Abstract
Aim
Multiple studies support the efficacy of combining a glucagon‐like peptide 1 receptor agonist (GLP‐1RA) with basal insulin in people with type 2 diabetes inadequately controlled on dual/triple oral therapy. Fixed‐ratio combinations of basal insulin + GLP‐1RA represent a further advance to facilitate management. We assessed the impact of fixed‐ratio combination basal insulin + GLP‐1RA treatment on β‐cell function.
Materials and Methods
We analysed data from 351 participants in the LixiLan‐G trial (NCT02787551) randomized to receive iGlarLixi (insulin glargine 100 U/ml + lixisenatide) or to continue daily/weekly GLP‐1RA, both on top of metformin. Participants received a 2‐h meal tolerance test before randomization and at study end (26 weeks), with timed plasma glucose and C‐peptide determinations. β‐cell function parameters were resolved using mathematical modelling.
Results
In the GLP‐1RA group (n = 162), both body weight and glycated haemoglobin decreased at week 26, yet none of the insulin secretion/β‐cell function parameters changed significantly. In contrast, in the iGlarLixi group (n = 189), glycated haemoglobin decreased significantly more than in the GLP‐1RA group (p < .0001) despite an increase in body weight (+1.7 ± 3.9 kg, p < .0001). Fasting and stimulated insulin secretion decreased at Week 26 (both p < .0001 vs. GLP‐1RA), while β‐cell glucose sensitivity increased by a median 35% (p = .0032 vs. GLP‐1RA). The incremental meal tolerance test glucose area showed a larger reduction with iGlarLixi versus GLP‐1RA (p < .0001).
Conclusions
In people with type 2 diabetes on metformin, 26‐week treatment with iGlarLixi resulted in a marked improvement in β‐cell function concomitant with sparing of endogenous insulin release and a reduction in meal absorption.
Keywords: β‐cell function, glucagon‐like peptide 1 receptor agonist, iGlarLixi, insulin secretion, mixed‐meal tolerance test
1. INTRODUCTION
People with type 2 diabetes often require multiple therapies to achieve and maintain satisfactory glycaemic control. There is wide consensus that glucagon‐like peptide 1 receptor agonists (GLP‐1RA) are a suitable initial injectable therapy in many adults with type 2 diabetes inadequately controlled by dual or triple oral therapy. 1 , 2 Combinations of GLP‐1RA with basal insulin as separate injectables have been shown to be effective, 3 , 4 , 5 and fixed‐ratio combinations of basal insulin plus GLP‐1RAs clearly represent a practical advantage to facilitate management. 6 , 7 The recent LixiLan‐G trial (NCT02787551) showed that the titratable fixed‐ratio combination of lixisenatide (a short‐acting GLP‐1RA) and insulin glargine 100 U/ml (iGlarLixi) substantially improved glucose control in adults with type 2 diabetes switched from previous daily/weekly GLP‐1RA treatment compared with those continuing such treatment. 8 However, it is not known whether the drug combination affects β‐cell dysfunction, which is the main defect of type 2 diabetes. In the present exploratory analysis of the LixiLan‐G trial, we set out to measure endogenous insulin secretion and β‐cell function in adults with type 2 diabetes randomized to single GLP‐1RA therapy or iGlarLixi.
