Abstract
Systemic sclerosis (SSc) is a rare multisystem autoimmune disease, characterized by fibrosis, vasculopathy, and autoimmunity. Recent advances have highlighted the significant implications of B-cells in SSc. B-cells are present in affected organs, their subpopulations are disrupted, and they display an activated phenotype, and the regulatory capacities of B-cells are impaired, as illustrated by the decrease in the IL-10+ producing B-cell subpopulation or the inhibitory membrane co-receptor density. Recent multi-omics evidence highlights the role of B-cells mainly in the early stage of SSc and preferentially during severe organ involvement. This dysregulated homeostasis partly explains the synthesis of anti-endothelial cell autoantibodies (AECAs) or anti-fibroblast autoantibodies (AFAs), proinflammatory or profibrotic cytokines (interleukin-6 and transforming growth factor-β) produced by B and plasma cells. That is associated with cell-to-cell interactions with endothelial cells, fibroblasts, vascular smooth muscle cells, and other immune cells, altogether leading to cell activation and proliferation, cell resistance to apoptosis, the impairment of regulatory mechanisms, and causing fibrosis of several organs encountered in the SSc. Finally, alongside these exploratory data, treatments targeting B-cells, through their depletion by cytotoxicity (anti-CD20 monoclonal antibody), or the cytokines produced by the B-cell, or their costimulation molecules, seem interesting, probably in certain profiles of early patients with severe organic damage.
Keywords: systemic sclerosis, B-cell, pathogenesis, B-cell receptor (BCR), interleukine 6 (IL-6), autoantibodies, transcriptomic, rituximab
Highlights
B-cells are key players in SSc, implicated in fibrosis and autoimmunity
B-cell subsets are disrupted
B-cells are activated and lose their regulation properties
B-cells produce autoantibodies and cytokines
B-cells crosstalk with numerous cells including fibroblasts, endothelial cells, T cells, dendritic cells, and other immune cells
Treatments targeting B-cells, through their depletion by cytotoxicity, or the cytokines produced by the B-cell, or their costimulation molecules seem interesting, probably in certain profiles of early patients with severe organic damage
1 Introduction
Systemic sclerosis (SSc) is a rare multisystem autoimmune disease of unknown cause characterized by fibrosis, vasculopathy, and autoimmune features (1, 2). SSc is responsible for visceral manifestations such as interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH), which represent the two main causes of death (3). SSc is also responsible for significant disability, handicap, and a hampered quality of life.
The pathogenesis of SSc is not fully understood. Still, significant progress has been made in the last 20 years in the understanding of the mechanisms contributing to the occurrence of vasculopathy and fibrosis. In patients with SSc, the vascular tone is dysregulated, leading to perturbed interactions between endothelial cells, vascular smooth muscle cells (VSMC), and extra-cellular matrix (ECM) components participating in vascular remodeling and occlusion. ECM accumulation is mostly the consequence of fibroblast activation (4). Since skin fibrosis is the main hallmark of this disease, research initially focused on the fibroblast. However, the demonstration in the early state of the disease of cutaneous lymphocyte infiltration before any process of fibrosis (5), the presence of auto-antibodies, imbalances in the B-cell subsets, and a hyperactivated state repositions the B-cell as an actor in the pathogenesis of SSc.
We herein provide an overview of B-cells in the pathogenesis of SSc from human data and animal models. We review B-cell distribution, B-cell dysfunction, and B-cell participation in inflammatory and fibrotic lesions, with emphasis on recently published data and their implication for patient prognosis. Lastly, we discuss the different therapeutic options targeting B-cells or pathways involved in B-cell activation.
2 B-cells Are Found in Affected Organs
Early presence of various innate or adaptative immune cells infiltrates which B and plasma cells have been demonstrated in the skin, lung, or gastrointestinal tract of SSc patients (6–10). Recent genetic and immunohistochemical data reinforce the implication of B-cells in the skin of SSc patients, from the early stages of the disease, in particular with the demonstration of the presence of CD20+ cells in the skin of patients and an activated B-cell genetic signature (11, 12) and illustrated in Figure 1. Moreover, in SSc patients, the presence of CD20+ B-cells in clinically affected skin seems to be greater compared to unaffected skin. Based on immunohistochemical data, the presence of inflammatory cells, including B-cells, in cutaneous samples from areas clinically involved and uninvolved by fibrosis may suggest the possibility of detecting pathological changes before the clinical onset of skin fibrosis (12).
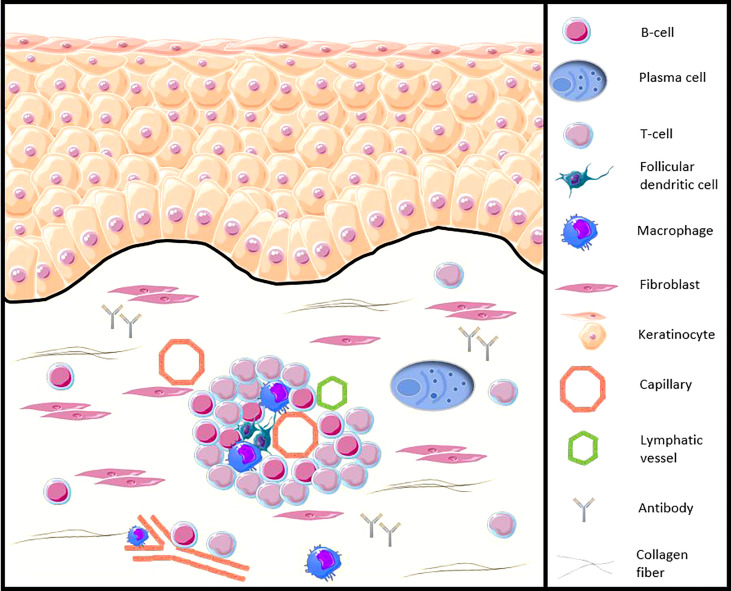
Figure 1.
Illustration of the B-cell presence in the SSc patients skin (adapted from Fetter et al. Cells, 2020 [13)]. CD20+ B-cells and plasma cells infiltrate the skin of SSc patients, from the early stages of the disease, especially around blood vessels, accompanied by other peripheral blood mononuclear cells, such as CD3+ T cells or follicular dendritic cells. B-cells participate in the functions of antigen-presenting cells, production of autoantibodies and proinflammatory and profibrotic cytokines, as well as direct contact with other cells such as the fibroblast.
Concerning the lung, a higher percentage of CD19+ B-cells in bronchoalveolar lavage fluid was found in SSc patients with ILD and could be correlated with worsening of the DLCO during pulmonary function tests (14). The occurrence of patchy lymphocytes and plasma cells or real lymphoid follicle organization in the lung examination of SSc-related ILD or PAH patients has also been described (15, 16).
3 B-cells in SSc Exhibit an Activated Phenotype in Some Unbalanced Subsets
3.1 Distribution of B-Cell Subsets in SSc
Peripheral CD3- CD19+ B-cells represent around 10% of total white blood cells (WBCs) and the last 30 years allowed us to isolate several peripheral B-cell subsets with specific phenotypes, including naïve, marginal zone, switched memory B-cells, and plasma cells (17). In SSc patients, the frequency of B-cells (relative count) among peripheral blood mononuclear cells is increased compared to healthy controls (18, 19). Contradictorily, data vary dramatically in the absolute count of peripheral B-cells in SSc patients according to the authors, reported to be increased (18, 20) similar (21, 22), or decreased (23) compared to healthy controls. These differences could be explained by differences in the methods used, the threshold definitions, and/or the use of glucocorticoids and/or immunosuppressant drugs. Thus, B-cell subsets in SSc patients are unbalanced, with an expansion of naive and transitional B-cells with a decrease in memory B-cells, switched memory B-cells, and IL-10+-producing regulatory B-cells (Bregs), particularly in steroid-untreated patients (16, 18–20, 22, 24–27), with a higher circulating plasma cell molecular signature (28). Transitional B-cells represent a maturing subset undergoing peripheral selection, whereas switched memory B-cells are long-lived cells persisting in a quiescent state (17). These immune dysregulations lead to inadequate B-cell homeostasis and favor the emergence of autoimmune reactions.
3.2 B-Cells Overexpress Activated Phenotype in SSc
In SSc patients, the transitional and memory B-cells are constantly activated compared with controls. The expression levels of the co-stimulatory molecules CD80, CD86, and CD95 are increased, with an increased proportion of IL-6+-and IL-6 receptor+ (IL6R) B-cells (4, 18–20). The overexpression of CD69, CD5, CD86, CD95 markers, and the IL6R is associated with the diffuse cutaneous SSc (dcSSc) form and the early stage of disease, suggesting the implication of the activated B-cells in the active and aggressive forms of SSc (16). The decrease in the memory B-cell compartment found in SSc compared to healthy controls is probably due to increased apoptosis (18). B-cells are not only a cellular precursor of differentiated secreting-antibody cells. The B-cells are involved in various essential regulatory immune processes like the antigen presentation, T-cell maturation and differentiation, the organization of the architecture of the lymphoid organ, and the cytokine synthesis (29). The upregulation of the CD80/86–CD28 costimulatory molecules contributes to activating autoreactive T cells and increasing the secretion of profibrotic cytokines in SSc (30). Simultaneously, the ability of B-cells to produce IL-10 is altered, participating in downregulating Breg (20, 22). Additionally, B-cells from SSc patients express increased programmed death-1 (PD-1) and PD-1 ligand 2 (PD-L2), a co-stimulatory CD28/B7 family system, which contributes to the impairment of immune tolerance (31). This disrupted homeostasis of peripheral B-cell subsets in SSc could be secondary to an overexpression of activation markers in certain subsets, counterbalanced by increased CD95-mediated apoptosis in memory B-cells (18). This disrupted homeostasis encountered in B-cells in SSc patients probably influences many of their regulatory immune functions.
4 B-cell Receptor, Intracellular Signaling and Intrinsic Pathways of B-cells Are Disrupted in SSc
4.1 B-Cell Receptor and Its Co-Receptors
The activation of B-cells mediated by antigen implicates the B-cell receptor (BCR), many co-receptors, and coactivation molecules (Figure 2). Several components of this axis are altered during the SSc. In SSc patients, the BCR diversity is altered, which is related to the alteration of B-cell development. In particular, the VDJ rearrangement is altered, leading to an excess of membrane IgD diversity and a defective mutation load identified as a SSc-related PAH signature (33). In humans, the membrane density of the CD19 co-receptor at the membrane of peripheral B-cells in SSc patients was rated 20% higher than that in healthy controls, which seems to be specific to SSc since it is not the case in SLE (18, 21). Additionally, the expression of CD21, another positive regulator of the BCR signal, is also higher at around 20% (18, 21). Several membrane inhibitors, such as CD22 or CD35, have been found decreased on B-cells, close to a factor of 2 for CD35, especially on memory B-cells in SSc patients with peripheral vascular modified Medsger scale altered (≥1), with diffuse cutaneous SSc (dcSSc), and ILD (19, 34).
