Abstract
Background
Acute thrombotic thrombocytopenic purpura (TTP) is a life‐threatening emergency and plasma exchange (PEX) is the initial treatment shown to reduce acute mortality.
Objectives
To compare current practice in the United Kingdom (UK) against the standards set out in the 2012 British Society of Haematology guideline, and to better understand the issues affecting prompt initiation of PEX.
Patients/Methods
The trainee research network HaemSTAR conducted a retrospective nationwide review of adults presenting to UK hospitals with a first episode of acute TTP.
Results
Data on 148 patients treated at 80 UK hospitals between 2014 and 2019 demonstrated that 64.8% of patients received PEX within 24 h. Diagnostic uncertainty was the most commonly cited reason for delayed treatment. Conversely, a shorter time to PEX occurred in patients who had red cell fragments or severe thrombocytopenia identified on their first complete blood count. Availability of on‐site PEX was associated with a greater proportion of patients receiving PEX within 8 h compared to patients transferred, but by 24 h there was no difference between the two groups and two‐thirds of all patients had received their first PEX. The mortality rate for patients that received PEX was 9.2%, with 27.8% of deaths linked to delayed treatment initiation.
Conclusions
This is the first multi‐center evaluation of treatment delays in acute TTP and it will inform targeted pathways to improve prompt access to life‐saving intervention.
Keywords: immune thrombotic thrombocytopenic purpura, plasma exchange, quality improvement, service evaluation, thrombocytopenia
ESSENTIALS.
We review the causes for delays to plasma exchange for acute thrombotic thrombocytopenic purpura.
The treatment of adults in the United Kingdom was compared to national standards.
Of patients treated between 2014 and 2019, 64.8% received plasma exchange within 24 h.
Diagnostic uncertainty caused delayed treatment. Education is required to improve performance.
1. INTRODUCTION
Thrombotic thrombocytopenic purpura (TTP) is a very rare, life‐threatening condition precipitated by an acquired (immune‐mediated) or hereditary deficiency of A Disintigren And Metalloprotease with ThromboSponin‐type 1 motif, member 13 (ADAMTS‐13). TTP presents with a variable combination of fever, thrombocytopenia, microangiopathic hemolytic anemia, renal failure, neurological impairment, or other organ dysfunction. It is a recognized medical emergency and prompt diagnosis and initiation of therapy is imperative for survival. In cases of immune TTP, the introduction of plasma exchange (PEX) using fresh frozen plasma (FFP) in the 1990s transformed TTP from a near‐universally fatal disease into one with survival rates approaching 80%. 1 In the UK plasma exchange for TTP is performed using FFP that has been solvent detergent treated (SD‐FFP) to achieve viral inactivation, and this is sourced as the commercial product Octaplas. Both acquired and hereditary cases of TTP will receive urgent PEX at first presentation until the underlying cause is differentiated.
The addition of immunosuppression with steroids and rituximab further improved outcomes, but PEX remains the cornerstone of treatment shown to significantly impact acute mortality, and early treatment initiation is critical as up to 50% of TTP deaths occur within 24 h of presentation. 2 , 3 , 4 Caplacizumab, the monoclonal anti‐Von Willebrand factor (VWF) nanobody, was approved for use combined with PEX for the treatment of acute TTP in the UK in December 2020. While it is acknowledged that some international centers are moving to the use of caplacizumab to the exclusion of PEX, this is not the current standard of care in the UK.
Prompt initiation of PEX faces many logistical challenges. The average UK National Health Service (NHS) Trust may diagnose only one case of TTP per year, presenting symptoms are often non‐specific, and first point of contact is normally with non‐hematologists in the primary or secondary care setting. Laboratory biomedical scientists and/or laboratory technicians are often the first to propose the diagnosis of TTP after identifying thrombocytopenia and red cell fragmentation on blood film examination. Confirmation of the diagnosis is made by uniting the patient’s clinical presentation with the complete blood count and an ADAMTS‐13 activity of <10 iu/dl. ADAMTS‐13 activity is a specialist hemostasis assay that is only available at tertiary centers, with a turnaround time of 4–6 h, and is usually only offered during regular working hours.
Once TTP is recognized, arrangements for PEX must be made. Approximately 40 hospitals offer this service in the UK, typically the larger tertiary centers, with a maximum travel time of around 2 h for patients requiring transfer to one of these sites (excluding rare circumstances such as island residents who may need air ambulance). Where a 24‐h PEX service is not available either local ad hoc arrangements are made, or transfer to a tertiary center must be co‐ordinated, which can be a complex and time‐consuming task.
