Abstract
A powerful motivation to seek opioids remains after drug cessation and intensifies during extended periods of abstinence. Unfortunately, biomarkers associated with continued drug seeking have not been described. Moreover, previous studies have focused on the effects of early abstinence with little exploration into the long‐term drug‐induced mechanisms that occur after extended abstinence. Here we demonstrated that 30 days (D) of forced abstinence results in a time‐dependent increase in morphine seeking in a rat model of morphine self‐administration (SA). We measured expression of known drug‐responsive microRNAs (miRNAs) in the nucleus accumbens, an area critical for reward‐related plasticity, during early or late abstinence in animals that underwent either a cue‐induced relapse test or no relapse test. miRNAs are small noncoding RNAs that represent suitable biomarker candidates due to their long‐lasting nature. mir‐32‐5p levels during early abstinence negatively correlated with active lever pressing in both cue‐exposed and cue‐naïve animals. mir‐1298‐5p positively correlated with drug SA history after a relapse test during late abstinence. When animals underwent acute abstinence with no relapse test, mir‐1298‐5p correlated with drug infusions and active lever pressing during SA. In late abstinence with no relapse test, mir‐137‐3p negatively correlated with drug infusions. Regulation of mir‐32‐5p target genes and significant correlation of target gene mRNA with mir‐32‐5p was observed after abstinence. These results indicate that lasting regulation of miRNA expression is associated with drug intake following morphine SA. In addition, we conclude that the miRNA profile undergoes regulation from early to late abstinence and miRNA expression may indicate past drug history.
Keywords: craving, noncoding RNA, opioids, relapse, self‐administration
Abbreviations
- BTG Anti‐Proliferation Factor 2
(Btg2)
- CD69 Molecule
(Cd69)
- Claudin 11
(Cldn11)
- day
(D)
- DDB1 and CUL4 associated factor 6
(Dcaf6)
- Dual specificity phosphatase 5
(Dusp5)
- fixed ratio
(FR)
- microRNAs
(miRNAs)
- nucleus accumbens core
(NAc core)
- opioid use disorder
(OUD)
- Self‐administration
(SA)
- standard error of the mean
(SEM)
1. INTRODUCTION
Overdose deaths involving opioid narcotics are at an all‐time high in the United States (Hedegaard et al., 2020). While more than half of opioid use disorder (OUD) patients seek treatment each year, patients are plagued by high relapse rates, even long after seemingly successful rehabilitation (Center for Behavioral Health Statistics and Quality, 2018; Grella & Lovinger, 2011). Patients that undergo withdrawal from opioids experience many uncomfortable symptoms such as insomnia, irritability, anxiety, tachycardia and most importantly, a strong motivation to seek drug or cravings (Hodding et al., 1980). Although these overt withdrawal symptoms subside after detoxification, the cravings linger and can grow progressively stronger for a period of time, a phenomenon referred to as the ‘incubation of craving’ (Grimm et al., 2001; McHugh et al., 2014; Pickens et al., 2011). Exposure to drug‐associated cues and stressors during the drug‐free period precipitates drug craving in opioid‐dependent individuals (Saraiya et al., 2021). This perpetual drug‐seeking behaviour is a huge barrier to full recovery as our understanding of the molecular events that underlie craving at various stages of abstinence is extremely limited. However, rodent models of self‐administration (SA) have demonstrated incubation behaviour for various classes of drugs (Pickens et al., 2011) and represent a valuable tool to provide insight into the molecular mechanisms that sustain perseverant drug seeking.
In this study, we sought to characterise the relationship between microRNAs (miRNAs) and drug seeking for the opioid morphine after acute (1 day [D], 1D) or extended (30 days, 30D) forced abstinence in a rat model of morphine SA. miRNAs are a class of small, noncoding RNAs that repress protein translation through direct binding to the 3′‐UTR of target mRNAs and deadenylation (Bartel, 2004; Eulalio et al., 2009). With hundreds of predicted targets, a single miRNA has a wide degree of flexibility and genomic range, capable of modulating a host of cellular processes (Scott et al., 2012) and is poised to mediate complex behaviours (Hollander et al., 2010). The limited exploration into the role of microRNAs in substance use disorders has identified these small noncoding RNAs as both drug‐responsive and regulators of drug‐associated phenotypes. Functional manipulation of select miRNAs in discrete brain regions has demonstrated a role of miRNAs in modulating morphine analgesic tolerance (He et al., 2010; Hu et al., 2016). A handful of individual brain miRNAs have been shown to regulate seeking for alcohol, cocaine and opioids (Bastle et al., 2018; Hollander et al., 2010; Mavrikaki et al., 2019; Most et al., 2019; Tapocik et al., 2014; Xu et al., 2019, 2021; Yan et al., 2017). Exposure to opioid receptor agonists regulates miRNA expression in cultured neurons as well as brain regions implicated in the neuropathology of addiction, including the striatum, ventral tegmental area and hippocampus (He et al., 2010; Kim et al., 2018; Lu et al., 2014; Qiu et al., 2015; Sanchez‐Simon et al., 2010; Tapocik et al., 2013; Wu et al., 2013; Zheng et al., 2010). Some miRNAs, such as mir‐23b and mir‐339, appear to have regulatory feedback roles on opioid receptor expression after morphine treatment (Lu et al., 2014; Wu et al., 2009, 2013). These studies indicate that miRNAs participate in molecular signalling cascades to modulate both the behavioural phenotypes and the cellular neuroadaptations induced by opioid exposure. However, the relationship between the expression of miRNA during abstinence from opioids and drug‐seeking behaviour has not been described. Moreover, little is known about how the brain develops an increase for drug seeking during extended abstinence to promote relapse.
According to mirBase (Kozomara et al., 2019), more than 700 miRNAs are expressed in the mammalian brain and thus far only a small portion have been studied in OUD models. While a vast amount of work remains to be done to fully understand what noncoding RNAs do in the nervous system and in OUD, existing data from the literature have highlighted miRNAs that may contribute to drug‐induced plasticity during abstinence (see Kim et al., 2018; Quinn et al., 2018; Yan et al., 2017). In the current study, we chose six such miRNAs to investigate, based on their previously described opioid responsivity in the literature (Kim et al., 2018; Quinn et al., 2018; Yan et al., 2017), in the nucleus accumbens core (NAc core), a critical area for opioid‐induced molecular signalling (Sillivan et al., 2013) of animals that self‐administered morphine. We hypothesised that expression of these opioid‐responsive miRNAs may be associated with drug‐seeking behaviour or history of drug intake. We identified relationships between drug taking during SA and lasting changes in miRNA expression after both 1 and 30 D of abstinence. We conclude that abstinence from morphine SA induces long‐lasting regulation of unique sets of miRNAs at different stages of abstinence that are related to drug intake volume and motivation for drug.
2. METHODS
2.1. Subjects
Fifty‐seven adult male Sprague Dawley rats (60–180D old) were obtained from Taconic. Rats were pair‐housed in a reverse 12‐h light/dark cycle colony room for the duration of the experiments. All animals were handled daily for 2–5 min for at least 5D prior to the start of any behavioural procedure or test. Animals were fed ad libitum. All behavioural experiments and tissue collection were performed during the dark phase. All procedures followed the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and were approved by Temple University's Institutional Animal Care and Use Committee.
2.2. Drugs
Morphine sulphate (Spectrum Chemical, Gardena, CA) was dissolved in sterile 0.9% saline. The unit dose was 0.75 mg/kg/infusion for SA.
2.3. Intravenous surgery
Rats were anesthetised using an intraperitoneal injection of 80 mg/kg ketamine and 12 mg/kg xylazine prior to surgery. An indwelling silastic catheter was threaded subcutaneously over the shoulder blade, inserted in the jugular vein, and sutured in place. The catheter routed to a mesh back mount platform (Strategic Applications Inc., Lake Villa, IL) which was sutured below the skin between the shoulder blades. About 5 mg/kg of Meloxicam was administered subcutaneously daily after surgery for 3 D post‐operatively to relieve pain. Rats recovered in their colony room home cages for 7D prior to SA training. Catheters were flushed daily with 0.2 ml of timentin (0.93 mg/ml) dissolved in heparinised saline and sealed with plastic obturators when not in use, to prevent clogging.