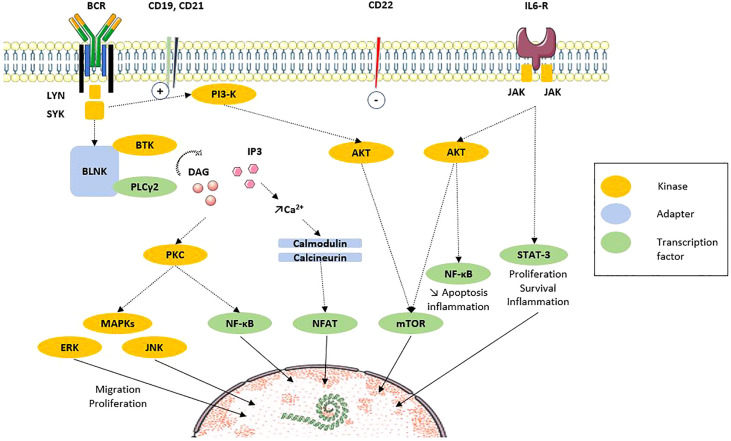
Figure 2.
B-cell signaling via BCR and costimulation molecules (adapted from Dal Porto et al. Mol Immunol, 2004 [32)]. After ligation of antigen on the B-cell antigen receptor (BCR) at the membrane, signals are transduced and propagated by several protein phosphorylations, modifications and interactions. The end point is the regulation of transcription factors that regulate the expression of genes. Recent results highlight the participation of intracellular signaling perturbations in B-cell including defective phosphorylation of MAPK, STAT3 or mTOR molecules. Solid line: translocation. Dotted line: direct action. AKT, protein kinase B; BCR, B-cell receptor; BLNK, B-cell linker protein; BTK, Bruton’s tyrosine kinase; CD, cluster of differentiation; DAG, diacylglycerol; ERK, extracellular signal-regulated kinases; IL, interleukin; IL6-R, IL6 receptor; IP3, inositol triphosphate; JAK, Janus kinases; JNK, c-Jun N-terminal kinase; Lyn, Lck/Yes novel tyrosine kinase; MAPK, mitogen associated protein kinase; mTOR, mammalian target of rapamycin; NFAT, Nuclear factor of activated T cells; PI3K, phosphoinositide 3-kinase; PLCγ, phospholipase Cγ2; PKC, protein kinase C; STAT3, signal transducer and activator of transcription 3; SYK, spleen tyrosine kinase.
4.2 B-Cell Activating Factors
Independently of antigen-mediated mechanisms, BAFF and APRIL pathways are implicated in the pathogenesis of SSc. B-cell activating factor (BAFF) and APRIL pathways, both members of the tumor necrosis factor (TNF) superfamily, play a critical role in the activation, survival, and maturation of B-cells, and are involved in many disorders like transplantation/graft versus host diseases (GVHD) and autoimmune diseases (35).
First, in the type 1 tight skin mouse model (TSK/+), an increase in serum BAFF levels has been reported. Its blockage inhibits the development of skin fibrosis and autoantibody production or TGF-β and IL6 mRNA levels in the skin (36). Thus, in humans, serum BAFF and APRIL levels are higher in SSc patients compared to healthy controls by about 25 to 50% for BAFF (22, 37–39), as well as the amount of one of its specific receptors (BAFFR) at the surface of B-cells (37). Positive correlations between serum levels of BAFF and APRIL and modified Rodnan skin score (mRSS), early dcSSc, ILD and IL-6, serum IgG level, and anti-Topo I Ab titers are reported (22, 37–42).
4.3 Intracellular Signaling and Intrinsic Pathways of B-Cells Are Upregulated in SSc
Beyond alterations targeting extrinsic activators, defects in intracellular B-cell signaling, mainly tyrosine kinases, have been documented in many autoimmune diseases, including SLE, rheumatoid arthritis (RA), and SSc. The involvement of calcium storage has also been reported.
In TSK/+ B-cells, the CD19-mediated intracellular response to stimulation appears to be exacerbated, with an increase in intracellular calcium [Ca2+]i storage (43, 44). Then, spleen tyrosine kinase (SYK) has been reported as hyperphosphorylated in B-cells of murine sclerodermatous chronic graft-versus-host disease (Scl-cGVHD) (45). Experimental use of fostamatinib, an inhibitor of SYK in Scl-cGVHD or BLM models, appears to contribute to decreasing phospho-SYK, TGF-β expression, and tissue fibrosis (45, 46).
Thus, Lyn kinase, the first signal transducing kinase involved after BCR activation, is downregulated in CD22low B-cells from SSc patients (34). Moreover, after activation, defective phosphorylation of downstream MAPK, STAT3 (20, 25) or mammalian target of rapamycin (mTOR) (22) has been reported, especially in CD27+ transitional or memory B-cells by some authors (20, 25).
4.4 Genomic, Transcriptomic, and Proteomic Data Argue a B-Cell Signature in SSc
Large-cohort genomic studies have identified associations between genes involved in B-cell signaling and the SSc phenotype, including B-cell scaffold protein with ankyrin repeats 1 (BANK1), B lymphoid kinase protein (BLK), c-src tyrosine kinase (CSK) or intracellular protein tyrosine phosphatase (PTNP22) genes (47).
All studies on global gene expression in peripheral blood cells from SSc patients revealed the interferon IFN-inducible signature (48). Moreover, the profiles show a heterogeneity of the signatures according to certain clinical characteristics. The presence of an anti-topoisomerase or anti-U1 RNP antibody is associated with a high IFN-inducible signature, in contrast to the presence of an anti-centromere antibody or digital ulcer (49, 50).
Transcriptomic analysis of skin biopsies of SSc patients has found a B-cell signature in 69% of cases (51), with an increased expression of B-cell-related genes like genes related to light and heavy chains of immunoglobulins, with genes that contribute to increased extracellular matrix synthesis or activation of T cells and correlated with skin thickness. These results are confirmed by immunohistochemistry staining on skin biopsy (11, 51). Whole skin transcriptomic analysis of SSc patients also showed discrimination according to the profiles of antinuclear antibodies (ANA) (52). Interestingly, adaptive immune signature, especially B-cell signature in the skin, was associated with early and diffuse SSc (51), suggesting once more time his early implication in disease and highlighting a potential therapeutic window for the use of B-cell targeting therapies.
Proteomic analyses in early dcSSc patients showed a difference in the longitudinal course of serum proteins according to the ANA status (52), confirming a B-cell implication with a continuum of differential signature from the genetic level to the protein level.
5 Functional Characteristics
B-cells contribute in many ways to the pathogenesis of SSc, namely, autoantibody synthesis, proinflammatory or profibrotic cytokine production, reduction of immunomodulatory cytokines, and/or through direct interactions with other cell types (Figure 3).
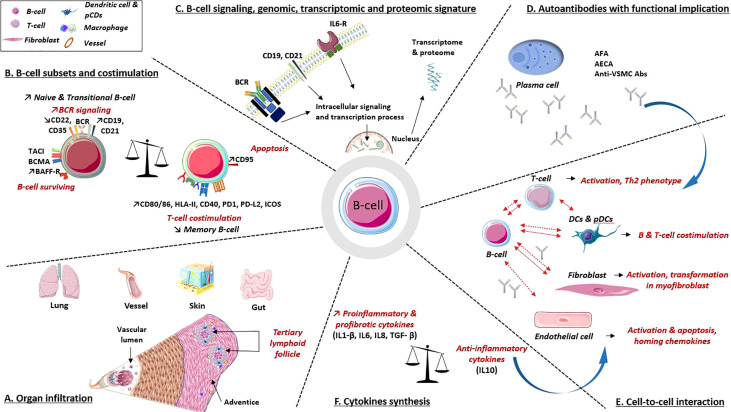
Figure 3.
B-cell implications in the pathogenic processes involved in systemic sclerosis. (A) Organ infiltration: B-cell infiltrates are present in various organs involved in SSc. These infiltrates combine B and T cells, plasma cells, dendritic cells and macrophages, underlying their interactions. (B) B-cell subpopulations: B-cells express at their surfaces multiple molecules involved in activation or surviving pathway, as well as decreased levels of inhibitors coreceptors. B-cells also express co-receptors required for interaction with T cell. (C) B-cell intracellular signaling, genomic, transcriptomic and proteomic data argue a B-cell signature in SSc. (D) Autoantibodies. Several autoantibodies have demonstrated their involvement in cell activation, apoptosis induction, proinflammatory and profibrotic processes. (E, F) Cell to cell interactions and cytokines synthesis: B-cells interact with various immune cells, fibroblast and endothelial cell through direct (solid line) and indirect contact (i.e., cytokines and antibodies; dotted line). All participate in profibrotic, proinflammatory processes, vascular remodeling and immune dysregulations. Abs, antibodies; AECA, anti-endothelial cell Abs; AFA, anti-fibroblast Abs; anti-VSMC Abs, anti-vascular smooth muscle cell Abs; BAFF-R, calcium-modulator and cyclophilin ligand-interactor and BAFF receptor; BCMA, B-cell survival membrane receptors: B-cell maturation protein; BCR, B-cell receptor; CD, cluster of differentiation; DCs, dendritic cells; HLA-II, human leukocyte antigen class II; ICOS, inducible T-cell costimulatory; IL, interleukin; IL6-R, IL6 receptorPD-1/PD-L2: programmed death/programmed death ligand immune checkpoint; pDCs, plasmacytoid dendritic cells; TACI, trans-membrane activator.
5.1 Autoantibodies Contribute to Cell Activation, Inflammation, Fibrosis, and Vasculopathy
The prevalence of anti-nuclear antibodies (Ab) in SSc patients is greater than 90% (53). Despite their important role in disease classification and prognosis staging, specific autoantibodies (mostly identified for centromeric proteins (CENP), topoisomerase I and RNA polymerase III) do not seem to play a major role in SSc pathogenesis (Table 1) (54, 69).
Table 1.
