Abstract
Early data suggest fecal microbiota transplant (FMT) may treat hepatic encephalopathy (HE). Optimal FMT donor and recipient characteristics are unknown. We assessed the safety and efficacy of FMT in patients with prior overt HE, comparing five FMT donors. We performed an open‐label study of FMT capsules, administered 5 times over 3 weeks. Primary outcomes were change in psychometric HE score (PHES) and serious adverse events (SAEs). Serial stool samples underwent shallow shotgun metagenomic sequencing. Ten patients completed FMT administration and 6‐month follow‐up. Model for End‐Stage Liver Disease (MELD) score did not change after FMT (14 versus 14, p = 0.51). Thirteen minor adverse events and three serious adverse events (two unrelated to FMT) were reported. One SAE was extended‐spectrum beta‐lactamase Escherichia coli bacteremia. The PHES improved after three doses of FMT (+2.1, p < 0.05), after five doses of FMT (+2.9, p = 0.007), and 4 weeks after the fifth dose of FMT (+3.1, p = 0.02). Mean change in the PHES ranged from −1 to +6 by donor. Two taxa were identified by random forest analysis and confirmed by linear regression to predict the PHES— Bifidobacterium adolescentis (adjusted R 2 = 0.27) and B. angulatum (adjusted R 2 = 0.25)—both short‐chain fatty acid (SCFA) producers. Patients who responded to FMT had higher levels of Bifidobacterium as well as other known beneficial taxa at baseline and throughout the study. The FMT donor with poorest cognitive outcomes in recipients had the lowest fecal SCFA levels. Conclusion: FMT capsules improved cognition in HE, with an effect varying by donor and recipient factors (NCT03420482).
In this trial, fecal microbiota transplant (FMT) from multiple different donors were used to treat hepatic encephalopathy. To our knowledge, this is the first study to evaluate FMT donor and recipient characteristics that influence response to therapy.

INTRODUCTION
Hepatic encephalopathy (HE) is a common complication of cirrhosis that is characterized by neuropsychiatric and motor dysfunction. HE leads to poor quality of life and increased mortality.[ 1 , 2 , 3 ] Currently available HE treatments have limited efficacy and carry risk of diarrhea, dehydration, and patient discomfort.[ 4 ] More effective and better tolerated therapies are needed to prevent overt HE episodes and treat subclinical HE that persists after overt episodes.
Growing evidence links the gut microbiome to HE pathogenesis.[ 5 ] Microbiome‐targeted therapies could treat HE by influencing host–microbiome metabolism (including ammonia generation), improving intestinal barrier function, and decreasing systemic immune activation.[ 6 ]
Fecal microbiota transplant (FMT) is the transfer of processed stool from a healthy donor to a recipient, with well‐documented efficacy for the treatment of recurrent Clostridium difficile infection.[ 7 ] Two randomized controlled trials have confirmed the safety of FMT enema and oral FMT capsules in patients with recurrent HE.[ 8 , 9 ] A single dose of FMT capsules from one donor improved cognitive function on one psychometric test but not another and did not change the fecal microbiome. Patients with cirrhosis require 2–3 times more oral FMT capsules than patients without cirrhosis to treat recurrent C. difficile infection so may also require additional FMT to overcome resident microbial dysbiosis and treat HE.[ 10 ] The ideal FMT donor and number of doses to treat HE remain unknown.
We conducted an open‐label trial to assess the safety and efficacy of multiple doses of oral FMT capsules to improve cognitive function in patients with a history of overt HE and compared the efficacy of different FMT donors. Secondarily, we aimed to identify recipient microbiome and metabolic features that predicted cognitive improvement with FMT.
MATERIALS AND METHODS
Study patients
Eligible patients were at least 18 years old, carried a diagnosis of cirrhosis, had at least one prior episode of overt HE, were taking both lactulose and rifaximin at least daily, and were not recently on additional antibiotics or consuming alcohol. Outpatients were enrolled from a single academic center. Only patients with ongoing neurocognitive dysfunction, defined as psychometric HE score (PHES) of less than 0, were enrolled. In November 2019, after a serious adverse event (SAE) related to FMT, the following exclusion criteria were added: Model for End‐Stage Liver Disease (MELD) >17, history of low‐protein ascites, and history of spontaneous bacterial peritonitis.[ 11 ] Complete inclusion and exclusion criteria are detailed in Table S1.
Study design and procedures
The study protocol was approved by the local institutional review board, and an Investigational New Drug application was filed with the the US Food and Drug Administration (FDA; IND 17895). Ten patients were enrolled in this open‐label pilot study of FMT. Once enrolled, patients received 15 oral FMT capsules on days 1, 2, 7, 14, and 21 (Figure 1). The dosing schedule was based on the authors’ prior study showing that patients with cirrhosis often require 4–6 doses of FMT capsules to achieve successful C. difficile treatment.[ 10 ] Pretreatment antibiotics were not used to avoid confounding.
FIGURE 1.