2.4. Morphine SA
Following the 7D recovery period, rats were placed in operant chambers and underwent 10 daily sessions of 12 h/day of morphine SA (0.75 mg/kg/infusion over 5 s) on a fixed ratio 1 (FR1) schedule. The dosage and protocol for SA were chosen based on previous morphine SA studies in the literature (Madsen et al., 2012; Vassoler et al., 2017). Responses on the active lever resulted in one morphine infusion, accompanied by a 5‐s light cue. A subsequent 20‐s timeout period followed each infusion, during which the house light was off and lever presses were recorded but had no contingent drug infusion. An inactive lever with no programmed consequence was also present to determine whether animals could specifically associate the active lever with reward delivery. Yoked saline animals underwent catherisation surgery and received all identical contextual, visual and auditory cues during SA, but infusions of saline were delivered based on SA behaviour of morphine animals. Following 10D of morphine SA, all rats underwent either 1 or 30D of forced abstinence in a between‐subjects design. During the respective abstinence period, rats lived in their home cages in colony rooms with ad libitum access to food and water but had no access to the SA chambers nor any drug access. After 1 or 30D of abstinence, rats were either euthanised with no re‐exposure to the drug‐paired environment or exposed to a 60‐min relapse test in the SA chamber to assess changes in miRNA expression after cue re‐exposure. During the relapse test, responses on the previously drug‐paired active lever resulted in illumination of the cue light and activation of the drug pump but no infusion of morphine. Responses on the active lever were used to quantify craving. Animals that underwent the relapse test were euthanised immediately after the test. Three animals did not acquire morphine SA due to lack of patency and were excluded from the study.
2.5. NAc RNA extraction
Animals were euthanised by live decapitation with no anaesthesia after 1 or 30D of forced abstinence. Whole brains were quickly flash frozen. The NAc core was dissected by gross microdissection on dry ice. Total RNA was extracted from NAc core tissue using the Mirvana Paris Protein & RNA Isolation System (Thermo Fisher Scientific, Waltham, MA) according to manufacturer's instructions as previously described (Sillivan et al., 2019). The RNA was suspended in RNase free water, and the concentration of the RNA was measured using the Qubit HS Assay Kit (Life Technologies Corporation of Thermo Fisher Scientific, Frederick, Maryland). One animal was excluded from the molecular analysis due to low RNA yield.
2.6. Quantitative polymerase chain reaction (qPCR)
For measurement of miRNAs with qPCR, 50 ng of RNA was reverse transcribed into cDNA using the miRCURY LNA RT KIT (Qiagen) according to the manufacturer's instructions. cDNA was diluted 1:60 and used as a template for qPCR with the miRcury LNA SYBR Green PCR Kit (Qiagen) and the following LNA miRCury SYBR green assays (Qiagen): rno‐mir‐101b‐3p (Assay ID: YP00205072), rno‐mir‐32‐5p (Assay ID: YP00204792), rno‐mir‐24‐1‐5p (Assay ID: YP00204357), rno‐mir‐137‐3p (Assay ID: YP00206062), rno‐mir‐181a‐1‐3p (Assay ID: YP00204110) and rno‐mir‐1298‐5p (Assay ID: YP02108491). The small RNA Rnu5g (Assay ID: YP00203908) was used as an endogenous control gene. For mRNA qPCR, 25 ng of RNA was reverse transcribed into cDNA using qScript XLT cDNA SuperMix (Quantbio, Beverly, MA) in a MiniAmp Thermal Cycler (Thermo Fisher Scientific). cDNA was diluted 1:5 in nuclease free water and used as a template for qPCR reactions with Perfecta Fastmix II (Quantbio) and the following Taqman assays (Thermo Fisher Scientific): BTG Anti‐Proliferation Factor 2 (Btg2, Assay ID: Rn00568504_m1), CD69 Molecule (Cd69, Assay ID: Rn01459575_m1), Claudin 11 (Cldn11, Assay ID: Rn00584941_m1), DDB1 and CUL4 associated factor 6 (Dcaf6, Assay ID: Rn01491143_m1) and Dual specificity phosphatase 5 (Dusp5, Assay ID: Rn00592122_m1). The housekeeping gene Beta‐2‐microglobulin (B2m, Assay ID: Rn00560865) was used as endogenous control for mRNA. Expression levels were calculated using the 2−ΔΔCt method (Livak & Schmittgen, 2001) for individual biological replicate samples, and no samples were pooled. All reactions for each sample were performed in triplicate, and statistical analyses were performed on resulting ΔΔCt expression levels.
2.7. Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Two‐way analysis of variance (ANOVAs) were used to analyse the change in morphine consumption across days of intravenous SA, as well as potential differences in consumption between rats that eventually underwent 1D versus 30D of forced abstinence. Unpaired student's t tests were used to analyse differences between active lever presses during early and late abstinence cue tests. Pearson correlations were used to measure the relationship between the ΔΔCt of miRNA expression and morphine SA behaviour or mRNA expression of miRNA target genes. T tests were used to compare the difference in mir‐1298‐5p expression between high and low morphine SA animals and to compare miRNA or mRNA expression in saline versus morphine animals. A p‐value of less than 0.05 (p < 0.05) was considered statistically significant. All analyses were performed using the GraphPad software package (Prism version 8; GraphPad, San Diego, California, USA).
3. RESULTS
3.1. Extended forced abstinence results in incubation of morphine craving
Adult male rats self‐administered 0.75 mg/kg/infusion morphine in 12‐h daily sessions for 10 consecutive days (Figure 1). Animals increased the number of morphine infusions taken over 10D of SA, and a significant main effect of time was observed (two‐way ANOVA, main effect of time: [F 9,90 = 4.739, p < 0.0001]; Figure 1). Animals were matched for drug infusions administered during SA, and no significant differences were observed between the two forced abstinence groups (two‐way ANOVA, main effect of abstinence day: [F 1,50 = 2.079, p = 0.1556]; time by abstinence day interaction: [F 9,50 = 0.3777, p = 0.9404]; Figure 1). Active lever presses increased over 10D of SA, and there were no differences in active lever presses between animals that underwent 1D versus 30D of abstinence (two‐way ANOVA, main effect of time [F 9,144 = 3.401, p = 0.0008]; main effect of abstinence day [F 1,16 = 1.357, p = 0.2611]; time by abstinence day interaction [F 9,144 = 0.9577, p = 0.4776]; Figure 1). To determine time‐dependent alterations in cue‐induced drug‐seeking behaviours, animals underwent a single 60‐min relapse test in SA chambers on either abstinence day 1 or day 30 following the 10th SA session. The relapse test contained identical contextual and visual cues used during acquisition; however, active lever presses did not result in any morphine infusions. Consistent with literature on incubation of craving for other opioids (Airavaara et al., 2011; Gyawali et al., 2020; Reiner et al., 2020; Theberge et al., 2013; Zanda et al., 2021), extended forced abstinence from morphine resulted in a time‐dependent increase in drug‐seeking behaviour, as measured by a significant increase in active lever presses at 30D (unpaired t test, 1D vs. 30D relapse test: t(14) = 4.405, p = 0.0006; Figure 1). Subsequent analyses were performed with this experimental group to provide insight into how miRNA expression is affected by cue re‐exposure in early and late abstinence.
FIGURE 1.