The recognized problems in acute TTP management have provoked recent proposals for a national service with appointed TTP specialist centers and provision for rapid hospital transfer. 5
There is currently no comprehensive record of treatment delays experienced by patients presenting with acute TTP in UK hospitals and these data would be valuable in the design of new or improved services. To understand issues affecting practice the trainee research network HaemSTAR conducted a retrospective nationwide audit of patients presenting to UK hospitals with TTP against British Society of Haematology (BSH) clinical guidelines. 6 HaemSTAR is a UK‐wide network of clinical hematology registrars supported by the National Institute of Health Research (NIHR) non‐malignant Clinical Research Network (CRN). 7
2. METHODS
2.1. Patients and data collection
The study protocol is appended as supporting information. Study registration and identification of eligible patients occurred August to October 2019 followed by data collection and submission October to December 2019. Adults ≥18 years presenting to hospital between June 1, 2014 and June 1, 2019 with first episode acute TTP and ADAMTS‐13 level <10 iu/dl were identified by local clinicians by cross‐referencing clinical records with local and central laboratory databases of ADAMTS‐13 results. Results of ADAMTS‐13 inhibitor screens were not recorded and acquired versus hereditary cases were not differentiated. Anonymized data were submitted via a secure online server maintained by the Birmingham Centre for Observational and Prospective Studies (BiCOPS). Where patients were transferred between sites, data were linked by matching admission dates and times.
2.2. Primary objective and audit standards
The primary objective was to assess the proportion of patients initiating therapeutic plasma exchange within 8 h of presentation—“Time to PEX” (defined below)—per BSH guidelines. 6 Secondary objectives included the evaluation of the remaining BSH recommendations as follows: the administration of steroids within 24 h, rituximab within 48 h in presence of central nervous system (CNS) and/or cardiac involvement, FFP transfusion in the event of delayed PEX, and the avoidance of platelet transfusions in the absence of major hemorrhage.
2.3. Definition of “Time to PEX”
The time from receipt of the first complete blood count (CBC) sample in the laboratory for this episode to the release of FFP from the blood bank for PEX was used as the surrogate definition of time from hospital presentation to initiation of PEX. These points were chosen as they represent consistent electronic time stamps comparable across multiple sites. However, the first CBC will not always represent the time that TTP was first considered as a diagnosis.
2.4. Additional objectives
In addition to the BSH 8‐h target, the proportion of patients initiating PEX within 24 h was also assessed as a key performance target on the basis that up to 50% of TTP deaths occur within 24 h of presentation. Common blood tests used to diagnose TTP were recorded to investigate whether equivocal or borderline results influenced rapidity of treatment. To evaluate the contributory factors for delay in patients starting PEX after more than 24 h clinicians were asked to review case notes, categorize the causes for delay, and rank the three most significant in each individual case. There was a subdivision of analysis of the results between 2014–2016 and 2017–2019. This was based on the initiation of a proposal for regional centers nationally in England at that time. The hypothesis was that the requirement for transfer to a treatment center would be a prominent factor in delays to treatment, so patients were grouped in three cohorts for data analysis: Those who received their whole treatment at a single center (“Single Center”), those who were transferred to a tertiary center for treatment following diagnosis (“Transferred”), and all patients (“All”).
2.5. Data collection window
The June 1, 2019 cut‐off predates the introduction of caplacizumab into UK clinical practice. Caplacizumab is an anti‐VWF nanobody used in addition to PEX and immunosuppression for the treatment of TTP and a recent phase III trial has shown benefit in reducing time to platelet count recovery and thereby reducing the number of exchanges and total days in hospital. 8 It is now standard of care in the management of TTP following confirmation of TTP by ADAMTS‐13 analysis and may be the subject of future audit.
2.6. Statistics
Results are reported as absolute numbers with percentages for binary states, and otherwise by median with interquartile range (IQR, from first to third quartile). The Mann‐Whitney U test was used to assess significance of differences in time to PEX between patient subgroups. The Chi‐square test was used to assess significance of difference between subgroups in meeting the 8‐ and 24‐h targets for PEX initiation. The travel distance between hospitals sites was calculated using the shortest available road route identified by Google maps. Travel distance and time to PEX were analyzed using a Pearson’s R correlation co‐efficient. Multivariate analysis was performed by binomial logistic regression to assess variables associated with receiving PEX in the 8‐h target. An alpha value of <0.05 was considered statistically significant. Analyses were performed in Microsoft Excel and SPSS Statistics 27 (IBM).
