Abstract
Objective
The coronavirus disease 2019 (COVID-19) vaccines are considered to be an effective way to prevent the spread of the infection. Our previous study has shown that about 75% of healthcare workers (HCWs) in China were willing to receive the vaccine when it became available. Here, we examined the acceptance of a third booster dose among Chinese people and identified the influencing factors.
Methods
A cross-sectional online survey was conducted and the snowball sampling method was utilized. An online questionnaire was provided to all the participants in the form of a quick response (QR) code. The questionnaire included general demographic information, views on vaccines, the General Health Questionnaire-12 (GHQ-12), and the Depression, Anxiety, and Stress Scale-21 (DASS-21). The univariate analysis was done between all the variables and our dependent variable. Then, we used the multivariate logistic regression model to examine the influencing factors of the third booster dose acceptance.
Results
We collected 1,062 complete answers. Of these, 90.39% (n = 960) declared that they would accept the booster dose. Knowing more about the vaccine and recognizing the efficacy of vaccines were significantly associated with greater acceptance of the booster dose. People willing to take the booster dose had better psychological health. A belief that the booster dose could prevent severe infection caused by COVID-19 and enhance the effectiveness of the first two doses were the main contributing factors to vaccine acceptance. Vaccine hesitancy was mainly due to a low perceived risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and rapid mutation of SARS-CoV-2.
Conclusion
This study revealed that Chinese people were very receptive to the third booster dose, which is an inspiring result. More positive attitudes regarding COVID-19 vaccination were supported by its efficacy and few side effects.
Keywords: COVID-19, vaccine, acceptance, third booster dose, Chinese people
Introduction
The ongoing pandemic of coronavirus disease 2019 (COVID-19) has caused extensive damage worldwide. Despite established supportive therapies and the development of new antiviral drugs, vaccination is considered an effective method to prevent infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), particularly for at-risk populations. In China, the COVID-19 vaccines started to be used in priority groups on 15 December 2020. The Chinese government accelerated free vaccination for all the Chinese citizens starting in late March 2021 (1). As of 18 February 2022, more than three billion COVID-19 vaccine doses had been given nationwide and more than 1.2 people had completed the two-dose regimen (2).
However, typically, vaccine-induced immunity diminishes over time. Also, newly emerging SARS-CoV-2 variants can evade the immunity primed with vaccines developed against older variants (3). Hence, the need for a third dose of the COVID-19 vaccine has been discussed (4).
Increasing numbers of studies have suggested that a third booster dose could induce robust cellular and humoral immunity, thereby mitigating the fading of neutralizing antibodies after inoculation with two doses and reducing the risk of SARS-CoV-2 infection (5–9). A systematic review of 30 published studies on the efficacy and safety of the third dose of the COVID-19 vaccine has suggested that the reduction in the risk of infection ranged from 88 to 92% and conversion rates for immunoglobulin G ranged from 95 to 100% (10). Also, in cancer cases or immunocompromised patients, a significant increase in the antibody titer was noted (10). Studies have also shown that the third dose of the COVID-19 vaccine can reduce the risk of severe illness (5). Meanwhile, the safety of the third dose of the COVID-19 vaccine has been shown in several studies. A study about the safety and immunogenicity of the third dose in Chinese adults indicated that the third dose with either homologous or heterologous vaccine showed favorable safety profiles (3). A randomized controlled trial has shown that there were no serious side effects within 28 days after the third dose (9). Alasdair Munro et al. have assessed the safety of seven COVID-19 vaccines as a third dose and found that serious adverse events were uncommon and similar in active vaccine and control groups (11). Although all the studies mentioned above revealed the safety and immunogenicity of the third dose of a COVID-19 vaccine, whether people are willing to receive the third dose of the COVID-19 vaccine is still unknown. A total of 54.6% of Chinese people reported “probably yes” regarding their intent to accept the COVID-19 vaccine before it was developed (12). We previously reported that only one-third of healthcare workers (HCWs) in China were willing to take the COVID-19 vaccine when it became available (13). A total of 58.2% of medical students reported vaccine hesitancy months after the COVID-19 vaccine became available for use (14). Here, we examined the acceptance of the third booster dose among Chinese people and assessed the factors associated with its acceptance.
