Abstract
There is growing interest in, but limited data about, intestinal bile acid transport inhibitors as treatment for cholestatic liver disease. The current analyses combine two similar randomized placebo‐controlled trials with subsequent extension phases investigating the impact of maralixibat in children with severe cholestasis secondary to Alagille Syndrome (n = 57). The primary outcomes were measures of pruritus (ItchRO[Obs]) and clinician scratch scale (CSS), both increasing in severity from 0 to 4) and quality of life (QoL) (Parent PedsQL and Multidimensional Fatigue Scale module [MFS] scaled 0–100 with increased QoL) at week 48 of the extension phase relative to the baseline of the placebo‐controlled trials (week 13). Secondary assessments included other clinical and biochemical parameters assessed in participants at week 72 or end of treatment (after week 48). At week 48, statistically and clinically significant least square mean (95% CI) improvements in pruritus and QoL were observed (ItchRO[Obs] −1.59 [−1.81, −1.36], CSS −1.36 [−1.67, −1.05], PedsQL +10.17 [4.48, 15.86], and multidimension fatigue [MFS] +13.97 [7.85, 20.08]). At week 48, serum bile acids, platelet count, and cholesterol decreased, whereas alanine aminotransferase (ALT) increased and total bilirubin (TB) and albumin were stable. Changes were durable at week 72 and end of treatment. There were no deaths; 2 participants underwent liver transplantation. Study drug was discontinued in 9 participants after treatment‐emergent adverse events, 6 of which were events of increased ALT or TB. Conclusion: Maralixibat administration was associated with marked improvement in pruritus and QoL. Interpretation of these findings is complicated by the complex natural history of severe cholestasis in Alagille syndrome.
Potential attribution of an impact of maralixibat or other IBATi on disease progression in pediatric cholestasis may require continued long‐term follow‐up of individuals receiving IBATi and a relevant contemporaneous natural history cohort. Future investigations of IBATi in cholestatic liver disease may need to delineate the mechanisms of action including effects on bile acid composition, the intestinal microbiome and the gut‐liver axis.

INTRODUCTION
Alagille syndrome (ALGS) is a multisystem disorder, with cholestatic liver disease as a cardinal feature. The clinically most prevalent extrahepatic manifestations are right‐sided cardiac defects, skeletal abnormalities, characteristic ocular and facial findings, and restricted growth. The cholestasis is strongly associated with a reduction of intrahepatic bile duct radicals on light microscopy. Underlying all of this appears to be a reduction in NOTCH signaling, as >95% of affected individuals carry a heterozygous mutation in either JAG1 or NOTCH2. Other family members, however, frequently carry the same genetic defect but may manifest some, or no, features of the syndrome. The features that greatly affect the patient's quality of life (QoL) are the heart defects and the cholestasis.[ 1 ] The pruritus caused by the latter is without doubt the overall most distressing symptom.
Numerous off‐label medications are used in the management of pruritus in ALGS and other pediatric cholestatic disorders, including ursodeoxycholic acid, rifampicin, antihistamines, opiate antagonists, and serotonin reuptake inhibitors.[ 2 ] Through failure of medical management, up to 10% of patients undergo partial external biliary diversion. Despite this, a recent US multicenter series showed that >40% of patients with ALGS underwent liver transplantation before the age of 5 years, and overall only 24% survived to adulthood with their native liver.[ 3 ] Although some transplants in later childhood are undertaken for end‐stage liver disease, the vast majority of early transplants are the result of unremitting pruritus.
Partial external biliary diversion has been used successfully for the treatment for pediatric cholestatic liver disease.[ 4 ] The rationale for this procedure is to reduce the bile salt pool size, such that the flux of bile salts through the liver is reduced, ideally to below capacity of the liver to transport bile salts into bile. It has been shown to work in some patients with ALGS.[ 4 ] Recently a number of drugs have been tested in both adults and children with cholestatic liver disease, with the aim of pharmacologically reproducing this surgical procedure.[ 5 ] Maralixibat, an inhibitor of the apical sodium–dependent bile acid transporter present in the terminal ileum (ileal bile acid transporter inhibitor [IBATi]) was initially developed as a potential treatment for hyperlipidemia.[ 6 ] Although effective, it has not been licensed for that purpose. By inhibiting bile acid uptake in the small intestine, causing the bile acids to be subsequently lost in the feces, this treatment has the potential to reduce the bile salt pool size, and consequently alleviate cholestasis.
Two randomized double‐blind placebo‐controlled trials of maralixibat in participants with ALGS have been conducted[ 6 ] (NCT 01903460 and 02057692). Both studies lasted for 13 weeks and were followed by extension studies (NCT 02047318 and 02117713). The data from all four studies have been combined, and the analysis of long‐term outcomes and safety is now presented.
EXPERIMENTAL PROCEDURES
Detailed experimental procedures are found in Text S1.
Study population and design
This analysis pools data from four clinical trials (IMAGO, ITCH, IMAGINE, and IMAGINE II). The primary results of ITCH have been published.[ 6 ] IMAGO and its extension study, IMAGINE, were conducted by evolving sponsors, concluding with Mirum Pharmaceuticals at three sites in the United Kingdom, while ITCH and its extension study, IMAGINE II, were conducted by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)–funded Childhood Liver Disease Research Network (ChiLDReN) in collaboration and through a cooperative research and development agreement between the NIDDK and the same evolving sponsors. The studies conducted in the United Kingdom and by ChiLDReN were very similar in design, with earlier initiation of the studies in the United Kingdom (Table S1). Inclusion and exclusion criteria for IMAGO and ITCH were essentially the same (Table S2A,B), enrolling children between the ages of 2 and 18 inclusive, who had ALGS, evidence of cholestasis, intractable pruritus, and compensated liver disease. Entry criteria included significant pruritus as assessed by the ItchRO instrument with a requirement of a mean daily ItchRO(Obs) score of ≥2 for two consecutive weeks as previously described.[ 6 , 7 ] IMAGO and ITCH were randomized placebo‐controlled trials to investigate the safety and efficacy of maralixibat using multiple dosing regimens, which have been previously described for ITCH and were similar in IMAGO[ 6 ] (Figure 1, Table S1, and Figure S1A,B). Participants who completed IMAGO and ITCH were provided the opportunity to enroll in follow‐up studies of long‐term safety and durability of response in IMAGINE and IMAGINE II, respectively. The complex pattern of adjustment in dosing of maralixibat is seen in Figure S1A,B, capturing dose escalation and dose optimization, which was completed by week 12. Participants who had received placebo started maralixibat in a dose‐escalation manner in the extension studies, whereas those on maralixibat remained on their final dose and were escalated to their maximum tolerated doses in the extension studies. Due to procedural issues in the transition of the protocols to the long‐term follow‐up, some participants had a pause in study drug administration, which was followed by a blinded dose escalation over 4 weeks at the re‐initiation of study drug administration (Figure S1A,B). A priori, enhanced monitoring criteria and stopping guidelines for total bilirubin (TB) and alanine aminotransferase (ALT) levels were established for potential drug induced liver injury in the setting of pre‐existing liver disease (Table S3).