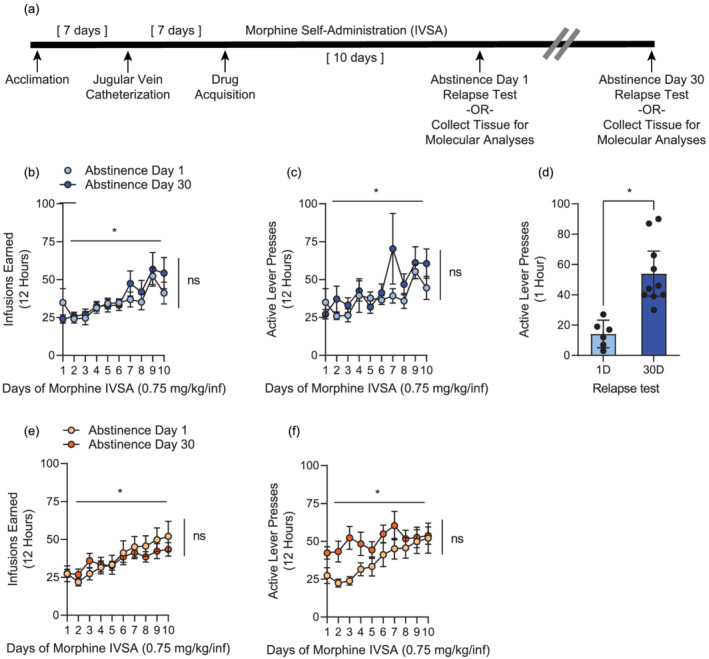
Self‐administration (SA) and incubation of morphine craving behaviour. (a) Experimental timeline of intravenous morphine SA, followed by either 1 or 30 days (D) of forced abstinence. Molecular analyses were performed on separate groups of animals that did (b–d) or did not (e–f) undergo a relapse test. Shown are morphine infusions (b, e) and active lever pressing (c, f) over 10 daily SA sessions. (d) Active lever presses during a cue‐induced drug‐seeking test performed after 1 or 30D of forced abstinence. *p < 0.05. Error ± standard error of the mean (SEM). 1D relapse n = 6; 30D relapse n = 10; 1D no relapse n = 16, 30D no relapse n = 15
A separate group of animals underwent morphine SA followed by 1 or 30D of abstinence with no relapse test to examine whether there is a relationship between the magnitude of drug intake and miRNA expression at each of the abstinence time points. Animals increased the number of morphine infusions taken over 10D of SA, and a significant main effect of time was observed (two‐way ANOVA, main effect of time: [F 9,300 = 4.154, p < 0.0001]; Figure 1). Animals in these groups were also balanced for morphine infusion quantity, and no differences in morphine consumption were observed between animals that underwent 1D versus 30D of abstinence (two‐way ANOVA, main effect of abstinence day: [F 1,300 = 0.3512, p = 0.5539]; time by abstinence day interaction [F 9,300 = 0.5044, p = 0.8710]; Figure 1). In animals that did not undergo a relapse test, active lever presses increased over 10D of SA, and there was no significant difference between active lever pressing behaviour in rats that underwent 1D versus 30D of abstinence (two‐way ANOVA, main effect of time [F 9,270 = 5.505, p < 0.0001]; main effect of abstinence day [F 1,30 = 3.629, p = 0.0664]; time by abstinence day interaction [F 9,270 = 1.572, p = 0.1236]; Figure 1).
Animals were euthanised after the indicated period of abstinence, and NAc core tissue was collected to analyse the molecular profile of drug‐associated miRNAs after acute or extended abstinence.
3.2. miRNA expression correlates with drug history but not relapse behaviour after extended abstinence
To assess whether morphine‐seeking behaviour was associated with miRNA expression during forced abstinence, we used qPCR to measure the expression levels of six putative opioid‐responsive miRNAs (Kim et al., 2018; Quinn et al., 2018; Yan et al., 2017) in the NAc core of animals that underwent 1 or 30D of abstinence, either with (Table 1) or without (Table 2) a relapse test. In animals that underwent the relapse test at 1D of abstinence, we observed a significant positive correlation between ΔΔCt expression levels of mir‐32‐5p and the average active lever presses during 10D of SA (Pearson r = 867, p = 0.025; Table 1). No significant correlations were observed between miRNA expression or drug infusion quantity after 1D of forced abstinence. When animals underwent 30D of forced abstinence with a relapse test, we observed a significant negative relationship between ΔΔCt expression of mir‐1298‐5p and the average number of morphine infusions during SA (Pearson r = −0.688, p = 0.040; Table 1), indicating that higher amounts of morphine infusions are associated with an increase in mir‐1298‐5p expression. No significant correlations were observed between relapse test behaviour and miRNA expression at either time point.
TABLE 1.
Correlation between morphine self‐administration behaviour and miRNA expression after 1 or 30D of abstinence with cue relapse test
| Cue relapse test | Avg infusions, last 3D | D10 active LP | Avg active LP over 10D | Avg infusions over 10D | Relapse test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1D | 30D | 1D | 30D | 1D | 30D | 1D | 30D | 1D | 30D | ||
| rno‐mir‐24‐1‐5p | p‐value | 0.425 | 0.881 | 0.555 | 0.640 | 0.078 | 0.545 | 0.460 | 0.786 | 0.311 | 0.337 |
| Pearson r | −0.405 | −0.059 | 0.306 | −0.181 | 0.762 | 0.234 | −0.378 | −0.106 | −0.501 | 0.363 | |
| rno‐mir‐32‐5p | p‐value | 0.817 | 0.442 | 0.534 | 0.781 | * 0.025 | 0.542 | 0.999 | 0.420 | 0.407 | 0.827 |
| Pearson r | −0.123 | 0.294 | 0.322 | 0.109 | * 0.867 | 0.235 | 0.001 | 0.308 | −0.420 | 0.085 | |
| rno‐mir‐101b‐3p | p‐value | 0.248 | 0.170 | 0.539 | 0.133 | 0.143 | 0.300 | 0.231 | 0.058 | 0.147 | 0.703 |
| Pearson r | −0.560 | −0.500 | 0.318 | −0.541 | 0.673 | −0.389 | −0.577 | −0.650 | −0.668 | 0.149 | |
| rno‐mir‐137‐3p | p‐value | 0.272 | 0.125 | 0.482 | 0.163 | 0.142 | 0.764 | 0.230 | 0.052 | 0.179 | 0.183 |
| Pearson r | −0.537 | −0.551 | 0.361 | −0.507 | 0.674 | −0.117 | −0.577 | −0.663 | −0.631 | 0.488 | |
| rno‐mir‐181a‐3p | p‐value | 0.743 | 0.264 | 0.483 | 0.763 | 0.057 | 0.818 | 0.786 | 0.314 | 0.453 | 0.641 |
| Pearson r | −0.173 | 0.417 | 0.360 | 0.118 | 0.798 | 0.090 | −0.144 | 0.379 | −0.383 | −0.181 | |
| rno‐mir‐1298‐5p | p‐value | 0.340 | 0.097 | 0.380 | 0.149 | 0.163 | 0.183 | 0.241 | * 0.040 | 0.152 | 0.957 |
| Pearson r | −0.476 | −0.586 | 0.442 | −0.522 | 0.649 | −0.487 | −0.566 | * ‐0.688 | −0.663 | 0.021 | |
Note: The bold/italics represent statistical significance.
Defines statistical significance.
TABLE 2.
Correlation between morphine self‐administration behaviour and miRNA expression after 1 or 30D of abstinence and no relapse test
| No relapse test | Avg infusions, last 3D | D10 active LP | Avg active LP over 10D | Avg infusions over 10D | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1D | 30D | 1D | 30D | 1D | 30D | 1D | 30D | ||
| rno‐mir‐24‐1‐5p | p‐value | 0.443 | 0.062 | 0.259 | 0.654 | 0.119 | 0.755 | 0.497 | 0.099 |
| Pearson r | 0.207 | 0.493 | 0.300 | 0.126 | 0.406 | −0.088 | 0.183 | 0.442 | |
| Rno‐mir‐32‐5p | p‐value | 0.263 | 0.178 | * 0.030 | 0.822 | 0.248 | 0.823 | 0.589 | 0.079 |
| Pearson r | 0.309 | 0.368 | * 0.561 | 0.064 | 0.318 | 0.063 | 0.152 | 0.467 | |
| rno‐mir‐101b‐3p | p‐value | 0.648 | 0.102 | 0.807 | 0.649 | 0.949 | 0.810 | 0.613 | 0.172 |
| Pearson r | −0.124 | 0.438 | 0.066 | 0.128 | 0.017 | −0.068 | −0.137 | 0.372 | |
| rno‐mir‐137‐3p | p‐value | 0.750 | * 0.030 | 0.160 | 0.521 | 0.855 | 0.857 | 0.844 | 0.057 |
| Pearson r | 0.087 | * 0.560 | 0.369 | 0.180 | 0.050 | −0.051 | −0.053 | 0.502 | |
| rno‐mir‐181a‐3p | p‐value | 0.838 | 0.058 | 0.775 | 0.517 | 0.460 | 0.945 | 0.842 | 0.080 |
| Pearson r | 0.056 | 0.500 | 0.078 | 0.182 | 0.199 | 0.020 | 0.054 | 0.465 | |
| rno‐mir‐1298‐5p | p‐value | * 0.005 | 0.243 | 0.140 | 0.897 | * 0.014 | 0.795 | * 0.003 | 0.390 |
| Pearson r | * −0.682 | 0.322 | −0.399 | 0.036 | * −0.619 | −0.073 | * −0.707 | 0.239 | |
Note: The bold/italics represent statistical significance.
