Abstract
Geothermal energy has been harnessed and used for domestic heating in Iceland. In wells that are typically drilled to a depth of 1,500 to 2,000 m, the temperature of the source water is 50 to 130°C. The bottoms of the boreholes can therefore be regarded as subterranean hot springs and provide a unique opportunity to study the subterranean biosphere. Large volumes of geothermal fluid from five wells and a mixture of geothermal water from 50 geothermal wells (hot tap water) were sampled and concentrated through a 0.2-μm-pore-size filter. Cells were observed in wells RG-39 (91.4°C) and MG-18 (71.8°C) and in hot tap water (76°C), but no cells were detected in wells SN-4, SN-5 (95 to 117°C), and RV-5 (130°C). Archaea and Bacteria were detected by whole-cell fluorescent in situ hybridization. DNAs were extracted from the biomass, and small-subunit rRNA genes (16S rDNAs) were amplified by PCR using primers specific for the Archaea and Bacteria domains. The PCR products were cloned and sequenced. The sequence analysis showed 11 new operational taxonomic units (OTUs) out of 14, 3 of which were affiliated with known surface OTUs. Samples from RG-39 and hot tap water were inoculated into enrichment media and incubated at 65 and 85°C. Growth was observed only in media based on geothermal water. 16S rDNA analysis showed enrichments dominated with Desulfurococcales relatives. Two strains belonging to Desulfurococcus mobilis and to the Thermus/Deinococcus group were isolated from borehole RG-39. The results indicate that subsurface volcanic zones are an environment that provides a rich subsurface for novel thermophiles.
Natural environments for thermophilic microorganisms are widespread on Earth's surface. The most common and accessible thermal habitats are hot springs, sulfatara, and geothermally heated soils. Thermophiles have also been found in less accessible biotopes, like terrestrial and oceanic deep-subsurface environments (2, 4, 7, 28, 31, 33, 34, 39). The existence of microorganisms in the deep terrestrial subsurface has been noted for a long time, but only in the past decade has there been an increasing interest toward exploring subterranean microbial life in deep-surface environments (27).
The subsurface environment can be divided into nonvolcanic areas that are cold and volcanic areas that are hot. Sometimes the thermal energy is visible on the surface as hot springs and sulfatara fields, and sometimes it is visible in the form of volcanic eruptions. The temperature at nonvolcanic areas increases by 25°C for each kilometer of depth; therefore, thermophilic microorganisms should thrive in the deep subsurface. A number of thermophiles and hyperthermophiles within the domains of Bacteria and Archaea have been isolated from continental and deep-sea oil reservoirs (12, 21, 28) and from other deep wells where the temperature does not exceed 113°C (27). In volcanic areas, however, heat from the mantle is transported to the surface by conduction (heat flow), by volcanic eruptions, and by water in geothermal systems. Such energy has been harnessed as geothermal energy and is used in Iceland for such applications as heating and electricity production. The temperature of the source water is generally 50 to 130°C in wells that are typically drilled to a depth of 1,500 to 2,000 m. The bottoms of the boreholes could therefore be regarded as subterranean hot springs, the boreholes could be regarded as windows into the springs, and pipelines could be regarded as extensions, allowing for access to the subterranean springs. These environments have been minimally investigated (15, 23, 33), while terrestrial surface hot springs are one of the most extensively studied natural environments for thermophiles (3, 4, 17).
Here, we report the diversity of microbes associated within the geothermal fluid of boreholes that reach about 2,000 m below the surface, with reservoir temperatures being below 100°C. Culture-dependent and culture-independent molecular phylogenetic surveys showed that some of the community members are shared with terrestrial thermal springs but that some of the diversity appears to be unique to this subsurface biosphere. We present here the existence of an indigenous thermophilic subterranean community in the subsurface in Iceland, and we suggest that nonendemic thermophiles may be disseminated in the subsurface.
MATERIALS AND METHODS
Study site and sample collection.
