Abstract
Background:
In the phase III ASCLEPIOS I and II trials, participants with relapsing multiple sclerosis receiving ofatumumab had significantly better clinical and magnetic resonance imaging (MRI) outcomes than those receiving teriflunomide.
Objectives:
To assess the efficacy and safety of ofatumumab versus teriflunomide in recently diagnosed, treatment-naive (RDTN) participants from ASCLEPIOS.
Methods:
Participants were randomized to receive ofatumumab (20 mg subcutaneously every 4 weeks) or teriflunomide (14 mg orally once daily) for up to 30 months. Endpoints analysed post hoc in the protocol-defined RDTN population included annualized relapse rate (ARR), confirmed disability worsening (CDW), progression independent of relapse activity (PIRA) and adverse events.
Results:
Data were analysed from 615 RDTN participants (ofatumumab: n = 314; teriflunomide: n = 301). Compared with teriflunomide, ofatumumab reduced ARR by 50% (rate ratio (95% confidence interval (CI)): 0.50 (0.33, 0.74); p < 0.001), and delayed 6-month CDW by 46% (hazard ratio (HR; 95% CI): 0.54 (0.30, 0.98); p = 0.044) and 6-month PIRA by 56% (HR: 0.44 (0.20, 1.00); p = 0.049). Safety findings were manageable and consistent with those of the overall ASCLEPIOS population.
Conclusion:
The favourable benefit–risk profile of ofatumumab versus teriflunomide supports its consideration as a first-line therapy in RDTN patients.
ASCLEPIOS I and II are registered at ClinicalTrials.gov (NCT02792218 and NCT02792231).
Keywords: Relapsing multiple sclerosis, recently diagnosed, treatment-naive, progression independent of relapse activity, no evidence of disease activity, neurofilament light chain
Introduction
In young adults, MS is the most common chronic, inflammatory, demyelinating and neurodegenerative CNS disease, 1 and is a leading cause of non-traumatic disability. 2 In patients with relapsing MS (RMS), disability accrual was previously thought to be a sequential process, driven by poor recovery from relapses during the initial relapsing stage, followed by relapse-independent progression in the secondary progressive stage. 3 However, accumulating clinical evidence indicates that relapses with incomplete recovery and progression independent of relapse activity (PIRA) both contribute to disability accrual from disease onset, albeit in different proportions.4–6 Consistent with PIRA occurring from MS onset, findings from neuropathology, biomarker and imaging studies suggest that neuroaxonal loss, the main driver of neurodegeneration and irreversible progression in advanced MS, may already be prominent in early RMS.7–9 Younger patients with RMS have higher clinical and MRI disease activity, as well as more pronounced acute axonal damage, 7 than older patients. 10 Moreover, neuronal and brain volume loss begin early in the disease.11,12 High levels of disability, high lesion load and low brain volume are associated with poor MS prognosis. 13
The effect of disease-modifying therapies (DMTs) on worsening of MS disability is age-dependent, with younger patients and those earlier in the disease course showing the greatest benefit.10,14–16 Therefore, early treatment with high-efficacy DMTs that can slow disability accrual is essential.17,18 However, there are barriers to early intervention with high-efficacy DMTs. Uncertainty early in the disease course regarding MS severity means that some patients are reluctant to accept long-term treatment with any DMT, including high-efficacy DMTs. Also, safety concerns may cause clinicians to delay high-efficacy DMT use, resulting in subclinical CNS damage compromising repair and compensation capacity, poor symptom control and eventually accrual of irreversible disability. Moreover, some high-efficacy therapies are restricted to later treatment lines by regulators, payers and healthcare management organizations.
Ofatumumab, a fully human anti-CD20 monoclonal antibody that selectively depletes B cells, 19 is approved in the USA, across Europe and in several other countries for the treatment of adults with RMS.20,21 In the ASCLEPIOS I and II phase III trials, ofatumumab (20 mg subcutaneously every 4 weeks) was superior to teriflunomide (14 mg orally once daily) in participants with RMS, significantly reducing relapse rate, disability worsening and MRI lesion activity with a favourable safety and tolerability profile that allowed for home administration without premedication. 22 The ASCLEPIOS trials and comparison across trials via network meta-analyses have shown that ofatumumab is among the most highly efficacious treatments for MS.22,23
In this study, we assessed the benefit–risk profile of ofatumumab versus that of teriflunomide, analysing clinical and MRI data in a subpopulation of participants with early RMS (recently diagnosed and treatment-naive (RDTN)) from the combined ASCLEPIOS I and II trial populations.
Methods
Trial design and participants
Details of the ASCLEPIOS I (ClinicalTrials.gov identifier: NCT02792218) and II (NCT02792231) trials have been reported. 22 ASCLEPIOS I and II were randomized, double-blind, double-dummy, active-controlled, multicentre trials of identical design conducted concurrently in participants with RMS. Participants were randomized (1:1) to ofatumumab 20 mg subcutaneously every 4 weeks (starting at week 4, after initial dosing of 20 mg on days 1, 7 and 14) or teriflunomide 14 mg orally once daily for up to 30 months. 22 Protocols were approved by the relevant institutional review board or ethics committee at each trial site, and all participants provided written informed consent.
Analysis populations
Unless otherwise specified, analyses were performed in the protocol-defined RDTN subpopulation of participants from the pooled full analysis set (FAS; all randomized participants with assigned treatments) from ASCLEPIOS I and II. The full inclusion and key exclusion criteria for the FAS in ASCLEPIOS I and II are listed in Supplementary Text 1. This sub-population was defined as those participants who had received an RMS diagnosis within the 36-month period before screening and had no prior treatment with a DMT. As reported elsewhere, 24 a modified FAS was used for the analysis of no evidence of disease activity (NEDA) and it included all participants in the FAS according to the intent-to-treat principle, but excluded those who discontinued treatment early for reasons other than ‘lack of efficacy’ or ‘death’ and who had NEDA before early discontinuation. The safety set included all participants who received trial drugs.
Endpoints and definitions
Efficacy endpoints to assess the benefit–risk of ofatumumab versus teriflunomide treatment were: annualized relapse rate (ARR); confirmed disability worsening (CDW) at 3 months (3mCDW) and at 6 months (6mCDW); PIRA at 3 months (3mPIRA) and at 6 months (6mPIRA); the number of gadolinium-enhancing (Gd+) T1 lesions per MRI scan; the number of new or enlarging T2 lesions per year; annual rate of brain volume loss; the three-parameter NEDA (NEDA-3); and neurofilament light chain (NfL) concentration. Quantitation of NfL in human serum was done using the Quanterix Simoa NF-light assay advantage kit, which is a two-step quantitative digital immunoassay. A technical assessment was performed to validate the performance claims of the Quanterix Simoa NfL kit in a serum matrix for use as a clinical trial assay.