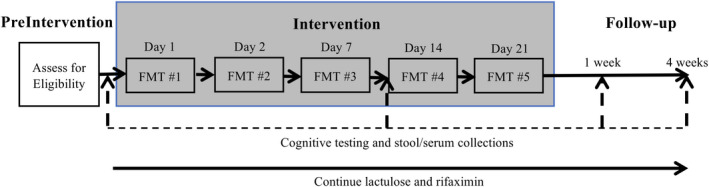
Study design. Patients received 15 oral FMT capsules on 5 days over 3 weeks. Cognitive testing and serum and stool collections occurred at 4 time points. Standard of care with lactulose and rifaximin were continued throughout the study. Abbreviation: FMT, fecal microbiota transplant
FMT donors were healthy adults with normal body mass index who were selected through a previously published rigorous screening process.[ 12 , 13 ] FMT capsules were generated using established protocols and approved by the local institutional review board and the FDA.[ 13 ] Donated fecal matter was blenderized, sieved, centrifuged, suspended in sterile saline with 40% glycerol, and double encapsulated with an acid‐resistant capsule. On average, 15 capsules contained 24 g of fecal matter. Processing was performed under ambient air, and capsules were stored in −80°C until use. The plan was for five donors to provide stool for FMT for two patients each. Patients received FMT derived from one donor. However, due to FMT capsule availability, one donor supplied FMT to three patients and another donor supplied to one patient.
Efficacy and safety assessments
Safety was assessed at 8 time points until 6 months after FMT, and cognitive function was assessed 4 times over the study period. Stool and serum were obtained for sequencing, inflammatory markers, and metabolomic analysis at 4 time points (Figure 1).
Clinical efficacy was primarily assessed by change in the PHES. The PHES is a validated assessment tool specifically designed for HE trials to test cognitive and psychomotor processing speed and visuomotor coordination (copyright by Hanover Medical School).[ 14 , 15 , 16 ] Prior work has demonstrated no learning effect or improving scores when tests are 14 days apart in patients with cirrhosis and a history of overt HE.[ 17 ] Any potential learning effect was mitigated by using four different PHES versions. A secondary efficacy outcome was assessed by change in the EncephalApp Stroop test, also validated in HE.[ 17 ] Short form health survey 36 (SF‐36) was performed to assess quality of life.
Adverse events were recorded and graded based on the Common Terminology Criteria for Adverse Events, V.4.03. The definition of an SAE is outlined in the Code of Federal Regulations Title 21 (312.32).
Stool analysis
Fresh stool was collected at 4 time points and kept at 4°C for <24 hours before being stored at −80°C. All samples were analyzed in a single batch at the completion of the study. For full microbiome analysis details, see the [Link], [Link]. Samples were analyzed using the SHOGUN pipeline.[ 18 ] Every input sequence was compared to every reference sequence in Diversigen’s Venti database, using fully gapped alignment with BURST. Statistical analyses of microbiome data were performed in R (R Core Team, 2017). The HMP package[ 19 ] was used to determine group mean relative abundance values by fitting the sample relative abundances to a Dirichlet‐multinomial distribution, using a maximum likelihood method. Alpha diversity was calculated as the Shannon index.[ 20 ] Beta diversity was calculated using the Bray‐Curtis dissimilarity index and mapped onto two‐dimensional space using multidimensional scaling.[ 19 ] Feature selection was performed with the R package Boruta.[ 21 ] Linear regression using the R stats package was used to determine significant associations of taxa identified as important with PHES. Antimicrobial resistance genes in the data set were identified by alignment of the FASTQ files to MEGARes 2.0.[ 22 ]
Inflammatory biomarkers
Cytokine profiling of serum samples was performed on a Luminex 12‐plex plate.
Statistical analysis
Efficacy data were analyzed by intention‐to‐treat. The primary outcome was change in the PHES from day 1 to 1 week after the last day of FMT. Secondary outcomes included the number of adverse events and change in Stroop test results, SF‐36, venous ammonia level, and microbiome features.
We planned to perform a paired t test to compare PHES scores if the data were normally distributed and a Wilcoxon rank sum test if not normally distributed. This testing strategy was also used for the continuous secondary outcome variables.
Post hoc, we categorized patients as responders versus nonresponders. Responders had an improvement in the PHES from day 1 to 1 week after the last FMT and did not have an episode of overt HE in 6 months of follow‐up.
During the coronavirus disease 2019 pandemic, most study visits were converted to virtual video visits. Due to the remote nature of those study visits, two patients could not provide serum samples for inflammatory biomarker analysis or Stroop test results at some time points.
All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Of 132 patients screened, 10 patients with cirrhosis and a history of overt HE were enrolled between May 2018 and May 2020 (Figure 2). All 10 patients received five doses of 15 FMT capsules and completed study activities through 6 months of follow‐up. The median age was 61 years (range, 53–72), six (60%) were men, four (40%) had alcohol‐associated cirrhosis, three (30%) had nonalcoholic steatohepatitis cirrhosis, and four (40%) had undergone transjugular intrahepatic portosystemic shunt (TIPS) (Table 1). Median MELD at screening was 14 (range, 9–18).
FIGURE 2.
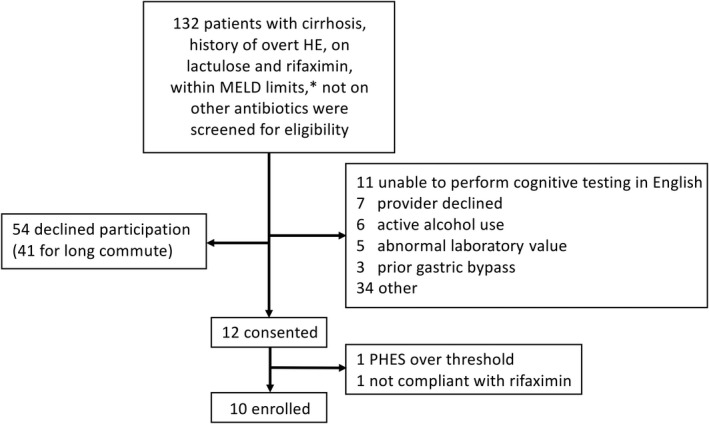
Subject enrollment flowchart. *For the first five subjects, MELD >17 was excluded. Per protocol, after the first five patients, MELD >20 was excluded. However, after a serious adverse event, MELD >17 was again excluded. Abbreviations: HE, hepatic encephalopathy; MELD, Model for End‐Stage Liver Disease; PHES, psychometric hepatic encephalopathy score
TABLE 1.