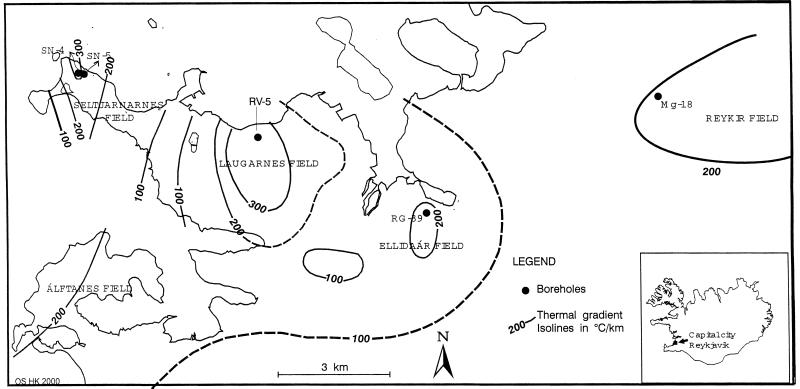
Samples were collected from five geothermal wells (Table 1) located in four separate geothermal fields in the Reykjavík region (Fig. 1) and from a reservoir whose geothermal fluid was a mixture of geothermal water transported from 50 geothermal wells (hot tap water). The fields are located in the same Quaternary rock section within the Kjalarnes caldera. All the fields appear to have a local heat source, different chemical properties, and water recharge (20). The production characteristics of the fields are similar except for the fields with wells SN-4 and SN-5, which have higher salinity in their geothermal fluids and fluctuation in temperature.
TABLE 1.
Description of sampling sites
| Well or hot tap water | Temp (°C) | pH at 22°C | Amt of Cl (mg/liter) | Depth (m) | Yr drilled | Flow rate (liters/s) | Filtration time (h) | Sample vol (liters) | Presence of microbes |
|---|---|---|---|---|---|---|---|---|---|
| RG-39a | 91.4 | 9.5 | 20 | 2,100 | 1984 | 58.3 | 3.8 | 55.7 | Yes |
| MG-18a | 71.8 | 12 | 2,043 | 1973 | 46.6 | 3.3 | 106 | Yes | |
| RV-5a | 129.2 | 9.2 | 6 | 741 | 1959 | 52.3 | 24 | 1,872 | No |
| SN-4b | 84.3 | 8.1 | 2,362 | 2,025 | 1972 | >30 | 2.52 | 117.9 | No |
| SN-5c | 99.2 | 8.5 | 1,265 | 2,207 | 1981 | >30 | 3.2 | 139.9 | No |
| Hot tap waterd | 76 | 9.4 | 0.5–186 | 634–2,857 | 1959–1984 | >30 | 20 | 1,900 | Yes |
Data are from reference 17a. Temperature was measured during sample collection.
In well SN-4 the sampled water was 84.3°C, but this well has previously yielded water of temperatures up to 117°C. The well has cooled down due to the drilling and subsequent production of a deeper well nearby (20).
The temperature varies from 95 to 105°C.
The hot tap water was a mixture of geothermal fluid transported from 50 operating geothermal wells into geothermal water tanks and then delivered into the laboratory through the one-way district heating system. The fluid from each well varied in chemical composition and temperature before it was mixed in the water tanks.
FIG. 1.
Shown are the locations of the three geothermal fields. From west to east are Seltjarnernes (boreholes SN-4 and SN-5), Laugarnes (borehole RV-5), Ellidaar (borehole RG-39) in the Reykjavík area and the fourth geothermal field in the east, and Reykir (borehole MG-18). The inset shows the location of the Reykjavík area in Iceland.
The borehole samples were collected from the wellhead and concentrated in situ by cross-flow filtration through sterile hollow fiber cartridges (0.2-μm-pore-size filter; Amicon). Hot reservoir fluid (50 to 100 liters) was flushed out of the wellhead faucet before sampling of the biomass. One end of a sterile stainless steel tube (5 mm diameter) was connected to a sampling faucet located on the wellhead in all boreholes and the other end was connected to the filter unit. The total length of the tube between the faucet and the filter unit was about 23 m. The tube formed two 10-m-long spirals that were immersed in cool water baths to reduce the hot geothermal fluid down to 40 to 50°C before filtration. The cells trapped inside the filter cartridge (250 ml) were concentrated later by centrifugation at 8,000 rpm (Sorval 5RC centrifuge) for 30 min at 4°C at the laboratory.
Microscopy.
Samples and cultures were viewed with a Leica DM LB light microscope equipped with a phase-contrast oil immersion objective (magnification, ×100). A Petroff-Hausser chamber (depth, 0.02 mm) was used for counting cells. In situ hybridization was performed as described by Harmsen et al. (13). Fixed cells were hybridized with the ARCH915 and EUB338 fluorescein-labeled probes. Cells were stained with 0.01% (wt/vol) acridine orange (14).
Enrichments and isolation.
