Abstract
A simple blood-derived biomarker is desirable in the routine management of multiple sclerosis (MS) patients and serum neurofilament light chain (sNfL) is the most promising candidate. Although its utility was first shown in cerebrospinal fluid (CSF), technological advancements have enabled reliable detection in serum and less frequently plasma, obviating the need for repeated lumbar punctures. In this review, after defining the knowledge gap in MS management that many hope sNfL could fill, we summarize salient studies demonstrating associations of sNfL levels with outcomes of interest. We group these outcomes into inflammatory activity, progression, treatment response, and prediction/prognosis. Where possible we focus on data from real-world perspective observational cohorts. While acknowledging the limitations of sNfL and highlighting key areas for ongoing work, we conclude with our opinion of the role for sNfL as an objective, convenient, and cost-effective adjunct to clinical assessment. Paving the way for other promising biomarkers both blood-derived and otherwise, sNfL is an incremental step toward precision medicine for MS patients.
Keywords: Neurofilament light chain, biomarkers, multiple sclerosis
The need for better biomarkers in MS
Multiple sclerosis (MS) is known for its varied clinical presentations and somewhat unpredictable clinical course, producing a spectrum of disease from mild or even benign forms of illness to the very severe, rapidly progressive type. There has been an expansion in the number of treatments available to target MS inflammation, and it is increasingly recognized that early optimal control of inflammatory disease activity offers the best long-term outcome. 1 As a result, the threshold for escalation to higher efficacy therapies, should treatment produce a sub-optimal response, is reducing, and physicians are even considering higher efficacy treatments up front in some cases. However, it is still clear that some patients have a much more indolent course and can remain well for years on the safer but lower-efficacy therapies; 2 in fact, some of these patients may not require treatment at all. In an era when “no evidence of disease activity” seems attainable for many patients, individualisation of treatment principally involves identifying the minimum intensity treatment capable of achieving disease quiescence. However, despite the identification of reasonable diagnostic and safety biomarkers in MS (e.g. magnetic resonance imaging (MRI), oligoclonal bands, JC virus antibody titres), the development of objective and clinically applicable surrogates of disease outcomes has not kept pace. 3 Without better, more accessible biomarkers such as those obtained from blood or other accessible body fluids, our ability to identify and respond to damaging disease activity is hampered, and timely interventions to limit irreversible neurological damage are delayed.
As it relates to MS, biomarkers can take various forms including imaging measures by MRI measures or optical coherence tomography and soluble markers. As surrogates underlying biology, varied roles include diagnosis, monitoring, prediction, and prognostication. Annual MRI scans looking for new lesions are currently the current gold standard of disease activity monitoring. 4 However, annual MRI has shortcomings including: missing clinically silent disease activity and spinal cord lesions; 5 variation in acquisition and analysis quality; high healthcare utilization costs (the scan plus any dye infusion); inconvenience and labor intensity; and may expose patients to the unknown effects of frequent gadolinium (Gd) use. 6 Clinical translation of a convenient and cost-effective marker which objectively reflects the emergence/disappearance of disease activity when monitored serially, as well as predicting future disease is highly desirable.
Serum NfL as a biomarker in MS
Neurofilaments are neuronal-specific heteropolymers assembled from five different intermediate filaments in different combinations and concentrations depending on the type of neuron, location in the axon and stage of development. 7 In mature myelinated axons, neurofilaments are the single most abundant protein where their primary role is to support axonal structure. Physiologic turnover and/or damage to neurons is thought to be the origin of neurofilaments measurable in the cerebrospinal fluid (CSF) and eventually the blood. Neurofilament light chain (NfL) levels in serum have garnered the most interest.
