Abstract
Objectives
To investigate the association between intraprostatic, intratumoral maximum standardised uptake values (SUVmax) on prostate‐specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer (PCa) prior to robot‐assisted radical prostatectomy (RARP) and pathology outcomes, including pathological International Society of Urological Pathology score (pISUP) and lymph node (LN) status (pN0/pN1).
Patients and Methods
A bi‐centric, secondary analysis of two previous, prospective cohort studies was performed in 318 patients with biopsy confirmed PCa and who were scheduled for RARP. Before surgery, patients received a PSMA PET/CT with either 68Ga‐PSMA‐11 (59% of the patients) or 18F‐PSMA (DCFPyL; 41%) as radiotracer. PET/CT images were analysed both visually and semi‐quantitatively by measuring the SUVmax of the most intense suspect lesion in the prostate. The association between the SUVmax of the primary tumour and pre‐ and postoperative variables was analysed.
Results
The SUVmax was associated with clinical and biopsy preoperative variables, as well as with pISUP score and pathological tumour stage. Patients with a pISUP of ≤2 showed significantly lower SUVmax compared to patients with a pISUP of >2 for both tracers (SUVmax 18F‐PSMA: median 5.1 vs 9.6, P = 0.002; SUVmax 68Ga‐PSMA‐11: 6.6 vs 8.6, P = 0.003). Moreover, patients with pN1 had significantly higher median SUVmax than those with pN0/pNx for both tracers (SUVmax 18F‐PSMA: 7.9 vs 12.3, P = 0.04; SUVmax 68Ga‐PSMA‐11: 7.6 vs 12.0, P < 0.001). On multivariable logistic regression analysis, the intraprostatic SUVmax was an independent predictor of pN1 for both 68Ga‐PSMA‐11 (per doubling: odds ratio [OR] 1.96, 95% confidence interval [CI] 1.27–3.01)) and 18F‐PSMA (per doubling: OR 1.79, 95% CI 1.06–3.03).
Conclusion
Intraprostatic, intratumoral PSMA intensity on PET/CT, as semi‐quantitatively expressed by SUVmax, may be a valuable innovative biomarker in patients with localised PCa, as it is highly associated with known conventional prognostic factors, such as pISUP and LN status.
Keywords: 18F‐DCFPyL, 68Ga‐PSMA‐11, prostate cancer, standardised uptake values, biomarkers, #uroonc, #PCSM, #ProstateCancer
Abbreviations
- bISUP
biopsy ISUP score
- cT
clinical tumour stage
- EANM
European Association of Nuclear Medicine
- EARL
EANM Research Ltd
- EAU
European Association of Urology
- E‐PSMA
EANM standardised reporting guidelines for PSMA‐PET
- IQR
interquartile range
- ISUP
International Society of Urological Pathology: LN
lymph node
- (eP)LND
(extended pelvic) LN dissection
- mi
molecular imaging
- mpMRI
multiparametric MRI
- MSKCC
Memorial Sloan Kettering Cancer Center
- NCI
Netherlands Cancer Institute
- (cs)PCa
(clinically significant) prostate cancer
- PET
positron emission tomography
- pISUP
pathological ISUP score
- pN
pathological LN status
- PSMA
prostate‐specific membrane antigen
- pT
pathological tumour stage
- (RA)RP
robot‐assisted radical prostatectomy
- SUV(max)
(maximum) standardised uptake values
- VOI
volume of interest
- VUmc
Amsterdam University Medical Center
Introduction
Prostate cancer (PCa) is the most frequently diagnosed cancer in men around the world [1]. Curative therapy for patients with clinically significant and localised PCa includes robot‐assisted radical prostatectomy (RARP), brachytherapy, and external beam radiation therapy [2]. Along with RARP, an extended pelvic lymph node dissection (ePLND) is usually indicated for patients with intermediate‐ and high‐risk disease stage, as a staging method for lymph node (LN) involvement [2]. The most common prognostic variables to predict the outcome of patients with PCa after treatment include the PSA level at the time of diagnosis, the biopsy International Society of Urological Pathology (bISUP) score, the percentage of positive biopsies, and the clinical tumour stage [2, 3, 4]. These variables have prognostic ability on a group level but not for the individual patient [5, 6].
Besides common clinical and pathological prognostic parameters, different imaging modalities may assist clinicians to assess outcome of disease in patients with PCa. Recently, prostate‐specific membrane antigen (PSMA) positron‐emission tomography (PET)/CT has been introduced as a valuable alternative for conventional imaging. PSMA is usually labelled with 68Gallium (e.g. 68Ga‐PSMA‐11) or 18Fluorine (e.g. 18F‐DCFPyL [PSMA]) and is highly overexpressed in PCa cells [7, 8]. PSMA PET/CT has shown diagnostic superiority in detecting PCa metastases, compared to bone scintigraphy and CT scan, especially in the recurrent stage setting of the disease [9, 10, 11]. For primary staging purposes, PSMA PET/CT has found its place in the armamentarium of the urologist to select patients for different treatment options [12, 13].
Besides visual interpretation of PSMA‐PET/CT images, semi‐quantitative measurements of PSMA expression, such as by the measurement of standardised uptake values (SUV), can be extracted from the PET/CT scan, conforming with the European Association of Nuclear Medicine (EANM) standardised reporting guidelines for PSMA‐PET (E‐PSMA) [14]. SUV represents the amount of the tracer uptake in a pre‐defined anatomical region on the PET/CT, that usually normalises the lesion activity to the body weight and the injected activity of tracer [15]. Histological studies have shown that increased immunohistochemical PSMA expression is associated with higher tumour grade and disease progression [16, 17]. The semi‐quantitative measurement of the 18F‐PSMA uptake in the dominant intraprostatic lesion on PET/CT, expressed as SUV, might therefore be an alternative imaging biomarker, that, like the immunohistochemical expression of PSMA, may be associated with tumour characteristics and clinical outcome [18].
