Abstract
Venous thromboembolism is a very common and costly health problem worldwide. Anticoagulant treatment for VTE is imperfect: all have the potential for significant bleeding, and none prevent the development of post thrombotic syndrome after deep vein thrombosis or chronic thromboembolic pulmonary hypertension after pulmonary embolism. For these reasons, alternate forms of therapy with improved efficacy and decreased bleeding are needed. Selectins are a family (P‐selectin, E‐selectin, L‐selectin) of glycoproteins that facilitate and augment thrombosis, modulating neutrophil, monocyte, and platelet activity. P‐ and E‐selectin have been investigated as potential biomarkers for thrombosis. Inhibition of P‐selectin and E‐selectin decrease thrombosis and vein wall fibrosis, with no increase in bleeding. Selectin inhibition is a promising avenue of future study as either a stand‐alone treatment for VTE or as an adjunct to standard anticoagulation therapies.
Keywords: inflammation, pulmonary embolism, selectins, venous thromboembolism, venous thrombosis
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), may affect up to 900 000 patients per year, with over 300 000 deaths per year in the United States. 1 The incidence has remained constant over the past 30 years and is increasing. DVT has been increasing with the age of the population – those 85–89 years old have an incidence as high as 310/100 000 population. 2 Treatment costs run in billions of dollars per year and is increasing with the widespread adoption of the more expensive direct oral anticoagulants (DOACs). 3
1. UNMET NEEDS IN CARE OF THE POST DVT PATIENT
All current and planned therapies involve targeting portions of the coagulation system, resulting in bleeding risk. The current standard of therapy, DOACs, target either factor Xa or factor IIa. The incidence of major bleeding with DOACs has been reported between 0.6% and 1.4%, while major plus clinically non‐major bleeding has been noted in 4.3%–9.4% of patients. 4 A more recent real‐world comparison of bleeding risks among >60 000 non‐valvular atrial fibrillation patients demonstrated rates of any bleeding 11%–16% for the different DOACs, and major bleeding 1.4%–2.1%. 5 Case fatality rates for major bleeding in 13 randomized studies with over 100 000 cases are 7.6% for DOACs and 11% for warfarin, and 50% if >90 years. 6 , 7 For patients who do not qualify or cannot afford DOACs, the mainstay of therapy is low molecular weight heparin (LMWH) or vitamin K antagonists (VKAs). The incidence of major bleeding with the LMWH enoxaparin has been reported between 1.7% and 2.1% for DVT prophylaxis and treatment, 8 while the incidence of major bleeding in trials with warfarin is between 1.6% and 2.0%. 4
The complications of DVT treatment extend beyond bleeding. A significant proportion of patients with significant iliofemoral and femoral‐popliteal DVT will develop post thrombotic syndrome (PTS), 9 manifested as leg pain, swelling, varicosities and even venous ulceration. In the setting of iliofemoral DVT, significant PTS occurs in up to 50%–80% of patients. 10 Patients who suffer from PE find that dyspnea and poor physical performance is very common after “adequately treated PE” along with the development of chronic thromboembolic pulmonary hypertension (CTEPH). 11 The burden of VTE disease has been recognized, with the Surgeon General's call to action against VTE in 2008, yet the overall incidence of VTE and development of complications has not decreased.
2. SELECTINS AND THEIR LIGANDS
A full history of the mechanisms and functions of selectins and their ligands is beyond the scope of this concise review. However, this topic is covered in more detail by previous literature. 12 , 13 , 14 , 15
2.1. P‐selectin
For over 150 years, venous thrombogenesis has been driven by the concept of Virchow's triad of circulatory stasis, endothelial injury, and blood hypercoagulability. In 1992, inflammation was implicated in thrombogenesis as it was shown that if the glycoprotein P‐selectin was inhibited, thrombosis was decreased in a primate model of an arteriovenous fistula. 16 Selectins, including P‐selectin, E‐selectin, and L‐selectin, are a family of calcium‐dependent lectins (glycoproteins) that are expressed on the surface of platelets, endothelial cells and leukocytes. They are important mediators of leukocyte and immune cell adherence and transmigration into sites of inflammation and facilitate and augment thrombosis by modulating neutrophil and monocyte activity. 17
P‐selectin is a type‐1 transmembrane protein, encoded by the Selectin‐P (SELP) gene in humans. It is produced in megakaryocytes and endothelial cells, and is packaged in platelet alpha granules and endothelial cell Weibel‐Palade bodies. 17 On stimulation, P‐selectin is rapidly mobilized to the surface of the cell and then externalized. Just as rapidly P‐selectin can be re‐internalized and destroyed or recycled. The main ligand for P‐selectin is P‐selectin glycoprotein ligand‐1 (PSGL‐1), present on most leukocytes. P‐selectin also binds platelet glycoprotein (Gp)1b, facilitating both leukocyte and platelet rolling and adhesion. P‐selectin additionally triggers the release of procoagulant microparticles 18 and increases tissue factor expression on monocytes. 19 Both of these actions augment thrombosis. PSGL‐1 plus microvesicles, originating from monocytes and endothelial cells, are elevated in patients with unprovoked VTE. 20 P‐selectin expression on platelets also stabilizes initial glycoprotein IIb/IIIa‐fibrinogen interactions, leading to the formation of large stable platelet aggregates. 21
In a mouse model of complete inferior vena cava (IVC) stasis with IVC ligation, animals with elevated soluble P‐selectin (sP‐selectin) had a 50% increase in thrombus mass (thrombus weight/IVC length), while those animals with selectins gene deleted demonstrated a significant decrease in thrombus mass. 22 The increased thrombus mass in mice with elevated sP‐selectin was associated with the release of procoagulant microparticles and neutrophil extracellular traps. 23 (Figure 1) Similar findings have been noted with a model of IVC stasis with partial IVC ligation. 24 The fact that similar findings have been found in these different models is important, as areas of complete stasis and partial stasis often exist simultaneously in the pathophysiology of clinical DVT. In another study using the model of complete IVC stasis, when mice were inoculated with klebsiella pneumoniae to induce pneumonia, they were found to have increased levels of circulating P‐selectin. 25 Since elevated sP‐selectin is associated with DVT risk and sepsis increases risk for VTE, the role of P‐selectin in sepsis associated DVT was explored. When sepsis was induced experimentally in mice with the endotoxin lipopolysaccharide, P‐selectin was up‐regulated at the vein wall. 26 These findings suggests that P‐selectin also plays a role in VTE in the inflammatory setting of infection. Additionally, inflammatory cell entrance into the vein wall and surrounding tissue contributes to vein wall inflammation and eventually vein wall and valve fibrosis. These changes contribute to PTS development.
FIGURE 1.
