Abstract
Angioimmunoblastic T‐cell lymphoma (AITL) is a common type of peripheral T‐cell lymphoma (PTCL) with a poor prognosis, and an effective first‐line therapy is lacking. Chidamide is a selective histone deacetylase inhibitor and has been approved by the China Food and Drug Administration for relapsed or refractory PTCL. We conducted a multicenter phase II clinical trial combining chidamide with prednisone, etoposide, and thalidomide (CPET regimen) for a total of eight cycles in untreated AITL patients in China. The primary objectives were the overall response rate (ORR) and complete remission (CR) rate after eight cycles of the CPET regimen. The secondary endpoints were progression‐free survival (PFS) and safety. Of the 71 enrolled patients, 51 completed the eight cycles of the CPET regimen. The ORR and CR of the 51 patients were 90.2 and 54.9%, respectively. After a median follow‐up of 11.4 months (95% confidence interval [CI], 9.9–17.0), the median PFS of the 51 patients was 42.6 months (95% CI, 27.7—not reached) and the median overall survival (OS) was not reached. The 2‐year PFS rate and OS rate were 66.5 and 82.2%, respectively. Sixty‐eight patients received at least one cycle of CPET regimen and were included as the safety assessment population. The most common grade 3/4 adverse event was neutropenia (n = 22, 32.3%). Twelve patients showed treatment‐related infections and recovered from antibiotic therapy; the other adverse events were mostly mild and reversible. The oral CPET regimen is an effective, tolerable, and economical choice for untreated AITL in a Chinese population. This trial was registered in www.clinicaltrials.gov as NCT03273452.
1. INTRODUCTION
Angioimmunoblastic T‐cell lymphoma (AITL) is a mature T‐cell lymphoma as defined by the World Health Organization (WHO) 2016 Classification. 1 Globally, AITL accounts for 1–2% of non‐Hodgkin lymphomas and 15–20% of peripheral T‐cell lymphoma (PTCL), and AITL is the second most common PTCL after PTCL not otherwise specified. 2 AITL accounted for 1.6% of non‐Hodgkin lymphomas and 37.4% of PTCL in a Chinese population. 3 AITL is an aggressive disease that preferentially affects older patients and is characterized by generalized lymphadenopathy, hepatosplenomegaly, bone marrow involvement, and lymphoma‐related symptoms. 4 , 5
Currently, there is no consensus on the first‐line treatment for patients with AITL. The most common treatments for AITL are cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or a similar regimen. 2 The complete remission (CR) rates of AITL are 28.6–60.6%, 6 and AITL shows a high progression–relapse rate of 82%. 1 Therefore, exploration of new treatments for AITL is critical.
Chidamide is an oral selective histone deacetylase inhibitor (HDACi) that has been approved for the treatment of relapsed or refractory PTCL (R/R PTCL) in China with tolerable toxicity. 7 Thalidomide is an immunomodulatory drug (IMiD) effective for AITL, 8 , 9 , 10 and numerous studies showed the synergistic effect of IMiD with HDACi. 11 , 12 Etoposide, a topoisomerase II inhibitor, also showed synergetic effects with HDACi. 13 Prednisone has been used in combination with HDACi in regimes for the treatment of PTCL. 14
We conducted a prospective single‐arm phase II clinical trial to investigate the efficacy and safety of an all‐oral regimen chidamide plus prednisone, etoposide, and thalidomide (CPET) regimen in untreated patients with AITL in a Chinese population.
2. PATIENTS AND METHODS
2.1. Study design and participants
This was a multicenter prospective single‐arm phase II clinical trial carried out at seven hospitals in China. This study (NCT03273452) was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the Affiliated Hospital of Qingdao University. All patients signed informed consent before joining the study.
Patients who met the following criteria were recruited: (i) pathologically diagnosed as AITL based on the 2016 WHO classification 15 with at least one measurable focus, which was confirmed by a central review process with hematopathological experts; (ii) age 18–85 years; and (iii) Eastern Cooperative Oncology Group score 0–3 with an expected survival longer than 3 months. Criteria of laboratory findings included peripheral blood neutrophil counts ≥1.5 × 109/L, platelet count ≥20 × 109/L, hemoglobin ≥70 g/L, and proper organ function: total bilirubin, aspartate aminotransferase and aspartate aminotransferase ≤ two times the upper limit of normal (three times for patients with liver infiltration), and blood creatinine ≤ two times the upper limit of normal. Patients were excluded if they had central nervous system involvement, severe acute infection, significant cardiac abnormalities, thrombosis or embolism, or organ transplantation. Women of child‐bearing age were screened by β‐HCG within 7 days before enrollment.