ARR was the number of confirmed MS relapses observed on the study, standardized to 1 year. 3mCDW and 6mCDW were increased from baseline Expanded Disability Status Scale (EDSS) score (by ⩾1.5 points for a score of 0, by ⩾1 point for scores of 1–5 and by ⩾0.5 points for a score ⩾5.5) sustained for at least 3 or 6 months, respectively; 3mPIRA and 6mPIRA used the same EDSS criteria as 3mCDW and 6mCDW, but included only cases in which the onset of progression did not occur during an investigator-reported relapse. NEDA-3 criteria were no confirmed relapses, no 6mCDW and no MRI activity (i.e. Gd+T1 lesions or enlarging T2 lesions on any MRI scan vs. baseline).
Safety endpoints to assess the benefit–risk of ofatumumab versus teriflunomide treatment were adverse events (AEs), AEs leading to study discontinuation, and serious AEs (SAEs); AEs were recorded at all visits and graded using the Common Terminology Criteria for Adverse Events. 25
Statistical analyses
Participant characteristics at baseline were summarized descriptively by treatment in the RDTN subpopulation and in the pooled FAS. Percentage compliance was calculated in the safety set as the duration of exposure to study drug (days)/treatment duration (days).
ARR was analysed using a negative binomial regression model, with an offset to adjust for variable study duration in years. Disability-related endpoints (CDW and PIRA) were analysed using a Cox proportional hazards model. 3mPIRA and 6mPIRA were analysed in: (1) RDTN participants with no confirmed on-study relapses and (2) RDTN participants with no confirmed relapses on study or before a CDW event. More stringent sensitivity analyses of these two groups included only participants without confirmed or unconfirmed on-study relapses, and with CDW defined such that confirmation could not be within 90 days of a relapse. Numbers of Gd+T1 lesions and of new or enlarging T2 lesions were assessed using negative binomial regression models; the number of available MRI scans was the offset for analysis of Gd+T1 lesions; and the time in years between baseline scan and last available scan was the offset for analysis of T2 lesions. The annual rate of brain volume loss was estimated as the marginal slope estimate from a random coefficient model with random intercept and slope based on assessments of brain volume percentage change from baseline at month 12, month 24 and end of treatment and/or trials. NEDA-3 was analysed in the modified FAS using logistic regression. Serum NfL concentration was measured at months 3, 12 and 24, and analysed using a repeated-measures model after log-transformation of the data; the treatment effect (i.e. percentage reduction in NfL concentration) was the ratio of geometric means (ofatumumab vs. teriflunomide) × 100.
Safety data were collected during the treatment period (screening to the end of the trial) and the safety follow-up period until a participant’s last visit. After the last treatment dose, participants were followed up for at least 9 months. Data collected on or before 100 days after the last dose of study medication were included in the analysis except for SAEs, for which all data collected until the end of the trial were included.
Analyses of individual endpoints in RDTN participants were undertaken post hoc.
Results
Participants
Of the 1882 participants randomly assigned to treatment in ASCLEPIOS I and II, 615 (32.7%) were RDTN (ofatumumab, 314; teriflunomide, 301). RDTN participants had a median of 0.35 and 0.36 years from diagnosis for the ofatumumab and teriflunomide treated patients, respectively, with a range of 0.1–2.9 years from diagnosis for both groups. Demographic and disease characteristics were similar between treatment groups and across trials (Table 1). Comparison by treatment group with the overall ASCLEPIOS population showed that RDTN participants in both groups were – as expected – younger with lower disability scores and lower total T2 lesion volume.
Table 1.
Participant characteristics and demographics in RDTN participants and in the overall population from the ASCLEPIOS I and II trials (FAS).
| Characteristic | RDTN participants from ASCLEPIOS I
and II
a
|
All participants from ASCLEPIOS I
and II |
||
|---|---|---|---|---|
| Ofatumumab (n = 314) | Teriflunomide (n = 301) | Ofatumumab (N = 946) | Teriflunomide (N = 936) | |
| Age, mean (SD) b , years | 36.8 (9.4) | 35.7 (9.0) | 38.4 (9.0) | 38.0 (9.2) |
| Women, n (%) | 217 (69.1) | 195 (64.8) | 637 (67.3) | 636 (67.9) |
| Type of MS, n (%) | ||||
| RRMS | 311 (99.0) | 296 (98.3) | 890 (94.1) | 884 (94.4) |
| SPMS | 3 (1.0) | 5 (1.7) | 56 (5.9) | 52 (5.6) |
| Time since diagnosis, mean (SD), years | 0.58 (0.63) | 0.53 (0.51) | 5.68 (6.21) | 5.56 (6.10) |
| Relapses in previous 12 months, mean (SD) | 1.3 (0.7) | 1.4 (0.7) | 1.2 (0.7) | 1.3 (0.7) |
| Relapses in previous 12–24 months, mean (SD) | 0.6 (0.8) | 0.5 (1.0) | 0.8 (1.0) | 0.9 (1.1) |
| EDSS score at baseline, mean (SD) c | 2.30 (1.20) | 2.28 (1.20) | 2.93 (1.35) | 2.90 (1.37) |
| Participants with Gd+T1 lesions, mean (SD) |
n = 306 1.8 (4.4) |
n = 298 1.4 (2.8) |
n = 923 1.7 (4.5) |
n = 922 1.3 (3.4) |
| Participants with Gd+T1 lesions, n (%) | 141 (44.9) | 130 (43.2) | 385 (40.7) | 352 (37.6) |
| Total volume of T2 lesions, mean (SD), cm3 |
n = 311 10.11 (12.23) |
n = 300 8.31 (8.83) |
n = 934 13.72 (13.80) |
n = 930 12.55 (13.81) |
| NfL concentration, mean (SD), pg/mL |
n = 297 15.19 (18.57) |
n = 279 13.66 (14.52) |
n = 893 13.98 (15.86) |
n = 853 12.54 (11.94) |
| Normalized brain volume, mean (SD), cm3 |
n = 310 1472.6 (72.3) |
n = 300 1472.9 (66.1) |
n = 929 1439.8 (78.9) |
n = 927 1444.0 (77.8) |
EDSS: Expanded Disability Status Scale; FAS: full analysis set; Gd+: gadolinium-enhancing; NfL: neurofilament light chain; RDTN, recently diagnosed, treatment-naive; RRMS: relapsing–remitting MS; SD: standard deviation; SPMS: secondary progressive MS.
Unless otherwise stated in individual rows, the number of participants with available data at baseline is indicated in the column header.
RDTN were those who had not received a prior MS disease-modifying therapy and who had received a diagnosis in the 36 months before screening.
Age at baseline was calculated from the date of the first administration of trial drug and the birth year (no exact birth date was captured for data privacy reasons). Eligibility for trial entry was assessed at the screening visit.
EDSS score at baseline was defined as the EDSS score at the last assessment before administration of the first dose of trial drug. EDSS scores range from 0 to 10.0, with higher scores indicating a higher degree of disability.
