Abstract
Plasmid transfection of mammalian cells is the dominant platform used to produce adeno‐associated virus (AAV) vectors for clinical and research applications. Low yields from this platform currently make it difficult to supply these activities with adequate material. In an effort to better understand the current limitations of transfection‐based manufacturing, this study examines what proportion of cells in a model transfection produce appreciable amounts of assembled AAV capsid. Using conformation‐specific antibody staining and flow cytometry, we report the surprising result that despite obtaining high transfection efficiencies and nominal vector yields in our model system, only 5%–10% of cells appear to produce measurable levels of assembled AAV capsids. This finding implies that considerable increases in vector titer could be realized through increasing the proportion of productive cells. Furthermore, we suggest that the flow cytometry assay used here to quantify productive cells may be a useful metric for future optimization of transfection‐based AAV vector manufacturing platforms.
Keywords: adeno‐associated virus, gene therapy, transfection, viral vectors
Transfection efficiencies during adeno‐associated virus (AAV) vector production by mammalian cell transfection commonly approach 60%, but what proportion of these cells are actually producing AAV vectors? In this study, the authors report the surprising result that <10% of cells produce assembled AAV capsid. Critically, results also suggest this is not an inherent consequence of the transfection process itself, and that significant gains in vector yield could be obtained by increasing the proportion of productive cells.
1. INTRODUCTION
Adeno‐associated viruses (AAV) have proven to be safe and efficacious gene transfer vectors, with 149 completed or ongoing clinical trials and 5 approved therapies for various forms of cell and gene therapy (Kuzmin et al., 2021). The bulk of AAV vectors used for research, clinical trials, and approved therapies are currently produced by multiplasmid transfection of mammalian cells (Clement & Grieger, 2016; Wang et al., 2019). Though variations on the process exist, production of AAV by transfection typically uses three plasmids: a transfer plasmid encoding the gene of interest flanked by viral inverted terminal repeat elements; a helper plasmid encoding the minimal helper virus genes necessary for the AAV lifecycle; and a packaging plasmid containing the AAV REP and CAP genes (D. Sharon & Kamen, 2018; Wang et al., 2019).
In this study, HEK‐293SF cells producing an AAV2‐GFP vector were used as a model to determine what proportion of transfected cells generate fully assembled capsids during vector production. Commonly used cationic transfection reagents (polyethyleneimine, calcium phosphate, lipofectamine, etc.) coprecipitate heterogeneous plasmid mixtures into larger complexes for transit across the cell membrane, and so in theory, all successfully transfected cells should contain the genetic elements necessary to produce AAV (Cardarelli et al., 2016; Erbacher et al., 2004; Fus‐Kujawa et al., 2021). Reported transfection efficiencies (measured by the expression of a fluorescent marker) for well‐optimized processes range from 40% to 60% (Chahal et al., 2014; Nguyen et al., 2021). However, the degree to which transfection efficiency corresponds with the proportion of productive cells in the culture remains largely untested. Clarifying this will give an indication of how much of the cell biomass is being utilized with current AAV manufacturing protocols, and, in turn, how much those protocols might be improved.
To establish a model for subsequent experiments, a triple plasmid transfection to produce AAV2‐GFP was carried out on HEK‐293SF cells in suspension, based on an optimized process developed by Chahal et al. (2014). As seen in Figure 1a, cell density was relatively stable up to the harvest point at 48 hours posttransfection (hpt), the previously determined optimal harvest point for this process (Chahal et al., 2014). The mean vector yield 48 hpt was measured at 2.08 × 108 VG/ml, in line with previous studies where AAV vectors were produced by transfection in HEK‐293 or derivative cell lines (Figure 1b). Two controls used in subsequent experiments were also assayed for AAV particle yield; transfection with an AAV infectious clone and helper plasmid to generate replication‐competent AAV (rcAAV) (1.26 × 109 VG/ml), as well as cells infected with rcAAV2 and a human adenovirus type 5 (hAd5) helper virus (2.97 × 109 VG/ml) (Figure 1b). It should be highlighted that the relatively crude measures of AAV yield used here do not fully capture the differences between rcAAV and AAV vectors; nearly 100% of rcAAV particles are infectious, whereas <1% of AAV vector particles are able to successfully transduce cells. The reasons behind this are poorly understood, but likely stem from cis‐acting sequences within the REP and CAP genes necessary for efficient particle maturation (Zeltner et al., 2010).