Materials and Methods
Study Participants
The study protocol was approved (2022-S-27) by the Ethics Committee of Third People's Hospital of Chengdu (Chengdu, China). Informed consent was obtained before study enrollment. This was a cross-sectional online survey using a social media platform (WeChat™)-based survey program “Questionnaire Star” between 1 December and 31 December 2021. The online questionnaire was provided to all the participants in the form of a quick response (QR) code via WeChat. The snowball sampling method was utilized; thus, agreeable participants could send the QR code to their respective WeChat friends in the same way. Participants answered the questions on the questionnaire by scanning the QR code. Participation was voluntary and the responses were anonymous. All the adults (>18 years of age), regardless of region, occupation, or status, were eligible to participate in our study.
Questionnaire Design and Data Collection
Before initiating the study, we first consulted psychologists working at the Third People's Hospital of Chengdu and the psychologists recommended two commonly used scales, as described below, suitable for measuring psychological status. The questionnaire gathered information on: (i) general demographics (e.g., sex, age, education level, occupation, marital status, children, and income); (ii) whether or not to accept the third booster dose; and (iii) the reasons for being willing or not willing to be vaccinated. Moreover, this questionnaire included the General Health Questionnaire-12 (GHQ-12) and the Depression, Anxiety, and Stress Scale-21 (DASS-21) to investigate the psychological health of respondents.
Participants were asked if they would accept the third booster dose of the COVID-19 vaccine. They rated the item regarding their attitudes toward a booster dose on a four-point scale (1 = strongly agree, 2 = agree, 3 = disagree, and 4 = strongly disagree). Those answering 1 or 2 were identified as the vaccine-agree group. Those who answered 3 or 4 were identified as the vaccine-disagree group. Then, we listed common or possible causes of people's willingness or unwillingness to be vaccinated. People who agreed to take the vaccine could voluntarily select factors promoting their willingness to be vaccinated. Those who did not agree to take the vaccine could voluntarily choose the factors causing their resistance. The percentage of people who chose different factors was calculated.
With the help of a consultant psychologist, we chose the scores for the GHQ-12 and the DASS-21 to measure participants' mental health. The GHQ-12 is widely used to identify common psychiatric conditions (15, 16). The questionnaire consists of 12 items, where each is assessed with a four-point Likert scale ranging from “never” to “often” and is used with the 0–0–1–1 scoring method. The total score ranges between 0 and 12 points (poor mental health was defined as a total score ≥3) (17). The higher the score, the more significant the mental problem. The DASS-21 is a popular measure of mental health (18, 19). It consists of the subscales of depression, anxiety, and stress with 21 items (seven items for each subscale). Each seven-item subscale is rated on a four-point Likert scale ranging from 0 (“Did not apply to me at all”) to 3 (“Applied to me very much”). The higher the score, the more significant is the mental problem (20, 21).
Only complete questionnaires could be collected and incomplete data could not be submitted through the Questionnaire Star. The Questionnaire Star automatically collected data. We could convert all the data into text format and numeric form and export them to spreadsheets.
Statistical Analysis
Descriptive analyses were conducted on all the study variables, which were reported as the mean, SD, number (n), and percentage. We used the univariate analysis with the t-test or the chi-squared test to compare the two groups (agree and disagree) to identify the factors associated with vaccine hesitancy. Then, the multivariate logistic regression model was employed to examine and identify the factors associated with the acceptance of the COVID-19 vaccine. Statistical analyses were performed using SPSS version 23.0 (IBM Incorporation, Armonk, New York, USA). p ≤ 0.05 was considered statistically significant.
Results
Acceptance of the Third Booster Dose
We collected 1,062 responses. Of these, 960 responders (90.39%) declared that they would accept a booster dose. Of those who were willing to accept the third booster dose, 884 responders (83.2%) were strongly willing and 76 responders (7.1%) were willing but waiting to review more data. Of the 1,062 participants, only 102 (9.6%) participants were not willing to accept the third booster dose. Of them, 71 (6.7%) participants did not plan to receive the COVID-19 vaccine and 31 (2.9%) participants were strongly against taking the booster dose. We found that 95.6% (1,016) of respondents had completed the two-dose regimen and 97.3% (935) of respondents who had completed the two doses were willing to receive the third dose (Table 1).
Table 1.