Concentrated water samples from borehole RG-39 and hot tap water were enriched in different media for chemolithotrophic and chemorganotrophic organisms under anaerobic conditions. Samples from other boreholes were not tested. The enrichments were incubated at 65 and 85°C. Enrichments were performed in standard medium for thermophilic microorganisms (9, 13, 25) and in modified media with different pHs. The following modified medium was used: medium prepared with hot tap water and supplemented with 0.4% (wt/vol) yeast extract, 0.1% (wt/vol) peptone, 10 ml of vitamin solution per liter, 10 ml of element trace solution (1) per liter, and 5 g of S0 liter−1. The pH was adjusted to 9.0, and N2 or H2-CO2 (80%/20%) was used as the gas phase in enrichments. Strain isolation was performed with serial dilutions in Hungate culture tubes.
DNA extraction and PCR amplification.
DNA was extracted from the biomass obtained by filtration of the geothermal fluid, from enrichments, and from the isolated strains (24). DNA extractions and 16S rRNA gene (rDNA) PCR amplifications were performed as described before (24, 30). One microliter was used as the template in PCR amplifications with both archaeal (23 FPL and 1391R [4]) and bacterial (F9, R1544, and R805 [30]) primers.
Cloning and sequencing of 16S rDNA.
The PCR products from the biomass were cloned directly by the TA cloning method by using a TOPO TA cloning kit according to the instruction of the manufacturer (Invitrogen). Plasmid DNA from single colonies was isolated and sequenced by using reverse and forward M13 vector primers and R805 on an ABI 377 DNA sequencer by using a Big Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems). The following specific Archaea sequencing primers were used: FPL23, 765FA, R1391, 340RA, 774RA, and R805 for sequencing more than 400 bp of the 16S rDNAs and F9, F338, F515, F1392, R357, R805, R1195, and R1544 as specific primers for the bacterial genes (30). PCR products from isolates were partially sequenced on an ABI 377 sequencer using the R805 sequencing primer.
Phylogenetic analysis.
All sequences were manually aligned (400 to 500 bp) with closely related sequences obtained from the Ribosomal Database Project (22) after BLAST searches. Sequence alignments and phylogenetic analysis were performed with the ARB program (http:/www.mikro.biologie.tu-muenchen.de), with regions of sequence ambiguity being omitted. The phylogenetic trees based on three algorithms (neighbor joining, maximum parsimony, and maximum likelihood) were constructed using 300 to 400 bp and 800 to 1,390 bp of the 16S rDNA. Distance trees were constructed by using neighbor-joining algorithms with the Jukes and Cantor correction, and maximum-likelihood trees were constructed by the fastDNAml software included in the ARB package. Homologous nucleotide positions, based on the filter of the ARB database, were included in the alignment and used for the comparison analysis. The CHECK-CHIMERA program of the Ribosomal Database Project server was used for searches of chimera artifacts (22).
Nucleotide sequence accession numbers.
All small-subunit rRNA sequences were deposited in the GenBank database under the following accession numbers: AF361206 (SUBT-2), AF361207 (SUBT-3), AF361208 (SUBT-10), AF361209 (SUBT-12), AF361210 (SUBT-6), AF3612011 (SUBT-14), AF361212 (SUBT-13), AF361213 (SUBT-9), AF361214 (SUBT-11), AF361215 (SUBT-7), AF361216 (SUBT-5), AF361217 (SUBT-1), and AF361218 (SUBT-4).
RESULTS
Sample collection.
As listed in Table 1, 55 to 1,900 liters of geothermal fluid with different chemical characteristics was filtered from wells and hot tap water.
Microscopic observations.
Both coccoid and rod-shaped cells were observed in samples from boreholes RG-39 and MG-18 and the hot tap water. The cells were thin rods of various lengths, from short rods up to long filaments. Some of the rods had spherical structures within them or on their ends, and some rods were bundled together as narrow pins. To estimate the original population densities of microorganisms in emergent reservoir fluid from borehole RG-39, we collected four sequential 20-liter samples and one additional 20-liter sample after 180 liters had been discarded. Cells were counted in the range of 4 × 106 to 7 × 106 cells liter−1 in each sample. Similar cell counts in each sample portion taken in well RG-39 could indicate constant numbers of cells dispersed by the well fluid. No cells were detected in the two geothermal field of Seltjarnarnes (SN-4 and SN-5) and Laugarnes (RV-5) (water above 100°C). Domain-specific 16S rRNA-targeted oligonucleotide probes were used to identify Bacteria and Archaea and revealed coccoid and rod-shaped cells belonging to both domains. Long rod-shaped cells or filaments were observed with the Archaea-specific probes in well RG-39 (data not shown).
Enrichments, isolations, and identification.
