Abstract
Hepatic steatosis (HS) related to nonalcoholic fatty liver disease (NAFLD) is increasing globally. In people living with human immunodeficiency virus (PLWH) risk factors of HS are increased. The impact of HS on outcomes and in particular health‐related quality of life (HRQL) in PLWH remains unknown. The aim of this cross‐sectional cohort study (FLASH, Prevalence of Advanced Fibrosis in Patients Living With HIV) was to determine the contribution of HS on HRQL in PLWH and to identify confounders on HRQL. A total of 245 PLWH were prospectively enrolled. HS was assessed using vibration‐controlled transient elastography and defined as a controlled attenuation parameter (CAP) of ≥ 275 dB/m. The analysis was performed between CAP < 275 and ≥ 275 dB/m. The generic European Quality‐of‐Life 5‐Dimension 5‐Level questionnaire was used to determine differences in the HRQL. Univariable and multivariable linear regression models were applied to identify predictors with impaired HRQL in both groups. In this cohort, 65% (n = 160) presented without and 35% (n = 85) with HS, of whom most had NAFLD (n = 65, 76.5%). The HRQL (UI‐value) was significantly lower in PLWH and steatosis (0.86 ± 0.18) in comparison with no steatosis (0.92 ± 0.13). Unemployment (p = 0.025) and waist circumference (p = 0.017) remained independent predictors of a poor HRQL in the steatosis subgroup. In turn, age (p = 0.045), female sex (p = 0.030), body mass index (p = 0.010), and arterial hypertension (p = 0.025) were independent predictors of a low HRQL in the subgroup without steatosis. Conclusion: HS and metabolic comorbidities negatively affect the HRQL. Addressing these factors may improve patient‐reported and liver‐related outcomes in PLWH.
Despite a high prevalence of hepatic steatosis and NAFLD in people living with HIV (PLWH), the impact on health‐related quality of life (HRQL) remains unknown to date. We analyzed the HRQL in PLWH and hepatic steatosis using the generic EQ‐5D‐5L questionnaire and observed a lower HRQL compared to PLWH without steatosis. Addressing hepatic steatosis and its metabolic comorbidities may improve patient reported and liver‐related outcomes in PLWH.

INTRODUCTION
Since the introduction of antiretroviral therapy (ART), significant improvements in the life expectancy of people living with HIV (PLWH) and a reduced incidence of human immunodeficiency virus (HIV)–related complications have been achieved.[ 1 ] The burden of disease in PLWH may nowadays be affected by age‐related and metabolic comorbidities as seen in the general population.[ 2 ] More notably, these noninfectious comorbidities are all numerically more prevalent in PLWH compared with uninfected individuals.[ 3 ] An increasingly sedentary lifestyle and changing dietary patterns have led to a dramatic rise in obesity in the general population and in PLWH. Nevertheless, weight gain has also been discussed in the context of HIV infection itself and ART regimens.[ 4 , 5 ]
An often‐overlooked comorbidity in this context is hepatic steatosis (HS), more commonly referred as nonalcoholic fatty liver disease (NAFLD), in the absence of high alcohol intake and other secondary causes.[ 6 , 7 ] In a previous analysis, we and others were able to show a high prevalence of NAFLD and its association with metabolic risk factors in PLWH.[ 8 ] If left undiagnosed and untreated, NAFLD and its inflammatory subtype, nonalcoholic steatohepatitis, can progress to severe liver‐related complications with a high economic burden and increased mortality.[ 9 , 10 , 11 ] Current research is aimed at evaluating noninvasive tests (NITs), such as vibration‐controlled transient elastography (VCTE), to screen and identify patients at risk without harmful side effects.[ 12 ]
Despite these objective findings, health‐related quality of life (HRQL) is an important aspect of a patient’s well‐being and thus in the assessment of overall disease burden. Patients with NAFLD and other chronic liver diseases show impaired HRQL.[ 13 , 14 , 15 ] To quantify a patient’s HRQL, several generic[ 16 ] and disease‐specific questionnaires[ 17 ] have been implemented in the case of HIV. The European Quality‐of‐Life 5‐Dimension 5‐Level (EQ‐5D‐5L) is a generic measure of HRQL; it is a widely used questionnaire in different diseases and in PLWH.[ 18 ] The questionnaire consists of five different dimensions, an overall value (utility index [UI] value), and the visual analog scale (VAS) to assess the patient’s current health state.[ 16 ] A perception of poor health may negatively influence self‐care and adherence to treatment, and in this context an HRQL assessment may predict survival in PLWH.[ 19 ] Previous studies have shown that symptomatic HIV infection and comorbidities can profoundly affect the HRQL.[ 20 , 21 , 22 ] Furthermore, HRQL is declining with the number of coexisting comorbidities.[ 23 ] However, none of these studies have looked at HS, despite the high prevalence of metabolic risk factors and the metabolic side effects of ART in PLWH.[ 5 , 24 ]
To date, the impact of HS as a liver‐related comorbidity on HRQL in PLWH remains unknown. Moreover, no data exist from the generic EQ‐5D‐5L questionnaire related to HRQL in PLWH from Germany, although country‐specific differences may exist.[ 21 , 25 ] Therefore, the aim of this prospective study was to determine differences in HRQL between PLWH with and without HS, and to identify treatable predictors of an impaired HRQL in both groups.
METHODS
Patients
A total of 302 PLWH were approached between 2018 and 2021 for this prospectively enrolling single‐center cohort study (FLASH, Prevalence of Advanced Fibrosis in Patients Living With HIV; NCT04066608) after informed consent was obtained at the outpatient clinic of the Metabolic Liver Disease Research Program at the University Medical Center Mainz in Germany. Of these patients, 57 did not meet the inclusion criteria and had to be excluded from the analysis. A final number of 245 PLWH were eligible for study inclusion. Participants with an active malignancy or below 18 years of age were not included. A flow diagram is seen in Figure 1. Medical and treatment history was available from the electronic health care records. Education was categorized in higher and lower education. Higher education means that individuals had at least a high school diploma. Number of medications is the total number and does not include the number of pills taken. HIV‐RNA viral load was divided into two groups: below and above the 50 copies/ml threshold. In the case of incomplete data, the total number (n) of analyzed data is provided for the respective variables.
FIGURE 1.