2.7. Ethics and governance
Due to the rarity of TTP, particular attention was given to protecting patient confidentiality. The audit and data management plans were reviewed by the Birmingham Clinical Trials Unit, the Cancer Research UK Clinical Trials Unit, Information Governance at University Hospitals Birmingham NHS Foundation Trust, and the Caldicott Guardian at University Hospitals Plymouth NHS Trust. All were satisfied that the protocol is an audit and does not compromise patient anonymity. To ensure the perspective of patients was included we consulted the TTP Network, the largest UK charity for patients with TTP. The protocol was reviewed by charity members via a patient forum. Feedback from patients was supportive of the audit’s aims and no concerns regarding patient anonymity were raised.
3. RESULTS
Data on 251 patients treated at 80 UK hospitals sites were submitted and after data clearing 148 patient records were sufficiently complete for final analysis. The primary reason for patient exclusion was the inability to retrieve data from the patient’s initial site of presentation (Figure 1).
FIGURE 1.
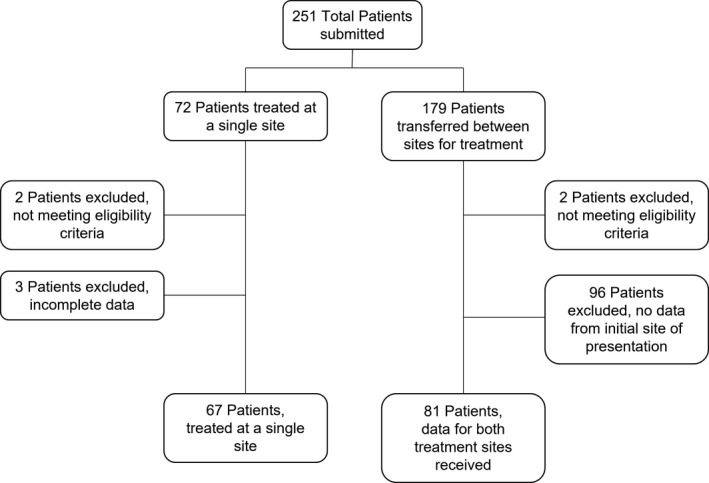
Data clearing of submitted patients
3.1. Baseline patient characteristics
Baseline patient characteristics were broadly similar between those treated at a single site and those requiring transfer, though transferred patients tended to be older (29.6% of transferred patients >60 years, vs. 18.0% of single‐center patients; Table 1).
TABLE 1.
Patient characteristics
| Patients treated at a single site | Patients transferred to another site for treatment | All patients | |
|---|---|---|---|
| Total no. of patients | 67 | 81 | 148 |
| ≥18 years ‐ <40 years | 35 (52.2%) | 23 (28.4%) | 58 (39.2%) |
| ≥40 years ‐ <50 years | 11 (16.4%) | 16 (19.8%) | 27 (18.2%) |
| ≥50 years ‐ <60 years | 9 (13.4%) | 18 (22.2%) | 27 (18.2%) |
| ≥60 years ‐ <70 years | 4 (6%) | 9 (11.1%) | 13 (8.8%) |
| ≥70 years ‐ <80 years | 4 (6%) | 12 (14.8%) | 16 (10.8%) |
| ≥80 years | 4 (6%) | 3 (3.7%) | 7 (4.7%) |
| No. pregnant | 3 (4.5%) | 3 (3.7%) | 6 (4.1%) |
| Laboratory values at presentation: | |||
| Platelet count, ×109/l, median (IQR) | 15 (10–27.5) | 11 (7–22) | 13 (8–24) |
| Hemoglobin, g/l, median (IQR) | 92 (83.5–108) | 94 (76–110) | 92 (80–110) |
| Creatinine, µmol/l, median (IQR) | 82 (69–108) | 101 (77–138) | 92 (70–129) |
| No. with schistocytes reported on first blood film: | 57 (85.1%) | 65 (80.2%) | 122 (82.4%) |
| No. with CNS signs or symptoms in first 48 h: | 28 (41.8%) | 51 (63%) | 79 (53.4%) |
| No. with troponin raised above ULN: | 42 (62.7%) | 60 (74.1%) | 102 (68.9%) |
| Mortality at 30 days: | 12 (17.9%) | 6 (7.4%) | 18 (12.2%) |
| Length of stay in days for patients surviving to discharge, median (IQR): | 12 (8–21) | 15 (10–25.5) | 14 (9–22) |
Abbreviations: CNS, central nervous system; IQR, interquartile range; PEX, plasma exchange; ULN, upper limit of normal.
3.2. Performance against audit standards
One hundred forty‐two patients (95.9%) received PEX, and of these 38 (26.8%) did so within the recommended 8 h from first presentation to hospital (Figure 2), with a statistically significant difference between patients treated at a single center versus those transferred (43.5% vs. 13.8%, P=< .001; Table 2). By 24 h, there was no statistical difference between the two groups (69.4% vs. 61.3% had received PEX, P = .316; Figure 3). Rates of timely administration of steroids (within 24 h, 69.8% single site vs. 61.5% transferred) and rituximab (within 48 h, 37.8% vs. 33.3%) were broadly similar. FFP was transfused in 5 of 16 patients (31.3%) whose PEX was delayed due to difficulty in arranging hospital transfer.