Figure 1.
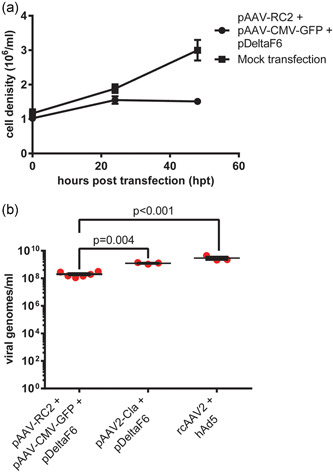
Production of rAAV2‐GFP by plasmid transfection HEK‐293SF cells in serum‐free suspension were transfected with equimolar amounts of pAdDeltaF6, pAAV‐RC2, and pAAV‐CMV‐GFP to produce AAV2 vectors carrying a GFP transgene. (a) Cell density in transfected and mock‐transfected cells tracked until harvest at 48 hpt. (b) Volumetric vector yield in terms of nuclease‐resistant vector genomes measured by droplet digital PCR. Also shown are two controls; transfection with equimolar amounts of pAdDeltaF6 + pAAV2‐Cla to produce replication‐competent AAV (rcAAC) by transfection, and infection with rcAAV and hAd5 at an MOI of 10 to produce rcAAV. Error bars represent SEM. Significance was determined using a nonparametric t‐test with Welch's correction. AAV, adeno‐associated virus; GFP, green fluorescent protein; hAd5, human adenovirus type 5; hpt, hours post‐transfection; MOI, multiplicity of infection; PCR, polymerase chain reaction
To determine what proportion of cells in our triple transfection model produce fully assembled vector capsids, transfected cells were stained with a conformation‐specific antibody that binds only to assembled viral capsids, allowing the measurement of this subpopulation by flow cytometry (Wobus et al., 2000; Xiao et al., 2002). The presence of a green fluorescent protein (GFP) expression cassette on the transfer plasmid allowed simultaneous assessment of transfection efficiency. Cells infected with rcAAV2 and hAd5 helper virus were used as a positive control for capsid assembly, while cells transfected with an infectious AAV2 clone and helper plasmid were used to determine how capsid assembly is impacted by transfection and/or the recombination of AAV2 genes. Results are shown in Figure 2.
Figure 2.
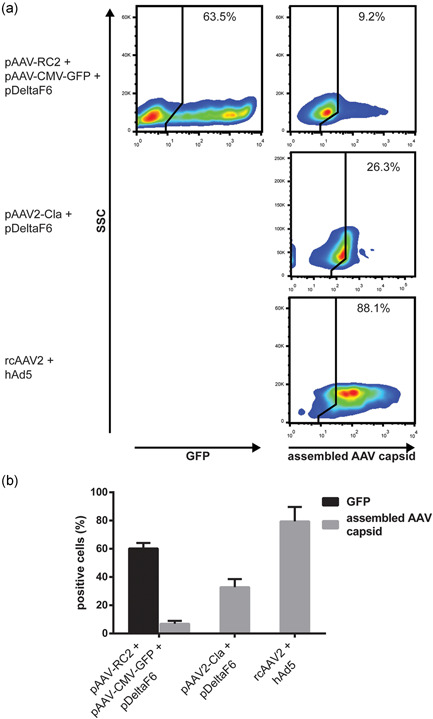
Comparison of transfection efficiency and assembled AAV capsid production in transfected cells producing AAV2‐GFP vectors. HEK‐293SF cells producing AAV2‐GFP vectors by transfection were fixed 48 hpt and stained for assembled AAV capsids. (a) Gating for transfection marker (GFP) and assembled AAV capsids following exclusion of debris and singlet gating. Positive gates were set using mock‐transfected control set to 1%–2% positive (not shown). (b) Also shown are a positive control for capsid assembly consisting of cells infected with rcAAV2 and a hAd5 helper virus, and cells transfected with pAdDeltaF6 + pAAV2‐Cla. Quantification of flow cytometry results from n = 3 biological replicates. Error bars represent SEM. AAV, adeno‐associated virus; GFP, green fluorescent protein; hAd5, human adenovirus type 5; hpt, hours post‐transfection; rcAAV2, replication‐competent AAV2
As expected, nearly all cells in the rcAAV2 and helper virus‐infected control are positive for assembled AAV capsid. However, despite a transfection efficiency of approximately 60%, only a small fraction (∼7%) of cells in our triple transfection model produced measurable amounts of assembled AAV capsid. Interestingly, transfection with an AAV2 infectious clone and helper plasmid improves the proportion of cells positive for assembled AAV capsid by fourfold–fivefold (Figure 2), commensurate with the differences in particle yield observed in Figure 1b. Critically, this indicates that the process of transfection is not inherently detrimental to AAV capsid assembly.