2.2. Procedures
The CPET regimen was given as follows: chidamide 30 mg orally twice a week, prednisone 100 mg orally d1—5, etoposide 100 mg orally d1—5, and thalidomide 100 mg orally d1—14 at bedtime; the regimen was administered for 3 weeks as one therapeutic period for a total of eight cycles. Considering that venous thromboembolism is a known complication of thalidomide, especially combined with prednisone or chemotherapy, the enrolled patients were requested to take aspirin 100 mg per night during the study to prevent thrombosis; aspirin administration was stopped if the platelet count was less than 50 × 109/L or the patient presented obvious signs of bleeding.
The dose reductions were determined according to grade and type of toxicity. Hematological toxicities attributable to 25% dose reduction of etoposide and thalidomide were ≥ grade‐3 thrombocytopenia or grade‐4 neutropenia during days 1 to 21. There was no plan for dose reduction of chidamide and prednisone.
The dose reductions due to non‐hematological toxicities were decided by physicians in practice.
2.3. Assessment and criteria
The assessments of response were conducted every four cycles by individual site investigators according to the Cheson 2014 criteria. 16 The assessments included review of radiology data and clinical data such as physical examination and laboratory data.
The primary endpoints of this study were ORR and CR rate. The secondary endpoints were PFS, OS, and safety. PFS was calculated from the date of the first dose of CPET regimen to the first date of disease progression, relapse, death for any reason, or the last date of follow‐up. OS was calculated from the date of the first dose of CPET regimen to the date of death for any reason or the date of the last follow‐up.
Safety assessment included evaluation of treatment‐related adverse events (TRAEs), and serious adverse events (SAEs) were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events Version 4.0. Symptoms of respiratory tract and digestive system were collected by self‐assessment reported to doctors during the visits. Complete blood count and serum biochemistry were detected once every 3 weeks and other safety indexes like ultrasound were detected once every 6 weeks. Prophylactic use of granulocyte colony‐stimulating factor (G‐CSF) was permitted, and G‐CSF was also given for subjects with grade 3/4 neutropenia during the CPET treatment period.
2.4. Statistical analysis
2.4.1. Sample size
Considering the CR rate of AITL treated by CHOP and CHOP‐like regimen was 33.0%, 6 we hypothesized that 55.0% of patients would respond to the CPET regimen and calculated the sample size with 80% power at an overall 5% (one‐sided) significance level to detect a CR rate improvement from 33.0 to 55%. Using the online sample calculators (powerandsamplesize.com; Chow S, Shao J, Wang H. 2008. Sample Size Calculations in Clinical Research. 2nd Ed. Chapman & Hall/CRC Biostatistics Series. page 89), 60 patients were acquired. Taking a 10% drop‐out rate into account, the final sample size was 66.
All descriptive statistical analyses were performed by SPSS software version 23.0. PFS and OS were evaluated using Kaplan–Meier methods and analyzed with the log‐rank test. Differences between groups were estimated with the χ2 test and Fisher's exact test as appropriate. p < .05 indicated statistical significance.
3. RESULTS
3.1. Patient and clinical characteristics
From October 2017 to November 2020, 71 patients were initially enrolled in this study. One patient showed hemophagocytic syndrome and two patients dropped out before receiving the treatment. Sixty‐eight patients participated in the efficacy and safety assessments. Two patients who confirmed progression after two cycles of the CPET regimen by the two‐cycle examination and discontinued the study were excluded. Another 15 patients discontinued at the fifth to eighth cycles of treatment for the following reasons: 9 patients experienced disease progression (13.2%); 3 patients showed SAEs (4.4%), one with severe lung infection (grade 4), one with incomplete intestinal obstruction (grade 3) accompanied with granulocytopenia (grade 3) and anemia (grade 2), the other with interstitial pneumonia (grade 3) accompanied with fever (grade 3) and granulocytopenia (grade 3); 3 patients withdrew consent after receiving at least four cycles of the CPET regimen (4.4%). A total of 51 patients (75.0%) completed the eight cycles of CPET regimen (Figure S1).
Three patients had to reduce the dose of etoposide because of AEs. One patients received 75 mg etoposide after 2nd cycle and complete 8 cycles in total, one patient progressed after reduction (total 4 cycles), while the other one could not tolerate the AEs (grade 3 pneumonitis and grade 4neutropenia) after second reduction of etoposide and discontinued the following treatment.
The clinical characteristics of the 68 patients are listed in Table 1. The median patient age was 63 years with a range of 25–83 years. Of the 68 patients, 49 were male and 19 were female. Half of the patients (n = 34) showed B symptoms. Approximately 82.4% of patients progressed to advanced stage and 45.6% of patients had a high or high‐intermediate international prognostic index risk score. Severe thrombocytopenia (PLT count <50 × 109/L) was detected in 7 patients (10.3%), while 36 patients (52.9%) showed anemia, and 54 patients (79.4%) had hypoalbuminemia before initiation of the first CPET regimen cycle.
TABLE 1.