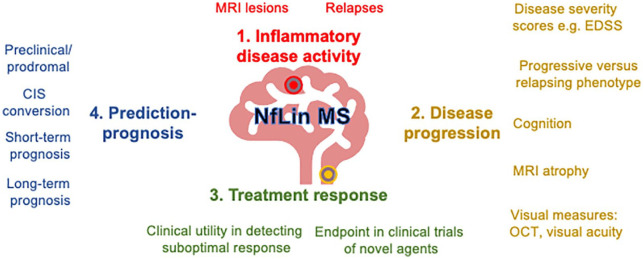
While initial studies focused on CSF NfL, advancements in ultrasensitive detection technologies have enabled reliable measurement in blood (serum/plasma), which correlates with CSF. 8 While most studies investigating blood-derived neurofilaments have focused on serum (sNfL), a minority report associations with closely-related plasma levels. Although strongly correlated, plasma levels are around 25% lower than paired serum levels using the single molecule array (SiMoA) platform and thus may not be directly comparable. 9 In this review, we focus on sNfL unless explicitly stated otherwise. Associations between sNfL and MS can be broadly summarized in terms of inflammatory disease activity, disease progression, treatment response, and prognosis (Figure 1).
Figure 1.
Associations and proposed clinical utility blood neurofilament light chain in multiple sclerosis.
EDSS: expanded disability severity score; OCT: optical coherence tomography; CIS: clinical isolated syndrome.
Serum NfL and inflammatory disease activity in MS
Firing the starting gun for sNfL associations of MS patients, in a seminal 2016 paper, 10 Kuhle and colleagues found that sNfL was elevated in MS patients and correlated with white matter lesion volume using an electrochemiluminescence assay. Since then, the development of the higher sensitivity SiMoA digital immunoassay 8 has allowed for multiple cross-sectional studies to substantiate group-level findings that increase in sNfL is temporally associated with impending or recent clinical relapse, as well as the relapse rate, 11 the number and volume of new T1 Gd-enhancing lesions,11,12 and new T2 lesions.12,13 Comparable associations have also been demonstrated in distinct groups of MS patients including children 14 and pregnant patients. 15 In preliminary evidence involving a large Swiss data set of 1336 patients, relapse in the preceding 4 months was associated with 22% higher log normalized sNfL compared to patients who had not had a relapse. 16
In an effort to individualize group-level associations of sNfL and inflammatory demyelinating activity, several groups have looked for more individualized metrics derived from prospective observational cohorts. In milder relapsing disease, in a cohort of 34 patients on first-line agents sampled serially over 2 years, sNfL levels stayed low or dropped in stable patients who did not experience relapses, remaining significantly lower than relapsing patients. 17 In more active disease, in a cohort of patients serially monitored after alemtuzumab treatment, an sNfL increase was seen 5 months prior to new relapses, peaking at clinical onset, with recovery to baseline within 4–5 months of remission. 18 In this study, patients meeting criteria for “no evidence of disease activity” (NEDA-3, that is, no relapse, MRI activity or disease worsening as measured by the Expanded Disability Status Scale (EDSS) 19 ) consistently had lower levels of sNfL. Here, “peak” levels of sNfL (>3 SD above steady state levels) were associated with clinical and MRI disease activity in 27 out of 34 events. 18 Concordantly, in a study of annual sNfL levels, individual increases of approximately 30% were seen within a 3-month window of new Gd-enhancing lesions. 20 While the percentage change in sNfL strongly associated with T1 and T2 lesion burden accumulation, 21 low sNfL levels (<30th percentile) helped identify patients with a very low probability of having experienced radiologic disease activity in the preceding year. 22 Accordingly, there is an emerging consensus that the primary role of sNfL in clinical practice may be as a serial monitoring tool for subclinical disease activity.
Serum NfL and disease progression in MS
Insidious neuroaxonal loss is one presumptive underpinning of progression in MS, which we expect to be reflected in sNfL levels. In one of the first reports of sNfL in MS, levels were 52.2% higher in patients with progressive compared to relapsing clinical subtypes, with the difference remaining significant after adjustment for age (beta coefficient = 1.205). 13 sNfL has emerged as a leading candidate to help objectify progression in MS, where such a marker is sorely needed as an endpoint in the accelerating pace of treatment trials in this area. 23 Of course, given the dynamic sNfL changes that occur with inflammatory activity, a particular challenge with this use of NfL is to tease-apart progressive and inflammatory disease when they happen concurrently.
