87–90. [DOI] [PubMed] [Google Scholar]
- Arnold, J.W. (2008) Colorimetric assay for biofilms in wet processing conditions. Journal of Industrial Microbiology & Biotechnology, 35, 1475–1480. [DOI] [PubMed] [Google Scholar]
- Aul, J.J. , Anderson, K.W. , Wadowsky, R.M. , Doyle, W.J. , Kingsley, L.A. , Post, J.C. et al. (1998) Comparative evaluation of culture and PCR for the detection and determination of persistence of bacterial strains and DNAs in the Chinchilla laniger model of otitis media. Annals of Otology, Rhinology & Laryngology, 107, 508–513. [DOI] [PubMed] [Google Scholar]
- Azarpazhooh, A. , Sgro, A. , Cardoso, E. , Elbarbary, M. , Laghapour Lighvan, N. , Badewy, R. et al. (2022) A scoping review of 4 decades of outcomes in nonsurgical root canal treatment, nonsurgical retreatment, and apexification studies‐part 2: outcome measures. Journal of Endodontics, 48, 29–39. [DOI] [PubMed] [Google Scholar]
- Azeredo, J. , Azevedo, N.F. , Briandet, R. , Cerca, N. , Coenye, T. , Costa, A.R. et al. (2017) Critical review on biofilm methods. Critical Reviews in Microbiology, 43, 313–351. [DOI] [PubMed] [Google Scholar]
- Azim, A.A. , Aksel, H. , Margaret Jefferson, M. & Huang, G.T. (2018) Comparison of sodium hypochlorite extrusion by five irrigation systems using an artificial root socket model and a quantitative chemical method. Clinical Oral Investigations, 22, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Baca, P. , Junco, P. , Arias‐Moliz, M.T. , González‐Rodríguez, M.P. & Ferrer‐Luque, C.M. (2011) Residual and antimicrobial activity of final irrigation protocols on Enterococcus faecalis biofilm in dentin. Journal of Endodontics, 37, 363–366. [DOI] [PubMed] [Google Scholar]
- Baca, P. , Junco, P. , Arias‐Moliz, M.T. , Castillo, F. , Rodríguez‐Archilla, A. & Ferrer‐Luque, C.M. (2012) Antimicrobial substantivity over time of chlorhexidine and cetrimide. Journal of Endodontics, 38, 927–930. [DOI] [PubMed] [Google Scholar]
- Ballal, N.V. , Ivica, A. , Meneses, P. , Narkedamalli, R.K. , Attin, T. & Zehnder, M. (2021) Influence of 1‐hydroxyethylidene‐1,1‐diphosphonic acid on the soft tissue‐dissolving and gelatinolytic effect of ultrasonically activated sodium hypochlorite in simulated endodontic environments. Materials, 14, 2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin, E. , Brady, K.M. & Greenberg, E.P. (2006) Chelator‐induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Applied and Environmental Microbiology, 72, 2064–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios, R. , Ferrer‐Luque, C.M. , Arias‐Moliz, M.T. , Ruiz‐Linares, M. , Bravo, M. & Baca, P. (2013) Antimicrobial substantivity of alexidine and chlorhexidine in dentin. Journal of Endodontics, 39, 1413–1415. [DOI] [PubMed] [Google Scholar]
- Baumgartner, J.C. & Cuenin, P.R. (1992) Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. Journal of Endodontics, 18, 605–612. [DOI] [PubMed] [Google Scholar]
- Baumgartner, J.C. , Johal, S. & Marshall, J.G. (2007) Comparison of the antimicrobial efficacy of 1.3% NaOCl/BioPure MTAD to 5.25% NaOCl/15% EDTA for root canal irrigation. Journal of Endodontics, 33, 48–51. [DOI] [PubMed] [Google Scholar]
- Baumgartner, J.C. & Mader, C.L. (1987) A scanning electron microscopic evaluation of four root canal irrigation regimens. Journal of Endodontics, 13, 147–157. [DOI] [PubMed] [Google Scholar]
- Bergmans, L. , Moisiadis, P. , van Meerbeek, B. , Quirynen, M. & Lambrechts, P. (2005) Microscopic observation of bacteria: review highlighting the use of environmental SEM. International Endodontic Journal, 38, 775–788. [DOI] [PubMed] [Google Scholar]
- Bergmans, L. , Moisiadis, P. , Huybrechts, B. , van Meerbeek, B. , Quirynen, M. & Lambrechts, P. (2008) Effect of photo‐activated disinfection on endodontic pathogens ex vivo . International Endodontic Journal, 41, 227–239. [DOI] [PubMed] [Google Scholar]
- Beumer, R.R. , de Vries, J. & Rombouts, F.M. (1992) Campylobacter jejuni non‐culturable coccoid cells. International Journal of Food Microbiology, 15, 153–163. [DOI] [PubMed] [Google Scholar]
- Bhuva, B. , Patel, S. , Wilson, R. , Niazi, S. , Beighton, D. & Mannocci, F. (2010) The effectiveness of passive ultrasonic irrigation on intraradicular Enterococcus faecalis biofilms in extracted single‐rooted human teeth. International Endodontic Journal, 43, 241–250. [DOI] [PubMed] [Google Scholar]
- Blome, B. , Braun, A. , Sobarzo, V. & Jepsen, S. (2008) Molecular identification and quantification of bacteria from endodontic infections using real‐time polymerase chain reaction. Oral Microbiology and Immunology, 23, 384–390. [DOI] [PubMed] [Google Scholar]
- de Boer, M.R. , Waterlander, W.E. , Kuijper, L.D. , Steenhuis, I.H. & Twisk, J.W. (2015) Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate. International Journal of Behavioral Nutrition and Physical Activity, 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonev, B. , Hooper, J. & Parisot, J. (2008) Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. Journal of Antimicrobial Chemotherapy, 61, 1295–1301. [DOI] [PubMed] [Google Scholar]
- Bouillaguet, S. , Manoil, D. , Girard, M. , Louis, J. , Gaïa, N. , Leo, S. et al. (2018) Root microbiota in primary and secondary apical periodontitis. Frontiers in Microbiology, 9, 2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutsioukis, C. (2019) Internal tooth anatomy and root canal irrigation. In: Versiani, M.A. , Basrani, B. & Sousa‐Neto, M.D. (Eds.) The root canal anatomy in permanent dentition. New York, NY, USA: Springer, pp. 303–321. [Google Scholar]
- Boutsioukis, C. , Gogos, C. , Verhaagen, B. , Versluis, M. , Kastrinakis, E. & van der Sluis, L.W. (2010b) The effect of apical preparation size on irrigant flow in root canals evaluated using an unsteady Computational Fluid Dynamics model. International Endodontic Journal, 43, 874–881. [DOI] [PubMed] [Google Scholar]
- Boutsioukis, C. & Gutierrez Nova, P. (2021) Syringe irrigation in minimally shaped root canals using 3 endodontic needles: a Computational Fluid Dynamics study. Journal of Endodontics, 47, 1487–1495. [DOI] [PubMed] [Google Scholar]
- Boutsioukis, C. , Kastrinakis, E. , Lambrianidis, T. , Verhaagen, B. , Versluis, M. & van der Sluis, L.W. (2014) Formation and removal of apical vapor lock during syringe irrigation: a combined experimental and Computational Fluid Dynamics approach. International Endodontic Journal, 47, 191–201. [DOI] [PubMed] [Google Scholar]
- Boutsioukis, C. , Lambrianidis, T. & Kastrinakis, E. (2009) Irrigant flow within a prepared root canal using different flow rates: a Computational Fluid Dynamics study. International Endodontic Journal, 42, 144–155. [DOI] [PubMed] [Google Scholar]
- Boutsioukis, C. , Lambrianidis, T. , Verhaagen, B. , Versluis, M. , Kastrinakis, E. , Wesselink, P.R. et al. (2010c) The effect of needle‐insertion depth on the irrigant flow in the root canal: evaluation using an unsteady Computational Fluid Dynamics model. Journal of Endodontics, 36, 1664–1668. [DOI] [PubMed] [Google Scholar]
- Boutsioukis, C. , Psimma, Z. & van der Sluis, L.W. (2013) Factors affecting irrigant extrusion during root canal irrigation: a systematic review. International Endodontic Journal, 46, 599–618. [DOI] [PubMed] [Google Scholar]
- Boutsioukis, C. & Tzimpoulas, N. (2016) Uncontrolled removal of dentin during in vitro ultrasonic irrigant activation. Journal of Endodontics, 42¸, 289–293. [DOI] [PubMed] [Google Scholar]
- Boutsioukis, C. , Verhaagen, B. , Versluis, M. , Kastrinakis, E. & van der Sluis, L.W. (2010a) Irrigant flow in the root canal: experimental validation of an unsteady Computational Fluid Dynamics model using high‐speed imaging. International Endodontic Journal, 43, 393–403. [DOI] [PubMed] [Google Scholar]
- Braissant, O. , Astasov‐Frauenhoffer, M. , Waltimo, T. & Bonkat, G. (2020) A review of methods to determine viability, vitality, and metabolic rates in microbiology. Frontiers in Microbiology, 11, 547458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronnec, F. , Bouillaguet, S. & Machtou, P. (2010) Ex vivo assessment of irrigant penetration and renewal during the cleaning and shaping of root canals: a digital subtraction radiographic study. International Endodontic Journal, 43, 275–282. [DOI] [PubMed] [Google Scholar]
- Brundin, M. , Figdor, D. , Johansson, A. & Sjögren, U. (2014) Preservation of bacterial DNA by human dentin. Journal of Endodontics, 40, 241–245. [DOI] [PubMed] [Google Scholar]
- Brundin, M. , Figdor, D. , Sundqvist, G. & Sjögren, U. (2015) Preservation of Fusobacterium nucleatum and Peptostreptococcus anaerobius DNA after loss of cell viability. International Endodontic Journal, 48, 37–45. [DOI] [PubMed] [Google Scholar]
- Bryce, G. , MacBeth, N. , Gulabivala, K. & Ng, Y.L. (2018) The efficacy of supplementary sonic irrigation using the EndoActivator® system determined by removal of a collagen film from an ex vivo model. International Endodontic Journal, 51, 489–497. [DOI] [PubMed] [Google Scholar]
- Burleson, A. , Nusstein, J. , Reader, A. & Beck, M. (2007) The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. Journal of Endodontics, 33, 782–787. [DOI] [PubMed] [Google Scholar]
- Busanello, F.H. , Petridis, X. , So, M.V.R. , Dijkstra, R.J.B. , Sharma, P.K. & van der Sluis, L.W.M. (2019) Chemical biofilm removal capacity of endodontic irrigants as a function of biofilm structure: optical coherence tomography, confocal microscopy and viscoelasticity determination as integrated assessment tools. International Endodontic Journal, 52, 461–474. [DOI] [PubMed] [Google Scholar]
- Cai, X. , Wang, X.‐Y. , Santarcangelo, F. , Schoeffel, G.J. , Bergeron, B.E. , Tay, F.R. et al. (2018) Effect of simulated intraosseous sinusoidal pressure on NaOCl extrusion. Journal of Dentistry, 78, 46–50. [DOI] [PubMed] [Google Scholar]
- Camargo, C.H. , Siviero, M. , Camargo, S.E. , de Oliveira, S.H. , Carvalho, C.A. & Valera, M.C. (2007) Topographical, diametral, and quantitative analysis of dentin tubules in the root canals of human and bovine teeth. Journal of Endodontics, 33, 422–426. [DOI] [PubMed] [Google Scholar]
- Camilleri, J. , Arias Moliz, T. , Bettencourt, A. , Costa, J. , Martins, F. , Rabadijeva, D. et al. (2020) Standardization of antimicrobial testing of dental devices. Dental Materials, 36, e59–e73. [DOI] [PubMed] [Google Scholar]
- Camps, J. , Pommel, L. & Aubut, V. (2009) Shelf life, dissolving action, and antibacterial activity of a neutralized 2.5% sodium hypochlorite solution. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 108, e66–e73. [DOI] [PubMed] [Google Scholar]
- Carrigan, P.J. , Morse, D.R. , Furst, M.L. & Sinai, I.H. (1984) A scanning electron microscopic evaluation of human dentinal tubules according to age and location. Journal of Endodontics, 10, 359–363. [DOI] [PubMed] [Google Scholar]
- Ceri, H. , Olson, M.E. , Stremick, C. , Read, R.R. , Morck, D. & Buret, A. (1999) The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. Journal of Clinical Microbiology, 37, 1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara, K. , Friedman, S. , Sherman, A. , Kishen, A. , Malkhassian, G. , Khakpour, M. et al. (2016) Assessment of apical extrusion during root canal irrigation with the novel GentleWave system in a simulated apical environment. Journal of Endodontics, 42, 135–139. [DOI] [PubMed] [Google Scholar]
- Chau, N.P.T. , Chung, N.H. & Jeon, J.G. (2015) Relationships between the antibacterial activity of sodium hypochlorite and treatment time and biofilm age in early Enterococcus faecalis biofilms. International Endodontic Journal, 48, 782–789. [DOI] [PubMed] [Google Scholar]
- Chávez de Paz, L.E. (2007) Redefining the persistent infection in root canals: possible role of biofilm communities. Journal of Endodontics, 33, 652–662. [DOI] [PubMed] [Google Scholar]
- Chávez de Paz, L.E. (2009) Image analysis software based on color segmentation for characterization of viability and physiological activity of biofilms. Applied and Environmental Microbiology, 75, 1734–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez de Paz, L.E. (2012) Development of a multispecies biofilm community by four root canal bacteria. Journal of Endodontics, 38, 318–323. [DOI] [PubMed] [Google Scholar]
