Abstract
Public health initiatives aim to improve health outcomes for populations by preventing disease and ill‐health consequences of environmental hazards and natural or human‐made disasters. Whilst public health initiatives have been used successfully to modify behaviours for chronic diseases, many initiatives targeting reduced dementia risk in older adults suffer from conceptual and statistical flaws that greatly limit their usefulness. The limited success in modifying lifestyle dementia risk factors has led us to fall short in building a successful roadmap to dementia risk reduction. Here we argue for adopting a population‐level, holistic approach to dementia risk reduction strategies across the lifespan. This approach is supplemented by 10 strategies that focus on improving social policies, harnessing existing policy, legislature and incentive schemes, and identifying feasible approaches to increase recreational and transport‐related physical activity to creating best practice health care that supports healthy brain ageing for all.
Keywords: dementia, geriatrics, Health policy, health services, public health
Policy Impact
Dementia is the greatest cause of disability in older Australians, with significant individual, societal, economical and health‐care impacts. This paper provides a brief overview of existing public health initiatives targeting dementia prevention, and argues that a complex, multi‐faceted, socio‐technical approach supported by the fiscal policy is required for translational change.
Practice Impact
This paper explores the topic of dementia prevention, and existing innovative interventions targeting awareness of dementia risk factors and reducing dementia risk. It builds on current knowledge by reframing existing theory and experimental studies to further apply effective initiatives that can work.
1. INTRODUCTION
Preventative health is a critical element of national dementia strategic health planning. 1 With the prevalence of dementia projected on current trends to reach 152 million people by 2050 and the cost of dementia care set to outstrip any other health condition, it is timely that dementia takes its place in national preventative health planning. 2 In 2017, the World Health Organisation presented their dementia global action plan and risk reduction guidelines. 3 In 2018, the United Kingdom introduced dementia risk as a part of the NHS Health Check programme, with GPs now able to discuss with patients how they can reduce their dementia risk, which is a promising start. In 2019, Australia commenced producing evidence‐based guidelines targeting better dementia care and dementia prevention with resources on communication and diagnosis readily available for primary‐care stakeholders. 4 However, further efforts in implementing dementia risk reduction in national health programs in many countries is still underway. 5 , 6
This lack of attention may be due to the perceptions that (i) dementia is seen as an aged care rather than a chronic health issue, (ii) dementia is a non‐curable condition where nothing can be done, and (iii) the need from the general public to be informed about a brain‐healthy lifestyle is not expediated. 7 Indeed, while most government expenditure on dementia justly relates to the care of people with dementia, there is limited recognition that prevention may play an important role in reducing the escalating costs of this major disease in the future. 7
In this article, we outline five common problems associated with adopting healthy brain lifestyles on a population level. We use examples from different countries and suggest possible strategies based on health‐care systems and implementation science principles to target improvements across the life course. Our core argument for prompting better outcomes relies on changes operating beyond individual control and focussing more on wider societal and policy changes.
2. BEHAVIOUR CHANGE INTERVENTIONS TARGETING BETTER BRAIN HEALTH MAY WORK
The Lancet Commission's work on Dementia Prevention, Intervention, and Care highlighted 14 modifiable dementia risk factors 2 , 8 and suggests that higher levels of education (early life), absence of hypertension, peripheral hearing loss or obesity and avoidance of excessive alcohol consumption (>21 units/week) or head injuries are protective factors against dementia during mid‐life. Smoking, poor mental health, diabetes, air pollution, physical inactivity and lack of social engagement are risk factors for the onset of dementia in later life. 2 , 8 While evidence has been inconclusive in establishing a link between a single biopsychosocial risk factor and dementia prevalence, the general consensus is that optimising protective and modifying behaviours throughout the lifespan may have an impact in reducing dementia prevalence, the associated burden of care, and costs in years to come. 2 , 8
Experimental studies aimed at addressing modifiable dementia risk factors include the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), 9 which was a large‐scale multi‐domain lifestyle intervention study that harnessed goal setting and monitoring within individually tailored programs for physical exercise, dietary counselling, cardiovascular risk management, cognitive training, and social support in group discussions about lifestyle changes to improve brain health. Despite finding modest improvements in cognition composite scores in research participants, the FINGER study was a very resource‐intensive intervention, expensive to deliver and unlikely to be implemented on a large scale. 9
Other prominent intervention trials targeting multiple risk factors have been less successful. The Multidomain Alzheimer Preventive Trial (MAPT study) in France applied cognitive training, counselling on nutrition and facilitated small group training, but found no improvement in cognition composite scores. The Prevention of Dementia by Intensive Vascular care (preDIVA study) in the Netherlands, which used nurse‐led advice on health behaviours targeting vascular risk factors, found no overall change in dementia incidence but a subgroup effect in participants with untreated or sub‐optimally treated hypertension. 10 Whilst the behavioural science behind the selection of these study interventions is not immediately apparent, these large‐scale studies have put on track the conversation and emphasis towards current applications of dementia prevention in a short timeframe. Indeed, within Australia, there are several multidomain interventions currently underway (e.g., Body Brain Life, 11 Maintain Your Brain, 12 Better Brains, 13 Brain Bootcamp 14 ).