Autoantibodies associated with Systemic Sclerosis.
| Autoantibody target | Prevalence (%) | Clinical association | Vasculopathy | Fibrosis | Other | References |
|---|---|---|---|---|---|---|
| Centromere | 20–40 | lcSSc & PAH | − | − | – | (54) |
| Topoisomerase I | 9–42 | dcSSc & ILD | − | − | – | (54) |
| Fibrillarin (U3 RNP) |
3–20 | dcSSc | + | + | – | (54, 55) |
| RNA polymerase III | 4–11 | dcSSc, SRC & malignancy | − | − | – | (54) |
| Th/To | 2–5 | SRC | − | − | – | (54) |
| Ku | 2–5 | Overlap syndrome | − | − | – | (54) |
| Endothelial cell [anti-VCAM, ICAM-1, NAG-2] |
22–85 | PAH | + | − | Leukocyte adhesion | (56–58) |
| Angiotensin II type 1 Receptor (AT1R) |
85 | PAH & Digital ulcer | + | + | – | (56, 59) |
| Endothelin type A receptor (ETAR) | 85 | PAH ulcer | + | + | – | (56, 59) |
| M3R | 65–80 | Gastrointestinal involvement | − | − | Inhibition of type 3 muscarinic receptor | (60, 61) |
| Vascular smooth muscle cells | 80 | – | + | − | – | (56–58) |
| Fibroblast | 25–60 | dcSSc with PAH, ILD | − | + | Adhesive mechanism | (62–64) |
| MMP-1 & MMP-3 | 50 | dcSSc | − | + | – | (65) |
| PDGF-Receptor | 33 | – | − | + | – | (66) |
| MSRA | 33 | ILD & cardiac involvement | + | + | Reinforcement of oxidative stress | (67) |
| PRX I | 33 | ILD & cardiac involvement | + | + | – | (67) |
| CD22 | 22 | – | − | − | Antagonize inhibitory CD22 signal | (68) |
Anti-AT1-Rc Abs, anti-angiotensin II type 1 receptor Abs; anti-ETa-Rc Abs, anti-endothelin 1 type A receptor Abs; anti-M3R Abs, anti-muscarinic acetylcholine receptor M3; anti-MSRA Abs, anti-Methionine Sulfoxide Reductase A Abs; anti-PDGF-Receptor Abs, anti-platelet-derived growth factor receptor Abs; anti-Prx I Abs, peroxiredoxin I; anti-RNA polymerase III Abs, anti-ribonucleid acid polymerase enzyme III Abs; anti-U3-RNP Abs, anti-U3 ribonucleoprotein Abs, anti-topoisomerase 1 Ab; Anti-VCAM Abs, anti-vascular cell adhesion molecules Abs; anti-VSMC Abs, anti-vascular smooth muscle cell Abs; CCL, chemokine ligand; CD, cluster of differentiation; CENP, centromeric protein with a molecular weight of 17 (CENP-A), 80 (CENP-B) and 140 kDa (CENP-C); dcSSc, diffuse cutaneous form of systemic sclerosis; ICAM, intercellular adhesion molecules; ILD, interstitial lung disease; Ku, p70/p80 is DNA-binding protein involved in DNA repair and regulation; lcSSc, limited cutaneous form of systemic sclerosis; MMP, matrix metalloproteinases; PAH, pulmonary arterial hypertension; SRC, scleroderma renal crisis; TM4SF7 or Nag-2, cell surface tetraspanin transmembrane 4 superfamily molecules member 7; Th/To, 7-2 RNP and 8-2 RNP that are subunits of human mitochondrial RNase and RNase P ribonucleoprotein complexes.
The anti-Topo I Ab is associated with the highest incidence of ILD and the poorest survival, followed by the anti-RNA polymerase III Ab. The anti-RNA polymerase III Ab is also associated with the highest incidence of scleroderma renal crisis (SRC). In contrast, SSc patients with an anti-U3 RNP Ab have the greatest incidence of pulmonary hypertension and SSc-related cardiac involvement (70, 71). Unbiased clustering methods incorporating autoantibody status also show their involvement in grading long-term disease prognosis (72).
Conversely, other autoantibodies, targeting cell surface receptors or ubiquitous molecules with potential direct pathogenic effects, have been identified in skin and pulmonary fibrosis, bowel involvement and/or vascular remodeling. The three main target cells are endothelial cells, fibroblasts, and vascular smooth muscle cells (VSMC). These autoantibodies induce activation and a proinflammatory or pro-adhesive phenotype of their cellular targets, and sometimes apoptosis (73). Thus, a direct pathogenic effect has been documented for certain anti-endothelial cells (AECA) and anti-fibroblast antibodies (AFA) (62–64, 74–76). This is the case for AECA targeting angiotensin II type I receptor or endothelin-1 type A receptor, which induce activation and apoptosis of endothelial cells with fibroblast activation, and secretion of profibrotic TGF-β cytokine (56, 77). Anti-platelet derived growth factor (PDGF)-receptor antibodies activate human VSMCs and fibroblasts and their transition into myofibroblasts, leading to fibrotic and vascular injury (66, 75). In the first publication, the presence of these anti-PDGF-receptor stimulatory antibodies was found in 100% of patients (n = 46). Their functional activity has been demonstrated through the involvement of the Ha-Ras-ERK1/2 activating cascade downstream of the PDFG receptor, the induction of ROS accumulation in a dose-dependent manner in in vitro experiments, and the promotion of type I collagen-related genes (66). Similar results have been demonstrated on human arterial smooth muscle cells (n = 11) with activation by anti-PDGF receptor antibodies of cell growth, migration ability, the generation of ROS, type I collagen gene expression, and the involvement of the NOX4 and mTORC1 pathways (75). These data must be relativized slightly because the sample sizes are low and, thus far, these results have not been replicated. Proteomic studies have identified numerous antigenic targets, such as Stress-Induced-Phosphoprotein 1 (STIP 1) or alpha-enolase, two proteins involved in the contraction of vascular smooth muscle cells, with an antibody-mediated direct pathogenic effect with contraction induced by incubation with purified IgG from SSc patients (78).
5.2 Imbalance in Proinflammatory, Profibrotic and Anti-Inflammatory Cytokines Produced by B-Cells in SSc
In SSc patients, activated B-cells secrete high levels of IL-6 and TGF-β. The IL-6 levels in the supernatant of purified B-cells from SSc patients are correlated with the mRSS, suggesting a pivotal role of B-cells in the fibrosis process (16). IL-6 stimulates B and T-cell proliferation, increases the resistance to apoptosis, activates fibroblasts and reduces the regulatory functions of Tregs. The IL-6 signaling induced indirectly the collagen synthesis by the fibroblast, through the phosphorylation of STAT3, the activation of downstream targeted genes, and via Gremlin-1, a Bone Morphogenetic Protein (BMP) antagonist, which activates the non-canonical TGF-β pathway via smad3 (79). Furthermore, the highest serum levels of IL-6 are reported in the early dcSSc compared to the other groups of patients (80). Fibroblasts are also important producers of IL-6. Fibroblasts from affected skin areas in SSc patients produce significantly more IL-6 compared with fibroblasts from unaffected skin areas, or from healthy controls (81). There is, therefore, a loop between the fibroblast and the B-cell that is partly dependent on IL-6. Noteworthy, IL-6 and IL-10 serum levels correlated with skin and pulmonary fibrosis (82, 83). Otherwise, circulating B-cells secrete increased amounts of IL-8, IL-1β, BAFF, and CXCL13, which are proinflammatory, profibrotic cytokines or chemo-attractant molecules, both in scleroderma mouse models and SSc patients (20, 22, 37, 84). Many of these cytokines contribute to polarizing CD4+ T cells into IL-4 producing Th2 cells (85), T follicular helper (Tfh) and/or Th17 cells. In SSc, a Th2 lymphocyte phenotype is observed in the early stages of disease (86), and Th2 cytokines enhance antibody production by plasma cells and contribute to stimulating collagen production by fibroblasts.
Among regulatory cytokines, a particular interest has recently been given to IL-10, produced by Breg. Bregs can decrease inflammatory and autoimmune processes (87). These cells exert suppressive effects on a large variety of cells, including autoreactive T cells, dendritic cells, or macrophages through IL-10 production/synthesis. A reduction of IL-10 synthesis has been documented, both in mouse models and in SSc patients, and is correlated with the decrease of IL-10+ B-cells (19, 27, 84, 88). The decrease in these cells encountered in SSc patients is associated with the presence of ILD and inversely correlated with autoantibodies levels, and with Th17 T cells (26) suggesting their high implication in the control of the profibrotic environment (26).
5.3 B and T Cells Co-Interact and Self-Activate
The identification of B- and T-cell infiltrates in SSc patient skin biopsies led to arguments for their strong interaction (12, 89) and predominantly in the early stages of disease. As expected, the production of anti-topoisomerase I Ab requires direct contact and restriction to HLA-DR and CD40/CD40-ligand costimulatory molecules in collaboration with CD4+ T cells (90, 91). Follicular helper T cells (Tfhs) are crucial for the activation and differentiation of B-cells into plasma cells (92). Many data highlighted the interaction of Tfhs and B-cells through direct contact or via the intermediate of cytokines (IL-21) in the fibrotic process encountered in SSc patients or murine models (93, 94). Tfhs are hyperactivated in SSc patients through the higher expression of activation proteins like inducible T-cell costimulatory (ICOS) and PD-1 on their surface compared to healthy controls (93). Compared to healthy controls, circulating Tfh from SSc patients secreted higher IL-21 levels, promoting the differentiation of naïve B-cells into plasma cells and proliferation, and Ig secretion, especially in dcSSc and PAH (94).
B-cells interact with other T-cell subsets in SSc. The transmembrane glycoprotein T-cell Ig and Mucin domain protein 1 (TIM-1), a regulatory marker at the Breg surface, is implicated in the interaction between B and T cells in SSc. The frequency of peripheral blood TIM-1+ IL-10+ Breg is decreased in SSc patients compared with healthy controls, with a further defective ability to suppress CD4+ T-cell activation and inflammatory cytokine secretion (IFN-γ, TNF-α, IL-17) (27). However, the CD70 promoter is demethylated, favoring its overexpression on CD4+ T cells and seems to have contributed to the autoimmune response via its costimulatory activity of the B-cell during the B–T cell interaction (95). CD8+ effector T cells, Th17-cells and Treg are also involved in the synthesis of many proinflammatory and profibrotic cytokines in SSc (IL-4, IL-5, IL-9, IL-13, IL-17, TGF-β, and TNF-α) (96–98).
5.4 B-Cells Interact With Dendritic Cells and Other Innate Immune Cells
Very little data are available on the B-cell/dendritic cell (DC) interaction in the SSc. Activated B-cells induce the maturation of DCs in a contact-dependent manner (CD83, CD80, CD86, CD40, and HLA-DR) through BCR signaling and the BAFF receptor. B-cell-matured DCs secondary promote polarization and activation of naive CD4+ T cells into Th2 cells, producing increased amounts of IL-4, IL-5, and IL-13 (99).
Otherwise, proteome-wide analysis demonstrated a high level of CXCL4 in the blood and skin of SSc patients, and primarily synthesis by the plasmacytoid DCs (pDCs). CXCL4 levels were correlated with skin and lung fibrosis and with PAH (100). Similar data were shown in the BLM model with an increased number of pDCs in the affected skin and lungs compared to wild-type mice (101). The pDCs express the BAFF ligand (35), supporting the argument for an interaction between B-cells and pDCs in the SSc.
B-cells interact with other innate immune cells such as the macrophages. Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF)+ B effector cells stimulate macrophages through GM-CSF synthesis to induce inflammatory and fibrotic lesions. These cells belong to the memory B-cell subset and strongly produce IL-6 and TNF-α. These cells have been reported to be increased in SSc patients, especially in dcSSc and associated with ILD, suggesting their role in the pathogenesis of SSc (102).
5.5 B-Cells Activate Fibroblasts
In cellular culture, cell-to-cell contact between B-cells and dermal fibroblasts induces an increase in collagen, α-smooth muscle actin, and metalloproteinase synthesis, or cytokines and chemokines (IL-6, TGF-β1, CCL2) (40, 41).