FIGURE 1.
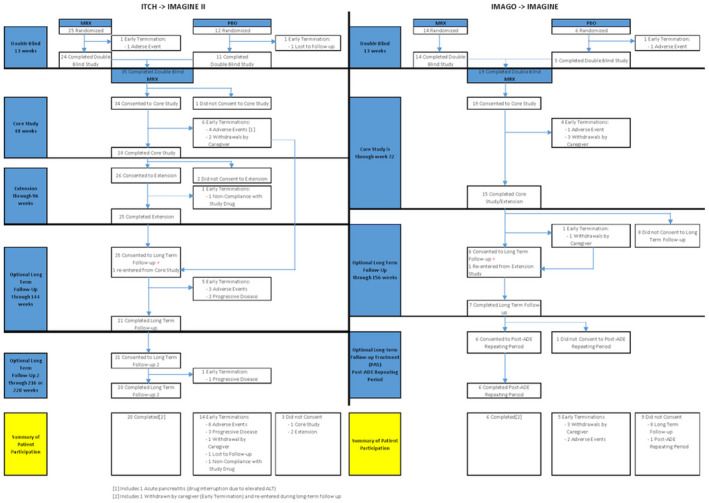
Flow diagram of study schemas and subject disposition. Left: ITCH transitioning to IMAGINE II; right: IMAGO transitioning to IMAGINE.
Written informed consent was obtained from caregivers, and assent was obtained when appropriate from the child according to local institutional review board (IRB) rules. These studies were approved by local IRBs and Ethics Committees and complied with the Declaration of Helsinki and Good Clinical Practice Guidelines. The studies were registered at ClinicalTrials.gov.
A number of endpoints were examined during the 13‐week double‐blind trials and up to 220 (IMAGINE II) or 288 weeks (IMAGINE) in the extension studies at approximately 12‐week intervals. Week 0 was set at the initiation of IMAGINE or IMAGINE II; therefore, the initiation of study drug administration in ITCH and IMAGO occurred at week −13. Endpoints included change in pruritus as measured by ItchRO(Obs) and the clinician scratch scale (CSS). Change in QoL was assessed using PedsQL total score‐parent and subscores for the multidimension fatigue (MFS) and family impact total scale modules, which may be major factors impacted by pruritus in ALGS.[ 8 ] A priori, clinically significant improvements in ItchRO(Obs) and CSS were set at ≤−1 and at ≥+10 for the QoL measurements. Other endpoints included total serum bile acids (SBA), total serum cholesterol, ALT, aspartate aminotransferase (AST), gamma‐glutamyl transpeptidase (GGT), TB, platelet count, AST‐to–platelet ratio index, and albumin. Growth and nutriture were assessed by examining changes in weight, height, and body mass index (BMI) z‐scores, along with fat soluble vitamin levels assessed as previously described.[ 9 ]
Treatment‐emergent adverse events (AEs), serious adverse events (SAEs), treatment discontinuations due to AEs, and AEs of special interest (e.g., gastrointestinal [GI] symptoms, liver injury, fat‐soluble vitamin level abnormalities) were analyzed to characterize the safety and tolerability of maralixibat.
Statistical methods
The focus of analyses is on the long‐term effect of maralixibat on efficacy and safety outcomes during the extension studies; thus, most of the summaries pool data from all 4 studies across all doses of maralixibat (ranging from 140 to 560 μg/kg/d) without consideration of the original randomized treatment given during the 13‐week double‐blind studies.
Changes from baseline (pretreatment at Week −13) to Weeks 48, 72, and end‐of‐treatment (EOT) efficacy outcomes (after week 48) in the extension studies are summarized descriptively and graphically using observed cases. These 3 time points were selected because (1) Week 48 reflects the approximate 1 year experience on maralixibat, avoids drug interruptions due to protocol administrative delays, which affected 18 participants, and allows sufficient maralixibat drug exposure for participants originally randomized to placebo to respond; (2) Week 72 reflects the end of the original follow‐up period in IMAGINE, after which a number of participants declined further participation, and the middle of the second follow‐up period in IMAGINE II; and (3) EOT reflects the experience of participants who have at least 48 weeks of treatment in IMAGINE or IMAGINE II and provides the most long‐term measures of drug response.
To estimate and test the significance of the change from baseline to Week 48, we fit linear mixed‐effects models with random subject‐specific intercepts and slopes for changes from baseline controlling for study (ITCH/IMAGINE II or IMAGO/IMAGINE), week (0, 2, 4, 8, 12, 24, 36, and 48), age at baseline (years), and baseline level of the dependent variable for each efficacy outcome. Least squares (adjusted) mean, SEM, 95% confidence interval, and p value are reported for each outcome. Multiple imputation was used to address missing data.
For the safety analysis, treatment‐emergent AEs, SAEs, AEs resulting in early discontinuations, and AEs of special interest are summarized as the number of events, number of participants with at least 1 event, and rates (per person‐year of follow‐up) during the course of the studies. Potential impact on markers of liver injury were assessed by examining TB and ALT over time and by the application and modification of evaluation of drug‐induced serious hepatotoxicity (DISH) plots.[ 10 ] To address the relationship of pruritus measures and serum bile acid, scatterplots and Spearman correlations (with p value based on Fisher's z transformation) were provided. Mean ± SD are reported unless otherwise specified. Analyses were performed using SAS 9.4 (Cary, NC) or R 4.0.5 (Vienna, Austria).