Growth was not significant in any of the enrichments, except in medium prepared with reservoir water, complex nutrients, and elemental sulfur. Mainly coccoid cells and a few rods were observed in the enrichments at 65 and 85°C. In order to analyze the growing communities, the enrichments were mixed together before 16S rDNA analysis. The majority (97%) of the sequences were identified by BLAST search as belonging to Desulfurococcus mobilis (19, 39), and 3% belonged to Aquificales clone SRI-48 (30). Coccoid cells forming H2S were isolated after seven sequential subcultures at 85°C and identified as D. mobilis (99% similarity by BLAST search) by 16S rDNA sequencing (450 bp). Straight rods that did not form spores or H2S were isolated at 65°C. The rods were identified by 16S rDNA analysis as a new member of the Thermus (also called Deinococcus) group.
Phylogenetic analysis.
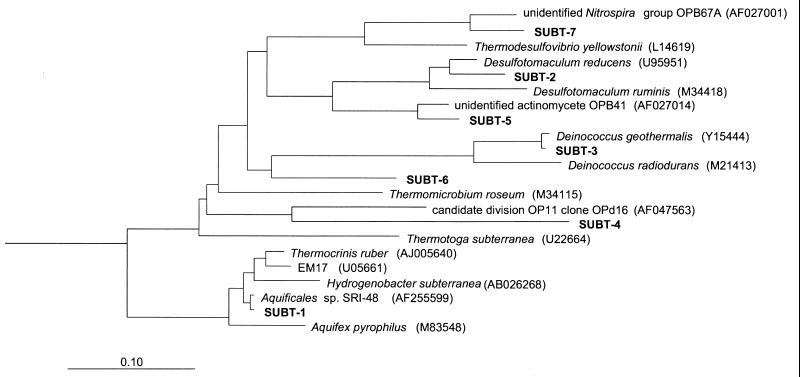
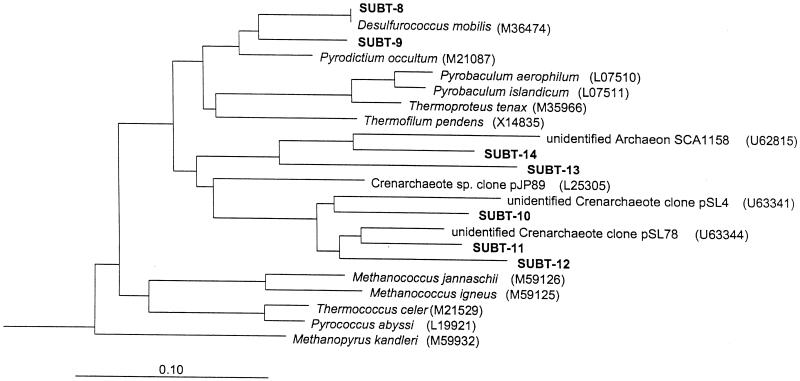
Both bacterial and archaeal sequences were obtained from all biomass samples by PCR amplifications of 16S rDNAs. One hundred thirteen clones (73 bacterial and 40 archaeal) from borehole MG-18, 74 clones (57 bacterial and 17 archaeal) from borehole RG-39, and 76 clones (20 bacterial and 56 archaeal) from the hot tap water were successfully sequenced. The results obtained from bacterial clones are presented in Table 2 and in Fig. 2. The bacterial sequence libraries revealed seven phylogenetically distinct lineages: type sequences SUBT-1, -2, -3, -4, -5, -6, and -7. Two operational taxonomic units (OTUs) (SUBT-1 and SUBT-7) were found in all samples, and their closest database matches were the Aquificales (30) and Nitrospira (5) groups. SUBT-2, -3, and SUBT-5 were found only in hot tap water. These OTUs were most closely related to Desulfotomaculum reducens (35), to Deinococcus geothermalis (37), and to an unidentified actinomycete OPB41 (17). Borehole RG-39 contained two OTUs (SUBT-4 and SUBT-6) whose closest database matches were to OP11 and OP8 (17) and which were not found in the other samples. The results obtained from the archaeal clones are presented in Table 3 and in Fig. 3. The archaeal sequence libraries revealed seven phylogeneticly distinct lineages, type sequences SUBT-8, -9, -10, -11, -12, -13, and -14. All OTUs, except two (SUBT-8 and -9) belonged to the candidate division Crenarchaeota (3–5). Only one OTU (SUBT-9) was found in all samples, and one other (SUBT-11) was found in both boreholes. One OTU (SUBT-8) was found only in hot tap water, one (SUBT-12) was found in borehole RG-39, and three (SUBT-10, -13 and -14) were found in borehole MG-18. Although different numbers of bases (350 to 1,390) were used in the phylogenetic analysis, the topology in trees did not significantly change within the bacterial or archaeal OTUs.