The aim of this study was to examine if intraprostatic SUV measured from both 68Ga‐PSMA‐11 and 18F‐PSMA PET/CT is associated with well‐established prognostic tumour markers, such as pathological ISUP score (pISUP), pathological tumour stage (pT) and pathological LN status (pN).
Patients and Methods
Study Design and Patient Population
This study was a bi‐centric, secondary analysis of two previous prospective cohort studies. All consecutive patients with histologically confirmed intermediate‐ to high‐risk PCa received a PSMA‐PET/CT scan before RARP, with or without an additional ePLND. Patients were included from August 2016 until August 2020. An ePLND was performed based on a ≥8% risk of LN involvement, as predicted by the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram [19], or in the presence of high‐risk features: PSA level of >20 ng/mL, ISUP score 4 and 5, or suspicion of clinical tumour stage (cT)2c or higher [2]. Patients were included in two reference centres of the Prostate Cancer Network Netherlands, the Amsterdam University Medical Center (location VUmc) and the Netherlands Cancer Institute (NCI). This study encompasses a secondary analysis based on two studies that were approved by the local medical ethical committees (review number 2017.543 [VUmc] and review number IRBdm19‐348 [NCI]). All patients signed informed consent when enrolled in the original studies, explicitly allowing secondary analysis of their study data.
Preoperative and Postoperative Variables
Preoperative parameters that were assessed included age, initial PSA level, cT, European Association of Urology (EAU) risk classification [2], bISUP, and number of (positive) cores [3]. Postoperative parameters that were assessed included: pISUP as determined in the RARP specimen, pT, pN, and surgical margin status.
PSMA‐PET/CT Imaging Protocol
For the VUmc patients, PSMA‐PET/CT imaging was performed with 18F‐DCFPyL (PSMA), a second‐generation fluorinated PSMA radiotracer. The scanner used was a Philips Ingenuity (Philips®, the Netherlands/USA) PET/CT system. 18F‐DCFPyL (PSMA) was synthesised at the on‐site cyclotron facility according to Good Manufacturing Practices and was also provided to the NCI for imaging purposes. At the NCI, PSMA‐PET/CT imaging was performed with both 68Ga‐PSMA‐11 and 18F‐PSMA tracers, using a Philips Gemini TF‐II or Vereos Digital PET/CT (Philips®, the Netherlands/USA). The 68Ga‐PSMA‐11 was radiolabelled in‐house using a fully automated system (Scintomics GmbH, Germany). All PET‐images were combined with either a low‐dose CT scan (120–140 kV, 40–80 mA) or a diagnostic CT scan (130 kV, 110 mA), for attenuation correction and anatomical localisation. All PET images were corrected for scatter, decay, and random coincidences and were conducted according to EANM Research Ltd. (EARL) standards [20].
Image Interpretation of PSMA‐PET/CT Imaging
At both centres, 18F‐PSMA and 68Ga‐PSMA‐PET/CT scans were assessed by nuclear medicine physicians (D.O., M.D.) with ample experience of reading PSMA‐PET/CT scans. The PSMA‐PET/CT scan results used in this study were based on clinical reports, which were structured in line with the E‐PSMA guidelines [14]. This means it included the location of the primary prostate lesion and possible secondary lesions, molecular imaging (mi)T stage and the presence of LN, bone or visceral metastases [21]. Imaging results were primarily based on visual interpretation, relating PSMA uptake to background uptake in the blood, liver, and salivary glands on a visual scale (0–3), as recently proposed [14].
Scan Assessment, SUV Assessment
For semi‐quantitative analysis, the SUVmax was measured for the most clinically suspect prostatic lesion of each patient and was normalised for body weight. SUVmax was chosen because it does not require exact tumour borders as compared to SUVmean [22] and therefore is clinically most used. Suspect lesions were delineated according to the available clinical reports describing the dominant intraprostatic lesion. SUVmax was measured according to the E‐PSMA criteria and was compliant with EARL standards [14, 15]. Volumes‐of‐interest (VOIs) were manually drawn at least 1.5 cm in diameter over the index lesion, carefully omitting physiological activity from the urethra or bladder. If no PSMA expression suspect for PCa was detected by the nuclear medicine physician, a VOI was drawn over the prostate location corresponding with a suspect lesion on multiparametric MRI (mpMRI), when available. Available clinical software of the Intellispace Portal (Philips®, the Netherlands/USA) and Osirix MD (Pixmeo SARL, Switzerland) were used to calculate the SUVmax. To cross‐validate both measuring software programs, identical scans from four patients were analysed with both programs, with 100% agreement.
Statistical Analysis
The primary outcome of this study was to determine if intraprostatic, intratumoral SUVmax is a prognostic variable associated with known pathological prognosticators of PCa, such as pISUP score, pT stage, and pN stage. To compare the medians of SUVmax with ISUP grade, pT stage, surgical margin status, and pN stage, the Mann–Whitney‐Wilcoxon test was used for two groups; and the Kruskal–Wallis test was used for multiple groups. The intraprostatic SUVmax was compared to initial PSA level by linear regression analysis (R 2) using Pearson’s correlation coefficient. A multivariable logistic regression analysis with predefined preoperative variables was performed for both tracers to predict pN1 status after RARP and ePLND, including SUVmax of the dominant intraprostatic lesion. For the preoperative analysis, initial PSA level, bISUP score and cT stage were used. Numerical variables were assessed for normality using histogram analysis and were summarised with median values and interquartile ranges (IQRs), categorical variables with proportions. Significance level was set at P < 0.05. Statistical analysis was performed using the IBM® Statistical Package for the Social Sciences (SPSS®) for Windows®, version 26 (IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
A total of 318 patients were included in this study. All patients received a PSMA‐PET/CT before RARP of whom 288/318 (91%) underwent ePLND. Included patients had a median (IQR) age of 68.5 (62.4–72.5) years, and a median (IQR) initial PSA‐level of 10.4 (7.2–19.8) ng/mL. According to EAU guidelines, 76/318 (23.9%) patients had intermediate‐risk PCa and 242/318 (76%) high‐risk PCa. The median (IQR) MSKCC risk for pelvic LN metastases was 15% (9.7–34%). Preoperative characteristics of included patients are listed in Table 1.