Clinical characteristics of patients with AITL (n = 68)
| Characteristics | N (n = 68) | % |
|---|---|---|
| Gender M/F | 2.6:1 | |
| Median age (range) | 63 (25,83) | |
| ≥60 | 34.6 | |
| ECOG score | ||
| 0–1 | 53 | 77.9 |
| ≥2 | 15 | 22.1 |
| B symptoms | 34 | 50.0 |
| Ann Arbor stage | ||
| I‐II | 12 | 17.6 |
| III‐IV | 56 | 82.4 |
| IPI risk | ||
| Low | 14 | 20.6 |
| Low‐intermediate | 23 | 33.8 |
| High‐intermediate | 16 | 23.5 |
| High | 15 | 22.1 |
| Anemia | 36 | 52.9 |
| Thrombocytopenia | 7 | 10.3 |
| Elevated LDH (≥245) | 31 | 45.6 |
| Elevated β2‐MG (≥1.8) n = 65 | 58 | 89.2 |
| Elevated D‐dimer | 34 | 50.0 |
| Hypoalbuminemia | 54 | 79.4 |
| Number of lymph node groups | ||
| 0–5 | 12 | 17.7 |
| 6–10 | 21 | 30.9 |
| ≥11 | 35 | 51.5 |
| BM involvement | 17 | 25.0 |
| Splenomegaly (n = 62) | 29 | 46.8 |
| Hepatomegaly (n = 56) | 4 | 7.1 |
3.2. Clinical efficacy
Of the enrolled 68 patients, 66 patients received at least four cycles of CPET therapy. The ORR after four cycles was 83.8%; 16 (23.5%) patients reached CR, while 2 patients experienced disease progression after only two cycles of CPET regimen. As described above, 15 patients discontinued the treatment, and the efficacy population included 51 patients who completed eight cycles of the CPET regimen and the final ORR was 90.2% (54.9% CR–unconfirmed complete response [CRu]) (Table 2). The final ORR was similar between the subgroups stratified by age or the involvement of bone marrow (Table S1). Seven patients had concomitant thrombocytopenia at baseline, which is considered a typical feature of AITL associated with autoimmune manifestations. Platelet counts of six patients returned to normal after receiving two cycles of CPET regimen.
TABLE 2.
Response of patients with AITL to CPET regimen
| Response after 4 cycles (n = 68) | Final response (n = 68) | Final response of 8 cycles (n = 51) | |
|---|---|---|---|
| ORR% | 83.8 | 76.5 | 90.2 |
| CR n/% | 16/23.5 | 29/42.6 | 28/54.9 |
| PR n/% | 41/60.3 | 23/33.8 | 18/35.3 |
| SD n/% | 9/13.2 | 1/1.5 | 1/1.9 |
| PD n/% | 2/2.9 | 15/22.1 | 4/7.8 |
During the median follow‐up of 11.4 months (95% CI, 9.9–17.0), 28 patients had disease progression; the median PFS for all patients was 27.7 months (95% CI, 13.2–42.3) and the median OS was not reached. The estimated 2‐year PFS rate and OS rate of patients who received at least one dose of study medication (n = 68) were 52.8 and 78.9%, respectively. The median duration of response (DOR) was 24.7 months (95% CI, 10.8–not reached) (Figure 1). For the patients who completed eight cycles of the CPET regimen (n = 51), the estimated median PFS was 42.6 months (95% CI, 27.7—not reached), which was superior to that of the patients who discontinued treatment (4.4 months; 95% CI, 3.5–8.3; p < .001). The estimated 2‐year PFS rate and OS rate of patients who completed the study were 66.5 and 82.2%, respectively (Figure S2A,B). Furthermore, patients who responded to the regimen (n = 57) had a significantly longer PFS than those who did not (n = 11), with a median PFS of 28.0 months (95% CI, 27.7–NA) compared with 4.9 months (95% CI, 3.5–NA), respectively (p < .001) (Figure S2C,D).
FIGURE 1.