Demographics of study population and the univariate analysis between the agree and disagree groups (n = 1,062).
| Variable | n = 1,062 (percentage) |
Agree group (n = 960)
(percentage) |
Disagree group (n = 102)
(Percentage) |
χ2/t | p-Value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 406 (38.2) | 371 (38.6) | 35 (34.3) | 0.733 | 0.392 |
| Female | 656 (61.8) | 589 (61.4) | 67 (65.7) | ||
| Age (years) | |||||
| <18 | 3 (0.3) | 2 (0.2) | 1 (1.0) | 3.825 | 0.407 |
| 18–30 | 377 (35.5) | 346 (36.1) | 31 (30.4) | ||
| 31–40 | 305 (28.7) | 272 (28.4) | 33 (32.4) | ||
| 41–50 | 196 (18.5) | 177 (18.4) | 19 (18.6) | ||
| >50 | 181 (17.0) | 163 (16.9) | 18 (17.6) | ||
| Healthcare workers | |||||
| Yes | 497 (46.7) | 457 (47.6) | 40 (39.2) | 2.606 | 0.106 |
| No | 565 (53.3) | 503 (52.4) | 62 (60.8) | ||
| Education | |||||
| Junior high and below | 93 (8.7) | 86 (8.9) | 7 (6.9) | 4.785 | 0.187 |
| Senior school | 97 (9.1) | 90 (9.3) | 7 (6.9) | ||
| Bachelor | 724 (68.1) | 657 (68.5) | 67 (65.7) | ||
| Postgraduate | 148 (13.9) | 127 (13.3) | 21 (20.5) | ||
| Marital status | |||||
| Married | 725 (68.2) | 652 (67.9) | 73 (71.6) | 0.568 | 0.451 |
| Single | 337 (31.8) | 308 (32.1) | 29 (28.4) | ||
| Children | |||||
| Yes | 682 (64.2) | 621 (64.6) | 61 (59.8) | 0.957 | 0.328 |
| No | 380 (35.8) | 339 (35.4) | 41 (40.2) | ||
| Living with elderly individuals | |||||
| Yes | 538 (50.6) | 493 (51.3) | 45 (44.1) | 1.932 | 0.165 |
| No | 524 (49.4) | 467 (48.6) | 57 (55.9) | ||
| Influenza vaccination in 2020 | |||||
| Yes | 525 (49.4) | 482 (50.2) | 43 (42.2) | 2.391 | 0.122 |
| No | 537 (50.6) | 478 (4.8) | 59 (57.8) | ||
| Worried about experiencing COVID-19 | |||||
| Yes | 666 (62.7) | 613 (63.8) | 53 (52.0) | 5.577 | 0.018* |
| No | 396 (37.3) | 347 (36.2) | 49 (48.0) | ||
| Understanding how the vaccine works | |||||
| Yes | 1,017 (4.3) | 924 (96.2) | 93 (91.2) | 5.849 | 0.016* |
| No | 45 (95.7) | 36 (3.8) | 9 (8.8) | ||
| Completion of COVID-19 vaccination with a two-dose regimen | |||||
| Yes | 1,016 (95.6) | 935 (97.3) | 81 (79.4) | 71.965 | <0.001** |
| No | 46 (4.4) | 25 (2.7) | 21 (20.6) | ||
| The vaccine is effective | |||||
| Yes | 925 (87.1) | 867 (90.3) | 58 (56.9) | 91.816 | <0.001** |
| No | 137 (12.9) | 93 (9.7) | 44 (43.1) | ||
| COVID contact or living in a high-risk area | |||||
| Yes | 86 (8.1) | 81 (8.4) | 5 (4.9) | 1.549 | 0.213 |
| No | 976 (91.9) | 879 (91.6) | 97 (95.1) | ||
| Income (RMB) | |||||
| <5,000 | 412 (38.8) | 381 (39.6) | 31 (30.4) | 4.034 | 0.134 |
| 5,000–10,000 | 465 (43.7) | 417 (43.5) | 48 (47.1) | ||
| >10,000 | 185 (17.5) | 162 (16.9) | 23 (22.5) | ||
| Effect of COVID-19 | |||||
| Not at al | 40 (3.7) | 38 (3.9) | 2 (2.0) | 6.842 | 0.086 |
| Mild | 67 (6.3) | 55 (5.7) | 12 (11.8) | ||
| Moderate | 581 (54.7) | 528 (55.1) | 53 (51.9) | ||
| Severe | 374 (35.3) | 339 (35.3) | 35 (34.3) | ||
| GHQ-12 score | |||||
| ≥3 | 105 (9.9) | 88 (9.2) | 17 (16.7) | 5.821 | 0.016* |
| <3 | 957 (90.1) | 872 (90.8) | 85 (83.3) | ||
| DASS-21 | |||||
| Depression (mean ± SD) | 1,062 | 3.61 ± 3.18 | 5.75 ± 4.10 | 9.378 | 0.002* |
| Anxiety (mean ± SD) | 1,062 | 2.71 ± 3.25 | 3.93 ± 4.33 | 17.03 | <0.001** |
| Stress (mean ± SD) | 1,062 | 3.73 ± 3.77 | 4.96 ± 4.21 | 2.475 | 0.116 |
*p < 0.05.