Table 1.
Baseline characteristics of patients undergoing PSMA PET/CT at initial staging for 129 included patients who received a 18F‐PSMA PET/CT and 189 patients who received a 68Ga‐PSMA‐11 PET/CT.
| Baseline characteristics | ||
|---|---|---|
| Median (IQR) | 18F‐DCFPyL (N = 129) | 68Ga‐PSMA‐11 (N = 189) |
| Age, years | 67 (62–71) | 69 (65–74) |
| Prostate volume, mL | 40 (33–60) | 45 (38–61) |
| Initial PSA level, ng/mL | 10.5 (7.2–20.0) | 10.3 (7.3–19.3) |
| Positive biopsy cores, % of total cores | 42 (25–71) | 38 (13–67) |
| MSKCC risk of LN metastases | 16 (11–31) | 15 (9–36) |
| N (%) | ||
|---|---|---|
| EAU risk category [2], n (%) | ||
| Intermediate | 40 (31) | 36 (19) |
| High | 89 (69) | 153 (81) |
| Total | 129 (100) | 189 (100) |
| bISUP score * , n (%) | ||
| 1 | 3 (2) | 16 (9) |
| 2 | 32 (25) | 31 (16) |
| 3 | 39 (30) | 41 (22) |
| 4 | 35 (27) | 67 (35) |
| 5 | 20 (16) | 34 (18) |
| Total | 129 (100) | 189 (100) |
| cT stage, n (%) | ||
| 1c | 46 (36) | 68 (36) |
| 2a/b | 29 (23) | 41 (22) |
| 2c | 21 (16) | 51 (27) |
| 3a | 25 (19) | 17 (9) |
| 3b | 7 (5) | 12 (6) |
| Total | 129 (100) | 189 (100) |
| miN stage, n (%) | ||
| miN0 | 108 (84) | 164 (87) |
| miN1 | 21 (16) | 25 (13) |
| Total | 129 (100) | 189 (100) |
ISUP 1 = Gleason score 3 + 3 = 6.
ISUP 2 = Gleason score 3 + 4 = 7.
ISUP 3 = Gleason score 4 + 3 = 7.
ISUP 4 = Gleason score 4 + 4 = 8/Gleason score 3 + 5 = 8 /Gleason score 5 + 3 = 8.
ISUP 5 = Gleason score 4 + 5 = 9/Gleason score 5 + 4 = 9/Gleason score 5 + 5 = 10.
ISUP Definition.
Scan Characteristics
In total, 129/318 (40%) patients received a 18F‐PSMA‐PET/CT scan before surgery and 189/318 (59%) patients received a 68Ga‐PSMA‐11‐PET/CT. The 18F‐PSMA‐PET/CT images were acquired at a median (IQR) of 118 (90–123) min after intravenous injection of a median (IQR) dose of 305.4 (240.2–318.2) MBq. A median (IQR) dose of 98.7 (92.4–104.5) MBq 68Ga‐PSMA‐11 was administered, and scanning started at a median (IQR) of 48 (44–53) min after injection.
Postoperative Tumour Features
The pathological features after RARP and ePLND are listed in Table 2. When comparing bISUP score to pISUP score, there was under grading of the bISUP in 121/318 (38%) patients, and over grading of bISUP in 59/318 (19%). A total of 112/318 (35%) patients who underwent RARP had positive surgical margins (R1), vs 203/318 (64%) who had free surgical margins (R0). In 68/288 (24%) patients undergoing ePLND, pN1 status was detected at pathological examination. An overview of the diagnostic accuracy of 18F‐PSMA PET/CT and 68Ga‐PSMA PET/CT is depicted in Table S1.
Table 2.
Characteristics of patients undergoing RARP and EPLND for 129 included patients who received a 18F‐DCFPyL PET/CT and 189 patients who received a 68Ga‐PSMA‐11 PET/CT.
| Pathology results |
18F‐PSMA N (%) |
68Ga‐PSMA‐11 N (%) |
|---|---|---|
| Pathological ISUP score [2] * | ||
| 1 | 3 (2.3) | 3 (1.6) |
| 2 | 44 (34) | 67 (35) |
| 3 | 49 (38) | 68 (36) |
| 4 | 7 (5.4) | 20 (10) |
| 5 | 24 (19) | 30 (16) |
| n.a. † | 2 (1.6) | 2 (1.1) |
| Total | 129 (100) | 189 (100) |
| pT stage | ||
| pT2 | 58 (45) | 87 (46) |
| pT3a | 43 (33) | 54 (29) |
| pT3b | 25 (19) | 48 (25) |
| n.a. † | 2 (1.6) | 2 (1.1) |
| Total | 129 (100) | 189 (100) |
| pN stage | ||
| N0 | 102 (79) | 118 (62) |
| N1 | 21 (16) | 47 (25) |
| Nx | 6 (4.7) | 24 (13) |
| Total | 129 (100) | 189 (100) |
| Surgical margin status (R) | ||
| R0 | 85 (63) | 119 (63) |
| R1 | 42 (31) | 70 (25) |
| n.a. † | 2 (1.6) | 2 (1.1) |
| Total | 129 (100) | 189 (100) |
n.a., not available.