As a static measure, several cross-sectional comparisons have reported group-level associations between higher sNfL levels and poorer performance on clinical measures of disability progression including EDSS, conversion to a secondary progressive phenotype, MRI brain volume loss, and measures of cognitive function.11,13,24,25 A one step higher in EDSS corresponds to a 5%–10% higher sNfL. 24 Concurrently, several groups have reported that higher sNfL levels associate with MRI brain volume loss13,21,25 as well as optical coherence tomography (OCT) measures, particularly peripapillary retinal nerve fiber thinning. 26 Studies to-date have not found associations between sNfL levels and fatigue. 27
Serum NfL and treatment response in MS
Given the association of sNfL with inflammatory disease activity, it is not surprising that following immunomodulatory/immunosuppressive treatment, levels should decrease. In a 2017 study, treatment with any disease-modifying therapy was associated with 29% lower sNfL levels compared to untreated individuals. 24 Subsequently, multiple studies have reported longitudinal reductions in sNfL following most approved MS treatments including injectable therapies, 24 dimethyl fumarate, 9 fingolimod, 28 natalizumab, 24 rituximab (plasma NfL), 29 ocrelizumab, 30 ofatumumab, 31 alemtuzumab, 18 and hematopoietic stem cell transplantation. 32 Interestingly, reductions in sNfL levels have not been convincingly reported following teriflunomide or glatiramer acetate, and several groups reported no longitudinal reduction following high-dose vitamin D3 initiation.33,34
In real-world cohorts comparing different treatments in MS patients, treatment escalation, as opposed to similar efficacy treatment switch, resulted in reductions in sNfL levels. 11 , 35 In a study of 1261 unselected Swedish patients started on one of six disease-modifying therapies (alemtuzumab, dimethyl fumarate, fingolimod, natalizumab, rituximab, and teriflunomide), it was shown that the largest reductions of log-normalized plasma NfL occurred in patients treated with alemtuzumab (48%), and the smallest reduction for teriflunomide (7%, not significant), with the other agents falling in the middle. 36
In data presented on 1366 Swiss MS patients in an open-label setting, log normalized sNfL levels in patients on platform therapies, oral therapies, and monoclonal therapies were respectively, 10.4%, 14.2%, and 19.7% lower than untreated patients. 16 While it is encouraging that post-treatment reductions fall in line with widely perceived treatment efficacy, a confounder in these studies relates to indication bias: patients selected for more intensive treatments have higher initial sNfL levels presumably due to more aggressive disease. 24
In progressive MS, given the relative lack of other objective endpoints and limitations of practical MRI atrophy measurements, sNfL is increasingly recognized as an outcome measure and has been quantified in several phase II and III studies. For secondary progressive disease, reductions in sNfL have been shown following siponimod, 37 ocrelizumab, 38 and natalizumab; 39 in the latter, reductions were seen despite the study not reaching its primary endpoint of slowing EDSS progression. 39 In primary progressive disease, reductions in levels were seen following fingolimod 40 and ocrelizumab. 38 No reduction was seen following ibudilast, a treatment that slowed the progression of MRI brain atrophy but not clinical worsening. 41
Thus, in both relapsing and active progressive disease, there is growing excitement that sNfL could serve both an objective endpoint in clinical trials and also as a convenient clinical tool to monitor patients on treatment for efficacy.
Serum NfL and prediction/prognosis in MS
Early significant burden of inflammatory disease and rapid rate of disease progression are key predictors of poor outcome in MS. 42 Since NfL integrates both inflammation and neurodegeneration in a single metric, it stands to reason that we might expect sNfL to be of prognostic merit at various stages of the disease.