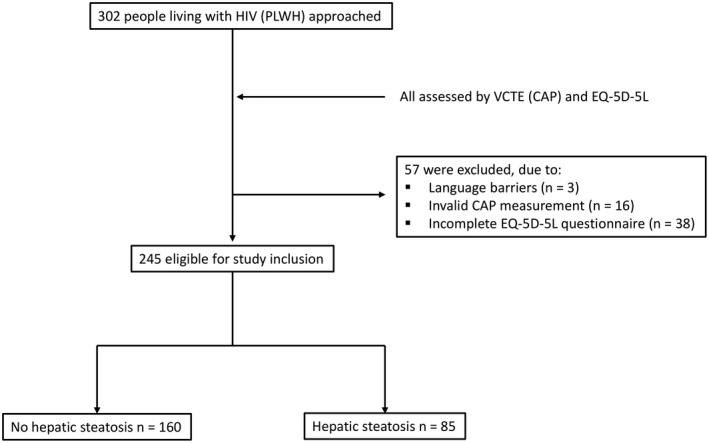
Flow diagram showing the exclusion of ineligible participants. CAP, controlled attenuation parameter; EQ‐5D‐5L, European Quality‐of‐Life 5‐Dimension 5‐Level; HIV, human immunodeficiency virus; PLWH, people living with HIV; VCTE, vibration‐controlled transient elastography
Assessment of HS
Hepatic steatosis was noninvasively assessed by measuring the controlled attenuation parameter (CAP, dB/m) using VCTE (FibroScan 430 mini; SMART Exam was introduced in 2020; Echosens).[ 26 ] A CAP of ≥ 275 dB/m was chosen to define HS in this analysis, according to current practice guidelines.[ 27 ] In most cases, the M probe was used (n = 222, 90.6%), and in cases of severe obesity the XL probe (n = 23, 9.4%) was used. Fibrosis was measured using liver stiffness measurement (LSM, kPa) with VCTE. A LSM ≥ 8.2 kPa defined significant fibrosis (≥F2 fibrosis).[ 28 ] Only measurements with an interquartile range–to–median ratio <30% and a success rate >70% were considered valid.[ 29 ] A total of 16 patients were excluded due to invalid measurements. NAFLD was defined according to current practice guidelines.[ 30 ]
Assessment of metabolic risk factors
The metabolic syndrome and its associated risk factors, including waist circumference (males ≥ 94 cm, females ≥ 80 cm), diabetes mellitus (previously diagnosed type 2 diabetes), elevated triglycerides (≥150 mg/dl, or treatment of this condition), low high‐density lipoprotein cholesterol (males < 40 mg/dl; females < 50 mg/dl), and arterial hypertension (systolic ≥ 130 mm Hg or diastolic ≥ 85 mm Hg), were defined according to the criteria of the International Diabetes Federation.[ 31 ] Body mass index (BMI) was calculated using height and weight (BMI = weight [kg]/height² [m²]). Waist circumference (cm) was measured at study inclusion. The amount of alcohol consumption (g/day) was determined clinically. Higher alcohol intake was defined as 20 g/day and 10 g/day for males and females, respectively. Laboratory values were assessed at baseline at study inclusion.
Assessment of HRQL
For the assessment of HRQL in PLWH, the validated German version of the EQ‐5D‐5L questionnaire was used under standardized conditions. The EQ‐5D‐5L represents a generic measure of the quality of life,[ 16 ] which has previously been used in PLWH[ 21 ] and in patients with chronic liver disease.[ 32 ] It contains five dimensions to capture different aspects of someone’s HRQL. The five dimensions include mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. Each of the dimensions have five response levels ranging from no problems, slight problems, moderate problems, severe problems, to extreme problems. Numbers from 1 (“no problems”) to 5 (“extreme problems”) quantify the different response levels numerically. The overall current health is reflected in the EQ VAS ranging from 0 (the worst health) to 100 (the best health). The UI indicates an overall score based on the five dimensions with the use of a country‐specific value set. For the analysis of the UI‐value in this study, the value set for Germany was used.[ 33 ] Higher scores on the five dimensions indicate a poorer HRQL, whereas higher scores on the UI‐value and VAS indicate a better HRQL.
Statistical analysis
Descriptive and categorical variables are presented as median values with interquartile ranges (IQR 25th; 75th) or mean values with SD and frequencies with percentages. For the comparison of differences between groups of categorical variables, an unpaired t test or the Mann‐Whitney U test was used. The chi‐squared test was applied for the comparison of two or more patient‐groups. All tests were two‐tailed; statistically significant values were defined as p < 0.05. Univariable linear regression was used to examine correlations between demographic and clinical variables with the UI‐value of the EQ‐5D‐5L. All variables with a p value of < 0.05 in the univariable analysis were then analyzed in a multivariable linear regression model based on a stepwise variable selection process. For all data analyses and statistical tests, IBM SPSS Statistic Version 23.0 (IBM Corp.) was used. For all figures, Microsoft Excel 2016 (Microsoft Corp.) was used.
RESULTS
Demographic and clinical characteristics
A total of 245 PLWH were prospectively enrolled, of whom 35% (n = 85) were classified as having HS as defined by a CAP of ≥ 275 dB/m on VCTE. The median LSM was 4.6 kPa in the entire cohort with a higher median value in the steatosis group (5.0 kPa). Significant fibrosis was detected in a total of 16 PLWH (6.5%). The remaining 65% (n = 160) of participants had no HS. In the total cohort, 27.2% (n = 65) were classified as having NAFLD. The median age was 52, and metabolic comorbidities were highly prevalent in the entire cohort. PLWH presenting with HS were older (p = 0.041), and more males were affected (p = 0.027). Overall, metabolic risk factors were more common in the HS group, with a median BMI of 28.2 kg/m² (overweight) and a median waist circumference of 104 cm. Additionally, median alanine aminotransferase, gamma‐glutamyltransferase, and triglyceride levels significantly differed between both groups. No differences were seen in HIV‐related characteristics, including CD4+ cell count and HIV RNA. The prevalence of HIV stages was not significantly different between both groups. Demographic and clinical characteristics and a comparison between PLWH presenting with and without HS are summarized in Table 1.
TABLE 1.