Defines statistical significance.
3.3. miRNA expression correlates with drug‐seeking history after acute and extended abstinence
We next examined miRNA expression in the NAc of animals that underwent 1 or 30D forced abstinence without a relapse test. After 1D of forced abstinence, the ΔΔCt expression levels of mir‐1298‐5p were negatively correlated with the average infusion number or active lever response over the 10 drug SA sessions as well as the average number of infusions in the last three drug sessions (average all infusions: Pearson's r = −0.707, p = 0.003; average active lever all sessions: Pearson's r = −0.619, p = 0.014; last three sessions: Pearson's r = −0.682, p = 0.005; Table 2). Conversely, ΔΔCt expression levels of mir‐32‐5p were positively correlated with the average number of active lever presses on the last day of SA after 1D of forced abstinence (Pearson's r = 0.561, p = 0.030; Table 1). After 30D of abstinence, these associations were no longer observed, but instead, mir‐137‐3p ΔΔCt expression levels were positively correlated with average infusions in the last three drug sessions (total infusions: Pearson's r = 0.560, p = 0.030; Table 1). No significant associations between drug SA behaviour and miRNA expression were observed for mir‐24‐1‐5p, mir‐101b‐3p or mir‐181a‐3p at any timepoint examined. The ΔΔCt values for all animals can be found in supporting information Table S1.
3.4. NAc mir‐1298‐5p is dose‐dependently regulated during abstinence
NAc ΔΔCt mir‐1298‐5p expression after 30D forced abstinence with a relapse test, as well as 1D forced abstinence with no relapse test, was significantly correlated to the total number of morphine infusions that animals received during SA (30D with relapse test: Pearson r = −0.688, p = 0.040; 1D, no relapse test: Pearson's r = −0.707, p = 0.003; Figure 2b). The strong relationship between mir‐1298‐5p expression and drug history at two abstinence timepoints suggested that this miRNA may be regulated in a dose‐dependent manner. To examine this potential miRNA‐morphine relationship, we segregated animals into two groups based on their average number of infusions during SA by performing a median split as previously described (Brabant et al., 2010; Puhl et al., 2009; Zanda et al., 2021). Animals that self‐administered less than the median (35 infusions) were considered ‘low morphine’, while animals that self‐administered more than the median were considered ‘high morphine’. The fold change expression level of NAc mir‐1298‐5p was significantly higher in ‘high morphine’ animals compared with ‘low morphine’ animals at 30D abstinence with a relapse test and 1D abstinence with no relapse test (unpaired t tests; 30D with relapse test: t(7) = 3.120, p = 0.017; 1D, no relapse test: t(13) = 3.059, p = 0.009; Figure 2c,d). However, this pattern was not observed in animals that underwent 30D forced abstinence with no relapse test (unpaired t test: t(13) = 0.415; Figure 2e).
FIGURE 2.
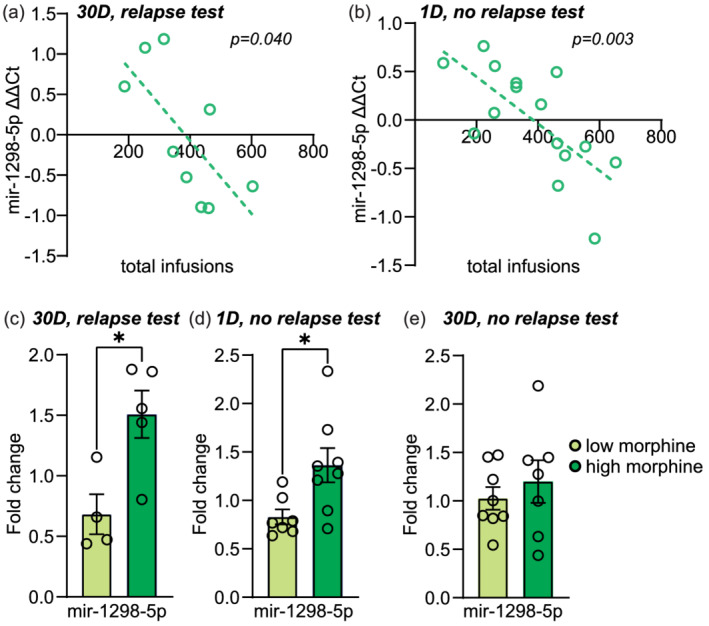
NAc mir‐1298‐5p expression during forced abstinence is associated with morphine history. Correlation analyses of calculated ΔΔCt mir‐1298‐5p expression in the NAc with the total number of morphine infusions over 10 days (D) of Self‐administration (SA) after 30D forced abstinence with a relapse test (a) or after 1D forced abstinence with no relapse test (b). The line of best fit is displayed with a dotted line. (c–e) Fold change values of mir‐1298‐5p expression in animals that self‐administered lower or higher amounts of morphine during SA at 30D forced abstinence with a relapse test (c), 1D abstinence with no relapse test (d) and 30D abstinence with no relapse test (e). *p < 0.05. Error ± standard error of the mean (SEM)
Lastly, we compared the expression of mir‐24‐1‐5p, mir‐32‐5p, mir‐101b‐3p, mir‐137‐3p, mir‐181a‐3p and mir‐1298‐5p in the NAc of morphine SA animals to that of yoked saline animals that also underwent 1 of 30D of forced abstinence with no relapse test. Expression levels of miRNAs in morphine treated animals did not differ from yoked saline animals (Figure 3); however, a trend of downregulation was observed in the morphine group for mir‐32‐5p (p = 0.08). Given the strong correlation between mir‐32‐5p and drug taking behaviour observed in Tables 1 and 2 and Figure 2, we measured the mRNA expression of downstream mir‐32‐5p target genes. mRNA expression of BTG Anti‐Proliferation Factor 2 (Btg2), CD69 Molecule (Cd69), Claudin 11 (Cldn11), DDB1 and CUL4 associated factor 6 (Dcaf6) and Dual specificity phosphatase 5 (Dusp5) was measured with qPCR in the NAc of animals that underwent either 1 or 30D of forced abstinence with no relapse test (supporting information Table S2 and Figure 3). Amplification of Cd69 could not be detected due to low levels of the gene in the NAc (data not shown). A significant upregulation of Btg2 was observed in animals that underwent 1D of forced abstinence from morphine relative to yoked saline animals (unpaired t test: t(8) = 2.40, p = 0.043; Figure 3). No significant differences were observed in levels of Cldn11, Dcaf6 or Dusp5 in animals that underwent 1D of forced abstinence from morphine. In animals that underwent 30D of forced abstinence, a significant downregulation of Dusp5 was observed in morphine animals (unpaired t test: t(7) = 3.70, p = 0.008; Figure 3). Similar nonsignificant trends of downregulation were observed in morphine animals for Btg2 (p = 0.08) and Cldn11 (p = 0.06). A significant correlation was observed between mir‐32‐5p ΔΔCt levels at 30D forced abstinence with mRNA ΔΔCt levels of Btg2 (Pearson r = 0.827, p = 0.011; Figure 3), Cldn11 (Pearson r = 0.718, p = 0.045; Figure 3) and Dcaf6 (Pearson r = 0.801, p = 0.017; Figure 3).
FIGURE 3.