TABLE 2.
Summary of the bacterial 16S rDNA sequences identified in wells and hot tap water
| Type sequencea | % of clones in indicated borehole or HTWb
|
Closest division | Closest database match (%) | Reference | ||
|---|---|---|---|---|---|---|
| MG-18 | RG-39 | HTW | ||||
| SUBT-1 | 5 | 94 | 80 | Aquificales | SRI-48 (98–99) | 30 |
| SUBT-2 | 1 | Firmicutes | Desulfotomaculum reducens (95) | 35 | ||
| SUBT-3 | 1 | Thermus/Deinococcus group | Deinococcus geothermalis (99) | 37 | ||
| SUBT-4 | 2 | Candidate division OP11 | Clone Opd16 (64) | Hugenholtz et al.c | ||
| SUBT-5 | 4 | High-G+C-content gram-positive organisms | Clone OPB41 (95) | 17 | ||
| SUBT-6 | 2 | Candidate division OP11 | Clone OPB23 (86) | 17 | ||
| SUBT-7 | 95 | 2 | 14 | Nitrospira group | Clone OPB67A (90) | 5 |
The type 16S rDNA sequence was used in the maximum-likelihood analysis. Sequence similarities of other sequenced representatives were more than 98%.
HTW, hot tap water.
P. Hugenholtz, K. L. Hershberger, J. L. Flanagan, B. Kimmel, and N. R. Pace, Abstr. 97th Gen. Meet. Am. Soc. Microbiol., abstr. N-23, 1997.
FIG. 2.
Phylogenetic relationships of bacterial 16S rRNA sequences as determined by maximum-likelihood analysis. Sequences from the subterranean hot springs are marked in bold with the abbreviation SUBT. Accession numbers of reference sequences are in parentheses. Sulfolobus acidocaldarius was used as the outgroup. The scale bar indicates the estimated number of base changes per nucleotide sequence position.
TABLE 3.
Summary of the archaeal 16S rDNA sequences identified in wells and hot tap water
| Type sequencea | % of clones in indicated borehole or HTWb
|
Closest division | Closest database match (%) | Reference | ||
|---|---|---|---|---|---|---|
| MG-18 | RG-39 | HTW | ||||
| SUBT-8 | 97 | Desulfurococcales | Desulfurococcus mobilis (99) | 19 | ||
| SUBT-9 | 3 | 22 | 3 | Pyrodictiales | Pyrodictium occultum (94) | 18 |
| SUBT-10 | 2 | New division Crenachaeota? | Clone pSL4 (88) | 3 | ||
| SUBT-11 | 76 | 73 | New division Crenachaeota? | Clone pSL78 (92) | 3 | |
| SUBT-12 | 5 | New division Crenachaeota? | Clone pSL78 (87) | 3 | ||
| SUBT-13 | 2 | New division Crenachaeota? | Clone pJP33 (80) | 4 | ||
| SUBT-14 | 17 | New division Crenachaeota? | Clone SCA1173 (87) | 5 | ||
The type 16S rDNA sequence was used in the maximum-likelihood analysis. The sequence similarities of other sequenced representatives were more than 98%.
HTW, hot tap water.
FIG. 3.
Phylogenetic relationships of archaeal 16S rRNA sequences as determined by maximum-likelihood analysis. Sequences from the subterranean hot springs are marked in bold with the abbreviation SUBT. Accession numbers of reference sequences are in parentheses. Thermotoga maritima was used as the outgroup. The scale bar indicates the estimated number of base changes per nucleotide sequence position.
DISCUSSION
We have shown that two deep subsurface geothermal fields in Iceland with temperatures below 100°C harbor a diverse community of unknown thermophiles. In situ whole-cell hybridization with fluorescently labeled 16S rRNA-based oligonucleotide probes indicated that the archaeal and bacterial cells were still intact. From enrichment cultures it was confirmed that cells were thermophilic, growing at high temperatures. Direct 16S rDNA analysis showed that all sequences could be affiliated with thermophilic divisions and that almost all OTUs were new and have not yet been obtained in pure cultures. Despite the use of various media and enrichment conditions, growth was observed only in media with source borehole water. The cultures were dominated by the thermophilic archeon Desulfurococcus mobilis (19, 39) and a few bacterial sequences closely related to Aquificales clone SRI-48 (30). This may suggest that subsurface growth conditions are needed for growing the noncultivated subterranean cells detected in samples.