ISUP 1 = Gleason score 3 + 3 = 6.
ISUP 2 = Gleason score 3 + 4 = 7.
ISUP 3 = Gleason score 4 + 3 = 7.
ISUP 4 = Gleason score 4 + 4 = 8/Gleason score 3 + 5 = 8/Gleason score 5 + 3 = 8.
ISUP 5 = Gleason score 4 + 5 = 9/Gleason score 5 + 4 = 9/Gleason score 5 + 5 = 10.
ISUP definition.
In two patients, ePLND was successfully performed, yet surgical removal of the prostate proved unfeasible due to extensive intraoperative bleeding.
Associating Intraprostatic SUVmax with Initial PSA level and Postoperative Outcomes
Patients who received an 18F‐PSMA‐PET/CT scan before surgery had a median (IQR) SUVmax of the intraprostatic dominant lesion of 7.8 (5.8–13.8). A clinical example of a patient receiving both a 18F‐PSMA‐PET/CT and RARP with corresponding intraprostatic SUVmax is shown in Fig. 1. For patients who received 68Ga‐PSMA‐11‐PET/CT, the median (IQR) SUVmax of the intraprostatic dominant lesion was 8.1 (4.9–14.5). On univariable analysis, initial PSA level showed a statistically significant, but weak correlation with SUVmax for both tracers ((18F‐PSMA: R 2 = 0.09, P < 0.001; 68Ga‐PSMA‐11: R 2 = 0.02, P < 0.03), as shown in Fig. 2.
Fig. 1.
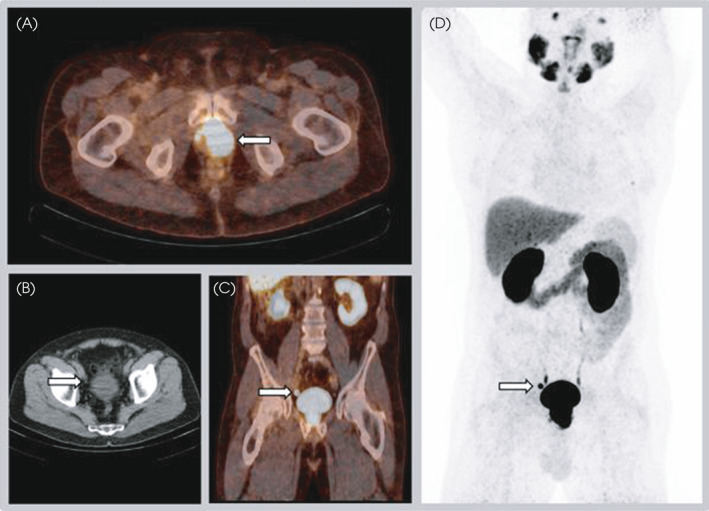
A 70‐year‐old man with cT2a, ISUP 2 (systematic TRUS biopsy) PCa and initial PSA level of 11 ng/mL was considered a candidate for RP with ePLND. The MSKCC nomogram showed an 8% risk of LN involvement. Fused 18F‐PSMA PET/CT transversal view of the pelvic region (A) revealed high local PSMA expression mostly in the left side of the prostate from the apex to base, without seminal vesicle involvement. The SUVmax of this index lesion was 36.5. Transversal view CT only (B), fused PET/CT coronal view (C), and maximum intensity projection image (D) show a right sided para‐iliacal LN measuring 7 mm, suspicious for a PCa metastasis. No suspicion of a PCa‐LN metastasis on the left side existed. Histopathological analysis showed a pT3a, pISUP 5 PCa in the RP specimen. Two LN metastases were found after histopathological analysis of 26 resected LNs, one left‐sided in the iliac region and one right‐sided iliac LN. [Colour figure can be viewed at wileyonlinelibrary.com]
Fig. 2.
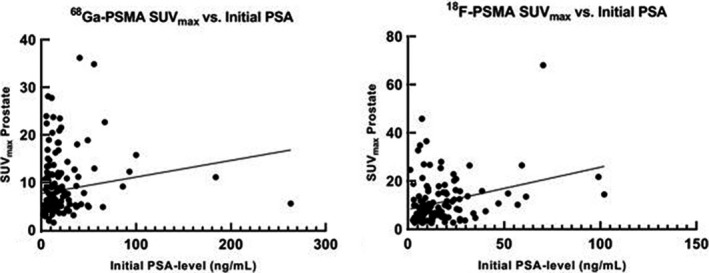
Association between initial PSA‐level and both 68Ga‐PSMA‐11 and 18F‐PSMA SUVmax. The scatter plots show the correlation between PSA level and SUVmax value of the prostate, which shows low correlation for both tracers (18F‐DCFPyL, R 2 = 0.09, P < 0.001; 68Ga‐PSMA‐11, R 2 = 0.02; P < 0.03).
When assessing pISUP, patients with pISUP ≤2 had a significantly lower SUVmax compared to patients with pISUP >2 for both tracers (SUVmax 18F‐PSMA: 5.1 vs 9.6, P = 0.002; SUVmax 68Ga‐PSMA‐11: 6.6 vs 8.6, P < 0.001). Overall, the intraprostatic SUVmax scores were statistically different for pISUP for both tracers (SUVmax 18F‐PSMA, P = 0.01; SUVmax 68Ga‐PSMA‐11, P = 0.007), as shown in Fig. 3A.
Fig. 3.