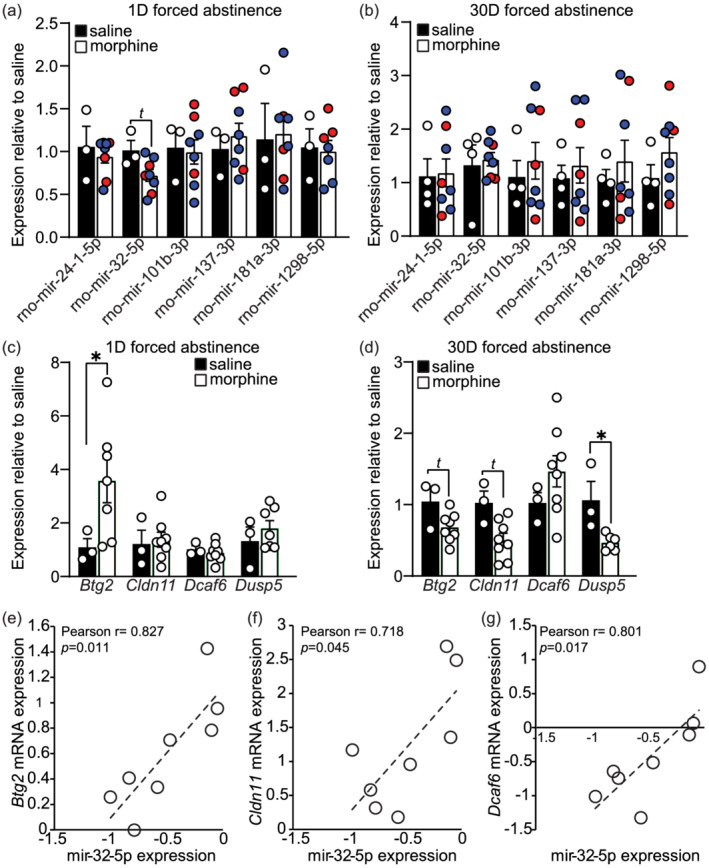
mir‐32‐5p target genes are regulated after forced abstinence and correlate with mir‐32‐5p expression levels. miRNA expression in yoked saline and morphine animals after 1 day (D) (a) or 30D (b) of forced abstinence with no relapse test. Displayed are fold change values relative to saline animals. Animals that self‐administered high amounts of morphine (35 + infusions per day) are indicated with red circles or low amounts of morphine (<35 infusions per day) are indicated with blue circles. (c, d) mRNA expression of predicted mir‐32‐5p target genes Btg2, Cldn11, Dcaf6, and Dusp5 in animals that received yoked saline or morphine SA and underwent 1D (c) or 30D (d) forced abstinence with no relapse test. (f, g) Correlation analyses of calculated ΔΔCt mir‐32‐5p expression in the NAc with ΔΔCt levels of the target genes Btg2 (e), Cldn11 (f), and Dcaf6 (g) in animals that underwent 30D of forced abstinence from morphine SA. t indicates trend for statistical significance 0.05 < p < 0.1. *p < 0.05. Error ± standard error of the mean (SEM)
4. DISCUSSION
Understanding the mechanisms of opioid seeking that underlie the continued motivation to use opioids, even after periods of abstinence, is critical for combating the opioid epidemic. Opioids induce adaptations in the brain that perpetuate the drive to seek more drug during abstinence in a time‐dependent manner, referred to as the incubation of craving (Pickens et al., 2011). Long‐lasting motivation to seek opioids has been well‐described for heroin, fentanyl and oxycodone (Altshuler et al., 2021; Gyawali et al., 2020; Reiner et al., 2020). However, few studies have described incubation of morphine‐seeking behaviour in rats after extended forced abstinence or the molecular mechanisms that sustain morphine‐seeking behaviour (Mayberry et al., 2022). Thus, the rat model of morphine SA can be used as a tool to investigate the molecular mechanisms that are affected during incubation.
We investigated morphine‐induced regulation of miRNAs during forced abstinence in the NAc, a brain region that mediates the rewarding properties of addictive substances (Volkow et al., 2019). We chose this region because previous studies have reported that overexpression of mir‐9 in the NAc impacts oxycodone SA, and manipulation of NAc mir‐181a can modulate incubation of heroin seeking after extended abstinence, indicating that regulation of miRNA expression in the NAc is sufficient to impact opioid seeking behaviour (Mavrikaki et al., 2019; Xu et al., 2021). miRNA changes in various brain regions, including the NAc, dorsal striatum and ventral tegmental area, have been reported after exposure to opioid receptor agonists and stimulants (Kim et al., 2018; Quinn et al., 2018; Yan et al., 2017). We used those studies as a guide to identify putative morphine‐responsive miRNAs in the NAc that were currently understudied in the literature. Among those, mir‐32‐5p was downregulated in the NAc of male animals by chronic intraperitoneal injections of heroin, although the timing of when the tissue was collected relative to drug exposure was not described (Yan et al., 2017). In contrast, chronic morphine SA upregulated mir‐32‐5p in the NAc of male animals 1D after the last drug session (Kim et al., 2018). Indeed, a study of miRNA expression quantitative loci (eQTL) in the mouse brain identified the genomic region of mir‐32‐3p as a significant eQTL and reported that addiction pathways are enriched in mir‐32 predicted target genes, including ‘morphine addiction’ (Kordas et al., 2019). Our data demonstrate a link between NAc mir‐32‐5p levels only after 1D of abstinence, only in the absence of cue exposure, and active lever pressing on the final day of morphine SA. This indicates that mir‐32‐5p expression may be a reflection of drug history only on the last day of morphine SA. Conversely, mir‐32‐5p levels in the 1D animals that underwent relapse only correlated with the average lever pressing over the entire 10D of SA. One interpretation of these findings is that the relapse test triggers a drug‐associated memory that encompasses the entire drug‐seeking experience that correlates with mir‐32‐5p, rather than only the last day of SA. These data, combined with the literature, indicate that NAc mir‐32‐5p may be responsive to opioids in general, but the route of administration may impact the direction. In addition, mir‐32‐5p regulation by morphine appears to be transient and not a long‐lasting marker of drug exposure.
mir‐1298‐5p expression in the NAc was positively associated with multiple measures of morphine seeking at both 1D and 30D abstinence and may represent a suitable biomarker of drug history. The correlation of mir‐1298‐5p with both infusions and active lever pressing indicates that this miRNA may be related to both the motivation to obtain the drug and the degree of drug intoxication. mir‐1298‐5p was regulated in a dose‐dependent manner, with significantly lower levels of mir‐1298‐5p observed in animals that self‐administered lower amounts of morphine. This pattern was not observed for any other miRNA examined in our study. Most intriguingly, mir‐1298‐5p expression remained correlated to morphine infusions at 30D abstinence only after a relapse test, indicating that re‐exposure to the drug‐paired environment may trigger a molecular memory response as this effect was not observed in animals that endured 30D forced abstinence with no relapse test. miRNA biogenesis can occur extremely rapidly, with some cells generating hundreds of mature miRNAs within minutes, which is orders of magnitude faster than mRNA synthesis (Reichholf et al., 2019). Additionally, some miRNAs, such as mir‐485‐5p, have been reported to be activity dependent on a short time scale (<1 h) and function within the synapse of the cell to participate in modulation of synaptic plasticity (Cohen et al., 2011). Thus, association of mir‐1298‐5p expression with drug history at 30D only after a 30D relapse test may be the result of rapid miRNA synthesis that was triggered by a memory response of the cue relapse test. This could also explain why no miRNAs examined were correlated with relapse behaviour but instead were correlated with drug history, as the relapse event results in changes in miRNA biogenesis and/or degradation. We selected mir‐1298‐5p for analyses in our study because it was downregulated in the NAc after chronic morphine SA in a short‐access mouse model (Kim et al., 2018). In contrast, our data suggest that longer access to morphine SA may result in higher levels of NAc mir‐1298‐5p that are associated with perseverant morphine‐seeking behaviour. Synaptic levels of mir‐1298‐5p were significantly decreased in both the ventral tegmental area and the substantia nigra of mice after chronic alcohol exposure, indicating that mir‐1298‐5p is present in the synaptoneurosomes compartment of neurons (Most et al., 2019) and may be poised to regulate drug‐seeking behaviour.