- Chávez de Paz, L.E. , Bergenholtz, G. , Dahlén, G. & Svensäter, G. (2007) Response to alkaline stress by root canal bacteria in biofilms. International Endodontic Journal, 40, 344–355. [DOI] [PubMed] [Google Scholar]
- Chávez de Paz, L.E. , Bergenholtz, G. & Svensäter, G. (2010) The effects of antimicrobials on endodontic biofilm bacteria. Journal of Endodontics, 36, 70–77. [DOI] [PubMed] [Google Scholar]
- Chávez de Paz, L.E. , Davies, J.R. , Bergenholtz, G. & Svensäter, G. (2015) Strains of Enterococcus faecalis differ in their ability to coexist in biofilms with other root canal bacteria. International Endodontic Journal, 48, 916–925. [DOI] [PubMed] [Google Scholar]
- Chávez de Paz, L.E. & Marsh, P.D. (2015) Ecology and physiology of root canal microbial biofilm communities. In: Chávez de Paz, L. , Sedgley, C.M. & Kishen, A. (Eds.) The root canal biofilm. New York, NY: Springer, pp. 3–22. [Google Scholar]
- Chávez de Paz, L.E. & Ordinola Zapata, R. (2019) Challenges for root canal irrigation: microbial biofilms and root canal anatomy. ENDO‐Endodontic Practice Today, 13, 91–100. [Google Scholar]
- Chen, B. , Shen, Y. , Ma, J. & Haapasalo, M. (2021) Effect of apical size on apical pressure during syringe‐needle and multisonic negative pressure irrigation. Odontology, 109, 625–631. [DOI] [PubMed] [Google Scholar]
- Chen, J.E. , Nurbakhsh, B. , Layton, G. , Bussmann, M. & Kishen, A. (2014) Irrigation dynamics associated with positive pressure, apical negative pressure and passive ultrasonic irrigations: a computational fluid dynamics analysis. Australian Endodontic Journal, 40, 54–60. [DOI] [PubMed] [Google Scholar]
- Chin, M.Y. , Busscher, H.J. , Evans, R. , Noar, J. & Pratten, J. (2006) Early biofilm formation and the effects of antimicrobial agents on orthodontic bonding materials in a parallel plate flow chamber. European Journal of Orthodontics, 28, 1–7. [DOI] [PubMed] [Google Scholar]
- Chivatxaranukul, P. , Dashper, S.G. & Messer, H.H. (2008) Dentinal tubule invasion and adherence by Enterococcus faecalis . International Endodontic Journal, 41, 873–882. [DOI] [PubMed] [Google Scholar]
- Choi, Y.S. , Kim, C. , Moon, J.H. & Lee, J.Y. (2018) Removal and killing of multispecies endodontic biofilms by N‐acetylcysteine. Brazilian Journal of Microbiology, 49, 184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, G.D. , Simpson, W.A. , Younger, J.J. , Baddour, L.M. , Barrett, F.F. , Melton, D.M. et al. (1985) Adherence of coagulase‐negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. Journal of Clinical Microbiology, 22, 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, A. (2010) Penetration of sodium hypochlorite into periapical lesions in necrotic dog teeth. Journal of Endodontics, 36, 558. [Google Scholar]
- Clarkson, R.M. , Kidd, B. , Evans, G.E. & Moule, A.J. (2012) The effect of surfactant on the dissolution of porcine pulpal tissue by sodium hypochlorite solutions. Journal of Endodontics, 38, 1257–1260. [DOI] [PubMed] [Google Scholar]
- Clegg, M.S. , Vertucci, F.J. , Walker, C. , Belanger, M. & Britto, L.R. (2006) The effect of exposure to irrigant solutions on apical dentin biofilms in vitro. Journal of Endodontics, 32, 434–437. [DOI] [PubMed] [Google Scholar]
- Codony, F. , Dinh‐Thanh, M. & Agustí, G. (2020) Key factors for removing bias in viability PCR‐based methods: a review. Current Microbiology, 77, 682–687. [DOI] [PubMed] [Google Scholar]
- Cohenca, N. , Heilborn, C. , Johnson, J.D. , Flores, D.S. , Ito, I.Y. & da Silva, L.A. (2010) Apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing on root canal disinfection in dog teeth. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 109, e42–e46. [DOI] [PubMed] [Google Scholar]
- Collins, S.P. , Pope, R.K. , Scheetz, R.W. , Ray, R.I. , Wagner, P.A. & Little, B.J. (1993) Advantages of environmental scanning electron microscopy in studies of microorganisms. Microscopy Research and Technique, 25, 398–405. [DOI] [PubMed] [Google Scholar]
- Conard, M.C. (2012) A prospective study of fluid pressures of irrigation during root canal therapy. Master Thesis. Columbus, OH: Ohio State University, pp. 1–113. [Google Scholar]
- Conde, A.J. , Estevez, R. , Loroño, G. , Valencia de Pablo, Ó. , Rossi‐Fedele, G. & Cisneros, R. (2017) Effect of sonic and ultrasonic activation on organic tissue dissolution from simulated grooves in root canals using sodium hypochlorite and EDTA. International Endodontic Journal, 50, 976–982. [DOI] [PubMed] [Google Scholar]
- Costerton, J.W. , Stewart, P.S. & Greenberg, E.P. (1999) Bacterial biofilms: a common cause of persistent infections. Science, 21, 1318–1322. [DOI] [PubMed] [Google Scholar]
- Cullen, J.K. , Wealleans, J.A. , Kirkpatrick, T.C. & Yaccino, J.M. (2015) The effect of 8.25% sodium hypochlorite on dental pulp dissolution and dentin flexural strength and modulus. Journal of Endodontics, 41, 920–924. [DOI] [PubMed] [Google Scholar]
- Daddi Oubekka, S. , Briandet, R. , Fontaine‐Aupart, M.P. & Steenkeste, K. (2012) Correlative time‐resolved fluorescence microscopy to assess antibiotic diffusion‐reaction in biofilms. Antimicrobial Agents and Chemotherapy, 56, 3349–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De‐Deus, G. (2012) Research that matters—root canal filling and leakage studies. International Endodontic Journal, 45, 1063–1064. [DOI] [PubMed] [Google Scholar]
- De‐Deus, G. , Belladonna, F.G. , Cavalcante, D.M. , Simões‐Carvalho, M. , Silva, E.J.N.L. , Carvalhal, J.C.A. et al. (2021) Contrast‐enhanced micro‐CT to assess dental pulp tissue debridement in root canals of extracted teeth: a series of cascading experiments towards method validation. International Endodontic Journal, 54, 279–293. [DOI] [PubMed] [Google Scholar]
- De‐Deus, G. , de Berredo Pinho, M.A. , Reis, C. , Fidel, S. , Souza, E. & Zehnder, M. (2013) Sodium hypochlorite with reduced surface tension does not improve in situ pulp tissue dissolution. Journal of Endodontics, 39, 1039–1043. [DOI] [PubMed] [Google Scholar]
- De‐Deus, G. , Paciornik, S. , Pinho Mauricio, M.H. & Prioli, R. (2006) Real‐time atomic force microscopy of root dentine during demineralization when subjected to chelating agents. International Endodontic Journal, 39, 683–692. [DOI] [PubMed] [Google Scholar]
- De‐Deus, G. , Reis, C.M. , Fidel, R.A. , Fidel, S.R. & Paciornik, S. (2007) Co‐site digital optical microscopy and image analysis: an approach to evaluate the process of dentine demineralization. International Endodontic Journal, 40, 441–452. [DOI] [PubMed] [Google Scholar]
- De‐Deus, G. , Reis, C. & Paciornik, S. (2011) Critical appraisal of published smear layer‐removal studies: methodological issues. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 112, 531–543. [DOI] [PubMed] [Google Scholar]
- del Carpio‐Perochena, A. , Bramante, C.M. , de Andrade, F.B. , Maliza, A.G.A. , Cavenago, B.C. , Marciano, M.A. et al. (2015) Antibacterial and dissolution ability of sodium hypochlorite in different pHs on multi‐species biofilms. Clinical Oral Investigations, 19, 2067–2073. [DOI] [PubMed] [Google Scholar]
- Desai, P. & Himel, V. (2009) Comparative safety of various intracanal irrigation systems. Journal of Endodontics, 35, 545–549. [DOI] [PubMed] [Google Scholar]
- Dominici, J.T. , Eleazer, P.D. , Clark, S.J. , Staat, R.H. & Scheetz, J.P. (2001) Disinfection/sterilization of extracted teeth for dental student use. Journal of Dental Education, 65, 1278–1280. [PubMed] [Google Scholar]
- Döring, G. , Unertl, K. & Heininger, A. (2008) Validation criteria for nucleic acid amplification techniques for bacterial infections. Clinical Chemistry and Laboratory Medicine, 46, 909–918. [DOI] [PubMed] [Google Scholar]
- dos Reis, S. , Cruz, V.M. , Hungaro Duarte, M.A. , da Silveira Bueno, C.E. , Vivan, R.R. , Pelegrine, R.A. et al. (2020) Volumetric analysis of irrigant extrusion in immature teeth after different final agitation techniques. Journal of Endodontics, 46, 682–687. [DOI] [PubMed] [Google Scholar]
- Du, T. , Shi, Q. , Shen, Y. , Cao, Y. , Ma, J. , Lu, X. et al. (2013) Effect of modified nonequilibrium plasma with chlorhexidine digluconate against endodontic biofilms in vitro . Journal of Endodontics, 39, 1438–1443. [DOI] [PubMed] [Google Scholar]
- Du, T. , Wang, Z. , Shen, Y. , Ma, J. , Cao, Y. & Haapasalo, M. (2014) Effect of long‐term exposure to endodontic disinfecting solutions on young and old Enterococcus faecalis biofilms in dentin canals. Journal of Endodontics, 40, 509–514. [DOI] [PubMed] [Google Scholar]
- Dutner, J. , Mines, P. & Anderson, A. (2012) Irrigation trends among American Association of Endodontists members: a web‐based survey. Journal of Endodontics, 38, 37–40. [DOI] [PubMed] [Google Scholar]
- Editorial Board of the Journal of Endodontics . (2007) Wanted: a base of evidence. Journal of Endodontics, 33, 1401–1402. [DOI] [PubMed] [Google Scholar]
- Eldarrat, A.H. , High, A.S. & Kale, G.M. (2010) Age‐related changes in ac‐impedance spectroscopy studies of normal human dentine: further investigations. Journal of Materials Science: Materials in Medicine, 21, 45–51. [DOI] [PubMed] [Google Scholar]
- Enrich‐Essvein, T. , Benavides‐Reyes, C. , Álvarez‐Lloret, P. , Bolaños‐Carmona, M.V. , Rodríguez‐Navarro, A.B. & González‐López, S. (2021) Influence of de‐remineralization process on chemical, microstructural, and mechanical properties of human and bovine dentin. Clinical Oral Investigations, 25, 841–849. [DOI] [PubMed] [Google Scholar]
- Ercan, E. , Ozekinci, T. , Atakul, F. & Gül, K. (2004) Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: in vivo study. Journal of Endodontics, 30, 84–87. [DOI] [PubMed] [Google Scholar]
- Fairbourn, D.R. , McWalter, G.M. & Montgomery, S. (1987) The effect of four preparation techniques on the amount of apically extruded debris. Journal of Endodontics, 113, 102–108. [DOI] [PubMed] [Google Scholar]
- Fang, F.C. & Casadevall, A. (2010) Lost in translation–basic science in the era of translational research. Infection and Immunity, 78, 563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer‐Luque, C.M. , Arias‐Moliz, M.T. , González‐Rodríguez, M.P. & Baca, P. (2010) Antimicrobial activity of maleic acid and combinations of cetrimide with chelating agents against Enterococcus faecalis biofilm. Journal of Endodontics, 36, 1673–1675. [DOI] [PubMed] [Google Scholar]
- Ferrer‐Luque, C.M. , Bejarano, I. , Ruiz‐Linares, M. & Baca, P. (2014) Reduction in Enteroccocus faecalis counts—a comparison between rotary and reciprocating systems. International Endodontic Journal, 47, 380–386. [DOI] [PubMed] [Google Scholar]
- Figdor, D. & Brundin, M. (2016) Contamination controls for analysis of root canal samples by molecular methods: an overlooked and unsolved problem. Journal of Endodontics, 42, 1003–1008. [DOI] [PubMed] [Google Scholar]
- Folkesson, A. , Haagensen, J.A. , Zampaloni, C. , Sternberg, C. & Molin, S. (2008) Biofilm induced tolerance towards antimicrobial peptides. PLoS One, 3, e1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto, Y. , Kikuchi, I. , Yoshioka, T. , Kobayashi, C. & Suda, H. (2006) An ex vivo evaluation of a new root canal irrigation technique with intracanal aspiration. International Endodontic Journal, 39, 93–99. [DOI] [PubMed] [Google Scholar]
- Gao, R. , Liao, X. , Zhao, X. , Liu, D. & Ding, T. (2021) The diagnostic tools for viable but nonculturable pathogens in the food industry: current status and future prospects. Comprehensive Reviews in Food Science and Food Safety, 20, 2146–2175. [DOI] [PubMed] [Google Scholar]
- Garcia de Paula‐Silva, F.W. , Hassan, B. , Bezerra da Silva, L.A. , Leonardo, M.R. & Wu, M.K. (2009) Outcome of root canal treatment in dogs determined by periapical radiography and cone‐beam computed tomography scans. Journal of Endodontics, 35, 723–726. [DOI] [PubMed] [Google Scholar]