The median duration of exposure to study treatment was 1.7 years for those receiving ofatumumab, and 1.6 years for those receiving teriflunomide. 90% of patients received study treatment for more than 1 year, and more than 25% of participants received treatment for more than 2 years (Supplementary Figure 1).
Compliance in RDTN participants was high (ofatumumab, 98.8%; teriflunomide, 98.9%), 100% compliance being achieved by 171 of 314 (54.5%) and 176 of 301 (58.5%) in the two groups, respectively; at least 90% compliance was achieved by 307 of 314 (97.8%) ofatumumab-treated participants.
Efficacy in RDTN participants
Ofatumumab reduced ARR by 50% versus teriflunomide (ARR: 0.09 vs. 0.18; rate ratio (95% confidence interval; CI): 0.50 (0.33, 0.74); p < 0.001) (Table 2).
Table 2.
Clinical, MRI and biomarker outcomes in the subpopulation of RDTN participants from the ASCLEPIOS I and II trials (FAS).
| Outcome | RDTN participants a | ||
|---|---|---|---|
| Ofatumumab | Teriflunomide | p value | |
| Relapses | |||
| No. of participants evaluated | 314 | 301 | |
| Total no. of relapses | 45 | 88 | |
| No. of patient-years | 509 | 494 | |
| Adjusted ARR (95% CI) | 0.09 (0.07, 0.12) | 0.18 (0.14, 0.23) | |
| Rate ratio (95% CI) | 0.50 (0.33, 0.74) | <0.001 | |
| Disability-related outcomes | |||
| 3mCDW | |||
| No. of events during the trial/no. of participants (%) | 24/312 (7.7) | 37/300 (12.3) | |
| HR (95% CI) | 0.62 (0.37, 1.03) | 0.065 | |
| 6mCDW | |||
| No. of events during the trial/no. of participants (%) | 17/312 (5.4) | 30/300 (10.0) | |
| HR (95% CI) | 0.54 (0.30, 0.98) | 0.044 | |
| MRI-related outcomes | |||
| Gd+T1 lesions | |||
| No. of participants evaluated | 296 | 284 | |
| No. of Gd+lesions | 10 | 212 | |
| No of evaluable scans | 561 | 540 | |
| Mean no. of lesions per scan (95% CI) | 0.02 (<0.01, 0.04) | 0.39 (0.28, 0.53) | |
| Rate ratio (95% CI) | 0.05 (0.02, 0.10) | <0.001 | |
| New or enlarging T2 lesions | |||
| No. of participants evaluated | 300 | 287 | |
| No. of new or enlarging T2 lesions | 418 | 2179 | |
| No. of patient-years | 481 | 469 | |
| Mean no. of lesions per year (95% CI) | 0.86 (0.70, 1.05) | 4.78 (3.97, 5.76) | |
| Rate ratio (95% CI) | 0.18 (0.14, 0.24) | <0.001 | |
| Brain volume change | |||
| No. of participants evaluated | 295 | 280 | |
| Annual rate of change b (95% CI) | –0.30 (–0.37, –0.23) | –0.31 (–0.38, –0.24) | |
| Difference in percentage points (95% CI) | 0.01 (–0.10, 0.11) | 0.9 | |
| NEDA-3 | |||
| Months 0–12 | |||
| No. of participants achieving NEDA-3/no. of participants c (%) | 134/285 (47.0) | 71/288 (24.7) | |
| Odds ratio (95% CI) | 3.31 (2.24, 4.90) | <0.001 | |
| Months 12–24 | |||
| No. of participants achieving NEDA-3/no. of participants c (%) | 258/280 (92.1) | 131/280 (46.8) | |
| Odds ratio (95% CI) | 14.68 (8.76, 24.61) | <0.001 | |
| Months 0–24 | |||
| No. of participants achieving NEDA-3/no. of participants c (%) | 127/285 (44.6) | 51/288 (17.7) | |
| Odds ratio (95% CI) | 4.63 (3.05, 7.03) | <0.001 | |
| Biomarker outcomes | |||
| Serum NfL concentration | |||
| At 3 months | |||
| No. of participants evaluated | 294 | 280 | |
| Geometric mean (95% CI), pg/mL | 8.72 (8.20, 9.26) | 9.13 (8.58, 9.72) | |
| Geometric mean ratio (95% CI) | 0.95 (0.88, 1.03) | 0.258 | |
| At 12 months | |||
| No. of participants evaluated | 285 | 274 | |
| Geometric mean (95% CI), pg/mL | 6.60 (6.25, 6.98) | 8.61 (8.14, 9.11) | |
| Geometric mean ratio (95% CI) | 0.77 (0.71, 0.83) | <0.001 | |
| At 24 months | |||
| No. of participants evaluated | 254 | 253 | |
| Geometric mean (95% CI), pg/mL | 6.47 (6.11, 6.85) | 8.10 (7.64, 8.58) | |
| Geometric mean ratio (95% CI) | 0.80 (0.74, 0.86) | <0.001 | |
3mCDW: 3-month confirmed disability worsening; 6mCDW: 6-month confirmed disability worsening; ARR: annualized relapse rate; CI: confidence interval; FAS: full analysis set; Gd+: gadolinium-enhancing; HR: hazard ratio; NEDA: no evidence of disease activity; NfL: neurofilament light chain; RDTN: recently diagnosed, treatment-naive.
RDTN participants were those who had not received a prior disease-modifying therapy and who had received a diagnosis in the 36 months before screening.
The annual rate of brain volume change was estimated according to the slope from a random coefficient model based on assessment of the percentage change from baseline in brain volume performed at month 12, month 24 and the end of treatment and/or trial.
The total number of participants in the treatment group for whom the response variable was defined.