Antibody-mediated effects are illustrated with AFA, from the serum of SSc patients, which binds skin and lung fibroblasts, leading to proadhesive (ICAM-1), proinflammatory (reactive oxygen species (ROS)) and profibrotic (collagen) processes (66, 75, 103, 104) (Table 1).
Paracrine-mediated effects are illustrated by the ability of B-cell supernatant purified from SSc patients, rich in IL-6 and TGF-β, to induce fibroblast proliferation, collagen synthesis, and fibroblast-myofibroblast transition.
5.6 B-Cells Participate in Endothelial Cell Activation and Endothelial Damages
Interactions between B-cells, autoantibodies that they produce, and endothelial cells lead to their activation, immune cell homing, and apoptosis (56, 77). Purified IgG from AECA-positive SSc, PAH, or SLE patients is able to upregulate cell expression of various chemoattractant molecules (ICAM-1, VCAM-1, E-selectin, CCL2, or CXCL8), ROS production, interleukins (IL-6, -8) (56–58) and promotes intima hyperplasia and contributes to vascular injury (75).
6 B-cell as Therapeutic Target
Many data argue for the involvement of the B-cell in the early phase of SSc, or during some specific SSc-related involvement. Overall, the therapies directly targeting the B-cells, the cytokines produced by the B-cells during SSc, or these costimulation pathways therefore do not work in all patients, despite interesting effects (105). This suggests the potential interest of combined therapies, which have been developed in a few years. Over the past decade, interventional trials of biotherapies have been steadily increasing, representing about one-third of all therapeutic trials.
6.1 Depleting Therapies of B-Cell in SSc
6.1.1 Direct Cytotoxicity of B-cell: The Anti-CD20 Monoclonal Antibody
B-cell depletion in SSc patients is associated with a decrease of the TGF-β pathway, the collagen and myofibroblast accumulation, and the attenuation of PDGFR expression and activation in the skin (106–108). In newborn TSK mice, B-cell depletion prevents skin fibrosis and autoantibody production (88).
Impact of anti-CD20 monoclonal antibody (rituximab RTX) on the course of SSc has been reported in two small randomized and controlled trials, respectively in 16 (versus placebo, ratio 1:1) and in 60 patients (versus cyclophosphamide, ratio 1:1) with early SSc (<2 years of duration) (109, 110). Despite the small number and the need for caution in interpretation, FVC improved slightly in the RTX group compared to the decrease in the cyclophosphamide group, as well as an improvement in mRSS in the RTX group (110). The same trends have been highlighted in RA-related ILD (111, 112). A recent systematic review with meta-analysis has assessed the impact of RTX on the changes in FVC and DLCO parameters in the SSc-ILD from 20 studies (2 RCT, 6 prospective studies, 5 retrospective studies, and 7 conference abstracts) representing 575 SSc patients. In summary, RTX slightly improves FVC and DLCO at 6 and 12 months of follow-up compared to baseline. In the 2 trials comparing RTX to another immunosuppressant, RTX was similar to controls on FVC and DLCO, but exposed to a lower risk of infection (113). Data from the RECITAL RCT (NCT01862926) evaluating RTX versus cyclophosphamide (CYC) in ILD related to SSc (N = 39, 38.6%), an idiopathic inflammatory myositis (N = 45, 44.6%), or mixed connective tissue disease (N = 17, 16.8%), were recently presented at the American Thoracic Society International Conference (ATS). Patients were randomized 1:1 to receive CYC (N = 50) or RTX (N = 51). Concerning the primary endpoint at 24 weeks, both RTX and CYC improved the FVC without the superiority of the RTX but with fewer adverse events and a reduction in corticosteroid exposure compared to CYC (114). Many trials are ongoing or published testing rituximab in SSc-related ILD alone, or in association with mycophenolate mofetil, MMF (EvER-ILD NCT02990286), or SSc-associated PAH (115). This suggests a potential place for rituximab in the therapeutic arsenal of early forms with skin or ILD manifestations. The results of larger randomized and controlled trials are awaited.
6.1.2 Direct Cytotoxicity of B-Cell: The Anti-CD19 Monoclonal Antibody
A phase I, randomized, placebo-controlled trial testing inebilizumab, an anti-CD19 monoclonal antibody in 28 SSc patients (24 in inebilizumab and 4 in the placebo group), has shown a depletion of peripheral B and plasma cells, with a possible effect on skin fibrosis (mRSS), but 2 serious adverse effects in the inebilizumab group led to the discontinuation of the trial (116).
6.1.3 Impact of Autologous Hematopoietic Stem Cell Transplantation (AHSCT) on B-Cell Homeostasis
Several data have shown an encouraging impact of AHSCT in patients with SSc (117–119). Post transplantation SSc patients have a reduced B-cell division rate, a restoration of the B-cell homeostasis, and a tolerant adaptative immune status. This is supported by post-transplant Breg levels rise, an increased Breg/memory B-cell ratio, with a long-term increase of IL10 production and recover their ability to suppress synthesis of Th1 cytokines by CD4+ T cells (120, 121). After transplant, the B-cell subsets show an increase of CD27/IgD+ naive B-cell percentage associated with a decrease of CD27+/IgD+ pre-switch memory, CD27+/IgD− post-switched memory B-cells (122), extinction of interferon and inflammation transcriptomic molecular signatures (123), a decrease of IL-6 and TGF-β-producing B-cells (121).
6.2 Therapy Targeting Cytokines Produced by B-Cell
Serum IL-6 levels are increased in SSc patients, especially in cases of aggressive disease (16, 124). The neutralization of IL-6 by monoclonal antibodies or by immunization against a peptide from murine IL-6 in BLM mice has induced a reduction in dermal thickness (124). Additionally, the anti-IL-6 receptor monoclonal antibody (tocilizumab) induces a slightly reduction of collagen fiber bundles in the skin (125).
Based on these preliminary data, two multicenter, randomized, double-blind, placebo-controlled trials assessed the efficacy of tocilizumab on mRSS (primary endpoint) in SSc patients: the phase 2 faSScinate trial [n = 87 (126)] and the phase 3 focuSSced trial [n = 212 (127)]. Both of them failed on the primary endpoint, the difference in the mean change in mRSS. Secondary analysis showed a less consistent decline in predicted FVC in favor of the tocilizumab group. As expected, a shorter disease course and the presence of inflammatory biomarkers were associated with the best response. Its therapeutic place remains to be clarified, and further trials are necessary. The Food and Drug Administration (FDA) has just approved its use in SSc-ILD.
TGF-β appears as a central mediator in organ fibrosis and it is mostly produced by the B-cell in SSc. Its main target is the fibroblast (4). Data from SSc patients and TGF-β transgenic mice support the rationale for targeting TGF-β (128). Fresolimumab, a pan-specific anti-TGF-β antibody, has been tested in an open-label study on early and diffuse SSc patients. The expression of TGF-β-related genes in the skin of SSc patients decreases after treatment with fresolimumab, as with myofibroblast infiltration and the skin fibrosis (129). These are very preliminary results.
6.3 Therapy Targeting Cytokine Activating B-Cell: BAFF
Belimumab is a recombinant monoclonal antibody targeting soluble BAFF that promotes the B-cell apoptosis. In a TSK mouse model, BAFF antagonist inhibited the synthesis of autoantibodies and skin fibrosis (36). One double-blind randomized and controlled trial, assessed versus placebo in association with MMF, failed on significantly improving mRSS. It showed a decrease in genetic pathways, including BCR, TLR signaling, and profibrotic genes like TGF-β which were overexpressed in improved patients (130). Combined sequential therapy after a rituximab course is currently being studied in dcSSc patients (NCT03844061).
6.4 Proteasome Inhibitor Targeting Plasma Cell: The Bortezomib
Bortezomib, the first-in-class proteasome inhibitor, suppresses activated B-cells, plasma cells, and DC maturation and promotes immune-modulatory effects. This therapeutic class, used particularly in multiple myeloma, is very active and attractive. A human phase 2 trial is being recruited, testing a combination of bortezomib and MMF in SSc-related ILD patients (NCT02370693). In preliminary data on BLM, TSK/+, or Scl-cGVHD mouse models, bortezomib reduced skin infiltration of B-cells and the number of germinal centers, preventing dermal fibrosis with antifibrotic and anti-inflammatory efficacy as well vas a T-cell polarization toward a regulatory phenotype (104, 131, 132).
6.5 Others Drugs Targeting B-Cell Intracellular Signaling and Metabolism
Several other treatments are under study in SSc and can target B-cells. For example, some immunosuppressive therapies like pomalidomide are tested in SSc-related ILD in phase 2, randomized, placebo-controlled trials (133). Currently, no studies are underway regarding the assessment of SYK inhibitors in SSc patients. Encouraging studies have been conducted in several murine models (45, 46, 134).
7 Conclusion
Many advances have been made in understanding the involvement of B-cells in the pathogenesis of SSc. Besides autoantibody production, B-cells produce profibrotic and proinflammatory cytokines and interact by direct contact with the fibroblast or other immune cells. This is associated with a disorder of the homeostasis of B-cell subsets, membrane or intracellular signaling, arguing for its implication in the proinflammatory and profibrotic mechanisms. Many of these B-cell abnormalities encountered in SSc patients are associated with specific prognostic profiles. Treatments targeting B-cells, through their depletion by cytotoxicity, or the cytokines produced by the B-cells, or their costimulation molecules, seem interesting, probably in certain profiles of early patients with severe organic damage. This requires additional randomized controlled trials.