- Gazzaneo, I. , Vieira, G.C.S. , Pérez, A.R. , Alves, F.R.F. , Gonçalves, L.S. , Mdala, I. et al. (2019) Root canal disinfection by single‐ and multiple‐instrument systems: effects of sodium hypochlorite volume, concentration, and retention time. Journal of Endodontics, 45, 736–741. [DOI] [PubMed] [Google Scholar]
- Genc Sen, O. & Kaya, M. (2018) Comparative safety of needle, endoactivator, and laser‐activated irrigation in overinstrumented root canals. Photomedicine and Laser Surgery, 36, 198–202. [DOI] [PubMed] [Google Scholar]
- Generali, L. , Bertoldi, C. , Bidossi, A. , Cassinelli, C. , Morra, M. , Del Fabbro, M. et al. (2020) Evaluation of cytotoxicity and antibacterial activity of a new class of silver citrate‐based compounds as endodontic irrigants. Materials, 6, 5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, R. & Walsh, L.J. (2008) Apical extrusion of root canal irrigants when using Er:YAG and Er, Cr:YSGG lasers with optical fibers: an in vitro dye study. Journal of Endodontics, 34, 706–708. [DOI] [PubMed] [Google Scholar]
- Giardino, L. , Bidossi, A. , Del Fabbro, M. , Savadori, P. , Maddalone, M. , Ferrari, L. et al. (2020a) Antimicrobial activity, toxicity and accumulated hard‐tissue debris (AHTD) removal efficacy of several chelating agents. International Endodontic Journal, 53, 1093–1110. [DOI] [PubMed] [Google Scholar]
- Giardino, L. , Savadori, P. , Generali, L. , Mohammadi, Z. , Del Fabbro, M. , De Vecchi, E. et al. (2020b) Antimicrobial effectiveness of etidronate powder (Dual Rinse® HEDP) and two EDTA preparations against Enterococcus faecalis: a preliminary laboratory study. Odontology, 108, 396–405. [DOI] [PubMed] [Google Scholar]
- Gomes, B.P. , Berber, V.B. , Kokaras, A.S. , Chen, T. & Paster, B.J. (2015) Microbiomes of endodontic‐periodontal lesions before and after chemomechanical preparation. Journal of Endodontics, 41, 1975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, B.P. , Souza, S.F. , Ferraz, C.C. , Teixeira, F.B. , Zaia, A.A. , Valdrighi, L. et al. (2003) Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro . International Endodontic Journal, 36, 267–275. [DOI] [PubMed] [Google Scholar]
- de Gregorio, C. , Estevez, R. , Cisneros, R. , Heilborn, C. & Cohenca, N. (2009) Effect of EDTA, sonic, and ultrasonic activation on the penetration of sodium hypochlorite into simulated lateral canals: an in vitro study. Journal of Endodontics, 35, 891–895. [DOI] [PubMed] [Google Scholar]
- Guivarc'h, M. , Ordioni, U. , Ahmed, H.M. , Cohen, S. , Catherine, J.H. & Bukiet, F. (2017) Sodium hypochlorite accident: a systematic review. Journal of Endodontics, 43, 16–24. [DOI] [PubMed] [Google Scholar]
- Gulabivala, K. , Patel, B. , Evans, G. & Ng, Y.L. (2005) Effects of mechanical and chemical procedures on root canal surfaces. Endodontic Topics, 10, 103–122. [Google Scholar]
- Guneser, M.B. , Arslan, D. & Usumez, A. (2015) Tissue dissolution ability of sodium hypochlorite activated by photon‐initiated photoacoustic streaming technique. Journal of Endodontics, 41, 729–732. [DOI] [PubMed] [Google Scholar]
- Gutarts, R. , Nusstein, J. , Reader, A. & Beck, M. (2005) In vivo debridement efficacy of ultrasonic irrigation following hand‐rotary instrumentation in human mandibular molars. Journal of Endodontics, 31, 166–170. [DOI] [PubMed] [Google Scholar]
- Haapasalo, M. (2016) Level of evidence in endodontics: what does it mean? Endodontic Topics, 34, 30–41. [Google Scholar]
- Haapasalo, M. & Ørstavik, D. (1987) In vitro infection and disinfection of dentinal tubules. Journal of Dental Research, 66, 1375–1379. [DOI] [PubMed] [Google Scholar]
- Haapasalo, M. , Qian, W. , Portenier, I. & Waltimo, T. (2007) Effects of dentin on the antimicrobial properties of endodontic medicaments. Journal of Endodontics, 33, 917–925. [DOI] [PubMed] [Google Scholar]
- Haapasalo, M. , Qian, W. & Shen, Y. (2012) Irrigation: beyond the smear layer. Endodontic Topics, 27, 35–53. [Google Scholar]
- Haapasalo, M. , Shen, Y.A. , Wang, Z. , Park, E. , Curtis, A. , Patel, P. et al. (2016) Apical pressure created during irrigation with the GentleWave system compared to conventional syringe irrigation. Clinical Oral Investigations, 20, 1525–1534. [DOI] [PubMed] [Google Scholar]
- Haapasalo, M. , Wang, Z. , Shen, Y. , Curtis, A. , Patel, P. & Khakpour, M. (2014) Tissue dissolution by a novel multisonic ultracleaning system and sodium hypochlorite. Journal of Endodontics, 40, 1178–1181. [DOI] [PubMed] [Google Scholar]
- Hall‐Stoodley, L. , Costerton, J.W. & Stoodley, P. (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews Microbiology, 2, 95–108. [DOI] [PubMed] [Google Scholar]
- Hand, R.E. , Smith, M.L. & Harrison, J.W. (1978) Analysis of the effect of dilution on the necrotic tissue dissolution property of sodium hypochlorite. Journal of Endodontics, 4, 60–64. [DOI] [PubMed] [Google Scholar]
- Hannig, C. , Follo, M. , Hellwig, E. & Al‐Ahmad, A. (2010) Visualization of adherent micro‐organisms using different techniques. Journal of Medical Microbiology, 59, 1–7. [DOI] [PubMed] [Google Scholar]
- Hannig, C. , Hannig, M. , Rehmer, O. , Braun, G. , Hellwig, E. & Al‐Ahmad, A. (2007) Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ . Archives of Oral Biology, 52, 1048–1056. [DOI] [PubMed] [Google Scholar]
- Harvey, L.A. (2018) Statistical testing for baseline differences between randomised groups is not meaningful. Spinal Cord, 56, 919. [DOI] [PubMed] [Google Scholar]
- Hauser, V. , Braun, A. & Frentzen, M. (2007) Penetration depth of a dye marker into dentine using a novel hydrodynamic system (RinsEndo). International Endodontic Journal, 40, 644–652. [DOI] [PubMed] [Google Scholar]
- Hildebrand, T. , Nogueira, L. , Sunde, P.T. , Ørstavik, D. , Glasmacher, B. & Haugen, H.J. (2021) Contrast‐enhanced nano‐CT reveals soft dental tissues and cellular layers. International Endodontic Journal, 54, 1275–1288. [DOI] [PubMed] [Google Scholar]
- Holland, G.R. (1992) Periapical innervation of the ferret canine one year after pulpectomy. Journal of Dental Research, 71, 470–474. [DOI] [PubMed] [Google Scholar]
- Honraet, K. & Nelis, H.J. (2006) Use of the modified robbins device and fluorescent staining to screen plant extracts for the inhibition of S. mutans biofilm formation. Journal of Microbiological Methods, 64, 217–224. [DOI] [PubMed] [Google Scholar]
- Hope, C.K. , Clements, D. & Wilson, M. (2002) Determining the spatial distribution of viable and nonviable bacteria in hydrated microcosm dental plaques by viability profiling. Journal of Applied Microbiology, 93, 448–455. [DOI] [PubMed] [Google Scholar]
- Horz, H.P. , Vianna, M.E. , Gomes, B.P. & Conrads, G. (2005) Evaluation of universal probes and primer sets for assessing total bacterial load in clinical samples: general implications and practical use in endodontic antimicrobial therapy. Journal of Clinical Microbiology, 43, 5332–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, R.K. , Kirkpatrick, T.C. , Rutledge, R.E. & Yaccino, J.M. (2011) Comparison of debris removal with three different irrigation techniques. Journal of Endodontics, 37, 1301–1305. [DOI] [PubMed] [Google Scholar]
- Hsieh, Y.D. , Gau, C.H. , Kung Wu, S.F. , Shen, E.C. , Hsu, P.W. & Fu, E. (2007) Dynamic recording of irrigating fluid distribution in root canals using thermal image analysis. International Endodontic Journal, 40, 11–17. [DOI] [PubMed] [Google Scholar]
- Huang, T.Y. , Gulabivala, K. & Ng, Y.L. (2008) A bio‐molecular film ex‐vivo model to evaluate the influence of canal dimensions and irrigation variables on the efficacy of irrigation. International Endodontic Journal, 41, 60–71. [DOI] [PubMed] [Google Scholar]
- Huang, X. , Zhang, J. , Huang, C. , Wang, Y. & Pei, D. (2012) Effect of intracanal dentine wettability on human dental pulp cell attachment. International Endodontic Journal, 45, 346–353. [DOI] [PubMed] [Google Scholar]
- Hülsmann, M. & Hahn, W. (2000) Complications during root canal irrigation–literature review and case reports. International Endodontic Journal, 33, 186–193. [DOI] [PubMed] [Google Scholar]
- Hülsmann, M. , Peters, O.A. & Dummer, P.M.H. (2005) Mechanical preparation of root canals: shaping goals, techniques and means. Endodontic Topics, 10, 30–76. [Google Scholar]
- İriboz, E. , Arıcan Öztürk, B. , Kolukırık, M. , Karacan, I. & Sazak Öveçoğlu, H. (2018) Detection of the unknown components of the oral microflora of teeth with periapical radiolucencies in a Turkish population using next‐generation sequencing techniques. International Endodontic Journal, 51, 1349–1357. [DOI] [PubMed] [Google Scholar]
- James, S.A. , Hilal, N. & Wright, C.J. (2017) Atomic force microscopy studies of bioprocess engineering surfaces ‐ imaging, interactions and mechanical properties mediating bacterial adhesion. Biotechnology Journal, 12, 1600698. [DOI] [PubMed] [Google Scholar]
- Jameson, M.W. , Tidmarsh, B.G. & Hood, J.A. (1994) Effect of storage media on subsequent water loss and regain by human and bovine dentine and on mechanical properties of human dentine in vitro . Archives of Oral Biology, 39, 759–767. [DOI] [PubMed] [Google Scholar]
- Jiang, L.M. , Hoogenkamp, M.A. , van der Sluis, L.W. , Wesselink, P.R. , Crielaard, W. & Deng, D.M. (2011a) Resazurin metabolism assay for root canal disinfectant evaluation on dual‐species biofilms. Journal of Endodontics, 37, 31–35. [DOI] [PubMed] [Google Scholar]
- Jiang, L.M. , Verhaagen, B. , Versluis, M. & van der Sluis, L.W. (2010) Evaluation of a sonic device designed to activate irrigant in the root canal. Journal of Endodontics, 36, 143–146. [DOI] [PubMed] [Google Scholar]
- Jiang, L.M. , Verhaagen, B. , Versluis, M. , Langedijk, J. , Wesselink, P. & van der Sluis, L.W.M. (2011b) The influence of the ultrasonic intensity on the cleaning efficacy of passive ultrasonic irrigation. Journal of Endodontics, 37, 688–692. [DOI] [PubMed] [Google Scholar]
- Jiang, R. , Xu, Y. & Lin, H. (2019) Effects of two disinfection/sterilization methods for dentin specimens on dentin permeability. Clinical Oral Investigations, 23, 899–904. [DOI] [PubMed] [Google Scholar]
- Kachlany, S.C. , Levery, S.B. , Kim, J.S. , Reuhs, B.L. , Lion, L.W. & Ghiorse, W.C. (2001) Structure and carbohydrate analysis of the exopolysaccharide capsule of Pseudomonas putida G7. Environmental Microbiology, 3, 774–784. [DOI] [PubMed] [Google Scholar]
- Kakehashi, S. , Stanley, H.R. & Fitzgerald, R.J. (1965) The effects of surgical exposures of dental pulps in germ‐free and conventional laboratory rats. Oral Surgery Oral Medicine Oral Pathology, 20, 340–349. [DOI] [PubMed] [Google Scholar]
- Kakoli, P. , Nandakumar, R. , Romberg, E. , Arola, D. & Fouad, A.F. (2009) The effect of age on bacterial penetration of radicular dentin. Journal of Endodontics, 35, 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan, C.G. , Pelegrine, R.A. , da Silveira Bueno, C.E. , Shimabuko, D.M. , Valamatos Pinto, N.M. & Kato, A.S. (2020) Can irrigant agitation lead to the formation of a smear layer? Journal of Endodontics, 46, 1120–1124. [DOI] [PubMed] [Google Scholar]
- Kapalas, A. , Spratt, D.A. , Ng, Y.L. & Gulabivala, K. (2011) An investigation of a potential confounder in ex vivo microbiological studies—the bulk flow of fluid through apical foramina during tooth extraction. International Endodontic Journal, 44, 534–542. [DOI] [PubMed] [Google Scholar]
- Kawane, K. , Motani, K. & Nagata, S. (2014) DNA degradation and its defects. Cold Spring Harbor Perspectives in Biology, 6, a016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaoglu, G. , Erten, H. & Ørstavik, D. (2005) Growth at high pH increases Enterococcus faecalis adhesion to collagen. International Endodontic Journal, 38, 389–396. [DOI] [PubMed] [Google Scholar]
- Kempsell, K.E. , Cox, C.J. , Hurle, M. , Wong, A. , Wilkie, S. , Zanders, E.D. et al. (2000) Reverse transcriptase‐PCR analysis of bacterial rRNA for detection and characterization of bacterial species in arthritis synovial tissue. Infection and Immunity, 68, 6012–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin, C. , Demiryürek, E.Ö. & Onuk, E.E. (2017) Pyrosequencing analysis of cryogenically ground samples from primary and secondary/persistent endodontic infections. Journal of Endodontics, 43, 1309–1316. [DOI] [PubMed] [Google Scholar]