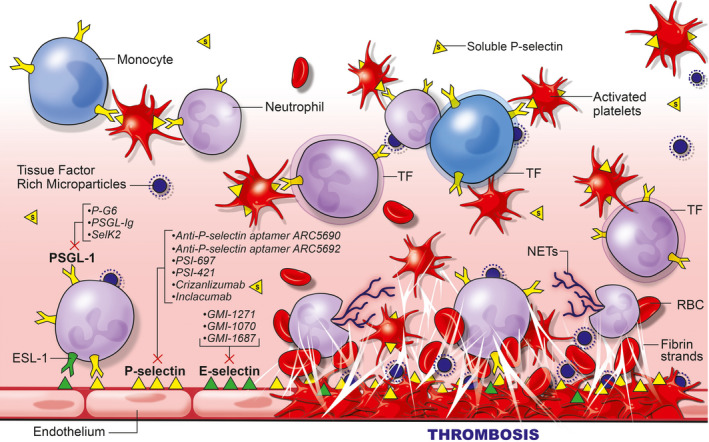
E‐selectin and P‐selectin involvement in thrombosis and inflammation. Upon activation of the endothelium or platelets, P‐selectin becomes exposed and E‐selectin becomes upregulated. These selectin glycoproteins, located on inflammatory cells (P‐ and E‐selectin) and platelets (P‐selectin) allow for leukocyte‐endothelial, leukocyte‐platelet, and leukocyte‐leukocyte interactions, the release of tissue factor rich microparticles from neutrophils, the upregulation of tissue factor on monocytes, and thus the facilitation of thrombosis and thrombosis amplification. At the same time, other inflammatory cells hone into the area of thrombosis. These include neutrophils which become activated to produce procoagulant neutrophil extracellular traps. These inflammatory cells bind to other receptors such as the MAC‐1 receptor, facilitating the production of fibrin, the accumulation of red blood cells, and the ultimate development of a very cellular thrombus. Additionally, inflammatory cell entrance into the vein wall and surrounding tissue contributes to vein wall inflammation and eventually vein wall and valve fibrosis. Note circulating sP‐selectin (s). The location of the primary mechanism of action for the inhibitors of P‐selectin (including inhibitors to the PSGL‐1 ligand) and E‐selectin are included
2.2. P‐selectin in human disease and as a biomarker
Although it is the membrane associated P‐selectin which primarily contributes to the pathogenesis of DVT, its soluble form has been evaluated in several studies as a biomarker for VTE. Membrane P‐selectin forms dimers or oligomers to increase leukocyte membrane binding leading to the strong inflammatory and thrombotic responses, where sP‐selectin is present as a monomer that is not sufficient to interact with its functional ligands. 27 , 28 sP‐selectin is derived primarily from the proteolytic cleavage of transmembrane P‐selectin, shed from activated platelets and endothelial cells. High levels of sP‐selectin are associated with VTE and have been proposed as a biomarker with better diagnostic performance than D‐dimer where it performed favorably compared with D‐dimer when combined with the Wells Score. 29 , 30 When combined with a Wells Score ≥2, sP‐selectin >90 ng/ml demonstrated a positive predictive value of 100% for the diagnosis of DVT in a prospective trial. Levels of sP‐selectin of <60 ng/ml with a low probability Wells Score ruled out DVT with a sensitivity of 99% and negative predictive value of 96%. 30 A 2014 meta‐analysis of sP‐selectin that compared 586 VTE patients with 1843 controls found significantly increased sP‐selectin in patients with VTE or with DVT alone and minimal heterogeneity among studies. 31 Pooled sensitivity and specificity for sP‐selectin were 0.57 (95% confidence interval, 0.30–0.82) and 0.73 (95% confidence interval, 0.51–0.90). A nonsignificant trend to higher sP‐selectin levels in proximal DVT compared with distal DVT has been demonstrated 32 and, similarly, a nonsignificant trend was shown for increased sP‐selectin levels with greater extent of thrombus. Patients who experienced recurrent VTE had significantly higher levels of sP‐selectin than patients who did not have a recurrent VTE. 33 544 patients were prospectively followed after their first unprovoked VTE and after finishing the corresponding anticoagulant. Patients with VTE recurrence had significantly higher levels of sP‐selectin than patients who did not have a VTE recurrence. Additionally, patients with a sP‐selectin level greater than the 75th percentile were 1.7 times more likely to have a VTE recurrence.
sP‐selectin has been widely studied as a biomarker for cancer, and has been identified as a biomarker of cancer‐associated thrombosis in a general cancer population. 34 Specific cancers that have been identified by sP‐selectin include non‐small cell lung cancer, 35 breast, lung, gastrointestinal tract, pancreas, kidney, prostate, brain and hematological malignancies. 36 , 37 sP‐selectin has also been implicated in VTE in infectious and inflammatory states. Sickle cell disease (SCD) is an inflammatory disease with elevated sP‐selectin and elevated DVT risk. 38 , 39 The reported cumulative risk for VTE in SCD ranges from 2.9% in children up to 25.0% in adults. Although clinical and laboratory risk factors for VTE are not clear, its clinical importance in SCD is highlighted by a 2.3‐ to 2.9‐fold increased risk of death in those with vs. without a VTE event. The genetic basis for VTE risk in SCD has not been previously reported, but variants implicated in other non‐SCD African American cohorts may be relevant.
Patients with human immunodeficiency virus (HIV) who also experienced VTE had increased levels of D‐dimer, hyaluronic acid, and sP‐selectin. 40 In a murine study, obesity was associated with increased sP‐selectin expression and formation of monocyte‐platelet conjugates. 41 As obesity is a risk factor for VTE, this finding suggests that sP‐selectin could play a causal role in VTE in obesity. As obstructive sleep apnea (OSA) is also associated with VTE, a meta‐analysis was conducted that explored levels of sP‐selectin in OSA. 42 In this analysis, sP‐selectin levels were higher in patients with moderate‐to‐severe OSA than in patients with mild or no OSA, regardless of patient body mass index (BMI) or blood source of plasma or serum. COVID‐19, the cause of the worldwide coronavirus pandemic, has also been associated with elevated rates of VTE. 43 Levels of sP‐selectin are significantly elevated in patients with COVID‐19 when compared to controls. It was also determined that sP‐selectin levels were positively correlated to COVID‐19 severity. Upregulation of P‐selectin on injured endothelium and activated platelets in COVID‐19 could contribute to immunothrombosis and thromboinflammation, the hallmarks of COVID‐19 coagulopathy. 17
2.3. P‐selectin inhibitors (Table 1)
TABLE 1.