Demographic and clinical characteristics of PLWH presenting with and without HS
| Variable | Total cohort (n = 245) | No HS (n = 160) | HS (n = 85) | p value |
|---|---|---|---|---|
| n (% or IQR) | n (% or IQR) | n (% or IQR) | ||
| General characteristics | ||||
| Age (years), n = 245 | 52 (42; 58) | 51 (41; 57) | 53 (47; 60) | 0.041 |
| Disease duration, n = 231 | 13 (7; 23) | 13 (7; 23) | 14 (6; 25.8) | 0.869 |
| Sex | 0.027 | |||
| Male | 174 (71) | 106 (66.3) | 68 (80) | |
| Female | 71 (29) | 54 (33.7) | 17 (20) | |
| Unemployment | 27 (11) | 16 (11.6) | 11 (13.9) | 0.617 |
| School education, n = 214 | 0.144 | |||
| Lower education | 146 (68.2) | 88 (64.7) | 58 (74.4) | |
| Higher education | 68 (31.8) | 48 (35.3) | 20 (25.6) | |
| Number of medications (>4), n = 234 | 36 (14.7) | 21 (13.9) | 15 (18.1) | 0.398 |
| VCTE (HS/fibrosis) | ||||
| CAP (dB/m) | 245 (214.5; 293) | 224 (203; 244) | 315 (292; 342) | <0.001 |
| LSM (kPa) | 4.6 (3.9; 5.7) | 4.5 (3.7; 5.6) | 5.0 (4.1; 6.2) | 0.036 |
| Fibrosis ≥ 8.2 kPa | 16 (6.5) | 10 (6.3) | 6 (7) | 0.807 |
| NAFLD | 65 (27.2) | 0 | 65 (76.5) | |
| Metabolic comorbidities | ||||
| Hyperlipidemia | 73 (29.8) | 39 (24.8) | 34 (40) | 0.013 |
| Arterial hypertension, n = 230 | 75 (30.6) | 42 (27.1) | 33 (41.2) | 0.027 |
| Type 2 diabetes | 27 (11) | 13 (8.1) | 14 (16.5) | 0.055 |
| Metabolic syndrome | 68 (27.8) | 31 (19.4) | 37 (43.5) | <0.001 |
| BMI (kg/m²), n = 241 | 25.1 (22.4; 28.5) | 23.5 (21.8; 26.4) | 28.2 (25.4; 32.6) | <0.001 |
| Waist circumference (cm), n = 238 | 96 (86.7; 104.3) | 92 (84; 100) | 104 (98; 114) | <0.001 |
| Higher alcohol intake, n = 239 | 28 (11.4) | 14 (8.7) | 14 (17.7) | 0.054 |
| Laboratory values | ||||
| ALT (U/l), n = 231 | 23 (18; 32) | 22 (17; 30) | 28 (19.5; 39.5) | <0.001 |
| AST (U/l), n = 231 | 26 (23; 31) | 26 (22; 30) | 26 (23; 33.5) | 0.248 |
| GGT (U/l), n = 226 | 27.5 (19; 44.3) | 25 (18; 36.8) | 34 (23.5; 55) | <0.001 |
| Triglycerides (mg/dl), n = 154 | 133 (90; 191.5) | 109.5 (82.8; 170.8) | 179 (115; 239) | <0.001 |
| Cholesterol (mg/dl), n = 159 | 202 (177; 229) | 197.5 (172; 226.3) | 207 (183; 233) | 0.124 |
| Leukocytes, n = 240 | 6.5 (5.3; 7.8) | 6.3 (5.1; 7.7) | 6.7 (5.5; 7.8) | 0.140 |
| Hemoglobin, n = 240 | 14.9 (13.6; 15.7) | 14.7 (13.4; 15.5) | 15.2 (14.5; 15.9) | 0.001 |
| HIV‐related characteristics | ||||
| CD4+ (cells/µl), n = 266 | 727 (516.8; 901.3) | 710 (515.5; 882) | 801 (529.5; 996) | 0.140 |
| HIV RNA, n = 238 | 0.236 | |||
| Below threshold | 166 (67.8) | 113 (72.4) | 53 (64.6) | |
| Above threshold | 72 (29.4) | 43 (27.6) | 29 (35.4) | |
| CDC stage, n = 155 | 0.335 | |||
| A | 69 (44.5) | 46 (47.9) | 23 (38.9) | |
| B | 40 (25.8) | 21 (21.8) | 19 (32.2) | |
| C | 46 (29.7) | 29 (30.2) | 17 (28.8) | |
| NRTI | 0.395 | |||
| TAF as part of ART | 155 (86.1) | 98 (54.4) | 57 (36.7) | |
| TDF as part of ART | 25 (13.8) | 18 (72) | 7 (28) | |
| PI, n = 241 | 39 (15.9) | 28 (17.8) | 11 (13.1) | 0.341 |
| INSTI, n = 241 | 161 (65.7) | 101 (64.3) | 60 (71.4) | 0.265 |
Data are expressed as numbers, median, percentage (%), or interquartile ranges (IQR 25th; 75th). p values refer to the comparison between no HS versus HS. Boldface indicates statistical significance. A p value < 0.05 was considered statistically significant.
Abbreviations: ALT, alanine‐aminotransaminase; ART, antiretroviral therapy; AST, aspartate‐aminotransaminase; BMI, body mass index; CDC, Centers for Disease Control and Prevention; GGT, gamma‐glutamyltransferase; INSTI, integrase inhibitor; LSM, liver stiffness measurement; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; NAFLD, nonalcoholic fatty liver disease; NRTI, nucleoside reverse‐transcriptase inhibitor; PI, protease inhibitor.
HRQL in PLWH
The mean EQ‐5D‐5L VAS score and UI value were 76.8 ± 19.5 and 0.90 ± 0.15, respectively. The pain/discomfort (1.72 ± 0.87) and anxiety/depression (1.59 ± 0.87) dimensions showed the highest mean scores, whereas the self‐care dimension had the lowest mean score (1.10 ± 0.44) (Table 2). Concomitantly, a high proportion of PLWH reported problems in the pain/discomfort (n = 122, 50.4%) and anxiety/depression (n = 96, 39.8%) dimensions. The distribution of each dimension (percentage) in the entire cohort is depicted in Figure S1. The UI‐value and the dimension mobility significantly differed between males (m) and females (f) (UI‐value: [m] 0.91 ± 0.12 vs. [f] 0.86 ± 0.20, p = 0.029; mobility: [m] 1.32 ± 0.72 vs. [f] 1.54 ± 0.97, p = 0.048). Although the dimension anxiety/depression was higher in females, no significant difference between males and females was seen (Table S1).
TABLE 2.