Patient characteristics at baseline
| Characteristics | Values (n = 10) |
|---|---|
| Age, years | 61 (53, 72) |
| Male sex, n (%) | 6 (60%) |
| MELD score | 14 (9, 18) |
| Etiology of cirrhosis, n (%) | |
| Alcohol | 4 (40%) |
| NASH | 3 (30%) |
| NASH and alpha‐1 antitrypsin deficiency | 1 (10%) |
| Viral | 1 (10%) |
| Cryptogenic | 1 (10%) |
| Body mass index | 30.5 (21, 39) |
| Diabetes diagnosis, n (%) | 7 (70%) |
| Number of patients with OHE episode in prior 6 months, n (%) | 3 (30%) |
| TIPS in place, n (%) | 4 (40%) |
| Presence of ascites, n (%) | 7 (70%) |
| History of hepatocellular carcinoma, n (%) | 1 (10%) |
| Active on liver transplant waitlist, n (%) | 5 (50%) |
| Total bilirubin, mg/dL | 2.0 (1.4, 4.4) |
| Creatinine, mg/dL | 0.8 (0.50, 1.31) |
| International normalized ratio | 1.4 (1.1, 1.9) |
Data are presented as median (range) unless mentioned otherwise.
Abbreviations: MELD, Model for End‐Stage Liver Disease; NASH, nonalcoholic steatohepatitis; OHE, overt hepatic encephalopathy; TIPS, transjugular intrahepatic portosystemic shunt.
Safety
Mean MELD score did not change from baseline to after the third dose of FMT (14 versus 14, p = 0.34), after the fifth dose of FMT (14 versus 14, p = 0.51), and 4 weeks after the fifth dose of FMT (14 versus 14, p = 1.0; Figure S1).
Thirteen minor adverse events were reported by patients (Table S2), including nausea, bloating, fatigue, and constipation. Four were judged as possibly related to FMT.
Three SAEs occurred during the study. One occurred before the administration of FMT. One SAE was transmission of extended‐spectrum beta‐lactamase (ESBL)‐producing Escherichia coli bacteremia through FMT, documented in detail in a prior report.[ 11 ] The bacteremia was diagnosed 17 days after the patient’s fifth dose of FMT. The patient was treated with piperacillin–tazobactam and then 14 days of meropenem when organism sensitivities were known. His clinical condition remained stable after discharge. A follow‐up stool sample was negative for ESBL‐producing organisms. The third SAE occurred 12 weeks after the final dose of FMT. The patient was admitted after missing at least one dose of lactulose and had fatigue, slurred speech, and a urinary tract infection, electrolyte abnormalities, and acute kidney injury. She was diagnosed with probable precipitated overt HE, which was deemed unrelated to FMT.
Cognitive changes with fecal microbiota transplant
Compared to baseline, the PHES improved after three doses of FMT (+2.1, p < 0.05), after five doses of FMT (+2.9, p = 0.007), and 4 weeks after the fifth dose of FMT (+3.1, p = 0.02; Figure 3A). For reference, improving from 33 seconds to 15 seconds on the number‐connection test can improve the PHES by 1 point. Mean change in PHES ranged from −1 to +6 by donor. The mean improvement in PHES did not vary by history of TIPS (TIPS +2.5 versus no TIPS +3.2, p = 0.72; Figure 3B). Raw scores of 3/5 PHES subtests improved after five doses of FMT (Figure S2).
FIGURE 3.
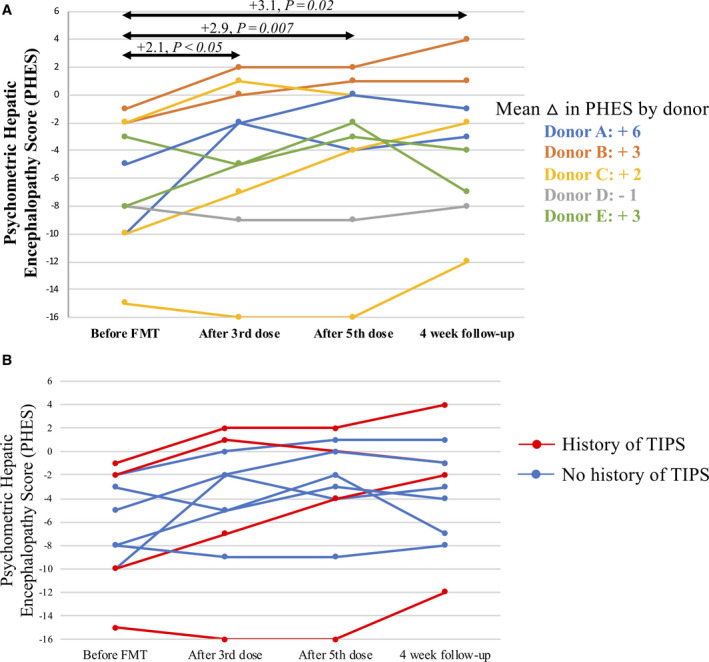
Illustration of PHES over time. The first time point was before FMT delivery, the second time point was day 14 (1 week after three doses of FMT), the third time point was day 21 + 1 week (1 week after the fifth FMT dose), and the fourth time point was day 21 + 4 weeks (4 weeks after the fifth FMT dose). (A) PHES over time for all patients and mean change in PHES by donor. (B) PHES over time by history of TIPS. Abbreviations: FMT, fecal microbiota transplant; PHES, psychometric hepatic encephalopathy score; TIPS, transjugular intrahepatic portosystemic shunt
Compared to baseline, Stroop test results did not improve after three doses of FMT (14.5 seconds improved, p = 0.40) but trended toward improvement after five doses of FMT (34.3 seconds improved, p = 0.06) and 4 weeks after the fifth dose of FMT (19.1 seconds improved, p = 0.05).