FIGURE 2.
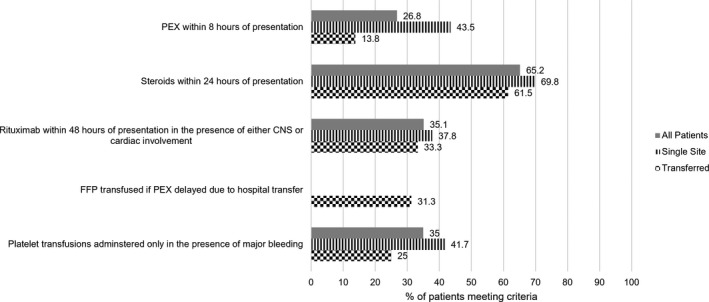
Graph of UK performance between 2014 and 2019 in the emergent management of new acute thrombotic thrombocytopenic purpura, measured against British Society of Haemotology guidelines. CNS, central nervous system; FFP, fresh frozen plasma; PEX, plasma exchange
TABLE 2.
Univariate analysis of factors affecting time to PEX with accompanying graphical representation
| Baseline characteristic | No. of patients | Median time to PEX, hours (IQR) | p | % PEX within 8 h | % PEX within 24 h | p | |
|---|---|---|---|---|---|---|---|
| All patients receiving PEX | 142 | 15 (8–40.75) | 26.8 | 64.8 | |||
| Site of PEX | |||||||
| Single center | 62 | 10 (4–41.75) | .002 | 43.5 | <.001 | 69.4 | .316 |
| Transferred | 80 | 19.5 (11–36.25) | 13.8 | 61.3 | |||
| Age, years | |||||||
| <60 | 109 | 13 (7–27) | .004 | 31.2 | .030 | 72.5 | <.001 |
| ≥60 | 33 | 34 (11–63) | 12.1 | 39.4 | |||
| Hemoglobin | |||||||
| <100 g/l | 88 | 11 (6.75–21.25) | <.001 | 34.1 | .018 | 78.4 | <.001 |
| ≥100 g/l | 54 | 31 (13–113.75) | 14.8 | 42.6 | |||
| Platelets | |||||||
| <30 × 10e9/l | 114 | 13 (7–25) | <.001 | 29.8 | .096 | 73.7 | <.001 |
| ≥30 × 10e9/l | 28 | 72 (20–183.75) | 14.3 | 28.6 | |||
| Fragments reported on first blood film | |||||||
| Yes | 118 | 13.5 (7–29) | .001 | 29.7 | .083 | 71.2 | <.001 |
| No | 24 | 98 (13–158) | 12.5 | 33.3 | |||
| Raised creatinine | |||||||
| Yes | 44 | 15.5 (10–32) | .904 | 22.7 | .467 | 68.2 | 0.322 |
| No | 98 | 15 (7–41.75) | 28.6 | 63.3 | |||
| Raised troponin | |||||||
| Yes | 101 | 14 (9–40) | 1.000 | 24.8 | .396 | 65.3 | .827 |
| No | 41 | 19 (5–41) | 31.7 | 63.4 | |||
| CNS symptoms | |||||||
| Yes | 78 | 16 (9.25–30.75) | .700 | 21.8 | .140 | 65.4 | .870 |
| No | 64 | 14 (5–78.5) | 32.8 | 64.1 | |||
| Admission after May 2017 | |||||||
| Yes | 81 | 12 (6–34) | .054 | 34.6 | .015 | 66.7 | .589 |
| No | 61 | 19 (11–63) | 16.4 | 62.3 | |||
Raised Creatinine / Troponin = value above upper limit of normal for local laboratory. Definition of CNS symptoms was at clinicians’ discretion.
Abbreviations: CNS, central nervous system; IQR, interquartile range; PEX, plasma exchange.
Twenty patients received platelet transfusion prior to PEX, with major bleeding identified as the indication for this in seven cases (35%). Where further details were available for the 13 patients transfused platelets in the absence of major bleeding the working diagnoses included acute sepsis (three cases), immune thrombocytopenia (two cases), coagulopathy of liver disease, and thrombocytopenia shortly following cardiothoracic surgery (one case each).