2. RESEARCH DESIGN AND METHODS
2.1. Study design
The present study is based on an already reported trial (LixiLan‐G), which was a randomized, open‐label, active‐controlled, parallel‐group, phase 3, 26‐week treatment study with a single‐arm 26‐week extension period on iGlarLixi only. 8 The study was designed and monitored in accordance with Good Clinical Practice guidelines, the International Conference on Harmonisation, and the Declaration of Helsinki. Institutional review boards or ethics committees at each study site approved the protocol. Each participant provided written informed consent. Briefly, eligible participants were adults with type 2 diabetes diagnosed for at least 1 year before screening, with glycated haemoglobin (HbA1c) of 7%‐9% (53‐75 mmol/mol), treated with the maximum tolerated dose of a GLP‐1RA, daily (60% on liraglutide once daily or exenatide twice daily) or weekly (40% on dulaglutide, exenatide extended release, or albiglutide) on a background of metformin ± pioglitazone (n = 22) ± a sodium‐glucose cotransporter 2 inhibitor (n = 42). Previous treatment with insulin in the year before the screening visit was an exclusion criterion. After a screening period of at least 2 weeks, participants were randomized in a 1:1 ratio either to continue with their current treatment with a GLP‐1RA or to switch from their current GLP‐1RA to iGlarLixi for 26 weeks. In both groups, existing oral agents were continued without modification. iGlarLixi treatment was initiated at a dose of 10 units (10 units insulin glargine 100 U/ml per 5 μg lixisenatide) and then titrated to reach and maintain a fasting self‐monitored plasma glucose target between 80 and 100 mg/dl (4.4 and 5.6 mmol/L), according to a detailed titration algorithm 8 (see Table S1 in Blonde et al. 8 ). GLP‐1RA comparator therapy was administered subcutaneously as per local labelling, with participants continuing the same dose regimen as before randomization. Basal insulin was not allowed as rescue therapy in the iGlarLixi arm. In the GLP‐1RA arm, the suggested rescue therapy was basal insulin at the investigator's discretion.
At baseline and 26 weeks, participants received a standardized mixed meal test (MMTT) as a liquid formula containing ~600 kcal (50%‐55% carbohydrate, 15%‐20% protein and 25%‐30% fat). iGlarLixi and exenatide twice daily were injected 30 min before the meal, and daily liraglutide was administered at any time of the day, as per label, while participants taking weekly GLP‐1RA injected their medication per their usual weekly schedule. The meal test was performed preferably within 3 days after the injection of the weekly GLP‐1RA. Blood samples were obtained at times −30, 0, 30, 60, 90 and 120 min into the MMTT for plasma glucose and C‐peptide determination. Plasma glucose was assayed using a Roche Cobas analyser, and plasma C‐peptide by a two‐site sandwich immunoassay and direct chemiluminescence method, with within‐ and between‐assay precision ranging from 3% to 5% (ADVIA Centaur C‐Peptide ReadyPack; Siemens Healthcare Diagnostics, Tarrytown, NY, USA).
2.2. Data analysis
β‐cell function was resolved by mathematical modelling of the plasma glucose and C‐peptide concentrations measured during the MMTT, as previously described. 9 The β‐cell function model consists of three blocks: (a) a model for fitting the plasma glucose concentration profile, the purpose of which is to smooth and interpolate plasma glucose concentrations; (b) a model describing the dependence of insulin (or C‐peptide) secretion on glucose concentration; and (c) a two‐exponential model of C‐peptide kinetics in which the model parameters are individually adjusted to the subject's anthropometric data. 10 The mean slope of the insulin secretion/plasma glucose dose‐response function is taken to represent β‐cell glucose sensitivity (in pmol/min/m2/mM). The model also yields an estimate of early insulin response (termed rate sensitivity, or response to plasma glucose rate of change) and a potentiation parameter, reflecting potentiation of insulin secretion by glucose and incretins. 11 The fasting insulin secretion rate and total insulin output (insulin secretion during 2 h post‐MMTT) were also calculated. Modelling analysis did not yield reliable results in 32 tests (19 in the iGlarLixi group, 13 in the GLP‐1RA group), as meal glucose excursions were minimal and not concordant with those of C‐peptide. These tests were excluded from the statistical analysis. Another 118 participants were excluded because the MMTT data at follow‐up were missing or incomplete. Overall, 66 cases were excluded from the iGlarLixi arm and 84 from the GLP‐1RA arm. Thus, from a total of 501 cases, 351 (189 iGlarLixi and 162 GLP‐1RA) were included in the present analysis; anthropometric and clinical characteristics did not differ between excluded and included subjects (Table S1).
Area under concentration curves (AUC) were calculated by the trapezium rule.