RESULTS
Baseline characteristics, study conduct, and participant retention
Fifty‐seven participants with ALGS with a mean age of 6.5 years were enrolled in randomized placebo‐controlled trials of maralixibat (IMAGO, n = 20; ITCH, n = 37) between August 20, 2013, and July 20, 2016 (Tables S4 and S5). The characteristics of the participants were similar in IMAGO and ITCH, with the exception of fewer Black and Hispanic participants in IMAGO. According to enrollment criteria, participants had severe pruritus at week −13 baseline (ItchRO[Obs] 2.9 ± 0.7 and CSS 2.9 ± 0.9), which was accompanied by markedly reduced QoL (Peds QL 62.7 ± 19.7, MFS 58.4 ± 20.8, and family impact total scale 61.2 ± 20.5). Serum liver biochemistries were typical for severe cholestasis in ALGS (TB 4.9 ± 5.7 mg/dl, cholesterol 430.2 ± 366.8 mg/dl, ALT 147.2 ± 79.1 IU/L, GGT 479.1 ± 363.8 IU/L, and SBA 234.7 ± 202.3 μM). Fat‐soluble vitamin insufficiency was frequent at baseline (47.2% with any insufficiency) (Figure 2 and Table S5). Symmetric reduction in height and weight for age (height z‐ score −1.7 ± 1.2, weight z‐score −1.4 ± 1.0), which is common in ALGS, was noted at baseline.
FIGURE 2.
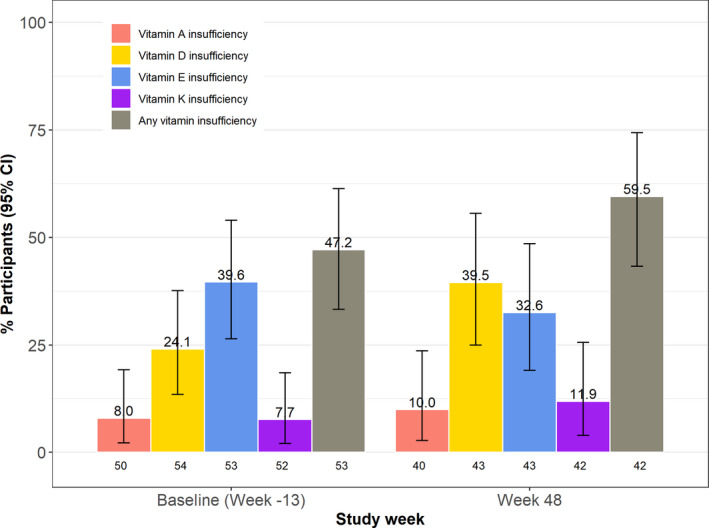
Fat‐soluble vitamin insufficiency at baseline and Week 48. The percentage of participants with the specified vitamin insufficiency is shown with 95% confidence intervals (CIs) indicated by the error bars.
Fifty‐three of the participants enrolled in the extension studies of maralixibat (IMAGO → IMAGINE n = 19; ITCH → IMAGINE II, n = 34; Figure 1). The complex pattern of adjustment in dosing of maralixibat is seen in Figure S1A,B. Study drug administration continued according to protocol for 45 of the 57 participants through week 48. After week 48 in IMAGINE II and week 72 in IMAGINE, 12 participants did not consent to the next follow‐up phase in the extension studies (IMAGINE, n = 10; IMAGINE II, n = 2). After week 48, 18 participants had interruption of study drug administration for between 3 and 55 weeks. Twenty‐six of the participants completed the study. Overall, the median follow‐up time in these studies was 3.9 years (minimum 0.5, maximum 6.7 years; Table S6). By 24 weeks, change in ItchRO and CSS relative to baseline was similar in participants who received either placebo or maralixibat in the placebo‐controlled phase of the study (Figure S2A,B). As such, participants originally receiving placebo and maralixibat were combined as a single group for analyses of efficacy outcomes after Week 24.
Week 48 outcomes
Clinically and statistically significant improvements in pruritus and QoL relative to baseline were observed at Week 48 using multiple imputation (Table 1 and Table S7). One point or greater reduction in ItchRO and CSS was observed in 73% and 68% of the participants, respectively, at Week 48 (Table S7 and Figure S3). Five of the 13 participants with cutaneous mutilation (CSS = 4) at baseline had a complete resolution to no pruritus (CSS = 0) at Week 48 (Figure 3). Clinically significant 10 point or greater increases in PedsQL and the multidimensional fatigue and family impact scales were observed in 45%, 52%, and 56% of participants, respectively (Table S7). Mean SBA and cholesterol levels were significantly reduced (−80 μM and −75 mg/dl, respectively) from baseline, while TB was unchanged. There was no correlation between change in SBA and either change in ItchRO or change in CSS (Figure S4A,B). Serum ALT increased significantly relative to baseline (+38 IU/L), whereas GGT did not change (Table 1 and Table S8). At Week 48, platelet count fell significantly (−38 × 103/μl). Height z‐score was marginally higher, although weight and BMI z‐scores were unchanged. Mean levels of the vitamins A, D, and E did not change, whereas there were some changes in sufficiency status over time (Figure 2; Tables S8 and S9).
TABLE 1.
