Abstract
The use of a diagnostic panel comprising multiple biomarkers has the potential to accurately diagnose Parkinson’s disease (PD). However, a panel consisting solely of plasma biomarkers to diagnose PD is not available. This study aimed to examine the diagnostic ability of plasma biomarker panels for de novo PD using novel digital ultrasensitive immunoassay technology. We recruited 45 patients with de novo PD and 20 healthy controls (HCs). The concentrations of plasma α‐synuclein (α‐syn), amyloid β‐42 (Aβ42), Aβ40, phosphorylated tau 181 (p‐tau181), neurofilament light (NFL), and glial fibrillary acidic protein (GFAP) were quantified using the ultrasensitive single molecule array (Simoa) platform. Patients with de novo PD had higher plasma levels of α‐syn and p‐tau181 than HCs, adjusting for age and sex. Plasma levels of α‐syn and p‐tau181 were positively correlated in de novo PD patients. Higher plasma α‐syn levels were significantly associated with worse Unified Parkinson’s Disease Rating Scale (UPDRS) Part III motor scores, modified Hoehn and Yahr (H‐Y) stages, and increased risk of PD with mild cognitive impairment (PD‐MCI). Higher plasma p‐tau181 concentrations were linked to worse H‐Y stages. The diagnostic panel using plasma α‐syn and p‐tau181, combined with age and sex, showed good performance in discriminating de novo PD patients from HCs (area under the curve = 0.806). These findings suggest that plasma α‐syn and p‐tau181 together may be a promising diagnostic biomarker panel for de novo PD patients.
![]()
Keywords: de novo, Parkinson’s disease, phosphorylated tau 181, plasma biomarker, ultrasensitive single‐molecule array, α‐synuclein
To examine the diagnostic ability of plasma biomarker panels for de novo Parkinson's disease (PD), we used the ultrasensitive single molecule array (Simoa) HD‐X Analyzer to quantify the concentrations of plasma α‐synuclein (α‐syn), amyloid β‐42 (Aβ42), Aβ40, phosphorylated tau 181 (p‐tau181), neurofilament light (NFL), and glial fibrillary acidic protein (GFAP). Data showed that the diagnostic panel using plasma α‐syn and p‐tau181, combined with age and sex, showed good performance in discriminating de novo PD patients from HC (area under the curve 0.806). Plasma α‐syn and p‐tau181 may be a promising diagnostic biomarker panel for de novo PD patients.
![]()
Abbreviations
- α‐syn
α‐synuclein
- Aβ
amyloid β
- AD
Alzheimer's disease
- AUC
area under the curve
- AVLT
Auditory Verbal Learning Test
- BBB
blood‐brain barrier
- BNT
Boston Naming Test
- CCI
cognitive complaints interview
- CDT
clock drawing test
- CI
confidence interval
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CV
coefficient of variation
- DST
Digit Span Backward Test
- EDTA
ethylenediaminetetraacetic acid
- GFAP
glial fibrillary acidic protein
- GWAS
genome‐wide association studies
- HCs
healthy controls
- H‐Y
Hoehn and Yahr
- HAMD
Hamilton Depression Scale
- HAMA
Hamilton Anxiety Scale
- HVOT
Hooper Visual Organization Test
- IMR
immunomagnetic reduction
- JLOT
Benton’s Judgment of Line Orientation Test
- LMT
Logical Memory Test
- LBs
lewy bodies
- LP
lumbar puncture
- MDS
movement disorder society
- MRI
magnetic resonance imaging
- MMSE
Mini‐Mental State Examination
- MoCA
Montreal Cognitive Assessment
- MCI
mild cognitive impairment
- NA
not available
- NFL
neurofilament light
- NMSs
non‐motor symptoms
- NMSQuest
non‐motor symptoms questionnaire
- p‐tau181
phosphorylated tau 181
- PD
Parkinson’s disease
- PD‐D
dementia associated with PD
- PET
positron emission tomography
- PDSS
Parkinson Disease Sleep Scale
- ROC
receiver operating characteristic
- SCWT
Stroop Color‐Word Test
- SPECT
single‐photon emission computed tomography
- Simoa
single molecule array
- SCCs
subjective cognitive complaints
- TMT‐A
Trail Making Test A
- TMT‐B
Trail Making Test B
- UPDRS
Unified Parkinson’s Disease Rating Scale
- VFT
Verbal Fluence Test
1. INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disorder worldwide and its incidence and prevalence have risen dramatically with the trend of population aging (Bloem et al., 2021). According to the latest statistics, approximately 6.1 million people globally were affected by PD in 2016 (Feigin et al., 2019). Currently, the diagnostic criteria for PD rely mainly on clinical motor manifestations, which do not appear until several years after the neurodegenerative process begins (Bloem et al., 2021). Even when the PD diagnostic criteria are correctly applied, the misdiagnosis rate is very high because of the large amount of clinical overlap between PD and other neurodegenerative diseases, especially during the early stages of the disease (Tolosa et al., 2006). Therefore, reliable biomarkers for early identification of PD patients are urgently needed.
Positron emission tomography (PET) and single‐photon emission computed tomography (SPECT) imaging are very sensitive at detecting decreases in the density of dopaminergic nerve terminals in the basal ganglia; however, these decreases are not unique to PD and these methods are expensive and expose patients to radiation (Verger et al., 2021). The proximity of cerebrospinal fluid (CSF) to the central nervous system (CNS) makes it an ideal source of diagnostic biomarkers for ongoing pathological processes. However, lumbar puncture (LP) is the only way to collect CSF and is unpopular because of its moderate invasiveness and potentially adverse effects (Duits et al., 2016). Peripheral biofluid samples mainly include saliva and blood (plasma/serum). As saliva is more accessible than blood, it is advantageous to measure biomarkers in saliva. However, there are several technical challenges of proteomic analysis of saliva samples, including low protein concentrations and high inter‐ and intra‐individual variability. Thus, blood appears to be more suitable for assessing biomarkers.
It must be noted that the presence of the blood‐brain barrier (BBB) contributes to a very low concentration range of PD blood biomarkers and an extensive body of complex blood matrix proteins further interferes with the measurement of low‐abundance target proteins, making it extremely challenging to accurately quantify PD blood biomarkers (Hansson, 2021). With advancements in ultrasensitive immunoassay technology in recent years, the ultrasensitive single‐molecule array (Simoa), which is based on the principle of digital protein detection, is 1000 times more sensitive than traditional immunoassay methods, making it the most sensitive protein detection technology available (Rissin et al., 2010). At present, ultrasensitive Simoa analysis kits have achieved successful quantitative detection of candidate biomarkers in plasma for the diagnosis of PD.
Candidate biomarkers for PD include α‐synuclein (α‐syn), amyloid β (Aβ), tau protein, neurofilament light (NFL), and glial fibrillary acidic protein (GFAP). Pathologically, PD is characterized by degeneration of the dopaminergic nigrostriatal system; the accumulation of α‐syn in Lewy bodies (LBs) and Lewy neurites; and various structural changes including amyloid pathology, tauopathy, and axonal damage (Jellinger, 2012). Notably, GFAP, which is a major structural component of astrocytes, is proven to be a useful blood biomarker of astrogliosis in neurodegenerative conditions (Ishiki et al., 2016; Yang & Wang, 2015).