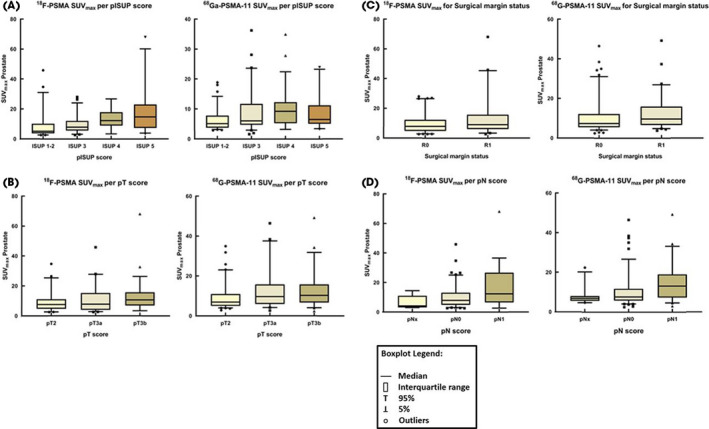
Box plots of SUVmax scores of the dominant intraprostatic lesion for both 18F‐PSMA PET/CT and 68Ga‐PSMA‐11 PET/CT, stratified by pathological ISUP scores (A), pT stage (B), surgical margin status (C), and pN stage (D). [Colour figure can be viewed at wileyonlinelibrary.com]
When assessing pT stage, patients with pT3a/b receiving 68Ga‐PSMA‐11‐PET/CT had a statistically significant higher median intraprostatic SUVmax than patients who had pT2 (6.9 vs 9.9, P = 0.01; Fig. 3B ). There was no significant difference in median SUVmax for different pT stages for 18F‐PSMA‐PET/CT (7.5 vs 8.6, P = 0.1). For patients receiving 68Ga‐PSMA‐11‐PET/CT, those with a positive surgical margin had a significantly higher median intraprostatic SUVmax than those who had negative surgical margins (9.6 vs 7.3, P = 0.009; Fig. 3C). No significant difference in median SUVmax for surgical margin status was found for 18F‐PSMA‐PET/CT (7.7 vs 9.0, P = 0.1).
Finally, patients with PCa with pN1 in the LND specimens had significantly higher median SUVmax than those with pN0/pNx for both tracers (SUVmax 18F‐PSMA: 7.9 vs 12.3, P = 0.04; SUVmax 68Ga‐PSMA‐11: 7.6 vs 12.0, P < 0.001; Fig. 3D).
The Prognostic Value of Intraprostatic SUVmax using Multivariable Analysis
When analysing preoperative parameters, including intraprostatic, intratumoral SUVmax, cT stage, PSA level, and bISUP grade, the only independent variables for the prediction of pN1 disease on multivariable analysis were 68Ga‐PSMA‐11‐PET/CT intraprostatic SUVmax (per doubling: odds ratio [OR] 1.96, 95% CI 1.27–3.01; P = 0.002), and 18F‐PSMA‐PET/CT intraprostatic SUVmax (per doubling: OR 1.79, 95% CI 1.06–3.03; P = 0.03; Table 3).
Table 3.
Multivariable logistic regression analysis to predict pN1 after RARP with ePLND in 318 patients with intermediate‐ and high‐risk PCa using predefined preoperative variables, including intraprostatic, intratumoral SUVmax, cT stage, PSA level, and bISUP grade. Effect sizes are presented as ORs with 95% CIs.
| 18F‐PSMA | 68Ga‐PSMA‐11 | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| log2 (Initial PSA value) | 0.76 (0.47–1.23) | 0.3 | 1.02 (0.74–1.41) | 0.9 |
| log2 (SUVmax prostate) | 1.79 (1.06–3.03) | 0.03 | 1.96 (1.27–3.01) | 0.002 |
| Prostate biopsy grade group according to ISUP * | ||||
| 1–2 | ||||
| 3 | 1.10 (0.26–4.68) | 0.9 | 1.07 (0.33–3.53) | 0.9 |
| ≥4 | 1.57 (0.43–5.77) | 0.5 | 1.63 (0.62–4.28) | 0.3 |
| cT stage | ||||
| cT1 | ||||
| cT2 (a,b,c) | 0.56 (0.20–1.59) | 0.3 | 1.74 (0.73–4.13) | 0.2 |
| cT3(a,b) | 0.71 (0.06–8.50) | 0.8 | 1.77 (0.59–5.35) | 0.3 |
ISUP 1 = Gleason score 3 + 3 = 6.
ISUP 2 = Gleason score 3 + 4 = 7.
ISUP 3 = Gleason score 4 + 3 = 7.
ISUP 4 = Gleason score 4 + 4 = 8/Gleason score 3 + 5 = 8/Gleason score 5 + 3 = 8.
ISUP 5 = Gleason score 4 + 5 = 9/Gleason score 5 + 4 = 9/Gleason score 5 + 5 = 10.
ISUP definition.
Discussion
The aim of this study was to examine if intraprostatic intratumoral PSMA uptake, as determined by SUVmax on PSMA‐PET/CT, is associated with conventional prognostic variables in patients with intermediate‐ to high risk primary PCa undergoing RARP. Two commonly used PSMA tracers (i.e. 68Ga‐PSMA‐11 and 18F‐PSMA) were analysed. In this study, significant associations were found between PSMA‐PET/CT median SUVmax of the dominant intraprostatic lesion and pISUP for both studied tracers, and between SUVmax and surgical margin status and pT stage for 68Ga‐PSMA‐11. Additionally, a significantly higher median PET/CT lesional SUVmax was found for positive pelvic LN status (pN1) compared to negative pelvic LN status (pN0) for both tracers. On multivariable analysis, when multiple preoperative variables were assessed for their ability to predict LN metastatic disease after surgery, the median PET/CT SUVmax of the dominant intraprostatic lesion proved to be an independent prognostic factor for pN1 disease for both tracers.