P‐selectin inhibitors investigated for use in VTE and other disorders
| Agent | Drug class | MOA | Preclinical model evaluated | Completed clinical trials | Ongoing clinical trials | FDA approval |
|---|---|---|---|---|---|---|
| PSI−697 44 , 49 , 57 , 58 , 60 and PSI−421 48 | Small molecule | Selective P‐selectin small molecule antagonists |
PSI−697: VTE, SCD, atherosclerosis (arterial injury) PSI−421: VTE |
PSI−697: Smokers and Scleritis | No | |
| PSGL‐Ig 45 , 46 , 47 , 61 , 62 , 63 , 64 , 65 , 66 | Conjugate | A PSGL−1 trap | VTE, thrombolysis, restenosis, MI, liver transplantation, and ischemia/reperfusion | Phase II:MI size reduction, liver transplantation | No | |
| ARC5692, 51 ARC5690 51 , 67 | Aptamer | Pegylated anti‐P‐selectin aptamer | VTE, SCD | No | ||
| PEG40‐GSnP−6 (P‐G6) 54 , 55 | Glycomimetic | Pegylated glycomimetic of the N terminus of PSGL−1 | VTE | No | ||
| Inclacumab 68 , 69 , 70 , 71 | Antibody | Recombinant monoclonal antibody against P‐selectin | SCD | ACS; MI; Peripheral vascular disorders | Phase 3: VOC in SCD | No |
| Crizanlizumab 72 , 73 , 74 | Antibody | Monoclonal antibody against P‐selectin | SCD, myelofibrosis |
Phase 1/2: Myelofibrosis Phase 2: Priapism; RVCL Phase 2, 3, 4: SCD Phase 4 COVID−19 |
Yes: SCD, VOC | |
| SelK2 56 | Antibody | Monoclonal antibody against PSGL−1 | VTE | DVT, COPD | No |
The ability of multiple P‐selectin inhibitors including small molecule inhibitors and receptor antagonists to limit thrombosis has been demonstrated in two different primate models, including IVC thrombosis and iliac vein thrombosis, and in rodent models of IVC thrombosis. 44 A meta‐analysis of five published primate studies revealed that vein re‐opening was significantly greater with inhibitors to both P‐selectin and its receptor PSGL‐1 compared to saline, similar to results obtains with the LMWH enoxaparin. 45 , 46 , 47 , 48 , 49 , 50 Inflammation, measured by Gadolinium enhancement in the vein wall by magnetic resonance venography, was significantly decreased in the anti‐P‐selectin treated group when compared to saline, with no significant differences compared to enoxaparin treated animals. In addition, there was less prolongation in thrombin clotting time (TCT) with P‐selectin/PSGL‐1 inhibitors compared to enoxaparin, suggesting a lower bleeding potential (p < .0001). 50
In a primate iliac vein thrombosis model, anti‐P‐selectin aptamer (which blocks soluble and bound P‐selectin/PSGL‐1 interactions) was compared to an aptamer which blocks von Willebrand factor (vWF). 51 In a treatment protocol where agents were given 2 days after thrombus formation, the anti‐P‐selectin aptamer resulted in the most open iliac vein lumen 21 days after thrombosis with 72% vein opening compared to 50% for enoxaparin, 13% for control, and 0% for the anti‐VWF aptamer. In a prophylaxis protocol where the agent was circulating at the time of thrombosis, both aptamers produced similar results, although neither totally prevented initial thrombus formation. 51 Importantly, P‐selectin inhibition resulted in less vein wall fibrosis measured by vein wall collagen immunostaining (a signal for a potential decrease in post‐thrombotic vein wall fibrosis and PTS). P‐selectin inhibition produced no significant increase in coagulation times, again suggesting less bleeding potential, even though the potential exists for bleeding as P‐selectin deletion and not E‐selectin deletion (in mice) has been found to cause defects in megakaryocytopoiesis 52 and increase bleeding. 53
Recently, a pegylated glycomimetic of the N terminus of PSGL‐1 has been developed, PEG40‐GSnP‐6 (P‐G6). 54 This agent was found to inhibit both mouse and human platelet‐neutrophil and platelet‐monocyte aggregations in vitro and to block platelet‐leukocyte interactions in vivo, interactions known to be dependent on PSGL‐1‐P‐selectin binding. 55 The agent was then tested in the mouse model of electrolytic injury to the IVC and found to be equivalent to LMWH with no increase in bleeding time. Administration resulted in a decrease in vein wall infiltrating Ly6G+ neutrophils and CD68+ macrophages 48 h after thrombus formation. Although this agent is very promising and warrants further study, a trial in thromboprophylaxis of elective knee surgery was negative using SELK2, an inhibitory antibody against PSGL‐1, alone regarding total VTE and bleeding after surgery, suggesting either a difference in mechanism or target between inhibiting only PSGL‐1 as opposed to the entire P‐selectin–PSGL‐1 axis. 56 However, data available on ClinicalTrials.gov reported that the combination of SelK2 plus Enoxaparin appeared favorable compared to SelK2 alone and Enoxaparin alone for prevention of DVT, but not PE without procedural hemorrhage (https://www.clinicaltrials.gov/ct2/show/NCT03812328?term=selk2&draw=2&rank=1; data non peer‐reviewed) when used for DVT prophylaxis in elective knee surgery.
P‐selectin inhibitors have also been explored in contexts other than VTE, as P‐selectin has been shown to contribute to states of pathological inflammation and thrombosis including atherosclerosis, arterial thrombosis, and ischemia‐reperfusion injury, among others. 15 PSI‐697, a selective P‐selectin small molecule antagonist, has been evaluated in a clinical trial of healthy smokers. 57 This agent was found not to inhibit platelet‐monocyte aggregate formation in humans, despite the fact that in an ex‐vivo model of thrombosis, PSI‐697 resulted in a reduction in thrombus formation at both high and low shear. 58 This agent has also been studied in experimental SCD, where it was shown to improve parameters associated with vaso‐occlusion when given in a prophylactic fashion 59 and in a rat arterial injury model where it was shown to decrease intima/media ratios compared to vehicle controls (in addition to it positive effects in a rat venous thrombosis model in the same manuscript) 60 rPSGL‐Ig, a P‐selectin trap, has been found effective to augment thrombolysis in animal models 61 and decrease restenosis in a swine angioplasty model. 62 In a canine coronary artery balloon occlusion model, rPSGL‐Ig decreased myocardial injury and inflammation for at least 7 days after reperfusion of ischemic myocardium. 63 However, it failed in two phase II clinical trials to reduce myocardial infarction (MI) infarct size. 64 , 65 It has also been studied in the field of liver transplantation and found to decrease ischemia and reperfusion injury in a single center phase II study. 66
The anti‐P‐selectin aptamer ARC5690 has been studied in the context of SCD where it was found to inhibit the adhesion of sickle RBCs and leukocytes to endothelial cells by 90% and 80%, respectively, in mice. It also increased microvascular flow velocities, reduced leukocyte rolling flux, and reduced mortality in mice. 67 Inclacumab, a monoclonal antibody against P‐selectin, has been studied in a first in human investigation, was well tolerated in 56 volunteers and did not affect bleeding or platelet aggregation. 68 Inclacumab has also been shown to inhibit platelet‐leukocyte interaction in a concentration dependent manner in healthy volunteers. 69 In the context of cardiac disease including acute coronary syndrome (ACS) and MI, Inclacumab reduced myocardial damage after percutaneous coronary intervention (PCI) in patients with non–ST‐segment elevation MI, 70 and results were best if the agent was administered <3 h before the PCI event. 71 However, this agent has been discontinued for this indication. There are ongoing phase three clinical trials in vaso‐occlusive crisis (VOC) and SCD. Table 2 Crizanlizumab, a humanized monoclonal antibody against P‐selectin, has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of VOC in SCD. 72 , 73 When used in patients with SCD, Crizanilzumab decreased the annual occurrence of VOC by 45%. 72 , 74 Currently, this agent is in a Phase 1/2 trial for myelofibrosis, Phase 2 trials for priapism, retinal vasculopathy and cerebral leukoencephalopathy (RVCL), Phase 2, 3, and 4 trials for SCD, and a Phase 2/4 trial for COVID‐19. Table 2 Finally, the inhibitory antibody against PSGL‐1, SelK2, is also being investigated for chronic obstructive pulmonary disease (COPD) and asthma.
TABLE 2.