Mean scores of the EQ‐5D‐5L questionnaire and a comparison of PLWH and no HS versus HS
| Variable | Total cohort (n = 245) | No HS (n = 160) | HS (n = 85) | p value |
|---|---|---|---|---|
| EQ‐5D‐5L | ||||
| Mobility, n = 238 | 1.38 ± 0.80 | 1.29 ± 0.69 | 1.55 ± 0.96 | 0.016 |
| Self‐care, n = 241 | 1.10 ± 0.44 | 1.08 ± 0.46 | 1.13 ± 0.44 | 0.472 |
| Usual activities, n = 239 | 1.33 ± 0.73 | 1.28 ± 0.64 | 1.43 ± 0.86 | 0.124 |
| Pain/discomfort, n = 242 | 1.72 ± 0.87 | 1.63 ± 0.81 | 1.91 ± 0.95 | 0.012 |
| Anxiety/depression, n = 241 | 1.59 ± 0.87 | 1.57 ± 0.77 | 1.63 ± 1.03 | 0.629 |
| VAS, n = 245 | 76.8 ± 19.5 | 77.8 ± 18.6 | 74.7 ± 20.9 | 0.225 |
| UI‐value, n = 236 | 0.90 ± 0.15 | 0.92 ± 0.13 | 0.86 ± 0.18 | 0.013 |
Data are expressed as means with SD. Boldface indicates statistical significance. A p value < 0.05 was considered statistically significant.
Abbreviations: UI, utility index; VAS, visual analog scale.
Comparison of the HRQL between PLWH presenting with and without HS
PLWH presenting with HS had an overall lower UI‐value in comparison to PLWH with no HS (no HS: 0.92 ± 0.13 vs. HS: 0.86 ± 0.18, p = 0.013) (Table 2). The scores of the mobility (1.55 ± 0.96, p = 0.016) and pain/discomfort (1.91 ± 0.95, p = 0.012) dimensions were significantly higher in the HS group. The dimension anxiety/depression showed high values in both groups. Furthermore, no differences were seen in the self‐care and usual activities dimensions as well as in the VAS. More than half of PLWH in the steatosis subgroup reported problems in the pain/discomfort (58.3%) dimension. A high proportion of participants (no HS: 42.4%; HS: 34.9%) reported problems in the anxiety/depression dimension. The comparison of the distribution of each dimension (%) between both groups is shown in Figure 2.
FIGURE 2.
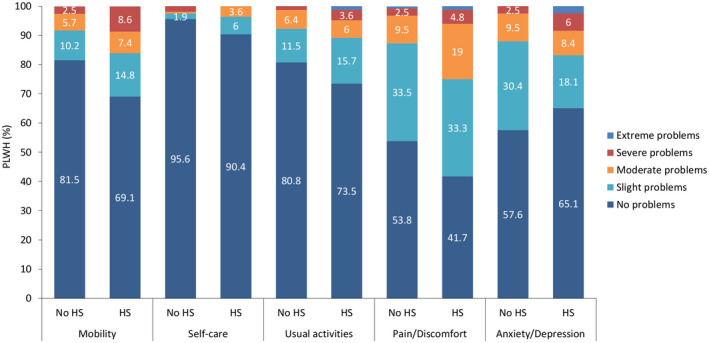
Distribution of the EQ‐5D‐5L dimensions in PLWH and without hepatic steatosis (HS) or with HS. The EQ‐5D‐5L questionnaire consists of five dimensions: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. Each of these dimensions is divided into five levels of perceived problems: no problems, slight problems, moderate problems, severe problems, and extreme problems
Clinical predictors of impaired HRQL in PLWH and without HS
The factors age (β: −0.254; 95% confidence interval [CI] −0.414, −0.101), disease duration (β: −0.183; 95% CI −0.347, −0.021), BMI (β: −0.251; 95% CI −0.408, −0.095), waist circumference (β: −0.272; 95% CI −0.425, −0.115), arterial hypertension (β: −0.306; 95% CI −0.466, −0.153), and triglyceride levels (β: −0.236; 95% CI −0.448, −0.032) were all associated with a lower HRQL. In a multivariable linear regression analysis, age (β: −0.168; 95% CI −0.369, −0.005), female sex (β: −0.173; 95% CI −0.348, −0.018), BMI (−0.214; 95% CI −0.391, −0.054), and arterial hypertension (β: −0.194; 95% CI −0.378, −0.025) remained independent predictors of a worse HRQL in PLWH and without HS (Table 3).
TABLE 3.
Clinical predictors of impaired health‐related quality of life in PLWH and without HS
| Variable | EQ‐5D‐5L UI‐value | |||||
|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis a | |||||
| β | 95% CI | p value | β | 95% CI | p value | |
| General characteristics | ||||||
| Age | −0.254 | −0.414, −0.101 | 0.001 | −0.168 | −0.369, −0.005 | 0.045 |
| Disease duration | −0.183 | −0.347, −0.021 | 0.027 | |||
| Sex, female | −0.150 | −0.311, 0.009 | 0.063 | −0.173 | −0.348, −0.018 | 0.030 |
| Unemployment | −0.077 | −0.240, 0.092 | 0.378 | |||
| Higher education | 0.169 | −0.002, 0.324 | 0.053 | |||
| Number of medications (>4) | −0.117 | −0.281, 0.047 | 0.160 | |||
| Fibrosis ≥ 8.2 kPa | −0.056 | −0.213, 0.101 | 0.485 | |||
| Metabolic comorbidities | ||||||
| BMI | −0.251 | −0.408, −0.095 | 0.002 | −0.214 | −0.391, −0.054 | 0.010 |
| Waist circumference | −0.272 | −0.425, −0.115 | 0.001 | |||
| Higher alcohol intake | 0.009 | −0.148, 0.167 | 0.907 | |||
| Type 2 diabetes | −0.148 | −0.301, 0.010 | 0.067 | |||
| Arterial hypertension | −0.306 | −0.466, −0.153 | <0.001 | −0.194 | −0.378, −0.025 | 0.025 |
| Hyperlipidemia | 0.118 | −0.043, 0.280 | 0.148 | |||
| Metabolic syndrome | −0.148 | −0.310, 0.010 | 0.066 | |||
| Laboratory values | ||||||
| ALT (U/l) | 0.083 | −0.076, 0.229 | 0.322 | |||
| AST (U/l) | 0.079 | −0.080, 0.226 | 0.346 | |||
| Triglycerides | −0.236 | −0.448, −0.032 | 0.024 | |||
| Cholesterol | 0.095 | −0.128, 0.350 | 0.358 | |||
| Hemoglobin | 0.068 | −0.086, 0.212 | 0.404 | |||
| Leukocytes | 0.005 | −0.145, 0.153 | 0.955 | |||
| HIV‐related characteristics | ||||||
| CDC stage C | 0.058 | −0.139, 0.247 | 0.580 | |||
| HIV RNA | 0.052 | −0.102, 0.198 | 0.529 | |||
| CD4 cells/µl | 0.076 | −0.081, 0.224 | 0.356 | |||
| TAF vs. TDF | 0.081 | −0.102, 0.255 | 0.399 | |||
| PI | −0.028 | −0.191, 0.134 | 0.728 | |||
| INSTI | 0.053 | −0.109, 0.216 | 0.514 | |||
Univariable and multivariable linear regression analyses were done. With all factors showing a p value < 0.05 and the clinical parameters of age and sex, a multivariable linear regression model was built. Confidence interval (CI) and Beta (β) show each of the standardized values. Boldface indicates statistical significance. A p value < 0.05 was considered statistically significant.