Change from baseline to Week 48 in key outcomes (combined studies and multiple imputation)
| Characteristics | Baseline a | Adjusted b change from baseline to Week 48 (multiple imputation) c (n = 57) d | p value |
|---|---|---|---|
| ItchRO(Obs) | 2.7 [2.4, 3.3] | −1.59 (0.11) [−1.81, −1.36] | <0.0001 |
| CSS | 3.0 [2.0, 4.0] | −1.36 (0.16) [−1.67, −1.05] | <0.0001 |
| Serum bile acid (umol/L) | 181.1 [83.4, 329.0] | −79.88 (17.60) [−114.57, −45.19] | <0.0001 |
| PedsQL total, parent | 63.5 [47.8, 78.2] | 10.17 (2.88) [4.48, 15.86] | 0.0006 |
| Multidimensional fatigue scale | 58.6 [45.8, 72.2] | 13.97 (3.09) [7.85, 20.08] | <0.0001 |
| TB (mg/dl) | 2.1 [0.9, 7.4] | 0.26 (0.58) [−0.89, 1.41] | 0.6567 |
| Total cholesterol (mg/dl) | 309.5 [234.0, 443.0] | −74.78 (30.58) [−135.66, −13.91] | 0.0167 |
| ALT (U/L) | 130.0 [91.0–189.0] | 38.13 (10.69) [17.08, 59.18] | 0.0004 |
| Albumin (g/dl) | 4.5 [4.3–4.7] | 0.04 (0.05) [−0.06, 0.14] | 0.423 |
| Platelet (103/ul) | 267.0 [217.0–382.0] | −38.53 (14.88) [−68.82, −8.23] | 0.0143 |
| Height z‐score | −1.6 [−2.5, −1.0] | 0.34 (0.16) [0.01, 0.67] | 0.0463 |
| Weight z‐score | −1.3 [−2.0, −0.6] | 0.20 (0.13) [−0.06, 0.46] | 0.1245 |
Median [Q1, Q3].
Least squares mean, SEM, 95% CI, and p value based on separate linear mixed models (random intercept and slope for each subject) for each characteristic, with dependent variable as the change from baseline controlling for study (ITCH/IMAGINE II or IMAGO/IMAGINE), week (0, 2, 4, 8, 12, 24, 36, and 48), age at baseline, and baseline level of the dependent variable as covariates.
Ten imputed data sets were generated using the multivariate normal distribution (MVN) method for continuous measures and the fully conditional specification (FCS) method for categorical methods. The multiple imputation model included all characteristics at weeks −13, 0, 2, 4, 8, 12, 24, 36, and 48, study (ITCH/IMAGINE II or IMAGO/IMAGINE), age at baseline (years), and sex.
Mean (SEM) [95% CI].
FIGURE 3.
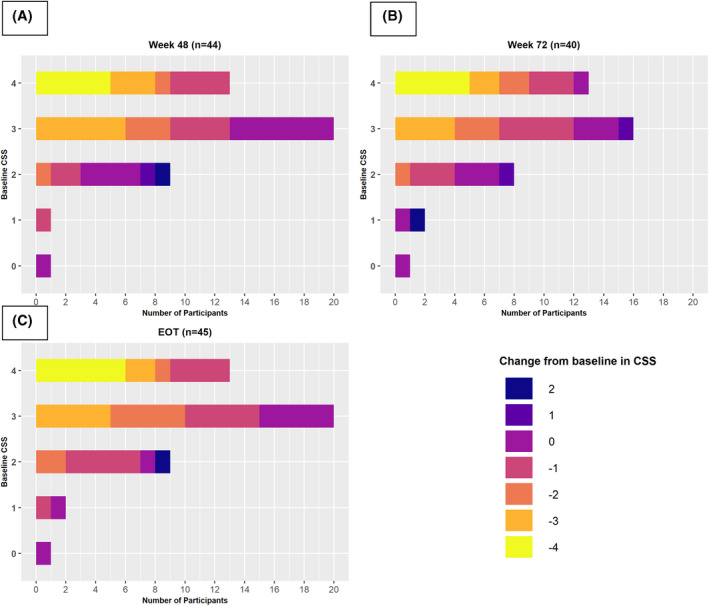
Heatmap of change in clinician scratch score (CSS) at Weeks 48 (A) and 72 (B) and end of treatment (EOT) (C) by baseline CSS. The number of participants with changes as noted by the legend are relative to differing baseline CSS.
Extended treatment responses
The changes that were observed at Week 48 were essentially maintained by Week 72 or at EOT (Table 2 and Table S10). Among observed cases, the mean reduction in ItchRO(Obs) extended from Week 48 to Week 72 [ItchRO[Obs] −1.61 [Week 48], −2.00 [Week 72]). There were changes in vitamin sufficiency status at the EOT relative to baseline (sufficient → insufficient: vitamin A, 8 of 37; vitamin E, 1 of 29; vitamin D, 5 of 34; vitamin K, 7 of 39; and insufficient → sufficient: vitamin A, 2 of 3; vitamin E, 4 of 13; vitamin D, 1 of 9; vitamin K, 0 of 4) (Table S9). At EOT, most (n = 34) of the 45 participants at stable dosing after Week 48 were receiving 280 mcg/kg/d of maralixibat, whereas 6 and 5 participants, respectively, were receiving lower or higher doses (Table S11). There were monotonic increases in the change in ItchRO(Obs), cholesterol, ALT, height z‐score, and weight z‐score with increasing dosage of maralixibat at EOT. Conversely, monotonic decreases in the change of CSS and SBA with increasing dosage of maralixibat were observed at EOT.
TABLE 2.