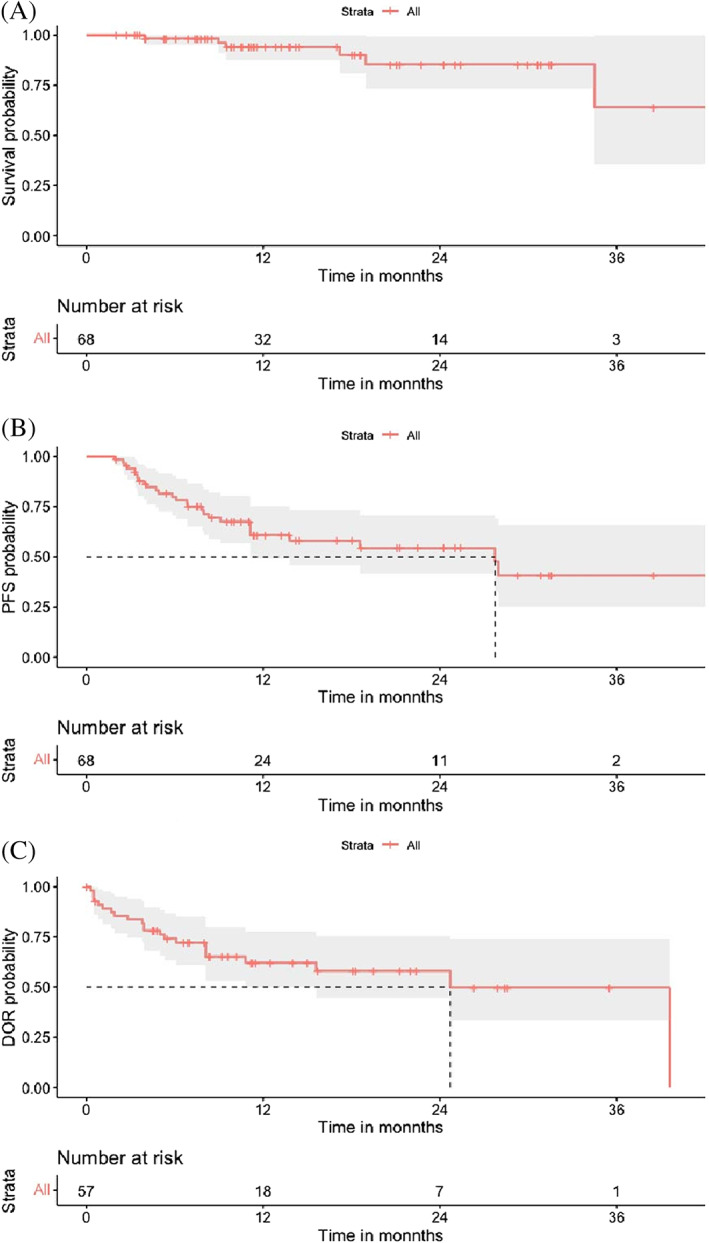
Kaplan–Meier analysis of overall survival (panel A, n = 68), progression‐free survival (panel B, n = 68) in all patients, and Kaplan–Meier analysis of the median duration of response (panel C, n = 57) [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Safety
The 68 patients who received at least two cycles of CPET regimen were included for the safety analysis. The most common hematologic TRAE was neutropenia (n = 36, 52.9%), and the proportion of grade 3/4 AEs was 32.3%. The degrees of AEs in most patients who experienced anemia (26.5%) and thrombopenia (26.5%) were mild, with only 3.0% of anemia and 5.9% of thrombopenia cases categorized as grade 3/4 AEs. Twelve patients showed treatment‐related infection including pneumonitis (n = 10, 8.8%), CMV infection (n = 1, 1.5%), and urinary tract infection (n = 1, 1.5%). All patients recovered with oral or intravenous anti‐infection therapy. The most common non‐hematologic TRAE was transaminase increase (n = 17, 25.0%), and almost all cases were grade 1/2 AEs (Table 3). Five patients discontinued the CPET regimen treatment because of severe AEs, including grade 4 neutropenia (n = 3), grade 3 pneumonitis (n = 1), and grade 3 ileus. No treatment‐related death was observed during the treatment period.
TABLE 3.
Treatment‐related adverse events of CPET regimen
| Any grade (n/%) | Grade 1–2 (n/%) | Grade 3–4 (n/%) | Grade 2–4 (n/%) | |
|---|---|---|---|---|
| Hematologic AEs | ||||
| Anemia | 18/26.5 | 16/23.5 | 2/2.9 | 11/16.2 |
| Neutropenia | 36/52.9 | 14/20.6 | 22/32.3 | 30/44.1 |
| Lymphopenia | 14/20.6 | 10/14.8 | 4/5.8 | 9/13.2 |
| Thrombopenia | 18/26.5 | 14/20.6 | 4/5.9 | 11/16.2 |
| Non‐hematologic AEs | ||||
| Infection | 12/17.6 | 7/10.3 | 5/7.4 | 12/17.6 |
| Pneumonitis | 10/8.8 | 7/10.3 | 3/4.4 | 10/14.7 |
| CMV | 1/1.5 | 0/0 | 1/1.5 | 1/1.5 |
| Urinary tract infection | 1/1.5 | 0/0 | 1/1.5 | 1/1.5 |
| Hepatic dysfunction | 17/25.0 | 16/23.5 | 1/1.5 | 5/7.4 |
| Edema | 7/10.3 | 6/8.8 | 1/1.5 | 3/4.4 |
| Gastrointestinal disorder | 6/8.8 | 1/1.5 | 5/7.4 | 6/8.8 |
| Diarrhea | 2/2.9 | 1/1.5 | 1/1.5 | 2/2.9 |
| Constipation | 1/1.5 | 0/0 | 1/1.5 | 1/1.5 |
| Vomiting | 1/1.5 | 1/1.5 | 0/0 | 1/1.5 |
| Ileus | 1/1.5 | 0/0 | 1/1.5 | 1/1.5 |
| Rash | 1/1.5 | 1/1.5 | 0/0 | 0/ |
| Pruritus | 1/1.5 | 1/1.5 | 0/0 | 0/ |
| Venous thrombosis | 1/1.5 | 0/0 | 1/1.5 | 1/1.5 |
| Oral mucositis | 1/1.5 | 1/1.5 | 0/0 | 1/1.5 |
4. DISCUSSION
To the best of our knowledge, this study is the largest trial in patients with AITL in China. Our results indicated that the oral CPET regimen was effective and feasible in patients with AITL from a Chinese population, especially for participants who completed 8 cycles treatments, with an ORR of 90.2% (54.9% CR–CRu) and a median PFS of 42.6 months.