3.3. Variables affecting the time to plasma exchange
The overall median time to PEX was 15 h (IQR 8–40.75). Univariate analysis identified on‐site availability of PEX, age <60 years, hemoglobin (Hb) <100 g/l, platelet count <30x109/l, the presence of fragments reported on first blood film, and admissions occurring after May 2017 to be statistically significant predictors for PEX initiation within 8 hrs (Table 2). Availability of on‐site PEX was associated with significantly earlier treatment initiation with median time to PEX for those treated on site 10 hrs (IQR 4–41.75) versus 19.5 h (IQR 11–36.25) for patients transferred (P = .002; Figure 3). A blood film comment of red cell fragments significantly impacted time to treatment, this being 13.5 h (IQR 7–29) in cases in which fragments were reported versus 98 h (IQR 13–158) in the 24 cases in which fragments were not reported (Figure 3). The presence of a raised serum creatinine, raised troponin, or CNS abnormalities did not correlate with time to PEX. Patients requiring transfer by road had to travel a median of 22 miles (range 1–169, IQR 9.5–43.5) to reach an apheresis center. In patients treated within 24 h, travel distance between hospital sites did not correlate with time to PEX (R 2 = 0.058, P = .61). Using the significant variables from univariate analysis, on‐site availability of PEX (P < .001, odds ratio [OR] 4.77, 95% confidence interval [CI] 1.97–11.56) and admission occurring after 2017 (P = .043, OR 2.55, 95% CI 1.03–6.30) remained significant.
FIGURE 3.
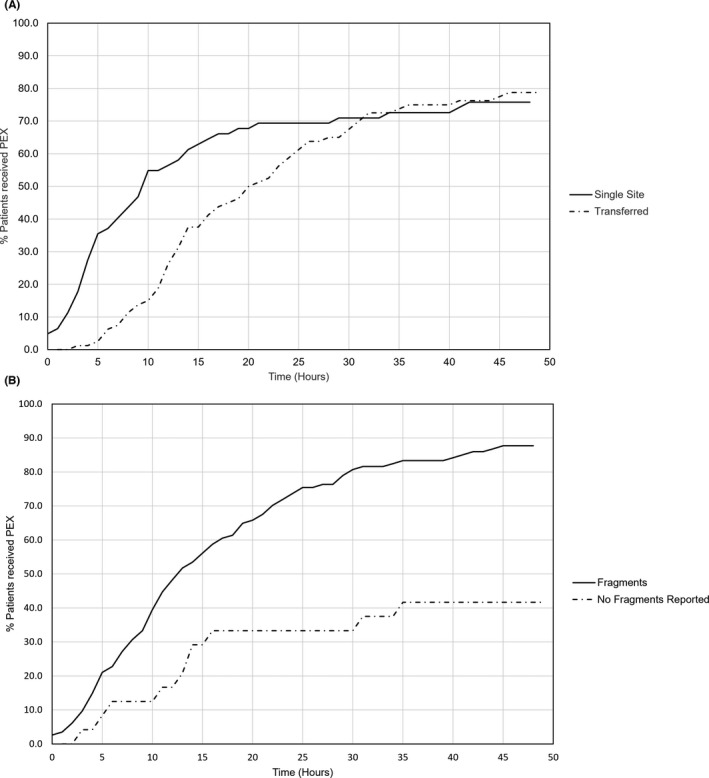
Graphical representation of univariate analysis showing percentage of patients commencing plasma exchange against time from first full blood count. A, Single site versus transferred patients. B, Red cell fragments reported on first blood film versus not reported
3.4. Clinical review of case notes
Fifty patients (35.2%) received PEX more than 24 h after first CBC. Diagnostic uncertainty was the most frequently cited factor in the assessment of causes for delayed treatment (Table 3). Where patients were initially managed for an alternative diagnosis, this resulted from both clinical and laboratory findings (e.g., stroke in nine cases and immune thrombocytopenia in 16 cases). In other cases, the diagnosis of TTP was suspected but the level of suspicion was considered low enough to wait for ADAMTS‐13 levels before arranging hospital transfer (nine cases). Difficulty in arranging hospital transfer was most frequently attributed to bed availability and the need for consultant‐to‐consultant referrals (four cases each).
TABLE 3.
Number of times prespecified factors were identified as contributing to the delayed treatment of the 50 patients who received PEX after more than 24 h
| Reason given for treatment delay | Single center | Transfer |
|---|---|---|
| Delayed or uncertain Diagnosis | 36 | 59 |
| Difficulty co‐ordinating hospital transfer | – | 16 |
| Waited for ADAMTS‐13 before transferring patient | – | 9 |
| Central line insertion | 5 | 2 |
| Coordinating PEX service | 4 | 1 |
| Other | 15 | 20 |
Abbreviations: ADAMTS‐13, A Disintigren And Metalloprotease with ThromboSponin‐type 1 motif, member 13; PEX, plasma exchange.