The observation that only ∼7% of transfected cells appeared to produce assembled AAV capsids raised the question as to what proportion of cells were expressing the necessary factors for AAV vector production. The three transfected plasmids collectively encode ∼17 protein and RNA elements, making measurement of every factor impractical (D. Sharon & Kamen, 2018; Wang et al., 2019). Instead, a subset of proteins from each of the three transfected plasmids was visualized in our triple transfection model and controls via immunofluorescence.
As shown in Figure 3, GFP was broadly expressed in our triple transfection model, consistent with the flow cytometry results in Figure 2. Interestingly though, GFP expression does not seem to reliably indicate coexpression of AAV capsid monomers or the hAd5 E2A helper factor, both of which were expressed at detectable levels in a much smaller subset of cells. This may partially explain the observation in Figure 2b that only ∼7% of transfected cells contain assembled AAV capsids, despite an apparent transfection efficiency nearly tenfold higher. Transfection with an infectious AAV2 clone and helper plasmid dramatically increased expression of AAV capsid monomers compared to our triple transfection model; a predictable result given that CAP copy number is static during vector production, but exponentially increasing in systems with rcAAV. In contrast, hAd5 E2A was weakly expressed in both transfection systems compared to cells infected with rcAAV and hAd5 virus. This is intriguing given that the two transfection systems show substantial differences in particle yield (Figure 1b) and proportion of cells positive for capsid assembly (Figure 2). Speculatively, this could indicate that the threshold for effective helper factor expression is low relative to other factors necessary for AAV vector production, or highlight the importance of cis‐acting elements within the REP and CAP genes in capsid assembly.
Figure 3.
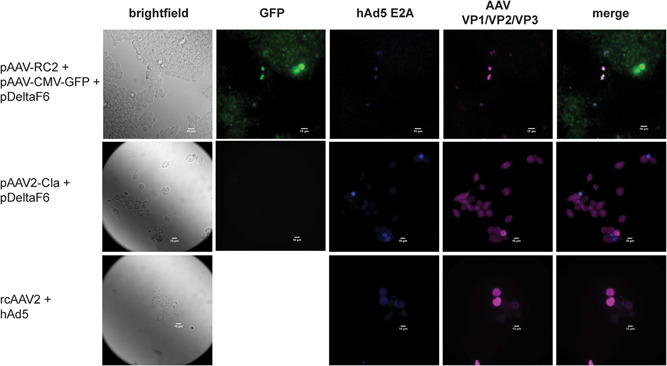
Expression of transfected plasmids during AAV2‐GFP vector production. Immunofluorescence of transfected cells producing AAV2‐GFP vectors 48 hpt. Images show the expression of GFP from the transfer plasmid, hAd5 E2A from the helper plasmid, and AAV capsid monomers VP1, VP2, and VP3 from the packaging plasmid. Also shown is a positive control consisting of cells infected with rcAAV2 and a hAd5 helper virus, and cells transfected with pAdDeltaF6 + pAAV2‐Cla. Images are taken at ×40 magnification. Scale bar = 10 µm. AAV, adeno‐associated virus; GFP, green fluorescent protein; hAd5, human adenovirus type 5; hpt, hours post‐htransfection
Low yields in transfection‐based AAV vector production platforms are a long‐standing issue, and have spurred the development of numerous alternatives. Plasmid‐free systems, such as baculovirus expression vectors, herpesvirus vectors, and more recent self‐silencing adenoviral systems boast significantly increased yields and are far more amenable to scaleup (Cawood, 2020; D. Sharon & Kamen, 2018). There have also been attempts both within academia and industry to develop a stable producer cell line, with Cevec Pharmaceuticals' Elevecta platform garnering particular attention in recent years (Tan et al., 2021). While these platforms may supplant transfection for late‐stage and approved therapies, the unparalleled speed and simplicity of transfection‐based manufacturing mean it is likely to remain a mainstay of AAV vector production for early clinical and research applications in the foreseeable future.