Ongoing clinical trials for P‐selectin inhibitors (modified from clinicaltrials.gov)
| Ongoing: ClinicalTrials.Gov study numbers for Inclacumab | |||
| VOC in SCD | NCT04935879 | Phase 3 | Global blood therapeutics |
| Recurrent VOC in SCD | NCT04927247 | Phase 3 | Global blood therapeutics |
| Ongoing: ClinicalTrials.Gov study numbers for Crizanlizumab | |||
| COVID−19 | NCT04505774 | Phase 4 | University of Pittsburgh |
| Myelofibrosis | NCT04097821 | Phase 1/2 | Novartis |
| Priapism | NCT03938454 | Phase 2 | Novartis |
| RVCL | NCT04611880 | Phase 2 | Washington University |
| SCD (Pediatrics) | NCT03474965 | Phase 2 | Novartis |
| SCD | NCT04053764 | Phase 2 | Novartis |
| SCD | NCT03264989 | Phase 2 | Novartis |
| SCD | NCT03814746 | Phase 3 | Novartis |
| SCD | NCT04657822 | Phase 4 | Novartis |
| SCD | NCT04662931 | Phase 4 | Novartis |
2.4. E‐selectin
E‐selectin is also a key regulator of thrombus formation and fibrin content. E‐selectin is a glycoprotein expressed from activated endothelium that facilitates thrombosis, directly modulating neutrophil and monocyte activity. Figure 1 E‐selectin resides primarily in the endothelium and associates closely with P‐selectin to recruit leukocytes to sites of inflammation. 15 E‐selectin can use the same receptor as P‐selectin, but also has other additional leukocyte receptors. Although P‐selectin is exposed at the vein wall as early as 6 h after DVT formation, E‐selectin is upregulated and expressed later, approximately 2 days after DVT formation. 75
E‐selectin is an important regulator of thrombus formation and fibrin content in a mouse venous thrombosis model. P/E‐selectin double‐knockout mice had less thrombus burden and less inflammation when thrombosis was induced 75 and E‐selectin knockout mice had decreased fibrin content of the thrombus and less vein wall inflammation and fibrosis. 50 , 51 , 76 E‐selectin has been shown to be efficient at raising the affinity and avidity of CD18 Macrophage‐1 antigen (Mac‐1) integrins which support neutrophil trafficking to sites of acute inflammation and recruit platelets and red blood cells. 77 Sialyl‐Lewis X (sLex), expressed on L‐selectin on leukocytes, is aggressively bound by E‐selectin and on ligation, initiates the secretion of myeloid‐related protein 8 (MRP8) and myeloid‐related protein 14 (MRP14) that bind the toll‐like receptor‐4 (TLR4) to elicit the extension of β2‐integrin receptor to an intermediate affinity state. 78 Neutrophil rolling over E‐selectin at venous shear rates then transmits tension and catch‐bond formation, resulting in a distinct signal to upshift the β2‐integrin receptor to a high‐affinity state, allowing leukocytes to stop rolling and extravasates into the vein wall, thrombus and local environment. These findings are bolstered by a study that explored the degree of leukocyte rolling, as a marker of endothelial inflammation and damage, in mice with and without E‐selectin gene deletion. Mice IVCs were treated with tumor necrosis factor alpha (TNFα) to induce inflammation in both groups. As would be expected based on prior studies, increased leukocyte rolling velocity and rolling flux was observed in E‐selectin gene‐deleted mice as compared to wild type mice, while leukocyte adhesion was attenuated. 79
2.5. E‐selectin in human disease and as a potential biomarker
Endotoxin induced tissue factor mediated coagulation is enhanced in humans carrying the S128R E‐selectin allele. 80 Patients homozygous for the S128R E‐selectin allele have an increased risk for VTE recurrence, highlighting the importance of E‐selectin in venous thrombosis. 81 The importance of E‐selectin inhibition in decreasing vein wall intimal thickness and fibrosis has been supported by a recent case‐control study of 124 patients with DVT, of which 31 had severe PTS, 62 mild/no PTS, and 31 were healthy controls. 82 Patients with severe PTS demonstrated elevated levels of C‐reactive protein (CRP), soluble intercellular adhesion molecule‐1 (sICAM‐1), soluble E‐selectin (sE‐selectin), and decreased matrix metalloproteinase (MMP‐9), and monocyte chemoattractant protein‐1 (MCP‐1) levels when compared to patients with mild/no PTS, suggesting that patients with PTS present an altered inflammatory state many months after an acute thrombotic event. The increase in the levels of sE‐selectin were statistically higher in patients with PTS compared to those with mild/no PTS.
Although the above discussion supports the role of endothelial‐derived E‐selectin in venous thrombogenesis and PTS, there have been studies that have found sE‐selectin levels to be lower in DVT patients than controls 83 , 84 or not significantly elevated, 85 , 86 and not elevated in patients with PTS, although levels of sE‐selectin tended to be higher in the more severe case groups. 87 This is likely due to the fact that E‐selectin is an endothelial cell associated glycoprotein which may not be readily released into the circulation. Thus, it has suggested that sE‐selectin is not as good a biomarker for diagnosis of DVT as sP‐selectin, which tends to be rapidly released into the circulation on activation of platelets and endothelial cells and is a suboptimal biomarker with mixed results compared to the current standard of care. However, levels of sE‐selectin have recently been suggested to be a good biomarker for patients with COVID‐19 pneumonia requiring ICU care, likely related to the endothelial activation and inflammation. 88 Research by other authors has furthered the notion that E‐selectin plays a significant role in VTE formation. Twenty‐five patients with VTE were found to have both elevations in sE‐selectin (EMP62E) and a greater percentage of monocytes positive for E‐selectin than patients without VTE, highlighting the evidence that E‐selectin's release and role in the inflammatory pathway are significant in VTE. 89
Venous thromboembolism is a common complication in other vascular pathologies. sE‐selectin was studied in the setting of VTE in post‐operative surgical patients with abdominal malignancies compared to those that did not undergo surgery nor have a malignancy. 90 It was concluded that sE‐selectin levels were higher in patients in the former group. The authors again further suggested that sE‐selectin could be utilized as a diagnostic tool for predicting DVT. In replicating the inflammatory reaction due to shear stress that occurs in saphenous vein graft failure for coronary artery bypass grafts, the role that E‐selectin plays in endothelial cell dysfunction has been highlighted. 91 Veins that were put under high stretch in vitro, in order to replicate vein arterialization and initiate inflammation, were found to have an increase in E‐selectin endothelial surface markers when compared to veins that underwent low stretch, suggesting that E‐selectin has a role in the inflammation of arterialized saphenous vein grafts. E‐selectin has also been studied in the context of pulmonary arterial hypertension (PAH). 92 Patients with PAH were found to have significantly higher levels of sE‐selectin than patients with normal pulmonary arterial pressure. The role of E‐selectin in VTE has also been explored in sex differences. E‐selectin endothelial cell‐derived microparticle concentration was determined to be higher in females than males. 93 This higher E‐selectin level was noted during the luteal phase of the menstrual cycle. The authors hypothesized that this finding could play a role in the hypercoagulable state of premenopausal women.
2.6. E‐selectin inhibitors (Table 3)
TABLE 3.
E‐selectin inhibitors investigated for use in VTE and other disorders
| Agent | Drug class | MOA | Preclinical model evaluated | Completed clinical trials | Ongoing clinical trials | FDA approval |
|---|---|---|---|---|---|---|
| GMI−1271 94 , 95 , 96 , 97 | Small molecule | E‐selectin antagonist | DVT, AML, MM, ALL, PDAC, CML, PC, HSCT | DVT, AML, MM |
Phase 1: AML, MDS, MPAL Phase 1/2: AML, COVID−19 pneumonia Phase 2: Auto‐HCT for MM Phase 2/3: AML Phase 3: AML (see details below, Table 4) |
Ongoing |
| GMI‐1687 98 | Small molecule | E‐selectin antagonist | DVT, AML, VOC in SCD, CNV, MM | Pre‐clinical | ||
| GMI−1070 99 | Small molecule | E‐selectin (primary) P‐, L‐selectin inhibitor (secondary) | VOC in SCD | SCD VOC | No |
Abbreviations: ALL, acute lymphocytic leukemia; CML, chronic myelogenous leukemia; CNV, corneal neovascularization; PC, prostate cancer; PDAC, pancreatic ductal adenocarcinoma.