Change from baseline to Week 48, Week 72, and EOT in key outcomes (combined studies and observed cases)
| Characteristics | Change from baseline to Week 48 (observed cases) (n = 45) Mean (SEM) 95% CI | Change from baseline to Week 72 a (observed cases) (n = 41) | Change from baseline to EOT b (observed cases) (n = 45) |
|---|---|---|---|
| ItchRO(Obs) | 42 | 16 | 45 |
| −1.61 (0.16) | −2.00 (0.25) | −1.88 (0.15) | |
| −1.92, −1.30 | −2.48, −1.52 | −2.18, −1.58 | |
| CSS | 44 | 40 | 45 |
| −1.48 (0.23) | −1.43 (0.25) | −1.64 (0.21) | |
| −1.93, −1.02 | −1.91, −0.94 | −2.06, −1.23 | |
| Serum bile acid (umol/L) | 43 | 37 | 45 |
| −62.43 (15.82) | −57.61 (16.50) | −74.68 (15.05) | |
| −93.43, −31.43 | −89.95, −25.27 | −104.18, −45.18 | |
| PedsQL total, parent | 42 | 30 | 44 |
| 10.15 (2.57) | 10.69 (2.63) | 8.31 (2.61) | |
| 5.10, 15.19 | 5.54, 15.85 | 3.21, 13.42 | |
| Multidimensional fatigue scale | 35 | 25 | 39 |
| 14.33 (3.16) | 12.97 (2.70) | 11.27 (3.07) | |
| 8.14, 20.51 | 7.69, 18.26 | 5.25, 17.29 | |
| Bilirubin (mg/dl) | 44 | 37 | 45 |
| 0.18 (0.33) | −0.30 (0.25) | −0.05 (0.47) | |
| −0.46, 0.83 | −0.79, 0.19 | −0.98, 0.87 | |
| Cholesterol (mg/dl) | 42 | 35 | 43 |
| −31.19 (14.82) | −38.57 (21.14) | −64.58 (17.76) | |
| −60.23, −2.15 | −80.00, 2.86 | −99.39, −29.77 | |
| ALT (U/L) | 44 | 37 | 45 |
| 49.57 (11.34) | 50.19 (10.18) | 42.22 (14.20) | |
| 27.35, 71.79 | 30.23, 70.15 | 14.40, 70.05 | |
| Albumin (g/dl) | 44 | 37 | 45 |
| −0.04 (0.05) | −0.02 (0.05) | −0.10 (0.06) | |
| −0.14, 0.06 | −0.11, 0.07 | −0.21, 0.02 | |
| Platelet (103/ul) | 42 | 36 | 45 |
| −32.24 (10.59) | −37.28 (9.80) | −62.53 (13.85) | |
| −53.00, −11.47 | −56.49, −18.07 | −89.68, −35.38 | |
| Height z‐score | 44 | 39 | 45 |
| 0.22 (0.06) | 0.29 (0.08) | 0.29 (0.09) | |
| 0.11, 0.34 | 0.13, 0.44 | 0.11, 0.47 | |
| Weight z‐score | 44 | 39 | 45 |
| 0.13 (0.08) | 0.17 (0.10) | 0.13 (0.13) | |
| −0.02, 0.28 | −0.02, 0.37 | −0.12, 0.38 |
Week 72 is observed week.
For EOT analysis, only participants who have at least 48 weeks in IMAGINE or IMAGINE II are included. The EOT data were obtained as the last value that is before the date of last treatment dose +7 days.
Safety
During the median follow‐up of 3.9 years, there were no deaths and two liver transplants in the four studies. Fifty‐two participants (91%) had treatment‐emergent AEs; and in IMAGINE and IMAGINE II, participants averaged seven AEs per person year (Table 3). Treatment‐emergent GI AEs occurred in 42 participants (74%); 90% were mild, and the rate in the randomized phase of the studies was identical in participants receiving placebo or maralixibat (Table S12). There were a total of 33 treatment‐emergent SAEs in the extension studies (Table 3 and Table S13). Maralixibat was discontinued in response to 10 treatment‐emergent AEs in 9 participants in all studies (Tables S14 and S15). In 8 of 10 AEs, it was determined by the investigator that the AE was at least possibly related to study treatment. Liver test abnormalities led to discontinuation in 6 of 9 participants (Tables S14 and S15). Serum ALT levels were quite variable during the course of these studies (Figure S5A–H). Applying DISH plots to baseline parameters, 44% of participants started in the Hy's Law range, whereas 32% were in the Temple's corollary range (Figure 4A), making the use of eDISH problematic as a means of assessing potential serious hepatotoxicity (Figure 4B and Figure S6B). Given the high baseline ALT and TB for many participants, the modified hybrid DISH plot approach revealed no participants in the Hy's Law quadrant, although some moved to the Temple's corollary quadrant (Figure 4C,D and Figure S6C,D). The relationship of the timing of study drug discontinuation to the DISH plots and related changes in ItchRO(Obs) and CSS is found in Table S16. During the course of the studies, 42% of the participants met the a priori defined enhanced monitoring criteria for ALT or TB (Table S17). Three participants met the a priori defined liver test–related stopping guidelines, and study drug was discontinued in 1 participant (Figure S7A–C).
TABLE 3.
Treatment‐emergent serious adverse events and adverse events by treatment (combined studies)
| Characteristic | ITCH/IMAGO | IMAGINE II/IMAGINE | Combined IMAGINE II/IMAGINE | ||
|---|---|---|---|---|---|
| MRX | PBO | MRX‐MRX | PBO‐MRX | MRX | |
| N | 39 | 18 | 37 | 16 | 53 |
| SAEs (n) | 1 | 0 | 13 | 20 | 33 |
| Mean SAEs per subject | 0.0 | 0.0 | 0.4 | 1.3 | 0.6 |
| SAE rate (per person‐year of follow‐up) | 0.1 | 0.0 | 0.1 | 0.4 | 0.2 |
| Participants with > 1 SAE (n [%]) | 1 (2.6) | 0 (0.0) | 8 (21.6) | 4 (25.0) | 12 (22.6) |
| AEs (n) | 175 | 71 | 846 | 353 | 1,199 |
| Mean AEs per subject | 4.5 | 3.9 | 22.9 | 22.1 | 22.6 |
| AE rate (per person‐year of follow‐up) | 15.8 | 14.8 | 7.7 | 7.2 | 7.5 |
| Participants with > 1 AE (n [%]) a | 35 (89.7) | 16 (88.9) | 36 (97.3) | 16 (100.0) | 52 (98.1) |
Abbreviations: AE, adverse event; SAE serious adverse event.
If a participant started an AE/SAE on ITCH or IMAGO that continued during the extension period, the AE/SAE is counted in the ITCH or IMAGO study only. All AEs/SAEs in extension studies are included, even those after 48 weeks.
FIGURE 4.