In a rare insight into the prodromal/preclinical phase of MS, in a nested case–control study of routine blood tests obtained from US military personnel, sNfL was elevated 6 years prior to the clinical onset of MS. 43 In both adult and pediatric populations diagnosed with clinically isolated syndrome, higher sNfL was associated with faster conversion to clinically definite MS independent of other prognostic factors. 44 In a prospective longitudinal cohort of early MS (early relapsing or clinically isolated syndrome), patients who remained treatment naïve had lower sNfL than those who needed disease-modifying treatment within 4 years: subsequent to treatment initiation the sNfL levels reduced. 35
Several other studies have shown that in patients with confirmed relapsing or progressive MS, baseline sNfL predicts short-term outcomes (up to 5 years) including relapses, MRI disease activity, disability worsening, MRI brain and spinal cord atrophy, and poorer cognitive outcomes.24,45 The Swiss group have presented preliminary but important evidence from 1366 MS patients followed for a median of 5 years. 16 Log-normalized levels were converted into age-adjusted z scores derived from a cohort of 8865 healthy controls. Patients with an age-adjusted z score >1 at baseline (i.e. sNfL level in the top 16% of healthy control levels, representing 30% of MS patients) had a 41% increased risk of relapse or EDSS worsening and 90% increase risk of new/enlarging T2 lesion the following year compared to those with a z score of <1. sNfL z score was predictive of clinical events in the next year independently of preceding EDSS change, new relapses, or new MRI T2/Gd lesions. Finally, they showed that in patients with NEDA-3, higher NfL correlated with an increased rate of disease activity in the next year: 9.4% of the whole NEDA-3 group had activity within 1 year, increasing to 25% of NEDA-3 patients with sNfL z score >2. Thus, sNfL seems to provide independent value in detecting subclinical disease activity in NEDA-3 patients within the next year. This demonstrates that sNfL could be considered for inclusion in NEDA to further define this evolving concept.
Baseline measurements of sNfL obtained close to disease onset could be of long-term prognostic value. In the longest such follow-up report to date, our group recently reported that sNfL sampled within the first 5 years of MS symptom onset was shown to independently predict worsening EDSS score and risk of developing progressive MS in patients followed longitudinally for 15–26 years. 46 Those with the baseline sNfL less 7.6 pg/mL were seven times less likely to develop progressive MS compared to those above the cutoff. In the Boston CLIMB cohort 47 and UCSF EPIC cohort, 48 respectively, averaged annualized and baseline NfL levels were in multivariate models attributable for 15% and 18% of 10-year brain atrophy, although the prognostic power for relapse activity and long-term disability progression was limited.
The future of sNfL in MS
Higher sNfL is associated with more relapses, worsening EDSS, lesions on MRI scans and atrophy of both the brain and spinal cord. Although sNfL is already being used as a surrogate of MS-related disease in some centers and as an endpoint in clinical trials, a number of challenges remain before this test can be readily adopted as a routine measurement. Key issues of biological validity relate to the overlap of levels in MS patients and controls and the non-specific nature of sNfL in MS. While age is the most significant factor to be accounted for especially over the age of 60, 49 confounding comorbidities include head injuries, 50 vascular risk factors, renal function, 51 and high body mass index (BMI) leading to increased volume of distribution, 52 which is discussed at greater length elsewhere. 53 Associations of sNfL levels and disease metrics seems easily demonstrable in groups of patients; however, the availability of robust normative data sets which can adjust for age is important to bring meaning to sNfL in individuals. Neurologists will require education in the interpretation of sNfL, where careful consideration must be made to the clinical context . Hurdles relating to analytical validity relate to assay standardization, currently being addressed by ongoing multisite validation efforts as well as consensus guidelines.