In the GELA study, 17 AITL patients younger than 60 years with normal lactate dehydrogenase values benefited from intensive chemotherapy following autologous stem cell transplantation, while older patients did not benefit from the intensive regimen because of treatment‐related toxicity. In a retrospective report in Chinese patients (n = 84), 18 the ORR of patients with AITL treated with the etoposide, cyclophosphamide, doxorubicin, vincristine, and prednisolone (EPOCH) regimen was 68.8%, but the CR rate was only 18.8% with a short PFS. Moreover, 71.4% of patients relapsed after autologous stem cell transplantation within 10 months. Honghuangming et al 19 reported a similar outcome in a retrospective study. Untreated AITL patients (n = 59) who received cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone (CHOPE) showed an ORR of 60% (45% CR), which was not superior to that in the CHOP regimen group. These results indicated that AITL patients might not benefit from intensive chemotherapy.
As the pathogenesis of AITL has been elucidated in the last decade, new targeted drugs have been introduced into the traditional CHOP regimen. 14 , 20 , 21 , 22 , 23 , 24 , 25 A clinicobiological study of the GELA 20 combined rituximab and CHOP to treat newly diagnosed AITL patients (n = 25) and reported a CR rate of 44.0%, a median PFS of 16 months, and an OS of 14 months. In the REVAIL phase 2 clinical trial, 21 untreated patients with AILT (60–80 years old) received eight cycles of lenalidomide plus CHOP. The complete metabolic response rate of patients who completed the study (n = 44) was 42.1%, and the 2‐year PFS rate and OS rate were 42.1% (95% CI, 30.9–52.8%) and 59.2% (95% CI, 47.3–69.3%), respectively. These results showed the lack of benefit from the combination of rituximab or lenalidomide to CHOP regimen in elderly patients with AITL.
AITL has been associated with several gene mutations, 26 including recurrent genetic mutations in ras homolog family member A (RHOA, 50–79%), 27 , 28 , 29 tet methylcytosine dioxygenase 2 (TET2, 68–80%), 29 , 30 , 31 , 32 DNA methyltransferase 3 alpha (DNMT3A, 20–30%), 28 , 29 and isocitrate dehydrogenase [NADP(+)] 2 (IDH2, 20–30%), 28 , 29 which are regulated by acetylation. 33 , 34 Histone deacetylases (HDACs) act as epigenetic modifiers that regulate signaling pathways via the deacetylation of histone proteins and non‐histone proteins. 35 Histone deacetylase inhibitors (HDACi) have shown efficacy in the treatment of AITL and presented promising results. 36
Approximately 47% of AITL patients carry a mutation in TET2, which encodes a protein that is regulated by acetylation. 34 HDAC1 and HDAC2 mediate deacetylation of TET2, leading to its ubiquitination and proteasome‐mediated degradation. 37 In this scenario, HDACi may help maintain acetylated and active TET, leading to DNA demethylation. 38 The hypothetical mechanism could explain the activity of chidamide in our study, which is a novel benzamide class of HDACi that selectively inhibits the activity of HDAC1, −2, −3, and −10 and has been approved by the China Food and Drug Administration for R/R PTCL in 2013. 7 Some studies have examined HDACi as a single agent and in combination therapies for PTCL, and AITL appears to respond better than PTCL not otherwise specified to HDACi such as romidepsin or belinostat, which are approved by the FDA. 14 , 24 , 39 , 40 , 41 , 42 , 43 However, the sample size of patients with AITL in these studies was too small to make a conclusion.