- Khademi, A.A. , Mohammadi, Z. & Havaee, A. (2006) Evaluation of the antibacterial substantivity of several intra‐canal agents. Australian Endodontic Journal, 32, 112–115. [DOI] [PubMed] [Google Scholar]
- Khan, S. , Niu, L.‐N. , Eid, A.A. , Looney, S.W. , Didato, A. , Roberts, S. et al. (2013) Periapical pressures developed by nonbinding irrigation needles at various irrigation delivery rates. Journal of Endodontics, 39, 529–533. [DOI] [PubMed] [Google Scholar]
- Kho, P. & Baumgartner, J.C. (2006) A comparison of the antimicrobial efficacy of NaOCl/Biopure MTAD versus NaOCl/EDTA against Enterococcus faecalis . Journal of Endodontics, 32, 652–655. [DOI] [PubMed] [Google Scholar]
- Kishen, A. & Haapasalo, M. (2012) Biofilm models and methods of biofilm assessment. Endodontic Topics, 22, 58–78. [Google Scholar]
- Kishen, A. , Sum, C.P. , Mathew, S. & Lim, C.T. (2008) Influence of irrigation regimens on the adherence of Enterococcus faecalis to root canal dentin. Journal of Endodontics, 34, 850–854. [DOI] [PubMed] [Google Scholar]
- Klein, M.I. , Scott‐Anne, K.M. , Gregoire, S. , Rosalen, P.L. & Koo, H. (2012) Molecular approaches for viable bacterial population and transcriptional analyses in a rodent model of dental caries. Molecular Oral Microbiology, 27, 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyn, S.L. , Kirkpatrick, T.C. & Rutledge, R.E. (2010) In vitro comparisons of debris removal of the EndoActivator system, the F file, ultrasonic irrigation, and NaOCl irrigation alone after hand‐rotary instrumentation in human mandibular molars. Journal of Endodontics, 36, 1367–1371. [DOI] [PubMed] [Google Scholar]
- Koch, J.D. , Jaramillo, D.E. , DiVito, E. & Peters, O.A. (2016) Irrigant flow during photon‐induced photoacoustic streaming (PIPS) using Particle Image Velocimetry (PIV). Clinical Oral Investigations, 20, 381–386. [DOI] [PubMed] [Google Scholar]
- Koch, J.D. , Smith, N.A. , Garces, D. , Gao, L. & Olsen, F.K. (2014) In vitro particle image velocity measurements in a model root canal: flow around a polymer rotary finishing file. Journal of Endodontics, 40, 412–416. [DOI] [PubMed] [Google Scholar]
- Komorowski, R. , Grad, H. , Wu, X.Y. & Friedman, S. (2000) Antimicrobial substantivity of chlorhexidine‐treated bovine root dentin. Journal of Endodontics, 26, 315–317. [DOI] [PubMed] [Google Scholar]
- Koskinen, K.P. , Stenvall, H. & Uitto, V.J. (1980) Dissolution of bovine pulp tissue by endodontic solutions. Scandinavian Journal of Dental Research, 88, 406–411. [DOI] [PubMed] [Google Scholar]
- Lawrence, J.R. , Korber, D.R. , Hoyle, B.D. , Costerton, J.W. & Caldwell, D.E. (1991) Optical sectioning of microbial biofilms. Journal of Bacteriology, 173, 6558–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton, G. , Wu, W.I. , Selvaganapathy, P.R. , Friedman, S. & Kishen, A. (2015) Fluid dynamics and biofilm removal generated by syringe‐delivered and 2 ultrasonic‐assisted irrigation methods: a novel experimental approach. Journal of Endodontics, 41, 884–889. [DOI] [PubMed] [Google Scholar]
- Lee, S.J. , Wu, M.K. & Wesselink, P.R. (2004) The efficacy of ultrasonic irrigation to remove artificially placed dentine debris from different‐sized simulated plastic root canals. International Endodontic Journal, 37, 607–612. [DOI] [PubMed] [Google Scholar]
- Leoni, G.B. , Versiani, M.A. , Silva‐Sousa, Y.T. , Bruniera, J.F. , Pécora, J.D. & Sousa‐Neto, M.D. (2017) Ex vivo evaluation of four final irrigation protocols on the removal of hard‐tissue debris from the mesial root canal system of mandibular first molars. International Endodontic Journal, 50, 398–406. [DOI] [PubMed] [Google Scholar]
- Lerminiaux, N.A. & Cameron, A.D.S. (2019) Horizontal transfer of antibiotic resistance genes in clinical environments. Canadian Journal of Microbiology, 65, 34–44. [DOI] [PubMed] [Google Scholar]
- Lewis, K. (2007) Persister cells, dormancy and infectious disease. Nature Reviews Microbiology, 5, 48–56. [DOI] [PubMed] [Google Scholar]
- Li, L. , Mendis, N. , Trigui, H. , Oliver, J.D. & Faucher, S.P. (2014) The importance of the viable but non‐culturable state in human bacterial pathogens. Frontiers in Microbiology, 5, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.H. & Bowden, G.H. (1994) Characteristics of accumulation of oral gram‐positive bacteria on mucin‐conditioned glass surfaces in a model system. Oral Microbiology and Immunology, 9, 1–11. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Wang, Y. , Chen, X. , Jiang, W. , Jiang, X. , Zeng, Y. et al. (2020) Antimicrobial peptide GH12 as root canal irrigant inhibits biofilm and virulence of Enterococcus faecalis . International Endodontic Journal, 53, 948–961. [DOI] [PubMed] [Google Scholar]
- Liang, Y.‐H. , Jiang, L.‐M. , Jiang, L. , Chen, X.‐B. , Liu, Y.‐Y. , Tian, F.‐C. et al. (2013) Radiographic healing after a root canal treatment performed in single‐rooted teeth with and without ultrasonic activation of the irrigant: a randomized controlled trial. Journal of Endodontics, 39, 1218–1225. [DOI] [PubMed] [Google Scholar]
- Lim, Z. , Cheng, J.L. , Lim, T.W. , Teo, E.G. , Wong, J. , George, S. et al. (2009) Light activated disinfection: an alternative endodontic disinfection strategy. Australian Dental Journal, 54, 108–114. [DOI] [PubMed] [Google Scholar]
- Little, B. , Wagner, P. , Ray, R. , Pope, R. & Scheetz, R. (1991) Biofilms: an ESEM evaluation of artifacts introduced during SEM preparation. Journal of Industrial Microbiology, 8, 213–221. [Google Scholar]
- Liu, H. , Wei, X. , Ling, J. , Wang, W. & Huang, X. (2010) Biofilm formation capability of Enterococcus faecalis cells in starvation phase and its susceptibility to sodium hypochlorite. Journal of Endodontics, 36, 630–635. [DOI] [PubMed] [Google Scholar]
- López, F.U. , Kopper, P.M. , Bona, A.D. , Steier, L. , de Figueiredo, J.A. & Vier‐Pelisser, F.V. (2015) Effect of different irrigating solutions and photo‐activated therapy for in vivo root canal treatment. Brazilian Dental Journal, 26, 228–233. [DOI] [PubMed] [Google Scholar]
- Loroño, G. , Zaldivar, J.R. , Arias, A. , Cisneros, R. , Dorado, S. & Jimenez‐Octavio, J.R. (2020) Positive and negative pressure irrigation in oval root canals with apical ramifications: a computational fluid dynamics evaluation in micro‐CT scanned real teeth. International Endodontic Journal, 53, 671–679. [DOI] [PubMed] [Google Scholar]
- Lottanti, S. , Gautschi, H. , Sener, B. & Zehnder, M. (2009) Effects of ethylenediaminetetraacetic, etidronic and peracetic acid irrigation on human root dentine and the smear layer. International Endodontic Journal, 42, 335–343. [DOI] [PubMed] [Google Scholar]
- Love, R.M. (2012) Biofilm‐substrate interaction: from initial adhesion to complex interactions and biofilm maturity. Endodontic Topics, 22, 50–57. [Google Scholar]
- Love, R.M. & Jenkinson, H.F. (2002) Invasion of dentinal tubules by oral bacteria. Critical Reviews in Oral Biology & Medicine, 13, 171–183. [DOI] [PubMed] [Google Scholar]
- Lukic, D. , Karygianni, L. , Flury, M. , Attin, T. & Thurnheer, T. (2020) Endodontic‐like oral biofilms as models for multispecies interactions in endodontic diseases. Microorganisms, 8, 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom, J.R. , Williamson, A.E. , Villhauer, A.L. , Dawson, D.V. & Drake, D.R. (2010) Bactericidal activity of stabilized chlorine dioxide as an endodontic irrigant in a polymicrobial biofilm tooth model system. Journal of Endodontics, 36, 1874–1878. [DOI] [PubMed] [Google Scholar]
- Ma, J. , Wang, Z. , Shen, Y. & Haapasalo, M. (2011) A new noninvasive model to study the effectiveness of dentin disinfection using confocal laser scanning microscopy. Journal of Endodontics, 37, 1380–1385. [DOI] [PubMed] [Google Scholar]
- Macedo, R.G. , Robinson, J.P. , Verhaagen, B. , Walmsley, A.D. , Versluis, M. , Cooper, P.R. et al. (2014a) A novel methodology providing insights into removal of biofilm‐mimicking hydrogel from lateral morphological features of the root canal during irrigation procedures. International Endodontic Journal, 47, 1040–1051. [DOI] [PubMed] [Google Scholar]
- Macedo, R.G. , Verhaagen, B. , Fernandez Rivas, D. , Gardeniers, J. , van der Sluis, L. , Wesselink, P.R. et al. (2014b) Sonochemical and high‐speed optical characterization of cavitation generated by an ultrasonically oscillating dental file in root canal models. Ultrasonics Sonochemistry, 21, 324–335. [DOI] [PubMed] [Google Scholar]
- Macedo, R.G. , Wesselink, P.R. , Zaccheo, F. , Fanali, D. & van der Sluis, L.W. (2010) Reaction rate of NaOCl in contact with bovine dentine: effect of activation, exposure time, concentration and pH. International Endodontic Journal, 43, 1108–1115. [DOI] [PubMed] [Google Scholar]
- Magni, E. , Jäggi, M. , Eggmann, F. , Weiger, R. & Connert, T. (2021) Apical pressures generated by several canal irrigation methods: a laboratory study in a maxillary central incisor with an open apex. International Endodontic Journal, 54, 1937–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista, S.E. , Barthold, S.W. & Persing, D.H. (1994) Fate of Borrelia burgdorferi DNA in tissues of infected mice after antibiotic treatment. The Journal of Infectious Diseases, 170, 1312–1316. [DOI] [PubMed] [Google Scholar]
- Malentacca, A. , Uccioli, U. , Zangari, D. , Lajolo, C. & Fabiani, C. (2012) Efficacy and safety of various active irrigation devices when used with either positive or negative pressure: an in vitro study. Journal of Endodontics, 38, 1622–1626. [DOI] [PubMed] [Google Scholar]
- Malki, M. , Verhaagen, B. , Jiang, L.‐M. , Nehme, W. , Naaman, A. , Versluis, M. et al. (2012) Irrigant flow beyond the insertion depth of an ultrasonically oscillating file in straight and curved root canals: visualization and cleaning efficacy. Journal of Endodontics, 38, 657–661. [DOI] [PubMed] [Google Scholar]
- Mangalam, S. , Rao, C.V.N. & Lakshminarayanan, L. (2002) Evaluation of apically extruded debris and irrigant using three instrumentation techniques. Endodontology, 14, 19–23. [Google Scholar]
- Manoil, D. , Al‐Manei, K. & Belibasakis, G.N. (2020) A systematic review of the root canal microbiota associated with apical periodontitis: lessons from next‐generation sequencing. Proteomics ‐ Clinical Applications, 14, e1900060. [DOI] [PubMed] [Google Scholar]
- Marinković, J. , Ćulafić, D.M. , Nikolić, B. , Đukanović, S. , Marković, T. , Tasić, G. et al. (2020) Antimicrobial potential of irrigants based on essential oils of Cymbopogon martinii and Thymus zygis towards in vitro multispecies biofilm cultured in ex vivo root canals. Archives of Oral Biology, 117, 104842. [DOI] [PubMed] [Google Scholar]
- Marshall, G.W. Jr. , Marshall, S.J. , Kinney, J.H. & Balooch, M. (1997) The dentin substrate: structure and properties related to bonding. Journal of Dentistry, 25, 441–458. [DOI] [PubMed] [Google Scholar]
- Masood, M. , Masood, Y. & Newton, J.T. (2015) The clustering effects of surfaces within the tooth and teeth within individuals. Journal of Dental Research, 94, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb, D. & Smith, D.C. (1975) A preliminary scanning electron microscopic study of root canals after endodontic procedures. Journal of Endodontics, 1, 238–242. [DOI] [PubMed] [Google Scholar]
- McGill, S. , Gulabivala, K. , Mordan, N. & Ng, Y.L. (2008) The efficacy of dynamic irrigation using a commercially available system (RinsEndo) determined by removal of a collagen ‘bio‐molecular film’ from an ex vivo model. International Endodontic Journal, 41, 602–608. [DOI] [PubMed] [Google Scholar]
- McKinlay, K.J. , Allison, F.J. , Scotchford, C.A. , Grant, D.M. , Oliver, J.M. , King, J.R. et al. (2004) Comparison of environmental scanning electron microscopy with high vacuum scanning electron microscopy as applied to the assessment of cell morphology. Journal of Biomedical Materials Research Part A, 69, 359–366. [DOI] [PubMed] [Google Scholar]