Despite its shortcomings, sNfL is a success story of a blood-based biomarker on the cusp of clinical translation in MS. It integrates and objectifies both inflammatory and neurodegenerative disease in a single marker which can be readily trended in real-time. While we expect that sNfL in early-stage disease will be of use in prognostication and initial treatment selection, the main utility of sNfL will be as a serial measure to objectively quantify treatable subclinical inflammatory disease activity. We envisage that levels will be measured serially every 3–6 months and compared to either age-adjusted normative data or compared to “baseline” sNfL levels from the same patient during a time of clinical and MRI disease quiescence. Acting as a trigger for neurologists to consider expedited reassessment, this would be at reduced cost and increased frequency in comparison to the current standard of care, the annual MRI. While serially low levels will be reassuring to neurologists and patients in remission, many hope sNfL will provide and earlier marker in patients with subclinical disease activity and poorer prognosis that is not otherwise as apparent when following clinical or MRI activity alone. Through more individualized treatment, sNfL could have the power to modify the trajectory of disease and improve outcomes. In trials of MS treatments, sNfL is already being considered as an exploratory endpoint. We foresee this having particular utility in assessing for progressive MS, where benefit of treatment can be difficult to appreciate in the absence of an untreated comparator group.
The cost of sNfL testing will eventually reduce with several assay developers already vying for market share. Accepted assay standards will become widely adopted as multisite validation efforts come to fruition. We believe that serial measurement of sNfL could supplant the need for annual MRI scans for routine monitoring of stable treated patients. This will be particularly important in areas where obtaining regular MRIs is burdensome on a publicly funded system and may be limited in terms of sheer MRI availability. In that way, MRI might be called upon only if there are substantial rises in a patient’s sNfL.
sNfL represents a “first in class” blood-derived biomarker reaching the threshold for clinical use. We think that sNfL should be considered in future definitions of “no evidence of disease activity” with a substantiative basis of evidence already in circulation. Subsequent biomarkers will inevitably emerge and improve our ability to monitor and predict disease. Enabled by large collaborative data sets and advanced computational analytics, sNfL may form part of a composite panel of biomarkers which collectively help us best understand the patient in front of us. sNfL represents an important, incremental, and iterative step toward precision medicine in MS.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Simon Thebault  https://orcid.org/0000-0003-0395-3395
https://orcid.org/0000-0003-0395-3395
Gauruv Bose  https://orcid.org/0000-0002-5204-6348
https://orcid.org/0000-0002-5204-6348
Contributor Information
Simon Thebault, The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Gauruv Bose, The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Ronald Booth, The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, ON, Canada/The University of Ottawa, Ottawa, ON, Canada.
Mark S Freedman, The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, ON, Canada.
References
- 1. Giovannoni G. Disease-modifying treatments for early and advanced multiple sclerosis. Curr Opin Neurol 2018; 31: 233–243. [DOI] [PubMed] [Google Scholar]
- 2. Sartori A, Abdoli M, Freedman MS. Can we predict benign multiple sclerosis? Results of a 20-year long-term follow-up study. J Neurol 2017; 264(6): 1068–1075 [DOI] [PubMed] [Google Scholar]
- 3. Comabella M, Montalban X. Body fluid biomarkers in multiple sclerosis. Lancet Neurol 2014; 13(1): 113–126. [DOI] [PubMed] [Google Scholar]
- 4. Wattjes MP, Rovira Miller ÀD, Yousry TA, et al. Evidence—based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—Establishing disease prognosis and monitoring patients. Nature Rev Neurol 2015; 11: 597–606. [DOI] [PubMed] [Google Scholar]
- 5. Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: An update. Clin Neuroradiol 2015; 25: 157–165. [DOI] [PubMed] [Google Scholar]