The results presented here indicate that current transfection‐based AAV vector production protocols utilize only a fraction of the available cell biomass. Critically, we also show that this issue is not an inherent consequence of transfection itself, suggesting dramatic increases in vector yield may yet be realized. To this end, the innovation and optimization of transfection‐based AAV vector manufacturing remain an active area of research. Computational work modeling plasmid uptake, expression, and vector assembly kinetics in HEK‐293 have demonstrated utility in identifying molecular bottlenecks to improve vector yield and quality (Nguyen et al., 2021). Design of experiment approaches to optimize process parameters have also recently been shown to be effective in increasing AAV vector yields across a wide range of serotypes (Zhao et al., 2020). Incorporation of cis‐acting elements within the viral genome into new vector designs also has the potential to increase vector yield and quality, but it remains to be seen how this would be accomplished.
The results of Figure 2 also demonstrate that the expression of a transfection marker does not necessarily imply a cell is producing AAV vector particles. While transfection efficiency remains an important process development metric, the confirmation‐specific antibody staining against assembled AAV capsids that were used here may also prove useful in the future development and optimization of transfection‐based AAV vector platforms.
2. METHODS
2.1. Cell culture and transfection
HEK‐293SF cells were maintained in serum‐free suspension as previously described (D. M. Sharon et al., 2020). All transfections were performed at a cell density of 106 cell/ml using linear polyethylenimine with a mean molecular weight of 25,000 Da (Polysciences) complexed with plasmid DNA at a 1:2 ratio. The final concentration of plasmid DNA in all cases was 1 µg/ml.
2.2. Plasmids
For the generation of rAAV2, plasmids pAdDeltaF6 (Addgene; #112867), pAAV‐RC2 (Cell Biolabs, Inc.), and pAAV‐CMV‐GFP (Addgene; #67634) were transfected in a 1:1:1 molar ratio. pAdDeltaF6 was a gift from James M. Wilson and pAAV‐CMV‐GFP was a gift from Connie Cepko (Xiong et al., 2015). Stocks of rcAAV2 were generated by equimolar transfection of pAdDeltaF6 and pAAV2‐Cla. pAAV2‐Cla, which contains a sequence identical to wildtype AAV2 apart from a point mutation to generate a Cla1 restriction site in the 3′‐untranslated region. pAV2‐Cla (called pAAV‐Cla in this paper) was generously provided by Dr. Thomas Weber.
2.3. rcAAV2 infection
Initial stocks of rcAAV2 were generated via plasmid transfection and titrated by digital polymerase chain reaction (dPCR). When using rcAAV2 infection as a positive control for capsid assembly, HEK‐293SF cells at an initial cell density of 106 cell/ml were infected with rcAAV2 stocks and hAd5 and a multiplicity of infection of 10.
2.4. Flow cytometry
Cells were fixed and permeabilized as previously described (Kerviel et al., 2016). Flow cytometry was carried out on the BD FACSJazz (BD Biosciences) or the BD LSRFortessa (BD Biosciences) and analyzed on FlowJo v10. Assembled particles of AAV2 were detected by staining with anti A20R (Progen) labeled with AlexaFluor 594 (Invitrogen).
2.5. Microscopy
HEK‐293SF cells were seeded at the low confluence on a 35 mm plate with a coverslip (MatTek). Twenty‐four hours post‐seeding, the cells were transfected for the production of AAV2‐GFP. Seventy‐two hours postseeding, the media was removed, and the cells fixed and stained as previously described (Kerviel et al., 2016). AAV capsid monomers were detected with anti‐VP1/VP2/VP3 (Progen) labeled with AlexaFluor 700 (Invitrogen), hAd5 E2A by anti‐E2A labeled with AlexaFluor 350 (Invitrogen). Anti‐E2A was a gift from Arnold J. Levine (Reich et al., 1983). Antibodies were incubated at room temperature for 1 h. Cells were then imaged using an Olympus IX‐83 confocal microscope. The images were analyzed using FIJI v1.53 (Schindelin et al., 2012).