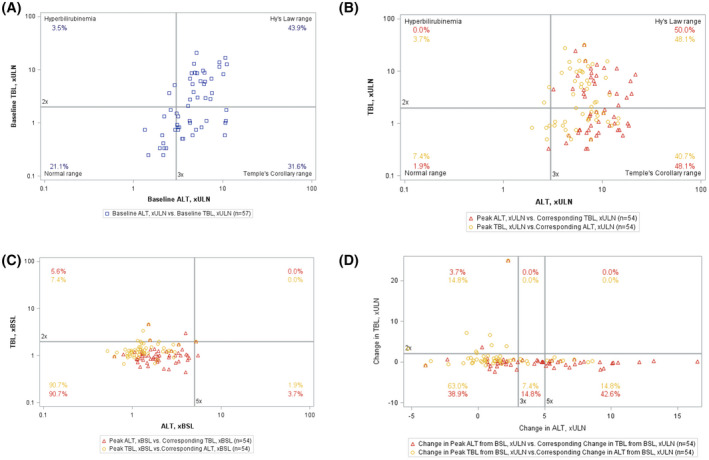
Drug‐induced serious hepatotoxicity (DISH) plot of changes in alanine aminotransferase (ALT) and total bilirubin (TB) during the course of the study. Each symbol represents a single value for a participant: Two values are possible per participant based on the peak ALT (triangle) or peak TB (circle), with co‐existing TB and ALT, respectively, used to plot the position of the box on the DISH plot. (A) Baseline values. There is only one box per participant representing TB and ALT at week −13. At baseline, more than 75% of the participants values were in either the Hy's Law or Temple's Corollary ranges. (B) eDISH plot. Values are shown as multiples of the upper limit of normal (ULN). The percentage of values in the Hy's Law or Temple's Corollary ranges increases to approximately 90% of the participants. (C) mDISH plot. Values are shown as multiples of baseline values. In this representation, there are no cases in the Hy's Law range quadrant and very few in the Temple's Corollary quadrant. (D) hDISH plot. Values are shown as the change in multiples of the ULN above the baseline value. In this representation, there are no cases in the Hy's Law range quadrant, but significant numbers of cases in the Temple's Corollary quadrant
DISCUSSION
This analysis represents combined data from two randomized double‐blind placebo‐controlled trials and their extension studies of maralixibat in children with ALGS and cholestatic pruritus. Clinically and statistically significant improvements in pruritus were observed at Week 48, which represented 60 weeks of therapy for those who received active drug in the placebo‐controlled phase of the study. A one‐point reduction in ItchRO and CSS, which is considered clinically meaningful, was observed in 73% and 68% of the observed cases, respectively, at Week 48. Furthermore, clinically meaningful increases in QoL scales (10 points or more) were observed in approximately half of the study participants. The improvement in pruritus scores in ALGS children receiving maralixibat was durable and maintained until the end of treatment, which averaged over 3 years. By the EOT in observed cases, one‐third of the children with severe pruritus (as indicated by CSS scores of 3 or 4) had complete resolution of their pruritus.
There is a growing experience with the impact of inhibiting intestinal reclamation of bile acids on pruritus in cholestatic liver disease. This is a complex area of investigation with two main stumbling blocks: limitations in the ability to precisely quantify pruritus, and the significant potential for placebo effects on symptomatology related to pruritus.[ 11 , 12 ] A cross‐over double‐blinded study demonstrated significant effects of GSK2330672 using three different measures of pruritus in adults with primary biliary cholangitis.[ 13 ] A randomized placebo‐controlled trial in adults with primary biliary cholangitis did not show a difference between maralixibat and placebo.[ 14 ] ITCH, the double‐blind placebo controlled trial in ALGS, which was part of this analysis, suggested a beneficial effect on pruritus as assessed by ItchRO(Obs).[ 6 ] A randomized placebo‐controlled withdrawal study of maralixibat in ALGS has shown a pronounced effect on serum bile acid levels and pruritus.[ 15 ] In 2021, the US Food and Drug Administration (FDA) approved the IBATi BYLVAY (odevixibat) and LIVMARLI (maralixibat) for the treatment of pruritus in progressive familial intrahepatic cholestasis and ALGS, respectively.
Laboratory and nutritional parameters were assessed as additional key outcome measures during the study period. As expected, based on the known mechanism of maralixibat to induce intestinal wasting of bile acids, total SBA and cholesterol levels were reduced at week 48. The lack of correlation of change in bile acids with measures of pruritus suggest that bile acids may not have a direct link to pruritus.[ 16 ] ALT and AST increased by approximately 20% relative to baseline, although TB and GGT were unchanged. Although direct controls are lacking for the extension period, levels of ALT were stable in a real‐world cohort of children with ALGS who met entry criteria for these studies.[ 17 ] Therefore, the increase in serum ALT in children receiving maralixibat is notable. The pathophysiology of this is unknown; it may reflect alteration in the speciation of the bile acid pool, the hepatic response to increasing bile acid synthesis in the setting of cholestasis, or alterations in the gut–liver axis signaling. A direct hepatotoxic effect is less likely, as maralixibat has very limited systemic absorption.[ 18 ] More importantly, it is not known whether there are any clinical consequences to the increase in ALT. Total bilirubin did not rise during the study period and liver failure did not occur, although 3 participants withdrew with progressive liver disease. The latter may be the natural history of severely cholestatic ALGS.[ 17 ] At Week 48, platelet count fell by 30,000 to 40,000/μl, which is in keeping with natural history data from multicenter study and real‐world data, likely reflecting progressive portal hypertension related to the underlying cholestatic liver disease.[ 3 , 17 ] Growth, as assessed by height, weight and BMI z‐scores, was not significantly altered for children receiving maralixibat. Fat‐soluble vitamin insufficiency is common in ALGS, and extended maralixibat treatment was not associated with generally increased frequencies of that insufficiency. Significant gastrointestinal side effects were uncommon in this study population during extended treatment with maralixibat and cannot be compared with a placebo control.
Liver injury is a major concern in the development of any new therapeutic regimen, especially with new pharmacologic agents. The science of the identification of drug‐induced liver injury is a somewhat new discipline and is particularly complicated in the setting of pre‐existing liver disease.[ 10 , 19 , 20 ] The cholestasis that characterized the participants in these clinical trials was more severe than most any other studies, which further complicated the approaches to monitoring and managing potential liver injury.[ 21 ] Given the known very limited systemic absorption of orally administered maralixibat, classical idiosyncratic drug hepatotoxicity was not expected. In this study, expert opinion was used to develop the a priori–enhanced monitoring and stopping guidelines. As there was little existing information on the effect of maralixibat on children with severe cholestasis, particularly in the setting of ALGS, a conservative approach was taken in these trials to limit potential adverse effects on children. A granular understanding of the variability of ALT levels during routine clinical care of children with ALGS was not available at the outset of these studies, which also impacted the interpretation of liver tests during the trials. In these studies, ALT variability over time was high. The percentage of participants who met enhanced monitoring criteria and who had study drug discontinued for increased liver biochemical testing results, reflected in part these limitations and a concerted effort to protect this vulnerable population.