Our findings are in agreement with recent demonstrations of other deep subsurface environments such as hot subterranean biospheres in continental oil reservoir aquifers (30, 29, 32), groundwater from a borehole in granite rock (10, 27), seafloor diking eruptive events (8), and marine sediments (26). The occurrence of these thermophiles in the subsurface is thought to be due to their deposition with the original sediment and their survival over geological time. The origin of the subterranean hot spring thermophiles is at present unclear. Nevertheless, our result could suggest an indigenous biosphere. In this study we have sequenced many new and diverse OTUs that are phylogenetically distinct lineages. At least 11 type sequences out of 14 were distantly related or showed less than 95% similarity to OTUs that have been detected in surface hot springs. Furthermore, eight of these type sequences were distantly related (64 to 95%) to candidates of new divisions originated from Obsidian Pool, Yellowstone Park. If the OTUs were introduced to the geothermal water during drilling, we would have expected to detect more OTUs closely related to known subsurface OTUs. The boreholes were drilled in the late 1970s, which is a relatively short time to form so many divergent population groups from contaminants. Closely related OTUs with more than 98% similarity to each other were found in wells RG-39, MG-18, and hot tap water. Their dominance in wells varied with the in situ temperature, and their presence was correlated to some extent to the known temperature range of their closest relatives (Tables 2 and 3). Identical OTUs within wells could suggest a subsurface geographical interconnection between wells (Fig. 2 and 3). However, several type sequences were found to be site restricted to wells and were not detected in the other wells. The absence of similar or identical OTUs within wells could possibly be explained by their low abundance in wells caused by differences in water chemistry and temperature, and therefore they were not detected.
The Seltjarnarnes field (SN-4 and SN-5) had water temperatures that fluctuated between 95 to 117°C, which is in the range of the upper temperature limit for life (6). However, no intact cells or DNA could be detected in this field, suggesting that it is not possible to find deep subsurface life everywhere. The reason could be that the field is geographically isolated from the other fields and without a terrestrial subsurface interconnecting flow. The Seltjarnarnes field is surrounded on four sides by the sea, and a hydrological barrier has been suggested between the field (Fig. 1) and the other fields (36). Furthermore, the nearest geothermal field, RV-5, is a high-temperature field with temperatures above 130°C, which is lethal for any known life form and could therefore possibly function as a dispersal barrier for subsurface migrating microbes. High chloride concentrations in SN-4 and SN-5 could also have an inhibiting effect on migrating salt-sensitive terrestrial microbes.
We have shown that geothermal reservoirs are a novel, volcanic, high-temperature environment for thermophilic microorganisms that extends the known ecological habitats of such groups of organisms. Iceland is one of the world's largest subsurface hot spots; its crust is very permeable, and therefore there are ideal paths through interconnected subsurface conduits for microorganisms that are able to survive over geological time in subsurface environments. The extent of subsurface dissemination depends on dispersal barriers, such as seawater and temperature, or hydrological barriers. The results highlight a new indigenous microbial biosphere and indicate that thermophilic microorganisms disseminate in the subsurface within volcanic zones through subsurface conduits, as has already been observed with meteoric groundwater (24) and on the surface in cold aquifers (16).
ACKNOWLEDGMENTS
This work could not have been done without the cooperation of Hitaveita Reykjavíkur and Hitaveita Seltjarnarnes; in particular, we thank G. Ivarsson for assistance in sampling. Thanks are also due to S. Ólafsdóttir for technical assistance. We thank Anna-Louise Reysenbach for critically reading the manuscript.
This work was supported by a grant from the Icelandic National Research Council.