We have done a pilot study with CPET regime in 13 AITL patients with median age of 67 years, and found CPET was effective and safe in AITL patients. 44 The median PFS and OS were 16 months (95% CI, 4–51) and 21 month (95% CI, 4–51), respectively. 44 In the present study, we expanded sample and enrolled 71 newly diagnosed AITL patients who did not tolerate or were not willing to take traditional CHOP or CHOP‐like regimens due to thrombocytopenia, anemia, Eastern Cooperative Oncology Group (ECOG score) >3, or inconvenience of hospitalization. Among the 71 patients, 46 completed eight cycles of CPET regimen therapy with a high response and durable efficacy. HDACi, prednisone, and etoposide target different aspects of the cell cycle with unique mechanisms of antitumor functions, creating a synergistic effect. 8 , 9 , 10 , 45 , 46 As the first clinically used IMiD, thalidomide disrupts the interaction between lymphoma cells and the tumor microenvironment and exhibits an anti‐angiogenesis effect. 10 Thus, thalidomide is especially effective in AITL, 8 , 9 , 10 which is characterized by a prominent tumor microenvironment associated with vascular hyperplasia. 2 A preclinical study showed that the combination of romidepsin and IMiDs induced reactive oxygen species in 53.0–61.8% of cells in a T‐cell lymphoma cell line, while the induction rates were 17.0–23.0% and 11.0% with romidepsin and IMiDs alone, respectively. The preclinical data suggested that the combination of HDACi and IMiDs induced cytotoxicity through mitochondria‐mediated apoptosis. 11 A phase I trial of vorinostat plus lenalidomide and dexamethasone in R/R PTCL revealed an ORR of 25%, and the median PFS and OS were 2.2 and 6.7 months, respectively. 12 A phase II study is ongoing that is investigating the combination of romidepsin and lenalidomide in untreated PTCL (NCT02232516). HDACi also showed synergetic effects with topoisomerase II inhibitors, including etoposide. 13 Prednisone, another drug in the CPET regimen, contributes to the suppression of autoimmune‐like manifestations and to the regression of tumors. 47
The side‐effect profiles of these drugs are well characterized. Thalidomide is associated with peripheral neuropathy, birth defects, gastrointestinal complications, and thrombosis. 48 , 49 Etoposide is associated with alopecia, myelosuppression, and gastrointestinal toxicity like nausea, vomiting, and stomatitis, which is relatively mild and dose‐limiting. 50 Prednisone is associated with dermatologic, metabolic, and immunologic effects. 51 As the metabolic profiles are different among chidamide, prednisone, etoposide, and thalidomide, the overlapping toxicity was mild. In this trial, the most frequent grade 3/4 AE was neutropenia (n = 22, 32.3%); other grade 3/4 AEs were less than 10% of the total grade 3/4 AEs. All AEs were self‐relieved or relieved after symptomatic treatments. In general, the CPET regimen was well‐tolerated and manageable.
All of the included drugs were taken orally. During the trial, patients visited their physician in the outpatient department without hospitalization. Therefore, it was convenient for patients to adhere to the regimen.
In conclusion, the present study demonstrated that the oral regimen of CPET was effective for untreated AITL patients with tolerable toxicity in the Chinese population. However, our study lacked independent response assessment and the efficacy population was small. Thus, further studies with larger sample size and sufficient follow‐up in AITL patients are needed.
CONFLICT OF INTEREST
The authors declare that there is no conflicts of interest.
AUTHOR CONTRIBUTIONS
Yawen Wang was responsible for data collection and interpretation, statistical analysis, literature research, and manuscript writing. Mingzhi Zhang, Wei Song, Qingqing Cai, Liling Zhang, Xiuhua Sun, Liqun Zou, and Huilai Zhang were responsible for data collection and patient care. Lili Wang was responsible for pathological consultation. Hongwei Xue designed the study and approved the final version. All authors reviewed the manuscript.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGMENTS
We thank Gabrielle White Wolf, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Wang Y, Zhang M, Song W, et al. Chidamide plus prednisone, etoposide, and thalidomide for untreated angioimmunoblastic T‐cell lymphoma in a Chinese population: A multicenter phase II trial. Am J Hematol. 2022;97(5):623-629. doi: 10.1002/ajh.26499
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Vose J, Armitage J, Weisenburger D. International peripheral T‐cell and natural killer/T‐cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124‐4130. [DOI] [PubMed] [Google Scholar]
- 2. Lunning MA, Vose JM. Angioimmunoblastic T‐cell lymphoma: the many‐faced lymphoma. Blood. 2017;129(9):1095‐1102. [DOI] [PubMed] [Google Scholar]
- 3. Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138(3):429‐434. [DOI] [PubMed] [Google Scholar]