The Physical Component Summary (p = 0.77) and Mental Component Summary (p = 0.64) of the SF‐36 did not change after five doses of FMT.
Unplanned antibiotic administration
Two patients received unplanned non‐rifaximin antibiotics during the study period. Removing patients with non‐rifaximin antibiotics from the analysis did not meaningfully change the primary analysis that the PHES improved after five doses of FMT (+2.6, p = 0.02).
Microbiome changes with fecal microbiota transplant
There was no significant change in alpha diversity between baseline and subsequent post‐FMT days (Figure S3). In beta‐diversity analysis, patients did not clearly remodel toward the donors over time (Figure S4).
Taxa that predict cognitive outcomes
In a random forest analysis, 22 variables were deemed important in predicting the PHES (Figure 4). Of these, six variables were found to be significantly associated with PHES by linear regression (Table 2). Two taxa were positively associated with PHES scores, Bifidobacterium adolescentis and B. angulatum, both short‐chain fatty acid (SCFA) producers. Two taxa were negatively correlated with PHES scores—Enterobacter asburiae and B. breve—although the significant association with B. breve disappeared when one outlier patient was removed (Figure S5).
FIGURE 4.
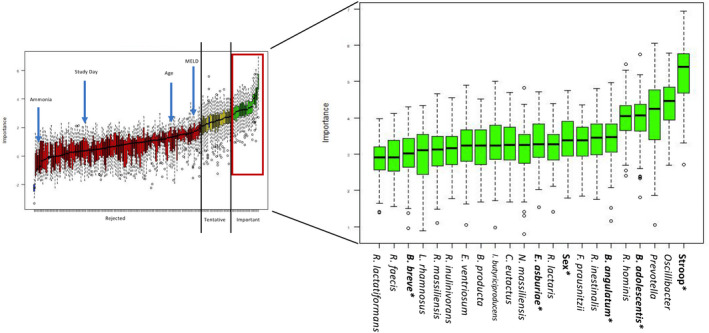
In a random forest analysis, variables were ranked by importance in predicting PHES. Of the important variables, those bolded and starred were additionally found to be significantly associated with PHES by linear regression. Abbreviations: MELD, Model for End‐Stage Liver Disease; PHES, psychometric hepatic encephalopathy score; B. of B. adolescentis, B. angulatum, and B. breve, Bifidobacterium; B. producta, Blautia producta; R. of R. hominis, R. intestinalis, R. faecis, and R. inulinivorans, Roseburia; R. lactaris, Ruminococcus lactaris; R. massiliensis, Raoultibacter massiliensis; R. lactatiformans, Ruthenibacterium lactatiformans; F. praustnitzii, Faecalibacterium prausnitzi; E. asburiae, Enterobacter asburiae; N. massiliensis, Negativibacillus massiliensis; C. eutactus, Coprococcus eutactus; I. butyriciproducens, Intestinimonas butyriciproducens; E. ventriosum, Eubacterium ventriosum; L. rhamnosus, Lactobacillus rhamnosus
TABLE 2.
Variables significantly associated with PHES
| Attribute | Mean importance (random forest) | Correlation with PHES (regression) | Adjusted R 2 |
|---|---|---|---|
| Stroop test | 5.17 | Negative a | N/A |
| Bifidobacterium adolescentis | 3.98 | Positive | 0.27 |
| Bifidobacterium angulatum | 3.41 | Positive | 0.25 |
| Sex | 3.31 | N/A | N/A |
| Enterobacter asburiae | 3.29 | Negative | 0.19 |
| Bifidobacterium breve | 2.97 | Negative | 0.39 |
Abbreviations: N/A, not applicable; PHES, psychometric hepatic encephalopathy score.
Higher Stroop test results (On + Off time in seconds) is associated with poorer cognition, whereas the inverse is true of PHES, where higher score is associated with better cognition.
Comparing fecal microbiota transplant donors
FMT donors did not vary by age (24–34 years old) or diet type (all omnivores) but did vary in their impact on recipient cognitive changes, secondary to primary bile acid ratios, and total normalized SCFA levels. Donor D was associated with the worst cognitive outcomes as well as the lowest secondary to primary bile acid ratio and normalized SCFA level (Table S3). Donor microbiomes generally shared the same genera but varied by relative abundance (Figure S6).