- 6. Cohan S, Chen C, Baraban E, et al. MRI utility in the detection of disease activity in clinically stable patients with multiple sclerosis: A retrospective analysis of a community based cohort. BMC Neurol 2016; 16: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuan A, Rao MV, Veeranna, et al. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 2017; 9(4): a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016; 54(10): 1655–1661. [DOI] [PubMed] [Google Scholar]
- 9. Sejbaek T, Nielsen HH, Penner N, et al. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naïve relapsing MS patients. J Neurol Neurosurg Psychiatry 2019; 90(12): 1324–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler 2016; 22(12): 1550–1559 [DOI] [PubMed] [Google Scholar]
- 11. Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017; 89(22): 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varhaug KN, Barro C, Bjørnevik K, et al. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol NeuroInflammation 2018; 5(1): e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92(10): E1007–E1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reinert MC, Benkert P, Wuerfel J, et al. Serum neurofilament light chain is a useful biomarker in pediatric multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2020; 7(4): 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuello JP, Martinez Gines ML, Kuhle J, et al. Neurofilament light chain levels in pregnant multiple sclerosis patients: A prospective cohort study. Eur J Neurol 2019; 26(9): 1200–1204 [DOI] [PubMed] [Google Scholar]
- 16. Yaldizli Benkert ÖP, Maceski A, Barakovic M, et al. Value of serum neurofilament light chain levels as a biomarker of suboptimal treatment response in MS clinical practice. Mult Scler 2018; 24(Suppl. 2): 97–98. [Google Scholar]
- 17. Huss A, Senel M, Abdelhak A, et al. Longitudinal serum neurofilament levels of multiple sclerosis patients before and after treatment with first-line immunomodulatory therapies. Biomedicines 2020; 8(9): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akgün K, Kretschmann N, Haase R, et al. Profiling individual clinical responses by high-frequency serum neurofilament assessment in MS. Neurol Neuroimmunol NeuroInflammation 2019; 6(3): e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lublin FD. Disease activity free status in MS. Mult Scler Relat Disord 2012; 1(1): 6–7. [DOI] [PubMed] [Google Scholar]
- 20. Rosso M, Gonzalez CT, Healy BC, et al. Temporal association of sNfL and gad-enhancing lesions in multiple sclerosis. Ann Clin Transl Neurol 2020; 7(6): 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Srpova B, Uher T, Hrnciarova T, et al. Serum neurofilament light chain reflects inflammation-driven neurodegeneration and predicts delayed brain volume loss in early stage of multiple sclerosis. Mult Scler J 2020; 27: 52–60. [DOI] [PubMed] [Google Scholar]
- 22. Uher T, Schaedelin S, Srpova B, et al. Monitoring of radiologic disease activity by serum neurofilaments in MS. Neurol Neuroimmunol Neuroinflammation 2020; 7(4): 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kapoor R, Smith KE, Allegretta M, et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology 2020; 95: 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81(6): 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filippi P, Vestenicka V, Siarnik P, et al. Neurofilament light chain and MRI volume parameters as markers of neurodegeneration in multiple sclerosis. Neuro Endocrinol Lett 2020; 41(1): 17–26. [PubMed] [Google Scholar]
- 26. Bsteh G, Berek K, Hegen H, et al. Serum neurofilament levels correlate with retinal nerve fiber layer thinning in multiple sclerosis. Mult Scler J 2020; 26: 1682–1690. [DOI] [PubMed] [Google Scholar]
- 27. Håkansson I, Johansson L, Dahle C, et al. Fatigue scores correlate with other self-assessment data, but not with clinical and biomarker parameters, in CIS and RRMS. Mult Scler Relat Disord 2019; 36: 101424. [DOI] [PubMed] [Google Scholar]
- 28. Jakimovski D, Zivadinov R, Ramanthan M, et al. Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: A longitudinal retrospective 5-year study. Mult Scler J 2019; 26: 1670–1681. [DOI] [PubMed] [Google Scholar]
- 29. de Flon P, Laurell K, Sundstrom P, et al. Comparison of plasma and cerebrospinal fluid neurofilament light in a multiple sclerosis trial. Acta Neurol Scand 2019; 139(5): 462–468 [DOI] [PubMed] [Google Scholar]
- 30. Cross A. Ocrelizumab treatment reduced levels of neurofilament light chain and numbers of B cells in the cerebrospinal fluid of patients with relapsing multiple sclerosis in the OBOE study S56.008. Neurology 2019; 92(15Supplement): 52. [Google Scholar]