When prior studies have looked for biomarkers that distinguish PD patients from healthy controls (HCs), it has been found that the level of a single biomarker exhibits considerable overlap between the two groups. Considering that PD is a multifactorial condition, a panel of biomarkers reflecting different pathological processes may be better at distinguishing PD patients from controls (Parnetti et al., 2019). A recent study demonstrated that the combination of CSF α‐syn species, classic Alzheimer's disease (AD) biomarkers, and plasma NFL can achieve significant diagnostic accuracy in PD (Oosterveld et al., 2020). However, the disadvantage of the panel used is that it requires some CSF biomarkers obtained by LP.
To the best of our knowledge, a panel solely composed of plasma biomarkers to distinguish between PD patients and HCs has not been investigated using the ultrasensitive Simoa HD‐X platform. To address these gaps, we assessed candidate biomarkers in plasma, including α‐syn, Aβ42, Aβ40, phosphorylated tau 181 (p‐tau181), NFL, and GFAP, to constitute a plasma biomarker panel that can discriminate de novo PD patients from HCs. We hypothesized that a plasma biomarker panel can differentiate de novo PD patients from HCs.
2. MATERIALS AND METHODS
2.1. Participants
The study population included 45 patients with de novo PD recruited from the Department of Neurology, Affiliated Brain Hospital of Nanjing Medical University, and 20 sex‐ and age‐matched HCs free of obvious neurological, psychiatric, or systemic diseases recruited from the community through advertisements between 2018 and 2021. Patients were defined as “de novo PD” according to the following inclusion criteria: (i) newly diagnosed with PD based on the United Kingdom Parkinson’s Disease Society Brain Bank clinical diagnostic criteria (Gibb & Lees, 1988); (ii) drug naive; (iii) early‐ or middle‐stage PD (modified Hoehn and Yahr (H‐Y) stage ≤3); (iv) follow‐up for at least 1 year; and (v) detailed clinical assessment information. The exclusion criteria were: (i) atypical or secondary parkinsonism disorders; (ii) a diagnosis of dementia associated with PD (PD‐D) based on the Movement Disorder Society (MDS) clinical diagnostic criteria (Emre et al., 2007); (iii) brain magnetic resonance imaging (MRI) scans indicating obvious clinically significant lesions; and (iv) serious chronic diseases including cardiac failure and diabetes with complications.
This exploratory study was not pre‐registered. We also did not perform blinding or conduct sample calculation. The sample size was based on previous similar studies exploring PD biomarkers (Delgado‐Alvarado et al., 2017; Schulz et al., 2021). Ethics approval was obtained from the Medical Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University (2015‐KY030 and 2019‐KY019‐01) for the use of human subjects in the present study. All participants provided written informed consent.
2.2. Clinical evaluation
Demographics, motor symptoms, and non‐motor symptoms (NMSs), including comprehensive neuropsychological assessments for five cognitive domains, were collected for all PD patients. The demographic data included age, sex, years of formal education, age of PD onset, and disease duration. Age of onset was defined as the age at which subjective motor symptoms first appeared, while disease duration was quantified as the time from first subjective motor symptoms until the time of blood sampling for this study. The severity of motor symptoms and stage of disease were rated using the UPDRS part II, UPDRS part III, and the modified H‐Y stage, respectively. NMSs were evaluated using the non‐motor symptoms questionnaire (NMSQuest) (Chaudhuri et al., 2006). Emotional state and sleep problems were assessed using the Hamilton Depression Scale (HAMD), Hamilton Anxiety Scale (HAMA), and Parkinson’s Disease Sleep Scale (PDSS). The cognitive complaints interview (CCI) was used to assess subjective cognitive complaints (SCCs) (Thomas‐Antérion et al., 2006). Global cognition was assessed using the Mini‐Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005). To correct for educational effect, if PD patients were educated for ≤12 years, the MoCA scores (if <30) were increased by 1 point. Detailed neuropsychological tests based on MDS recommendations (Litvan et al., 2012) were used to evaluate the following five cognitive domains: (i) attention/working memory: Digit Span Backward Test (DST), Trail Making Test A (TMT‐A), and Stroop Color‐Word Test (SCWT; comprising three tasks); (ii) executive function: Trail Making Test B (TMT‐B), Clock Drawing Test (CDT), and Verbal Fluence Test (VFT); (iii) language: Wechsler Adult Intelligence Scale III (WAIS‐III) Similarities Test and Boston Naming Test (BNT); (iv) memory: Auditory Verbal Learning Test (AVLT) and Logical Memory Test (LMT); and (v) visuospatial function: Benton’s Judgment of Line Orientation Test (JLOT) and Hooper Visual Organization Test (HVOT).
De novo PD patients were classified as PD with normal cognitive function (PD‐NC) or PD with mild cognitive impairment (PD‐MCI). The PD‐MCI diagnostic criteria proposed by MDS level 2 were applied (Litvan et al., 2012): (i) impaired on at least two tests within a single cognitive domain or across different cognitive domains and (ii) with cognitive performance of 1.5 standard deviations (SDs) below the normative sample.
2.3. Measurement of plasma α‐syn, Aβ42, Aβ40, p‐tau181, NFL, and GFAP
Venous blood samples were collected from all participants (45 PD patients and 20 HCs) using 9‐ml K3‐ethylenediamine tetraacetic acid (EDTA) tubes, and centrifuged at 2500 g for 15 min at 15–25℃ within 1 h of collection to obtain plasma. Plasma samples were then aliquoted and stored at −80°C until testing. Hemolysis of plasma samples was excluded.
Plasma concentrations of α‐syn, Aβ42, Aβ40, p‐tau181, NFL, and GFAP were measured using the ultrasensitive Simoa Human α‐syn Discovery Kit (α‐syn; Cat. No. 102233), Neurology 4‐Plex E Advantage Kit (Aβ40, Aβ42, GFAP, and NFL; Cat. No. 103670), p‐tau 181 V2 Advantage Kit (p‐tau181; Cat. No. 103714), and Simoa HD‐X Analyzer, in accordance with the manufacturer’s recommended protocol. Samples were measured in duplicate. The coefficient of variation (CV) was less than 10%, demonstrating the stability and reliability of the results. The two quality control samples with high and low concentrations provided as part of the kit were included in each run and the values were all within the expected range.