Author Contributions
BT, BC, and LM have made contributions to conception and design of this review, drafting and revising it. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum (2013) 65:2737–47. doi: 10.1002/art.38098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsou P-S, Varga J, O’Reilly S. Advances in Epigenetics in Systemic Sclerosis: Molecular Mechanisms and Therapeutic Potential. Nat Rev Rheumatol (2021) 17:596–607. doi: 10.1038/s41584-021-00683-2 [DOI] [PubMed] [Google Scholar]
- 3. Elhai M, Meune C, Boubaya M, Avouac J, Hachulla E, Balbir-Gurman A, et al. Mapping and Predicting Mortality From Systemic Sclerosis. Ann Rheum Dis (2017) 76:1897–905. doi: 10.1136/annrheumdis-2017-211448 [DOI] [PubMed] [Google Scholar]
- 4. Thoreau B, Chaigne B, Renaud A, Mouthon L. Pathophysiology of Systemic Sclerosis. Presse Med (2021) 50:104087. doi: 10.1016/j.lpm.2021.104087 [DOI] [PubMed] [Google Scholar]
- 5. Hügle T, O’Reilly S, Simpson R, Kraaij MD, Bigley V, Collin M, et al. Tumor Necrosis Factor-Costimulated T Lymphocytes From Patients With Systemic Sclerosis Trigger Collagen Production in Fibroblasts. Arthritis Rheum (2013) 65:481–91. doi: 10.1002/art.37738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roumm AD, Whiteside TL, Medsger TA, Rodnan GP. Lymphocytes in the Skin of Patients With Progressive Systemic Sclerosis. Quantification, Subtyping, and Clinical Correlations. Arthritis Rheum (1984) 27:645–53. doi: 10.1002/art.1780270607 [DOI] [PubMed] [Google Scholar]
- 7. Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P. Sequential Dermal Microvascular and Perivascular Changes in the Development of Scleroderma. J Pathol (1992) 166:255–63. doi: 10.1002/path.1711660307 [DOI] [PubMed] [Google Scholar]
- 8. Harrison NK, Myers AR, Corrin B, Soosay G, Dewar A, Black CM, et al. Structural Features of Interstitial Lung Disease in Systemic Sclerosis. Am Rev Respir Dis (1991) 144:706–13. doi: 10.1164/ajrccm/144.3_Pt_1.706 [DOI] [PubMed] [Google Scholar]
- 9. Manetti M, Neumann E, Müller A, Schmeiser T, Saar P, Milia AF, et al. Endothelial/lymphocyte Activation Leads to Prominent CD4+ T Cell Infiltration in the Gastric Mucosa of Patients With Systemic Sclerosis. Arthritis Rheum (2008) 58:2866–73. doi: 10.1002/art.23806 [DOI] [PubMed] [Google Scholar]
- 10. Laurent P, Sisirak V, Lazaro E, Richez C, Duffau P, Blanco P, et al. Innate Immunity in Systemic Sclerosis Fibrosis: Recent Advances. Front Immunol (2018) 9:1702. doi: 10.3389/fimmu.2018.01702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whitfield ML, Finlay DR, Murray JI, Troyanskaya OG, Chi J-T, Pergamenschikov A, et al. Systemic and Cell Type-Specific Gene Expression Patterns in Scleroderma Skin. Proc Natl Acad Sci USA (2003) 100:12319–24. doi: 10.1073/pnas.1635114100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bosello S, Angelucci C, Lama G, Alivernini S, Proietti G, Tolusso B, et al. Characterization of Inflammatory Cell Infiltrate of Scleroderma Skin: B-cells and Skin Score Progression. Arthritis Res Ther (2018) 20:75. doi: 10.1186/s13075-018-1569-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fetter T, Niebel D, Braegelmann C, Wenzel J. Skin-Associated B-cells in the Pathogenesis of Cutaneous Autoimmune Diseases-Implications for Therapeutic Approaches. Cells (2020) 9:E2627. doi: 10.3390/cells9122627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Santis M, Bosello SL, Peluso G, Pinnelli M, Alivernini S, Zizzo G, et al. Bronchoalveolar Lavage Fluid and Progression of Scleroderma Interstitial Lung Disease. Clin Respir J (2012) 6:9–17. doi: 10.1111/j.1752-699X.2010.00228.x [DOI] [PubMed] [Google Scholar]
- 15. Lafyatis R, O’Hara C, Feghali-Bostwick CA, Matteson E. B-cell Infiltration in Systemic Sclerosis-Associated Interstitial Lung Disease. Arthritis Rheum (2007) 56:3167–8. doi: 10.1002/art.22847 [DOI] [PubMed] [Google Scholar]
- 16. Dumoitier N, Chaigne B, Régent A, Lofek S, Mhibik M, Dorfmüller P, et al. Scleroderma Peripheral B Lymphocytes Secrete Interleukin-6 and Transforming Growth Factor β and Activate Fibroblasts. Arthritis Rheumatol (Hoboken NJ) (2017) 69:1078–89. doi: 10.1002/art.40016 [DOI] [PubMed] [Google Scholar]
- 17. Allman D, Pillai S. Peripheral B-cell Subsets. Curr Opin Immunol (2008) 20:149–57. doi: 10.1016/j.coi.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sato S, Fujimoto M, Hasegawa M, Takehara K. Altered Blood B Lymphocyte Homeostasis in Systemic Sclerosis: Expanded Naive B-cells and Diminished But Activated Memory B-cells. Arthritis Rheum (2004) 50:1918–27. doi: 10.1002/art.20274 [DOI] [PubMed] [Google Scholar]
- 19. Soto L, Ferrier A, Aravena O, Fonseca E, Berendsen J, Biere A, et al. Systemic Sclerosis Patients Present Alterations in the Expression of Molecules Involved in B-cell Regulation. Front Immunol (2015) 6:496. doi: 10.3389/fimmu.2015.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taher TE, Ong VH, Bystrom J, Hillion S, Simon Q, Denton CP, et al. Association of Defective Regulation of Autoreactive Interleukin-6-Producing Transitional B Lymphocytes With Disease in Patients With Systemic Sclerosis. Arthritis Rheumatol (Hoboken NJ) (2018) 70:450–61. doi: 10.1002/art.40390 [DOI] [PubMed] [Google Scholar]
- 21. Sato S, Hasegawa M, Fujimoto M, Tedder TF, Takehara K. Quantitative Genetic Variation in CD19 Expression Correlates With Autoimmunity. J Immunol (2000) 165:6635–43. doi: 10.4049/jimmunol.165.11.6635 [DOI] [PubMed] [Google Scholar]
- 22. Forestier A, Guerrier T, Jouvray M, Giovannelli J, Lefèvre G, Sobanski V, et al. Altered B Lymphocyte Homeostasis and Functions in Systemic Sclerosis. Autoimmun Rev (2018) 17:244–55. doi: 10.1016/j.autrev.2017.10.015 [DOI] [PubMed] [Google Scholar]
- 23. Gambichler T, Tigges C, Burkert B, Höxtermann S, Altmeyer P, Kreuter A. Absolute Count of T and B Lymphocyte Subsets is Decreased in Systemic Sclerosis. Eur J Med Res (2010) 15:44–6. doi: 10.1186/2047-783X-15-1-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simon D, Balogh P, Bognár A, Kellermayer Z, Engelmann P, Németh P, et al. Reduced non-Switched Memory B-cell Subsets Cause Imbalance in B-cell Repertoire in Systemic Sclerosis. Clin Exp Rheumatol (2016) 34 (Suppl 100):30–6. [PubMed] [Google Scholar]
- 25. Mavropoulos A, Simopoulou T, Varna A, Liaskos C, Katsiari CG, Bogdanos DP, et al. Breg Cells Are Numerically Decreased and Functionally Impaired in Patients With Systemic Sclerosis. Arthritis Rheumatol (Hoboken NJ) (2016) 68:494–504. doi: 10.1002/art.39437 [DOI] [PubMed] [Google Scholar]
- 26. Matsushita T, Hamaguchi Y, Hasegawa M, Takehara K, Fujimoto M. Decreased Levels of Regulatory B-cells in Patients With Systemic Sclerosis: Association With Autoantibody Production and Disease Activity. Rheumatol (Oxford) (2016) 55:263–7. doi: 10.1093/rheumatology/kev331 [DOI] [PubMed] [Google Scholar]
- 27. Aravena O, Ferrier A, Menon M, Mauri C, Aguillón JC, Soto L, et al. TIM-1 Defines a Human Regulatory B-cell Population That is Altered in Frequency and Function in Systemic Sclerosis Patients. Arthritis Res Ther (2017) 19:8. doi: 10.1186/s13075-016-1213-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Streicher K, Sridhar S, Kuziora M, Morehouse CA, Higgs BW, Sebastian Y, et al. Baseline Plasma Cell Gene Signature Predicts Improvement in Systemic Sclerosis Skin Scores Following Treatment With Inebilizumab (MEDI-551) and Correlates With Disease Activity in Systemic Lupus Erythematosus and Chronic Obstructive Pulmonary Disease. Arthritis Rheumatol (Hoboken NJ) (2018) 70:2087–95. doi: 10.1002/art.40656 [DOI] [PubMed] [Google Scholar]
- 29. Lipsky PE. Systemic Lupus Erythematosus: An Autoimmune Disease of B-cell Hyperactivity. Nat Immunol (2001) 2:764–6. doi: 10.1038/ni0901-764 [DOI] [PubMed] [Google Scholar]
- 30. O’Reilly S, Hügle T, van Laar JM. T Cells in Systemic Sclerosis: A Reappraisal. Rheumatol (Oxford) (2012) 51:1540–9. doi: 10.1093/rheumatology/kes090 [DOI] [PubMed] [Google Scholar]
- 31. Fukasawa T, Yoshizaki A, Ebata S, Nakamura K, Saigusa R, Miura S, et al. Contribution of Soluble Forms of Programmed Death 1 and Programmed Death Ligand 2 to Disease Severity and Progression in Systemic Sclerosis. Arthritis Rheumatol (Hoboken NJ) (2017) 69:1879–90. doi: 10.1002/art.40164 [DOI] [PubMed] [Google Scholar]
- 32. Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B-cell Antigen Receptor Signaling 101. Mol Immunol (2004) 41:599–613. doi: 10.1016/j.molimm.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 33. de Bourcy CFA, Dekker CL, Davis MM, Nicolls MR, Quake SR. Dynamics of the Human Antibody Repertoire After B-cell Depletion in Systemic Sclerosis. Sci Immunol (2017) 2:1–8. doi: 10.1126/sciimmunol.aan8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Melissaropoulos K, Liossis S-N. Decreased CD22 Expression and Intracellular Signaling Aberrations in B-cells of Patients With Systemic Sclerosis. Rheumatol Int (2018) 38:1225–34. doi: 10.1007/s00296-018-4076-3 [DOI] [PubMed] [Google Scholar]
- 35. Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL System: Emerging Functions Beyond B-cell Biology and Autoimmunity. Cytokine Growth Factor Rev (2013) 24:203–15. doi: 10.1016/j.cytogfr.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsushita T, Fujimoto M, Hasegawa M, Matsushita Y, Komura K, Ogawa F, et al. BAFF Antagonist Attenuates the Development of Skin Fibrosis in Tight-Skin Mice. J Invest Dermatol (2007) 127:2772–80. doi: 10.1038/sj.jid.5700919 [DOI] [PubMed] [Google Scholar]