- 31. Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med 2020; 383(6): 546–557. [DOI] [PubMed] [Google Scholar]
- 32. Thebault S, Tessier D, Lee H, et al. High serum neurofilament light chain normalises after haematopoietic stem cell transplant for MS. Neurol—Neuroimmunol Neuroinflammation 2019; 6(5): e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Røsjø E, Lindstrøm JC, Holmøy T, et al. Natural variation of Vitamin D and neurofilament light chain in relapsing-remitting multiple sclerosis. Front Neurol 2020; 11: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smolders J, Mimpen M, Oechtering J, et al. Vitamin D3 supplementation and neurofilament light chain in multiple sclerosis. Acta Neurol Scand 2020; 141(1): 77–80. [DOI] [PubMed] [Google Scholar]
- 35. Bittner S, Steffen F, Uphaus T, et al. Clinical implications of serum neurofilament in newly diagnosed MS patients: A longitudinal multicentre cohort study. Ebiomedicine 2020; 56: 102807–102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Delcoigne B, Manouchehrinia A, Barro C, et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology 2020; 94(11): e1201–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuhle J, Kropshofer HCB, Meinert R, et al. Siponimod reduces neurofilament light chain blood levels in secondary progressive multiple sclerosis patients. Neurology 2018; 90(15): 51–67.29247075 [Google Scholar]
- 38. Bar-Or A. Blood neurofilament light levels are lowered to a healthy donor range in patients with RMS and PPMS following ocrelizumab treatment. ECTRIMS Online Libr 2019; 152: 279451. [Google Scholar]
- 39. Kapoor R, Sellebjerg F, Hartung H-P, et al. Natalizumab reduced serum levels of neurofilament light chain in secondary progressive multiple sclerosis patients from the phase 3 ASCEND study. Mult Scler J 2018; 24(2): 988, 10.1177/1352458518799980 [DOI] [Google Scholar]
- 40. Kuhle J, Kropshofer H, Haring DA, et al. Neurofilament light levels in the blood of patients with secondary progressive MS are higher than in primary progressive MS and may predict brain atrophy in both MS subtypes. Mult Scler J 2018; 24(2 Suppl.): 111, http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed19&NEWS=N&AN=629481148 [Google Scholar]
- 41. Fox R, Karafa M, Konig V, et al. Effect of ibudilast on neurofilament-light chain in progressive ms: Analysis from a phase II trial. Neurology 2019; 92(15): 7831, https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01987831/full [Google Scholar]
- 42. Confavreux C, Vukusic S, Moreau T, et al. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2002; 343(20): 1430–1438. [DOI] [PubMed] [Google Scholar]
- 43. Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol 2020; 77(1): 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Vuurst de Vries RM, Wong YYM, Mescheriakova JY, et al. High neurofilament levels are associated with clinically definite multiple sclerosis in children and adults with clinically isolated syndrome. Mult Scler J 2018; 25(7): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141(8): 2382–2391. [DOI] [PubMed] [Google Scholar]
- 46. Thebault S, Abdoli M, Fereshtehnejad S-MM, Tessier D, et al. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Natue Sci Reports 2019; 10: 10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chitnis T, Gonzalez C, Healy BC, et al. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol 2018; 5(12): 1478–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 Years. JAMA Neurol 2019; 76(11): 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 2020; 11(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shahim P, Zetterberg H, Tegner Y, et al. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017; 88(19): 1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Korley FK, Goldstick J, Mastali M, et al. Serum NfL (Neurofilament Light Chain) levels and incident stroke in adults with diabetes mellitus. Stroke 2019; 50(7): 1669–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manouchehrinia A, Piehl F, Hillert J, et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann Clin Transl Neurol 2020; 7(1): 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barro C, Chitnis T, Weiner HL. Blood neurofilament light: A critical review of its application to neurologic disease. Ann Clin Transl Neurol 2020; 7: 2508–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]