An E‐selectin inhibitor (GMI‐1271) was equivalent to enoxaparin for limiting thrombosis in a mouse model of thrombosis that used stasis with some preserved flow, with a concurrent significant reduction in tail vein bleeding time. 94 A phase I and II clinical trial was then performed with GMI‐1271 given to normal volunteers in a dose‐dependent fashion as a one‐time dose, then as a daily dose for 5 consecutive days, compared to enoxaparin or saline, and then used to treat calf vein DVT (sponsored by the National Institutes of Health [NIH] Vascular Interventions/Innovations and Therapies Advances [VITA] program). Biomarkers of inflammation and coagulation were measured, along with cell adhesion and leukocyte and platelet activation markers. 95 In this study, findings included no serious adverse events. The inhibitor did not affect thromboelastrographic parameters, lower levels of sE‐selectin were found in GMI‐1271 treated volunteers, and lower leukocyte and platelet activation were seen in GMI‐1271 treated volunteers (indicated by reduced myeloperoxidase [MPO] and MAC‐1 levels). Two patients with calf vein DVT treated with 5 days of GMI‐1271 had immediate relief of pain and significant increase in vein recanalization by day 19. In these patients, levels of TNFα decreased from baseline to day 4 (6.84 ng/ml to 0), while CRP, D‐dimer and tissue factor levels all trended lower. 95 Following this investigation, a primate study was performed of E‐selectin inhibition with GMI‐1271 alone and in combination with LMWH and LMWH alone. Iliac vein thrombosis was induced by balloon occlusion of the iliac vein for 6 h. Starting on day 2 after thrombosis, animals began treatment in two phases. In phase one, non‐treated controls received no treatment (n = 5) vs. animals treated with the E‐selectin inhibitor GMI‐1271, 25 mg/kg, subcutaneous (SC), once daily (n = 4) for 21 days. 96 In Phase 2, animals were treated with GMI‐1271 plus a combination of LMWH 1.5 mg/kg or 40 mg (GMI +LMWHc), SC, once daily (n = 8) for 19 days; and animals treated with LMWH 1.5 mg/kg or 40 mg (LMWHc) alone, SC once daily (n = 6) for 19 days. Percent vein recanalization by magnetic resonance venography was highest in the GMI‐1271 alone group followed by GMI‐1271 plus LMWHc, both significantly different from control. On ultrasound examination, animals treated with GMI‐1271 alone had no decrease in open vein lumen by day 21, whereas decreases were observed in groups GMI‐1271 plus LMWHc (−26%), LMWHc alone (−27%), and controls (−80%). Vein wall inflammation decreased significantly in all treated groups. Intimal fibrosis and intimal thickness were best preserved in the GMI‐1271 alone group. An analysis of total vein wall collagen revealed a trend in all treated groups of decreasing vein wall collagen content. No clinically significant bleeding events were noted in any group. The LMWH groups trended to have prolonged coagulation test values, whereas E‐selectin inhibition with GMI‐1271 did not cause clinically significant changes in coagulation measures. 97 Thus, E‐selectin inhibition alone was the best therapy in this model.
E‐selectin inhibitors have also studied in other indications. They have been shown to be successful in treating VOC in SCD. Additionally, GMI‐1271 is in ongoing Phase I studies for acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and mixed phenotype acute leukemia (MPAL), Phase 1/2 studies for AML and COVID‐19 pneumonia, Phase 2 studies for autogenous hematopoietic cell transplantation (Auto‐HCT) for Multiple Myeloma (MM), and Phase 2/3 and Phase 3 studies for AML. Table 4 A new highly potent agent has recently been produced, GMI‐1687, that demonstrates high bioavailability following SC administration in preclinical models previously reported for GMI‐1271, but at approximately 250‐fold lower dose. 98 Finally, GMI‐1070, a pan‐selectin inhibitor, was shown to improve blood flow and survival in mice with SCD by inhibiting the binding of leukocytes and erythrocytes. 99 Furthermore, it was shown to be safe and well‐tolerated in humans with an increase in red blood cell velocity in a subset of patients.
TABLE 4.
Ongoing clinical trials for E‐selectin inhibitors (modified from clinicaltrials.gov)
| Ongoing: ClinicalTrials.Gov study numbers for GMI−1271 (Uproleselan) | |||
| AML (front line treatment) | NCT04964505 | Phase 1 | University of California, Davis |
| AML (relapsed/refractory) | NCT05054543 | Phase 3 | Apollomics, China |
| AML (treated secondary) | NCT04848974 | Phase 1b/2 | MD Anderson |
| AML (relapsed/refractory) | NCT03616470 | Phase 3 | Glycomimetics |
| AML (front line AML, >60 years) | NCT03701308 | Phase 2/3 | NCI/Alliance |
| AML, MDS, MPAL | NCT05146739 | Phase 1 | Children's Oncology Group |
| COVID−19 Pneumonia | NCT05057221 | Phase 1/2 | University of Michigan, Ann Arbor |
| Auto‐HCT for MM | NCT04682405 | Phase 2 | Washington University, St Louis |
3. FUTURE OF SELECTIN INHIBITORS AND VTE
While P‐selectin holds promise as an effective biomarker for the diagnosis of VTE, both P‐selectin and E‐selectin should make attractive alternatives to standard anticoagulants as effective agents for the prophylaxis and treatment of VTE and for the prevention of PTS (either as a stand‐alone treatment for VTE, or as an adjunct to standard therapies). This is by virtue of the fact that inhibition of these selectins decreases thrombosis, limits fibrosis, and produces no significant increases in tests that are associated with bleeding. For these agents to make the next steps for use in clinical VTE, larger trials to compare them head‐to‐head to current therapies are needed. Another area where selectin inhibitors could be very useful is in conjunction with stents in the venous system, including the new nitinol venous stents used especially in the iliac veins and IVC, where adjunctive anticoagulants have not been standardized. 100 Finally, drug delivery directly into the venous wall or the thrombus, to obtain more targeted therapy to the places where needed, should be pursued. For example, using a selectin inhibitor in conjunction with clot extraction could improve the results of aggressive interventional strategies for DVT, where these interventions have not proven robust enough to suggest them as standard of care therapies. The use of selectin inhibitors could be a great adjunctive therapy to extend the benefits of the “open‐vein hypothesis”.
CONFLICT OF INTEREST
All authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors have contributed to the writing and editing of this manuscript.
INFORMED CONSENT
All the authors have read and approved the submission. Full informed consent was obtained for this article from all the co‐authors.
ACKNOWLEDGEMENTS
We thank Robert G. Schaub PhD, and John L. Magnani PhD (Chief Science Officer and Senior Vice President, GlycoMimetics, Inc.) for their review of tables, Lorie Gavulic for Figure 1, and Mary Bergeron for her help with the preparation of the manuscript.