- 37. Matsushita T, Hasegawa M, Yanaba K, Kodera M, Takehara K, Sato S. Elevated Serum BAFF Levels in Patients With Systemic Sclerosis: Enhanced BAFF Signaling in Systemic Sclerosis B Lymphocytes. Arthritis Rheum (2006) 54:192–201. doi: 10.1002/art.21526 [DOI] [PubMed] [Google Scholar]
- 38. Matsushita T, Fujimoto M, Hasegawa M, Tanaka C, Kumada S, Ogawa F, et al. Elevated Serum APRIL Levels in Patients With Systemic Sclerosis: Distinct Profiles of Systemic Sclerosis Categorized by APRIL and BAFF. J Rheumatol (2007) 34:2056–62. [PubMed] [Google Scholar]
- 39. Abdo MS, Mohammed RHA, Raslan HM, Gaber SM. Serum B-cell Activating Factor Assessment in a Population Of Egyptian Patients With Systemic Sclerosis. Int J Rheum Dis (2013) 16:148–56. doi: 10.1111/1756-185x.12019 [DOI] [PubMed] [Google Scholar]
- 40. Matsushita T, Kobayashi T, Mizumaki K, Kano M, Sawada T, Tennichi M, et al. BAFF Inhibition Attenuates Fibrosis in Scleroderma by Modulating the Regulatory and Effector B-cell Balance. Sci Adv (2018) 4:eaas9944. doi: 10.1126/sciadv.aas9944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. François A, Chatelus E, Wachsmann D, Sibilia J, Bahram S, Alsaleh G, et al. B Lymphocytes and B-cell Activating Factor Promote Collagen and Profibrotic Markers Expression by Dermal Fibroblasts in Systemic Sclerosis. Arthritis Res Ther (2013) 15:R168. doi: 10.1186/ar4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bielecki M, Kowal K, Lapinska A, Bernatowicz P, Chyczewski L, Kowal-Bielecka O. Increased Production of a Proliferation-Inducing Ligand (APRIL) by Peripheral Blood Mononuclear Cells is Associated With Antitopoisomerase I Antibody and More Severe Disease in Systemic Sclerosis. J Rheumatol (2010) 37:2286–9. doi: 10.3899/jrheum.100454 [DOI] [PubMed] [Google Scholar]
- 43. Saito E, Fujimoto M, Hasegawa M, Komura K, Hamaguchi Y, Kaburagi Y, et al. CD19-Dependent B Lymphocyte Signaling Thresholds Influence Skin Fibrosis and Autoimmunity in the Tight-Skin Mouse. J Clin Invest (2002) 109:1453–62. doi: 10.1172/JCI15078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Asano N, Fujimoto M, Yazawa N, Shirasawa S, Hasegawa M, Okochi H, et al. B Lymphocyte Signaling Established by the CD19/CD22 Loop Regulates Autoimmunity in the Tight-Skin Mouse. Am J Pathol (2004) 165:641–50. doi: 10.1016/S0002-9440(10)63328-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Le Huu D, Kimura H, Date M, Hamaguchi Y, Hasegawa M, Hau KT, et al. Blockade of Syk Ameliorates the Development of Murine Sclerodermatous Chronic Graft-Versus-Host Disease. J Dermatol Sci (2014) 74:214–21. doi: 10.1016/j.jdermsci.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 46. Pamuk ON, Can G, Ayvaz S, Karaca T, Pamuk GE, Demirtas S, et al. Spleen Tyrosine Kinase (Syk) Inhibitor Fostamatinib Limits Tissue Damage and Fibrosis in a Bleomycin-Induced Scleroderma Mouse Model. Clin Exp Rheumatol (2015) 33:S15–22. [PubMed] [Google Scholar]
- 47. Wu M, Mohan C. B-cells in Systemic Sclerosis: Emerging Evidence From Genetics to Phenotypes. Curr Opin Rheumatol (2015) 27:537–41. doi: 10.1097/BOR.0000000000000215 [DOI] [PubMed] [Google Scholar]
- 48. Zuo X, Zhang L, Luo H, Li Y, Zhu H. Systematic Approach to Understanding the Pathogenesis of Systemic Sclerosis. Clin Genet (2017) 92:365–71. doi: 10.1111/cge.12946 [DOI] [PubMed] [Google Scholar]
- 49. Bos CL, van Baarsen LGM, Timmer TCG, Overbeek MJ, Basoski NM, Rustenburg F, et al. Molecular Subtypes of Systemic Sclerosis in Association With Anti-Centromere Antibodies and Digital Ulcers. Genes Immun (2009) 10:210–8. doi: 10.1038/gene.2008.98 [DOI] [PubMed] [Google Scholar]
- 50. Assassi S, Mayes MD, Arnett FC, Gourh P, Agarwal SK, McNearney TA, et al. Systemic Sclerosis and Lupus: Points in an Interferon-Mediated Continuum. Arthritis Rheum (2010) 62:589–98. doi: 10.1002/art.27224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Skaug B, Khanna D, Swindell WR, Hinchcliff ME, Frech TM, Steen VD, et al. Global Skin Gene Expression Analysis of Early Diffuse Cutaneous Systemic Sclerosis Shows a Prominent Innate and Adaptive Inflammatory Profile. Ann Rheum Dis (2019) 79(3):379–86. doi: 10.1136/annrheumdis-2019-215894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clark KEN, Campochiaro C, Csomor E, Taylor A, Nevin K, Galwey N, et al. Molecular Basis for Clinical Diversity Between Autoantibody Subsets in Diffuse Cutaneous Systemic Sclerosis. Ann Rheum Dis (2021) 80:1584–93. doi: 10.1136/annrheumdis-2021-220402 [DOI] [PubMed] [Google Scholar]
- 53. Guillevin L, Bérezné A, Seror R, Teixeira L, Pourrat J, Mahr A, et al. Scleroderma Renal Crisis: A Retrospective Multicentre Study on 91 Patients and 427 Controls. Rheumatol (Oxford) (2012) 51:460–7. doi: 10.1093/rheumatology/ker271 [DOI] [PubMed] [Google Scholar]
- 54. Stochmal A, Czuwara J, Trojanowska M, Rudnicka L. Antinuclear Antibodies in Systemic Sclerosis: An Update. Clin Rev Allergy Immunol (2019) 58(1):40–51. doi: 10.1007/s12016-018-8718-8 [DOI] [PubMed] [Google Scholar]
- 55. Okano Y, Steen VD, Medsger TA. Autoantibody to U3 Nucleolar Ribonucleoprotein (Fibrillarin) in Patients With Systemic Sclerosis. Arthritis Rheum (1992) 35:95–100. doi: 10.1002/art.1780350114 [DOI] [PubMed] [Google Scholar]
- 56. Kill A, Tabeling C, Undeutsch R, Kühl AA, Günther J, Radic M, et al. Autoantibodies to Angiotensin and Endothelin Receptors in Systemic Sclerosis Induce Cellular and Systemic Events Associated With Disease Pathogenesis. Arthritis Res Ther (2014) 16:R29. doi: 10.1186/ar4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arends SJ, Damoiseaux JGMC, Duijvestijn AM, Debrus-Palmans L, Boomars KA, Brunner-La Rocca H-P, et al. Functional Implications of IgG Anti-Endothelial Cell Antibodies in Pulmonary Arterial Hypertension. Autoimmunity (2013) 46:463–70. doi: 10.3109/08916934.2013.812080 [DOI] [PubMed] [Google Scholar]
- 58. Wolf SI, Howat S, Abraham DJ, Pearson JD, Lawson C. Agonistic Anti-ICAM-1 Antibodies in Scleroderma: Activation of Endothelial Pro-Inflammatory Cascades. Vascul Pharmacol (2013) 59:19–26. doi: 10.1016/j.vph.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Avouac J, Riemekasten G, Meune C, Ruiz B, Kahan A, Allanore Y. Autoantibodies Against Endothelin 1 Type A Receptor Are Strong Predictors of Digital Ulcers in Systemic Sclerosis. J Rheumatol (2015) 42:1801–7. doi: 10.3899/jrheum.150061 [DOI] [PubMed] [Google Scholar]
- 60. Goldblatt F, Gordon TP, Waterman SA. Antibody-Mediated Gastrointestinal Dysmotility in Scleroderma. Gastroenterology (2002) 123:1144–50. doi: 10.1053/gast.2002.36057 [DOI] [PubMed] [Google Scholar]
- 61. Kawaguchi Y, Nakamura Y, Matsumoto I, Nishimagi E, Satoh T, Kuwana M, et al. Muscarinic-3 Acetylcholine Receptor Autoantibody in Patients With Systemic Sclerosis: Contribution to Severe Gastrointestinal Tract Dysmotility. Ann Rheum Dis (2009) 68:710–4. doi: 10.1136/ard.2008.096545 [DOI] [PubMed] [Google Scholar]
- 62. Tamby MC, Humbert M, Guilpain P, Servettaz A, Dupin N, Christner JJ, et al. Antibodies to Fibroblasts in Idiopathic and Scleroderma-Associated Pulmonary Hypertension. Eur Respir J (2006) 28:799–807. doi: 10.1183/09031936.06.00152705 [DOI] [PubMed] [Google Scholar]
- 63. Terrier B, Tamby MC, Camoin L, Guilpain P, Broussard C, Bussone G, et al. Identification of Target Antigens of Antifibroblast Antibodies in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med (2008) 177:1128–34. doi: 10.1164/rccm.200707-1015OC [DOI] [PubMed] [Google Scholar]
- 64. Terrier B, Tamby MC, Camoin L, Guilpain P, Bérezné A, Tamas N, et al. Antifibroblast Antibodies From Systemic Sclerosis Patients Bind to {Alpha}-Enolase and are Associated With Interstitial Lung Disease. Ann Rheum Dis (2010) 69:428–33. doi: 10.1136/ard.2008.104299 [DOI] [PubMed] [Google Scholar]
- 65. Sato S, Hayakawa I, Hasegawa M, Fujimoto M, Takehara K. Function Blocking Autoantibodies Against Matrix Metalloproteinase-1 in Patients With Systemic Sclerosis. J Invest Dermatol (2003) 120:542–7. doi: 10.1046/j.1523-1747.2003.12097.x [DOI] [PubMed] [Google Scholar]
- 66. Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, et al. Stimulatory Autoantibodies to the PDGF Receptor in Systemic Sclerosis. N Engl J Med (2006) 354:2667–76. doi: 10.1056/NEJMoa052955 [DOI] [PubMed] [Google Scholar]
- 67. Ogawa F, Shimizu K, Hara T, Muroi E, Komura K, Takenaka M, et al. Autoantibody Against One of the Antioxidant Repair Enzymes, Methionine Sulfoxide Reductase A, in Systemic Sclerosis: Association With Pulmonary Fibrosis and Vascular Damage. Arch Dermatol Res (2010) 302:27–35. doi: 10.1007/s00403-009-0996-9 [DOI] [PubMed] [Google Scholar]
- 68. Odaka M, Hasegawa M, Hamaguchi Y, Ishiura N, Kumada S, Matsushita T, et al. Autoantibody-Mediated Regulation of B-cell Responses by Functional Anti-CD22 Autoantibodies in Patients With Systemic Sclerosis. Clin Exp Immunol (2010) 159:176–84. doi: 10.1111/j.1365-2249.2009.04059.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Berger M, Steen VD. Role of Anti-Receptor Autoantibodies in Pathophysiology of Scleroderma. Autoimmun Rev (2017) 16:1029–35. doi: 10.1016/j.autrev.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 70. Hesselstrand R, Scheja A, Shen GQ, Wiik A, Akesson A. The Association of Antinuclear Antibodies With Organ Involvement and Survival in Systemic Sclerosis. Rheumatol (Oxford) (2003) 42:534–40. doi: 10.1093/rheumatology/keg170 [DOI] [PubMed] [Google Scholar]