REFERENCES
- 1.Balch W E, Fox G E, Magrum L S, Woese C R, Wolfe R S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979;43:260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale S J, Goodman K, Rochelle P A, Marchesi J R, Fry J C, Weightman A J, Parkers R J. Desulfovibrio profundus sp. nov., a novel barophilic sulfate-reducing bacterium from deep sediment layers in the Japan Sea. Int J Syst Bacteriol. 1997;47:515–521. doi: 10.1099/00207713-47-2-515. [DOI] [PubMed] [Google Scholar]
- 3.Barns S M, Delwiche C F, Palmer J F, Pace N R. Perspective on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc Natl Acad Sci USA. 1996;93:9188–9193. doi: 10.1073/pnas.93.17.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barns S M, Fundyga R E, Jeffries M W, Pace N R. Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc Natl Acad Sci USA. 1994;91:1609–1613. doi: 10.1073/pnas.91.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bintrim S B, Donohue T J, Handelsman J, Roberts G P, Goodman R M. Molecular phylogeny of Archaea from soil. Proc Natl Acad Sci USA. 1997;94:277–282. doi: 10.1073/pnas.94.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blöchl E, Rachel R, Burggraf S, Hafenbradl D, Jannasch H W, Stetter K O. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113°C. Extremophiles. 1997;1:14–21. doi: 10.1007/s007920050010. [DOI] [PubMed] [Google Scholar]
- 7.Boone D R, Liu Y, Zhao Z-J, Balkwill D L, Drake G R, Stevens T O, Aldrich H C. Bacillus infernus sp. nov., an Fe(III)- and Mn(IV)-reducing anaerobe from the deep terrestrial subsurface. Int J Syst Bacteriol. 1995;45:441–448. doi: 10.1099/00207713-45-3-441. [DOI] [PubMed] [Google Scholar]
- 8.Delaney J R, Kelly D S, Lilley M D, Butterfield D A, Baross J A, Wilcock W S D, Embley R W, Summit M. The quantum event of oceanic crustal accretion. Impacts of diking at mid-ocean ridges. Science. 1998;281:222–230. [PubMed] [Google Scholar]
- 9.Erauso G, Reysenbach A-L, Godfroy A, Meunier J R, Crump B, Parensky F, Baross J A, Marteinsson V T, Barbier G, Pace N R, Prieur D. Pyrococcus abyssi sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Arch Microbiol. 1993;160:338–349. [Google Scholar]
- 10.Ghirose W C, Wilson J T. Microbial ecology of the terrestrial subsurface. Adv Appl Microbiol. 1988;33:107–172. doi: 10.1016/s0065-2164(08)70206-5. [DOI] [PubMed] [Google Scholar]
- 11.Gold T. The deep, hot biosphere. Proc Natl Acad Sci USA. 1992;89:6045–6049. doi: 10.1073/pnas.89.13.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene A C, Patel B K C, Sheehy A J. Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir. Int J Syst Bacteriol. 1997;47:505–509. doi: 10.1099/00207713-47-2-505. [DOI] [PubMed] [Google Scholar]
- 13.Harmsen H J M, Prieur D, Jeanthon C. Group-specific 16S rRNA-targeted oligonucleotide probes to identify thermophilic bacteria in marine hydrothermal vents. Appl Environ Microbiol. 1997;63:4061–4068. doi: 10.1128/aem.63.10.4061-4068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobbie J E, Dahney R J, Jasper S. Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber R, Krisjansson J K, Stetter K O. Pyrobaculum gen. nov., a new genus of neutrophilic, rod-shaped archaebacteria from continental solfataras growing optimally at 100°C. Arch Microbiol. 1987;149:95–101. [Google Scholar]
- 16.Huber R, Stoffers P, Cheminee J L, Richnow H H, Stetter K O. Hyperthermophilic archaebacteria within the crater and open-sea plume of erupting Macdonald Seamount. Nature. 1990;345:179–181. [Google Scholar]
- 17.Hugenholtz P, Pitulle C, Hershberger K L, Pace N R. Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 1998;180:366–376. doi: 10.1128/jb.180.2.366-376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Ivarsson G. Vatnsvinnslan 1994. Reykjavik, Iceland: Hitaveita Reykjavikur; 1995. [Google Scholar]
- 18.Kaine B P, Schurke C, Stetter K O. Genes for the 16S and 5S ribosomal RNAs and the 7S RNA of Pyrodictium occultum. Syst Appl Microbiol. 1989;12:8–14. [Google Scholar]
- 19.Kjems J, Garrett R A, Ansorge W. The sequence of the 16S RNA gene and its flanking region from the archaebacterium Desulfurococcus mobilis. Syst Appl Microbiol. 1987;9:22–28. [Google Scholar]
- 20.Kristmannsdóttir H, Tulinius H. Proceeding of the World Geothermal Congress 2000. 2000. The development of the Seltjarnarnes geothermal field, SW Iceland during thirty years exploitation; pp. 3465–3470. [Google Scholar]