2.4. Statistical analysis
Statistical analyses were performed using SPSS version 25.0 (SPSS, Inc.), and figures were created in GraphPad Prism version 8.3.0 (GraphPad Software) and OriginPro 2021 version 9.8.0.200 (OriginLab). The Shapiro–Wilk test was employed to assess the normal distribution of the data. Outliers were evaluated using the boxplot method with thresholds <Q1 ‐ 1.5 * IQR and > Q3 + 1.5 * IQR. No outliers or individual data points were excluded from the analysis. The mean and SD are presented for continuous variables, while numbers, and percentages are reported for categorical variables. The difference in sex composition between the HC and PD groups was assessed by the chi‐square test. Depending on the normality of the variables, the two‐sample t test, or Mann‐Whitney U test was performed to compare demographics other than sex and clinical characteristics between the HC and PD groups. Correlations between age and plasma biomarker levels were assessed using Spearman’s rank correlation or Pearman correlation analysis. Multivariable linear regression analysis was used to compare all plasma biomarker levels between PD and HC groups, adjusted for age, and sex. Correlations between plasma biomarkers in de novo PD patients were calculated using Spearman’s rank correlation analysis. For the multivariable linear or logistic regression analysis, Z‐score transformation of all plasma biomarkers was performed to achieve similar scales, and the association between biomarker levels and clinical outcomes (motor and cognitive) was then investigated controlling for age, sex, and disease duration as potential confounders. The ability of individual or combined biomarkers to predict PD diagnosis was quantified via receiver operating characteristic (ROC) analysis. A two‐tailed p < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Participant characteristics
Table 1 summarizes the demographic and clinical features of the 20 HCs and 45 de novo PD participants. PD patients and HCs were matched for age, sex, and formal education. The mean age of onset in the PD patients was 56.1 years old and the mean disease duration was 7.9 years. The PD patients had more severe motor symptoms (UPDRS part III) and NMSs (NMSQuest, HAMD, HAMA, CCI, and MoCA) than the HC group. For the neuropsychological tests, compared with the HC group, the PD patients performed significantly poorer on the SCWT‐A‐time, SCWT‐B‐time, CDT, AVLT‐delayed recall, AVLT‐recognition, JLOT, and HVOT. In PD patients, both plasma Aβ42 and p‐tau181 levels were positively correlated with age (rs = 0.315, p = 0.035; rs = 0.434, p = 0.003, respectively).
TABLE 1.
Demographic and clinical characteristics of healthy controls (HCs) and patients with de novo Parkinson’s disease (PD)
| Variables | HC (n = 20) | PD (n = 45) | p value |
|---|---|---|---|
| Age (years) | 61.4 ± 7.1 | 58.0 ± 8.0 | 0.112 |
| Sex (Male/Female) | 9/11 | 16/29 | 0.470 |
| Formal education (years) | 11.6 ± 2.4 | 10.0 ± 3.0 | 0.076 |
| Age at onset (years) | NA | 56.1 ± 7.9 | NA |
| Disease duration (years) | NA | 1.9 ± 1.1 | NA |
| UPDRS part II | NA | 7.2 ± 2.9 | NA |
| UPDRS part III | 0.5 ± 1.4 | 21.9 ± 10.3 | <0.001 |
| H‐Y stage | NA | 1.6 ± 0.5 | NA |
| NMSQuest | 1.8 ± 1.8 | 7.5 ± 3.7 | <0.001 |
| HAMD | 1.1 ± 2.4 | 8.6 ± 5.7 | <0.001 |
| HAMA | 0.6 ± 1.2 | 6.3 ± 4.4 | <0.001 |
| PDSS | NA | 128.3 ± 17.6 | NA |
| CCI | 1.7 ± 1.7 | 3.0 ± 2.4 | 0.030 |
| MMSE | 28.7 ± 1.1 | 27.7 ± 2.3 | 0.214 |
| MoCA | 27.7 ± 1.6 | 23.8 ± 3.4 | <0.001 |
| MCI | NA | 27 (60.0%) | NA |
| Attention/working memory | |||
| DST | 10.7 ± 2.1 | 11.9 ± 2.6 | 0.073 |
| DST‐forward | 6.7 ± 1.7 | 7.2 ± 1.4 | 0.119 |
| DST‐backward | 4.1 ± 1.1 | 4.6 ± 1.5 | 0.181 |
| TMT‐A (second) | 82.7 ± 24.2 | 97.4 ± 46.8 | 0.238 |
| SCWT‐A‐time (second) | 23.0 ± 4.1 | 28.8 ± 10.0 | 0.003 |
| SCWT‐B‐time (second) | 32.4 ± 6.3 | 40.6 ± 16.4 | 0.008 |
| SCWT‐C‐time (second) | 66.1 ± 14.5 | 73.1 ± 21.5 | 0.186 |
| SCWT‐A‐right | 50.0 ± 0.2 | 49.9 ± 0.4 | 0.785 |
| SCWT‐B‐right | 49.6 ± 1.0 | 48.8 ± 2.8 | 0.312 |
| SCWT‐C‐right | 47.4 ± 2.3 | 46.7 ± 5.9 | 0.194 |
| Executive function | |||
| TMT‐B (second) | 156.0 ± 38.4 | 203.1 ± 88.5 | 0.057 |
| CDT | 10.0 ± 0.0 | 8.2 ± 2.4 | <0.001 |
| VFT | 19.2 ± 2.9 | 18.2 ± 4.9 | 0.272 |
| Language | |||
| Similarities | 17.4 ± 3.4 | 15.6 ± 4.9 | 0.228 |
| BNT | 25.5 ± 2.5 | 23.4 ± 4.4 | 0.110 |
| Memory | |||
| AVLT‐delayed recall | 7.2 ± 2.5 | 5.7 ± 2.4 | 0.031 |
| AVLT‐recognition | 23.0 ± 1.1 | 22.0 ± 1.7 | 0.032 |
| LMT‐delayed recall | 6.9 ± 1.7 | 5.9 ± 2.4 | 0.109 |
| Visuospatial function | |||
| JLOT | 24.8 ± 2.5 | 22.7 ± 3.1 | 0.009 |
| HVOT | 16.9 ± 3.9 | 13.5 ± 4.9 | 0.008 |
Note: “n” refers to the number of participants.
Data are presented as the mean ± SD or n (%).
p‐values were calculated using the two‐sample t test, Mann‐Whitney U test, or Chi‐square test. Statistically significant values (p < 0.05) are indicated in bold.
Abbreviations: AVLT, Auditory Verbal Learning Test; BNT, Boston Naming Test; CCI, cognitive complaints interview; CDT, Clock Drawing Test; DST, Digit Span Backward Test; HAMA, Hamilton anxiety scale; HAMD, Hamilton depression scale; HVOT, Hooper Visual Organization Test; H‐Y, Hoehn and Yahr; JLOT, Benton’s Judgment of Line Orientation Test; LMT, Logical Memory Test; MCI, mild cognitive impairment; MMSE, Mini‐mental state examination; MoCA, Montreal cognitive assessment; NA, not available; NMSQuest, non‐motor symptoms questionnaire; PDSS, Parkinson disease sleep scale; SCWT, Stroop Color‐Word Test; TMT‐A, Trail Making Test A; TMT‐B, Trail Making Test B; UPDRS, Unified Parkinson’s disease rating scale; VFT, Verbal Fluence Test.