Multivariable linear regression analysis, stepwise selection (n = 137): age, disease duration, sex, BMI, and waist circumference; triglycerides were excluded due limited numbers available.
Clinical predictors of impaired HRQL in PLWH and with HS
The factors unemployment (β: −0.293; 95% CI −0.503, −0.069), waist circumference (β: −0.235; 95% CI −0.450, −0.014), arterial hypertension (β: −0.297; 95% CI −0.525, −0.079), and cholesterol (β: −0.343; 95% CI −0.523, −0.078) were all associated with a lower HRQL. In turn, a higher education (β: 0.268; 95% CI 0.041, 0.487) showed a positive correlation with a better HRQL. In a multivariable linear regression analysis, unemployment (β: −0.270; 95% CI −0.492, −0.035) and waist circumference (β: −0.289; 95% CI −0.492, −0.051) remained the two independent predictors of a worse HRQL in PLWH and with HS (Table 4). Although HS was associated with a lower HRQL in the analysis of the total cohort, it did not remain an independent predictor (Table S2).
TABLE 4.
Clinical predictors of impaired health‐related quality of life in PLWH and with HS
| Variable | EQ‐5D‐5L UI‐value | |||||
|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis a | |||||
| β | 95% CI | p value | β | 95% CI | p value | |
| General characteristics | ||||||
| Age | −0.002 | −0.229, 0.225 | 0.987 | |||
| Disease duration | 0.038 | −0.199, 0.277 | 0.746 | |||
| Sex, female | −0.198 | −0.410, −0.021 | 0.076 | |||
| Unemployment | −0.293 | −0.503, −0.069 | 0.011 | −0.270 | −0.492, −0.035 | 0.025 |
| Higher education | 0.268 | 0.041, 0.487 | 0.021 | |||
| Number of medications (>4) | −0.036 | −0.275, 0.199 | 0.751 | |||
| Fibrosis ≥ 8.2 kPa | −0.083 | −0.362, 0.165 | 0.460 | |||
| Metabolic comorbidities | ||||||
| BMI | −0.196 | −0.413, −0.024 | 0.081 | |||
| Waist circumference | −0.235 | −0.450, −0.014 | 0.037 | −0.289 | −0.492, −0.051 | 0.017 |
| Higher alcohol intake | −0.060 | −0.295, 0.174 | 0.609 | |||
| Type 2 diabetes | 0.073 | −0.157, 0.309 | 0.516 | |||
| Arterial hypertension | −0.297 | −0.525, −0.079 | 0.009 | |||
| Hyperlipidemia | 0.028 | −0.200, 0.257 | 0.803 | |||
| Metabolic syndrome | 0.057 | −0.168, 0.281 | 0.615 | |||
| Laboratory values | ||||||
| ALT (U/l) | 0.105 | −0.133, 0.364 | 0.358 | |||
| AST (U/l) | −0.058 | −0.431, 0.256 | 0.612 | |||
| Triglycerides | 0.181 | −0.074, 0.380 | 0.182 | |||
| Cholesterol | −0.343 | −0.523, −0.078 | 0.009 | |||
| Hemoglobin | 0.025 | −0.200, 0.249 | 0.829 | |||
| Leukocytes | −0.148 | −0.378, 0.077 | 0.191 | |||
| HIV‐related characteristics | ||||||
| CDC stage C | −0.172 | −0.467, 0.104 | 0.208 | |||
| HIV RNA | 0.030 | −0.202, 0.264 | 0.793 | |||
| CD4 cells/µl | −0.135 | −0.379, 0.096 | 0.238 | |||
| TAF vs. TDF | 0.170 | −0.093, 0.458 | 0.190 | |||
| PI | 0.089 | −0.139, 0.322 | 0.430 | |||
| INSTI | −0.181 | −0.404, 0.041 | 0.109 | |||
Univariable and multivariable linear regression analyses were done. With all factors showing a p value < 0.05 and the clinical parameters of age and sex, a multivariable linear regression model was built. CI and β show each of the standardized values. Boldface indicates statistical significance. A p value < 0.05 was considered statistically significant.
Multivariable linear regression analysis, stepwise selection (n = 64): age, sex, unemployment, higher education, waist circumference, and arterial hypertension; cholesterol was excluded due to limited numbers available.
Next, we investigated predictors of a poor HRQL in the dimension’s mobility and pain/discomfort within the HS subgroup. Higher education (β: −0.299; 95% CI −0.516, −0.086) was a predictor of a better mobility, whereas waist circumference (β: 0.314; 95% CI 0.085, 0.518) remained an independent predictor of a poor mobility (Table S3). In turn, arterial hypertension was the only independent predictor of an impaired pain/discomfort dimension (β: 0.301; 95% CI 0.084, 0.510) (Table S4).
DISCUSSION
In this study, HRQL was analyzed using the generic EQ‐5D‐5L questionnaire in PLWH presenting either with or without hepatic steatosis (HS). Despite the rising numbers of patients with chronic liver diseases and the detrimental effects on HRQL, no data related to HS and HRQL in PLWH currently exist. Here, we show that PLWH presenting with HS have a poorer HRQL. More specifically, the mobility and pain/discomfort dimensions were the most burdensome in this subgroup analysis. In the entire cohort, the anxiety/depression dimension was also a major aspect of HRQL. In the subgroup analysis of PLWH and without HS, older age, female sex, a high BMI, and arterial hypertension were negatively associated with HRQL. More importantly, in this study we identified potentially treatable comorbidities. Addressing these factors may not only enhance HRQL but also affect the resolution of HS and its related complications.