Purdy M, Obi A, Myers D, Wakefield T. P‐ and E‐ selectin in venous thrombosis and non‐venous pathologies. J Thromb Haemost. 2022;20:1056–1066. doi: 10.1111/jth.15689
Manuscript Handled by: Patricia Liaw
Final decision: Patricia Liaw, 22 February 2022
REFERENCES
- 1. Heit JA, Cohen A, Anderson FA Jr. Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the U.S. Blood. 2005;106(11):267a. [Google Scholar]
- 2. Heit JA, Silverstein MD, Mohr DN, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost. 2001;86(1):452‐463. [PubMed] [Google Scholar]
- 3. Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital‐acquired and preventable costs utilising long‐term attack rates. Thromb Haemost. 2012;108(2):291‐302. [DOI] [PubMed] [Google Scholar]
- 4. Yeh CH, Gross PL, Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood. 2014;124(7):1020‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tepper PG, Mardekian J, Masseria C, et al. Real‐world comparison of bleeding risks among non‐valvular atrial fibrillation patients prescribed apixaban, dabigatran, or rivaroxaban. PLoS One. 2018;13(11):e0205989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson I, Cifu AS. Management of bleeding in patients taking oral anticoagulants. JAMA. 2018;319(19):2032‐2033. [DOI] [PubMed] [Google Scholar]
- 7. Vasco B, Villalba JC, Lopez‐Jimenez L, et al. Venous thromboembolism in nonagenarians. Findings from the RIETE registry. Thromb Haemost. 2009;101(6):1112‐1118. [PubMed] [Google Scholar]
- 8. Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood. 2008;111(10):4871‐4879. [DOI] [PubMed] [Google Scholar]
- 9. Baldwin MJ, Moore HM, Rudarakanchana N, Gohel M, Davies AH. Post‐thrombotic syndrome: a clinical review. J Thromb Haemost. 2013;11(5):795‐805. [DOI] [PubMed] [Google Scholar]
- 10. Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long‐term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239(1):118‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matusov Y, Singh I, Yu YR, et al. Chronic thromboembolic pulmonary hypertension: the bedside. Curr Cardiol Rep. 2021;23(10):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91(16):7390‐7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79(1):181‐213. [DOI] [PubMed] [Google Scholar]
- 14. Magnani JL. The discovery, biology, and drug development of sialyl Lea and sialyl Lex. Arch Biochem Biophys. 2004;426(2):122‐131. [DOI] [PubMed] [Google Scholar]
- 15. McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107(3):331‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palabrica T, Lobb R, Furie BC, et al. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P‐selectin on adherent platelets. Nature. 1992;359(6398):848‐851. [DOI] [PubMed] [Google Scholar]
- 17. Agrati C, Sacchi A, Tartaglia E, et al. The role of P‐selectin in COVID‐19 coagulopathy: an updated review. Int J Mol Sci. 2021;22(15):7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andre P, Hartwell D, Hrachovinova I, Saffaripour S, Wagner DD. Pro‐coagulant state resulting from high levels of soluble P‐selectin in blood. Proc Natl Acad Sci USA. 2000;97(25):13835‐13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Celi A, Pellegrini G, Lorenzet R, et al. P‐selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci USA. 1994;91(19):8767‐8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jamaly S, Basavaraj MG, Starikova I, Olsen R, Braekkan SK, Hansen JB. Elevated plasma levels of P‐selectin glycoprotein ligand‐1‐positive microvesicles in patients with unprovoked venous thromboembolism. J Thromb Haemost. 2018;16(8):1546‐1554. [DOI] [PubMed] [Google Scholar]
- 21. Merten M, Thiagarajan P. P‐selectin expression on platelets determines size and stability of platelet aggregates. Circulation. 2000;102(16):1931‐1936. [DOI] [PubMed] [Google Scholar]
- 22. Myers DD, Hawley AE, Farris DM, et al. P‐selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38(5):1075‐1089. [DOI] [PubMed] [Google Scholar]
- 23. Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. P‐selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126(2):242‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yago T, Liu Z, Ahamed J, McEver RP. Cooperative PSGL‐1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice. Blood. 2018;132(13):1426‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obi AT, Andraska E, Kanthi Y, et al. Gram‐negative pneumonia alters large‐vein cell‐adhesion molecule profile and potentiates experimental stasis venous thrombosis. J Vasc Res. 2016;53(3–4):186‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Obi AT, Andraska E, Kanthi Y, et al. Endotoxaemia‐augmented murine venous thrombosis is dependent on TLR‐4 and ICAM‐1, and potentiated by neutropenia. Thromb Haemost. 2017;117(2):339‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Panicker SR, Mehta‐D'souza P, Zhang N, Klopocki AG, Shao B, McEver RP. Circulating soluble P‐selectin must dimerize to promote inflammation and coagulation in mice. Blood. 2017;130(2):181‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morikis VA, Hernandez AA, Magnani JL, Sperandio M, Simon SI. Targeting neutrophil adhesive events to address vaso‐occlusive crisis in sickle cell patients. Front Immunol. 2021;12:663886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vandy FC, Stabler C, Eliassen AM, et al. Soluble P‐selectin for the diagnosis of lower extremity deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2013;1(2):117‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramacciotti E, Blackburn S, Hawley AE, et al. Evaluation of soluble P‐selectin as a marker for the diagnosis of deep venous thrombosis. Clin Appl Thromb Hemost. 2011;17(4):425‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Antonopoulos CN, Sfyroeras GS, Kakisis JD, Moulakakis KG, Liapis CD. The role of soluble P selectin in the diagnosis of venous thromboembolism. Thromb Res. 2014;133(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 32. Gremmel T, Ay C, Seidinger D, Pabinger I, Panzer S, Koppensteiner R. Soluble p‐selectin, D‐dimer, and high‐sensitivity C‐reactive protein after acute deep vein thrombosis of the lower limb. J Vasc Surg. 2011;54(6 Suppl):48S‐55S. [DOI] [PubMed] [Google Scholar]
- 33. Kyrle PA, Hron G, Eichinger S, Wagner O. Circulating P‐selectin and the risk of recurrent venous thromboembolism. Thromb Haemost. 2007;97(6):880‐883. [PubMed] [Google Scholar]
- 34. Hisada Y, Mackman N. Cancer‐associated pathways and biomarkers of venous thrombosis. Blood. 2017;130(13):1499‐1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castellon Rubio VE, Segura PP, Munoz A, Farre AL, Ruiz LC, Lorente JA. High plasma levels of soluble P‐Selectin and factor VIII predict venous thromboembolism in non‐small cell lung cancer patients: the Thrombo‐Nsclc risk score. Thromb Res. 2020;196:349‐354. [DOI] [PubMed] [Google Scholar]
- 36. Ay C, Simanek R, Vormittag R, et al. High plasma levels of soluble P‐selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna cancer and thrombosis study (CATS). Blood. 2008;112(7):2703‐2708. [DOI] [PubMed] [Google Scholar]