- 4. Chiba S, Sakata‐Yanagimoto M. Advances in understanding of angioimmunoblastic T‐cell lymphoma. Leukemia. 2020;34(10):2592‐2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broccoli A, Zinzani PL. Angioimmunoblastic T‐cell lymphoma. Hematol Oncol Clin North Am. 2017;31(2):223‐238. [DOI] [PubMed] [Google Scholar]
- 6. Abouyabis AN, Shenoy PJ, Sinha R, Flowers CR, Lechowicz MJ. A systematic review and meta‐analysis of front‐line Anthracycline‐based chemotherapy regimens for peripheral T‐cell lymphoma. ISRN Hematol 2011;2011:623924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi Y, Dong M, Hong X, et al. Results from a multicenter, open‐label, pivotal phase II study of chidamide in relapsed or refractory peripheral T‐cell lymphoma. Ann Oncol. 2015;26(8):1766‐1771. [DOI] [PubMed] [Google Scholar]
- 8. Gottardi M, Danesin C, Canal F, et al. Complete remission induced by thalidomide in a case of angioimmunoblastic T‐cell lymphoma refractory to autologous stem cell transplantation. Leuk Lymphoma. 2008;49(9):1836‐1838. [DOI] [PubMed] [Google Scholar]
- 9. Ramasamy K, Lim Z, Pagliuca A, Salisbury JR, Mufti GJ, Devereux S. Successful treatment of refractory angioimmunoblastic T‐cell lymphoma with thalidomide and dexamethasone. Haematologica 2006;91(8 Suppl):ECR44. [PubMed] [Google Scholar]
- 10. Dogan A, Ngu LS, Ng SH, Cervi PL. Pathology and clinical features of angioimmunoblastic T‐cell lymphoma after successful treatment with thalidomide. Leukemia. 2005;19(5):873‐875. [DOI] [PubMed] [Google Scholar]
- 11. Cosenza M, Civallero M, Fiorcari S, et al. The histone deacetylase inhibitor romidepsin synergizes with lenalidomide and enhances tumor cell death in T‐cell lymphoma cell lines. Cancer Biol Ther. 2016;17(10):1094‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hopfinger G, Nösslinger T, Lang A, et al. Lenalidomide in combination with vorinostat and dexamethasone for the treatment of relapsed/refractory peripheral T cell lymphoma (PTCL): report of a phase I/II trial. Ann Hematol. 2014;93(3):459‐462. [DOI] [PubMed] [Google Scholar]
- 13. Marchion DC, Bicaku E, Daud AI, Sullivan DM, Munster PN. In vivo synergy between topoisomerase II and histone deacetylase inhibitors: predictive correlates. Mol Cancer Ther. 2005;4(12):1993‐2000. [DOI] [PubMed] [Google Scholar]
- 14. Johnston PB, Cashen AF, Nikolinakos PG, et al. Belinostat in combination with standard cyclophosphamide, doxorubicin, vincristine and prednisone as first‐line treatment for patients with newly diagnosed peripheral T‐cell lymphoma. Exp Hematol Oncol. 2021;10(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mourad N, Mounier N, Brière J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T‐cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood. 2008;111(9):4463‐4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li TT, Luo LT, Chen Y, Yang T, Hu JD. Clinical characteristics and prognosis in 84 patients with angioimmunoblastic T‐cell lymphoma: a single‐center analysis. Zhonghua Xue Ye Xue Za Zhi. 2020;41(11):915‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hong H, Fang X, Huang H, Wang Z, Lin T, Yao H. The derived neutrophil‐to‐lymphocyte ratio is an independent prognostic factor in patients with angioimmunoblastic T‐cell lymphoma. Br J Haematol. 2020;189(5):908‐912. [DOI] [PubMed] [Google Scholar]
- 20. Delfau‐Larue MH, de Leval L, Joly B, et al. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T‐cell lymphoma. A clinicobiological study of the GELA. Haematologica. 2012;97(10):1594‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemonnier F, Safar V, Beldi‐Ferchiou A, et al. Integrative analysis of a phase 2 trial combining lenalidomide with CHOP in angioimmunoblastic T‐cell lymphoma. Blood Adv. 2021;5(2):539‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gallamini A, Zaja F, Patti C, et al. Alemtuzumab (Campath‐1H) and CHOP chemotherapy as first‐line treatment of peripheral T‐cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110(7):2316‐2323. [DOI] [PubMed] [Google Scholar]
- 23. Kim SJ, Yoon DH, Kang HJ, et al. Bortezomib in combination with CHOP as first‐line treatment for patients with stage III/IV peripheral T‐cell lymphomas: a multicentre, single‐arm, phase 2 trial. Eur J Cancer (Oxford, England: 1990). 2012;48(17):3223‐3231. [DOI] [PubMed] [Google Scholar]
- 24. Dupuis J, Morschhauser F, Ghesquières H, et al. Combination of romidepsin with cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated patients with peripheral T‐cell lymphoma: a non‐randomised, phase 1b/2 study. Lancet Haematol. 2015;2(4):e160‐e165. [DOI] [PubMed] [Google Scholar]