3.2. Comparisons of plasma biomarkers between HC and PD groups
Comparisons of all plasma biomarker concentrations between HC and PD groups in multivariable analyses adjusted for age and sex are shown in Figure 1. There were no significant differences in terms of plasma Aβ42, Aβ40, NFL, or GFAP concentrations between the PD and HC groups (Aβ42: 6.7 ± 1.2 vs. 6.8 ± 1.6 pg/ml, p = 0.893; Aβ40: 93.3 ± 21.9 vs. 98.2 ± 25.0 pg/ml, p = 0.790; NFL: 13.3 ± 8.5 vs. 12.7 ± 5.0 pg/ml, p = 0.509; GFAP: 82.0 ± 28.1 vs. 84.3 ± 32.2 pg/ml, p = 0.990; Figure 1b, c, e, f). However, plasma α‐syn and p‐tau181 levels were significantly elevated in PD patients compared to the HC group (α‐syn: 8470.0 ± 7193.9 vs. 4568.4 ± 2878.2 pg/ml, p = 0.040; p‐tau181: 2.1 ± 1.3 vs. 1.5 ± 0.3 pg/ml, p = 0.015; Figure 1a, d).
FIGURE 1.
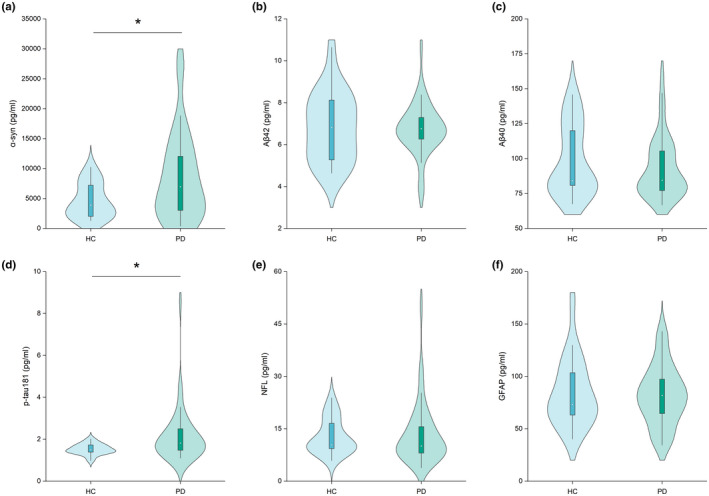
Violin plot of plasma biomarker levels between healthy controls (HCs) (n = 20) and patients with de novo Parkinson’s disease (PD) (n = 45). (a) α‐synuclein (α‐syn); plasma α‐syn concentration testing was unsuccessful for two HC participants; (b) amyloid β‐42 (Aβ42), (c) amyloid β‐40 (Aβ40); (d) phosphorylated tau 181 (p‐tau181); (e) neurofilament light chain protein (NFL); and (f) glial fibrillary acidic protein (GFAP). p‐values were assessed by multivariable linear regression analysis (adjusted for age and sex). *p < 0.05. “N” refers to the number of plasma samples
3.3. Correlations between plasma biomarkers in PD patients
Figure 2 shows the correlations between plasma biomarkers in the PD group. The plasma α‐syn level was positively correlated with plasma p‐tau181 concentration (rs = 0.34, p = 0.022), and the plasma Aβ42 level was positively associated with plasma Aβ40 concentration (rs = 0.66, p < 0.001). Moreover, the plasma NFL level was positively associated with plasma GFAP and Aβ40 concentrations (rs = 0.39, p = 0.008; rs = 0.52, p < 0.001, respectively).
FIGURE 2.
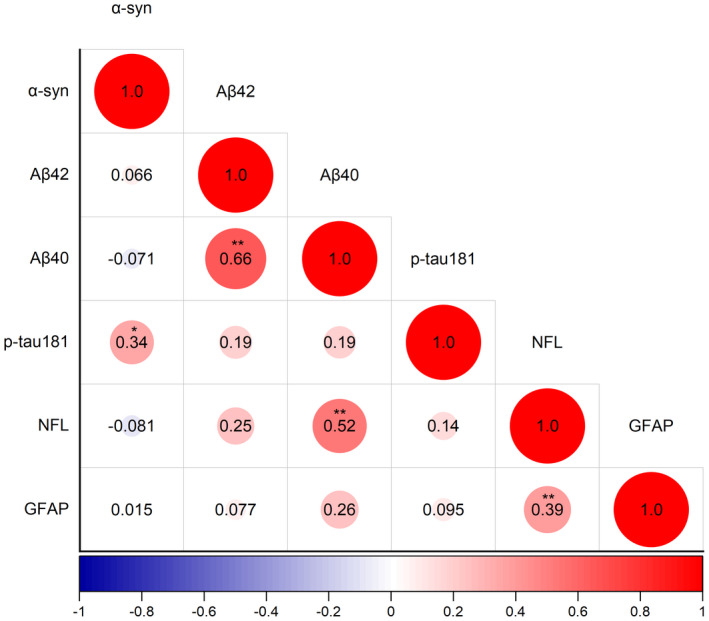
Correlations between plasma biomarkers in de novo Parkinson’s disease (PD) patients (n = 45). The size of the circle represents the relationship between the plasma biomarkers; the value in the circle is the correlation coefficient, calculated by Spearman’s rank correlation analysis. Negative correlations are marked in blue and positive correlations in red. *p < 0.05, **p < 0.01. “N” refers to the number of plasma samples. α‐syn, α‐synuclein; Aβ42, amyloid β‐42; Aβ40, amyloid β‐40; p‐tau181, phosphorylated tau 181; GFAP, glial fibrillary acidic protein; NFL, neurofilament light chain protein
3.4. Relationships between plasma biomarkers and clinical outcomes in PD patients
Next, we explored whether plasma biomarkers were related to motor or cognitive outcomes in de novo PD patients by multivariable regression analysis, controlling for age, sex, and disease duration (Table 2). Higher plasma α‐syn levels were significantly associated with worse UPDRS Part III motor scores (β = 3.105, 95% CI: 0.041–6.170, p = 0.047), worse modified H‐Y stages (β = 0.203, 95% CI: 0.056–0.350, p = 0.008), and increased risk of PD‐MCI (OR = 2.536, 95% CI: 1.030–6.245, p = 0.043). However, higher plasma p‐tau181 concentrations were only significantly related to worse H‐Y stages (β = 0.243, 95% CI: 0.091–0.394, p = 0.002).
TABLE 2.