- 37. Samuelson Bannow BT, Konkle BA. Laboratory biomarkers for venous thromboembolism risk in patients with hematologic malignancies: a review. Thromb Res. 2018;163:138‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Srisuwananukorn A, Raslan R, Zhang X, et al. Clinical, laboratory, and genetic risk factors for thrombosis in sickle cell disease. Blood Adv. 2020;4(9):1978‐1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lizarralde‐Iragorri MA, Shet AS. Sickle cell disease: a paradigm for venous thrombosis pathophysiology. Int J Mol Sci. 2020;21(15):5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Musselwhite LW, Sheikh V, Norton TD, et al. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS. 2011;25(6):787‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nishimura S, Manabe I, Nagasaki M, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118(2):710‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu D, Xu Z, Liu T, Li Y. Soluble P‐selectin levels in patients with obstructive sleep apnea: a systematic review and meta‐analysis. Eur Arch Otorhinolaryngol. 2021;278(12):4633‐4644. [DOI] [PubMed] [Google Scholar]
- 43. Comer SP, Cullivan S, Szklanna PB, et al. COVID‐19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021;19(2):e3001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Myers DD Jr, Rectenwald JE, Bedard PW, et al. Decreased venous thrombosis with an oral inhibitor of P selectin. J Vasc Surg. 2005;42(2):329‐336. [DOI] [PubMed] [Google Scholar]
- 45. Wakefield TW, Strieter RM, Schaub R, et al. Venous thrombosis prophylaxis by inflammatory inhibition without anticoagulation therapy. J Vasc Surg. 2000;31(2):309‐324. [DOI] [PubMed] [Google Scholar]
- 46. Myers DD Jr, Schaub R, Wrobleski SK, et al. P‐selectin antagonism causes dose‐dependent venous thrombosis inhibition. Thromb Haemost. 2001;85(3):423‐429. [PubMed] [Google Scholar]
- 47. Myers D, Wrobleski S, Londy F, et al. New and effective treatment of experimentally induced venous thrombosis with anti‐inflammatory rPSGL‐Ig. Thromb Haemost. 2002;87(3):374‐382. [PubMed] [Google Scholar]
- 48. Meier TR, Myers DD Jr, Wrobleski SK, et al. Prophylactic P‐selectin inhibition with PSI‐421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99(2):343‐351. [DOI] [PubMed] [Google Scholar]
- 49. Myers DD Jr, Wrobleski SK, Longo C, et al. Resolution of venous thrombosis using a novel oral small‐molecule inhibitor of P‐selectin (PSI‐697) without anticoagulation. Thromb Haemost. 2007;97(3):400‐407. [PubMed] [Google Scholar]
- 50. Ramacciotti E, Myers DD Jr, Wrobleski SK, et al. P‐selectin/ PSGL‐1 inhibitors versus enoxaparin in the resolution of venous thrombosis: a meta‐analysis. Thromb Res. 2010;125(4):e138‐e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Diaz JA, Wrobleski SK, Alvarado CM, et al. P‐selectin inhibition therapeutically promotes thrombus resolution and prevents vein wall fibrosis better than enoxaparin and an inhibitor to von Willebrand factor. Arterioscler Thromb Vasc Biol. 2015;35(4):829‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Banu N, Avraham S, Avraham HK. P‐selectin, and not E‐selectin, negatively regulates murine megakaryocytopoiesis. J Immunol. 2002;169(8):4579‐4585. [DOI] [PubMed] [Google Scholar]
- 53. Subramaniam M, Frenette PS, Saffaripour S, Johnson RC, Hynes RO, Wagner DD. Defects in hemostasis in P‐selectin‐deficient mice. Blood. 1996;87(4):1238‐1242. [PubMed] [Google Scholar]
- 54. Wong DJ, Park DD, Park SS, et al. A PSGL‐1 glycomimetic reduces thrombus burden without affecting hemostasis. Blood. 2021;138(13):1182‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Evangelista V, Manarini S, Sideri R, et al. Platelet/polymorphonuclear leukocyte interaction: P‐selectin triggers protein‐tyrosine phosphorylation‐dependent CD11b/CD18 adhesion: role of PSGL‐1 as a signaling molecule. Blood. 1999;93(3):876‐885. [PubMed] [Google Scholar]
- 56. Gross PL. A long‐half‐life, high‐affinity P‐selectin inhibitor. Blood. 2021;138(13):1096‐1097. [DOI] [PubMed] [Google Scholar]
- 57. Japp AG, Chelliah R, Tattersall L, et al. Effect of PSI‐697, a novel P‐selectin inhibitor, on platelet‐monocyte aggregate formation in humans. J Am Heart Assoc. 2013;2(1):e006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chelliah R, Lucking AJ, Tattersall L, et al. P‐selectin antagonism reduces thrombus formation in humans. J Thromb Haemost. 2009;7(11):1915‐1919. [DOI] [PubMed] [Google Scholar]
- 59. Jasuja R, Hett SP, Kaila N, Pittman D. Effect of PSI697, a small molecule inhibitor of P‐selectin, in the townes model of sickle cell disease. Blood. 2015;126(23):3391. [Google Scholar]
- 60. Bedard PW, Clerin V, Sushkova N, et al. Characterization of the novel P‐selectin inhibitor PSI‐697 [2‐(4‐chlorobenzyl)‐3‐hydroxy‐7,8,9,10‐tetrahydrobenzo[h] quinoline‐4‐carboxylic acid] in vitro and in rodent models of vascular inflammation and thrombosis. J Pharmacol Exp Ther. 2008;324(2):497‐506. [DOI] [PubMed] [Google Scholar]
- 61. Kumar A, Villani MP, Patel UK, Keith JC Jr, Schaub RG. Recombinant soluble form of PSGL‐1 accelerates thrombolysis and prevents reocclusion in a porcine model. Circulation. 1999;99(10):1363‐1369. [DOI] [PubMed] [Google Scholar]
- 62. Bienvenu JG, Tanguay JF, Theoret JF, Kumar A, Schaub RG, Merhi Y. Recombinant soluble p‐selectin glycoprotein ligand‐1‐Ig reduces restenosis through inhibition of platelet‐neutrophil adhesion after double angioplasty in swine. Circulation. 2001;103(8):1128‐1134. [DOI] [PubMed] [Google Scholar]
- 63. Wang K, Zhou X, Zhou Z, et al. Recombinant soluble P‐selectin glycoprotein ligand‐Ig (rPSGL‐Ig) attenuates infarct size and myeloperoxidase activity in a canine model of ischemia‐reperfusion. Thromb Haemost. 2002;88(1):149‐154. [PubMed] [Google Scholar]
- 64. Tanguay J‐F, Krucoff MW, Gibbons RJ, et al. Efficacy of a novel P‐selectin antagonist, rPSGL‐Ig for reperfusion therapy in acute myocardial infarction: the RAPSODY trial. J Am Coll Cardiol. 2003;41:404‐405. [Google Scholar]
- 65. Mertens P, Maes A, Nuyts J, et al. Recombinant P‐selectin glycoprotein ligand‐immunoglobulin, a P‐selectin antagonist, as an adjunct to thrombolysis in acute myocardial infarction. The P‐selectin antagonist limiting myonecrosis (PSALM) trial. Am Heart J. 2006;152(1):125.e1‐125.e8. [DOI] [PubMed] [Google Scholar]
- 66. Busuttil RW, Lipshutz GS, Kupiec‐Weglinski JW, et al. rPSGL‐Ig for improvement of early liver allograft function: a double‐blind, placebo‐controlled, single‐center phase II study. Am J Transplant. 2011;11(4):786‐797. [DOI] [PubMed] [Google Scholar]
- 67. Gutsaeva DR, Parkerson JB, Yerigenahally SD, et al. Inhibition of cell adhesion by anti‐P‐selectin aptamer: a new potential therapeutic agent for sickle cell disease. Blood. 2011;117(2):727‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schmitt C, Abt M, Ciorciaro C, et al. First‐in‐man study with inclacumab, a human monoclonal antibody against P‐selectin. J Cardiovasc Pharmacol. 2015;65(6):611‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kling D, Stucki C, Kronenberg S, et al. Pharmacological control of platelet‐leukocyte interactions by the human anti‐P‐selectin antibody inclacumab–preclinical and clinical studies. Thromb Res. 2013;131(5):401‐410. [DOI] [PubMed] [Google Scholar]