3.5. Patient outcomes
The median length of stay for all patients surviving to hospital discharge was 14 days (IQR 9–22). Eighteen patients died, giving a 30‐day mortality for all patients of 12.2% (9.2% for those that received PEX). Five patients who died did so after PEX was delayed for more than 24 h. In the three cases where further details were available, all were associated with delayed diagnosis of TTP due to another diagnosis being considered based on presenting clinical features (sepsis in two cases and acute stroke in the other). Six patients did not receive plasma exchange. Of these, five were considered too medically unstable for PEX and died within 10 days of presentation. Sixteen of the twenty patients who received platelet transfusions went on to receive plasma exchange and three of these patients died, giving a 30‐day mortality rate of 18.7%. No additional clinical information is available to implicate platelet transfusion related complications as the cause of death.
4. DISCUSSION
To our knowledge, worldwide this is the first multi‐center record of time to treatment from initial presentation of acute TTP. Our findings show that 64.8% of patients presenting with a new diagnosis of acute TTP to UK hospitals between 2014 and 2019 started PEX within 24 h of first CBC and that an 8‐h target is a challenging but achievable standard being met in 28.6% of cases. Performance improved, with an increased proportion of patients meeting the 8‐h target in the second half of our study window compared to the first. Early use of steroids and rituximab correlated with earlier use of PEX indicating that where timely diagnosis was made there was good compliance with guidelines.
Diagnostic uncertainty was cited as a cause of a delay in diagnosis and subsequently referral for treatment in 78% of reviewed cases, proving far more common than difficulty transferring patients between sites. In addition, while diagnostic uncertainty was identified in the majority of patients in both groups, it was more frequent in transferred patients (73% vs. 58% single center), which may indicate less awareness of TTP at hospitals without an apheresis service.
Older patients (>60 years), those with higher platelet counts (>30 × 109/l), higher Hb (>100 g/l), and absence of red cell fragments on film report were more likely to experience prolonged time to initiation of PEX. That 22% of patients initiated PEX over 48 h from admission indicates the issue is relatively common. Given the number of deaths occurring in this group, and the prevalence of diagnostic uncertainty as a cause of delayed treatment, we suggest initiatives to increase early diagnosis should be prioritized.
Targeted education could be used to raise awareness of this rare disease and we would suggest emergency and acute medicine health‐care professionals as those most likely to be the first to encounter a new diagnosis of TTP. A strength of the HaemSTAR network is that data were submitted by a hematolgoy trainee at each of the participating sites and they are well placed to present their local findings at departmental meetings to which the target audience can be invited. Word can also be spread nationally; abstracts will be submitted to acute medicine meetings to spread the word outside of our specialty bubble.
A change in laboratory practice to increase provision of ADAMTS‐13 testing by enabling out‐of‐hours testing and having access to it across a greater number of sites may reduce turnaround times and thereby accommodate a lower threshold of suspicion to instigate testing. This may identify atypical presentations more rapidly but can only be effective in the setting of an educated clinical team alert to the need for testing.
The establishment of commissioned UK TTP centers will allow for centralization of care, which has the potential to allow for more specialized care alongside improved audit and clinical governance processes. It could be further strengthened by offering outreach support to referring centers in making prompt diagnoses.
The impact of any such interventions described above will need to be assessed through re‐audit and we have made the full audit protocol available in the supporting information to facilitate this process for any center or group that choose to pursue this.
A 2021 paper retrospectively studied the treatment of 1096 adults, under the age of 60, treated for TTP in US hospitals between 2004 and 2015. 4 The investigators found that 28.8% of patients received PEX before their second day in the intensive care unit (ICU), median length of stay was 11 (7–20) days, and hospital mortality was 7.6%. A direct comparison with our UK data is not possible due to differences in the study window (2004–2015 vs. 2014–2019), patient population (age <60 treated in ICU vs. all adults), and definition of time to PEX (“before the second day of ICU admission” vs. as described above).
A recent French study reporting real‐world outcomes for patients receiving caplacizumab included comparison with an historical cohort of 180 patients treated with PEX, steroids, and rituximab between 2015 and 2018. 9 The authors report median length of stay and mortality for the historical cohort and the data are comparable with our findings (median length of stay 14 vs. 22 days and mortality 9.2% vs. 6.7%, for UK versus French data, respectively). These similarities support the reliability of our data and suggest UK and French practice is similar.