- Menezes, M.M. , Valera, M.C. , Jorge, A.O. , Koga‐Ito, C.Y. , Camargo, C.H. & Mancini, M.N. (2004) In vitro evaluation of the effectiveness of irrigants and intracanal medicaments on microorganisms within root canals. International Endodontic Journal, 37, 311–319. [DOI] [PubMed] [Google Scholar]
- Merritt, J.H. , Kadouri, D.E. & O’Toole, G.A. (2005) Growing and analyzing static biofilms. Current Protocols in Microbiology, 1B.1.1–1B.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, T.A. & Baumgartner, J.C. (2010) Comparison of the antimicrobial efficacy of irrigation using the EndoVac to endodontic needle delivery. Journal of Endodontics, 36, 509–511. [DOI] [PubMed] [Google Scholar]
- Miskin, I.P. , Farrimond, P. & Head, I.M. (1999) Identification of novel bacterial lineages as active members of microbial populations in a freshwater sediment using a rapid RNA extraction procedure and RT‐PCR. Microbiology, 145, 1977–1987. [DOI] [PubMed] [Google Scholar]
- Mitchell, R.P. , Baumgartner, J.C. & Sedgley, C.M. (2011) Apical extrusion of sodium hypochlorite using different root canal irrigation systems. Journal of Endodontics, 37, 1677–1681. [DOI] [PubMed] [Google Scholar]
- Mohmmed, S.A. , Vianna, M.E. , Penny, M.R. , Hilton, S.T. , Mordan, N. & Knowles, J.C. (2016) A novel experimental approach to investigate the effect of different agitation methods using sodium hypochlorite as an irrigant on the rate of bacterial biofilm removal from the wall of a simulated root canal model. Dental Materials, 32, 1289–1300. [DOI] [PubMed] [Google Scholar]
- Mohorn, H.W. , Dowson, J. & Blankenship, J.R. (1971a) Odontic periapical pressure following vital pulp extirpation. Oral Surgery, Oral Medicine, Oral Pathology, 31, 536–544. [DOI] [PubMed] [Google Scholar]
- Mohorn, H.W. , Dowson, J. & Blankenship, J.R. (1971b) Pressure exerted by odontic periapical lesions. Oral Surgery, Oral Medicine, Oral Pathology, 31, 810–818. [DOI] [PubMed] [Google Scholar]
- Moinzadeh, A.T. , Zerbst, W. , Boutsioukis, C. , Shemesh, H. & Zaslansky, P. (2015) Porosity distribution in root canals filled with gutta percha and calcium silicate cement. Dental Materials, 31, 1100–1108. [DOI] [PubMed] [Google Scholar]
- Möller, A.J. (1966) Microbiological examination of root canals and periapical tissues of human teeth. Methodological Studies. Odontologisk Tidskrift, 74(Suppl.), 1–380. [PubMed] [Google Scholar]
- Möller, A.J. , Fabricius, L. , Dahlén, G. , Ohman, A.E. & Heyden, G. (1981) Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scandinavian Journal of Dental Research, 89, 475–484. [DOI] [PubMed] [Google Scholar]
- Moorer, W.R. & Wesselink, P.R. (1982) Factors promoting the tissue dissolving capability of sodium hypochlorite. International Endodontic Journal, 15, 187–196. [DOI] [PubMed] [Google Scholar]
- Morago, A. , Ordinola‐Zapata, R. , Ferrer‐Luque, C.M. , Baca, P. , Ruiz‐Linares, M. & Arias‐Moliz, M.T. (2016) Influence of smear layer on the antimicrobial activity of a sodium hypochlorite/etidronic acid irrigating solution in infected dentin. Journal of Endodontics, 42, 1647–1650. [DOI] [PubMed] [Google Scholar]
- Morago, A. , Ruiz‐Linares, M. , Ferrer‐Luque, C.M. , Baca, P. , Rodríguez Archilla, A. & Arias‐Moliz, M.T. (2019) Dentine tubule disinfection by different irrigation protocols. Microscopy Research and Technique, 82, 558–563. [DOI] [PubMed] [Google Scholar]
- Müller, D.J. , Krieg, M. , Alsteens, D. & Dufrêne, Y.F. (2009) New frontiers in atomic force microscopy: analyzing interactions from single‐molecules to cells. Current Opinion in Biotechnology, 20, 4–13. [DOI] [PubMed] [Google Scholar]
- Mullis, K.B. , Ferré, F. & Gibbs, R.A. (1994) The polymerase chain reaction. Basel, Switzerland: Birkhäuser Verlag, pp. 1–432. [Google Scholar]
- Munoz, H.R. & Camacho‐Cuadra, K. (2012) In vivo efficacy of three different endodontic irrigation systems for irrigant delivery to working length of mesial canals of mandibular molars. Journal of Endodontics, 38, 445–448. [DOI] [PubMed] [Google Scholar]
- Myers, G.L. & Montgomery, S. (1991) A comparison of weights of debris extruded apically by conventional filing and Canal Master techniques. Journal of Endodontics, 17, 275–279. [DOI] [PubMed] [Google Scholar]
- Naenni, N. , Thoma, K. & Zehnder, M. (2004) Soft tissue dissolution capacity of currently used and potential endodontic irrigants. Journal of Endodontics, 30, 785–787. [DOI] [PubMed] [Google Scholar]
- Nagendrababu, V. , Kishen, A. , Chong, B.S. , Priya, E. , Duncan, H.F. , Rôças, I.N. et al. (2020) Preferred Reporting Items for study Designs in Endodontology (PRIDE): guiding authors to identify and correct reporting deficiencies in their manuscripts prior to peer review. International Endodontic Journal, 53, 589–590. [DOI] [PubMed] [Google Scholar]
- Nagendrababu, V. , Murray, P.E. , Ordinola‐Zapata, R. , Peters, O.A. , Rôças, I.N. , Siqueira, J.F. et al. (2021) PRILE 2021 guidelines for reporting laboratory studies in Endodontology: a consensus‐based development. International Endodontic Journal, 54, 1482–1490. [DOI] [PubMed] [Google Scholar]
- Nair, P.N. , Henry, S. , Cano, V. & Vera, J. (2005) Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after "one‐visit" endodontic treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 99, 231–252. [DOI] [PubMed] [Google Scholar]
- Nardello, L.C.L. , Amado, P.P.P. , Franco, D.C. , Cazares, R.X.R. , Nogales, C.G. , Mayer, M.P.A. et al. (2020) Next‐Generation Sequencing to assess potentially active bacteria in endodontic infections. Journal of Endodontics, 46, 1105–1112. [DOI] [PubMed] [Google Scholar]
- Nawrocka, A. & Łukomska‐Szymańska, M. (2019) Extracted human teeth and their utility in dental research. Recommendations on proper preservation: a literature review. Dental and Medical Problems, 56, 185–190. [DOI] [PubMed] [Google Scholar]
- Netuschil, L. , Auschill, T.M. , Sculean, A. & Arweiler, N.B. (2014) Confusion over live/dead stainings for the detection of vital microorganisms in oral biofilms—which stain is suitable? BMC Oral Health, 14, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu, T.R. & Lawrence, J.R. (2014) Investigation of microbial biofilm structure by laser scanning microscopy. Advances in Biochemical Engineering / Biotechnology, 146, 1–51. [DOI] [PubMed] [Google Scholar]
- Neuhaus, K.W. , Liebi, M. , Stauffacher, S. , Eick, S. & Lussi, A. (2016) Antibacterial efficacy of a new sonic irrigation device for root canal disinfection. Journal of Endodontics, 42, 1799–1803. [DOI] [PubMed] [Google Scholar]
- Nkuipou‐Kenfack, E. , Engel, H. , Fakih, S. & Nocker, A. (2013) Improving efficiency of viability‐PCR for selective detection of live cells. Journal of Microbiological Methods, 93, 20–24. [DOI] [PubMed] [Google Scholar]
- Nudera, W.J. , Fayad, M.I. , Johnson, B.R. , Zhu, M. , Wenckus, C.S. , BeGole, E.A. et al. (2007) Antimicrobial effect of triclosan and triclosan with Gantrez on five common endodontic pathogens. Journal of Endodontics, 33, 1239–1242. [DOI] [PubMed] [Google Scholar]
- Oberkampf, W.L. & Trucano, T.G. (2002) Verification and validation in computational fluid dynamics. Progress in Aerospace Sciences, 38, 209–272. [Google Scholar]
- O'Brien, J. , Wilson, I. , Orton, T. & Pognan, F. (2000) Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry, 267, 5421–5426. [DOI] [PubMed] [Google Scholar]
- Ordinola‐Zapata, R. , Bramante, C.M. , Brandão Garcia, R. , Bombarda de Andrade, F. , Bernardineli, N. , Gomes de Moraes, I. et al. (2013) The antimicrobial effect of new and conventional endodontic irrigants on intra‐orally infected dentin. Acta Odontologica Scandinavica, 71, 424–431. [DOI] [PubMed] [Google Scholar]
- Ordinola‐Zapata, R. , Crepps, J.T. , Arias, A. & Lin, F. (2021) In vitro apical pressure created by 2 irrigation needles and a multisonic system in mandibular molars. Restorative Dentistry and Endodontics, 46, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørstavik, D. (2019) Essential endodontology: prevention and treatment of apical periodontitis, 3rd edition. Oxford: Blackwell Science, pp. 1–10. [Google Scholar]
- Papa, J. , Cain, C. & Messer, H.H. (1994) Moisture content of vital vs endodontically treated teeth. Endodontics and Dental Traumatology, 10, 91–93. [DOI] [PubMed] [Google Scholar]
- Pappen, F.G. , Xavier, S.R. , Pilownic, K.J. , Santos, L.G.P. , Gomes, A.P.N. , Felix, A.C. et al. (2019) Impact of infected and noninfected human dentine debris on bone healing in rats. International Endodontic Journal, 52, 1679–1690. [DOI] [PubMed] [Google Scholar]
- Paqué, F. , Boessler, C. & Zehnder, M. (2011) Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps. International Endodontic Journal, 44, 148–153. [DOI] [PubMed] [Google Scholar]
- Paqué, F. , Laib, A. , Gautschi, H. & Zehnder, M. (2009) Hard‐tissue debris accumulation analysis by high‐resolution computed tomography scans. Journal of Endodontics, 35, 1044–1047. [DOI] [PubMed] [Google Scholar]
- Paqué, F. , Luder, H.U. , Sener, B. & Zehnder, M. (2006) Tubular sclerosis rather than the smear layer impedes dye penetration into the dentine of endodontically instrumented root canals. International Endodontic Journal, 39, 18–25. [DOI] [PubMed] [Google Scholar]
- Paqué, F. & Peters, O.A. (2011) Micro‐computed tomography evaluation of the preparation of long oval root canals in mandibular molars with the self‐adjusting file. Journal of Endodontics, 37, 517–521. [DOI] [PubMed] [Google Scholar]
- Paqué, F. , Zehnder, M. & Marending, M. (2010) Apical fit of initial K‐files in maxillary molars assessed by micro‐computed tomography. International Endodontic Journal, 43, 328–335. [DOI] [PubMed] [Google Scholar]
- Parente, J.M. , Loushine, R.J. , Susin, L. , Gu, L. , Looney, S.W. , Weller, R.N. et al. (2010) Root canal debridement using manual dynamic agitation or the EndoVac for final irrigation in a closed system and an open system. International Endodontic Journal, 43, 1001–1012. [DOI] [PubMed] [Google Scholar]
- Park, E. , Shen, Y. , Khakpour, M. & Haapasalo, M. (2013) Apical pressure and extent of irrigant flow beyond the needle tip during positive‐pressure irrigation in an in vitro root canal model. Journal of Endodontics, 39, 511–515. [DOI] [PubMed] [Google Scholar]
- Parsell, D.E. , Stewart, B.M. , Barker, J.R. , Nick, T.G. , Karns, L. & Johnson, R.B. (1998) The effect of steam sterilization on the physical properties and perceived cutting characteristics of extracted teeth. Journal of Dental Education, 62, 260–263. [PubMed] [Google Scholar]
- Parsons, G.J. , Patterson, S.S. , Miller, C.H. , Katz, S. , Kafrawy, A.H. & Newton, C.W. (1980) Uptake and release of chlorhexidine by bovine pulp and dentin specimens and their subsequent acquisition of antibacterial properties. Oral Surgery, Oral Medicine, Oral Pathology, 49, 455–459. [DOI] [PubMed] [Google Scholar]
- Pashley, E.L. , Tao, L. & Pashley, D.H. (1993) Sterilization of human teeth: its effect on permeability and bond strength. American Journal of Dentistry, 6, 189–191. [PubMed] [Google Scholar]
- Pavarina, A.C. , Dovigo, L.N. , Sanitá, P.V. , Machado, A.L. , Giampaolo, E.T. & Vergani, C.E. (2011) Dynamic models for in vitro biofilm formation. In: Bailey, W.C. (Ed.) Biofilms: formation, development and properties. São Paulo, Brazil: Nova Science Publishers, pp. 125–162. [Google Scholar]
- Peeters, E. , Nelis, H.J. & Coenye, T. (2008) Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. Journal of Microbiological Methods, 72, 157–165. [DOI] [PubMed] [Google Scholar]
- Peeters, H.H. & Gutknecht, N. (2014) Efficacy of laser‐driven irrigation versus ultrasonic in removing an airlock from the apical third of a narrow root canal. Australian Endodontic Journal, 40, 47–53. [DOI] [PubMed] [Google Scholar]