To our knowledge, we report on the first series of patients comparing SUVmax on 18F‐PSMA‐PET/CT to clinical outcomes, and on the largest 68Ga‐PSMA‐11 cohort to date [18, 23, 24]. Moreover, this study reports on the first large series of patients describing the association of SUVmax with pN status for both nuclear tracers. When the SUVmax on PSMA PET/CT of the intraprostatic lesion was assessed, patients with pISUP 1–2 had statistically lower values than those with pISUP 3–5, for both tracers (SUVmax 18F‐PSMA: 5.1 vs 9.6, P = 0.002; SUVmax 68Ga‐PSMA‐11: 6.63 vs 8.63, P < 0.001). This outcome confirms previous immunohistochemical staining studies showing that increased PSMA protein expression is associated with higher tumour grade and disease progression [16, 17]. Demirci et al. [23] retrospectively evaluated 141 patients with intermediate‐ and high‐risk primary PCa, who received 68Ga‐PSMA‐11‐PET/CT imaging before RARP. In line with our results, that study group also reported a higher mean SUVmax for pISUP 3–5 patients compared to pISUP 1–2 patients (18.9 vs 7.2, P < 0.001) [23]. Unfortunately, one of the drawbacks of that study was that it had a bi‐centric set‐up that led to a wide range of the applied 68Ga‐PSMA‐11 doses (113–384 MBq). This again might have caused inaccuracies of interpretation, as SUVmax is highly dependent on dosage [25]. Again, in a recent retrospective study by Roberts et al. [18] evaluating 71 patients with biopsy confirmed PCa who received 68Ga‐PSMA‐11‐PET/CT before RP, a strong association between pISUP ≥3 and PET/CT intraprostatic SUVmax (P = 0.01) was observed.
An association of SUVmax and pISUP is important as it is known that the bISUP is not directly comparable to pISUP due to under and over grading of the biopsy Gleason score compared to that in the RP specimen [26]. Therefore, the assessment of SUVmax on diagnostic PSMA‐PET/CT imaging might predict the pISUP more reliably before treatment than when a prediction of pISUP is made based on the bISUP and other clinical variables alone. For instance, if SUVmax predicts high pISUP in those with a low bISUP and a low initial PSA level, a more aggressive approach could be followed, whereas in those with low SUVmax, a more conservative approach or an adaption of treatment could be made. The previous PRIMARY study by Emmett et al. [27] investigated the biopsy outcomes of patients with an increased risk of PCa. In that prospective trial including 296 men, patients received a 68Ga‐PSMA PET/CT and mpMRI before prostate biopsy. The 68Ga‐PSMA PET/CT and mpMRI improved the negative predictive value and sensitivity for detecting clinically significant (cs)PCa in a mpMRI‐triaged population. In that study, SUVmax was associated with higher bISUP (P < 0.001). In fact, all men with a SUVmax of ≥12 had csPCa on prostate biopsy, independent of mpMRI findings. Furthermore, in men with mpMRI Prostate Imaging‐Reporting and Data System (PI‐RADS) 4 or 5, a SUVmax of ≥9 classified csPCa with 100% specificity, meaning that patients with a positive mpMRI and high SUVmax on PSMA PET/CT might be omitted prostate biopsy. Although our study consisted of patients with biopsy confirmed patients undergoing RARP, like Emmett et al. [27], SUVmax was highly associated with pISUP. Future randomised studies will determine whether biopsy can safely be omitted in men with a high clinical suspicion of csPCa but with low SUVmax on diagnostic PSMA PET/CT.
In the present study, significantly higher median PET/CT SUVmax of the intraprostatic lesions was found in patients with pT3a/b disease, compared to patients with pT2 disease for the 68Ga‐PSMA‐11‐PET/CT cohort (9.9 vs 6.9, P = 0.01). Again, an adaption of the treatment plan could be made if a higher‐than‐expected median SUVmax of the dominant lesion was reported on diagnostic PSMA‐PET/CT. For example, the surgical plan could be changed to perform non‐nerve sparing surgery or an ePLND in those with suspicion of capsular penetration or invasion of the seminal vesicles on PSMA PET/CT and who also have a high SUVmax of the dominant lesion.
Moreover, higher intraprostatic SUVmax on 68Ga‐PSMA‐11‐PET/CT was found in patients with positive surgical margins (R1). This association was confirmed by the study of Roberts et al. [18] who showed an independent prognostic association between SUVmax and margin status on multivariable analysis (P < 0.001). In a study by Wang et al. [28], that studied 195 patients receiving a 68Ga‐PSMA‐11‐PET/CT for initial staging before RARP, an association was reported between SUVmax and surgical margin status on univariate analysis (P = 0.04). The SUVmax was not found to be an independent variable for surgical margin status when assessed along other imaging parameters such as tumour volume, miN status, miT stage. In a multivariate analysis, only miN status was found to be an independent predictive parameter. In the present study, no significant association was found between SUVmax in the 18F‐PSMA cohort for both pT stage and surgical margin status. These findings cannot be explained by a difference in demographical or surgical characteristics between the cohorts studied with the different tracers. Possibly, the smaller sample size could have been responsible for this lack of association. When analysing the difference between the two tracers in terms of outcomes based on visual interpretation, previous studies have shown no significant differences between 18F‐PSMA and 68Ga‐PSMA‐11 [11, 29, 30].