Improving the implementation of evidence‐based practice and public health depends on effective behaviour change. 15 Behaviour change interventions, broadly defined as organised sets of activities designed to modify specified behaviour patterns, are fundamental to effective public health practice (for example, the Australian Government's ‘Slip Slop Slap’ scheme, 16 and the UK Government's ‘Change4Life’ programme 15 ). Despite the attraction, there are challenges underpinning the evidence base, as health problems have complex determinants, systems‐level changes to determinants of health often require years to implement and changing individual behaviours with public health programs is contingent on multiple factors. 15 , 17 In general, public health interventions targeting dementia risk reduction should be underpinned by a model of behaviour and the factors that influence it. This firstly requires an understanding of what people already know about dementia and the preventative risk factors 18 before developing an intervention guided by theory.
Public health campaigns utilising behaviour change techniques (BCTs) are often effective in addressing risk factors for chronic health conditions and may have contributed to declining dementia prevalence over the past decades in some high‐income countries, 19 but has not always been well chosen and operationalised to address dementia prevention. Awareness campaigns in Japan, the United Kingdom, and more recently in the Netherlands and Belgium have all sought to raise the understanding of dementia through education. 20 In the UK, mass media has been used to increase awareness and normalise dementia, and in the Netherlands a health promotion campaign used both mass media techniques and a neighbourhood‐focussed approach to increase awareness about dementia risk reduction in middle‐aged community‐dwelling individuals. 21 The health promotion campaign also adopted a mobile application allowing participants to complete a survey to identify their personalised dementia risk profile to motivate behaviour change. 21 In Australia, “Your Brain Matters” and “Mind Your Mind” campaigns have further highlighted the role of social marketing to bring about community awareness of dementia risk; however, they did not continue beyond the funding period.
Challenges that have arisen in previous campaigns include not effectively targeting the ‘at‐risk’ population, using ineffective BCTs or using BCTs ineffectively, or misunderstanding the public's awareness and information about health consequences. 8 , 9 Critically, earlier campaigns typically use a limited number of behaviour change techniques (out of 93 possible techniques), and, whilst communication or marketing is one possible policy category, to fully utilise BCTs effectively, recommended structural enablers of healthy behaviour such as system level changes through policy, legislation, and taxation are required. 3
3. TARGETED STEPS
Adopting system‐level changes presents many challenges, and the common reasons for these are highlighted in Table 1. A feasible approach is to implement a targeted dementia prevention intervention, supported by achievable fiscal changes, to successfully reduce dementia risk across the age span. Achieving these objectives will initially require measuring population dementia risk, evaluating outcomes of specific BCTs and a plan for a wide‐scale population‐level implementation. 2 Although dementia prevention recommendations have been recently described, 5 our strategies target reduced dementia risk on a population level and are intended for changes to the health‐care system (see Figure 1). The strategies are further predicated on the recognition that there is a societal responsibility to act and will require the involvement of governments, the private sector, researchers, and communities to implement. Our highlighted suggestions are based on effective system‐level behaviour changes that can promote healthy behaviour and are further described according to three main categories. In light of an environment that is limited in resources, these categories are provided in order, whereby implementation of the first category is likely to result in faster and more impactful change.