Comorbidities have a significant impact on the HRQL in PLWH,[ 23 ] and chronic health conditions are more prominent in PLWH > 50 years or older compared with the general population.[ 34 ] In the assessment of comorbidities, however, HS is often overlooked, despite its high prevalence in this analysis (>35%) and in previous studies.[ 8 ] Moreover, HIV itself and certain ART regimens can have negative metabolic outcomes, which need additional consideration in the context of lifestyle‐associated factors.[ 35 ] In a previous analysis and in this current study we did not see any impact of these HIV‐related parameters on HS or HRQL in PLWH. A recent longitudinal analysis reported a higher risk of tenofovir alafenamide and integrase inhibitors for steatosis progression.[ 5 ] To detect and screen patients at a particular risk of developing HS, NITs such as VCTE with no harmful side effects have become valuable tools in the clinical routine.[ 12 ] In this study, PLWH with HS had an overall lower HRQL, and these patients were mostly affected in the mobility and pain/discomfort dimensions. Interestingly, Balderson et al. were able to show that physical functioning was the major variable to be affected by chronic diseases in PLWH.[ 34 ] Waist circumference remained an independent predictor of impaired mobility in the subgroup with HS. Metabolic risk factors such as a high BMI may also cause more orthopedic issues, especially in the knees, with additional limitations in mobility and the ability to exercise.[ 36 ] Indeed, exercise has been shown to have positive effects on the HRQL in PLWH.[ 37 ] This may also explain the burden of the pain/discomfort dimension in these patients in addition to other comorbidities, primarily arterial hypertension or chronic back pain.[ 3 , 34 ] However, older age has also been reported to affect the pain/discomfort dimension independent of HS.[ 21 ] In the context of HS, lifestyle interventions with a change in diet and physical exercise are cornerstones in the treatment of NAFLD.[ 38 ] Therefore, it is important to educate these patients about the positive effects of lifestyle interventions to reduce weight.
The mean UI‐value of the entire cohort was 0.90 ± 0.15 (median 0.91) in the current study, which is higher than the pooled UI‐value (0.84) from various countries in a recent meta‐analysis.[ 18 ] A cohort from Vietnam showed the lowest UI‐value, and HRQL was negatively affected in symptomatic and more advanced HIV stages.[ 22 ] Using a specific HRQL questionnaire (MOS‐HIV) for PLWH, CD4+ count and viral load showed no independent association in a study from Ireland,[ 39 ] which is consistent with our findings with the EQ‐5D‐5L. Our cohort was in large parts well controlled; most had suppressed viral loads, and the median CD4+ cell count was above >500 cells/µl. Overall, the differences in these HRQL findings may be multifactorial and country‐specific.[ 25 ] According to previous studies, low socioeconomic status and income are often associated with low HRQL.[ 40 ] We were not able to see an effect of unemployment or education level on the HRQL in PLWH and without HS. Interestingly, in the subgroup of PLWH with HS, unemployment remained an independent predictor of a poor HRQL and higher education was positively correlated with better HRQL. Obesity, a major risk factor for HS, is often associated with unemployment.[ 41 ] Only recently, a study identified lower risk of NAFLD in patients with higher education, partially mediated by a better diet quality and physical activity.[ 42 ] In this regard, HS may reflect lower socioeconomic status in PLWH.
In line with previous findings, anxiety/depression and pain/discomfort were found to be the most burdensome dimensions in PLWH.[ 21 ] Mental well‐being often coexists with chronic diseases that need lifelong treatment such as HIV[ 43 ] or chronic liver diseases.[ 44 ] Popping et al. recently found a high prevalence of anxiety/depression in PLWH compared with the general population, especially if patients were >60 years of age.[ 25 ] Notably, older age remained independently associated with low HRQL in the subgroup analysis without HS in this study. Although the anxiety/depression dimension was prominent in both subgroups, they were not significantly different from each other. Due to stigmatization and the need for lifelong adherence to ART, HIV infection itself may have a higher impact on mental health than other chronic diseases.[ 45 ] Besides anxiety and depression, fatigue is frequently present in patients with more advanced chronic liver disease.[ 46 ] However, the EQ‐5D‐5L does not specifically assess fatigue; therefore, liver‐specific questionnaires may need to be used to capture this aspect in patients with hepatic steatosis. Moreover, females were more negatively affected in their HRQL than males in this study, a finding that has been observed with other chronic conditions.[ 32 ] Previous studies have often looked at the number of comorbidities in general without addressing the burden of individual comorbidities.[ 23 ] In the subgroup without HS, a higher BMI and arterial hypertension remained independent predictors of lower HRQL. This was a surprise, as arterial hypertension is mostly clinically asymptomatic. On the other hand, the treatment of arterial hypertension involves the prescription of medications that add to the medication burden, and some (e.g., beta‐blockers) may negatively affect activity and energy. Although other studies have reported lower HRQL with a higher number of prescribed medications,[ 21 ] we were not able to replicate these findings. BMI and arterial hypertension are treatable conditions. If they are adequately treated, this may also prevent progression to HS.
Although HS correlated with a lower HRQL, it did not remain an independent predictor in the analysis of the entire cohort. We hypothesize that this may be associated with, in large part, “invisibility” of HS for patients. Therefore, waist circumference, arterial hypertension, and even unemployment are more visible and may have immediate effects on one’s perceived health. However, this creates an argument to screen for HS and target HS for treatment with, for example, lifestyle interventions.
This study has several limitations. No disease‐specific HRQL assessment was used in this analysis; instead we used a generic questionnaire. Therefore, disease‐related aspects, either in the case of HIV infection or HS, may be underreported. To capture more liver‐related aspects of HRQL, the Chronic Liver Disease Questionnaire (CLDQ) could have been used.[ 32 ] However, the CLDQ has not been validated in PLWH and without chronic liver diseases so far, and most of this cohort had no HS. Moreover, the current study is a single‐center analysis, with the potential for referral bias and the lack of generalizability to other populations. Another limitation is the large proportion of men in this cohort. However, the EQ‐5D‐5L is a widely used and validated questionnaire that provides a quick assessment of an individual’s HRQL.