Multivariable regression analysis of plasma biomarker levels with motor outcomes and cognitive function in de novo Parkinson’s disease (PD) patients
| UPDRS part III | H‐Y stage | MoCA | PD‐MCI | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) a | p | β (95% CI) a | p | β (95% CI) a | p | OR (95% CI) b | p | |
| α‐syn | 3.105 (0.041 to 6.170) | 0.047 | 0.203 (0.056 to 0.350) | 0.008 | −0.035 (−0.987 to 0.917) | 0.941 | 2.536 (1.030 to 6.245) | 0.043 |
| Aβ42 | −2.534 (−5.753 to 0.684) | 0.119 | −0.077 (−0.241 to 0.088) | 0.352 | −0.046 (−1.026 to 0.935) | 0.925 | 0.864 (0.417 to 1.787) | 0.693 |
| Aβ40 | −1.737 (−5.166 to 1.692) | 0.312 | −0.004 (−0.178 to 0.169) | 0.959 | −0.136 (−1.161 to 0.890) | 0.790 | 1.929 (0.768to 4.846) | 0.162 |
| p‐tau181 | 1.261 (−2.125 to 4.646) | 0.456 | 0.243 (0.091 to 0.394) | 0.002 | −0.665 (−1.649 to 0.320) | 0.180 | 2.161 (0.572 to 8.168) | 0.256 |
| NFL | 1.924 (−1.359 to 5.206) | 0.243 | 0.109 (−0.054 to 0.273) | 0.184 | 0.157 (−0.829 to 1.142) | 0.750 | 1.222 (0.568 to 2.629) | 0.609 |
| GFAP | 1.614 (−1.981 to 5.209) | 0.370 | −0.015 (−0.197 to 0.166) | 0.865 | 0.422 (−0.643 to 1.486) | 0.428 | 1.387 (0.646 to 2.979) | 0.402 |
Note: All plasma biomarkers were standardized by z‐score transformation to achieve comparable scales for the various outcomes of interest. Multivariable regression analysis was adjusted for age, sex, and disease duration. Bold values are statistically significant (p < 0.05).
Abbreviations: Aβ40, amyloid β‐40; Aβ42, amyloid β‐42; CI, confidence interval; GFAP, glial fibrillary acidic protein; H‐Y, Hoehn and Yahr; MCI, mild cognitive impairment; MoCA, Montreal cognitive assessment; NFL, neurofilament light chain protein; OR, odds ratio; p‐tau181, phosphorylated tau 181; UPDRS, Unified Parkinson’s disease rating scale; α‐syn, α‐synuclein.
In the multivariable linear regression analysis, the β coefficient was the change in the score of UPDRS part III, H‐Y stage, or MoCA by increasing one unit of standardized plasma biomarkers.
Multivariable logistic regression analysis was performed to determine the independent variables associated with the PD‐MCI.
3.5. Combined basic clinical features and plasma biomarkers for PD diagnosis
The ability of age, sex, and the aforementioned significant plasma α‐syn and p‐tau181 biomarkers to diagnose PD was expressed as the area under the curve (AUC) and its 95% confidence interval (CI) in the ROC analysis (Figure 3). Compared to the basic model (age and sex, AUC 0.646, 95% CI 0.497–0.794, sensitivity 55.6%, specificity 80.0%, p = 0.063), diagnostic ability increased when adding the plasma α‐syn or p‐tau181 biomarker (basic + α‐syn: AUC 0.719, 95% CI 0.588–0.849, sensitivity 55.6%, specificity 83.3%, p = 0.007; basic + p‐tau181: AUC 0.782, 95% CI 0.665–0.900, sensitivity 82.2%, specificity 70.0%, p < 0.001). The best discriminating panel comprised age, sex, plasma α‐syn, and p‐tau181 levels as the full model (AUC 0.806, 95% CI 0.690–0.922, sensitivity 82.2%, specificity 66.7%, p < 0.001).
FIGURE 3.
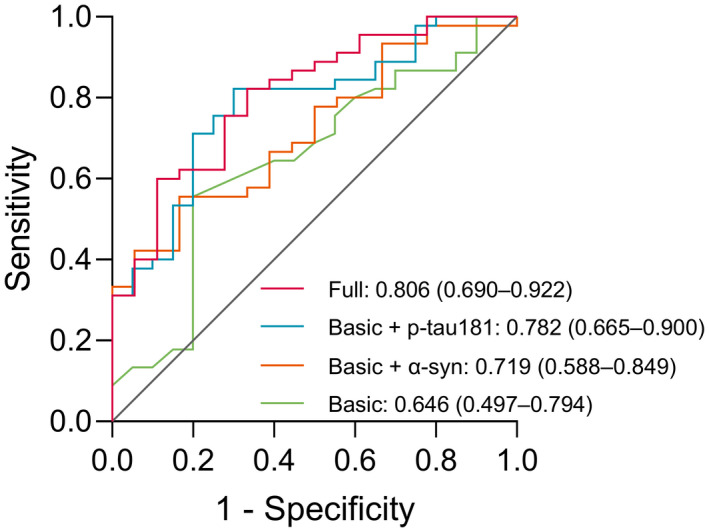
Receiver operating characteristic curves (ROC) for distinguishing Parkinson’s disease (PD) patients (n = 45) from healthy controls (n = 20). The AUC and 95% confidence interval for the ROC analysis are presented for the four models: (a) basic (age and sex): Green line; (b) basic + α‐syn: Orange line; (c) basic + p‐tau181: Blue line; and (d) full (age, sex, α‐syn, and p‐tau181): Red line. “N” refers to the number of plasma samples. α‐syn, α‐synuclein; p‐tau181, phosphorylated tau 181
4. DISCUSSION
This is the first study to evaluate the diagnostic value of plasma biomarkers to identify de novo PD using the ultrasensitive Simoa HD‐X platform. The results showed that plasma α‐syn and p‐tau181 levels were elevated in the de novo PD group compared to the HC group. Furthermore, higher plasma α‐syn levels were significantly associated with worse motor and cognitive impairments, and higher plasma p‐tau181 concentrations were related to motor symptoms. Importantly, the combination of age, sex, plasma α‐syn, and p‐tau181 showed good performance for discriminating de novo PD patients from HCs (AUC 0.806). Therefore, plasma α‐syn and p‐tau181 may serve as a promising biomarker panel for the discrimination of de novo PD patients.
The findings that plasma α‐syn levels are higher in PD patients compared with HCs, controlling for age and sex, using the ultrasensitive Simoa HD‐X platform, are consistent with previous research results (Ng et al., 2019; Youssef et al., 2021). Studies using other platform immunoassays, such as immunomagnetic reduction (IMR), Biolegend, and Mesoscale Discovery, also report elevated plasma α‐synuclein in PD patients compared with controls (Chang et al., 2019; Youssef et al., 2021), although there are some conflicting reports using similar platforms (Besong‐Agbo et al., 2013; Foulds et al., 2013). In view of our findings, it may be less important to use platforms with higher sensitivity and more important to consider pre‐analytical factors, including hemolysis and patients’ demographic data. However, even using the combination of age and sex with plasma α‐synuclein detected by ultrasensitive Simoa, the identification potential of this panel was only 0.719 (AUC), which indicates that plasma α‐synuclein level itself may not become a clear diagnostic biomarker of PD. Importantly, the mechanism of plasma α‐syn increase in PD patients remains unclear. Some researchers have suggested that plasma α‐syn is attributed to CSF efflux (Bates et al., 2015), while others believe that plasma α‐syn may derive from peripheral tissues, such as the enteric nerve plexus (Berg et al., 2021; Klingelhoefer & Reichmann, 2015). Furthermore, in our study, higher plasma α‐syn concentrations were significantly linked with worse motor and cognitive outcomes. Longitudinal follow‐up studies have confirmed that plasma α‐syn predicts PD motor and cognitive progression (Foulds et al., 2013; Niu et al., 2020; Wang et al., 2018). In contrast to the limited longitudinal follow‐up cohorts with plasma α‐syn data, there are many longitudinal assessments of CSF α‐syn. Notably, recent studies have found that CSF α‐syn does not change significantly over time, implying that it is not a biomarker of PD progression (Førland et al., 2018; Mollenhauer et al., 2016; Mollenhauer et al., 2017; Mollenhauer et al., 2019). Considering that CSF α‐syn level seems unrelated to the corresponding plasma α‐syn concentration (Goldman et al., 2018), the conclusions drawn from the longitudinal follow‐up cohorts of CSF α‐syn may not be applicable to whether plasma α‐synuclein is a useful biomarker of PD progression. Therefore, further longitudinal evaluation of plasma α‐synuclein is valuable and necessary.