We observed a significant positive relationship between NAc mir‐137‐3p ΔΔCt levels and morphine drug infusions during the end of SA in animals that underwent 30D of forced abstinence. We did not observe this relationship at any other timepoint examined or when animals were exposed to a relapse test, indicating that mir‐137‐3p may also be a marker of that last morphine SA session only. Active lever pressing is considered to be a measure of an animal's motivation to obtain a drug, and therefore, correlation between mir‐137‐3p and infusions, instead of active lever pressing, may be a relationship between the drug intoxication level and the miRNA, rather than a relationship between motivation for drug seeking and the miRNA. mir‐137‐3p is a highly expressed neuron‐enriched miRNA (Jovičić et al., 2013) that can regulate the expression of the dopamine transporter to impact dopamine levels (Jia et al., 2016). In a model of cocaine SA, NAc core mir‐137‐3p levels were significantly decreased 3 weeks after the last drug session but upregulated in the NAc shell of animals that were classified as ‘addiction prone’ following extinction and cue reinstatement tests (Quinn et al., 2018). Thus, mir‐137‐3p may be responsive to both opioids and psychostimulants and appears to be consistently regulated long after drug exposure, during the drug‐free period.
Interestingly, none of the miRNAs measured were regulated compared with yoked saline animals. Given the strong correlations between morphine taking and miRNA expression reported here, we examined the possibility of dose‐dependent regulation of miRNAs by separating animals into high (35 + infusions per day) or low (<35 infusions per day) groups. While we observed some patterns of potential dose‐dependent regulation of miRNAs, no robust significant differences were observed. miRNAs are under tight homeostatic regulation and small changes in miRNAs can have large impacts on the hundreds to thousands of target genes that each miRNA regulates. With this in mind, future studies may require larger sample sizes to detect small differences in some miRNAs that are under tight homeostatic regulation. We utilised the miRNA target prediction website Targetscan to identify putative mir‐32‐5p targets for investigation into the regulation of the mir‐32‐5p pathway after morphine abstinence (Friedman et al., 2009). The majority of miRNA targets are based on predictive algorithms, determined by a two‐ to nine‐nucleotide consensus region on the target mRNA where the miRNA should bind (Leitão et al., 2014), with scarce experimental evidence to confirm such numerous interactions. miRNA‐mediated protein inhibition in mammalian cells is primarily mediated through a blockade of translation, with mRNA degradation thought to be a consequence and one that does not always occur (Bartel, 2004). After 1D forced abstinence, we observed significant elevation of the mir‐32‐5p target gene Btg2, and trend for downregulation of mir‐32‐5p in morphine animals, suggesting that decreased mir‐32‐5p may lead to elevated Btg2. After 30D forced abstinence, however, mir‐32‐5p was not regulated and we observed significant downregulation of the mir‐32‐5p target Dusp5. A significant positive relationship between mir‐32‐5p expression and Btg2, Cldn11 and Dcaf6 expression was observed in 30D morphine abstinent animals, suggesting that the more mir‐32‐5p expression present, the higher mRNA levels of these three predicted target genes. More recently, it has come to light that miRNAs are not always antagonistic regulators of gene expression and the relationship between a miRNA and its target genes is dependent upon the target gene sequence. Indeed, miRNAs can bidirectionally regulate gene expression, with reports of some targets upregulated by a miRNA and others downregulated (Vasudevan et al., 2007). We observed only significant upregulation of mir‐32‐5p target genes at the mRNA level and positive correlations between some target genes and mir‐32‐5p expression. Such upregulation of mir‐32‐5p target genes could be through (i) mir‐32‐5p mediated upregulation of mRNA, (ii) compensatory upregulation after initial mir‐32‐5p mediated downregulation or (iii) a morphine‐induced mir‐32‐5p‐independent mechanism.
The presence of persistent miRNA expression associated with history of drug seeking indicates that measurement of miRNAs may serve as a biomarker to monitor past versus current usage or reduced drug seeking due to treatment responsiveness. Circulating miRNA levels in the blood have been shown to respond to treatment with the opioids hydromorphone and oxycodone, as well as nicotine and methamphetamine (Banerjee et al., 2015; Li et al., 2018; Takahashi et al., 2013; Toyama et al., 2017; Zhao et al., 2016). However, it is important to first characterise the brain region‐specific miRNA pathways regulated by opioids, such as morphine, so that studies restricted to peripheral miRNA measurements can be useful and informative. To fully understand the role of these miRNAs in the incubation of morphine seeking, future studies should investigate their contribution to addiction phenotypes at multiple stages of drug seeking, including during the escalation phase and relapse to drug seeking.
CONFLICT OF INTEREST
The authors declare that we have no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors have made substantial contribution to the work described in this manuscript and approved it for publication.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15650.
Supporting information
Table S1. ΔΔCt values measured for miRNAs in the NAc following morphine or yoked saline self‐administration.
ACKNOWLEDGEMENTS
We thank Siani Faison for technical support in performing data collection. This work was supported by the following NIDA/NIH grants: T32DA007237 (AG), R00DA041469 (SS, AG) and DP1 DA046537 (MEW), K01 DA039308 (MEW).
Gillespie, A. , Mayberry, H. L. , Wimmer, M. E. , & Sillivan, S. E. (2022). microRNA expression levels in the nucleus accumbens correlate with morphine‐taking but not morphine‐seeking behaviour in male rats. European Journal of Neuroscience, 55(7), 1742–1755. 10.1111/ejn.15650
Aria Gillespie and Hannah L. Mayberry have equal contribution.
Edited by: Venetia Zachariou
Funding information NIDA/NIH, Grant/Award Numbers: T32DA007237, R00DA041469, DP1DA046537, K01DA039308
DATA AVAILABILITY STATEMENT
Data that support the findings in this study are available from the authors upon reasonable request.