2.6. AAV genome quantification by droplet dPCR
HEK‐293SF cells in culture media were freeze‐thawed three times to lyse cells. Clarified lysates were then incubated with 5 U/ml Benzonase to digest unencapsulated DNA. Encapsulated genomes were then purified with the High Pure Viral DNA Extraction Kit (Roche Diagnostics) and quantified on the QX200 Droplet dPCR System (Bio‐Rad) as previously described (Furuta‐Hanawa et al., 2019).
ACKNOWLEDGMENTS
This study was supported by the National Research Council of Canada under Grant Number CGT‐602‐1. The authors would like to thank Ayyappasamy Sudalaiyadum Perumal, PhD, and Prof. Dan Nicolau for providing access to specialized microscopy equipment and support. They would also like to thank Prof. Jose Teodoro for producing antibodies used in this study.
Dash, S. , Sharon, D. M. , Mullick, A. , & Kamen, A. A. (2022). Only a small fraction of cells produce assembled capsids during transfection‐based manufacturing of adeno‐associated virus vectors. Biotechnology and Bioengineering, 119, 1685–1690. 10.1002/bit.28068
Shantoshini Dash and David M. Sharon contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Cardarelli, F. , Digiacomo, L. , Marchini, C. , Amici, A. , Salomone, F. , Fiume, G. , Rossetta, A. , Gratton, E. , Pozzi, D. , & Caracciolo, G. (2016). The intracellular trafficking mechanism of lipofectamine‐based transfection reagents and its implication for gene delivery. Scientific Reports, 6, 25879. 10.1038/srep25879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawood, R. (2020). Redefining AAV manufacturing: The time is now. Mary Ann Liebert. https://www.genengnews.com/magazine/redefining-aav-manufacturing-the-time-is-now/ [Google Scholar]
- Chahal, P. S. , Schulze, E. , Tran, R. , Montes, J. , & Kamen, A. A. (2014). Production of adeno‐associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. Journal of Virological Methods, 196, 163–173. 10.1016/j.jviromet.2013.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement, N. , & Grieger, J. C. (2016). Manufacturing of recombinant adeno‐associated viral vectors for clinical trials. Molecular Therapy. Methods & Clinical Development, 3, 16002. 10.1038/mtm.2016.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbacher, P. , Bettinger, T. , Brion, E. , Coll, J. L. , Plank, C. , Behr, J. P. , & Remy, J. S. (2004). Genuine DNA/polyethylenimine (PEI) complexes improve transfection properties and cell survival. Journal of Drug Targeting, 12(4), 223–236. 10.1080/10611860410001723487 [DOI] [PubMed] [Google Scholar]
- Furuta‐Hanawa, B. , Yamaguchi, T. , & Uchida, E. (2019). Two‐dimensional droplet digital PCR as a tool for titration and integrity evaluation of recombinant adeno‐associated viral vectors. Human Gene Therapy Methods, 30(4), 127–136. 10.1089/hgtb.2019.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fus‐Kujawa, A. , Prus, P. , Bajdak‐Rusinek, K. , Teper, P. , Gawron, K. , Kowalczuk, A. , & Sieron, A. L. (2021). An overview of methods and tools for transfection of eukaryotic cells in vitro. Frontiers in Bioengineering and Biotechnology, 9, 701031. 10.3389/fbioe.2021.701031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerviel, A. , Dash, S. , Moncorge, O. , Panthu, B. , Prchal, J. , Decimo, D. , Ohlmann, T. , Lina, B. , Favard, C. , Decroly, E. , Ottmann, M. , Roingeard, P. , & Muriaux, D. (2016). Involvement of an arginine triplet in M1 matrix protein interaction with membranes and in M1 recruitment into virus‐like particles of the influenza A (H1N1)pdm09 virus. PLoS One, 11(11), e0165421. 10.1371/journal.pone.0165421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin, D. A. , Shutova, M. V. , Johnston, N. R. , Smith, O. P. , Fedorin, V. V. , Kukushkin, Y. S. , van der Loo, J. C. M. , & Johnstone, E. C. (2021). The clinical landscape for AAV gene therapies. Nature Reviews. Drug Discovery, 20(3), 173–174. 10.1038/d41573-021-00017-7 [DOI] [PubMed] [Google Scholar]