The multivariable analysis comparing preoperative parameters to LN status showed that SUVmax remained an independent predictor for pN1 disease for both tracers (18F PET/CT per doubling: OR 1.96, 95% CI 1.27–3.01; and 68Ga PET/CT per doubling: OR 1.79, 95% CI 1.06–3.03). Preoperative parameters such as initial PSA and bISUP score were not prognostic of pN status. These findings seem to contrast with previous reports [19, 31]. The multivariable result that PSA was not a prognostic factor for positive LN status in the presence of SUVmax seems intuitive as the association between PSA values and SUVmax was only weak on univariate analysis for both tracers. This is in contrast with a study by Uprimny et al. [24], who studied 90 patients receiving a 68Ga‐PSMA‐11‐PET/CT for initial staging, and who found a stronger association between PET/CT intraprostatic SUVmax and the initial PSA level (R = 0.506, P < 0.001). When analysing the postoperative variables in the multivariable analysis in our study to predict pN1 status, significant predictors in the 68Ga‐PSMA‐11‐PET/CT cohort included the intraprostatic SUVmax as well as the known predictors such as positive surgical margin status, pISUP >3, and the presence of pT3. For the 18F‐PSMA‐PET/CT cohort, only pT3 stage was a significant predictor for the pN1 status, which can be explained by the discrepancies displayed in the cohort before.
As a limitation, the histopathological LN metastasis rate might have been underreported due to unresected LN metastases on surgical excision or due to undetected LNs on pathological examination. However, this is not a study‐specific limitation, but rather a general limitation that is inherent to the ePLND template and pathological examination. As a final point, the delineation of VOIs by two observers can result in inter‐observer variability, due to the manually drawn in mask around the PSMA‐avid dominant intraprostatic lesion to rule out bladder activity interference. The validation of the mask by a second observer was deployed to reduce inter‐observer variability. Also, as a validation of the method, the delineations were performed by both observers in four cases, which resulted in a 100% agreement of SUVmax level.
Based on present results, SUVmax could have a role in predicting pathological tumour characteristics and clinical outcomes in patients with intermediate‐ to high risk primary PCa undergoing PSMA‐PET/CT imaging before radical surgery. For future studies, intraprostatic SUVmax, as measured on PSMA‐PET/CT, could be an addition to future initial staging nomograms, thereby helping the clinician in treatment selection and shared decision‐making [19, 32].
Conclusion
This study evaluated the prognostic value of intraprostatic, intratumoral SUVmax for 68Ga‐PSMA‐11 and 18F‐PSMA‐PET/CT before RARP. An association was found between median intraprostatic SUVmax on PET/CT and conventional prognostic tumour characteristics, such as pISUP and pN stage in patients with primary intermediate‐ to high‐risk PCa. The SUVmax remained an independent predictive factor for pN1 status on multivariable analysis. Therefore, PSMA‐PET/CT has the potential to be of value for the preoperative prediction of intraprostatic tumour aggressiveness features. Further research is needed to examine the prognostic value of SUVmax by PSMA PET/CT as a future biomarker in the primary staging of intermediate‐ to high‐risk PCa.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Supporting information
Table S1. The diagnostic value of (A) 18F‐PSMA PET/CT and (B) 68Ga‐PSMA PET/CT for detecting lymph‐node metastatic disease, and (C) the size of PET/CT detected and missed LN metastases after PLND.
Acknowledgements
We gratefully acknowledge the patients for their participation in this study.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30 [DOI] [PubMed] [Google Scholar]
- 2. Mottet N, van den Bergh RCN, Briers E et al. EAU‐EANM‐ESTRO‐ESUR‐SIOG Guidelines on Prostate Cancer‐2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2021; 79: 243–62 [DOI] [PubMed] [Google Scholar]
- 3. Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 international society of urological pathology (ISUP) Consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 2016; 40: 244–52 [DOI] [PubMed] [Google Scholar]
- 4. Meijer D, van Leeuwen PJ, Donswijk ML et al. Predicting early outcomes in patients with intermediate‐ and high‐risk prostate cancer using prostate‐specific membrane antigen positron emission tomography and magnetic resonance imaging. BJU Int 2022; 129: 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Briganti A, Karnes RJ, Gandaglia G et al. Natural history of surgically treated high‐risk prostate cancer. Urol Oncol 2015; 33: 163.e7–13 [DOI] [PubMed] [Google Scholar]
- 6. Kupelian PA, Mahadevan A, Reddy CA, Reuther AM, Klein EA. Use of different definitions of biochemical failure after external beam radiotherapy changes conclusions about relative treatment efficacy for localized prostate cancer. Urology 2006; 68: 593–8 [DOI] [PubMed] [Google Scholar]
- 7. Perera M, Papa N, Roberts M et al. Gallium‐68 prostate‐specific membrane antigen positron emission tomography in advanced prostate cancer‐updated diagnostic utility, sensitivity, specificity, and distribution of prostate‐specific membrane antigen‐avid lesions: a systematic review and meta‐analysis. Eur Urol 2020; 77: 403–17 [DOI] [PubMed] [Google Scholar]
- 8. Dietlein F, Kobe C, Hohberg M et al. Intraindividual comparison of (18)F‐PSMA‐1007 with renally excreted PSMA ligands for PSMA PET imaging in patients with relapsed prostate cancer. J Nucl Med 2020; 61: 729–34 [DOI] [PubMed] [Google Scholar]