2.3. Statistical analysis
Data were summarized as mean ± standard deviation or median (interquartile range, IQR) for variables with a skewed distribution; the latter were transformed into their natural log for use in parametric testing. Change over time was calculated as week 26 minus baseline, so that negative values indicate a decrease and positive values correspond to an increase over time. Group differences were tested by Mann‐Whitney or χ2 test for continuous and dichotomic variables, respectively; differences between week 26 and baseline were tested by Wilcoxon signed rank test. Group differences over time were analysed by repeated‐measures multivariate analysis of variance, with treatment group as a fixed effect. Univariate and multivariate regressions were performed by standard methods. Multivariate logistic regression was reported as odds ratio and 95% confidence interval, and AUC receiver operating characteristic curve. Statistical significance was set as p < .05.
3. RESULTS
3.1. Baseline
This cohort featured the typical defects of β‐cell function in type 2 diabetes. Thus, by comparison with a sex‐, age‐ and body mass index (BMI)‐matched group of historical nondiabetic controls, 12 participants had increased fasting insulin secretion [median (interquartile range): 117 (57) vs. 95 (70) pmol/min/m2] and decreased total insulin secretion [29 (16) vs. 48 (24) nmol/m2]; glucose sensitivity was reduced by ~70% [34 (27) vs. 111 (73) pmol/min/m2/mM] and potentiation by 50% [1.07 (0.25) vs. 1.99 (1.29)], and the rate sensitivity was low. By multivariate regression of the pooled baseline data (n = 351), glucose sensitivity was independently related to BMI (directly, partial r = 0.21, p < .0001), diabetes duration (inversely, partial r = −0.11, p = .008) and HbA1c (inversely, partial r = −0.25, p < .0001) (Figure S1).
3.2. Treatment
Participants randomized to iGlarLixi or GLP‐1RA were well matched on all clinical characteristics (except for a slightly longer duration of metformin treatment in the GLP‐1RA group) (Table 1). Over 26 weeks, the mean ± SD daily dose of iGlarLixi (n = 184) was 44 ± 27 U of insulin and 17.0 ± 5.3 μg of lixisenatide, while the individual GLP‐1RA doses were maintained at pre‐randomization levels. None of the baseline metabolic parameters differed significantly between the two groups. Over the 26 weeks of follow‐up, all metabolic parameters changed differentially between the two treatments (Table 2). Thus, in the GLP‐1RA group, body weight, HbA1c, fasting and mean MMTT glucose, and incremental glucose AUC all declined significantly from baseline, whereas fasting and post‐MMTT C‐peptide levels (Figure 1) were superimposable at baseline and follow‐up; none of the ß‐cell functional parameters changed significantly.
TABLE 1.
Anthropometric and clinical characteristics of the study participants a
| iGlarLixi (n = 189) | GLP‐1RA (n = 162) | p b | |
|---|---|---|---|
| Male, % | 48 | 55 | NS |
|
Age, years Ethnicity |
59 ± 10 | 60 ± 10 | NS |
| Body weight, kg | 94 ± 16 | 95 ± 18 | NS |
| BMI, kg/m2 | 32.8 ± 4.4 | 32.9 ± 4.4 | NS |
| Diabetes duration, years | 10 (8) | 10 (8) | NS |
| Background antidiabetic therapy | |||
| Metformin duration, years | 6.4 (7.8) | 7.2 (7.2) | <0.04 |
| Metformin daily dose, mg | 1962 ± 430 | 2019 ± 505 | NS |
| GLP‐1RA type (L/D/A/E), % | 52/21/2/24 | 54/21/2/23 | NS |
| GLP‐1RA duration, years | 1.2 (1.9) | 1.1 (2.1) | NS |
| Retinopathy, % | 8.2 | 5.3 | NS |
| Nephropathy, % | 8.2 | 9.3 | NS |
| Neuropathy, % | 24 | 23 | NS |
| UACR, mg/g | 11 (19) | 10 (17) | NS |
| HbA1c, % | 7.77 ± 0.63 | 7.76 ± 0.54 | NS |
| HbA1c, mmol/mol | 61.4 ± 6.8 | 62.2 ± 6.2 | |
| eGFR, ml min−1 1.73 m−2 | 89 ± 25 | 86 ± 23 | NS |
Abbreviations: eGFR, estimated glomerular filtration rate; GLP‐1RA, glucagon‐like peptide 1 receptor agonist; HbA1c glycated haemoglobin A1C; iGlarLixi, insulin glargine 100 U/ml + lixisenatide; L/D/A/E, liraglutide, dulaglutide, albiglutide, exenatide; NS, not significant; UACR, urinary albumin/creatinine ratio.