- Nguyen, T. N. T. , Sha, S. , Hong, M. S. , Maloney, A. J. , Barone, P. W. , Neufeld, C. , Wolfrum, J. , Springs, S. L. , Sinskey, A. J. , & Braatz, R. D. (2021). Mechanistic model for production of recombinant adeno‐associated virus via triple transfection of HEK293 cells. Molecular Therapy. Methods & Clinical Development, 21, 642–655. 10.1016/j.omtm.2021.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich, N. C. , Sarnow, P. , Duprey, E. , & Levine, A. J. (1983). Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA‐binding protein. Virology, 128(2), 480–484. 10.1016/0042-6822(83)90274-x [DOI] [PubMed] [Google Scholar]
- Schindelin, J. , Arganda‐Carreras, I. , Frise, E. , Kaynig, V. , Longair, M. , Pietzsch, T. , Preibisch, S. , Rueden, C. , Saalfeld, S. , Schmid, B. , Tinevez, J. Y. , White, D. J. , Hartenstein, V. , Eliceiri, K. , Tomancak, P. , & Cardona, A. (2012). Fiji: An open‐source platform for biological‐image analysis. Nature Methods, 9(7), 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon, D. , & Kamen, A. (2018). Advancements in the design and scalable production of viral gene transfer vectors. Biotechnology and Bioengineering, 115(1), 25–40. 10.1002/bit.26461 [DOI] [PubMed] [Google Scholar]
- Sharon, D. M. , Nesdoly, S. , Yang, H. J. , Gelinas, J.‐F. , Xia, Y. , Ansorge, S. , & Kamen, A. A. (2020). A pooled genome‐wide screening strategy to identify and rank influenza host restriction factors in cell‐based vaccine production platforms. Scientific Reports, 10, 12166. 10.1038/s41598-020-68934-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, E. , Chin, C. S. H. , Lim, Z. F. S. , & Ng, S. K. (2021). HEK293 cell line as a platform to produce recombinant proteins and viral vectors. Frontiers in Bioengineering and Biotechnology, 9, 796991. 10.3389/fbioe.2021.796991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Tai, P. W. L. , & Gao, G. (2019). Adeno‐associated virus vector as a platform for gene therapy delivery. Nature Reviews. Drug Discovery, 18(5), 358–378. 10.1038/s41573-019-0012-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus, C. E. , Hugle‐Dorr, B. , Girod, A. , Petersen, G. , Hallek, M. , & Kleinschmidt, J. A. (2000). Monoclonal antibodies against the adeno‐associated virus type 2 (AAV‐2) capsid: Epitope mapping and identification of capsid domains involved in AAV‐2‐cell interaction and neutralization of AAV‐2 infection. Journal of Virology, 74(19), 9281–9293. 10.1128/jvi.74.19.9281-9293.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, W. , Warrington, K. H., Jr. , Hearing, P. , Hughes, J. , & Muzyczka, N. (2002). Adenovirus‐facilitated nuclear translocation of adeno‐associated virus type 2. Journal of Virology, 76(22), 11505–11517. 10.1128/jvi.76.22.11505-11517.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, W. , MacColl Garfinkel, A. E. , Li, Y. , Benowitz, L. I. , & Cepko, C. L. (2015). NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. Journal of Clinical Investigation, 125(4), 1433–1445. 10.1172/JCI79735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltner, N. , Kohlbrenner, E. , Clement, N. , Weber, T. , & Linden, R. M. (2010). Near‐perfect infectivity of wild‐type AAV as benchmark for infectivity of recombinant AAV vectors. Gene Therapy, 17(7), 872–879. 10.1038/gt.2010.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Lee, K. J. , Daris, M. , Lin, Y. , Wolfe, T. , Sheng, J. , Plewa, C. , Wang, S. , & Meisen, W. H. (2020). Creation of a high‐yield AAV vector production platform in suspension cells using a design‐of‐experiment approach. Molecular Therapy. Methods & Clinical Development, 18, 312–320. 10.1016/j.omtm.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