It is undeniable that there are few studies assessing p‐tau181 in plasma samples of PD patients, and most studies still focus on CSF samples. Our study is the first to report increased plasma p‐tau181 levels in PD patients compared to the HC group using the ultrasensitive Simoa detection technology, which is in line with previous studies using IMR (Lin et al., 2018; Yu et al., 2021). Similar to plasma α‐syn, increased plasma p‐tau181 is related to worse motor symptoms in PD, which may be because of the interaction between α‐syn and p‐tau181. The tau encoding gene MAPT and α‐syn encoding gene SNCA were both found to correlate with PD in a meta‐analysis of genome‐wide association studies (GWAS) (Nalls et al., 2011), and the PD risk alleles of MAPT were associated with global parkinsonism in a multicenter study (Shulman et al., 2014). In a postmortem study, co‐aggregation of tau protein and α‐syn was observed in LBs (Ishizawa et al., 2003). In various PD models, α‐syn has been shown to trigger focal tau pathology and promote the phosphorylation of tau; in addition, increased p‐tau concentrations have been found in the striatum of PD patients (Duka et al., 2009; Hadi et al., 2021; Haggerty et al., 2011). Moreover, longitudinal follow‐up studies have proven that high p‐tau levels in CSF are associated with worsening motor symptoms (Hall et al., 2015; Hall et al., 2016). These findings support the positive correlation between plasma α‐syn and p‐tau181 concentrations in this study and suggest that tau pathology plays an important role in motor progression in PD.
Although PD patients have higher plasma α‐syn and p‐tau181 levels than HCs adjusted for age and sex, we observed a certain degree of overlap in the concentration ranges of these two biomarkers between groups. Considering the positive correlation between plasma α‐syn and p‐tau181, as well as its impact on the clinical manifestations of PD, we used plasma α‐syn and p‐tau181 biomarkers in combination with age and sex to form a panel to identify de novo PD patients from HCs. As a result, the ability to diagnose PD significantly improved. Our ability to improve discrimination ability using the plasma biomarker panel confirms the recent view that a combination of biomarkers can more accurately distinguish PD patients from healthy controls than a single biomarker (Parnetti et al., 2019). Given the cost‐effectiveness and global availability of ultrasensitive Simoa assays, the use of plasma biomarker panels for diagnosing PD—whether in primary care settings or specialist clinics—is gradually approaching clinical application.
The strength of this study lies in the application of the ultrasensitive Simoa HD‐X platform to systematically quantify ultra‐low concentrations of PD‐related proteins to establish a panel of plasma biomarkers to distinguish de novo PD patients and HCs. However, there are some limitations. First, because of the cross‐sectional study design, we cannot test the predictive value of the panel for motor and cognition outcomes, nor can we obtain the trajectory of these plasma biomarkers over time, which will be important in future research. Therefore, longitudinal follow‐up studies are needed to track the biomarker profiles of PD patients to reveal changes in plasma biomarkers during disease progression and predictive performance for clinical outcomes. Second, in view of the potential side effects of LP, it is currently difficult to obtain CSF data to explore the correspondence between biomarkers in plasma and CSF. Third, because of difficulty in obtaining detailed information—especially neuropsychological test scores and qualified blood samples—the number of subjects in this study is limited. Therefore, our findings need to be verified in a larger cohort. Fourth, because of the limitations of detection technology, we did not explore α‐syn species, including oligomeric α‐syn, phosphorylated α‐syn, and α‐syn aggregates, which may have better diagnostic value (Parnetti et al., 2019).
In conclusion, the positively correlated plasma α‐syn and p‐tau181 are associated with motor and cognitive dysfunction in de novo PD patients. These two biomarkers, when combined with age and sex, show a good performance for discriminating de novo PD patients from HCs. Therefore, a biomarker panel that includes plasma α‐syn and p‐tau181 may be useful for distinguishing de novo PD patients. Larger multicenter longitudinal studies are essential for examining the mechanisms of plasma α‐syn and p‐tau181 in PD.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
AUTHOR CONTRIBUTIONS
WL organized the project and critically revised the manuscript. JR drafted the preliminary manuscript. JR, CP, YW, and CX collected data and performed statistical analysis. HL, JX, and HW critiqued the statistical analysis. WZ, PX, and YC critically revised the manuscript. All authors contributed to the article and approved the submitted manuscript.
ACKNOWLEDGEMENTS
The authors thank the patients for their active participation and their families for their cooperation.
All experiments were conducted in compliance with the ARRIVE guidelines.
Ren, J.P. , Pan, C. , Wang, Y. , Xue, C. , Lin, H. & Xu, J. et al. (2022) Plasma α‐synuclein and phosphorylated tau 181 as a diagnostic biomarker panel for de novo Parkinson’s disease. Journal of Neurochemistry, 161, 506–515. 10.1111/jnc.15601
DATA AVAILABILITY STATEMENT
The original data for the present study are available from the corresponding author.