The patients requiring transfer to a tertiary site tended to be older than those presenting directly to a treatment center. This difference may reflect the demographic differences between rural and urban areas. 10
This study has limitations inherent to its retrospective design and reliance on the accuracy of local clinician reporting. Published guidelines define time to PEX as starting from the point of consideration of a diagnosis of TTP. The choice of time of first complete blood count and time of FFP release from blood bank does not capture delayed sampling of patients, failure to collect FFP from blood bank in a timely manner, or register the time when TTP diagnosis was first considered. The authors recognize that the latter is difficult to retrospectively define. Importantly, however, they are objective, electronically recorded times that deliver a generalizable definition across multiple sites. It seems plausible that the 8‐h target would have been met in a higher proportion of the reviewed patients if it were possible to reliably mark the time of first consideration of a diagnosis of TTP.
It was not possible to access records for half of the transferred patients identified by clinicians at tertiary sites. The missing records were largely for patients initially treated at hospitals without hematology trainee representation or with otherwise limited hematology services. We therefore cannot comment on whether the performance data we present for transferred patients are generalizable to this unassessed cohort.
Our data did not differentiate acquired from hereditary cases of TTP, with the latter not expected to require PEX where simple top‐up transfusion of FFP will suffice to replace absent ADAMTS‐13. As this study examines only first episodes of TTP, it seems reasonable to include the whole patient cohort in our analysis as in the UK all patients will initial receive treatment on the presumption of an immune‐mediated process.
Other potential factors that were not assessed in this study design may have influenced time to PEX. Examples include the need for urgent neurological imaging, availability of suitably matched FFP, and the availability of equipment and staff to perform any of the steps along the treatment pathway.
In addition, this study exemplifies, for the second time, 11 the ability of the HaemSTAR network to mobilize trainee researchers across the nation and deliver projects on a scale that would be otherwise unachievable on the available budget.
In conclusion, most UK patients presenting with a new diagnosis of acute TTP receive prompt life‐saving treatment. Initiatives that increase early diagnosis are likely to yield the greatest gains and should be prioritized.
CONFLICTS OF INTEREST
TB has no conflicts of interest to declare. RM has received research funding and honorarium from Sanofi. AD has no conflicts of interest to declare. PLRN has received research grants from Novartis, Principia, and Rigel Pharmaceuticals, as well as honoraria from Bayer, Grifols, and Takeda. RS has no conflicts of interest to declare. AL has no conflicts of interest to declare. ZS has received honoraria from the British Journal of Cardiology. DT has been on advisory boards for Immunovant, Roche, Abbvie, and Novartis; as well as received honoraria for conferences from Amgen and Takeda. MS has received speaker’s fees and advisory boards for Sanofi, Takeda, Octapharma, Novartis, Alexion.
AUTHOR CONTRIBUTIONS
Tom Bull analyzed the data and wrote the manuscript. Rory McCulloch conceived the study and analyzed the data. Andrew Doyle analyzed data. Rory McCulloch, Phillip L. R. Nicolson, Rebecca J. Shaw, Alex Langridge, Tom Bull, Zara Sayar, David Tucker, Michala Pettitt, Rita Perry, and Marie Scully form the study management committee and designed the study. Michala Pettitt and Rita Perry also co‐ordinated data collection and generated data queries. Rory McCulloch, Phillip L. R. Nicolson, Andrew Doyle, and Marie Scully critically appraised the manuscript. All other contributors collected data.
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGMENTS
Sanofi provided a grant to support the running of this project but had no input on project or abstract design.
The HaemSTAR Collaborators . Diagnostic uncertainty presented barriers to the timely management of acute thrombotic thrombocytopenic purpura in the United Kingdom between 2014 and 2019. J Thromb Haemost. 2022;20:1428–1436. doi: 10.1111/jth.15681
The HaemSTAR Collaborators are listed in supporting information.
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 16 February 2022
Contributor Information
The HaemSTAR Collaborators, Email: t.p.bullncl@me.com.