- 21.L'Haridon S, Reysenbach A-L, Glénat P, Prieur D, Jeanthon C. Hot subterranean biosphere in a continental oil reservoir. Nature. 1995;377:223–224. [PubMed] [Google Scholar]
- 22.Maidak B L, Cole J R, Lilburn T G, Parker C T, Jr, Saxman P R, Stredwick J M, Garrity G M, Li B, Olsen G J, Pramanik S, Schmidt T M, Tiedje J M. The RDP (Ribosomal Database Project) continues. Nucleic Acids Res. 2000;28:173–174. doi: 10.1093/nar/28.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marteinsson V T, Kristjansson J K. Bakterier i varmtvannssystemer. Report 544. Copenhagen, Denmark: Nordistk Mineterraad; 1991. Enumeration of thermophilic bacteria in district heating system in Iceland. [Google Scholar]
- 24.Marteinsson V T, Kristjansson J K, Kristmannsdottir H, Dahlkvist M, Saemundsson K, Hannington M, Petursdottir S, Geptner A, Stoffers P. Discovery and description of giant submarine smectite cones on the seafloor in Eyjafjordur, northern Iceland, and a novel thermal microbial habitat. Appl Environ Microbiol. 2001;67:827–833. doi: 10.1128/AEM.67.2.827-833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marteinsson V T, Birrien J-L, Prieur D. In situ enrichment and isolation of thermophilic microorganisms from deep-sea environments. Can J Microbiol. 1997;43:694–697. [Google Scholar]
- 26.Parkers R J, Cragg B A, Bale S J, Getliff J M, Goodmann K, Rochelle P A, Fry J C, Weightman A J, Harvey S M. Deep bacterial biosphere in Pacific Ocean sediments. Nature. 1994;371:410–413. [Google Scholar]
- 27.Pedersen K. Exploration of deep intraterrestrial microbial life: current perspectives. FEMS Microbiol Lett. 2000;185:9–16. doi: 10.1111/j.1574-6968.2000.tb09033.x. [DOI] [PubMed] [Google Scholar]
- 28.Ravot G, Magot M, Fardeau M-L, Patel B K, Prensier G, Egan A, Garcia J-L, Ollivier B. Thermotoga elfii sp. nov., a novel thermophilic bacterium from an African oil-producing well. Int J Syst Bacteriol. 1995;45:308–314. doi: 10.1099/00207713-45-2-308. [DOI] [PubMed] [Google Scholar]
- 29.Rosnes J T, Torsvik T, Lien T. Spore-forming thermophilic sulfate-reducing bacteria isolated from North Sea oil field waters. Appl Environ Microbiol. 1991;57:2302–2307. doi: 10.1128/aem.57.8.2302-2307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skírnisdóttir S, Hreggvidsson G O, Hjörleifsdottir S, Marteinsson V T, Petursdottir S K, Holst O, Kristjansson J K. Influence of sulfide and temperature on species composition and community structure of hot spring microbial mats. Appl Environ Microbiol. 1999;66:2835–2841. doi: 10.1128/aem.66.7.2835-2841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens T O, McKinley J P. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science. 1995;270:450–454. [Google Scholar]
- 32.Szewzyk U, Szewzyk R, Stenström T-A. Thermophilic anaerobic bacteria isolated from deep borehole in granite in Sweden. Proc Natl Acad Sci USA. 1994;91:1810–1813. doi: 10.1073/pnas.91.5.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takai K, Horikoshi K. Molecular phylogenetic analysis of archaeal intron-containing genes coding for rRNA obtained from a deep-subsurface geothermal water pool. Appl Environ Microbiol. 1999;65:5586–5589. doi: 10.1128/aem.65.12.5586-5589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takami H, Inoue A, Fuji F, Horikoshi K. Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol Lett. 1997;152:279–285. doi: 10.1111/j.1574-6968.1997.tb10440.x. [DOI] [PubMed] [Google Scholar]
- 35.Tebo B M, Obraztsova A Y. Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiol Lett. 1998;162:193–198. [Google Scholar]
- 36.Thorsteinsson T, Eliasson J. Geohydrology of the Laugarnes hydrothermal system in Reykjavik. Geothermics. 1970;2:1191–1204. . (Special issue.) [Google Scholar]
- 37.Vaisanen O M, Weber A, Bennasar A, Rainey F A, Busse H J, Salkinoja-Salonen M S. Microbial communities of printing paper machines. J Appl Microbiol. 1998;84:1069–1084. doi: 10.1046/j.1365-2672.1998.00447.x. [DOI] [PubMed] [Google Scholar]
- 38.Whitmann W B, Coleman D C, Wiebe W J. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zillig W, Stetter K O, Prangishvilli D, Schafer W, Wunderl S, Jankekovic D, Holz I, Palm P. Desulfurococcaceae, the second family of the extremely thermophilic, anaerobic, sulfur-respiring Thermoproeales. Zentbl Bakteriol Hyg Abt 1 Orig C. 1981;3:304–317. [Google Scholar]