TABLE 1.
Five common problems associated with adopting healthy brain lifestyles on a population level
| 1. People (including health‐care professionals) generally are not aware of what the risk factors for dementia are 21 , 23 |
| 2. People want to change their behaviours but lack support for their motivations (e.g., being in an obesogenic environment) 24 |
| 3. Behaviour change interventions are poorly understood and not developed systematically by researchers and suffer from poor evaluation and minimal translation 24 |
| 4. The health issue is misunderstood and neglected at a policy level 25 |
| 5. Insufficient guidance provided to Governments on how to approach the issue 25 |
FIGURE 1.
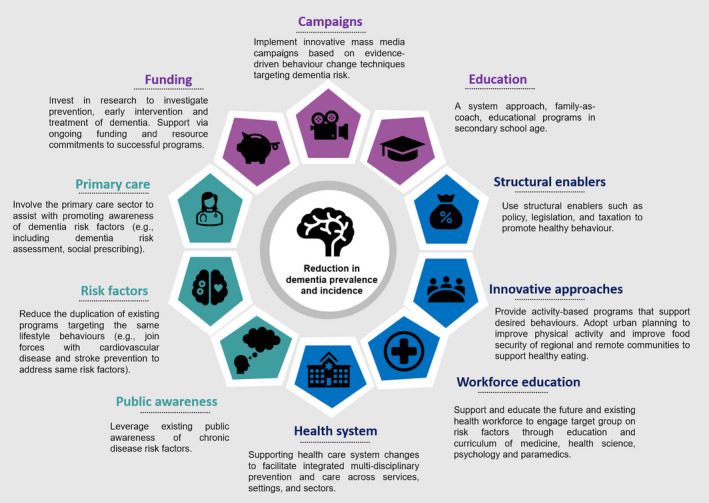
Three main approaches to achieving lower dementia risk reduction in older adults are presented. Purple represents adopting a population health approach, blue represents strategies towards building community capacity, and green represents how to integrate dementia prevention strategies and knowledge into existing public health behaviour change initiatives.
I) Adopting a population health approach to dementia 16 : There is a clear need to invest in research targeting prevention, early intervention and treatment of dementia. Whilst implementation of innovative mass media campaigns on dementia risk is a sensible approach, the effective framing of healthy brain ageing through healthy eating and/or physical activity messaging for parents and children, as well as specific messages for vulnerable groups and communities or other groups experiencing disproportionate harm is key.
At the very core, interventions need to utilise evidence‐based BCTs to reduce dementia risk and involve hard‐to‐reach groups (e.g., individuals with low health literacy, low SES, poor literacy) in the development of initiatives, as well as targeting middle‐aged individuals (40–75 years).
II) Building community capacity to optimise self‐management of risk factors 2 , 3 : Initiatives should use structural enablers such as policy, legislation, and taxation to promote healthy behaviour. For better diet, subsidising healthy foods, taxation of unhealthy foods (i.e., less nutritional value and are high in fat, sugar, and calories) alongside policy changes to regulate pricing of food and improving food security for at risk‐populations will reduce economic barriers to health eating. 3 For increased cognitive activity, better and equal access to education for all individuals is also crucial, especially for low socioeconomic families and individuals residing in rural locations. Adopting a system approach, family‐as‐coach, educational programs in secondary school age will lead to more accurate health beliefs and knowledge, and thus to better lifestyle choices. Furthermore, the stimulation of work can potentially have positive effects for cognitive health, and considerations around the retirement age or increasing opportunities for retired adults to keep cognitively active through community and social programs could further delay the onset of dementia.
To target better physical activity, adopting urban planning strategies (e.g., increase bicycle paths, footpaths, and covered landscapes) as well as additional contributions to subsidised or free public transport for older adults can facilitate access to public facilities such as senior parks, swimming pools, and sporting equipment, which can encourage physical activity. 3 Activity‐based programs that support desired behaviours for older adults should also be provided.