- Pereira, T.C. , Boutsioukis, C. , Dijkstra, R.J.B. , Petridis, X. , Versluis, M. , Andrade, F.B. et al. (2021a) Biofilm removal from a simulated isthmus and lateral canal during syringe irrigation at various flow rates: a combined experimental and Computational Fluid Dynamics approach. International Endodontic Journal, 54, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, T.C. , Dijkstra, R.J.B. , Petridis, X. , Meer, W.J. , Sharma, P.K. , de Andrade, F.B. et al. (2020) The influence of time and irrigant refreshment on biofilm removal from lateral morphological features of simulated root canals. International Endodontic Journal, 53, 1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, T.C. , Dijkstra, R.J.B. , Petridis, X. , Sharma, P.K. , Meer, W.J. , Sluis, L.W.M. et al. (2021b) Chemical and mechanical influence of root canal irrigation on biofilm removal from lateral morphological features of simulated root canals, dentine discs and dentinal tubules. International Endodontic Journal, 54, 112–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez, R. , Neves, A.A. , Belladonna, F.G. , Silva, E.J.N.L. , Souza, E.M. , Fidel, S. et al. (2017) Impact of needle insertion depth on the removal of hard‐tissue debris. International Endodontic Journal, 50, 560–568. [DOI] [PubMed] [Google Scholar]
- Peters, O.A. (2004) Current challenges and concepts in the preparation of root canal systems: a review. Journal of Endodontics, 30, 559–567. [DOI] [PubMed] [Google Scholar]
- Peters, O.A. , Bardsley, S. , Fong, J. , Pandher, G. & Divito, E. (2011) Disinfection of root canals with photon‐initiated photoacoustic streaming. Journal of Endodontics, 37, 1008–1012. [DOI] [PubMed] [Google Scholar]
- Peters, O.A. , Laib, A. , Rüegsegger, P. & Barbakow, F. (2000) Three‐dimensional analysis of root canal geometry by high‐resolution computed tomography. Journal of Dental Research, 79, 1405–1409. [DOI] [PubMed] [Google Scholar]
- Petridis, X. , Busanello, F.H. , So, M.V.R. , Dijkstra, R.J.B. , Sharma, P.K. & van der Sluis, L.W.M. (2019) Chemical efficacy of several NaOCl concentrations on biofilms of different architecture: new insights on NaOCl working mechanisms. International Endodontic Journal, 52, 1773–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit, R.K. , Weber, C.A. , Kean, M.J. , Hoffmann, H. , Pettit, G.R. , Tan, R. et al. (2005) Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrobial Agents and Chemotherapy, 49, 2612–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts, B. , Hamilton, M.A. , Zelver, N. & Stewart, P.S. (2003) A microtiter‐plate screening method for biofilm disinfection and removal. Journal of Microbiological Methods, 54, 269–276. [DOI] [PubMed] [Google Scholar]
- Priester, J.H. , Horst, A.M. , van de Werfhorst, L.C. , Saleta, J.L. , Mertes, L.A. & Holden, P.A. (2007) Enhanced visualization of microbial biofilms by staining and environmental scanning electron microscopy. Journal of Microbiological Methods, 68, 577–587. [DOI] [PubMed] [Google Scholar]
- Psimma, Z. , Boutsioukis, C. , Vasiliadis, L. & Kastrinakis, E. (2013a) A new method for real‐time quantification of irrigant extrusion during root canal irrigation ex vivo . International Endodontic Journal, 46, 619–631. [DOI] [PubMed] [Google Scholar]
- Psimma, Z. , Boutsioukis, C. , Kastrinakis, E. & Vasiliadis, L. (2013b) Effect of needle insertion depth and root canal curvature on irrigant extrusion ex vivo . Journal of Endodontics, 39, 521–524. [DOI] [PubMed] [Google Scholar]
- Psimma, Z. & Boutsioukis, C. (2019) A critical view on sodium hypochlorite accidents. ENDO‐Endodontic Practice Today, 13, 165–175. [Google Scholar]
- Ram, Z. (1977) Effectiveness of root canal irrigation. Oral Surgery, Oral Medicine, Oral Pathology, 44, 306–312. [DOI] [PubMed] [Google Scholar]
- Rana, M. , Jain, V. , Kaur, P. , Jalan, P. , Kapadia, J.M. & Shaikh, I. (2019) Assessment of impact of various root canal irrigants on the adherence of the gelatinase‐producing and the gelatinase‐deficient E. faecalis strains to dentin. The Journal of Contemporary Dental Practice, 20, 46–50. [PubMed] [Google Scholar]
- Reis, C. , De‐Deus, G. , Leal, F. , Azevedo, E. , Coutinho‐Filho, T. & Paciornik, S. (2008) Strong effect on dentin after the use of high concentrations of citric acid: an assessment with co‐site optical microscopy and ESEM. Dental Materials, 24, 1608–1615. [DOI] [PubMed] [Google Scholar]
- Retsas, A. , Koursoumis, A. , Tzimpoulas, N. & Boutsioukis, C. (2016) Uncontrolled removal of dentin during in vitro ultrasonic irrigant activation in curved root canals. Journal of Endodontics, 42, 1545–1549. [DOI] [PubMed] [Google Scholar]
- Ricucci, D. & Siqueira, J.F. Jr. (2008) Anatomic and microbiologic challenges to achieving success with endodontic treatment: a case report. Journal of Endodontics, 34, 1249–1254. [DOI] [PubMed] [Google Scholar]
- Robertson, D. , Leeb, I.J. , McKee, M. & Brewer, E. (1980) A clearing technique for the study of root canal systems. Journal of Endodontics, 6, 421–424. [DOI] [PubMed] [Google Scholar]
- Robinson, J.P. , Macedo, R.G. , Verhaagen, B. , Versluis, M. , Cooper, P.R. , van der Sluis, L.W.M. et al. (2018) Cleaning lateral morphological features of the root canal: the role of streaming and cavitation. International Endodontic Journal, 51(Suppl. 1), e55–e64. [DOI] [PubMed] [Google Scholar]
- Rödig, T. , Sedghi, M. , Konietschke, F. , Lange, K. , Ziebolz, D. & Hülsmann, M. (2010) Efficacy of syringe irrigation, RinsEndo and passive ultrasonic irrigation in removing debris from irregularities in root canals with different apical sizes. International Endodontic Journal, 43, 581–589. [DOI] [PubMed] [Google Scholar]
- Rodrigues, C.T. , de Andrade, F.B. , de Vasconcelos, L.R.S.M. , Midena, R.Z. , Pereira, T.C. , Kuga, M.C. et al. (2018) Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. International Endodontic Journal, 51, 901–911. [DOI] [PubMed] [Google Scholar]
- Rodrigues, C.T. , EzEldeen, M. , Jacobs, R. , Lambrechts, P. , Alcalde, M.P. & Hungaro Duarte, M.A. (2021) Cleaning efficacy and uncontrolled removal of dentin of two methods of irrigant activation in curved canals connected by an isthmus. Australian Endodontic Journal, 47, 631–638. [DOI] [PubMed] [Google Scholar]
- Rodrigues, R.C.V. , Zandi, H. , Kristoffersen, A.K. , Enersen, M. , Mdala, I. , Ørstavik, D. et al. (2017) Influence of the apical preparation size and the irrigant type on bacterial reduction in root canal‐treated teeth with apical periodontitis. Journal of Endodontics, 43, 1058–1063. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Figueroa, C. , McClanahan, S.B. & Bowles, W.R. (2014) Spectrophotometric determination of irrigant extrusion using passive ultrasonic irrigation, EndoActivator, or syringe irrigation. Journal of Endodontics, 40, 1622–1626. [DOI] [PubMed] [Google Scholar]
- Roehm, N.W. , Rodgers, G.H. , Hatfield, S.M. & Glasebrook, A.L. (1991) An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. Journal of Immunological Methods, 142, 257–265. [DOI] [PubMed] [Google Scholar]
- Rosales, J.I. , Marshall, G.W. , Marshall, S.J. , Watanabe, L.G. , Toledano, M. , Cabrerizo, M.A. et al. (1999) Acid‐etching and hydration influence on dentin roughness and wettability. Journal of Dental Research, 78, 1554–1559. [DOI] [PubMed] [Google Scholar]
- Rosenthal, S. , Spångberg, L. & Safavi, K. (2004) Chlorhexidine substantivity in root canal dentin. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 98, 488–492. [DOI] [PubMed] [Google Scholar]
- Rudney, J.D. , Chen, R. , Lenton, P. , Li, J. , Li, Y. , Jones, R.S. et al. (2012) A reproducible oral microcosm biofilm model for testing dental materials. Journal of Applied Microbiology, 113, 1540–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Linares, M. , Aguado‐Pérez, B. , Baca, P. , Arias‐Moliz, M.T. & Ferrer‐Luque, C.M. (2017) Efficacy of antimicrobial solutions against polymicrobial root canal biofilm. International Endodontic Journal, 50, 77–83. [DOI] [PubMed] [Google Scholar]
- Ruksakiet, K. , Hanák, L. , Farkas, N. , Hegyi, P. , Sadaeng, W. , Czumbel, L.M. et al. (2020) Antimicrobial efficacy of chlorhexidine and sodium hypochlorite in root canal disinfection: a systematic review and meta‐analysis of randomized controlled trials. Journal of Endodontics, 46, 1032–1041.e7. [DOI] [PubMed] [Google Scholar]
- Saini, H.R. , Tewari, S. , Sangwan, P. , Duhan, J. & Gupta, A. (2012) Effect of different apical preparation sizes on outcome of primary endodontic treatment: a randomized controlled trial. Journal of Endodontics, 38, 1309–1315. [DOI] [PubMed] [Google Scholar]
- Salzgeber, R.M. & Brilliant, J.D. (1977) An in vivo evaluation of the penetration of an irrigating solution in root canals. Journal of Endodontics, 3, 394–398. [DOI] [PubMed] [Google Scholar]
- Sánchez, M.C. , Llama‐Palacios, A. , Marin, M.J. , Figuero, E. , Leon, R. , Blanc, V. et al. (2013) Validation of ATP bioluminescence as a tool to assess antimicrobial effects of mouthrinses in an in vitro subgingival‐biofilm model. Medicina Oral, Patologia Oral, Cirugia Bucal, 18, e86–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Sanhueza, G. , Bello‐Toledo, H. , González‐Rocha, G. , Gonçalves, A.T. , Valenzuela, V. & Gallardo‐Escárate, C. (2018) Metagenomic study of bacterial microbiota in persistent endodontic infections using next‐generation sequencing. International Endodontic Journal, 51, 1336–1348. [DOI] [PubMed] [Google Scholar]
- Sandberg, M.E. , Schellmann, D. , Brunhofer, G. , Erker, T. , Busygin, I. , Leino, R. et al. (2009) Pros and cons of using resazurin staining for quantification of viable Staphylococcus aureus biofilms in a screening assay. Journal of Microbiological Methods, 78, 104–106. [DOI] [PubMed] [Google Scholar]
- Sandhu, S.V. , Tiwari, R. , Bhullar, R.K. , Bansal, H. , Bhandari, R. , Kakkar, T. et al. (2012) Sterilization of extracted human teeth: a comparative analysis. Journal of Oral Biology and Craniofacial Research, 2, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone, L.M. , Fidel, R.A. , Murad, C.F. , Fidel, S.R. & Hirata, R. Jr. (2008) Antimicrobial activity of sodium hypochlorite and chlorhexidine by two different tests. Australian Endodontic Journal, 34, 19–24. [DOI] [PubMed] [Google Scholar]
- Sathorn, C. , Parashos, P. & Messer, H.H. (2007) How useful is root canal culturing in predicting treatment outcome? Journal of Endodontics, 33, 220–225. [DOI] [PubMed] [Google Scholar]
- Sauer, K. , Camper, A.K. , Ehrlich, G.D. , Costerton, J.W. & Davies, D.G. (2002) Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. Journal of Bacteriology, 184, 1140–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, B.H. , Wesselink, P.R. & Türkün, M. (1995) The smear layer: a phenomenon in root canal therapy. International Endodontic Journal, 28, 141–148. [DOI] [PubMed] [Google Scholar]
- Shen, Y.A. , Gao, Y. , Qian, W. , Ruse, N.D. , Zhou, X. , Wu, H. et al. (2010b) Three‐dimensional numeric simulation of root canal irrigant flow with different irrigation needles. Journal of Endodontics, 36, 884–889. [DOI] [PubMed] [Google Scholar]
- Shen, Y. , Gao, Y. , Lin, J. , Ma, J. , Wang, Z. & Haapasalo, M. (2012) Methods and models to study irrigation. Endodontic Topics, 27, 3–34. [Google Scholar]
- Shen, Y. , Qian, W. , Chung, C. , Olsen, I. & Haapasalo, M. (2009) Evaluation of the effect of two chlorhexidine preparations on biofilm bacteria in vitro: a three‐dimensional quantitative analysis. Journal of Endodontics, 35, 981–985. [DOI] [PubMed] [Google Scholar]
- Shen, Y. , Stojicic, S. , Qian, W. , Olsen, I. & Haapasalo, M. (2010a) The synergistic antimicrobial effect by mechanical agitation and two chlorhexidine preparations on biofilm bacteria. Journal of Endodontics, 36, 100–104. [DOI] [PubMed] [Google Scholar]
- Shen, Y. , Stojicic, S. & Haapasalo, M. (2011) Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. Journal of Endodontics, 37, 657–661. [DOI] [PubMed] [Google Scholar]
- Silva, L.A. , Leonardo, M.R. , Assed, S. & Tanomaru Filho, M. (2004) Histological study of the effect of some irrigating solutions on bacterial endotoxin in dogs. Brazilian Dental Journal, 15, 109–114. [DOI] [PubMed] [Google Scholar]