REFERENCES
- Bates, C. A. , Fu, S. , Ysselstein, D. , Rochet, J. C. , & Zheng, W. (2015). Expression and transport of α‐synuclein at the blood‐cerebrospinal fluid barrier and effects of manganese exposure. Admet and Dmpk, 3, 15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, D. , Borghammer, P. , Fereshtehnejad, S. M. , Heinzel, S. , Horsager, J. , Schaeffer, E. , & Postuma, R. B. (2021). Prodromal Parkinson disease subtypes ‐ key to understanding heterogeneity. Nature Reviews Neurology, 17, 349–361. [DOI] [PubMed] [Google Scholar]
- Besong‐Agbo, D. , Wolf, E. , Jessen, F. , Oechsner, M. , Hametner, E. , Poewe, W. , Reindl, M. , Oertel, W. H. , Noelker, C. , Bacher, M. , & Dodel, R. (2013). Naturally occurring alpha‐synuclein autoantibody levels are lower in patients with Parkinson disease. Neurology, 80, 169–175. [DOI] [PubMed] [Google Scholar]
- Bloem, B. R. , Okun, M. S. , & Klein, C. (2021). Parkinson's disease. Lancet (London, England), 397, 2284–2303. [DOI] [PubMed] [Google Scholar]
- Chang, C. W. , Yang, S. Y. , Yang, C. C. , Chang, C. W. , & Wu, Y. R. (2019). Plasma and serum alpha‐synuclein as a biomarker of diagnosis in patients with Parkinson's disease. Frontiers in Neurology, 10, 1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, K. R. , Martinez‐Martin, P. , Schapira, A. H. V. , Stocchi, F. , Sethi, K. , Odin, P. , Brown, R. G. , Koller, W. , Barone, P. , MacPhee, G. , Kelly, L. , Rabey, M. , MacMahon, D. , Thomas, S. , Ondo, W. , Rye, D. , Forbes, A. , Tluk, S. , Dhawan, V. , … Olanow, C. W. (2006). International multicenter pilot study of the first comprehensive self‐completed nonmotor symptoms questionnaire for Parkinson's disease: The NMSQuest study. Movement Disorders: Official Journal of the Movement Disorder Society, 21, 916–923. [DOI] [PubMed] [Google Scholar]
- Delgado‐Alvarado, M. , Gago, B. , Gorostidi, A. , Jiménez‐Urbieta, H. , Dacosta‐Aguayo, R. , Navalpotro‐Gómez, I. , Ruiz‐Martínez, J. , Bergareche, A. , Martí‐Massó, J. F. , Martínez‐Lage, P. , Izagirre, A. , & Rodríguez‐Oroz, M. C. (2017). Tau/α‐synuclein ratio and inflammatory proteins in Parkinson's disease: An exploratory study. Movement Disorders: Official Journal of the Movement Disorder Society, 32, 1066–1073. [DOI] [PubMed] [Google Scholar]
- Duits, F. H. , Martinez‐Lage, P. , Paquet, C. , Engelborghs, S. , Lleó, A. , Hausner, L. , Molinuevo, J. L. , Stomrud, E. , Farotti, L. , Ramakers, I. H. G. B. , Tsolaki, M. , Skarsgård, C. , Åstrand, R. , Wallin, A. , Vyhnalek, M. , Holmber‐Clausen, M. , Forlenza, O. V. , Ghezzi, L. , Ingelsson, M. , … Blennow, K. (2016). Performance and complications of lumbar puncture in memory clinics: Results of the multicenter lumbar puncture feasibility study. Alzheimer's & Dementia: the Journal of the Alzheimer's Association, 12, 154–163. [DOI] [PubMed] [Google Scholar]
- Duka, T. , Duka, V. , Joyce, J. N. , & Sidhu, A. (2009). Alpha‐synuclein contributes to GSK‐3beta‐catalyzed tau phosphorylation in Parkinson's disease models. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 23, 2820–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre, M. , Aarsland, D. , Brown, R. , Burn, D. J. , Duyckaerts, C. , Mizuno, Y. , Broe, G. A. , Cummings, J. , Dickson, D. W. , Gauthier, S. , Goldman, J. , Goetz, C. , Korczyn, A. , Lees, A. , Levy, R. , Litvan, I. , McKeith, I. , Olanow, W. , Poewe, W. , … Dubois, B. (2007). Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society, 22, 1689–1707. [DOI] [PubMed] [Google Scholar]
- Feigin, V. L. , Nichols, E. , Alam, T. , Bannick, M. S. , Beghi, E. , Blake, N. , Culpepper, W. J. , Dorsey, E. R. , Elbaz, A. , Ellenbogen, R. G. , Fisher, J. L. , Fitzmaurice, C. , Giussani, G. , Glennie, L. , James, S. L. , Johnson, C. O. , Kassebaum, N. J. , Logroscino, G. , Marin, B. , … Vos, T. (2019). Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet. Neurology, 18, 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Førland, M. G. , Öhrfelt, A. , Dalen, I. , Tysnes, O. B. , Blennow, K. , Zetterberg, H. , Pedersen, K. F. , Alves, G. , & Lange, J. (2018). Evolution of cerebrospinal fluid total α‐synuclein in Parkinson's disease. Parkinsonism & Related Disorders, 49, 4–8. [DOI] [PubMed] [Google Scholar]
- Foulds, P. G. , Diggle, P. , Mitchell, J. D. , Parker, A. , Hasegawa, M. , Masuda‐Suzukake, M. , Mann, D. M. , & Allsop, D. (2013). A longitudinal study on α‐synuclein in blood plasma as a biomarker for Parkinson's disease. Scientific Reports, 3, 2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb, W. R. , & Lees, A. J. (1988). The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 51, 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, J. G. , Andrews, H. , Amara, A. , Naito, A. , Alcalay, R. N. , Shaw, L. M. , Taylor, P. , Xie, T. , Tuite, P. , Henchcliffe, C. , Hogarth, P. , Frank, S. , Saint‐Hilaire, M. H. , Frasier, M. , Arnedo, V. , Reimer, A. N. , Sutherland, M. , Swanson‐Fischer, C. , Gwinn, K. , & Kang, U. J. (2018). Cerebrospinal fluid, plasma, and saliva in the BioFIND study: Relationships among biomarkers and Parkinson's disease features. Movement Disorders: Official Journal of the Movement Disorder Society, 33, 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi, F. , Akrami, H. , Totonchi, M. , Barzegar, A. , Nabavi, S. M. , & Shahpasand, K. (2021). α‐Synuclein abnormalities trigger focal tau pathology, spreading to various brain areas in Parkinson disease. Journal of Neurochemistry, 157, 727–751. [DOI] [PubMed] [Google Scholar]
- Haggerty, T. , Credle, J. , Rodriguez, O. , Wills, J. , Oaks, A. W. , Masliah, E. , & Sidhu, A. (2011). Hyperphosphorylated tau in an α‐synuclein‐overexpressing transgenic model of Parkinson's disease. European Journal of Neuroscience, 33, 1598–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, S. , Surova, Y. , Öhrfelt, A. , Blennow, K. , Zetterberg, H. , & Hansson, O. (2016). Longitudinal measurements of cerebrospinal fluid biomarkers in Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society, 31, 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, S. , Surova, Y. , Öhrfelt, A. , Zetterberg, H. , Lindqvist, D. , & Hansson, O. (2015). CSF biomarkers and clinical progression of Parkinson disease. Neurology, 84, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson, O. (2021). Biomarkers for neurodegenerative diseases. Nature Medicine, 27, 954–963. [DOI] [PubMed] [Google Scholar]
- Ishiki, A. , Kamada, M. , Kawamura, Y. , Terao, C. , Shimoda, F. , Tomita, N. , Arai, H. , & Furukawa, K. (2016). Glial fibrillar acidic protein in the cerebrospinal fluid of Alzheimer's disease, dementia with Lewy bodies, and frontotemporal lobar degeneration. Journal of Neurochemistry, 136, 258–261. [DOI] [PubMed] [Google Scholar]
- Ishizawa, T. , Mattila, P. , Davies, P. , Wang, D. , & Dickson, D. W. (2003). Colocalization of tau and alpha‐synuclein epitopes in Lewy bodies. Journal of Neuropathology and Experimental Neurology, 62, 389–397. [DOI] [PubMed] [Google Scholar]