Comparing fecal microbiota transplant responders and nonresponders
The seven patients who clinically responded to FMT (improved PHES and no overt HE at 6 months) differed at baseline from the three patients who did not clinically respond to FMT. Bacterial families identified a priori as beneficial or harmful in HE were compared between FMT responders and nonresponders.[ 8 , 23 , 24 , 25 , 26 ] FMT responders appeared to have a higher abundance of beneficial families at baseline and across study time points, while FMT nonresponders had a higher abundance of harmful bacterial families (Figure 5). Bifidobacterium abundance in particular appeared to be higher in responders at baseline compared to nonresponders, as well as over the course of the study.
FIGURE 5.
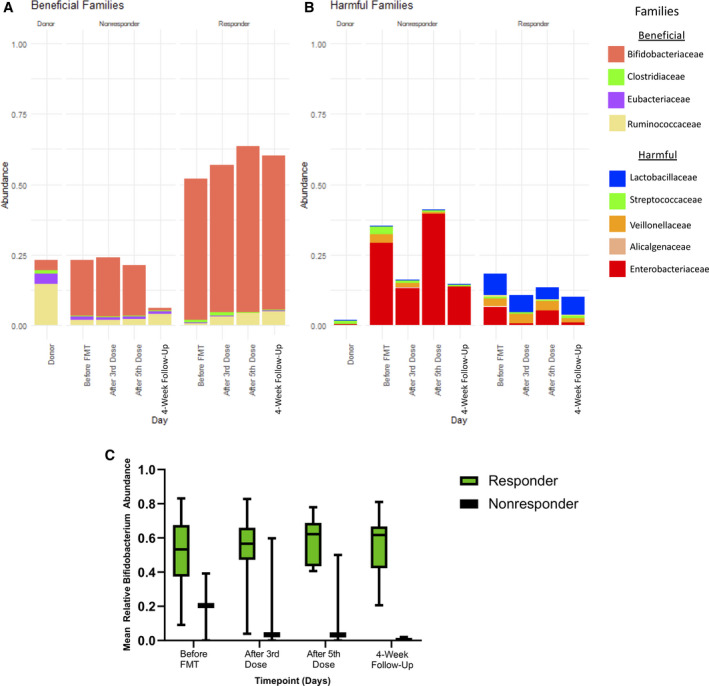
Comparison of bacterial families. (A,B) Bacterial families identified a priori as beneficial or harmful in HE were compared between FMT responders and nonresponders. (C) Bifidobacterium abundance appeared to be higher in responders compared to nonresponders. Abbreviations: FMT, fecal microbiota transplant; HE, hepatic encephalopathy
Antimicrobial resistance genes
Total antimicrobial resistance genes in patients’ fecal microbiome decreased from baseline to 4 weeks after the fifth FMT dose, approaching donor levels (Figure S7). The prevalence of the RNA polymerase β subunit (rpoB; resistance to rifampicin) gene was high in the cohort at baseline (present in seven of 10 subjects). One nonresponder appeared to obtain the rifampin resistance gene from their donor, whereas two responders appeared to lose rifampin resistance with FMT (Figure 6).
FIGURE 6.
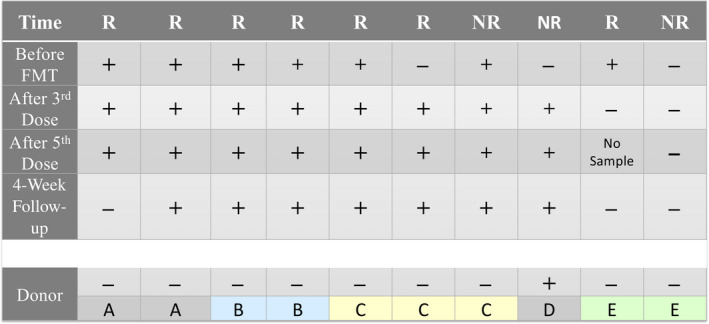
The presence of the rpoB gene over time. Each column represents a study subject. Donors (A–E) are show along the bottom. Abbreviations: +, presence of rpoB gene in that subject at that time point; −, absence of rpoB gene; FMT, fecal microbiota transplant; NR, nonresponder; R, fecal microbiota transplant responder; rpoB, RNA polymerase β subunit (resistance to rifampicin)
Metabolite changes with fecal microbiota transplant
In the entire group, total normalized SCFA levels did not change after five doses of FMT (p = 0.87; Figure S8). SCFA levels rose in four of seven responders and fell in two of three nonresponders. Only three of 10 patients developed an increase in secondary to primary bile acid ratios with FMT, and two of those were clinical nonresponders (Figure S9). Compared to baseline, venous ammonia did not change after five doses of FMT (73 µmol/L versus 75 µmol/L, p = 0.73; Figure S10).
Inflammatory markers with fecal microbiota transplant
Compared to baseline, serum tumor necrosis factor alpha (p = 0.09), interleukin‐6 (p = 0.55), and interferon‐gamma (p = 0.30) did not change after five doses of FMT (Figure S10).
DISCUSSION
Patients with a history of cirrhosis and overt HE developed improved cognitive function after five doses of oral FMT capsules given over 3 weeks. The mean improvement in the PHES 4 weeks after the last FMT dose was 3.1 points—a clinically relevant improvement. In addition, only one (10%) patient experienced an overt HE episode in 6 months of follow‐up. Similar patients in other studies experienced overt HE at 21% in 3 months or 30%–50% in 6 months.[ 8 , 9 , 27 ] Both Stroop scores and PHES improved between three and five doses of FMT, so it is possible that additional doses provide additional clinical benefit. A history of TIPS did not influence response to FMT.