Entries are mean ± SD or median (IQR).
By Mann‐Whitney test or χ2, as appropriate.
TABLE 2.
Metabolic parameters at baseline and their change at follow‐up a
| iGlarLixi (n = 189) | GLP‐1RA (n = 162) | p b | |
|---|---|---|---|
| Body weight, kg | |||
| Baseline | 94 ± 16 | 95 ± 18 | |
| Change at week 26 | 1.7 ± 3.9 b | −1.4 ± 3.1 b | <.0001 |
| HbA1c, % | |||
| Baseline | 7.77 ± 0.63 | 7.76 ± 0.54 | |
| Change at week 26 | −0.99 ± 0.79 b | −0.34 ± 0.79 b | <.0001 |
| HbA1c, mmol/mol | |||
| Baseline | 61.4 ± 6.9 | 61.3 ± 5.9 | |
| Change at week 26 | −10.8 ± 8.6 | −3.7 ± 8.6 | |
| Fasting glucose, mmol/L | |||
| Baseline | 9.16 ± 1.98 | 9.15 ± 1.71 | |
| Change at week 26 | −2.16 ± 2.35 c | −0.32 ± 2.05 b | <.0001 |
| Fasting ISR, pmol min−1 m−2 | |||
| Baseline | 117 (57) | 115 (59) | |
| Change at week 26 | −47 (46) b | 2 (−0.2) | <.0001 |
| Mean glucose, mmol/L | |||
| Baseline | 12.26 ± 2.54 | 12.16 ± 2.34 | |
| Change at week 26 | −2.89 ± 3.11 c | −0.56 ± 2.56 b | <.0001 |
| Incremental glucose AUC, mmol/L | |||
| Baseline | 3.2 (2.0) | 3.0 (1.9) | |
| Change at week 26 | −0.7 (2.5) c | −0.2 (1.8) b | <.0001 |
| Total insulin secretion, nmol/m2 | |||
| Baseline | 28.8 (14.9) | 28.8 (15.5) | |
| Change at week 26 | −3.5 (12.4) c | 0.1 (8.4) | <.0001 |
| Glucose sensitivity, pmol min−1 m−2 mM−1 | |||
| Baseline | 33.7 (25.2) | 33.7 (30.3) | |
| Change at week 26 | 12.6 (31.3) c | 0.9 (21.7) | .0032 |
| Rate sensitivity, pmol m−2 mM−1 | |||
| Baseline | 316 ± 610 | 319 ± 669 | |
| Change at week 26 | 152 ± 790 c | −76 ± 789 | .0073 |
| Potentiation ratio | |||
| Baseline | 1.07 (0.26) | 1.05 (0.24) | |
| Change at week 26 | 0.12 (0.54) c | 0.04 (0.38) | .0007 |
Abbreviations: AUC, area under the curve; ISR, insulin secretion rate.
Entries are mean ± SD or median (IQR).
For the difference between group changes by repeated‐measures ANOVA.
p ≤ .05 versus baseline by Wilcoxon signed rank test.
FIGURE 1.