**p < 0.001.
Variables Associated With Acceptance of the Third Booster Dose
The univariate analysis was used to decide significant differences between the two groups (the agree and disagree groups; Table 1). There were significant differences among the respondents in worrying about experiencing COVID-19, understanding how the vaccine works, completing a two-dose regimen, thinking that the vaccine works, and having good mental health. Results of the univariate analysis are shown in Table 1. Individuals willing to receive the booster dose were more worried about infection (χ2 = 5.577, p = 0.018), had a better understanding of how the vaccine works (χ2 = 5.849, p = 0.016), had nearly completed a two-dose regimen, were more likely to think that the vaccine works (χ2 = 91.816, p = 0.000), and had better mental health based on the GHQ-12 (χ2 = 14.805, p = 0.002; depression: F = 9.378, p = 0.002; anxiety: F = 17.03, p = 0.000). However, the two groups did not differ significantly in terms of sex, age, occupation, educational level, marital status, contact with a person with COVID-19, living in a high-risk area, or income.
To determine the factors associated with the willingness to be vaccinated, we used the multivariate logistic regression models. Results are given in Table 2. Five factors were significantly associated with the acceptance of the third booster dose: (a) worried about experiencing COVID-19; (b) understanding how the vaccine works; (c) completion of COVID-19 vaccination with a two-dose regimen; (d) the vaccine is effective; and (e) the GHQ-12 score. Those with a high perceived risk of being infected had twice the odds of vaccine acceptance compared with those with no perceived risk of being infected [odds ratio (OR) = 2.159; 95% CI = 1.331–3.501; p = 0.002]. Additionally, those who knew more about vaccine properties were almost three times more likely to accept the third booster dose (OR: 2.879; 95% CI: 1.143–7.148; p = 0.025). People who had completed the two-dose regimen were more likely to accept the third dose compared with those who had not completed the two-dose regimen (OR: 9.708; 95% CI: 4.474–21.069; p = 0.000). People who believed that the vaccine was effective had 7.949 times greater odds of accepting the third dose compared with those who believed that the vaccine was not effective (OR: 7.949; 95% CI: 4.769–13.251; p = 0.000). People with the lower GHQ-12 score were more likely to accept the third dose (OR: 2.903; 95% CI: 1.476–5.708; p = 0.002) compared to those with the higher GHQ-12.
Table 2.
The multivariate logistic regression analyses showing the factors associated with acceptance of a booster dose (n = 1,062).
| Variable | OR | 95%CI | p-Value |
|---|---|---|---|
| Sex | |||
| Male (reference) | 1 | ||
| Female | 0.673 | 0.401–1.130 | 0.134 |
| Age (years) | |||
| <18 (reference) | 1 | ||
| 18–30 | 0.372 | 0.021–6.517 | 0.498 |
| 31–40 | 1.409 | 0.539–3.687 | 0.484 |
| 41–50 | 1.201 | 0.556–2.596 | 0.641 |
| >50 | 0.862 | 0.388–1.915 | 0.716 |
| Healthcare workers | |||
| Yes (reference) | 1 | ||
| No | 0.694 | 0.409–1.180 | 0.177 |
| Education | |||
| Junior high and below (reference) | 1 | ||
| Senior school | 2.225 | 0.687–7.198 | 0.182 |
| Bachelor | 2.220 | 0.753–6.550 | 0.148 |
| Postgraduate | 1.611 | 0.868–2.991 | 0.131 |
| Marital status | |||
| Married (reference) | 1 | ||
| Single | 2.099 | 0.940–4.690 | 0.071 |
| Children | |||
| No (reference) | 1 | ||
| Yes | 1.913 | 0.864–2.488 | 0.122 |
| Living with elderly individuals | |||
| No (reference) | 1 | ||
| Yes | 1.466 | 0.824–2.448 | 0.156 |
| Influenza vaccination in 2020 | |||
| No (reference) | 1 | ||
| Yes | 1.215 | 0.756–1.950 | 0.421 |
| Worried about experiencing COVID-19 | |||
| No (reference) | 1 | ||
| Yes | 2.159 | 1.331–3.501 | 0.002* |
| Understanding of the vaccine | |||
| No (reference) | 1 | ||
| Yes | 2.879 | 1.143–7.248 | 0.025* |
| Completion of COVID-19 vaccination with a two-dose regimen | |||
| No (reference) | 1 | ||
| Yes | 9.708 | 4.474–21.069 | <0.001** |
| The vaccine is effective | |||
| No (reference) | 1 | ||
| Yes | 7.949 | 4.769–13.251 | <0.001** |
| COVID contact or living in a high risk area | |||
| No (reference) | 1 | ||
| Yes | 1.659 | 0.607–4.533 | 0.323 |
| Income (RMB) | |||
| <5,000 (reference) | 1 | ||
| 5,000–10,000 | 1.662 | 0.747–3.694 | 0.213 |
| >10,000 | 1.007 | 0.524–1.935 | 0.984 |
| GHQ score | |||
| ≥3 (reference) | 1 | ||
| <3 | 2.903 | 1.476–5.708 | 0.002* |
*p < 0.05.