In summary, the current analysis highlights that metabolic risk factors negatively affect HRQL using a generic quality‐of‐life tool in PLWH. Additionally, HS had a negative effect on HRQL, albeit not independently of metabolic comorbidities in this cohort. Identification of modifiable metabolic risk factors affecting both HS and HRQL may significantly improve patient‐reported outcomes in PLWH. More importantly, treatment of these factors may prevent the progression to more severe liver disease. However, longitudinal studies are warranted to verify these results.
CONFLICT OF INTEREST
Jörn M. Schattenberg consults for Boehringer Ingelheim, BMS, Genfit, Gilead Sciences, Intercept Pharmaceuticals, Madrigal, Novartis, Novo Nordisk, Nordic Bioscience, Pfizer, Roche, Sanofi, and Siemens Healthcare, received research funding from Gilead Sciences and Boehringer Ingelheim, and is on the speakers bureau for Falk Foundation MSD Sharp & Dohme. The other authors have no competing interests to declare.
AUTHOR CONTRIBUTIONS
Research: Maurice Michel, Malena Anders, Alisha Wahl, and Lisann Girolstein. Data acquisition: Maurice Michel, Christian Labenz, Malena Anders, Alisha Wahl, Lisann Girolstein, Leonard Kaps, Wolfgang M. Kremer, Yvonne Huber, Martin Sprinzl, and Jörn M. Schattenberg. Experiment design and data analysis: Maurice Michel and Jörn M. Schattenberg. Reagents, materials, and analysis tool contribution: Peter R. Galle and Jörn M. Schattenberg. Manuscript draft: Maurice Michel and Jörn M. Schattenberg. Manuscript revisions and editing: Maurice Michel and Jörn M. Schattenberg. Statistical analysis: Maurice Michel and Jörn M. Schattenberg. All authors approved the final version of the manuscript and the authorship list. Guarantor of the article: Jörn M. Schattenberg.
ETHICS
The study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). The study protocol was approved by the ethics committee of the Landesärztekammer Rhineland‐Palatine (No. 873.199.10 (7208)). All participants provided written informed consent.
INSTITUTIONAL REVIEW BOARD STATEMENT
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Landesärztekammer Rhineland‐Palatine No. 873.199.10 (7208).
INFORMED CONSENT STATEMENT
Informed consent was obtained from all patients involved in the study.
Supporting information
Fig S1
Supplementary Material
ACKNOWLEDGMENT
Maurice Michel is supported by the Clinician Scientist Fellowship “Else Kröner Research College: 2018_Kolleg.05.” This study contains parts of the medical thesis of Malena Anders, Alisha Wahl, and Lisann Girolstein.
Michel M, Labenz C, Anders M, Wahl A, Girolstein L, Kaps L, et al. Effect of hepatic steatosis and associated metabolic comorbidities on health‐related quality of life in people living with HIV. Hepatol Commun. 2022;6:2011–2021. doi: 10.1002/hep4.1958
Funding information
Supported in part by Gilead Sciences
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author.
REFERENCES
- 1. Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV‐1. Lancet. 1998;352:1725–30. [DOI] [PubMed] [Google Scholar]
- 2. Braithwaite RS, Justice AC, Chang C‐C, Fusco JS, Raffanti SR, Wong JB, et al. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med. 2005;118:890–8. [DOI] [PubMed] [Google Scholar]
- 3. Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, et al. Cross‐sectional comparison of the prevalence of age‐associated comorbidities and their risk factors between HIV‐infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–97. [DOI] [PubMed] [Google Scholar]
- 4. Bonfanti P, Giannattasio C, Ricci E, Facchetti R, Rosella E, Franzetti M, et al. HIV and metabolic syndrome: a comparison with the general population. JAIDS J Acquir Immune Defic Syndr. 2007;45:426–31. [DOI] [PubMed] [Google Scholar]
- 5. Bischoff J, Gu W, Schwarze‐Zander C, Boesecke C, Wasmuth J‐C, van Bremen K, et al. Stratifying the risk of NAFLD in patients with HIV under combination antiretroviral therapy (cART). EClinicalMedicine. 2021;40:101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 7. Liebe R, Esposito I, Bock HH, vom Dahl S, Stindt J, Baumann U, et al. Diagnosis and management of secondary causes of steatohepatitis. J Hepatol. 2021;74:1455–71. [DOI] [PubMed] [Google Scholar]
- 8. Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV‐monoinfection. AIDS (London, England). 2017;31:1621–32. [DOI] [PubMed] [Google Scholar]
- 9. Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy‐confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanyal AJ, Van Natta ML, Clark J, Neuschwander‐Tetri BA, Diehl AnnaMae, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schattenberg JM, Lazarus JV, Newsome PN, Serfaty L, Aghemo A, Augustin S, et al. Disease burden and economic impact of diagnosed non‐alcoholic steatohepatitis in five European countries in 2018: a cost‐of‐illness analysis. Liver Int. 2021;41:1227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Michel M, Schattenberg JM. Leberspezifische Diagnostik bei der nichtalkoholischen Fettlebererkrankung (NAFLD)—wann kann die Leberbiopsie ersetzt werden? Z Gastroenterol. 2020;58:1233–40. [DOI] [PubMed] [Google Scholar]
- 13. Huber Y, Boyle M, Hallsworth K, Tiniakos D, Straub BK, Labenz C, et al. Health‐related quality of life in nonalcoholic fatty liver disease associates with hepatic inflammation. Clin Gastroenterol Hepatol. 2019;17:2085–2092.e1. [DOI] [PubMed] [Google Scholar]
- 14. Hui Y, Li N, Yu Z, Li C, Wang X, Li Y, et al. Health‐related quality of life and its contributors according to a preference‐based generic instrument in cirrhosis. Hepatol Commun. 2022;6:610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Hara J, Finnegan A, Dhillon H, Ruiz‐Casas L, Pedra G, Franks B, et al. Cost of non‐alcoholic steatohepatitis in Europe and the USA: the GAIN study. JHEP Rep. 2020;2:100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devlin NJ, Brooks R. EQ‐5D and the EuroQol group: past, present and future. Appl Health Econ Health Policy. 2017;15:127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henderson WA, Schlenk EA, Kim KH, Hadigan CM, Martino AC, Sereika SM, et al. Validation of the MOS‐HIV as a measure of health‐related quality of life in persons living with HIV and liver disease. AIDS Care. 2010;22:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou T, Guan H, Wang L, Zhang Y, Rui M, Ma A. Health‐related quality of life in patients with different diseases measured with the EQ‐5D‐5L: a systematic review. Front Public Health. 2021;9:675523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Boer‐van der Kolk IM, Sprangers MAG, Prins JM, Smit C, de Wolf F, Nieuwkerk PT. Health‐related quality of life and survival among HIV‐infected patients receiving highly active antiretroviral therapy: a study of patients in the AIDS Therapy Evaluation in the Netherlands (ATHENA) cohort. Clin Infect Dis. 2010;50:255–63. [DOI] [PubMed] [Google Scholar]