FMT led to mild and brief gastrointestinal side effects in some patients. FMT also led to an SAE in one patient—ESBL‐producing E. coli bacteremia—the analysis of which has been published.[ 11 ] This is not the only report of pathogen transmission through FMT, with recent reports of Shiga toxin‐producing E. coli transmitted by FMT.[ 28 ] Despite these reports of FMT‐transmitted infections, a recent systematic review of 4241 patients found FMT to be overall safe, with a very low rate of microbiota‐related SAEs.[ 29 ] Even when investigating patients with cirrhosis specifically, a multicenter study found FMT to be safe, with no infection‐related SAEs.[ 30 ] FMT donor screening practices continue to evolve and incorporate enhanced screening for potential pathogens, including most recently severe acute respiratory syndrome coronavirus 2 virus.[ 31 ] Synthesizing available data, it appears that FMT is safe in some patients with cirrhosis; however, FMT screening practices must be rigorous, and some subgroups may warrant exclusion, such as those with high MELD, low‐protein ascites, or a history of spontaneous bacterial peritonitis.
FMT did not lead to wholesale fecal microbiome remodeling; rather, its therapeutic mechanism may have been through subtle or proximal gut changes in microbial composition and function. First, the microbial changes may have occurred in the proximal bowel, and this study sampled only stool. In a prior study of oral FMT capsules to treat HE, FMT did not change bacterial diversity in sigmoid or stool samples but did lead to composition and function changes in the proximal bowel mucosal microbiome.[ 9 ] Second, even in the distal bowel, it is possible that subtle changes in microbial composition and function influenced clinical outcomes. This study was designed in part to compare the efficacy of different FMT donors and thus introduced heterogeneity, which made summary assessment of microbiome changes challenging. It is possible that individual recipients acquired specific donor taxa that influenced cognitive outcomes without demonstrating significant changes in alpha and beta diversity. Finally, it is possible that co‐administration of rifaximin with FMT blunted microbiome remodeling.
While FMT has been highly effective in the treatment of C. difficile infection from nearly any healthy donor, clinical trials of FMT for inflammatory bowel disease have suggested a possible donor effect.[ 32 , 33 ] In our study, cognitive improvement in FMT recipients appeared to vary by donor. Prior trials of FMT for HE have selected donors based on abundance of potentially beneficial taxa.[ 8 , 9 ] Despite differences in clinical outcomes by donor, microbiome composition was fairly similar between donors. Ideal FMT donor selection for HE may be more related to microbial function than composition. FMT from donor D led to the worst recipient outcomes and notably had the lowest SCFA levels and secondary to primary bile acid ratios. Both SCFAs and bile acids (through different mechanisms) influence intestinal epithelial health and permeability.[ 34 ] Further study in larger cohorts should investigate possible FMT donor effects for this condition[ 35 ] and consider differentiating donors by microbiome metabolic activity as opposed to abundance alone.
Increasingly, recipient factors are being recognized as important in FMT success.[ 36 ] We found that patients who responded positively to FMT had a more beneficial baseline microbiome profile. In particular, FMT responders had higher baseline Bifidobacterium abundance compared to nonresponders. Two Bifidobacterium species significantly predicted cognitive scores. Bifidobacterium adolescentis is known to have beneficial qualities for the host, including increasing SCFA production, increasing tight junction protein production, decreasing intestinal permeability, and dampening systemic immune response.[ 37 , 38 , 39 ] Less is known of B. angulatum, but it has demonstrated SCFA‐producing abilities.[ 40 ] In alignment with these findings, total stool SCFA content rose in most FMT responders and fell in most nonresponders. Notably, venous ammonia levels did not change with FMT, nor were any of the taxa associated with cognitive scores involved in ammonia metabolism. Further study will be required to explore the role of Bifidobacterium in facilitating response to FMT, but the mechanism may involve known synergism between Bifidobacterium species and other taxa in fermentation and SCFA generation.[ 40 ]
Patients with cirrhosis, and especially those using rifaximin, have high prevalence of the rpoB gene, conferring resistance to rifaximin.[ 41 ] Our study found that FMT led to a decrease in total antimicrobial resistance genes in patients, nearly to healthy donor levels. Two FMT responders lost rifampicin resistance with FMT. These data support a prior finding of decreased rifaximin resistance after FMT in cirrhosis.[ 42 ] While the numbers are small, these findings raise the possibility that FMT exerts its effect by resensitizing the microbiota to conventional rifaximin therapy.
These results must be interpreted within the context of the study design. First, there was no control group in this study; therefore, definitive conclusions about efficacy and safety are not possible. Our study population was restricted by MELD and antibiotic use, thereby limiting the external validity of our results to sicker populations. Future, well‐powered, placebo‐controlled trials will be required for definitive evaluation of efficacy and safety. Second, FMT donors with high SCFA production should be strongly considered for future trials. Third, future trials should consider stratification or selection by recipient microbiome, including Bifidobacterium abundance. Fourth, future FMT studies should strive to perform strain‐level sequencing to better understand strain engraftment and impact on clinical outcomes. We did not find wholesale microbiome remodeling, but smaller community or strain‐level changes may have occurred. Fifth, the impact of TIPS, cirrhosis etiology, and metabolic disorders could not be explored in detail in this study design but should be investigated in future studies. Finally, this study does not explore the role of rifaximin after FMT, which will be important to investigate in future work, especially for patients who lose rifampicin resistance after FMT.