** p < 0.001. OR, odds ratio; CI, confidence interval.
Reasons for Willingness or Unwillingness to Be Vaccinated
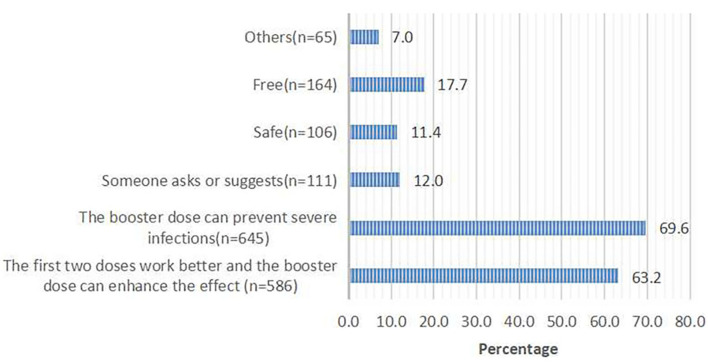
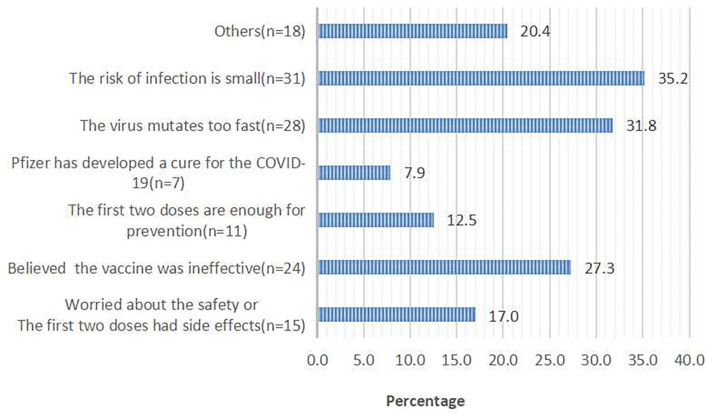
Of 960 respondents who were willing to receive the third dose, 927 (96.6%) respondents chose the reasons for accepting the third dose. Of 102 respondents who were not willing to receive the third dose, 88 (86.3%) respondents chose the reasons for rejecting the third dose. The top two reasons for accepting the third dose were that the booster dose can prevent severe infections (69.6%) and enhance the effect of the first two doses (63.2%; Figure 1). The top two reasons for rejecting the third dose were a perceived low risk of infection (35.2%) and rapid mutation of SARS-CoV-2 (31.8%; Figure 2).
Figure 1.
The main contributing factors for taking the third booster dose; responses from 960 participants who said they would accept the booster dose.
Figure 2.
Reasons for hesitancy in taking the third booster dose; responses from 102 participants who said they would refuse the booster dose.
Discussion
Despite newly approved antiviral drugs, the role of vaccination remains crucial. However, the efficacy of vaccination diminishes over time. Cohn and their colleagues have demonstrated that the efficacy of the COVID-19 vaccine against infection declined from 87 to 48% from February to October 2021 (22). Protection levels against SARS-CoV-2 (including the Delta variant) infection or symptomatic infection decline over time (23–25). Therefore, policymakers have begun to consider using the third booster dose to improve protection.
Most surveys on COVID-19 have focused on the safety and immunogenicity of the third dose. Only a few studies have assessed the acceptance of the third booster dose. Suman and their colleagues have found that nearly two-thirds of respondents were concerned that vaccination may be ineffective against new strains of SARS-CoV-2 and that booster doses may be required. However, acceptance by vaccine-hesitant respondents of a hypothetical booster dose was only 14.3% (26). Another study has indicated that 84.5% of medical students were willing to receive the third dose of the COVID-19 vaccine (4). Those studies were aimed at HCWs and were conducted from August to October 2021. However, after those studies, an increasing number of studies have demonstrated the efficacy and safety of the third booster dose. Hence, what are the attitudes of the general population toward the booster dose? What are the influencing factors? Our study provided some answers to those questions.