- 25. Horwitz S, O'Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30‐positive peripheral T‐cell lymphoma (ECHELON‐2): a global, double‐blind, randomised, phase 3 trial. Lancet (London, England). 2019;393(10168):229‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Butzmann ASK, Jangam D, Kumar J, et al. A comprehensive analysis of RHOA mutation positive and negative angioimmunoblastic T‐cell lymphomas by targeted deep sequencing, expression profiling and single cell digital image analysis. Int J Mol Med. 2020;46(4):1466‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palomero T, Couronné L, Khiabanian H, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014;46(2):166‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maura F, Agnelli L, Leongamornlert D, et al. Integration of transcriptional and mutational data simplifies the stratification of peripheral T‐cell lymphoma. Am J Hematol. 2019;94(6):628‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moskowitz AJ. Practical treatment approach for angioimmunoblastic T‐cell lymphoma. J Oncol Pract. 2019;15(3):137‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sakata‐Yanagimoto M, Enami T, Yoshida K, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46(2):171‐175. [DOI] [PubMed] [Google Scholar]
- 31. Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T‐cell lymphoma. Blood. 2014;123(9):1293‐1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yao WQ, Wu F, Zhang W, et al. Angioimmunoblastic T‐cell lymphoma contains multiple clonal T‐cell populations derived from a common TET2 mutant progenitor cell. J Pathol. 2020;250(3):346‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang P, Zhang M. Epigenetic alterations and advancement of treatment in peripheral T‐cell lymphoma. Clin Epigenetics. 2020;12(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quivoron C, Couronné L, Della Valle V, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20(1):25‐38. [DOI] [PubMed] [Google Scholar]
- 35. Mertsch SKO. The interplay between histone deacetylases and rho kinases is important for cancer and neurodegeneration. Cytokine Growth Factor Rev. 2017;37:29‐45. [DOI] [PubMed] [Google Scholar]
- 36. Tari GLF, Morschhauser F. Epigenetic focus on angioimmunoblastic T‐cell lymphoma: pathogenesis and treatment. Curr Opin Oncol. 2021;33(5):400‐405. [DOI] [PubMed] [Google Scholar]
- 37. Zhang YW, Wang Z, Xie W, et al. Acetylation enhances TET2 function in protecting against abnormal DNA methylation during oxidative stress. Mol Cell. 2017;65(2):323‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sawas A, Ma H, Shustov A, et al. Characterizing the belinostat response in patients with relapsed or refractory angioimmunoblastic T‐cell lymphoma. Leuk Lymphoma. 2020;61(8):2003‐2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Foss F, Horwitz S, Pro B, et al. Romidepsin for the treatment of relapsed/refractory peripheral T cell lymphoma: prolonged stable disease provides clinical benefits for patients in the pivotal trial. J Hematol Oncol. 2016;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T‐cell lymphoma: results of the pivotal phase II BELIEF (CLN‐19) study. J Clin Oncol. 2015;33(23):2492‐2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open‐label, phase II study of romidepsin in relapsed or refractory peripheral T‐cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631‐636. [DOI] [PubMed] [Google Scholar]
- 42. Pro B, Horwitz SM, Prince HM, et al. Romidepsin induces durable responses in patients with relapsed or refractory angioimmunoblastic T‐cell lymphoma. Hematol Oncol. 2017;35(4):914‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Connor OA, Falchi L, Lue JK, et al. Oral 5‐azacytidine and romidepsin exhibit marked activity in patients with PTCL: a multicenter phase 1 study. Blood. 2019;134(17):1395‐1405. [DOI] [PubMed] [Google Scholar]
- 44. Wei S. Clinical analysis of Chidamide combined with etoposide, prednisone and thalidomide (C‐PET) regimen in the treatment of AITL patients [in Chinese]. Qingdao University Master's Thesis 2019.
- 45. Koprinarova M, Schnekenburger M, Diederich M. Role of histone acetylation in cell cycle regulation. Curr Top Med Chem. 2016;16(7):732‐744. [DOI] [PubMed] [Google Scholar]
- 46. Luzhin AV, Velichko AK, Razin SV, Kantidze OL. Automated analysis of cell cycle phase‐specific DNA damage reveals phase‐specific differences in cell sensitivity to etoposide. J Cell Biochem. 2016;117(10):2209‐2214. [DOI] [PubMed] [Google Scholar]
- 47. Sionov RV, Spokoini R, Kfir‐Erenfeld S, Cohen O, Yefenof E. Mechanisms regulating the susceptibility of hematopoietic malignancies to glucocorticoid‐induced apoptosis. Adv Cancer Res. 2008;101:127‐248. [DOI] [PubMed] [Google Scholar]
- 48. Raza S, Safyan RA, Lentzsch S. Immunomodulatory drugs (IMiDs) in multiple myeloma. Curr Cancer Drug Targets. 2017;17(9):846‐857. [DOI] [PubMed] [Google Scholar]
- 49. Zajączkowska R, Kocot‐Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy‐induced peripheral neuropathy. Int J Mol Sci. 2019;20(6):1451‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sinkule JA. Etoposide: a semisynthetic epipodophyllotoxin. Chemistry, pharmacology, pharmacokinetics, adverse effects and use as an antineoplastic agent. Pharmacotherapy. 1984;4(2):61‐73. [DOI] [PubMed] [Google Scholar]
- 51. Griggs RC, Miller JP, Greenberg CR, et al. Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy. Neurology. 2016;87(20):2123‐2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