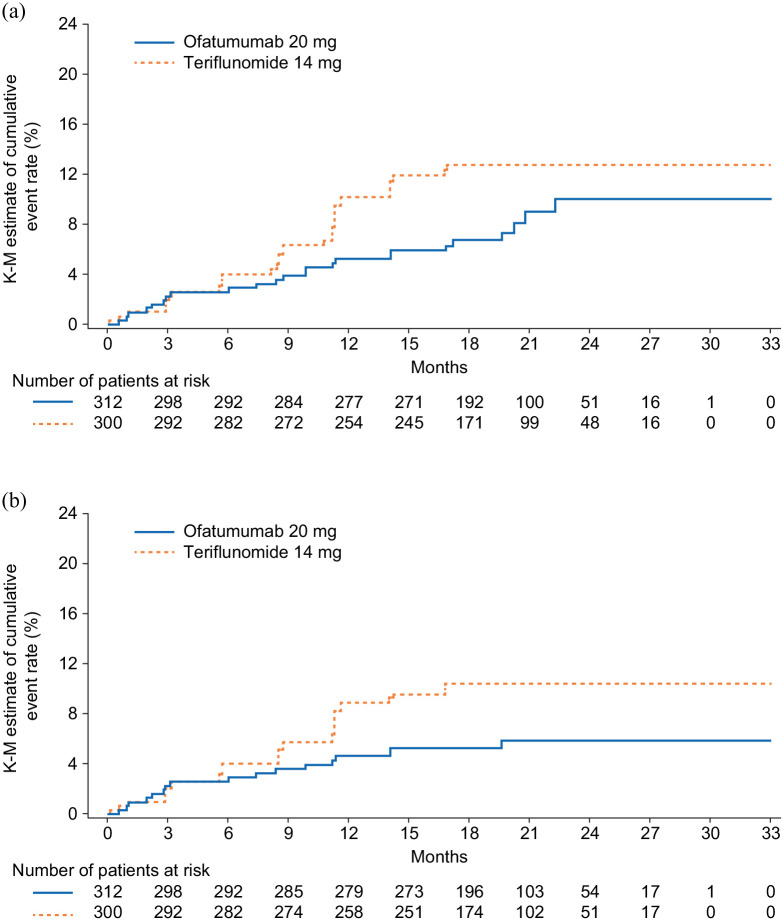
Ofatumumab reduced the risk of 3mCDW numerically by 38% (hazard ratio (HR) (95% CI): 0.62 (0.37, 1.03); p = 0.065) and of 6mCDW by 46% (HR (95% CI): 0.54 (0.30, 0.98); p = 0.044) versus teriflunomide (Figure 1 and Table 2).
Figure 1.
Kaplan–Meier estimates of percentage of patients with disability worsening confirmed at: (a) 3 and (b) 6 months.
Disability worsening confirmed at 3 or 6 months was defined as an increase from baseline in the Expanded Disability Status Scale (EDSS) score (on a scale from 0 to 10.0, with higher scores indicating worse disability) that was sustained for at least 3 or 6 months. For patients with a baseline EDSS score of 0, an increase in the EDSS score of at least 1.5 points was required; for patients with a baseline EDSS score of 1.0 to 5.0, the criterion was an increase of at least 1.0 points; and for patients with a baseline EDSS score of at least 5.5 points, the criterion was an increase of at least 0.5 points.
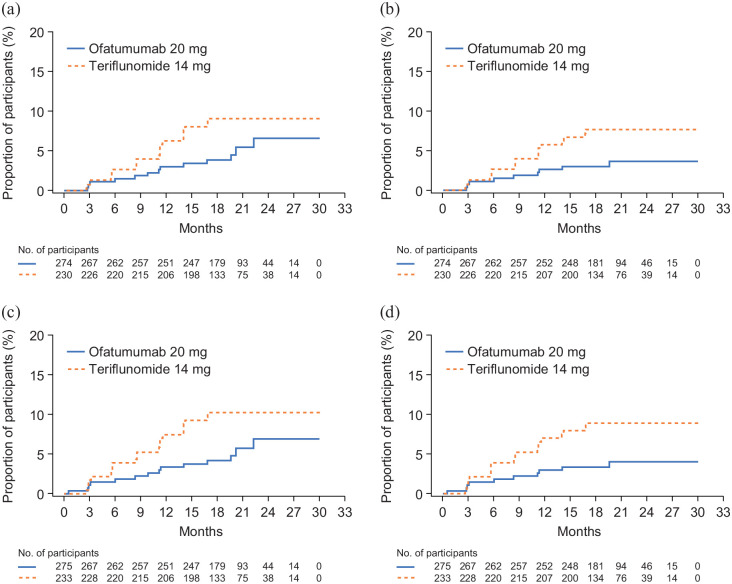
Over half of all 3mCDW events (ofatumumab, 13/24; teriflunomide, 20/37) and 6mCDW events (ofatumumab, 9/17; teriflunomide, 17/30) occurred in the absence of confirmed on-study relapses and were considered PIRA. In the subgroup of participants without confirmed on-study relapses, the proportion of participants with 3mPIRA events was numerically lower, and the proportion with 6mPIRA events significantly lower, with ofatumumab than with teriflunomide (3mPIRA: 6.6% vs. 9.1%; HR (95% CI): 0.55 (0.27, 1.11); p = 0.096); 6mPIRA: 3.6% vs. 7.7%; HR (95% CI): 0.44 (0.20, 1.00); p = 0.049) (Figure 2(a) and (b)). The findings in the subgroup without confirmed on-study relapses before 3mPIRA or 6mPIRA events were similar and significant (3mPIRA: 6.9% vs. 10.2%; HR (95% CI): 0.51 (0.26, 1.00); p = 0.049; 6mPIRA: 4.0% vs. 8.9%; HR (95% CI): 0.41 (0.19, 0.89); p = 0.023) (Figure 2(c) and (d)). In the sensitivity analyses (with PIRA defined such that CDW could not be within 90 days of a relapse), the hazard of a 3mPIRA and 6mPIRA event was numerically lower with ofatumumab than with teriflunomide (3mPIRA, HR (95% CI): 0.62 (0.27, 1.43); p = 0.263; 6mPIRA, HR (95% CI): 0.55 (0.23, 1.36); p = 0.197). Findings in the subgroup of participants without any confirmed on-study relapses before 3mPIRA or 6mPIRA were similar (3mPIRA, HR (95% CI): 0.63 (0.28, 1.40); p = 0.254; 6mPIRA, HR (95% CI): 0.57 (0.24, 1.33); p = 0.194).
Figure 2.
Kaplan–Meier analyses of time to PIRA in RDTN participants: (a) time to 3mPIRA in participants without confirmed relapses on study, (b) time to 6mPIRA in participants without confirmed relapses on study, (c) time to 3mPIRA in participants without confirmed relapses on study or before 3mCDW and (d) time to 6mPIRA in participants without confirmed relapses on study or before 6mCDW.
3mCDW: 3-month confirmed disability worsening; 3mPIRA: 3-month progression independent of relapse activity; 6mCDW: 6 month confirmed disability worsening; 6mPIRA: 6-month progression independent of relapse activity; PIRA: progression independent of relapse activity; RDTN: recently diagnosed, treatment-naive.