- 70. Tardif JC, Tanguay JF, Wright SR, et al. Effects of the P‐selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non‐ST‐segment elevation myocardial infarction: results of the SELECT‐ACS trial. J Am Coll Cardiol. 2013;61(20):2048‐2055. [DOI] [PubMed] [Google Scholar]
- 71. Stahli BE, Gebhard C, Duchatelle V, et al. Effects of the P‐selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention according to timing of infusion: insights from the SELECT‐ACS trial. J Am Heart Assoc. 2016;5(11):e004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Blair HA. Crizanlizumab: first approval. Drugs. 2020;80(1):79‐84. [DOI] [PubMed] [Google Scholar]
- 74. Gardner RV. Crizanlizumab in vaso‐occlusive crisis caused by sickle cell disease. Drugs Today (Barc). 2020;56(11):705‐714. [DOI] [PubMed] [Google Scholar]
- 75. Myers D Jr, Farris D, Hawley A, et al. Selectins influence thrombosis in a mouse model of experimental deep venous thrombosis. J Surg Res. 2002;108(2):212‐221. [DOI] [PubMed] [Google Scholar]
- 76. Sullivan VV, Hawley AE, Farris DM, et al. Decrease in fibrin content of venous thrombi in selectin‐deficient mice. J Surg Res. 2003;109(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 77. Chase SD, Magnani JL, Simon SI. E‐selectin ligands as mechanosensitive receptors on neutrophils in health and disease. Ann Biomed Eng. 2012;40(4):849‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Morikis VA, Chase S, Wun T, Chaikof EL, Magnani JL, Simon SI. Selectin catch‐bonds mechanotransduce integrin activation and neutrophil arrest on inflamed endothelium under shear flow. Blood. 2017;130(19):2101‐2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eriksson EE, Karlof E, Lundmark K, Rotzius P, Hedin U, Xie X. Powerful inflammatory properties of large vein endothelium in vivo. Arterioscler Thromb Vasc Biol. 2005;25(4):723‐728. [DOI] [PubMed] [Google Scholar]
- 80. Jilma B, Marsik C, Kovar F, Wagner OF, Jilma‐Stohlawetz P, Endler G. The single nucleotide polymorphism Ser128Arg in the E‐selectin gene is associated with enhanced coagulation during human endotoxemia. Blood. 2005;105(6):2380‐2383. [DOI] [PubMed] [Google Scholar]
- 81. Jilma B, Kovar FM, Hron G, et al. Homozygosity in the single nucleotide polymorphism Ser128Arg in the E‐selectin gene associated with recurrent venous thromboembolism. Arch Intern Med. 2006;166(15):1655‐1659. [DOI] [PubMed] [Google Scholar]
- 82. Bittar LF, Silva LQD, Orsi FLA, et al. Increased inflammation and endothelial markers in patients with late severe post‐thrombotic syndrome. PLoS One. 2020;15(1):e0227150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dzikowska‐Diduch O, Domienik‐Karlowicz J, Gorska E, Demkow U, Pruszczyk P, Kostrubiec M. E‐selectin and sICAM‐1, biomarkers of endothelial function, predict recurrence of venous thromboembolism. Thromb Res. 2017;157:173‐180. [DOI] [PubMed] [Google Scholar]
- 84. Torres C, Matos R, Morais S, Campos M, Lima M. Soluble endothelial cell molecules and circulating endothelial cells in patients with venous thromboembolism. Blood Coagul Fibrinolysis. 2017;28(8):589‐595. [DOI] [PubMed] [Google Scholar]
- 85. Mosevoll KA, Lindas R, Wendelbo O, Bruserud O, Reikvam H. Systemic levels of the endothelium‐derived soluble adhesion molecules endocan and E‐selectin in patients with suspected deep vein thrombosis. Springerplus. 2014;3:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bucek RA, Reiter M, Quehenberger P, Minar E, Baghestanian M. The role of soluble cell adhesion molecules in patients with suspected deep vein thrombosis. Blood Coagul Fibrinolysis. 2003;14(7):653‐657. [DOI] [PubMed] [Google Scholar]
- 87. Cushman M, Callas PW, Allison MA, Criqui MH. Inflammation and peripheral venous disease. The San Diego population study. Thromb Haemost. 2014;112(3):566‐572. [DOI] [PubMed] [Google Scholar]
- 88. Olivia A, Rando E, Al Ismail D, et al. Role of serum E‐selectin as a biomarker of infection severity in coronoavirus disease 2019. J Clin Med. 2021;10(17):4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chirinos JA, Heresi GA, Velasquez H, et al. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45(9):1467‐1471. [DOI] [PubMed] [Google Scholar]
- 90. Du T, Tan Z. Relationship between deep venous thrombosis and inflammatory cytokines in postoperative patients with malignant abdominal tumors. Braz J Med Biol Res. 2014;47(11):1003‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Girao‐Silva T, Fonseca‐Alaniz MH, Ribeiro‐Silva JC, et al. High stretch induces endothelial dysfunction accompanied by oxidative stress and actin remodeling in human saphenous vein endothelial cells. Sci Rep. 2021;11(1):13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Smadja DM, Mauge L, Sanchez O, et al. Distinct patterns of circulating endothelial cells in pulmonary hypertension. Eur Respir J. 2010;36(6):1284‐1293. [DOI] [PubMed] [Google Scholar]
- 93. Toth B, Nikolajek K, Rank A, et al. Gender‐specific and menstrual cycle dependent differences in circulating microparticles. Platelets. 2007;18(7):515‐521. [DOI] [PubMed] [Google Scholar]
- 94. Culmer DL, Dunbar ML, Hawley AE, et al. E‐selectin inhibition with GMI‐1271 decreases venous thrombosis without profoundly affecting tail vein bleeding in a mouse model. Thromb Haemost. 2017;117(6):1171‐1181. [DOI] [PubMed] [Google Scholar]
- 95. Devata S, Angelini DE, Blackburn S, et al. Use of GMI‐1271, an E‐selectin antagonist, in healthy subjects and in 2 patients with calf vein thrombosis. Res Pract Thromb Haemost. 2020;4(2):193‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Myers D Jr, Lester P, Adili R, et al. A new way to treat proximal deep venous thrombosis using E‐selectin inhibition. J Vasc Surg Venous Lymphat Disord. 2020;8(2):268‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Myers DD Jr, Ning J, Lester P, et al. E‐selectin inhibitor is superior to low‐molecular‐weight heparin for the treatment of experimental venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2021;10(1):211‐220. [DOI] [PubMed] [Google Scholar]
- 98. Peterson J, Baek M‐G, Locatelli‐Hoops S, et al. A novel and potent inhibitor of E‐selectin, GMI‐1687, attenuates thrombus formation and augments chemotherapeutic intervention of AML in preclinical models following subcutaneous administration. Blood. 2018;132:4678. [Google Scholar]
- 99. Wun T, Styles L, DeCastro L, et al. Phase 1 study of the E‐selectin inhibitor GMI 1070 in patients with sickle cell anemia. PLoS One. 2014;9(7):e101301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Williams ZF, Dillavou ED. A systematic review of venous stents for iliac and venacaval occlusive disease. J Vasc Surg Venous Lymphat Disord. 2020;8(1):145‐153. [DOI] [PubMed] [Google Scholar]