- Siqueira, J.F. Jr. (2008) On the issue of uncultivated bacteria and dead cell detection by molecular methods: reply to Dr. Nair's commentary. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 105, 8–10. [DOI] [PubMed] [Google Scholar]
- Siqueira, J.F. Jr. , Antunes, H.S. , Rôças, I.N. , Rachid, C.T. & Alves, F.R. (2016) Microbiome in the apical root canal system of teeth with post‐treatment apical periodontitis. PLoS One, 11, e0162887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira, J.F. , Pérez, A.R. , Marceliano‐Alves, M.F. , Provenzano, J.C. , Silva, S.G. , Pires, F.R. et al. (2018) What happens to unprepared root canal walls: a correlative analysis using micro‐computed tomography and histology/scanning electron microscopy. International Endodontic Journal, 51, 501–508. [DOI] [PubMed] [Google Scholar]
- Siqueira, J.F. Jr. , Rôças, I.N. , Favieri, A. & Lima, K.C. (2000) Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. Journal of Endodontics, 26, 331–334. [DOI] [PubMed] [Google Scholar]
- Siqueira, J.F. Jr. & Rôças, I.N. (2005a) Exploiting molecular methods to explore endodontic infections: part 1–current molecular technologies for microbiological diagnosis. Journal of Endodontics, 31, 411–423. [DOI] [PubMed] [Google Scholar]
- Siqueira, J.F. Jr. & Rôças, I.N. (2005b) Exploiting molecular methods to explore endodontic infections: part 2–redefining the endodontic microbiota. Journal of Endodontics, 31, 488–498. [DOI] [PubMed] [Google Scholar]
- Siqueira, J.F. & Rôças, I.N. (2014) Present status and future directions in endodontic microbiology. Endodontics Topics, 30, 3–22. [Google Scholar]
- Siqueira, J.F. & Rôças, I.N. (2017) Molecular analysis of endodontic infections. In: Fouad, A.F. (Ed.) Endodontic microbiology, 2nd edition. Hoboken, NJ, USA: John Wiley & Sons, pp. 81–128. [Google Scholar]
- Siren, E.K. , Haapasalo, M.P. , Ranta, K. , Salmi, P. & Kerosuo, E.N. (1997) Microbiological findings and clinical treatment procedures in endodontic cases selected for microbiological investigation. International Endodontic Journal, 30, 91–95. [PubMed] [Google Scholar]
- Sjögren, U. , Figdor, D. , Persson, S. & Sundqvist, G. (1997) Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. International Endodontic Journal, 30, 297–306. [DOI] [PubMed] [Google Scholar]
- van der Sluis, L.W.M. , Gambarini, G. , Wu, M.K. & Wesselink, P.R. (2006) The influence of volume, type of irrigant and flushing method on removing artificially placed dentine debris from the apical root canal during passive ultrasonic irrigation. International Endodontic Journal, 39, 472–476. [DOI] [PubMed] [Google Scholar]
- Slutzky‐Goldberg, I. , Hanut, A. , Matalon, S. , Baev, V. & Slutzky, H. (2013) The effect of dentin on the pulp tissue dissolution capacity of sodium hypochlorite and calcium hydroxide. Journal of Endodontics, 39, 980–983. [DOI] [PubMed] [Google Scholar]
- Snjaric, D. , Carija, Z. , Braut, A. , Halaji, A. , Kovacevic, M. & Kuis, D. (2012) Irrigation of human prepared root canal–ex vivo based computational fluid dynamics analysis. Croatian Medical Journal, 53, 470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, L.E. , Brugnera, A. Jr. , Zanin, F.A. , Santo, A.M. & Martin, A.A. (2011) Effects of heating by steam autoclaving and Er:YAG laser etching on dentin components. Lasers in Medical Science, 26, 605–613. [DOI] [PubMed] [Google Scholar]
- Solana, C. , Ruiz‐Linares, M. , Baca, P. , Valderrama, M.J. , Arias‐Moliz, M.T. & Ferrer‐Luque, C.M. (2017) Antibiofilm Activity of sodium hypochlorite and alkaline tetrasodium EDTA solutions. Journal of Endodontics, 43, 2093–2096. [DOI] [PubMed] [Google Scholar]
- Souza, E.M. , Campos, M.G. & Rosas Aguilar, R. (2021) Mapping the periapex anatomical pattern of teeth involved in sodium hypochlorite accidents: a cross‐sectional quasi‐experimental study. International Endodontic Journal, 54, 1212–1220. [DOI] [PubMed] [Google Scholar]
- Sperandio, C.B. , Silveira, L.F. , de Araújo, L.A. , Martos, J. & Malshe, A. (2008) Response of the periapical tissue of dogs’ teeth to the action of citric acid and EDTA. Journal of Applied Oral Science, 16, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanović, S. , Cirković, I. , Ranin, L. & Svabić‐Vlahović, M. (2004) Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Letters in Applied Microbiology, 38, 428–432. [DOI] [PubMed] [Google Scholar]
- Stewart, G.S. (1990) In vivo bioluminescence: new potentials for microbiology. Letters in Applied Microbiology, 10, 1–8. [DOI] [PubMed] [Google Scholar]
- Stock, S.R. (2008) Microcomputed tomography: methodology and applications, 1st edition. Boca Raton, FL: CRC Press, pp. 1–331. [Google Scholar]
- Stocks, S.M. (2004) Mechanism and use of the commercially available viability stain, BacLight. Cytometry Part A, 61, 189–195. [DOI] [PubMed] [Google Scholar]
- Stojicic, S. , Shen, Y. , Qian, W. , Johnson, B. & Haapasalo, M. (2012) Antibacterial and smear layer removal ability of a novel irrigant, QMiX. International Endodontic Journal, 45, 363–371. [DOI] [PubMed] [Google Scholar]
- Stojicic, S. , Shen, Y. & Haapasalo, M. (2013) Effect of the source of biofilm bacteria, level of biofilm maturation, and type of disinfecting agent on the susceptibility of biofilm bacteria to antibacterial agents. Journal of Endodontics, 39, 473–477. [DOI] [PubMed] [Google Scholar]
- Stojicic, S. , Zivkovic, S. , Qian, W. , Zhang, H. & Haapasalo, M. (2010) Tissue dissolution by sodium hypochlorite: effect of concentration, temperature, agitation, and surfactant. Journal of Endodontics, 36, 1558–1562. [DOI] [PubMed] [Google Scholar]
- Sunde, P.T. , Olsen, I. , Göbel, U.B. , Theegarten, D. , Winter, S. , Debelian, G.J. et al. (2003) Fluorescence In situ Hybridization (FISH) for direct visualization of bacteria in periapical lesions of asymptomatic root‐filled teeth. Microbiology, 149, 1095–1102. [DOI] [PubMed] [Google Scholar]
- Sundqvist, G. , Figdor, D. , Persson, S. & Sjögren, U. (1998) Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re‐treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 85, 86–93. [DOI] [PubMed] [Google Scholar]
- Svensäter, G. & Bergenholtz, G. (2004) Biofilms in endodontic infections. Endodontic Topics, 9, 27–36. [Google Scholar]
- Swimberghe, R.C.D. , Coenye, T. , De Moor, R.J.G. & Meire, M.A. (2019a) Biofilm model systems for root canal disinfection: a literature review. International Endodontic Journal, 52, 604–628. [DOI] [PubMed] [Google Scholar]
- Swimberghe, R.C.D. , Crabbé, A. , De Moor, R.J.G. , Coenye, T. & Meire, M.A. (2021) Model system parameters influence the sodium hypochlorite susceptibility of endodontic biofilms. International Endodontic Journal, 54, 1557–1570. [DOI] [PubMed] [Google Scholar]
- Swimberghe, R.C.D. , De Clercq, A. , De Moor, R.J.G. & Meire, M.A. (2019b) Efficacy of sonically, ultrasonically and laser‐activated irrigation in removing a biofilm‐mimicking hydrogel from an isthmus model. International Endodontic Journal, 52, 515–523. [DOI] [PubMed] [Google Scholar]
- Talwar, S. , Utneja, S. , Nawal, R.R. , Kaushik, A. , Srivastava, D. & Oberoy, S.S. (2016) Role of cone‐beam computed tomography in diagnosis of vertical root fractures: a systematic review and meta‐analysis. Journal of Endodontics, 42, 12–24. [DOI] [PubMed] [Google Scholar]
- Tan, C.H. , Lee, K.W. , Burmølle, M. , Kjelleberg, S. & Rice, S.A. (2017) All together now: experimental multispecies biofilm model systems. Environmental Microbiology, 19, 42–53. [DOI] [PubMed] [Google Scholar]
- Tan, K.S. , Yu, V.S. , Quah, S.Y. & Bergenholtz, G. (2015) Rapid method for the detection of root canal bacteria in endodontic therapy. Journal of Endodontics, 41, 447–450. [DOI] [PubMed] [Google Scholar]
- Tanomaru Filho, M. , Leonardo, M.R. & da Silva, L.A. (2002) Effect of irrigating solution and calcium hydroxide root canal dressing on the repair of apical and periapical tissues of teeth with periapical lesion. Journal of Endodontics, 28, 295–299. [DOI] [PubMed] [Google Scholar]
- Tartari, T. , Guimarães, B.M. , Amoras, L.S. , Duarte, M.A.H. , Silva e Souza, P.A.R. & Bramante, C.M. (2015) Etidronate causes minimal changes in the ability of sodium hypochlorite to dissolve organic matter. International Endodontic Journal, 48, 399–404. [DOI] [PubMed] [Google Scholar]
- Tartari, T. , Oda, D.F. , Zancan, R.F. , da Silva, T.L. , de Moraes, I.G. , Duarte, M.A.H. et al. (2017) Mixture of alkaline tetrasodium EDTA with sodium hypochlorite promotes in vitro smear layer removal and organic matter dissolution during biomechanical preparation. International Endodontic Journal, 50, 106–114. [DOI] [PubMed] [Google Scholar]
- Tay, F.R. , Gu, L.‐S. , Schoeffel, G.J. , Wimmer, C. , Susin, L. , Zhang, K. et al. (2010) Effect of vapor lock on root canal debridement by using a side‐vented needle for positive‐pressure irrigant delivery. Journal of Endodontics, 36, 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada, S. , Baca, P. , Ferrer‐Luque, C.M. , Ruiz‐Linares, M. , Valderrama, M.J. & Arias‐Moliz, M.T. (2019) Influence of dentine debris and organic tissue on the properties of sodium hypochlorite solutions. International Endodontic Journal, 52, 114–122. [DOI] [PubMed] [Google Scholar]
- Teplitsky, P.E. , Chenail, B.L. , Mack, B. & Machnee, C.H. (1987) Endodontic irrigation—a comparison of endosonic and syringe delivery systems. International Endodontic Journal, 20, 233–241. [DOI] [PubMed] [Google Scholar]
- Teske, A. , Wawer, C. , Muyzer, G. & Ramsing, N.B. (1996) Distribution of sulfate‐reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most‐probable‐number counts and denaturing gradient gel electrophoresis of PCR‐amplified ribosomal DNA fragments. Applied and Environmental Microbiology, 62, 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler, A. , Ebert, J. , Petschelt, A. & Pelka, M. (2008) Influence of tooth age and root section on root dentine dye penetration. International Endodontic Journal, 41, 1115–1122. [DOI] [PubMed] [Google Scholar]
- Thé, S.D. (1979) The solvent action of sodium hypochlorite on fixed and unfixed necrotic tissue. Oral Surgery, Oral Medicine, Oral Pathology, 47, 558–561. [DOI] [PubMed] [Google Scholar]
- Thomas, A.R. , Velmurugan, N. , Smita, S. & Jothilatha, S. (2014) Comparative evaluation of canal isthmus debridement efficacy of modified EndoVac technique with different irrigation systems. Journal of Endodontics, 40, 1676–1680. [DOI] [PubMed] [Google Scholar]
- Tobias, R.S. (1988) Antibacterial properties of dental restorative materials: a review. International Endodontic Journal, 21, 155–160. [DOI] [PubMed] [Google Scholar]
- Tolker‐Nielsen, T. & Sternberg, C. (2011) Growing and analyzing biofilms in flow chambers. Current Protocols in Microbiology, 21, 1B.2.1–1B.2.17. [DOI] [PubMed] [Google Scholar]
- Tong, Z. , Zhou, L. , Li, J. , Jiang, W. , Ma, L. & Ni, L. (2011) In vitro evaluation of the antibacterial activities of MTAD in combination with nisin against Enterococcus faecalis . Journal of Endodontics, 37, 1116–1120. [DOI] [PubMed] [Google Scholar]
- Topçuoğlu, H.S. , Topçuoğlu, G. & Arslan, H. (2018a) The effect of apical positive and negative pressure irrigation methods on postoperative pain in mandibular molar teeth with symptomatic irreversible pulpitis: a randomized clinical trial. Journal of Endodontics, 44, 1210–1215. [DOI] [PubMed] [Google Scholar]
- Topçuoğlu, H.S. , Topçuoğlu, G. & Arslan, H. (2018b) The effect of different irrigation agitation techniques on postoperative pain in mandibular molar teeth with symptomatic irreversible pulpitis: a randomized clinical trial. Journal of Endodontics, 44, 1451–1456. [DOI] [PubMed] [Google Scholar]
- Torabinejad, M. , Shabahang, S. , Aprecio, R.M. & Kettering, J.D. (2003) The antimicrobial effect of MTAD: an in vitro investigation. Journal of Endodontics, 29, 400–403. [DOI] [PubMed] [Google Scholar]
- Townsend, C. & Maki, J. (2009) An in vitro comparison of new irrigation and agitation techniques to ultrasonic agitation in removing bacteria from a simulated root canal. Journal of Endodontics, 35, 1040–1043. [DOI] [PubMed] [Google Scholar]