MRI-related outcomes
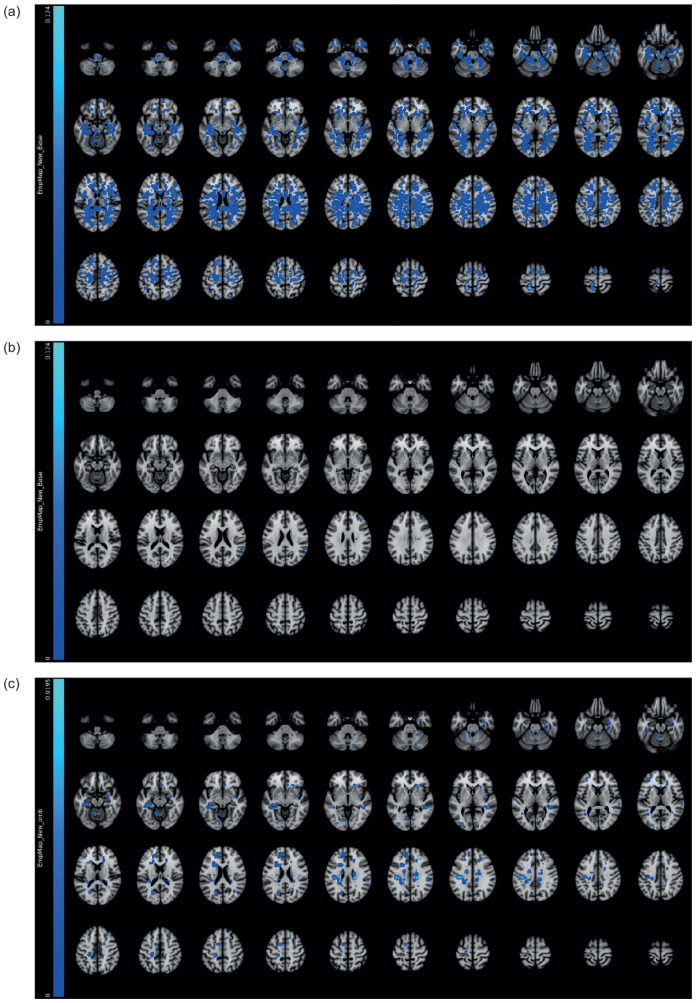
Ofatumumab reduced the mean number of Gd+T1 lesions per scan from baseline to the end of the trial by 95% versus teriflunomide (0.02 vs. 0.39; rate ratio (95% CI): 0.05 (0.02, 0.10); p < 0.001) (Table 2; Figure 3). Ofatumumab also reduced the annualized rate of new or enlarging T2 lesions by 82% versus teriflunomide (Table 2). There was no between-group difference in annual percentage change in brain volume from baseline (Table 2).
Figure 3.
Empirical lesion incidence maps for Gd+T1 lesions in all RDTN participants: (a) at baseline (N = 615); (b) 12 months after initiation of treatment with ofatumumab (N = 314); and (c) 12 months after initiation of treatment with teriflunomide (N = 301).
Gd+, gadolinium-enhancing; RDTN: recently diagnosed, treatment-naive.
No evidence of disease activity 3
Ofatumumab significantly increased the odds of achieving NEDA-3 versus teriflunomide in all study periods evaluated (Table 2). The proportions of ofatumumab-treated and teriflunomide-treated participants achieving NEDA-3 were: 47.0% and 24.7% in year 1, 92.1% and 46.8% in year 2 and 44.6% and 17.7% from baseline through year 2, respectively.
Serum NfL concentration
There was no significant difference in mean serum NfL concentration with ofatumumab and teriflunomide at month 3 (8.72 vs. 9.13 pg/mL; ratio (95% CI): 0.95 (0.88, 1.03); p = 0.258) (Table 2). However, mean serum NfL concentration was significantly lower (both p < 0.001) in the ofatumumab group than in the teriflunomide group at month 12 (6.60 vs. 8.61 pg/mL; ratio (95% CI): 0.77 (0.71, 0.83)) and month 24 (6.47 vs. 8.10 pg/mL; ratio (95% CI): 0.80 (0.74, 0.86)) (Table 2).
Safety in RDTN participants
Safety outcomes are summarized in Table 3; similar proportions of participants experienced AEs in both treatment groups (ofatumumab, 84.7%; teriflunomide, 86.0%). AEs that occurred in at least 10% of participants with ofatumumab were nasopharyngitis, injection-related systemic reactions, headache and upper respiratory tract infections; and with teriflunomide were nasopharyngitis, alopecia, upper respiratory tract infection, injection-related systemic reactions, headache and fatigue. SAEs were reported in 22 participants (7.0%) receiving ofatumumab and 16 (5.3%) receiving teriflunomide. There were no deaths.
Table 3.
AEs in RDTN participants from the ASCLEPIOS I and II trials (safety analysis set).
| Safety event | Ofatumumab (N = 314) | Teriflunomide (N = 301) |
|---|---|---|
| AEs | 266 (84.7) | 259 (86.0) |
| AEs leading to treatment discontinuation | 19 (6.1) | 7 (2.3) |
| Most common AEs (⩾10% in any group) | ||
| Nasopharyngitis | 78 (24.8) | 70 (23.3) |
| Injection-related systemic reaction | 63 (20.1) | 45 (15.0) |
| Headache | 45 (14.3) | 47 (15.6) |
| Upper respiratory tract infection | 40 (12.7) | 49 (16.3) |
| Fatigue | 28 (8.9) | 30 (10.0) |
| Alopecia | 16 (5.1) | 50 (16.6) |
| Infections (all) | 176 (56.1) | 170 (56.5) |
| SAEs | 22 (7.0) | 16 (5.3) |
| Infections a | 6 (1.9) | 2 (0.7) |
| Malignancy | 2 (0.6) b | 1 (0.3) c |
| Deaths | 0 (0.0) | 0 (0.0) |
AE: adverse event; N: total number of participants included in the analysis; RDTN: recently diagnosed, treatment-naive; SAE: serious AE.
Data are shown as the number of participants (%) with at least one event.
Three cases of appendicitis, one case of influenza, one case of neutropenic sepsis, one case of upper respiratory tract infection in the ofatumumab group, one case of appendicitis and one case of pneumonia in the teriflunomide group.
All malignancies were basal cell carcinomas.
One case of basal cell carcinoma was not listed as an SAE.
Injection-related reactions
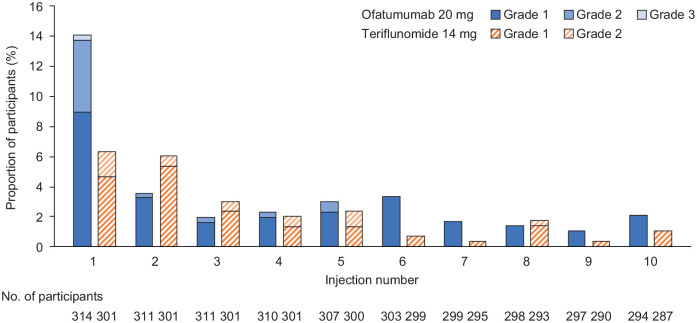
Injection-related systemic reactions were reported for 62 participants (20.1%) receiving ofatumumab and 45 participants (15.0%) in the teriflunomide group receiving placebo injections. Incidence of injection-site reactions was 14.0% (n = 44) and 7.0% (n = 21), respectively. After the first injection, the frequency of injection-related systemic reactions was similar in both groups (Figure 4). Nearly all (99.4%) injection-related reactions (systemic and site) were mild to moderate in severity (no Grade 4 injection-related reactions were reported). Only one participant receiving ofatumumab experienced a Grade 3, non-serious injection-related systemic reaction and discontinued the study treatment. This participant experienced abdominal pain, asthenia, pruritis general and urticaria that resolved within 1 day after treatment with an antihistamine. No anaphylaxis related to ofatumumab treatment was observed. Participants received training and self-administered ofatumumab at day 7, day 14 and month 1 visits, under the supervision of a healthcare provider. From the fifth injection, 48–95% of participants self-injected at home, increasing to 60%–95% from injection 10 onwards.