In conclusion, this study suggests that FMT may be effective in treating HE and is likely safe for select patients with intensive pathogen screening. Microbial manipulation with FMT or a defined consortium of beneficial bacteria may be a way to improve quality of life in patients with cirrhosis. This is the first study to explore donor and recipient factors that may lead to HE improvement with FMT; initial findings will be studied in future work.
CONFLICTS OF INTEREST
Patricia Bloom received a research grant from Vedanta Biosciences. Elizabeth Hohmann received a research grant from Seres Therapeutics and MicrobiomX and consults for Gilead. Raymond Chung has received research grants from Synlogic and Kaleido.
AUTHOR CONTRIBUTIONS
Planning and conducting the study, collecting and interpreting data, drafting the manuscript: Patricia Bloom. Conducting the study, collecting data: John Donlan, Mariam Torres Soto, and Michael Daidone. Planning and conducting the study, interpreting data, critical revisions of the manuscript: Elizabeth Hohmann and Raymond T. Chung. All authors approved the final version of the manuscript. Guarantor of the article: Patricia P. Bloom.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Fig S7
Fig S8
Fig S9
Fig S10
Table S1
Table S2
Table S3
Supplementary Material
ACKNOWLEDGMENTS
We thank Dana Walsh, Heidi Hau, Ken Blount, Bryan Fuchs, Romeo Papazyan, and Nicky Ferdyan at Ferring Pharmaceuticals for their assistance with microbiome and metabolomics analysis.
Bloom PP, Donlan J, Torres Soto M, Daidone M, Hohmann E, Chung RT. Fecal microbiota transplant improves cognition in hepatic encephalopathy and its effect varies by donor and recipient. Hepatol Commun. 2022;6:2079–2089. 10.1002/hep4.1950
Funding information
American College of Gastroenterology, Clinical Research Award
Contributor Information
Patricia P. Bloom, Email: ppbloom@med.umich.edu.
Raymond T. Chung, Email: chung.raymond@mgh.harvard.edu.
REFERENCES
- 1. Bajaj JS, O’Leary JG, Tandon P, Wong F, Garcia‐Tsao G, Kamath PS, et al. Hepatic encephalopathy is associated with mortality in patients with cirrhosis independent of other extrahepatic organ failures. Clin Gastroenterol Hepatol. 2017;15:565–574.e4. [DOI] [PubMed] [Google Scholar]
- 2. Rabiee A, Ximenes RO, Nikayin S, Hickner A, Juthani P, Rosen RH, et al. Factors associated with health‐related quality of life in patients with cirrhosis: a systematic review. Liver Int. 2021;41:6–15. [DOI] [PubMed] [Google Scholar]
- 3. Tapper EB, Aberasturi D, Zhao Z, Hsu CY, Parikh ND. Outcomes after hepatic encephalopathy in population‐based cohorts of patients with cirrhosis. Aliment Pharmacol Ther. 2020;51:1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gluud LL, Vilstrup H, Morgan MY. Non‐absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016;2016(5):Cd003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bajaj JS, Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol. 2020;72:1003–27. [DOI] [PubMed] [Google Scholar]
- 6. Bloom P, Tapper EB, Young VB, Lok AS. Microbiome therapeutics for hepatic encephalopathy. J Hepatol. 2021;75:1452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelly CR, Yen EF, Grinspan AM, Kahn SA, Atreja A, Lewis JD, et al. Fecal microbiota transplantation is highly effective in real‐world practice: initial results from the FMT national registry. Gastroenterology. 2021;160:183–192.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66:1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo‐controlled trial. Hepatology. 2019;70:1690–703. Erratum in: Hepatology. 2020;72:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pringle PL, Soto MT, Chung RT, Hohmann E. Patients with cirrhosis require more fecal microbiota capsules to cure refractory and recurrent Clostridium difficile infections. Clin Gastroenterol Hepatol. 2019;17:791–3. [DOI] [PubMed] [Google Scholar]
- 11. DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug‐resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381:2043–50. [DOI] [PubMed] [Google Scholar]
- 12. Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open‐label, controlled pilot study. Clin Infect Dis. 2014;58:1515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Youngster I, Mahabamunuge J, Systrom HK, Sauk J, Khalili H, Levin J, et al. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guerit J‐M, Amantini A, Fischer C, Kaplan PW, Mecarelli O, Schnitzler A, et al.; Members of the ISHEN Commission on Neurophysiological Investigations . Neurophysiological investigations of hepatic encephalopathy: ISHEN practice guidelines. Liver Int. 2009;29:789–96. [DOI] [PubMed] [Google Scholar]
- 15. Randolph C, Hilsabeck R, Kato A, Kharbanda P, Li Y‐Y, Mapelli D, et al.; International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) . Neuropsychological assessment of hepatic encephalopathy: ISHEN practice guidelines. Liver Int. 2009;29:629–35. [DOI] [PubMed] [Google Scholar]
- 16. Amodio P, Montagnese S. Clinical neurophysiology of hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(Suppl 1):S60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bajaj JS, Thacker LR, Heuman DM, Fuchs M, Sterling RK, Sanyal AJ, et al. The Stroop smartphone application is a short and valid method to screen for minimal hepatic encephalopathy. Hepatology. 2013;58:1122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hillmann B, Al‐Ghalith GA, Shields‐Cutler RR, Zhu Q, Knight R, Knights D. SHOGUN: a modular, accurate and scalable framework for microbiome quantification. Bioinformatics. 2020;36:4088–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. La Rosa PS, Brooks JP, Deych E, Boone EL, Edwards DJ, Wang Q, et al. Hypothesis testing and power calculations for taxonomic‐based human microbiome data. PLoS One. 2012;7:e52078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Legendre P, Legendre L. Numerical ecology. 3rd English ed. Amsterdam: Elsevier; 2012. [Google Scholar]