As behavioural risk factors are influenced by social determinants of health, supporting health‐care system changes to facilitate integrated multi‐disciplinary prevention and care across services, settings, and sectors is needed. Governments should support and educate the future and existing health workforce to engage target groups on risk factors through education and curriculum of medicine, health science, psychology and paramedics. We need to take an integrated healthcare approach by building partnerships between healthcare services in the public, private, and community sectors to facilitate a greater support of healthcare initiatives and choice for consumers accessing care. At the very basic level this approach should include communicating interventions to decision‐makers and the public. At the next level, measuring public health campaign reach, increases in referrals and real‐time monitoring and feedback about what works is absolutely paramount. The continual support through ongoing funding and resource commitments to successful programs is also necessary.
III) Integrate dementia prevention strategies and knowledge into existing public health behaviour change initiatives 3 , 6 : The majority of the Australian public recognise the symptoms of dementia and believe that dementia risk can be reduced. However, most are unaware of the association between dementia risk and cardiovascular factors. 18 We need to leverage existing public awareness of chronic disease risk factors, reduce the duplication of existing programs targeting the same lifestyle behaviours and join forces with cardiovascular disease and stroke prevention programs to address same risk factors. Such an approach will improve cost‐effectiveness of public health interventions by addressing risk factors for multiple stakeholders.
Connecting primary care stakeholders (e.g., general practitioners) to promote awareness of dementia risk factors for patients is vital. Actions could involve including dementia risk assessment within primary care by making it a part of the incentives scheme and employing social prescribed practices to help improve patients’ health and well‐being.
3.1. Ongoing evaluation of progress remains key
The extent to which a multi‐faceted approach envisaged here has achieved its potential, both in terms of process outcomes (e.g., whether newly and more widely applied preventive services have been used) and in terms of measurable, cost‐effective health improvement, is essential. Once the approach is determined efficacious, the next step is to address how the strategy can be effectively integrated into real‐world public health and clinical service systems. Implementation science will be required to untangle any complex, multiple evidence‐based intervention, identify strategies to encourage the provision and use of effective initiatives, promote the integration of evidence into policy and program decisions with the goal of adapting interventions to a range of diverse populations and settings, and identify approaches for scaling up effective initiatives to improve its delivery. 22
4. CONCLUSIONS
Reducing dementia risk in middle‐aged and older adults will inevitably be a complex, multi‐faceted, socio‐technical intervention that requires careful attention to design, implementation, and evaluation. Behaviour change interventions, whilst producing favourable outcomes in other public health areas, are complex and require many features in order to be successful and adhere fidelity. Public health initiatives to reduce dementia risk in adults require a greater understanding of the personal, environmental, cultural, and political determinants that influence health, and we still have a way to go. However, we are now in a unique position to leverage international research strengths and enhance partnerships between government, community, and private sector groups to deliver effective dementia mitigation in the future.
CONFLICTS OF INTEREST
The authors declare that Joyce Siette is Associate Editor for the Australasian Journal of Ageing. All authors declare no other relationships, including financial or professional, which may pose a competing interest. No conflicts of interest declared.
ACKNOWLEDGEMENTS
Open access publishing facilitated by Macquarie University, as part of the Wiley ‐ Macquarie University agreement via the Council of Australian University Librarians.
Siette J, Taylor N, Deckers K, et al. Advancing Australian public health initiatives targeting dementia risk reduction. Australas J Ageing. 2022;41:e190–e195. doi: 10.1111/ajag.13049
Funding information
None.
[Correction added on 10 May, after first online publication: CAUL funding statement has been added.]
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated.
REFERENCES
- 1. World Health Organization . Dementia: A Public Health Priority. World Health Organization; 2012. [Google Scholar]
- 2. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. The Lancet. 2017;390(10113):2673‐2734. [DOI] [PubMed] [Google Scholar]
- 3. Alzheimer's Disease International, Patterson C . World Alzheimer Report 2018. Alzheimer's Disease International, Patterson C; 2018. [Google Scholar]
- 4. Pond D, Phillips J, Day J, McNeill K, Evans L, Trollor J, Trollor J, Anstey KJ, Peters R. People with Dementia: A Care Guide for General Practice. 2019. NHMRC Partnership Centre for Dealing with Cognitive and Related Functional Decline in Older People (CDPC), Australia.