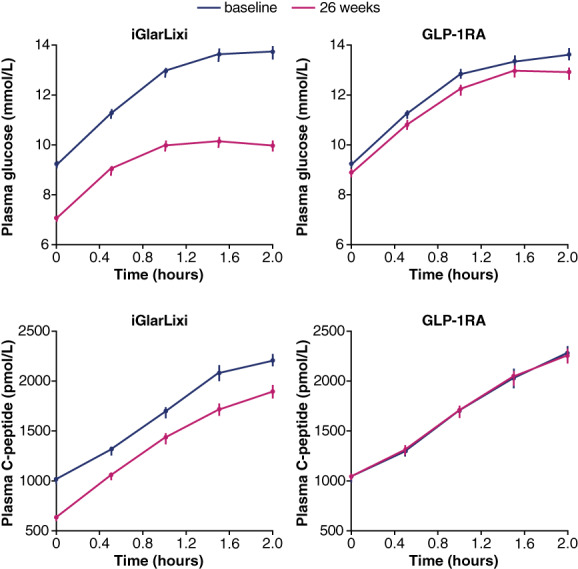
Plasma glucose and C‐peptide curves at baseline and 26 weeks in the two treatment arms. Plots are mean ± standard error of the mean. GLP‐1RA, glucagon‐like peptide 1 receptor agonist
In the iGlarLixi group, the HbA1c and glucose levels fell more than in the comparator group, as did the plasma C‐peptide concentrations (Figure 1) and fasting and total insulin secretion. In the face of diminished endogenous insulin release, the insulin secretion dose‐response function was shifted to the left (Figure 2), such that ß‐cell glucose sensitivity increased by a median 35%; rate sensitivity and potentiation also improved as compared with the GLP‐1RA group (Table 2).
FIGURE 2.

Insulin secretion‐plasma glucose dose‐response curves at baseline and 26 weeks in the iGlarLixi and GLP‐1RA arms. Plots are mean ± standard error of the mean. GLP‐1RA, glucagon‐like peptide 1 receptor agonist; iGlarLixi, insulin glargine 100 U/ml + lixisenatide
In the pooled data, treatment‐induced changes in glucose sensitivity were reciprocally related to the corresponding changes in body weight (Figure S2A) and to the changes in HbA1c (Figure S2B), with similar slopes in the two groups. The incremental MMTT glucose area showed a larger reduction with iGlarLixi as compared with GLP‐1RA (Table 2).
At week 26, 68% of participants in the iGlarLixi arm achieved an HbA1c of ≤7.0% (≤53 mmol/mol), and 33% of them achieved an HbA1c of <6.5% (<48 mmol/mol), versus 32% and 13% of participants in the GLP‐1RA arm, respectively (p < .0001 for both). In a multivariate logistic model including data from both arms, lower baseline HbA1c (a conditional element of any HbA1c goal), greater weight loss, larger increase in glucose sensitivity, and treatment with iGlarLixi v GLP‐1RA enhanced the probability of achieving an HbA1c of <6.5% (<48 mmol/mol) independently of each other (Figure 3); together, these four factors made up an AUC receiver operating characteristic of 0.78.
FIGURE 3.
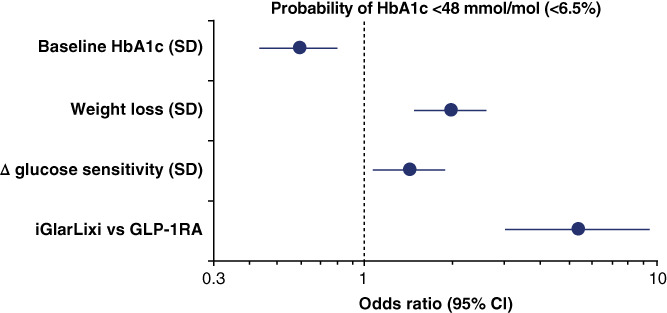
Multivariate logistic regression of the probability of an HbA1c <48 mmol/mol (<6.5%) at week 26. ∆ glucose sensitivity is the change in glucose sensitivity between week 26 and baseline. As a lower baseline HbA1c is associated with an increased probability of reaching an HbA1c of <48 mmol/mol (<6.5%), it has an odds ratio of <1. This is because as baseline HbA1c increases, the odds of reaching goal are reduced. Plots are odds ratio and 95% confidence intervals. GLP‐1RA, glucagon‐like peptide 1 receptor agonist; HbA1c glycated haemoglobin; iGlarLixi, insulin glargine 100 U/ml + lixisenatide; SD, standard deviation
4. DISCUSSION
The present study enrolled adults with type 2 diabetes of long duration and insufficient metabolic control, in whom β‐cell function was severely compromised compared with age‐ and weight‐matched nondiabetic controls. 12 The main finding is that switching from GLP‐1RA treatment to iGlarLixi resulted not only in superior glycaemic control compared with continuing GLP‐1RA therapy, but also in a marked improvement in global ß‐cell function. In terms of clinical outcome, over 26 weeks twice as many participants who switched to iGlarLixi achieved an HbA1c of ≤7.0% (≤53 mmol/mol), and three times as many of them (33 vs. 13%, p < .0001) reached optimal control [<6.5% (42 mmol/mol)] compared with participants who remained on their previous GLP‐1RA therapy; in the latter, body weight and glycaemic parameters improved (slightly, though significantly) but β‐cell function was unaffected.