- Ulusoy, Ö.I. , Savur, I.G. , Alaçam, T. & Çelik, B. (2018) The effectiveness of various irrigation protocols on organic tissue removal from simulated internal resorption defects. International Endodontic Journal, 51, 1030–1036. [DOI] [PubMed] [Google Scholar]
- Varela, P. , Souza, E. , de Deus, G. , Duran‐Sindreu, F. & Mercadé, M. (2019) Effectiveness of complementary irrigation routines in debriding pulp tissue from root canals instrumented with a single reciprocating file. International Endodontic Journal, 52, 475–483. [DOI] [PubMed] [Google Scholar]
- Vasiliadis, L. , Darling, A.I. & Levers, B.G. (1983a) The amount and distribution of sclerotic human root dentine. Archives of Oral Biology, 28, 645–649. [DOI] [PubMed] [Google Scholar]
- Vasiliadis, L. , Darling, A.I. & Levers, B.G. (1983b) The histology of sclerotic human root dentine. Archives of Oral Biology, 28, 693–700. [DOI] [PubMed] [Google Scholar]
- Venturi, M. , Prati, C. , Capelli, G. , Falconi, M. & Breschi, L. (2003) A preliminary analysis of the morphology of lateral canals after root canal filling using a tooth‐clearing technique. International Endodontic Journal, 36, 54–63. [DOI] [PubMed] [Google Scholar]
- Vera, J. , Arias, A. & Romero, M. (2012a) Dynamic movement of intracanal gas bubbles during cleaning and shaping procedures: the effect of maintaining apical patency on their presence in the middle and cervical thirds of human root canals‐an in vivo study. Journal of Endodontics, 38, 200–203. [DOI] [PubMed] [Google Scholar]
- Vera, J. , Siqueira, J.F. Jr. , Ricucci, D. , Loghin, S. , Fernández, N. , Flores, B. et al. (2012b) One‐ versus two‐visit endodontic treatment of teeth with apical periodontitis: a histobacteriologic study. Journal of Endodontics, 38, 1040–1052. [DOI] [PubMed] [Google Scholar]
- Verhaagen, B. , Boutsioukis, C. , Heijnen, G.L. , van der Sluis, L. & Versluis, M. (2012a) Role of the confinement of a root canal on jet impingement during endodontic irrigation. Experiments in Fluids, 53, 1841–1853. [Google Scholar]
- Verhaagen, B. , Boutsioukis, C. , van der Sluis, L.W. & Versluis, M. (2014a) Acoustic streaming induced by an ultrasonically oscillating endodontic file. Journal of the Acoustical Society of America, 135, 1717–1730. [DOI] [PubMed] [Google Scholar]
- Verhaagen, B. , Boutsioukis, C. , Sleutel, C.P. , Kastrinakis, E. , van der Sluis, L. & Versluis, M. (2014b) Irrigant transport into dental microchannels. Microfluidics and Nanofluidics, 16, 1165–1177. [Google Scholar]
- Verhaagen, B. , Lea, S.C. , de Bruin, G.J. , van der Sluis, L.W. , Walmsley, A.D. & Versluis, M. (2012b) Oscillation characteristics of endodontic files: numerical model and its validation. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 59, 2448–2459. [DOI] [PubMed] [Google Scholar]
- Versiani, M.A. , Alves, F.R.F. , Andrade‐Junior, C.V. , Marceliano‐Alves, M.F. , Provenzano, J.C. , Rôças, I.N. et al. (2016) Micro‐CT evaluation of the efficacy of hard tissue removal from the root canal and isthmus area by positive and negative pressure irrigation systems. International Endodontic Journal, 49, 1079–1087. [DOI] [PubMed] [Google Scholar]
- Versiani, M.A. , De‐Deus, G. , Vera, J. , Souza, E. , Steier, L. , Pécora, J.D. et al. (2015) 3D mapping of the irrigated areas of the root canal space using micro‐computed tomography. Clinical Oral Investigations, 19, 859–866. [DOI] [PubMed] [Google Scholar]
- Versluis, M. (2013) High‐speed imaging in fluids. Experiments in Fluids, 54, 1458. [Google Scholar]
- Vianna, M.E. , Horz, H.P. , Gomes, B.P. & Conrads, G. (2006) In vivo evaluation of microbial reduction after chemo‐mechanical preparation of human root canals containing necrotic pulp tissue. International Endodontic Journal, 39, 484–492. [DOI] [PubMed] [Google Scholar]
- Vidas, J. , Snjaric, D. , Braut, A. , Carija, Z. , Persic Bukmir, R. , De Moor, R.J.G. et al. (2020) Comparison of apical irrigant solution extrusion among conventional and laser‐activated endodontic irrigation. Lasers in Medical Science, 35, 205–211. [DOI] [PubMed] [Google Scholar]
- Villalta‐Briones, N. , Baca, P. , Bravo, M. , Solana, C. , Aguado‐Pérez, B. , Ruiz‐Linares, M. et al. (2021) A laboratory study of root canal and isthmus disinfection in extracted teeth using various activation methods with a mixture of sodium hypochlorite and etidronic acid. International Endodontic Journal, 54, 268–278. [DOI] [PubMed] [Google Scholar]
- Violante, T.L. , Haase, E.M. & Vickerman, M.M. (2013) Collagen‐binding streptococcal surface proteins influence the susceptibility of biofilm cells to endodontic antimicrobial solutions. Journal of Endodontics, 39, 370–374. [DOI] [PubMed] [Google Scholar]
- Wagner, M. & Horn, H. (2017) Optical coherence tomography in biofilm research: a comprehensive review. Biotechnology and Bioengineering, 114, 1386–1402. [DOI] [PubMed] [Google Scholar]
- Wang, R. , Shen, Y.A. , Ma, J. , Huang, D. , Zhou, X. , Gao, Y. et al. (2015) Evaluation of the effect of needle position on irrigant flow in the C‐shaped root canal using a Computational Fluid Dynamics model. Journal of Endodontics, 41, 931–936. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Shen, Y. & Haapasalo, M. (2012) Effectiveness of endodontic disinfecting solutions against young and old Enterococcus faecalis biofilms in dentin canals. Journal of Endodontics, 38, 1376–1379. [DOI] [PubMed] [Google Scholar]
- Weiger, R. , Bartha, T. , Kalwitzki, M. & Löst, C. (2006) A clinical method to determine the optimal apical preparation size. Part I. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 102, 686–691. [DOI] [PubMed] [Google Scholar]
- Westerweel, J. (1993) Digital particle image velocimetry ‐ theory and application. PhD Thesis. Delft, The Netherlands: Technical University of Delft, pp. 2–120. [Google Scholar]
- White, J.M. , Goodis, H.E. , Marshall, S.J. & Marshall, G.W. (1994) Sterilization of teeth by gamma radiation. Journal of Dental Research, 73, 1560–1567. [DOI] [PubMed] [Google Scholar]
- Willershausen, I. , Wolf, T.G. , Schmidtmann, I. , Berger, C. , Ehlers, V. , Willershausen, B. et al. (2015) Survey of root canal irrigating solutions used in dental practices within Germany. International Endodontic Journal, 48, 654–660. [DOI] [PubMed] [Google Scholar]
- Wilson, C.E. , Cathro, P.C. , Rogers, A.H. , Briggs, N. & Zilm, P.S. (2015) Clonal diversity in biofilm formation by Enterococcus faecalis in response to environmental stress associated with endodontic irrigants and medicaments. International Endodontic Journal, 48, 210–219. [DOI] [PubMed] [Google Scholar]
- Wright, P.P. , Cooper, C. , Kahler, B. & Walsh, L.J. (2021) Multiple assessment methodologies in determining the antibiofilm actions of sodium hypochlorite mixed with clodronate or etidronate in endodontic irrigation. Journal of Microbiological Methods, 180, 106107. [DOI] [PubMed] [Google Scholar]
- Wu, M.K. , Barkis, D. , Roris, A. & Wesselink, P.R. (2002) Does the first file to bind correspond to the diameter of the canal in the apical region? International Endodontic Journal, 35, 264–267. [DOI] [PubMed] [Google Scholar]
- Wu, M.K. & Wesselink, P.R. (1993) Endodontic leakage studies reconsidered. Part I. Methodology, application and relevance. International Endodontic Journal, 26, 37–43. [DOI] [PubMed] [Google Scholar]
- Xu, J. , He, J. , Shen, Y.A. , Zhou, X. , Huang, D. , Gao, Y. et al. (2019) Influence of endodontic procedure on the adherence of Enterococcus faecalis . Journal of Endodontics, 45, 943–949. [DOI] [PubMed] [Google Scholar]
- Yamada, R.S. , Armas, A. , Goldman, M. & Lin, P.S. (1983) A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: part 3. Journal of Endodontics, 9, 137–142. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Shen, Y.A. , Wang, Z. , Huang, X. , Maezono, H. , Ma, J. et al. (2016) Evaluation of the susceptibility of multispecies biofilms in dentinal tubules to disinfecting solutions. Journal of Endodontics, 42, 1246–1250. [DOI] [PubMed] [Google Scholar]
- Yassen, G.H. , Platt, J.A. & Hara, A.T. (2011) Bovine teeth as substitute for human teeth in dental research: a review of literature. Journal of Oral Science, 53, 273–282. [DOI] [PubMed] [Google Scholar]
- Ye, W.‐H. , Yeghiasarian, L. , Cutler, C.W. , Bergeron, B.E. , Sidow, S. , Xu, H.H.K. et al. (2019) Comparison of the use of d‐enantiomeric and l‐enantiomeric antimicrobial peptides incorporated in a calcium‐chelating irrigant against Enterococcus faecalis root canal wall biofilms. Journal of Dentistry, 91, 103231. [DOI] [PubMed] [Google Scholar]
- Yost, R.A. , Bergeron, B.E. , Kirkpatrick, T.C. , Roberts, M.D. , Roberts, H.W. , Himel, V.T. et al. (2015) Evaluation of 4 different irrigating systems for apical extrusion of sodium hypochlorite. Journal of Endodontics, 41, 1530–1534. [DOI] [PubMed] [Google Scholar]
- Young, G. , Turner, S. , Davies, J.K. , Sundqvist, G. & Figdor, D. (2007) Bacterial DNA persists for extended periods after cell death. Journal of Endodontics, 33, 1417–1420. [DOI] [PubMed] [Google Scholar]
- Zahran, S. , Witherden, E. , Mannocci, F. & Koller, G. (2021) Characterization of root canal microbiota in teeth diagnosed with irreversible pulpitis. Journal of Endodontics, 47, 415–423. [DOI] [PubMed] [Google Scholar]
- Zandi, H. , Kristoffersen, A.K. , Ørstavik, D. , Rôças, I.N. , Siqueira, J.F. Jr. & Enersen, M. (2018) Microbial analysis of endodontic infections in root‐filled teeth with apical periodontitis before and after irrigation using pyrosequencing. Journal of Endodontics, 44, 372–378. [DOI] [PubMed] [Google Scholar]
- Zandi, H. , Petronijevic, N. , Mdala, I. , Kristoffersen, A.K. , Enersen, M. , Rôças, I.N. et al. (2019) Outcome of endodontic retreatment using 2 root canal irrigants and influence of infection on healing as determined by a molecular method: a randomized clinical trial. Journal of Endodontics, 45, 1089–1098.e5. [DOI] [PubMed] [Google Scholar]
- Zandi, H. , Rodrigues, R.C.V. , Kristoffersen, A.K. , Enersen, M. , Mdala, I. , Ørstavik, D. et al. (2016) Antibacterial effectiveness of 2 root canal irrigants in root‐filled teeth with infection: a randomized clinical trial. Journal of Endodontics, 42, 1307–1313. [DOI] [PubMed] [Google Scholar]
- Zehnder, M. (2006) Root canal irrigants. Journal of Endodontics, 32, 389–398. [DOI] [PubMed] [Google Scholar]
- Zehnder, M. (2012) Editorial. International Endodontic Journal, 45, 961–962. [DOI] [PubMed] [Google Scholar]
- Zehnder, M. & Guggenheim, B. (2009) The mysterious appearance of enterococci in filled root canals. International Endodontic Journal, 42, 277–287. [DOI] [PubMed] [Google Scholar]
- Zehnder, M. , Kosicki, D. , Luder, H. , Sener, B. & Waltimo, T. (2002) Tissue‐dissolving capacity and antibacterial effect of buffered and unbuffered hypochlorite solutions. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 94, 756–762. [DOI] [PubMed] [Google Scholar]
- Zehnder, M. & Paqué, F. (2011) Disinfection of the root canal system during root canal re‐treatment. Endodontic Topics, 19, 58–73. [Google Scholar]
- Zeng, Y. , Li, X. , Feng, Z. , Luo, J. & Zhang, L. (2020) Antimicrobial peptide GH12 as root canal irrigant inhibits biofilm and virulence of Enterococcus faecalis . International Endodontic Journal, 53, 948–961. [DOI] [PubMed] [Google Scholar]
- Zhu, W.‐C. , Gyamfi, J. , Niu, L.‐N. , Schoeffel, G.J. , Liu, S.‐Y. , Santarcangelo, F. et al. (2013) Anatomy of sodium hypochlorite accidents involving facial ecchymosis—a review. Journal of Dentistry, 41, 935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotta, T. , Guidone, A. , Tremonte, P. , Parente, E. & Ricciardi, A. (2012) A comparison of fluorescent stains for the assessment of viability and metabolic activity of lactic acid bacteria. World Journal of Microbiology and Biotechnology, 28, 919–927. [DOI] [PubMed] [Google Scholar]
- Zou, L. , Shen, Y. , Li, W. & Haapasalo, M. (2010) Penetration of sodium hypochlorite into dentin. Journal of Endodontics, 36, 793–796. [DOI] [PubMed] [Google Scholar]