The HaemSTAR Collaborators:
Tom P. Bull, Rory McCulloch, Phillip L. R. Nicolson, Andrew J. Doyle, Rebecca J. Shaw, Alexander Langridge, Zara Sayar, David L. Tucker, Michala Pettit, Rita Perry, William Thomas, Catherine Page, Ioana Whalley, Tina Dutt, Louise Garth, Will Lester, Richard J. Buka, Mary Subhan, Victoria Ware, Rachel Rayment, Daniel Castle, Astrid Etherington, Luke Carter‐Brzezinski, Jayne Peters, Claire Corrigan, Narind Sharma, Gary Benson, Sarah Challenor, Thomas S. Skinner, Rui Zhao, Lyndsay A. G. McLeod‐Kennedy, Kenneth Douglas, Amy Knott, Sophie Smith, Julia Wolf, Sophie A. Todd, Vickie McDonald, Alexandros Rampotas, Christopher Dean, Gavinda Sangha, Sue Pavord, Nicholas Denny, Sarah Jaafar, David P. T. McLaughlin, Jennifer E. Ross, Mamatha Karanth, Sarah L. Beverstock, Lynn Mansonso, Samuel H. Burrows, David P. T. McLaughlin, Sudhir Tauro, Amir Shenouda, Benjamin M. Bailiff, Daniel Kajita, Joannes Hermans, Harshita Goradia, Emily M. Finan, Sarah Alford, Keir Pickard, Brigit Greystoke, Thomas Fail, Asmaa Abdussalam, Lara N Roberts, James B. Clark, Natalie Heeney, Jennifer Young, Jamie Maddox, Swathy Srinath, Jahanzeb Khawaja, Jayne Parkes, Samah Babiker, Beverley J. Hunt, Sarah L. Wheeldon, Paul Kerr, Molham Tahhan, Mark Vickers, Alexandra C. Pike, Quentin Hill, Nadreen Mustafa, Azza Almaremi, Emily Hughes, Sean J. F. McGoldrick, Eleana Loizou, Izabela James, Sara R. Boyce, Isabel Farmer, Murugaiyan Thanigaikumar, Sarah L. Wheeldon, Paul Kerr, Katherine Wickenden, Richard Gooding, Kathryn Thornton, Clare Kane, Adam Cole, JessicaC Griffin, Suzanne Docherty, Kiri I. Dixon, Josephine Crowe, Mathew Sheridan, Corinne De Lord, Amit Sud, Anna Austin, Nichola Coooper, Chris Bailey, Luke Attwell, Rachel Hall, Benjamin Gray, Salena R. Chauhan, Anand Lokare, Amy Gudger, Claire Horgan, Indrani Venkatadasari, Israa Kaddam, Claire L. Mapplebeck, Joost Van Veen, Maya Raj, Kanchana De Abrew, Edward Belsham, Cecilia Gyansah, Shalal Sadullah, Beena Salhan, Richard Murrin, Rhys L. Williams, Andrew Stewart, Naomi Cornish, Sophie Otton, Zeeshan Khan, Sam Ackroyd, Lucia Y. Chen, Nicholas P. Lafferty, Francesca Leonforte, Nicholas Pemberton, Emanal Rawi, Diana Triantafyllopoulou, Jagdish Adiyodi, Jun Yong, Elizabeth Jones, David Davies, Rachel C. Peck, Robson Philip, Thomas Seddon, Paul Cahalin, Catherine Prodger, David A. Dutton, Alexander J. Sternberg, Rajani Chengal, Paolo Polzella, and Marie Scully
REFERENCES
- 1. George J. TTP: the evolution of clinical practice. Blood. 2021;137(6):719‐720. [DOI] [PubMed] [Google Scholar]
- 2. Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393‐397. [DOI] [PubMed] [Google Scholar]
- 3. Scully M, Yarranton H, Liesner R, et al. Regional UK TTP registry: correlation with laboratory ADAMTS13 analysis and clinical features. Br J Haematol. 2008;142:819‐826. [DOI] [PubMed] [Google Scholar]
- 4. Louw A, Mariotte E, Darmon M, Cohrs A, Leslie D, Azoulay E. Outcomes in 1096 patients with severe thrombotic thrombocytopenic purpura before the caplacizumab era. PLoS One. 2021;16(8):e0256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dutt T, Scully M. A proposal: the need for thrombotic thrombocytopenic purpura Specialist Centres – providing better outcomes. Br J Haematol. 2015;170:737‐742. [DOI] [PubMed] [Google Scholar]
- 6. Scully M, Hunt BJ, Benjamin S, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158:323‐335. [DOI] [PubMed] [Google Scholar]
- 7. Nicolson PLR, Desborough MJR, Hart D, Biss TT, Lowe GC, Toh CH. A HaemSTAR is born; a trainee‐led, UK‐wide research network in haematology. Clin Med. 2019;19(6):532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scully M, Cataland SR, Peyvandi F, et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380:335‐346. [DOI] [PubMed] [Google Scholar]
- 9. Coppo P, Bubenheim M, Azoulay E, et al. A regimen with caplacizumab, immunosuppression and plasma exchange prevents unfavorable outcomes in immune‐mediated TTP. Blood. 2021;137(6):733‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rural Population 2014/15 Official Statistics, Department for Environment Food and Rural Affairs [Internet] . [cited 2021 Feb 08]. Available from: https://www.gov.uk/government/publications/rural‐population‐and‐migration/rural‐population‐201415
- 11. Nicolson PLR, Perry R, Buka R, et al. A single 1 g/kg dose of intravenous immunoglobulin is a safe and effective treatment for immune thrombocytopenia; results of the first HaemSTAR “Flash‐Mob” retrospective study incorporating 961 patients. Br J Haematol. 2021;196:433‐437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material