Because, by design, both participant groups had been on GLP‐1RA for ~2 years before randomization, it can be safely assumed that any benefits of continuing GLP‐1RA treatment (comparator arm) were carried over by the lixisenatide moiety in the iGlarLixi arm. The question then arises, what was the relative contribution of insulin and lixisenatide versus the GLP‐1RA to the incremental metabolic control? Clearly, the insulin moiety was a powerful antihyperglycaemic driver through its classical actions, inhibitory on endogenous glucose release and stimulatory on tissue glucose uptake. As a short‐acting GLP‐1RA, lixisenatide does not cause tachyphylaxis of the GLP‐1 receptor, and its effect on gastric emptying is greater than that of long‐acting GLP‐1RAs. 13 , 14 However, beneficial antihyperglycaemic effects have also been observed for IDegLira, the fixed‐ratio combination of insulin degludec and the long‐acting GLP‐1RA, liraglutide. 15
The current data confirm previous analyses (reviewed by Ferrannini 16 ) showing that HbA1c (or plasma glucose level) is very well correlated with β‐cell glucose sensitivity in a reciprocal fashion (Figure S1). Thus, reductions of HbA1c associate with increases in glucose sensitivity (by 12 ± 2 pmol min−1 m−2 mM−1 for each 1% HbA1c decrement in the present data set). The underlying biology is, at least in part, relief of glucose toxicity. 17 , 18 , 19 Perhaps less well appreciated is the independent direct association of glucose sensitivity with BMI (or other measures of adiposity), possibly because of a glucose‐independent stimulation of insulin secretion by endogenous free fatty acids. 19 , 20 , 21 Therefore, in the iGlarLixi group the lixisenatide component, which would per se lead to weight loss, as expected for the GLP‐1RA class in general, and observed in comparison with liraglutide, 14 , 22 restrained the weight gain expected from the insulin component, at the same time as it exerted an additional, if small, positive influence on glucose sensitivity (estimated at ~3.5 pmol min−1 m−2 mM−1 for each 10 kg of weight loss; Figure S2).
Another important consequence of iGlarLixi treatment was the reduction of both fasting and post‐MMTT (total) insulin secretion (Table 2). As plasma glucose is the dominant stimulus of insulin secretion under virtually all circumstances, 16 the large drop in glucose levels in the iGlarLixi arm was also the cause of the decreased insulin output. This apparently paradoxical combination of less absolute insulin release and better β‐cell glucose sensitivity is consistently seen with other effective antihyperglycaemic interventions (e.g. bariatric surgery 23 ). In type 2 diabetes, this physiological setup translates into a reduced secretory strain on an already defective β‐cell. In the current data, it can be estimated that iGlarLixi induced an average 25% sparing of endogenous insulin (18 of 76 U/day, assuming an average daily calorie intake of 2400 kcal).
A final major difference between the two arms of this study was the greater reduction of the incremental MMTT glucose area with iGlarLixi compared with GLP‐1RA (Table 2). This effect can be confidently ascribed to the ability of lixisenatide to slow down gastric emptying. In a previous study measuring gastric retention by combined scintigraphy and a double‐tracer technique, 24 a tight association was shown between slowing of gastric emptying and postprandial glucose lowering with lixisenatide versus placebo. Of note, while a strong delay in gastric emptying is observed shortly after lixisenatide injection at breakfast, this effect remains detectable during following meals. 25
Limitations of this study are that neither plasma insulin nor plasma glucagon measurements were available to estimate changes in insulin sensitivity and glucagon suppression, respectively, as contributors to the overall iGlarLixi benefit. In addition, a substantial number of participants either did not have an appropriate glycaemic response to the MMTT or the data were unavailable, which may represent a limitation. As detailed in the primary clinical trial, 8 , 26 the offset of the observed benefits in terms of adverse events was somewhat higher with iGlarLixi compared with GLP‐1RA, but was clinically judged to be mild to moderate in size and, altogether, reasonably well predictable. In conclusion, switching from GLP‐1RA to iGlarLixi resulted in better glycaemic control because there was not only insulin‐mediated abatement of glucose toxicity, but also enhanced β‐cell glucose sensitivity, endogenous insulin sparing, and a degree of slowing down of gastric emptying.