In our study, 90.39% of participants declared that they would accept the booster dose. This acceptance rate was higher than that of the first two doses in our previous study (76.63%) (13) among HCWs in the USA (36%) (27), among the general adult population in Kuwait (53.1%) (28), among citizens of the Democratic Republic of Congo (55.9%) (29), and among the general population in some other low- and middle-income countries in Asia and Africa (55%−80%) (30). Studies have found major barriers concerning the safety and efficacy of the COVID-19 vaccine and the rapid mutation of SARS-CoV-2 (13, 31). However, the first two factors became the motivator for the third booster dose in the present study. Having a low perceived risk of COVID-19 was an important factor for vaccine hesitancy, an issue not addressed in previous studies except in one review, in which the author has drawn a similar conclusion by analyzing some phenomena among Muslims (32). This perception may occur because people felt that they had already benefited from the first two doses and surveillance and control measures had been taken by the government. Additionally, worrying about the rapid mutation of SARS-CoV-2 was one of the primary factors making people hesitant about taking the booster dose. We included anti-COVID-19 drugs as a factor in the options and 7.9% of participants chose it as a factor, most of whom concurrently chose the factor “worried about the efficacy and safety of vaccines.” This finding suggested that some people prefer to believe that drugs, rather than vaccination, can be used to treat COVID-19, which may be related to the safety and side effects of vaccines and the uncertainty of the efficacy of vaccines.
Our study indicated that people with a high perceived risk of being infected had twice the odds of vaccine acceptance compared with those without a perceived risk of being infected. This concern is not surprising given that vaccines remain the main protection method against COVID-19. This result is similar to the findings noted by Harapan and collaborators and Rajamoorthy and coworkers (33, 34). Another study has also found that a high perceived risk of COVID-19 was associated with the acceptance of the COVID-19 vaccine among HCWs in China (35). We found that ~40% of respondents did not worry about experiencing COVID-19. This observation may be, at least in part, due to the effective control of the COVID-19 epidemic in China. In China, everyone was required to wear a face-covering mask outdoors during the COVID-19 epidemic. Especially during the COVID-19 epidemic outbreak, people were forbidden to gather in a public places, such as karaoke, bars, or movie venues, workers were encouraged to hold online meetings, and frontline HCWs were asked to take PCR tests twice a week. Hence, effective protective measures are also extremely important besides vaccination.
Our study indicated that learning more about the COVID-19 vaccine might have contributed to people being more willing to take the vaccine compared with those who had known less about the vaccine. This finding is consistent with data from our previous study, suggesting that greater education efforts toward vaccination against COVID-19 should be considered to increase public understanding of vaccines. People who believed that the COVID-19 vaccine was effective had 7.949 times greater odds of accepting the third dose compared with those who believed that the COVID-19 vaccine was not effective. Harapan and colleagues have reported similar findings (33). We found that approximately 90% of respondents thought that the COVID-19 vaccine was efficacious, showing that the efficacy of the COVID-19 vaccine was accepted widely. Importantly, people who had completed the two-dose regimen were more likely to accept the third dose compared with those who had not completed the two-dose regimen, as also suggested by Sugawara et al. (4). Those data suggest that: (i) the first two doses are widely approved and (ii) if there is sufficient evidence to demonstrate the efficacy and safety of the COVID-19 vaccine, then people will be more willing to be vaccinated. Wheelock and their coworkers have studied the psychological factors underlying adult behavior toward the influenza vaccine. They have revealed that a better understanding of the psychological aspects of vaccination across contexts and vaccines is a priority (36). However, no studies have linked psychological factors to the acceptance of COVID-19 vaccination except in our previous study. In the present study, we included the questionnaire internationally recognized scales to assess mental health. We found that people with the lower GHQ-12 score were more likely to accept the third dose. This observation suggests that people who were willing to take the booster dose had better psychological health. However, the low mental health status may be related to many factors (e.g., illness, living conditions, and work pressure), which may affect vaccine acceptance.
Our study had two major strengths. First, it was the first study to evaluate the acceptance of the third booster dose among the general population in China. Second, we evaluated mental health to investigate if it influenced vaccine hesitancy.
Our study had two main limitations. First, we employed an electronic questionnaire to collect data (instead of a face-to-face interview), which resulted in uncontrolled conditions during questionnaire completion. Second, as we used a snowball sampling method, some individuals in this sample have still not been found and some individuals are probably omitted by a provider, leading to a biased sample. Third, the sample size was small, limiting the generalizability of our findings.