REFERENCES
- Airavaara, M. , Pickens, C. L. , Stern, A. L. , Wihbey, K. A. , Harvey, B. K. , Bossert, J. M. , & Shaham, Y. (2011). Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addiction Biology, 16(2), 261–272. 10.1111/j.1369-1600.2010.00281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler, R. D. , Yang, E. S. , Garcia, K. T. , Davis, I. R. , Olaniran, A. , Haile, M. , Razavi, S. , & Li, X. (2021). Role of orbitofrontal cortex in incubation of oxycodone craving in male rats. Addiction Biology, 26(2), e12927. 10.1111/adb.12927 [DOI] [PubMed] [Google Scholar]
- Banerjee, A. , Waters, D. , Camacho, O. M. , & Minet, E. (2015). Quantification of plasma microRNAs in a group of healthy smokers, ex‐smokers and non‐smokers and correlation to biomarkers of tobacco exposure. Biomarkers, 20(2), 123–131. 10.3109/1354750X.2014.1000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel, D. P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell, 116(2), 281–297. 10.1016/s0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Bastle, R. M. , Oliver, R. J. , Gardiner, A. S. , Pentkowski, N. S. , Bolognani, F. , Allan, A. M. , Chaudhury, T. , Peter, M. S. , Galles, N. , Smith, C. , Neisewander, J. L. , & Perrone‐Bizzozero, N. I. (2018). In silico identification and in vivo validation of miR‐495 as a novel regulator of motivation for cocaine that targets multiple addiction‐related networks in the nucleus accumbens. Molecular Psychiatry, 23(2), 434–443. 10.1038/mp.2016.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant, C. , Kuschpel, A. S. , & Picciotto, M. R. (2010). Locomotion and self‐administration induced by cocaine in 129/OlaHsd mice lacking galanin. Behavioral Neuroscience, 124(6), 828–838. 10.1037/a0021221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. E. , Lee, P. R. , Chen, S. , Li, W. , & Fields, R. D. (2011). MicroRNA regulation of homeostatic synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America, 108(28), 11650–11655. 10.1073/pnas.1017576108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality . (2018). Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18‐5068, NSDUH Series H‐53). In. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/ [Google Scholar]
- Eulalio, A. , Huntzinger, E. , Nishihara, T. , Rehwinkel, J. , Fauser, M. , & Izaurralde, E. (2009). Deadenylation is a widespread effect of miRNA regulation. RNA, 15(1), 21–32. 10.1261/rna.1399509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, R. C. , Farh, K. K. , Burge, C. B. , & Bartel, D. P. (2009). Most mammalian mRNAs are conserved targets of microRNAs. Genome Research, 19(1), 92–105. 10.1101/gr.082701.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella, C. E. , & Lovinger, K. (2011). 30‐year trajectories of heroin and other drug use among men and women sampled from methadone treatment in California. Drug and Alcohol Dependence, 118(2–3), 251–258. 10.1016/j.drugalcdep.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, J. W. , Hope, B. T. , Wise, R. A. , & Shaham, Y. (2001). Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature, 412(6843), 141–142. 10.1038/35084134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyawali, U. , Martin, D. A. , Sulima, A. , Rice, K. C. , & Calu, D. J. (2020). Role of BNST CRFR1 receptors in incubation of fentanyl seeking. Frontiers in Behavioral Neuroscience, 14, 153. 10.3389/fnbeh.2020.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Yang, C. , Kirkmire, C. M. , & Wang, Z. J. (2010). Regulation of opioid tolerance by let‐7 family microRNA targeting the mu opioid receptor. The Journal of Neuroscience, 30(30), 10251–10258. 10.1523/jneurosci.2419-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard, H. , Miniño, A. M. , & Warner, M. (2020). Drug overdose deaths in the United States, 1999‐2019. NCHS Data Brief, (394), 1–8. [PubMed] [Google Scholar]
- Hodding, G. C. , Jann, M. , & Ackerman, I. P. (1980). Drug withdrawal syndromes‐‐ a literature review. The Western Journal of Medicine, 133(5), 383–391. [PMC free article] [PubMed] [Google Scholar]
- Hollander, J. A. , Im, H. I. , Amelio, A. L. , Kocerha, J. , Bali, P. , Lu, Q. , Willoughby, D. , Wahlestedt, C. , Conkright, M. D. , & Kenny, P. J. (2010). Striatal microRNA controls cocaine intake through CREB signalling. Nature, 466(7303), 197–202. 10.1038/nature09202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. M. , Cao, S. B. , Zhang, H. L. , Lyu, D. M. , Chen, L. P. , Xu, H. , Pan, Z. Q. , & Shen, W. (2016). Downregulation of miR‐219 enhances brain‐derived neurotrophic factor production in mouse dorsal root ganglia to mediate morphine analgesic tolerance by upregulating CaMKIIγ. Molecular Pain, 12, 1–12. 10.1177/1744806916666283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, X. , Wang, F. , Han, Y. , Geng, X. , Li, M. , Shi, Y. , Lu, L. , & Chen, Y. (2016). miR‐137 and miR‐491 negatively regulate dopamine transporter expression and function in neural cells. Neuroscience Bulletin, 32(6), 512–522. 10.1007/s12264-016-0061-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovičić, A. , Roshan, R. , Moisoi, N. , Pradervand, S. , Moser, R. , Pillai, B. , & Luthi‐Carter, R. (2013). Comprehensive expression analyses of neural cell‐type‐specific miRNAs identify new determinants of the specification and maintenance of neuronal phenotypes. The Journal of Neuroscience, 33(12), 5127–5137. 10.1523/jneurosci.0600-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Im, H. I. , & Moon, C. (2018). Intravenous morphine self‐administration alters accumbal microRNA profiles in the mouse brain. Neural Regeneration Research, 13(1), 77–85. 10.4103/1673-5374.224374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas, G. , Rudra, P. , Hendricks, A. , Saba, L. , & Kechris, K. (2019). Insight into genetic regulation of miRNA in mouse brain. BMC Genomics, 20(849). 10.1186/s12864-019-6110-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara, A. , Birgaoanu, M. , & Griffiths‐Jones, S. (2019). miRBase: From microRNA sequences to function. Nucleic Acids Research, 47(D1), D155–D162. 10.1093/nar/gky1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão, A. L. , Costa, M. C. , & Enguita, F. J. (2014). A guide for miRNA target prediction and analysis using web‐based applications. Methods in Molecular Biology, 1182, 265–277. 10.1007/978-1-4939-1062-5_23 [DOI] [PubMed] [Google Scholar]
- Li, H. , Li, C. , Zhou, Y. , Luo, C. , Ou, J. , Li, J. , & Mo, Z. (2018). Expression of microRNAs in the serum exosomes of methamphetamine‐dependent rats vs. ketamine‐dependent rats. Experimental and Therapeutic Medicine, 15(4), 3369–3375. 10.3892/etm.2018.5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K. J. , & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C[T]) Method. Methods, 25(4), 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu, Z. , Xu, J. , Xu, M. , Pasternak, G. W. , & Pan, Y. X. (2014). Morphine regulates expression of μ‐opioid receptor MOR‐1A, an intron‐retention carboxyl terminal splice variant of the μ‐opioid receptor (OPRM1) gene via miR‐103/miR‐107. Molecular Pharmacology, 85(2), 368–380. 10.1124/mol.113.089292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen, H. B. , Brown, R. M. , Short, J. L. , & Lawrence, A. J. (2012). Investigation of the neuroanatomical substrates of reward seeking following protracted abstinence in mice. The Journal of Physiology, 590(10), 2427–2442. 10.1113/jphysiol.2011.225219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrikaki, M. , Anastasiadou, E. , Ozdemir, R. A. , Potter, D. , Helmholz, C. , Slack, F. J. , & Chartoff, E. H. (2019). Overexpression of miR‐9 in the nucleus Accumbens increases oxycodone self‐administration. The International Journal of Neuropsychopharmacology, 22(6), 383–393. 10.1093/ijnp/pyz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry, H. L. , Bavley, C. C. , Karbalaei, R. , Peterson, D. R. , Bongiovanni, A. R. , Ellis, A. S. , Downey, S. H. , Toussaint, A. B. , & Wimmer, M. E. (2022). Transcriptomics in the nucleus accumbens shell reveal sex‐ and reinforcer‐specific signatures associated with morphine and sucrose craving. Neuropsychopharmacology. 10.1038/s41386-022-01289-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh, R. K. , Park, S. , & Weiss, R. D. (2014). Cue‐induced craving in dependence upon prescription opioids and heroin. The American Journal on Addictions, 23(5), 453–458. 10.1111/j.1521-0391.2014.12129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most, D. , Salem, N. A. , Tiwari, G. R. , Blednov, Y. A. , Mayfield, R. D. , & Harris, R. A. (2019). Silencing synaptic MicroRNA‐411 reduces voluntary alcohol consumption in mice. Addiction Biology, 24(4), 604–616. 10.1111/adb.12625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens, C. L. , Airavaara, M. , Theberge, F. , Fanous, S. , Hope, B. T. , & Shaham, Y. (2011). Neurobiology of the incubation of drug craving. Trends in Neurosciences, 34(8), 411–420. 10.1016/j.tins.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl, M. D. , Fang, J. , & Grigson, P. S. (2009). Acute sleep deprivation increases the rate and efficiency of cocaine self‐administration, but not the perceived value of cocaine reward in rats. Pharmacology, Biochemistry, and Behavior, 94(2), 262–270. 10.1016/j.pbb.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, S. , Feng, Y. , LeSage, G. , Zhang, Y. , Stuart, C. , He, L. , Li, Y. , Caudle, Y. , Peng, Y. , & Yin, D. (2015). Chronic morphine‐induced microRNA‐124 promotes microglial immunosuppression by modulating P65 and TRAF6. Journal of Immunology, 194(3), 1021–1030. 10.4049/jimmunol.1400106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, R. K. , James, M. H. , Hawkins, G. E. , Brown, A. L. , Heathcote, A. , Smith, D. W. , Cairns, M. J. , & Dayas, C. V. (2018). Temporally specific miRNA expression patterns in the dorsal and ventral striatum of addiction‐prone rats. Addiction Biology, 23(2), 631–642. 10.1111/adb.12520 [DOI] [PubMed] [Google Scholar]