CONFLICT OF INTEREST
EN, TD and SS are employees of, and have shares/stock options for Sanofi. EF is a member of Boehringer‐Ingelheim/Eli Lilly & Company and Lexicon advisory boards, has received speaker fees from Boehringer‐Ingelheim/Eli Lilly & Company and MSD, and research grant support from Janssen and Oramed, and does ad hoc consulting for AstraZeneca, Sanofi and Oramed. AM has received research grant support from Eli Lilly & Company.
AUTHOR CONTRIBUTIONS
EF and AM conducted the data analysis. EF wrote the manuscript. All authors reviewed and revised the manuscript, and all approved the final version for submission.
Supporting information
Figure S1. Simultaneous dependence of ß‐cell glucose sensitivity on BMI, diabetes duration and serum HbA1c in the whole cohort. BMI, body mass index; HbA1c, glycated haemoglobin A1C.
Figure S2. Regression of the changes in ß‐cell glucose sensitivity between baseline and week 26 against the corresponding changes in body weight (A) and HbA1c (B) by treatment group.
Table S1. Anthropometric and clinical characteristics of included and excluded study participants †
ACKNOWLEDGMENTS
The authors would like to thank the LixiLan‐G steering committee for their contribution to trial design and inclusion of measurements during the mixed‐meal tolerance tests, which allowed for the analyses described in this manuscript. This study was funded by Sanofi US. Editorial assistance in manuscript formatting and submission was provided by Helen Jones, PhD, CMPP, of Envision Scientific Solutions, Philadelphia, and funded by Sanofi US.
Ferrannini E, Niemoeller E, Dex T, Servera S, Mari A. Fixed‐ratio combination of insulin glargine plus lixisenatide (iGlarLixi) improves ß‐cell function in people with type 2 diabetes. Diabetes Obes Metab. 2022;24(6):1159‐1165. doi: 10.1111/dom.14688
Funding information Sanofi
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to patient‐level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient‐level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi's data‐sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com/.
REFERENCES
- 1. American Diabetes Association Professional Practice Committee . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2022. Diabetes Care. 2022;45(Supplement_1):S125‐S143. doi: 10.2337/dc22-s009 [DOI] [PubMed] [Google Scholar]
- 2. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2020;43(2):487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maiorino MI, Chiodini P, Bellastella G, et al. Free and fixed‐ratio combinations of basal insulin and GLP‐1 receptor agonists versus basal insulin intensification in type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20(9):2309‐2313. [DOI] [PubMed] [Google Scholar]
- 4. Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F. GLP‐1 receptor agonist added to insulin versus basal‐plus or basal‐bolus insulin therapy in type 2 diabetes: a systematic review and meta‐analysis. Diabetes Metab Res Rev. 2019;35(1):e3082. [DOI] [PubMed] [Google Scholar]
- 5. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728‐742. [DOI] [PubMed] [Google Scholar]
- 6. Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan‐L randomized trial. Diabetes Care. 2016;39(11):1972‐1980. [DOI] [PubMed] [Google Scholar]
- 7. Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan‐O randomized trial. Diabetes Care. 2016;39(11):2026‐2035. [DOI] [PubMed] [Google Scholar]
- 8. Blonde L, Rosenstock J, Del Prato S, et al. Switching to iGlarLixi versus continuing daily or weekly GLP‐1 RA in type 2 diabetes inadequately controlled by GLP‐1 RA and oral antihyperglycemic therapy: the LixiLan‐G randomized clinical trial. Diabetes Care. 2019;42(11):2108‐2116. [DOI] [PubMed] [Google Scholar]
- 9. Mari A, Tura A, Natali A, et al. Impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia. 2010;53(4):749‐756. [DOI] [PubMed] [Google Scholar]
- 10. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C‐peptide levels. Comparison of individual and standard kinetic parameters for C‐peptide clearance. Diabetes. 1992;41(3):368‐377. [DOI] [PubMed] [Google Scholar]