The interpretation of the changes in the liver biochemistries in these studies is complex and not definitive. A simultaneous and significant increase in ALT and TB, Hy's Law, is used to identify potentially significant hepatotoxicity. Unfortunately, the baseline ALT and TB levels in these participants precluded the standard application of eDISH and mDISH plots in the identification of Hy's Law cases. The hDISH plot used in these analyses suggests that Hy's Law–type injury did not occur in these studies. There were participants who had progressive liver disease during the studies, although that is not unexpected for this type of severe cholestatic liver disease in the setting of many years of follow‐up. The hDISH plot did reveal movement into the Temple's corollary quadrant, which is of unclear significance. This is reflected in the statistically significant increase in ALT levels at Week 48 of the studies. This increase in ALT levels over time was not seen in a cohort of children with ALGS who met entry criteria for ITCH.[ 17 ] The potential liver safety issues associated with IBATi use in treating pediatric cholestasis are reflected in FDA guidance in the prescribing information for odevixibat and maralixibat.
Several potential limitations of this analysis are noted. These four clinical trials were conducted independently in the United Kingdom and North America, without an a priori plan to combine the data. However, the study designs (both the placebo‐controlled and extension portions) were very similar with virtually identical inclusion/exclusion criteria, rendering this consolidated analytical approach valid. The study design included a maximal 30‐day follow‐up after study drug discontinuation, so it is difficult to ascertain longer‐term outcomes in participants in this study who underwent early study drug discontinuation. The results of unscheduled laboratory testing results, such as in response to enhanced monitoring for abnormal liver testing, were not available for analysis, thereby limiting some of the interpretations. Another limitation to note is the dose interruption that affected 18 participants, although this only occurred after 48 weeks of treatment, and therefore would not affect the primary analyses presented here. There was no long‐term placebo control for these studies, which makes inferential assessment of the outcomes complicated relative to the natural history of ALGS.[ 17 ] The mean follow‐up period in this study population was 3.5 years, which in the light of the natural history of ALGS makes assessment of the impact on outcomes like progressive liver disease, transplantation, and death challenging.
Here we present data supporting the efficacy and durability of maralixibat treatment in children with ALGS and cholestatic pruritus in an international cohort. Pruritus measurements improved in approximately 70% of children who were still receiving drug at 48 weeks. Half of these children also had clinically relevant improvements in QoL. In general, maralixibat was well tolerated with very few clinical side effects; however, 6 participants did discontinue treatment due to elevated serum transaminases. It is well accepted that cholestasis (symptoms and biochemical parameters) in ALGS improves in survivors with native liver during childhood. To fully appreciate the magnitude of benefit of maralixibat treatment on pruritus, an extended placebo study arm would be required. This would be unacceptable to families and an impossible study design. Therefore, a detailed natural history comparison of children with ALGS is needed to fully elucidate how much of the pruritus and QoL improvements in observed cases can be attributable to maralixibat and other IBATi treatments. Potential attribution of an impact of maralixibat or other IBATi on disease progression in pediatric cholestasis may require continued long‐term follow‐up of individuals receiving IBATi and a relevant contemporaneous natural history cohort. Future investigations of IBATi in cholestatic liver disease may need to delineate the mechanisms of action, including effects on bile acid composition, the intestinal microbiome, and the gut–liver axis.
CONFLICTS OF INTEREST
RT consults for and received grants from Mirum and Albireo. He owns stock in and consults for Rectify Therapeutics. He owns stock in Generation Bio. AB received grants from Albireo, assert Study, and PI. DK consults for, advises, is on the speaker's bureau of, and received grants from Mirum. AM and SH received grants from Mirum. RK and SK consults for Mirum, Alberio, and Intercept. KML consults for and received grants from Albireo and Mirum. She consults for Traverse. BK consults for and received grants from Mirum and Albireo. She consults for Audentes. DL advises Vertex and received grants from Abbvie, Mirum, and CF Foundation. He advises and receive grants from Gilead. RS consults for Albireo and Mirum. PR consults for MedinCell, Dicerna, Encoded, Audentes, BioMarin, Ambys, and Takeda/Vertext. He received grants from Merck, Abbvie, and Arrowhead. He consults for and received grants from Mirum, Gilead, Albiero, and Traverse.
Supporting information
Appendix S1 Supporting Figures
Appendix S2 Supporting Tables
Appendix S3 Experimental Procedures
ACKNOWLEDGMENT
Editorial assistance was provided by Shauna Leighton, a medical editor at Arbor Research Collaborative for Health.
Shneider BL, Spino CA, Kamath BM, Magee JC, Ignacio RV, Huang S, et al. Impact of long‐term administration of maralixibat on children with cholestasis secondary to Alagille syndrome. Hepatol Commun. 2022;6:1922–1933. 10.1002/hep4.1992
Funding information
Supported by Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] DK62436 and National Center for Advancing Translational Sciences [NCATS] UL1TR001422); Cincinnati Children's Hospital Medical Center, Cincinnati, OH (NIDDK DK62497 and NCATS UL1TR000077); Children's Hospital Colorado, Aurora, CO (NIDDK DK62453 and NCATS UL1TR002535); The Children's Hospital of Philadelphia, Philadelphia, PA (NIDDK DK62481); Children's Hospital of Pittsburgh, Pittsburgh, PA (NIDDK DK62466 and NCATS UL1TR000005); UCSF Children's Hospital, San Francisco, CA (NIDDK DK62500 and NCATS UL1TR000004); Saint Louis University School of Medicine, St. Louis, MO (NIDDK DK62453); Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, IN (NIDDK DK84536 and NCATS UL1TR001108); Seattle Children's Hospital, Seattle WA (NIDDK DK84575 and NCATS UL1TR000423); The Hospital for Sick Children, Toronto, Ontario (NIDDK DK103135); University of Utah, Salt Lake City, UT (NIDDK DK103140); Children's Hospital Los Angeles, Los Angeles, CA (NIDDK DK84538 and NCATS UL1TR000130); Children's Healthcare of Atlanta, Atlanta, GA (NIDDK DK062470 and NCATS UL1TR000454); Texas Children's Hospital, Houston, TX (NIDDK DK103149); King's College Hospital, London, UK; NIDDK, Bethesda, MD; and Scientific Data Coordinating Center, Ann Arbor, MI (NIDDK DK62456).