- 21. Kursa M, Rudnicki W. Feature selection with the Boruta package. J Stat Softw. 2010;36:1–13. [Google Scholar]
- 22. Doster E, Lakin SM, Dean CJ, Wolfe C, Young JG, Boucher C, et al. MEGARes 2.0: a database for classification of antimicrobial drug, biocide and metal resistance determinants in metagenomic sequence data. Nucleic Acids Res. 2020;48:D561–D569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sung CM, Lin Y‐F, Chen K‐F, Ke H‐M, Huang H‐Y, Gong Y‐N, et al. Predicting clinical outcomes of cirrhosis patients with hepatic encephalopathy from the fecal microbiome. Cell Mol Gastroenterol Hepatol. 2019;8:301–318.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bajaj JS, Fagan A, Sikaroodi M, White MB, Sterling RK, Gilles H, et al. Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver Transpl. 2017;23:907–14. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Z, Zhai H, Geng J, Yu R, Ren H, Fan H, et al. Large‐scale survey of gut microbiota associated with MHE Via 16S rRNA‐based pyrosequencing. Am J Gastroenterol. 2013;108:1601–11. [DOI] [PubMed] [Google Scholar]
- 26. Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol‐Gastrointestinal Liver Physiol. 2012;302:G168–G175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bajaj JS, O’Leary JG, Tandon P, Wong F, Kamath PS, Biggins SW, et al. Targets to improve quality of care for patients with hepatic encephalopathy: data from a multi‐centre cohort. Aliment Pharmacol Ther. 2019;49:1518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zellmer C, Sater MRA, Huntley MH, Osman M, Olesen SW, Ramakrishna B. Shiga toxin‐producing Escherichia coli transmission via fecal microbiota transplant. Clin Infect Dis. 2021;72:e876‐80. [DOI] [PubMed] [Google Scholar]
- 29. Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F. Systematic review: the global incidence of faecal microbiota transplantation‐related adverse events from 2000 to 2020. Aliment Pharmacol Ther. 2021;53:33–42. [DOI] [PubMed] [Google Scholar]
- 30. Cheng YW, Alhaffar D, Saha S, Khanna S, Bohm M, Phelps E, et al. Fecal microbiota transplantation is safe and effective in patients with Clostridioides difficile infection and cirrhosis. Clin Gastroenterol Hepatol. 2021;19:1627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ianiro G, Mullish BH, Kelly CR, Kassam Z, Kuijper EJ, Ng SC, et al. Reorganisation of faecal microbiota transplant services during the COVID‐19 pandemic. Gut. 2020;69:1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Olesen SW. Fecal microbiota transplantation “donor effects” are not clinically relevant for Clostridioides difficile infection. Gastroenterology. 2021;160:2635–6. [DOI] [PubMed] [Google Scholar]
- 33. Olesen SW, Gerardin Y. Re‐evaluating the evidence for faecal microbiota transplantation 'super‐donors' in inflammatory bowel disease. J Crohns Colitis. 2021;15:453–61. [DOI] [PubMed] [Google Scholar]
- 34. Woodhouse CA, Patel VC, Singanayagam A, Shawcross DL. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther. 2018;47:192–202. [DOI] [PubMed] [Google Scholar]
- 35. Olesen SW. Power calculations for detecting differences in efficacy of fecal microbiota donors. Contemp Clin Trials Commun. 2020;20:100674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Danne C, Rolhion N, Sokol H. Recipient factors in faecal microbiota transplantation: one stool does not fit all. Nat Rev Gastroenterol Hepatol. 2021;18:503–13. [DOI] [PubMed] [Google Scholar]
- 37. Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, et al. Two routes of metabolic cross‐feeding between Bifidobacterium adolescentis and butyrate‐producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72:3593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roberts JL, Liu G, Darby TM, Fernandes LM, Diaz‐Hernandez ME, Jones RM, et al. Bifidobacterium adolescentis supplementation attenuates fracture‐induced systemic sequelae. Biomed Pharmacother. 2020;132:110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Y, Lv L, Ye J, Fang D, Shi D, Wu W, et al. Bifidobacterium adolescentis CGMCC 15058 alleviates liver injury, enhances the intestinal barrier and modifies the gut microbiota in D‐galactosamine‐treated rats. Appl Microbiol Biotechnol. 2019;103:375–93. [DOI] [PubMed] [Google Scholar]
- 40. Falony G, Calmeyn T, Leroy F, De Vuyst L. Coculture fermentations of Bifidobacterium species and Bacteroides thetaiotaomicron reveal a mechanistic insight into the prebiotic effect of inulin‐type fructans. Appl Environ Microbiol. 2009;75:2312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patel VC, Williams R. Antimicrobial resistance in chronic liver disease. Hepatol Int. 2020;14:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bajaj JS, Shamsaddini A, Fagan A, Sterling RK, Gavis E, Khoruts A, et al. Fecal microbiota transplant in cirrhosis reduces gut microbial antibiotic resistance genes: analysis of two trials. Hepatol Commun. 2021;5:258–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Fig S7
Fig S8
Fig S9
Fig S10
Table S1
Table S2
Table S3
Supplementary Material