Figure 4.
Proportion of RDTN participants with injection-related systemic reactions following the first 10 injections in the study.
RDTN: recently diagnosed, treatment-naive.
Only Common Terminology Criteria for Adverse Events grades that were observed in the data are shown. Only reactions/symptoms occurring within 24 hours after injections are included (i.e. time to onset of reaction ⩽ 24 hours).
As teriflunomide is taken as an oral medication, injection-related systemic reactions in participants treated with teriflunomide are in response to placebo injections.
Infections
Similar proportions of participants in both treatment groups experienced infections (ofatumumab, 56.1%; teriflunomide, 56.5%) (Table 3). Nasopharyngitis and upper respiratory tract infection were the most common and were mostly mild to moderate in severity. Six participants (1.9%) receiving ofatumumab and two (0.7%) receiving teriflunomide had serious infections. No opportunistic infections were reported.
Other safety findings
Two malignancies (0.6%) were reported in the ofatumumab group, and one (0.3%) in the teriflunomide group (all basal cell carcinomas). No congenital abnormalities or birth defects were reported among participants exposed to either treatment during pregnancy (ofatumumab, two participants; teriflunomide, three participants). Neutropenia, a known risk associated with teriflunomide, occurred more frequently in the teriflunomide group (four participants) than in the ofatumumab group (two participants).
B- cell levels
B-cell depletion to below the lower limit of normal (LLN) (40 cells/µL) was achieved quickly with ofatumumab. By week 2, 97% of participants had B-cell levels below the LLN and this proportion remained constant until the end of the trial. B-cell depletion of less than or equal to 10 cells/µL was achieved for 90% of patients by week 4, and 98% of patients by week 12 (Supplementary Figure 2). The effect of ofatumumab on B-cell depletion was consistent across body weight quartiles. After the last ofatumumab dose in participants who stopped treatment for any reason, B-cell repletion (levels above the LLN) was observed in 12 of 27 participants (44%) by week 24, 13 of 21 (62%) by week 36, 6 of 8 (75%) by week 48 and 8 of 8 (100%) by week 60.
Discussion
In RDTN participants from the phase III ASCLEPIOS I and II trials, ofatumumab was superior to teriflunomide in reducing relapse rates, delaying all-cause disability worsening, including PIRA, with a near-complete abrogation of new focal inflammatory activity. These findings are consistent with those observed in the overall ASCLEPIOS population 22 and show that ofatumumab can delay disability worsening in early MS.
Among patients early in the MS disease course, disability accrual was previously thought to be exclusively attributable to incomplete recovery from relapses. However, in agreement with other studies of patients with RMS on DMT,4–6 we observed that, when relapses and acute symptoms were suppressed, proportionally more disability events occurred in the form of progression events detected in the course of regular 3-monthly neurological assessments by EDSS raters blinded to treatment. In the RDTN group, most patients (92.3% in the ofatumumab arm versus 87.7% in the teriflunomide arm) remained clinically stable and did not experience any 3mCDW events during the entire study. Approximately half of the observed CDW events in RDTN patients occurred in the absence of overt relapses, although an effect of subclinical relapse biology cannot be ruled out. Progression independent of relapse activity in early relapsing–remitting MS is thought to be related to neurodegenerative processes such as continuous neuroaxonal damage and brain volume loss processes that have been shown to be present from the initial stages of RMS.10,26 In patients with RMS, a high baseline T2 lesion volume has been consistently identified as an important risk factor for on-study brain volume 26 and neuronal 27 loss. This suggests that MS lesion prevention should be a target in the treatment of MS, as it is likely to underlie insidious disease progression.28,29
Ofatumumab delayed disability accrual compared with teriflunomide in RDTN participants by reducing the risk of CDW and PIRA, albeit not significantly in sensitivity analyses that used a more rigorous definition of PIRA requiring no relapses within 90 days of a disability event. The ASCLEPIOS studies were powered to show an effect in the overall combined trials, not in subgroups. Thus, the small sample size and relatively low number of events using the more stringent definitions may explain the lack of statistical significance despite a clinically meaningful effect size.
The findings of this comparative analysis of ofatumumab versus teriflunomide efficacy in early RMS are consistent with those from a network analysis that suggested a benefit for ofatumumab versus other first-line therapies. 23 ARR comparisons in RDTN participants suggest that, on average, patients would experience neurological symptoms manifesting in mostly temporary EDSS score changes once in 11 years with ofatumumab and once in 5.5 years with teriflunomide. MRI findings indicate almost complete abrogation of focal inflammatory disease with ofatumumab and a substantial associated reduction in annual accrual of lesion burden. Consistent with these clinical and radiological findings, RDTN participants receiving ofatumumab had a 3 and 15 times greater likelihood of achieving NEDA-3 during the first and second year of treatment, respectively, versus teriflunomide. Notably, 9 of 10 ofatumumab-treated participants achieved NEDA-3 during year 2, which might better reflect the long-term preventive effect on disease activity and worsening of disability. Achieving NEDA-3 during the first 2 years of treatment has been associated with lower odds of disability at 7–8 years.18,30
The safety and tolerability profile of ofatumumab in RDTN participants was consistent with that in the overall ASCLEPIOS population, 22 with no safety events that would prevent the use of ofatumumab early in MS. Injection-related systemic reactions were mostly mild to moderate and limited to the first injection with ofatumumab; reactions with subsequent injections were largely similar to those observed with placebo injections in the teriflunomide arm. After initial training, most participants self-injected at home. The short-term safety and tolerability profile of ofatumumab also seems to compare favourably with that of other treatments considered suitable for use in early MS, such as interferon-β and glatiramer acetate, although long-term safety data for ofatumumab are not yet available.31,32 Compliance with ofatumumab in RDTN participants was high, consistent with the rates seen in the overall ASCLEPIOS I and II population. The high compliance with ofatumumab in this study lasting 30 months, as compared with other injectable DMTs,33,34 might be explained by the low frequency of injections and lack of need for accompanying medications to prevent or mitigate injection-related adverse events.