REFERENCES
- 1. Kamath BM, Baker A, Houwen R, Todorova L, Kerkar N. Systematic review: the epidemiology, natural history, and burden of alagille syndrome. J Pediatr Gastroenterol Nutr. 2018;67:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dull MM, Kremer AE. Treatment of pruritus secondary to liver disease. Curr Gastroenterol Rep. 2019;21:48. [DOI] [PubMed] [Google Scholar]
- 3. Kamath BM, Ye W, Goodrich NP, Loomes KM, Romero R, Heubi JE, et al. Outcomes of childhood cholestasis in alagille syndrome: results of a multicenter observational study. Hepatol Commun. 2020;4:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang KS, Tiao G, Bass LM, Hertel PM, Mogul D, Kerkar N, et al. Analysis of surgical interruption of the enterohepatic circulation as a treatment for pediatric cholestasis. Hepatology. 2017;65:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamath BM, Stein P, Houwen RHJ, Verkade HJ. Potential of ileal bile acid transporter inhibition as a therapeutic target in Alagille syndrome and progressive familial intrahepatic cholestasis. Liver Int. 2020;40:1812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shneider BL, Spino C, Kamath BM, Magee JC, Bass LM, Setchell KD, et al. Placebo‐controlled randomized trial of an intestinal bile salt transport inhibitor for pruritus in alagille syndrome. Hepatol Commun. 2018;2:1184–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamath BM, Abetz‐Webb L, Kennedy C, Hepburn B, Gauthier M, Johnson N, et al. Development of a novel tool to assess the impact of itching in pediatric cholestasis. Patient. 2018;11:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamath BM, Spino C, McLain R, Magee JC, Fredericks EM, Setchell KD, et al. Unraveling the relationship between itching, scratch scales, and biomarkers in children with alagille syndrome. Hepatol Commun. 2020;4:1012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shneider BL, Magee JC, Bezerra JA, Haber B, Karpen SJ, Raghunathan T, et al. Efficacy of fat‐soluble vitamin supplementation in infants with biliary atresia. Pediatrics. 2012;130:e607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merz M, Lee KR, Kullak‐Ublick GA, Brueckner A, Watkins PB. Methodology to assess clinical liver safety data. Drug Saf. 2014;37((Suppl 1)):S33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartels DJ, van Laarhoven AI, van de Kerkhof PC, Evers AW. Placebo and nocebo effects on itch: effects, mechanisms, and predictors. Eur J Pain. 2016;20:8–13. [DOI] [PubMed] [Google Scholar]
- 12. Schoch D, Sommer R, Augustin M, Stander S, Blome C. Patient‐reported outcome measures in pruritus: a systematic review of measurement properties. J Invest Dermatol. 2017;137:2069–77. [DOI] [PubMed] [Google Scholar]
- 13. Hegade VS, Kendrick SF, Dobbins RL, Miller SR, Thompson D, Richards D, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double‐blind, randomised, placebo‐controlled, crossover, phase 2a study. Lancet. 2017;389:1114–23. [DOI] [PubMed] [Google Scholar]
- 14. Mayo MJ, Pockros PJ, Jones D, Bowlus CL, Levy C, Patanwala I, et al. A randomized, controlled, phase 2 study of maralixibat in the treatment of itching associated with primary biliary cholangitis. Hepatol Commun. 2019;3:365–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzales E, Hardikar W, Stormon M, Baker A, Hierro L, Gliwicz D, et al. Efficacy and safety of maralixibat treatment in patients with Alagille syndrome and cholestatic pruritus (ICONIC): a randomised phase 2 study. Lancet. 2021;398:1581–92. [DOI] [PubMed] [Google Scholar]
- 16. Chen Y, Wang ZL, Yeo M, Zhang QJ, Lopez‐Romero AE, Ding HP, et al. Epithelia‐sensory neuron cross talk underlies cholestatic itch induced by lysophosphatidylcholine. Gastroenterology. 2021;161:301–317.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shneider BL, Kamath BM, Magee JC, Goodrich NP, Loomes KM, Ye W, et al. for the Childhood Liver Disease Research Network (ChiLDReN). Use of funded multicenter prospective longitudinal databases to inform clinical trials in rare diseases—Examination of cholestatic liver disease in Alagille syndrome. Hepatol Commun. 2022; 1–12. 10.1002/hep4.1970 [DOI] [PMC free article] [PubMed]
- 18. Mirum Pharmaceuticals Inc . Livmarli prescribing information. Foster City, CA; 2021. [Google Scholar]
- 19. Chalasani N, Regev A. Drug‐induced liver injury in patients with preexisting chronic liver disease in drug development: how to identify and manage? Gastroenterology. 2016;151:1046–51. [DOI] [PubMed] [Google Scholar]
- 20. Hey‐Hadavi J, Seekins D, Palmer M, Coffey D, Caminis J, Abdullaev S, et al. Overview of causality assessment for drug‐induced liver injury (DILI) in clinical trials. Drug Saf. 2021;44:619–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palmer M, Regev A, Lindor K, Avigan MI, Dimick‐Santos L, Treem W, et al. Consensus guidelines: best practices for detection, assessment and management of suspected acute drug‐induced liver injury occurring during clinical trials in adults with chronic cholestatic liver disease. Aliment Pharmacol Ther. 2020;51:90–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Figures
Appendix S2 Supporting Tables
Appendix S3 Experimental Procedures