- 71. Nihtyanova SI, Sari A, Harvey JC, Leslie A, Derrett-Smith EC, Fonseca C, et al. Using Autoantibodies and Cutaneous Subset to Develop Outcome-Based Disease Classification in Systemic Sclerosis. Arthritis Rheumatol (2020) 72:465–76. doi: 10.1002/art.41153 [DOI] [PubMed] [Google Scholar]
- 72. Sobanski V, Giovannelli J, Allanore Y, Riemekasten G, Airò P, Vettori S, et al. Phenotypes Determined by Cluster Analysis and Their Survival in the Prospective European Scleroderma Trials and Research Cohort of Patients With Systemic Sclerosis. Arthritis Rheumatol (2019) 71:1553–70. doi: 10.1002/art.40906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moroncini G, Svegliati Baroni S, Gabrielli A. Agonistic Antibodies in Systemic Sclerosis. Immunol Lett (2018) 195:83–7. doi: 10.1016/j.imlet.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 74. Tamby MC, Chanseaud Y, Humbert M, Fermanian J, Guilpain P, Garcia-de-la-Peña-Lefebvre P, et al. Anti-Endothelial Cell Antibodies in Idiopathic and Systemic Sclerosis Associated Pulmonary Arterial Hypertension. Thorax (2005) 60:765–72. doi: 10.1136/thx.2004.029082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Svegliati S, Amico D, Spadoni T, Fischetti C, Finke D, Moroncini G, et al. Agonistic Anti-PDGF Receptor Autoantibodies From Patients With Systemic Sclerosis Impact Human Pulmonary Artery Smooth Muscle Cells Function. In Vitro Front Immunol (2017) 8:75. doi: 10.3389/fimmu.2017.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Weigold F, Günther J, Pfeiffenberger M, Cabral-Marques O, Siegert E, Dragun D, et al. Antibodies Against Chemokine Receptors CXCR3 and CXCR4 Predict Progressive Deterioration of Lung Function in Patients With Systemic Sclerosis. Arthritis Res Ther (2018) 20:52. doi: 10.1186/s13075-018-1545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sgonc R, Gruschwitz MS, Boeck G, Sepp N, Gruber J, Wick G. Endothelial Cell Apoptosis in Systemic Sclerosis is Induced by Antibody-Dependent Cell-Mediated Cytotoxicity via CD95. Arthritis Rheum (2000) 43:2550–62. doi: [DOI] [PubMed] [Google Scholar]
- 78. Bussone G, Tamby MC, Calzas C, Kherbeck N, Sahbatou Y, Sanson C, et al. IgG From Patients With Pulmonary Arterial Hypertension and/or Systemic Sclerosis Binds to Vascular Smooth Muscle Cells and Induces Cell Contraction. Ann Rheum Dis (2012) 71:596–605. doi: 10.1136/annrheumdis-2011-200195 [DOI] [PubMed] [Google Scholar]
- 79. O’Reilly S, Ciechomska M, Cant R, van Laar JM. Interleukin-6 (IL-6) Trans Signaling Drives a STAT3-Dependent Pathway That Leads to Hyperactive Transforming Growth Factor-β (TGF-β) Signaling Promoting SMAD3 Activation and Fibrosis via Gremlin Protein. J Biol Chem (2014) 289:9952–60. doi: 10.1074/jbc.M113.545822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hasegawa M, Sato S, Fujimoto M, Ihn H, Kikuchi K, Takehara K. Serum Levels of Interleukin 6 (IL-6), Oncostatin M, Soluble IL-6 Receptor, and Soluble Gp130 in Patients With Systemic Sclerosis. J Rheumatol (1998) 25:308–13. [PubMed] [Google Scholar]
- 81. Feghali CA, Bost KL, Boulware DW, Levy LS. Mechanisms of Pathogenesis in Scleroderma. I. Overproduction of Interleukin 6 by Fibroblasts Cultured From Affected Skin Sites of Patients With Scleroderma. J Rheumatol (1992) 19:1207–11. [PubMed] [Google Scholar]
- 82. Sato S, Hasegawa M, Takehara K. Serum Levels of Interleukin-6 and Interleukin-10 Correlate With Total Skin Thickness Score in Patients With Systemic Sclerosis. J Dermatol Sci (2001) 27:140–6. doi: 10.1016/S0923-1811(01)00128-1 [DOI] [PubMed] [Google Scholar]
- 83. Numajiri H, Yoshizaki A, Fukasawa T, Ebata S, Nakamura K, Yamashita T, et al. Rapid Alteration of Serum Interleukin-6 Levels may Predict the Reactivity of I.V. Cyclophosphamide Pulse Therapy in Systemic Sclerosis-Associated Interstitial Lung Disease. J Dermatol (2018) 45:1221–4. doi: 10.1111/1346-8138.14580 [DOI] [PubMed] [Google Scholar]
- 84. Sanges S, Jendoubi M, Kavian N, Hauspie C, Speca S, Crave J-C, et al. B-cell Homeostasis and Functional Properties Are Altered in an Hypochlorous Acid-Induced Murine Model of Systemic Sclerosis. Front Immunol (2017) 8:53. doi: 10.3389/fimmu.2017.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rincón M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 Directs the Differentiation of IL-4–Producing CD4+ T Cells. J Exp Med (1997) 185:461–70. doi: 10.1084/jem.185.3.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shah A, Storek J, Woolson R, Pinckney A, Keyes-Elstein L, Wallace PK, et al. Lymphocyte Subset Abnormalities in Early Severe Scleroderma Favor a Th2 Phenotype and are Not Altered by Prior Immunosuppressive Therapy. Rheumatol (Oxford) (2022) 2:keac015. doi: 10.1093/rheumatology/keac015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a Rare IL-10-Competent B-cell Subset in Humans That Parallels Mouse Regulatory B10 Cells. Blood (2011) 117:530–41. doi: 10.1182/blood-2010-07-294249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz J-D, Uchida J, Fujimoto M, et al. B-Lymphocyte Depletion Reduces Skin Fibrosis and Autoimmunity in the Tight-Skin Mouse Model for Systemic Sclerosis. Am J Pathol (2006) 169:954–66. doi: 10.2353/ajpath.2006.060205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fleischmajer R, Perlish JS, Reeves JR. Cellular Infiltrates in Scleroderma Skin. Arthritis Rheum (1977) 20:975–84. doi: 10.1002/art.1780200410 [DOI] [PubMed] [Google Scholar]
- 90. Kuwana M, Medsger TA, Wright TM. T and B-cell Collaboration is Essential for the Autoantibody Response to DNA Topoisomerase I in Systemic Sclerosis. J Immunol (1995) 155:2703–14. [PubMed] [Google Scholar]
- 91. Kuwana M, Medsger TA, Wright TM. Analysis of Soluble and Cell Surface Factors Regulating Anti-DNA Topoisomerase I Autoantibody Production Demonstrates Synergy Between Th1 and Th2 Autoreactive T Cells. J Immunol (2000) 164:6138–46. doi: 10.4049/jimmunol.164.12.6138 [DOI] [PubMed] [Google Scholar]
- 92. Ise W, Fujii K, Shiroguchi K, Ito A, Kometani K, Takeda K, et al. T Follicular Helper Cell-Germinal Center B-cell Interaction Strength Regulates Entry Into Plasma Cell or Recycling Germinal Center Cell Fate. Immunity (2018) 48:702–715.e4. doi: 10.1016/j.immuni.2018.03.027 [DOI] [PubMed] [Google Scholar]
- 93. Taylor DK, Mittereder N, Kuta E, Delaney T, Burwell T, Dacosta K, et al. T Follicular Helper-Like Cells Contribute to Skin Fibrosis. Sci Transl Med (2018) 10:1–13. doi: 10.1126/scitranslmed.aaf5307 [DOI] [PubMed] [Google Scholar]
- 94. Ricard L, Jachiet V, Malard F, Ye Y, Stocker N, Rivière S, et al. Circulating Follicular Helper T Cells are Increased in Systemic Sclerosis and Promote Plasmablast Differentiation Through the IL-21 Pathway Which can be Inhibited by Ruxolitinib. Ann Rheum Dis (2019) 78(4):539–50. doi: 10.1136/annrheumdis-2018-214382 [DOI] [PubMed] [Google Scholar]
- 95. Jiang H, Xiao R, Lian X, Kanekura T, Luo Y, Yin Y, et al. Demethylation of TNFSF7 Contributes to CD70 Overexpression in CD4+ T Cells From Patients With Systemic Sclerosis. Clin Immunol (2012) 143:39–44. doi: 10.1016/j.clim.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 96. Fuschiotti P. Current Perspectives on the Role of CD8+ T Cells in Systemic Sclerosis. Immunol Lett (2018) 195:55–60. doi: 10.1016/j.imlet.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Radstake TRDJ, van Bon L, Broen J, Hussiani A, Hesselstrand R, Wuttge DM, et al. The Pronounced Th17 Profile in Systemic Sclerosis (SSc) Together With Intracellular Expression of TGFbeta and IFNgamma Distinguishes SSc Phenotypes. PloS One (2009) 4:e5903. doi: 10.1371/journal.pone.0005903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. MacDonald KG, Dawson NAJ, Huang Q, Dunne JV, Levings MK, Broady R. Regulatory T Cells Produce Profibrotic Cytokines in the Skin of Patients With Systemic Sclerosis. J Allergy Clin Immunol (2015) 135:946–.e9. doi: 10.1016/j.jaci.2014.12.1932 [DOI] [PubMed] [Google Scholar]
- 99. Maddur MS, Sharma M, Hegde P, Stephen-Victor E, Pulendran B, Kaveri SV, et al. Human B-cells Induce Dendritic Cell Maturation and Favour Th2 Polarization by Inducing OX-40 Ligand. Nat Commun (2014) 5:4092. doi: 10.1038/ncomms5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van Bon L, Affandi AJ, Broen J, Christmann RB, Marijnissen RJ, Stawski L, et al. Proteome-Wide Analysis and CXCL4 as a Biomarker in Systemic Sclerosis. N Engl J Med (2014) 370:433–43. doi: 10.1056/NEJMoa1114576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kafaja S, Valera I, Divekar AA, Saggar R, Abtin F, Furst DE, et al. pDCs in Lung and Skin Fibrosis in a Bleomycin-Induced Model and Patients With Systemic Sclerosis. JCI Insight (2018) 3:1–17. doi: 10.1172/jci.insight.98380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Higashioka K, Kikushige Y, Ayano M, Kimoto Y, Mitoma H, Kikukawa M, et al. Generation of a Novel CD30+ B-cell Subset Producing GM-CSF and its Possible Link to the Pathogenesis of Systemic Sclerosis. Clin Exp Immunol (2020) 201:233–43. doi: 10.1111/cei.13477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hénault J, Robitaille G, Senécal J-L, Raymond Y. DNA Topoisomerase I Binding to Fibroblasts Induces Monocyte Adhesion and Activation in the Presence of Anti-Topoisomerase I Autoantibodies From Systemic Sclerosis Patients. Arthritis Rheum (2006) 54:963–73. doi: 10.1002/art.21646 [DOI] [PubMed] [Google Scholar]