- 11. Mari A, Tura A, Grespan E, Bizzotto R. Mathematical modeling for the physiological and clinical investigation of glucose homeostasis and diabetes. Front Physiol. 2020;11:575789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrannini E, Natali A, Camastra S, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62(5):1730‐1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quast DR, Schenker N, Menge BA, Nauck MA, Kapitza C, Meier JJ. Effects of lixisenatide versus liraglutide (short‐ and long‐acting GLP‐1 receptor agonists) on esophageal and gastric function in patients with type 2 diabetes. Diabetes Care. 2020;43(9):2137‐2145. [DOI] [PubMed] [Google Scholar]
- 14. Meier JJ, Rosenstock J, Hincelin‐Méry A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care. 2015;38(7):1263‐1273. [DOI] [PubMed] [Google Scholar]
- 15. Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open‐label, randomised, 26‐week, treat‐to‐target trial in insulin‐naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885‐893. [DOI] [PubMed] [Google Scholar]
- 16. Ferrannini E. A journey in diabetes: from clinical physiology to novel therapeutics: the 2020 Banting Medal for Scientific Achievement lecture. Diabetes. 2021;70(2):338‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13(6):610‐630. [DOI] [PubMed] [Google Scholar]
- 18. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard lecture 2009. Diabetologia. 2010;53(7):1270‐1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lytrivi M, Castell AL, Poitout V, Cnop M. Recent insights into mechanisms of β‐cell lipo‐ and glucolipotoxicity in type 2 diabetes. J Mol Biol. 2020;432(5):1514‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrannini E, Camastra S, Coppack SW, Fliser D, Golay A, Mitrakou A. Insulin action and non‐esterified fatty acids. Proc Nutr Soc. 1997;56(2):753‐761. [DOI] [PubMed] [Google Scholar]
- 21. Astiarraga B, Chueire VB, Souza AL, et al. Effects of acute NEFA manipulation on incretin‐induced insulin secretion in participants with and without type 2 diabetes. Diabetologia. 2018;61(8):1829‐1837. [DOI] [PubMed] [Google Scholar]
- 22. Kapitza C, Forst T, Coester HV, Poitiers F, Ruus P, Hincelin‐Méry A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab. 2013;15(7):642‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Camastra S, Muscelli E, Gastaldelli A, et al. Long‐term effects of bariatric surgery on meal disposal and β‐cell function in diabetic and nondiabetic patients. Diabetes. 2013;62(11):3709‐3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rayner CK, Watson LE, Phillips LK, et al. Effects of sustained treatment with lixisenatide on gastric emptying and postprandial glucose metabolism in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2020;43(8):1813‐1821. [DOI] [PubMed] [Google Scholar]
- 25. Lorenz M, Pfeiffer C, Steinstrasser A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes‐‐relationship to postprandial glycemia. Regul Pept. 2013;185:1‐8. [DOI] [PubMed] [Google Scholar]
- 26. Del Prato S, Frias JP, Blonde L, et al. Impact of disease duration and β‐cell reserve on the efficacy of switching to iGlarLixi in adults with type 2 diabetes on glucagon‐like peptide‐1 receptor agonist therapy: exploratory analyses from the LixiLan‐G trial. Diabetes Obes Metab. 2020;22(9):1567‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Simultaneous dependence of ß‐cell glucose sensitivity on BMI, diabetes duration and serum HbA1c in the whole cohort. BMI, body mass index; HbA1c, glycated haemoglobin A1C.
Figure S2. Regression of the changes in ß‐cell glucose sensitivity between baseline and week 26 against the corresponding changes in body weight (A) and HbA1c (B) by treatment group.
Table S1. Anthropometric and clinical characteristics of included and excluded study participants †
Data Availability Statement
Qualified researchers may request access to patient‐level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient‐level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi's data‐sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com/.