Conclusion
Ofatumumab had a superior benefit–risk profile in RDTN patients compared with teriflunomide, with an almost complete abrogation of inflammatory disease activity and no unexpected safety signals, supporting its use as a first-line treatment in early MS.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585221078825 for Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: Results from ASCLEPIOS I and II by Jutta Gärtner, Stephen L Hauser, Amit Bar-Or, Xavier Montalban, Jeffrey A Cohen, Anne H Cross, Kumaran Deiva, Habib Ganjgahi, Dieter A Häring, Bingbing Li, Ratnakar Pingili, Krishnan Ramanathan, Wendy Su, Roman Willi, Bernd Kieseier and Ludwig Kappos in Multiple Sclerosis Journal
Acknowledgments
The authors thank the participants for their involvement in and commitment to the ASCLEPIOS trials and the clinical study team for the conduct of the study. The authors are grateful to Angela Pozo Ramajo (Oxford PharmaGenesis, Oxford, UK) for providing medical writing support. These services were funded by Novartis Pharma AG.
Footnotes
Author Contributions: J. G. contributed to data interpretation; manuscript review for intellectual content; and final manuscript approval. S.L.H. contributed to trial design; data collection and responses to queries; data review; data interpretation; manuscript review for intellectual content; and final manuscript approval. A.B.-O. contributed to trial design; data collection and responses to queries; data review; data interpretation; manuscript review for intellectual content; and final manuscript approval. X.M. contributed to trial design; data collection and responses to queries; data review; data interpretation; manuscript review for intellectual content; and final manuscript approval. J.A.C. contributed to trial design; data collection and responses to queries; data review; data interpretation; manuscript review for intellectual content; and final manuscript approval. A.H.C. contributed to trial design; data collection and responses to queries; data review; data interpretation; manuscript review for intellectual content; and final manuscript approval. K.D. contributed to data interpretation; manuscript review for intellectual content; and final manuscript approval. H.G. contributed to data analysis; manuscript review for intellectual content; and final manuscript approval. D.A.H. contributed to trial design; statistical analysis; data review; data interpretation; planning of this manuscript; review of the manuscript for intellectual content; and final manuscript approval. B.L. contributed to trial design; statistical analysis; data review; data interpretation; review of the manuscript for intellectual content; and final manuscript approval. R.P. contributed to trial design; data review; data interpretation; review of the manuscript for intellectual content; and final manuscript approval. K.R. contributed to trial design; data review; data interpretation; review of the manuscript for intellectual content; and final manuscript approval. W.S. contributed to data review; data interpretation; review of the manuscript for intellectual content; and final manuscript approval. R.W. contributed to trial design; data review; data interpretation; review of the manuscript for intellectual content; and final manuscript approval. B.K. contributed to data interpretation; review of the manuscript for intellectual content; and final manuscript approval. L.K. contributed to trial design; data collection and responses to queries; data review; data interpretation; manuscript review for intellectual content; and final manuscript approval.
Data Availability Statement: ASCLEPIOS I and II trial data are available on reasonable request provided the reason for the request is in line with current ethical and intellectual property requirements surrounding the use of data. Requests should be directed through ClinicalStudyDataRequest.com.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: J.G., in the past 3 years, has received fees for lectures and consultancy fees from Bayer, Biogen, Merck, Novartis and Sanofi, as well as funding for a research project from Novartis. S.L.H. has received personal compensation from Alector, Annexon Biosciences, Bionure and Neurona Therapeutics; he has also received travel reimbursement from F. Hoffmann-La Roche and Novartis for CD20-related meetings and presentations. A.B.-O. has participated as a speaker in meetings sponsored by and received consulting fees and/or grant support from Atara Biotherapeutics, Biogen Idec, Celgene/Receptos, Janssen/Actelion, MedImmune, Merck/EMD Serono, Novartis, Roche/Genentech and Sanofi Genzyme. X.M. has received speaking fees and travel expenses for participation in scientific meetings, has been a steering committee member for clinical trials, or has participated in advisory boards for clinical trials in the past years with Actelion, Alexion Pharmaceuticals, Bayer, Biogen, Celgene, EMD Serono, EXCEMED, Genzyme, Immunic, MedDay, Merck, the MS International Federation, Mylan, NervGen Pharma, the National Multiple Sclerosis Society, Novartis, Roche, Sanofi Genzyme, Teva Pharmaceuticals and TG Therapeutics. J.A.C. has received personal compensation for consulting from Adamas Pharmaceuticals, Atara Biotherapeutics, Bristol Myers Squibb, Convelo Therapeutics, MedDay and Mylan, and for serving as an editor of the Multiple Sclerosis Journal. A.H.C. has consulted for Biogen, Celgene, EMD Serono, Genentech/Roche, Novartis and TG Therapeutics. K.D. has received personal compensation for speaker activities from Novartis and Sanofi. In the past 3 years, L.K.’s institution (University Hospital of Basel) has received steering committee, advisory board and consultancy fees used exclusively for research support in the department, as well as support of educational activities, from Actelion, Allergan, Almirall, Baxalta, Bayer, Biogen, Celgene/Receptos, CSL Behring, Desitin, Eisai, EXCEMED, F. Hoffmann-La Roche, Genzyme, Japan Tobacco, Merck, Minoryx Therapeutics, Novartis, Pfizer, Sanofi Aventis, Santhera Pharmaceuticals and Teva Pharmaceuticals, and license fees for Neurostatus-UHB products. Research at the MS Center in Basel has been supported by grants from Bayer, Biogen, the European Union, Inno-Suisse, Novartis, the Swiss MS Society, the Swiss National Research Foundation and Roche research foundations. D.A.H., K.R., R.W. and B.K. are employees of Novartis Pharma AG, Basel, Switzerland. B.L., R.P. and W.S. are employees of Novartis Pharmaceutical Corporation, East Hanover, NJ, USA.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was funded by Novartis Pharma AG, Basel, Switzerland. Novartis Pharma AG supported the development of this manuscript, provided data analyses according to the direction of the authors, and paid for medical writing support.
ORCID iDs: Stephen L Hauser  https://orcid.org/0000-0002-4932-4001
https://orcid.org/0000-0002-4932-4001
Jeffrey A Cohen  https://orcid.org/0000-0001-9245-9772
https://orcid.org/0000-0001-9245-9772
Anne H Cross  https://orcid.org/0000-0003-0829-7569
https://orcid.org/0000-0003-0829-7569
Roman Willi  https://orcid.org/0000-0001-9612-9877
https://orcid.org/0000-0001-9612-9877
Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jutta Gärtner, Department of Paediatrics and Adolescent Medicine, Division of Paediatric Neurology, University Medical Centre Göttingen, Georg August University Göttingen, Göttingen, Germany.
Stephen L Hauser, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California – San Francisco, San Francisco, CA, USA.
Amit Bar-Or, Center for Neuroinflammation and Experimental Therapeutics and Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Xavier Montalban, Department of Neurology-Neuroimmunology, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Hospital Universitari Vall d’Hebron, Barcelona, Spain.
Jeffrey A Cohen, Department of Neurology, Mellen MS Center, Neurological Institute, Cleveland Clinic, Cleveland, OH, USA.
Anne H Cross, Department of Neurology, Section of Neuroimmunology, Washington University School of Medicine, St Louis, MO, USA.