- Reichholf, B. , Herzog, V. A. , Fasching, N. , Manzenreither, R. A. , Sowemimo, I. , & Ameres, S. L. (2019). Time‐resolved small RNA sequencing unravels the molecular principles of MicroRNA homeostasis. Molecular Cell, 75(4), 756, e757–768. 10.1016/j.molcel.2019.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner, D. J. , Lofaro, O. M. , Applebey, S. V. , Korah, H. , Venniro, M. , Cifani, C. , Bossert, J. M. , & Shaham, Y. (2020). Role of projections between piriform cortex and orbitofrontal cortex in relapse to fentanyl seeking after palatable food choice‐induced voluntary abstinence. The Journal of Neuroscience, 40(12), 2485–2497. 10.1523/jneurosci.2693-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Simon, F. M. , Zhang, X. X. , Loh, H. H. , Law, P. Y. , & Rodriguez, R. E. (2010). Morphine regulates dopaminergic neuron differentiation via miR‐133b. Molecular Pharmacology, 78(5), 935–942. 10.1124/mol.110.066837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiya, T. C. , Jarnecke, A. M. , Jones, J. , Brown, D. G. , Brady, K. T. , & Back, S. E. (2021). Laboratory‐induced stress and craving predict opioid use during follow‐up among individuals with prescription opioid use disorder. Drug and Alcohol Dependence, 225, 108755. 10.1016/j.drugalcdep.2021.108755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, H. L. , Tamagnini, F. , Narduzzo, K. E. , Howarth, J. L. , Lee, Y. B. , Wong, L. F. , Brown, M. W. , Warburton, E. C. , Bashir, Z. I. , Uney, J. B. , & Uney, J. B. (2012). MicroRNA‐132 regulates recognition memory and synaptic plasticity in the perirhinal cortex [research support, non‐U.S. Gov't]. The European Journal of Neuroscience, 36(7), 2941–2948. 10.1111/j.1460-9568.2012.08220.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillivan, S. E. , Jones, M. E. , Jamieson, S. , Rumbaugh, G. , & Miller, C. A. (2019). Bioinformatic analysis of long‐lasting transcriptional and translational changes in the basolateral amygdala following acute stress. PLoS ONE, 14(1), e0209846. 10.1371/journal.pone.0209846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillivan, S. E. , Whittard, J. D. , Jacobs, M. M. , Ren, Y. , Mazloom, A. R. , Caputi, F. F. , Horvath, M. , Keller, E. , Ma'ayan, A. , Pan, Y.‐X. , Chiang, L. W. , & Hurd, Y. L. (2013). ELK1 transcription factor linked to dysregulated striatal mu opioid receptor signaling network and OPRM1 polymorphism in human heroin abusers. Biological Psychiatry, 74(7), 511–519. 10.1016/j.biopsych.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K. , Yokota, S. , Tatsumi, N. , Fukami, T. , Yokoi, T. , & Nakajima, M. (2013). Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicology and Applied Pharmacology, 272(1), 154–160. 10.1016/j.taap.2013.05.018 [DOI] [PubMed] [Google Scholar]
- Tapocik, J. D. , Barbier, E. , Flanigan, M. , Solomon, M. , Pincus, A. , Pilling, A. , Sun, H. , Schank, J. R. , King, C. , & Heilig, M. (2014). microRNA‐206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. The Journal of Neuroscience, 34(13), 4581–4588. 10.1523/JNEUROSCI.0445-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik, J. D. , Luu, T. V. , Mayo, C. L. , Wang, B. D. , Doyle, E. , Lee, A. D. , Lee, N. H. , Elmer, G. I. , & Elmer, G. I. (2013). Neuroplasticity, axonal guidance and micro‐RNA genes are associated with morphine self‐administration behavior. Addiction Biology, 18(3), 480–495. 10.1111/j.1369-1600.2012.00470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge, F. R. , Li, X. , Kambhampati, S. , Pickens, C. L. , St Laurent, R. , Bossert, J. M. , Baumann, M. H. , Hutchinson, M. R. , Rice, K. C. , Watkins, L. R. , & Shaham, Y. (2013). Effect of chronic delivery of the toll‐like receptor 4 antagonist (+)‐naltrexone on incubation of heroin craving. Biological Psychiatry, 73(8), 729–737. 10.1016/j.biopsych.2012.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama, K. , Kiyosawa, N. , Watanabe, K. , & Ishizuka, H. (2017). Identification of circulating miRNAs differentially regulated by opioid treatment. International Journal of Molecular Sciences, 18(9), 1991. 10.3390/ijms18091991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler, F. M. , Oliver, D. J. , Wyse, C. , Blau, A. , Shtutman, M. , Turner, J. R. , & Byrnes, E. M. (2017). Transgenerational attenuation of opioid self‐administration as a consequence of adolescent morphine exposure. Neuropharmacology, 113(Pt A), 271–280. 10.1016/j.neuropharm.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan, S. , Tong, Y. , & Steitz, J. A. (2007). Switching from repression to activation: microRNAs can up‐regulate translation. Science, 318(5858), 1931–1934. 10.1126/science.1149460 [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , Michaelides, M. , & Baler, R. (2019). The neuroscience of drug reward and addiction. Physiological Reviews, 99(4), 2115–2140. 10.1152/physrev.00014.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Q. , Hwang, C. K. , Zheng, H. , Wagley, Y. , Lin, H. Y. , Kim, D. K. , Law, P.‐Y. , Loh, H. H. , & Wei, L. N. (2013). MicroRNA 339 down‐regulates μ‐opioid receptor at the post‐transcriptional level in response to opioid treatment. The FASEB Journal, 27(2), 522–535. 10.1096/fj.12-213439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Q. , Zhang, L. , Law, P. Y. , Wei, L. N. , & Loh, H. H. (2009). Long‐term morphine treatment decreases the association of mu‐opioid receptor (MOR1) mRNA with polysomes through miRNA23b. Molecular Pharmacology, 75(4), 744–750. 10.1124/mol.108.053462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W. , Hong, Q. , Lin, Z. , Ma, H. , Chen, W. , Zhuang, D. , Zhu, H. , Lai, M. , Fu, D. , Zhou, W. , & Liu, H. (2021). Role of nucleus accumbens microRNA‐181a and MeCP2 in incubation of heroin craving in male rats. Psychopharmacology, 238(8), 2313–2324. 10.1007/s00213-021-05854-3 [DOI] [PubMed] [Google Scholar]
- Xu, X. , Pan, J. , Li, X. , Cui, Y. , Mao, Z. , Wu, B. , Xu, H. , Zhou, W. , & Liu, Y. (2019). Inhibition of methamphetamine self‐administration and reinstatement by central blockade of angiotensin II receptor in rats. The Journal of Pharmacology and Experimental Therapeutics, 369(2), 244–258. 10.1124/jpet.118.255729 [DOI] [PubMed] [Google Scholar]
- Yan, B. , Hu, Z. , Yao, W. , Le, Q. , Xu, B. , Liu, X. , & Ma, L. (2017). MiR‐218 targets MeCP2 and inhibits heroin seeking behavior. Scientific Reports, 7, 40413. 10.1038/srep40413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanda, M. T. , Floris, G. , & Sillivan, S. E. (2021). Drug‐associated cues and drug dosage contribute to increased opioid seeking after abstinence. Scientific Reports, 11(1), 14825. 10.1038/s41598-021-94214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Zhang, K. , Jiang, H. , Du, J. , Na, Z. , Hao, W. , Yu, S. , & Zhao, M. (2016). Decreased expression of plasma MicroRNA in patients with methamphetamine (MA) use disorder. Journal of Neuroimmune Pharmacology, 11(3), 542–548. 10.1007/s11481-016-9671-z [DOI] [PubMed] [Google Scholar]
- Zheng, H. , Zeng, Y. , Chu, J. , Kam, A. Y. , Loh, H. H. , & Law, P. Y. (2010). Modulations of NeuroD activity contribute to the differential effects of morphine and fentanyl on dendritic spine stability. The Journal of Neuroscience, 30(24), 8102–8110. 10.1523/jneurosci.6069-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ΔΔCt values measured for miRNAs in the NAc following morphine or yoked saline self‐administration.
Data Availability Statement
Data that support the findings in this study are available from the authors upon reasonable request.