Conclusion
In China, about 1.2 billion people have completed the two-dose regimen. The safety and efficacy of the COVID-19 vaccines have been recognized. People's acceptance of the booster dose has also been improved. Our study findings make us optimistic about the COVID-19 vaccination. We believe that the COVID-19 vaccination campaign will progress smoothly and will eventually provide herd immunity against COVID-19.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Third People's Hospital of Chengdu.
Author Contributions
YS, TX, PW, and XZ conceived and designed the questionnaire. JZ, DC, and YH recruited participants. YS, TX, and HD analyzed the data. YS wrote and revised the manuscript. All authors have approved the final version of the manuscript and agreed with submission to your esteemed journal.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the participants for their time and interest, as well as the editor and reviewers for their valuable feedback.
References
- 1.National Health Commission of the People's Republic of China (2021). Available online at: http://www.nhc.gov.cn/wjw/mtbd/202109/bf2cf785ce0544ae818a76ffbb18ca79.shtml (accessed September 19, 2021).
- 2.National Health Commission of the People's Republic of China (2022). Available online at: http://www.nhc.gov.cn/xcs/fkdt/202202/dcf93cbd72574c5295dcf4a546451064.shtml (accessed February 19, 2022).
- 3.Yun Z, Yun Y, Niu Q, Xue W, Ling D, Xiu Z, et al. Early assessment of the safety and immunogenicity of a third dose (booster) of COVID-19 immunization in Chinese adults. Front Med. (2022) 16:93–101. 10.1007/s11684-021-0914-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugawara N, Yasui-Furukor N, Fukushima A, Shimoda K. Attitudes of medical students toward COVID-19 vaccination: who is willing to receive a third dose of the vaccine? Vaccines. (2021) 9:1295. 10.3390/vaccines9111295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. (2021) 385:1393–400. 10.1056/NEJMoa2114255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. (2021) 385:661–2. 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue L, Xie T, Yang T, Zhou J, Chen H, Zhu H, et al. A third booster dose may be necessary to mitigate neutralizing antibody fading after inoculation with two doses of an inactivated SARS-CoV-2 vaccine. J Med Virol. (2022) 94:35–8. 10.1002/jmv.27334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Addi JR, Axel R, Svenja H, Dennis DG, Iiris V, Heike H, et al. Induction of robust cellular and humoral immunity against SARS-CoV-2 after a third dose of BNT162b2 vaccine in previously unresponsive older adults. Nat Microbiol. (2022) 7:195–9. 10.1038/s41564-021-01046-z [DOI] [PubMed] [Google Scholar]
- 9.Gang Z, Qian W, Hong P, Min L, Juan Y, Lin W, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. (2021) 22:483–95. 10.1016/S1473-3099(21)00681-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fausto P, Andrea L, Karen B, Mara G, Maria Chiara P, Daniela P, et al. Third dose of SARS-CoV-2 vaccine: a systematic review of 30 published studies. J Med Virol. (2022) 94:2837–44. 10.1002/jmv.27644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alasdair P, Leila J, Victoria C, Parvinder KA, Gavin B, David B, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. (2021) 398:2258–76. 10.1016/S0140-6736(21)02717-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y, Hu Z, Zhao Q, Alias H, Danaee M, Wong LP, et al. Understanding COVID-19 vaccine demand and hesitancy: s nationwide online survey in China. PLoS Negl Trop Dis. (2020) 12:14.e0008961. 10.1371/journal.pntd.0008961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu S, Xiao C, Min C, Tao X, Ji Z, Ping W, et al. Will healthcare workers accept a COVID-19 vaccine when it becomes available? A cross-sectional study in China. Front Public Health. (2021) 9:664905. 10.3389/fpubh.2021.664905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao A, Li H, He W, Zeng W. COVID-19 vaccine hesitancy among medical students: the next COVID-19 challenge in Wuhan, China. Disaster Med Public Health Prep. (2021) 2021:1–6. 10.1017/dmp.2021.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez-Salgado J, Andrés-Villas M, Domínguez-Salas S, Díaz-Milanés D, Ruiz-Frutos C. Related health factors of psychological distress during the COVID-19 pandemic in Spain. Int J Environ Res Public Health. (2020) 17:3947. 10.3390/ijerph17113947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang N, Li Y, Wang Q, Lei C, Liu Y, Zhu S, et al. Psychological impact of COVID- 19 pandemic on HCWs in China Xi'an central hospital. Brain Behav. (2021) 11:e02028. 10.1002/brb3.2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bizu G, Tadesse MG, Lohsoonthorn V, Lertmeharit S, Pensuksan WC, Sanchez S, et al. Psychometric properties and factor structure of the General Health Questionnaire as a screening tool for anxiety and depressive symptoms in a multi-national study of young adults. J Affect Disord. (2015) 187:197–202. 10.1016/j.jad.2015.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bibi A, Lin M, Zhang XC, Margraf J. Psychometric properties and measurement invariance of Depression, Anxiety and Stress Scales (DASS-21) across cultures. Int J Psychol. (2020) 55:916–25. 10.1002/ijop.12671 [DOI] [PubMed] [Google Scholar]