Kumaran Deiva, Department of Pediatric Neurology, University Hospitals Paris Saclay, Hôpital Bicêtre, National Reference Center for Rare Inflammatory Brain and Spinal Diseases, Le Kremlin-Bicêtre, France.
Habib Ganjgahi, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Population Health, University of Oxford, Oxford, UK/Statistics Department, University of Oxford, Oxford, UK.
Dieter A Häring, Novartis Pharma AG, Basel, Switzerland.
Bingbing Li, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Ratnakar Pingili, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Krishnan Ramanathan, Novartis Pharma AG, Basel, Switzerland.
Wendy Su, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Roman Willi, Novartis Pharma AG, Basel, Switzerland.
Bernd Kieseier, Novartis Pharma AG, Basel, Switzerland.
Ludwig Kappos, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB) and MS Center, and Departments of Medicine, Clinical Research, Biomedicine and Biomedical Engineering, University Hospital of Basel, University of Basel, Basel, Switzerland.
References
- 1. Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers 2018; 4(1): 43. [DOI] [PubMed] [Google Scholar]
- 2. Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010; 9(5): 520–532. [DOI] [PubMed] [Google Scholar]
- 3. Scott TF. Understanding the impact of relapses in the overall course of MS; refinement of the 2 stage natural history model. J Neuroimmunol 2017; 305: 162–166. [DOI] [PubMed] [Google Scholar]
- 4. Kappos L, Butzkueven H, Wiendl H, et al. Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler 2018; 24(7): 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 2020; 77: 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. University of California San Francisco MS-EPIC Team, Cree BAC, Hollenbach JA, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol 2019; 85(5): 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pfeifenbring S, Bunyan RF, Metz I, et al. Extensive acute axonal damage in pediatric multiple sclerosis lesions. Ann Neurol 2015; 77(4): 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141(8): 2382–2391. [DOI] [PubMed] [Google Scholar]
- 9. De Stefano N, Giorgio A, Battaglini M, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology 2010; 74(23): 1868–1876. [DOI] [PubMed] [Google Scholar]
- 10. Dahlke F, Arnold DL, Aarden P, et al. Characterisation of MS phenotypes across the age span using a novel data set integrating 34 clinical trials (NO.MS cohort): Age is a key contributor to presentation. Mult Scler 2021; 27(13): 2062–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andravizou A, Dardiotis E, Artemiadis A, et al. Brain atrophy in multiple sclerosis: Mechanisms, clinical relevance and treatment options. Auto Immun Highlights 2019; 10(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banwell B, Arnold DL, Tillema JM, et al. MRI in the evaluation of pediatric multiple sclerosis. Neurology 2016; 87(9 Suppl 2): S88–S96. [DOI] [PubMed] [Google Scholar]
- 13. Traboulsee AL, Cornelisse P, Sandberg-Wollheim M, et al. Prognostic factors for long-term outcomes in relapsing-remitting multiple sclerosis. Mult Scler J Exp Transl Clin 2016; 2: 2055217316666406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisher KS, Cuascut FX, Rivera VM, et al. Current advances in pediatric onset multiple sclerosis. Biomedicines 2020; 8(4): 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gärtner J, Chitnis T, Ghezzi A, et al. Relapse rate and MRI activity in young adult patients with multiple sclerosis: A post hoc analysis of phase 3 fingolimod trials. Mult Scler J Exp Transl Clin 2018; 4(2): 2055217318778610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghezzi A, Baroncini D, Zaffaroni M, et al. Pediatric versus adult MS: Similar or different? Mult Scler Demyelinating Disord 2017; 2(1): 5. [Google Scholar]
- 17. Merkel B, Butzkueven H, Traboulsee AL, et al. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: A systematic review. Autoimmun Rev 2017; 16(6): 658–665. [DOI] [PubMed] [Google Scholar]
- 18. Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72(2): 152–158. [DOI] [PubMed] [Google Scholar]
- 19. Engelberts PJ, Voorhorst M, Schuurman J, et al. Type I CD20 antibodies recruit the B cell receptor for complement-dependent lysis of malignant B cells. J Immunol 2016; 197(12): 4829–4837. [DOI] [PubMed] [Google Scholar]
- 20. Kesimpta® prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf (2020, accessed 15 December 2020).
- 21. Kesimpta® summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/kesimpta-epar-product-information_en.pdf (2021, accessed 23 April 2021).
- 22. Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med 2020; 383(6): 546–557. [DOI] [PubMed] [Google Scholar]
- 23. Samjoo IA, Worthington E, Drudge C, et al. Comparison of ofatumumab and other disease-modifying therapies for relapsing multiple sclerosis: A network meta-analysis. J Comp Eff Res 2020; 9: 1255–1274. [DOI] [PubMed] [Google Scholar]
- 24. Havrdová E, Arnold DL, Bar-Or A, et al. No evidence of disease activity (NEDA) analysis by epochs in patients with relapsing multiple sclerosis treated with ocrelizumab vs interferon beta-1a. Mult Scler J Exp Transl Clin 2018; 4(1): 2055217318760642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (2017, accessed 13 November 2020).
- 26. De Stefano N, Silva DG, Barnett MH. Effect of fingolimod on brain volume loss in patients with multiple sclerosis. CNS Drugs 2017; 31(4): 289–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology 2015; 84(8): 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krieger SC, Cook K, De Nino S, et al. The topographical model of multiple sclerosis: A dynamic visualization of disease course. Neurol Neuroimmunol Neuroinflamm 2016; 3(5): e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. University of California San Francisco MS-EPIC Team, Cree BA, Gourraud PA, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 2016; 80(4): 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology 2002; 59(9): 1412–1420. [DOI] [PubMed] [Google Scholar]
- 31. Bar-Or A, Grove RA, Austin DJ, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study. Neurology 2018; 90(20): e1805–e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. La Mantia L, Di Pietrantonj C, Rovaris M, et al. Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev 2016; 11: CD009333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cross AH, Naismith RT. Established and novel disease-modifying treatments in multiple sclerosis. J Intern Med 2014; 275(4): 350–363. [DOI] [PubMed] [Google Scholar]
- 34. Lebrun-Frenay C, Moulignier A, Pierrot-Deseilligny C, et al. Five-year outcome in the copaxone observatory: A nationwide cohort of patients with multiple sclerosis starting treatment with glatiramer acetate in France. J Neurol 2019; 266(4): 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585221078825 for Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: Results from ASCLEPIOS I and II by Jutta Gärtner, Stephen L Hauser, Amit Bar-Or, Xavier Montalban, Jeffrey A Cohen, Anne H Cross, Kumaran Deiva, Habib Ganjgahi, Dieter A Häring, Bingbing Li, Ratnakar Pingili, Krishnan Ramanathan, Wendy Su, Roman Willi, Bernd Kieseier and Ludwig Kappos in Multiple Sclerosis Journal