- 20. Anis AH, Nosyk B, Sun H, Guh DP, Bansback N, Li X, et al. Quality of life of patients with advanced HIV/AIDS: measuring the impact of both AIDS‐defining events and non‐AIDS serious adverse events. JAIDS J Acquir Immune Defic Syndr. 2009;51:631–9. [DOI] [PubMed] [Google Scholar]
- 21. Belay YB, Ali EE, Sander B, Gebretekle GB. Health‐related quality of life of patients with HIV/AIDS at a tertiary care teaching hospital in Ethiopia. Health Qual Life Outcomes. 2021;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tran BX, Ohinmaa A, Nguyen LT. Quality of life profile and psychometric properties of the EQ‐5D‐5L in HIV/AIDS patients. Health Qual Life Outcomes. 2012;10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Duin MJM, Conde R, Wijnen B, Evers SMAA, Gonzalez‐Rodriguez JL, Govers MJG, et al. The impact of comorbidities on costs, utilities and health‐related quality of life among HIV patients in a clinical setting in Bogotá. Expert Rev Pharmacoecon Outcomes Res. 2017;17:303–10. [DOI] [PubMed] [Google Scholar]
- 24. Vance DE, Mugavero M, Willig J, Raper JL, Saag MS. Aging with HIV: a cross‐sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care. 2011;22:17–25. [DOI] [PubMed] [Google Scholar]
- 25. Popping S, Kall M, Nichols BE, Stempher E, Versteegh L, van de Vijver DAMC, et al. Quality of life among people living with HIV in England and the Netherlands: a population‐based study. Lancet Reg Health Eur. 2021;8:100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wong V‐S, Vergniol J, Wong G‐H, Foucher J, Chan H‐Y, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–62. [DOI] [PubMed] [Google Scholar]
- 27. Berzigotti A, Boursier J, Castera L, Cazzagon N, Friedrich‐Rust M, Petta S, et al. EASL Clinical Practice Guidelines (Cpgs) on non‐invasive tests for evaluation of liver disease severity and prognosis—2020 update. J Hepatol. 2021. 10.1016/jhep.2021.05.025. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30. [DOI] [PubMed] [Google Scholar]
- 29. Pezzini MF, Cheinquer H, de Araujo A, Schmidt‐Cerski CT, Sprinz E, Herz‐Wolff F, et al. Hepatic steatosis among people living with HIV in Southern Brazil: prevalence and risk factors. Sci Rep. 2020;10:8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- 31. International Diabetes Federation (IDF) . The IDF consensus worldwide definition of the metabolic syndrome. https://www.idf.org/e‐library/consensus‐statements/60‐idfconsensus‐worldwide‐definitionof‐the‐metabolic‐syndrome.html. Accessed 1 Dec 2021.
- 32. Michel M, Spinelli F, Grambihler A, Labenz C, Nagel M, Kaps L, et al. Health‐related quality of life in patients with autoimmune hepatitis. Qual Life Res. 2021;30:2853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ludwig K, Graf von der Schulenburg J‐M, Greiner W. German value set for the EQ‐5D‐5L. Pharmacoeconomics. 2018;36:663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, Catz S. Chronic illness burden and quality of life in an aging HIV population. AIDS Care. 2013;25:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Surial B, Mugglin C, Calmy A, Cavassini M, Günthard HF, Stöckle M, et al. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann Intern Med. 2021;174:758–67. [DOI] [PubMed] [Google Scholar]
- 36. Raud B, Gay C, Guiguet‐Auclair C, Bonnin A, Gerbaud L, Pereira B, et al. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci Rep. 2020;10:3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin K, Naclerio F, Karsten B, Vera JH. Physical activity and quality of life in people living with HIV. AIDS Care. 2019;31:589–98. [DOI] [PubMed] [Google Scholar]
- 38. Michel M, Schattenberg JM. Effectiveness of lifestyle interventions in NAFLD (nonalcoholic fatty liver disease)—how are clinical trials affected? Expert Opin Investig Drugs. 2020;29:93–7. [DOI] [PubMed] [Google Scholar]
- 39. George S, Bergin C, Clarke S, Courtney G, Codd MB. Health‐related quality of life and associated factors in people with HIV: an Irish cohort study. Health Qual Life Outcomes. 2016;14:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Algaralleh A, Altwalbeh D, Al‐Tarawneh F. Health‐related quality of life among persons living with HIV/AIDS in Jordan: an exploratory study. HIV/AIDS (Auckland, N.Z.). 2020;12:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98. [DOI] [PubMed] [Google Scholar]
- 42. Vilar‐Gomez E, Nephew LD, Vuppalanchi R, Gawrieh S, Mladenovic A, Pike F, et al. High quality diet, physical activity and college education are associated with low risk of NAFLD among the U.S. population. Hepatology (Baltimore, MD). 2021. 10.1002/hep.32207. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43. Wang X, Luo H, Yao E, Tang R, Dong W, Liu F, et al. The role of personality, social economic and prevention strategy effects on health‐related quality of life among people living with HIV/AIDS. Infect Dis Poverty. 2021;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ladegaard Grønkjær L, Munk LM. Quality of life and unmet needs in patients with chronic liver disease: a mixed method systematic review. JHEP Rep. 2021;3:100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Engelhard EAN, Smit C, van Dijk PR, Kuijper TM, Wermeling PR, Weel AE, et al. Health‐related quality of life of people with HIV: an assessment of patient related factors and comparison with other chronic diseases. AIDS (London, England). 2018;32:103–12. [DOI] [PubMed] [Google Scholar]
- 46. Labenz C, Toenges G, Schattenberg JM, Nagel M, Huber Y, Marquardt JU, et al. Health‐related quality of life in patients with compensated and decompensated liver cirrhosis. Eur J Intern Med. 2019;70:54–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material
Data Availability Statement
The data presented in this study are available on request from the corresponding author.