- 19.Vaughan RS, Edwards EJ, MacIntyre TE. Mental health measurement in a post Covid-19 world: psychometric properties and invariance of the DASS-21 in athletes and non-athletes. Front Psychol. (2020) 11:590559. 10.3389/fpsyg.2020.590559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. Sydney, NSW: Psychology Foundation; (1995), p. 112–8. 10.1037/t01004-000 [DOI] [Google Scholar]
- 21.Oei TP, Sawang S, Goh YW, Mukhtar F. Using the Depression Anxiety Stress Scale 21 (DASS-21) across cultures. Int J Psychol. (2013) 48:1018–29. 10.1080/00207594.2012.755535 [DOI] [PubMed] [Google Scholar]
- 22.Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. (2021) 375:331–6. 10.1126/science.abm0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre D, Vihta KD, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. (2021) 27:2127–35. 10.1038/s41591-021-01548-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tartof SY, Slezak JM, Fischer H. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. (2021) 398:1407–16. 10.1016/S0140-6736(21)02183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chemaitelly H, Tang P, Hasan MR, Al Mukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. (2021) 385:e83. 10.1056/NEJMoa2114114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal S, Shekhar R, Kottewar S, Upadhyay S, Singh M, Pathak D, et al. COVID-19 vaccine hesitancy and attitude toward booster doses among us healthcare workers. Vaccines. (2021) 9:1358. 10.3390/vaccines9111358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shekhar R, Sheikh AB, Upadhyay S, Singh M, Kottewar S, Mir H, et al. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines. (2021) 9:119. 10.3390/vaccines9020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alqudeimat Y, Alenezi D, Al Hajri B, Alfouzan H, Almokhaizeem Z, Altamimi S, et al. Acceptance of a COVID-19 vaccine and its related determinants among the general adult population in Kuwait. Med Princ Pract. (2021) 30:262–71. 10.1159/000514636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ditekemena JD, Nkamba DM, Mutwadi A, Mavoko HM, SieweFodjo JN, Luhata C, et al. COVID-19 vaccine acceptance in the Democratic Republic of Congo: a cross-sectional survey. Vaccines. (2021) 9:153. 10.3390/vaccines9020153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dott FR, Samsul A, Amanda Y, Rashed YA, Mohajer IHI, Asma YI, et al. Acceptance of COVID-19 vaccination at different hypothetical efficacy and safety levels in ten countries in Asia, Africa, and South America. Narra J. (2021) 1. 10.52225/narra.v1i3.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulian Y, Zhaoliang W, Xinyu X, Meiqi J, Yanyan W, Zhiwen H. Unfolding determinants of COVID-19 vaccine acceptance in China. J Med Internet Res. (2021) 23:e26089. 10.2196/26089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wardah H, Syeda KK, Muhammad JT, Irfan U, Hibban AR, Marhami F, et al. Global acceptance and hesitancy of COVID-19 vaccination: a narrative review. Narra J. (2021) 1. 10.52225/narra.v1i3.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, et al. Acceptance of a COVID-19 vaccine in Southeast Asia: a cross-sectional study in Indonesia. Front Public Health. (2020) 8:381. 10.3389/fpubh.2020.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajamoorthy Y, Radam A, Taib NM, Rahim KA, Wagner AL, Mudatsir M, et al. The relationship between perceptions and self-paid hepatitis B vaccination: a structural equation modeling approach. PLoS ONE. (2018) 13:e0208402. 10.1371/journal.pone.0208402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu C, We Z, Pei S, Li S, Sun X, Liu P, et al. Acceptance and preference for COVID- 19 vaccination in health-care workers (HCWs). medRxiv. (2020). 10.1101/2020.04.09.20060103 [DOI] [Google Scholar]
- 36.Wheelock A, Thomson A, Sevdalis N. Social and psychological factors underlying adult vaccination behavior: lessons from seasonal influenza vaccination in the US and the UK. Expert Rev Vaccines. (2013) 12:893–901. 10.1586/14760584.2013.814841 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.