- 104. Fineschi S, Goffin L, Rezzonico R, Cozzi F, Dayer J-M, Meroni PL, et al. Antifibroblast Antibodies in Systemic Sclerosis Induce Fibroblasts to Produce Profibrotic Chemokines, With Partial Exploitation of Toll-Like Receptor 4. Arthritis Rheum (2008) 58:3913–23. doi: 10.1002/art.24049 [DOI] [PubMed] [Google Scholar]
- 105. Thoreau B, Chaigne B, Renaud A, Mouthon L. Treatment of Systemic Sclerosis. Presse Med (2021) 50:104088. doi: 10.1016/j.lpm.2021.104088 [DOI] [PubMed] [Google Scholar]
- 106. Smith V, Piette Y, van Praet JT, Decuman S, Deschepper E, Elewaut D, et al. Two-Year Results of an Open Pilot Study of a 2-Treatment Course With Rituximab in Patients With Early Systemic Sclerosis With Diffuse Skin Involvement. J Rheumatol (2013) 40:52–7. doi: 10.3899/jrheum.120778 [DOI] [PubMed] [Google Scholar]
- 107. Daoussis D, Tsamandas A, Antonopoulos I, Filippopoulou A, Papachristou DJ, Papachristou NI, et al. B-cell Depletion Therapy Upregulates Dkk-1 Skin Expression in Patients With Systemic Sclerosis: Association With Enhanced Resolution of Skin Fibrosis. Arthritis Res Ther (2016) 18:118. doi: 10.1186/s13075-016-1017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Daoussis D, Tsamandas AC, Liossis S-NC, Antonopoulos I, Karatza E, Yiannopoulos G, et al. B-cell Depletion Therapy in Patients With Diffuse Systemic Sclerosis Associates With a Significant Decrease in PDGFR Expression and Activation in Spindle-Like Cells in the Skin. Arthritis Res Ther (2012) 14:R145. doi: 10.1186/ar3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Boonstra M, Meijs J, Dorjée AL, Marsan NA, Schouffoer A, Ninaber MK, et al. Rituximab in Early Systemic Sclerosis. RMD Open (2017) 3:e000384. doi: 10.1136/rmdopen-2016-000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous Cyclophosphamide vs Rituximab for the Treatment of Early Diffuse Scleroderma Lung Disease: Open Label, Randomized, Controlled Trial. Rheumatol (Oxford) (2018) 57:2106–13. doi: 10.1093/rheumatology/key213 [DOI] [PubMed] [Google Scholar]
- 111. Sharp C, McCabe M, Dodds N, Edey A, Mayers L, Adamali H, et al. Rituximab in Autoimmune Connective Tissue Disease-Associated Interstitial Lung Disease. Rheumatol (Oxford) (2016) 55:1318–24. doi: 10.1093/rheumatology/kew195 [DOI] [PubMed] [Google Scholar]
- 112. Md Yusof MY, Kabia A, Darby M, Lettieri G, Beirne P, Vital EM, et al. Effect of Rituximab on the Progression of Rheumatoid Arthritis-Related Interstitial Lung Disease: 10 Years’ Experience at a Single Centre. Rheumatol (Oxford) (2017) 56:1348–57. doi: 10.1093/rheumatology/kex072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Goswami RP, Ray A, Chatterjee M, Mukherjee A, Sircar G, Ghosh P. Rituximab in the Treatment of Systemic Sclerosis-Related Interstitial Lung Disease: A Systematic Review and Meta-Analysis. Rheumatol (Oxford) (2021) 60:557–67. doi: 10.1093/rheumatology/keaa550 [DOI] [PubMed] [Google Scholar]
- 114. Maher TM, Tudor V, Saunders P, Gibbons M, Fletcher S, Parfrey H, et al. (2022). “Rituximab Versus Cyclophosphamide for the Treatment of Connective Tissue Disease Associated Interstitial Lung Disease (RECITAL): A Multi-Centre Randomised Controlled Trial.,”, in: C93. CLINICAL TRIALS IN CHRONIC LUNG DISEASE. American Thoracic Society International Conference Abstracts. American Thoracic Society, pp. A4779–9. doi: 10.1164/ajrccm-conference.2022.205.1_MeetingAbstracts.A4779 [DOI] [Google Scholar]
- 115. Zamanian RT, Badesch D, Chung L, Domsic RT, Medsger T, Pinckney A, et al. Safety and Efficacy of B-cell Depletion With Rituximab for the Treatment of Systemic Sclerosis Associated Pulmonary Arterial Hypertension: A Multi-Center, Double-Blind, Randomized, Placebo-Controlled Trial. Am J Respir Crit Care Med (2021) 204(2):209–21. doi: 10.1164/rccm.202009-3481OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schiopu E, Chatterjee S, Hsu V, Flor A, Cimbora D, Patra K, et al. Safety and Tolerability of an Anti-CD19 Monoclonal Antibody, MEDI-551, in Subjects With Systemic Sclerosis: A Phase I, Randomized, Placebo-Controlled, Escalating Single-Dose Study. Arthritis Res Ther (2016) 18:131. doi: 10.1186/s13075-016-1021-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-Myeloablative Haemopoietic Stem-Cell Transplantation Compared With Pulse Cyclophosphamide Once Per Month for Systemic Sclerosis (ASSIST): An Open-Label, Randomised Phase 2 Trial. Lancet (2011) 378:498–506. doi: 10.1016/S0140-6736(11)60982-3 [DOI] [PubMed] [Google Scholar]
- 118. van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous Hematopoietic Stem Cell Transplantation vs Intravenous Pulse Cyclophosphamide in Diffuse Cutaneous Systemic Sclerosis: A Randomized Clinical Trial. JAMA (2014) 311:2490–8. doi: 10.1001/jama.2014.6368 [DOI] [PubMed] [Google Scholar]
- 119. Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N Engl J Med (2018) 378:35–47. doi: 10.1056/NEJMoa1703327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Arruda LCM, Malmegrim KCR, Lima-Júnior JR, Clave E, Dias JBE, Moraes DA, et al. Immune Rebound Associates With a Favorable Clinical Response to Autologous HSCT in Systemic Sclerosis Patients. Blood Adv (2018) 2:126–41. doi: 10.1182/bloodadvances.2017011072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lima-Júnior JR, Arruda LCM, Gonçalves MS, Dias JBE, Moraes DA, Covas DT, et al. Autologous Hematopoietic Stem Cell Transplantation Restores the Suppressive Capacity of Regulatory B-cells in Systemic Sclerosis Patients. Rheumatol (Oxford) (2021) 60(12):5538–548. doi: 10.1093/rheumatology/keab257 [DOI] [PubMed] [Google Scholar]
- 122. Gernert M, Tony H-P, Schwaneck EC, Gadeholt O, Schmalzing M. Autologous Hematopoietic Stem Cell Transplantation in Systemic Sclerosis Induces Long-Lasting Changes in B-cell Homeostasis Toward an Anti-Inflammatory B-cell Cytokine Pattern. Arthritis Res Ther (2019) 21:106. doi: 10.1186/s13075-019-1889-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Assassi S, Wang X, Chen G, Goldmuntz E, Keyes-Elstein L, Ying J, et al. Myeloablation Followed by Autologous Stem Cell Transplantation Normalises Systemic Sclerosis Molecular Signatures. Ann Rheum Dis (2019) 78:1371–8. doi: 10.1136/annrheumdis-2019-215770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Desallais L, Avouac J, Fréchet M, Elhai M, Ratsimandresy R, Montes M, et al. Targeting IL-6 by Both Passive or Active Immunization Strategies Prevents Bleomycin-Induced Skin Fibrosis. Arthritis Res Ther (2014) 16:R157. doi: 10.1186/ar4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shima Y, Kuwahara Y, Murota H, Kitaba S, Kawai M, Hirano T, et al. The Skin of Patients With Systemic Sclerosis Softened During the Treatment With Anti-IL-6 Receptor Antibody Tocilizumab. Rheumatol (Oxford) (2010) 49:2408–12. doi: 10.1093/rheumatology/keq275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and Efficacy of Subcutaneous Tocilizumab in Adults With Systemic Sclerosis (Fasscinate): A Phase 2, Randomised, Controlled Trial. Lancet (2016) 387:2630–40. doi: 10.1016/S0140-6736(16)00232-4 [DOI] [PubMed] [Google Scholar]
- 127. Khanna D, Lin CJF, Furst DE, Goldin J, Kim G, Kuwana M, et al. Tocilizumab in Systemic Sclerosis: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir Med (2020) 8:963–74. doi: 10.1016/S2213-2600(20)30318-0 [DOI] [PubMed] [Google Scholar]
- 128. Sonnylal S, Denton CP, Zheng B, Keene DR, He R, Adams HP, et al. Postnatal Induction of Transforming Growth Factor Beta Signaling in Fibroblasts of Mice Recapitulates Clinical, Histologic, and Biochemical Features of Scleroderma. Arthritis Rheum (2007) 56:334–44. doi: 10.1002/art.22328 [DOI] [PubMed] [Google Scholar]
- 129. Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab Treatment Decreases Biomarkers and Improves Clinical Symptoms in Systemic Sclerosis Patients. J Clin Invest (2015) 125:2795–807. doi: 10.1172/JCI77958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gordon JK, Martyanov V, Franks JM, Bernstein EJ, Szymonifka J, Magro C, et al. Belimumab for the Treatment of Early Diffuse Systemic Sclerosis: Results of a Randomized, Double-Blind, Placebo-Controlled, Pilot Trial. Arthritis Rheumatol (Hoboken NJ) (2018) 70:308–16. doi: 10.1002/art.40358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Koca SS, Ozgen M, Dagli F, Tuzcu M, Ozercan IH, Sahin K, et al. Proteasome Inhibition Prevents Development of Experimental Dermal Fibrosis. Inflammation (2012) 35:810–7. doi: 10.1007/s10753-011-9380-y [DOI] [PubMed] [Google Scholar]
- 132. Pai C-CS, Chen M, Mirsoian A, Grossenbacher SK, Tellez J, Ames E, et al. Treatment of Chronic Graft-Versus-Host Disease With Bortezomib. Blood (2014) 124:1677–88. doi: 10.1182/blood-2014-02-554279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hsu VM, Denton CP, Domsic RT, Furst DE, Rischmueller M, Stanislav M, et al. Pomalidomide in Patients With Interstitial Lung Disease Due to Systemic Sclerosis: A Phase II, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. J Rheumatol (2018) 45:405–10. doi: 10.3899/jrheum.161040 [DOI] [PubMed] [Google Scholar]
- 134. Flynn R, Allen JL, Luznik L, MacDonald KP, Paz K, Alexander KA, et al. Targeting Syk-Activated B-cells in Murine and Human Chronic Graft-Versus-Host Disease. Blood (2015) 125:4085–94. doi: 10.1182/blood-2014-08-595470 [DOI] [PMC free article] [PubMed] [Google Scholar]