- 9. Rowe SP, Gorin MA, Allaf ME et al. PET imaging of prostate‐specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis 2016; 19: 223–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmadzadehfar H, Essler M. Prostate‐specific membrane antigen imaging: a game changer in prostate cancer diagnosis and therapy planning. Eur Urol 2020; 77: 418–9 [DOI] [PubMed] [Google Scholar]
- 11. Jansen BH, Bodar YJ, Zwezerijnen GJ et al. Pelvic lymph‐node staging with (18)F‐DCFPyL PET/CT prior to extended pelvic lymph‐node dissection in primary prostate cancer ‐ the SALT trial. Eur J Nucl Med Mol Imaging 2021; 48: 509–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hofman MS, Lawrentschuk N, Francis RJ et al. Prostate‐specific membrane antigen PET‐CT in patients with high‐risk prostate cancer before curative‐intent surgery or radiotherapy (proPSMA): a prospective, randomised, multi‐centre study. Lancet 2020; 395: 1208–16 [DOI] [PubMed] [Google Scholar]
- 13. Amiel T, Würnschimmel C, Heck M et al. Regional lymph node metastasis on prostate specific membrane antigen positron emission tomography correlates with decreased biochemical recurrence‐free and therapy‐free survival after radical prostatectomy: a retrospective single‐center single‐arm observational study. J Urol 2021; 205: 1663–70 [DOI] [PubMed] [Google Scholar]
- 14. Ceci F, Oprea‐Lager DE, Emmett L et al. E‐PSMA: the EANM standardized reporting guidelines v1.0 for PSMA‐PET. Eur J Nucl Med Mol Imaging 2021; 48: 1626–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boellaard R, Delgado‐Bolton R, Oyen WJ et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015; 42: 328–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon‐Cardo C. Prostate‐specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997; 3: 81–5 [PubMed] [Google Scholar]
- 17. Perner S, Hofer MD, Kim R et al. Prostate‐specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol 2007; 38: 696–701 [DOI] [PubMed] [Google Scholar]
- 18. Roberts MJ, Morton A, Donato P et al. (68)Ga‐PSMA PET/CT tumour intensity pre‐operatively predicts adverse pathological outcomes and progression‐free survival in localised prostate cancer. Eur J Nucl Med Mol Imaging 2021; 48: 477–82 [DOI] [PubMed] [Google Scholar]
- 19. Memorial Sloan Kettering Cancer Center. Dynamic Prostate Cancer Nomogram: Coefficients . 2016. Available at: https://www.mskcc.org/nomograms/prostate/pre_op/coefficients. Accessed 13 November 2017
- 20. Boellaard R, Willemsen AT, Arends B, Visser EP. EARL procedure for assessing PET/CT system specific patient FDG activity preparations for quantitative FDG PET/CT studies, 2013. Available at: http://earl.eanm.org/html/img/pool/EARL‐procedure‐for‐optimizing‐FDG‐activity‐for‐quantitative‐FDG‐PET‐studies_version_1_1.pdf. Accessed 2018 23 July
- 21. Eiber M, Herrmann K, Calais J et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA‐Ligand PET/CT. J Nucl Med 2018; 59: 469–78 [DOI] [PubMed] [Google Scholar]
- 22. Li X, Rowe SP, Leal JP et al. Quantitative parameters in PSMA‐targeted PET imaging with 18F‐DCFPyL: variability in normal organ uptake. J Nucl Med 2017; 58: 942–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Demirci E, Kabasakal L, Şahin OE et al. Can SUVmax values of Ga‐68‐PSMA PET/CT scan predict the clinically significant prostate cancer? Nucl Med Commun 2019; 40: 86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uprimny C, Kroiss AS, Decristoforo C et al. 68Ga‐PSMA‐11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging 2017; 44: 941–9 [DOI] [PubMed] [Google Scholar]
- 25. Svirydenka H, Muehlematter UJ, Nagel HW et al. (68)Ga‐PSMA‐11 dose reduction for dedicated pelvic imaging with simultaneous PET/MR using TOF BSREM reconstructions. Eur Radiol 2020; 30: 3188–97 [DOI] [PubMed] [Google Scholar]
- 26. Kasivisvanathan V, Rannikko AS, Borghi M et al. MRI‐Targeted or Standard Biopsy for Prostate‐Cancer Diagnosis. N Engl J Med 2018; 10: 1767–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Emmett L, Buteau J, Papa N et al. The additive diagnostic value of prostate‐specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): A Prospective Multicentre Study. Eur Urol 2021; 80: 682–9 [DOI] [PubMed] [Google Scholar]
- 28. Wang H, Amiel T, Würnschimmel C, et al. PSMA‐ligand uptake can serve as a novel biomarker in primary prostate cancer to predict outcome after radical prostatectomy. EJNMMI Research. 2021; 11(1). 10.1186/s13550-021-00818-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Kalmthout LW, van Melick HH, Lavalaye J et al. Prospective validation of gallium‐68 prostate specific membrane antigen‐positron emission tomography/computerized tomography in primary staging of patients with prostate cancer. J Urol 2020; 203: 537–45 [DOI] [PubMed] [Google Scholar]
- 30. Dietlein M, Kobe C, Kuhnert G et al. Comparison of [(18)F]DCFPyL and [ (68)Ga]Ga‐PSMA‐HBED‐CC for PSMA‐PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol 2015; 17: 575–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gandaglia G, Fossati N, Zaffuto E et al. Development and internal validation of a novel model to identify the candidates for extended pelvic lymph node dissection in prostate cancer. Eur Urol 2017; 72: 632–40 [DOI] [PubMed] [Google Scholar]
- 32. Meijer D, van Leeuwen PJ, Roberts MJ et al. External validation and addition of prostate‐specific membrane antigen positron emission tomography to the most frequently used nomograms for the prediction of pelvic lymph‐node metastases: an international multicenter study. Eur Urol 2021; 80: 234–42 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The diagnostic value of (A) 18F‐PSMA PET/CT and (B) 68Ga‐PSMA PET/CT for detecting lymph‐node metastatic disease, and (C) the size of PET/CT detected and missed LN metastases after PLND.