- 5. Chong TW, Macpherson H, Schaumberg MA, et al. Dementia prevention: the time to act is now. Med J Aust. 2021;214(7):302‐304.e1. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization . Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. World Health Organization; 2019. [PubMed] [Google Scholar]
- 7. Collins R, Silarova B, Clare L. Dementia primary prevention policies and strategies and their local implementation: A scoping review using England as a case study. J Alzheimers Dis. 2019;70:S303‐S318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. The Lancet. 2015;385(9984):2255‐2263. [DOI] [PubMed] [Google Scholar]
- 10. Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain interventions to prevent cognitive impairment, alzheimer's disease, and dementia: From FINGER to world‐wide FINGERS. J Prevent Alzheimer's Dis. 2020;7(1):29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McMaster M, Kim S, Clare L, Torres SJ, D'Este C, Anstey KJ. Body, Brain, Life for Cognitive Decline (BBL‐CD): protocol for a multidomain dementia risk reduction randomized controlled trial for subjective cognitive decline and mild cognitive impairment. Clin Interv Aging. 2018;13:2397‐2406. doi: 10.2147/CIA.S182046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heffernan M, Andrews G, Fiatarone Singh MA, et al. Maintain your brain: Protocol of a 3‐year randomized controlled trial of a personalized multi‐modal digital health intervention to prevent cognitive decline among community dwelling 55 to 77 year olds. J Alzheimers Dis. 2019;70(s1):S221‐S237. doi: 10.3233/JAD-180572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Better Brains . https://www.betterbrains.org.au/ Accessed January 25, 2022
- 14. Brain Bootcamp . https://www.brainbootcamp.com.au Accessed January 25, 2022
- 15. Michie S, van Stralen MM, West R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kite J, Thomas M, Grunseit A, Li V, Bellew W, Bauman A. Results of a mixed methods evaluation of the Make Healthy Normal campaign. Health Educ Res. 2020;35(5):418‐436. [DOI] [PubMed] [Google Scholar]
- 17. Wrieden WL, Levy LB. ‘Change4Life Smart Swaps’: quasi‐experimental evaluation of a natural experiment. Public Health Nutr. 2016;19(13):2388‐2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steyaert J, Deckers K, Smits C, et al. Putting primary prevention of dementia on everybody's agenda. Aging Ment Health. 2021;25(8):1376‐1380. [DOI] [PubMed] [Google Scholar]
- 19. Horstkötter D, Deckers K, Köhler S. Primary prevention of dementia: an ethical review. J Alzheimers Dis. 2021;79(2):467‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolters FJ, Chibnik LB, Waziry R, et al. Twenty‐seven‐year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology. 2020;95(5):e519‐e531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Asbroeck S, van Boxtel MPJ, Steyaert J, et al. Increasing knowledge on dementia risk reduction in the general population: Results of a public awareness campaign. Prev Med. 2021;147:106522. [DOI] [PubMed] [Google Scholar]
- 22. Heger I, Deckers K, van Boxtel M, et al. Dementia awareness and risk perception in middle‐aged and older individuals: baseline results of the MijnBreincoach survey on the association between lifestyle and brain health. BMC Public Health. 2019;19(1):678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Braithwaite J, Churruca K, Long JC, Ellis LA, Herkes J. When complexity science meets implementation science: a theoretical and empirical analysis of systems change. BMC Med. 2018;16(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parial LL, Lam SC, Ho JYS, Suen LKP, Leung AYM. Public knowledge of the influence of modifiable cardiovascular risk factors on dementia: a systematic literature review and meta‐analysis. Aging Ment Health. 2021;25(8):1395‐1409. [DOI] [PubMed] [Google Scholar]
- 25. Cations M, Radisic G, Crotty M, Laver KE. What does the general public understand about prevention and treatment of dementia? A systematic review of population‐based surveys. PLoS One. 2018;13(4):e0196085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated.