- Jellinger, K. A. (2012). Neuropathology of sporadic Parkinson's disease: Evaluation and changes of concepts. Movement Disorders: Official Journal of the Movement Disorder Society, 27, 8–30. [DOI] [PubMed] [Google Scholar]
- Klingelhoefer, L. , & Reichmann, H. (2015). Pathogenesis of Parkinson disease‐‐the gut‐brain axis and environmental factors. Nature Reviews Neurology, 11, 625–636. [DOI] [PubMed] [Google Scholar]
- Lin, C. H. , Yang, S. Y. , Horng, H. E. , Yang, C. C. , Chieh, J. J. , Chen, H. H. , Liu, B. H. , & Chiu, M. J. (2018). Plasma biomarkers differentiate Parkinson's disease from atypical parkinsonism syndromes. Frontiers in Aging Neuroscience, 10, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan, I. , Goldman, J. G. , Tröster, A. I. , Schmand, B. A. , Weintraub, D. , Petersen, R. C. , Mollenhauer, B. , Adler, C. H. , Marder, K. , Williams‐Gray, C. H. , Aarsland, D. , Kulisevsky, J. , Rodriguez‐Oroz, M. C. , Burn, D. J. , Barker, R. A. , & Emre, M. (2012). Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Movement Disorders: Official Journal of the Movement Disorder Society, 27, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer, B. , Caspell‐Garcia, C. J. , Coffey, C. S. , Taylor, P. , Shaw, L. M. , Trojanowski, J. Q. , Singleton, A. , Frasier, M. , Marek, K. , & Galasko, D. (2017). Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. Neurology, 89, 1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer, B. , Caspell‐Garcia, C. J. , Coffey, C. S. , Taylor, P. , Singleton, A. , Shaw, L. M. , Trojanowski, J. Q. , Frasier, M. , Simuni, T. , Iranzo, A. , Oertel, W. , Siderowf, A. , Weintraub, D. , Seibyl, J. , Toga, A. W. , Tanner, C. M. , Kieburtz, K. , Chahine, L. M. , Marek, K. , & Galasko, D. (2019). Longitudinal analyses of cerebrospinal fluid α‐synuclein in prodromal and early Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society, 34, 1354–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer, B. , Zimmermann, J. , Sixel‐Döring, F. , Focke, N. K. , Wicke, T. , Ebentheuer, J. , Schaumburg, M. , Lang, E. , Trautmann, E. , Zetterberg, H. , Taylor, P. , Friede, T. , & Trenkwalder, C. (2016). Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology, 87, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls, M. A. , Plagnol, V. , Hernandez, D. G. , et al. (2011). Imputation of sequence variants for identification of genetic risks for Parkinson's disease: A meta‐analysis of genome‐wide association studies. Lancet (London, England), 377, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips, N. A. , Bédirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , Cummings, J. L. , & Chertkow, H. (2005). The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Ng, A. S. L. , Tan, Y. J. , Lu, Z. , Ng, E. Y. L. , Ng, S. Y. E. , Chia, N. S. Y. , Setiawan, F. , Xu, Z. , Tay, K. Y. , Prakash, K. M. , Au, W. L. , Tan, E. K. , & Tan, L. C. S. (2019). Plasma alpha‐synuclein detected by single molecule array is increased in PD. Annals of Clinical and Translational Neurology, 6, 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, M. , Li, Y. , Li, G. , Zhou, L. , Luo, N. , Yao, M. , Kang, W. , & Liu, J. (2020). A longitudinal study on α‐synuclein in plasma neuronal exosomes as a biomarker for Parkinson's disease development and progression. European Journal of Neurology, 27, 967–974. [DOI] [PubMed] [Google Scholar]
- Oosterveld, L. P. , Verberk, I. M. W. , Majbour, N. K. , El‐Agnaf, O. M. , Weinstein, H. C. , Berendse, H. W. , Teunissen, C. E. , & van de Berg, W. D. J. (2020). CSF or serum neurofilament light added to α‐synuclein panel discriminates Parkinson's from controls. Movement Disorders: Official Journal of the Movement Disorder Society, 35, 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnetti, L. , Gaetani, L. , Eusebi, P. , Paciotti, S. , Hansson, O. , El‐Agnaf, O. , Mollenhauer, B. , Blennow, K. , & Calabresi, P. (2019). CSF and blood biomarkers for Parkinson's disease. The Lancet Neurology, 18, 573–586. [DOI] [PubMed] [Google Scholar]
- Rissin, D. M. , Kan, C. W. , Campbell, T. G. , Howes, S. C. , Fournier, D. R. , Song, L. , Piech, T. , Patel, P. P. , Chang, L. , Rivnak, A. J. , Ferrell, E. P. , Randall, J. D. , Provuncher, G. K. , Walt, D. R. , & Duffy, D. C. (2010). Single‐molecule enzyme‐linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nature Biotechnology, 28, 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, I. , Kruse, N. , Gera, R. G. , Kremer, T. , Cedarbaum, J. , Barbour, R. , Zago, W. , Schade, S. , Otte, B. , Bartl, M. , Hutten, S. J. , Trenkwalder, C. , & Mollenhauer, B. (2021). Systematic assessment of 10 biomarker candidates focusing on α‐synuclein‐related disorders. Movement Disorders: Official Journal of the Movement Disorder Society, 36, 2874–2887. [DOI] [PubMed] [Google Scholar]
- Shulman, J. M. , Yu, L. , Buchman, A. S. , Evans, D. A. , Schneider, J. A. , Bennett, D. A. , & De Jager, P. L. (2014). Association of Parkinson disease risk loci with mild parkinsonian signs in older persons. JAMA Neurology, 71, 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas‐Antérion, C. , Honoré‐Masson, S. , & Laurent, B. (2006). The cognitive complaint interview (CCI). Psychogeriatrics, 6, S18–S22. [Google Scholar]
- Tolosa, E. , Wenning, G. , & Poewe, W. (2006). The diagnosis of Parkinson's disease. The Lancet Neurology, 5, 75–86. [DOI] [PubMed] [Google Scholar]
- Verger, A. , Grimaldi, S. , Ribeiro, M. J. , Frismand, S. , & Guedj, E. (2021). Single photon emission computed tomography/positron emission tomography molecular imaging for parkinsonism: A fast‐developing field. Annals of Neurology, 90, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Atik, A. , Stewart, T. , Ginghina, C. , Aro, P. , Kerr, K. F. , Seibyl, J. , Jennings, D. , Jensen, P. H. , Marek, K. , Shi, M. , & Zhang, J. (2018). Plasma α‐synuclein and cognitive impairment in the Parkinson's associated risk syndrome: A pilot study. Neurobiology of Disease, 116, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , & Wang, K. K. (2015). Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends in Neurosciences, 38, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef, P. , Kim, W. S. , Halliday, G. M. , Lewis, S. J. G. , & Dzamko, N. (2021). Comparison of different platform immunoassays for the measurement of plasma alpha‐synuclein in Parkinson's disease patients. Journal of Parkinson's disease, 11, 1761–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C. C. , Lu, C. Y. , Chen, M. H. , Chen, Y. S. , Lu, C. H. , Lin, Y. Y. , Chou, K. H. , & Lin, W. C. (2021). Brain atrophy mediates the relationship between misfolded proteins deposition and cognitive impairment in Parkinson's disease. Journal of Personalized Medicine, 11, 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data for the present study are available from the corresponding author.


