Abstract
The AIM2 inflammasome is activated by host and pathogen DNA. Work from the past 5 years indicates that the AIM2 inflammasome has an important role in advanced atherosclerosis driven by clonal haematopoiesis and possibly in atherosclerosis accelerated by acute infection. Therefore, the AIM2 inflammasome might be an important target for precision medicine.
Subject terms: Cardiovascular diseases, Inflammatory diseases
Inflammasomes are large protein complexes that assemble in the cytoplasm after infection or tissue injury. Inflammasome activation initiates the maturation and secretion of pro-inflammatory cytokines such as IL-1β and IL-18, as well as the cleavage of gasdermin D, which triggers pyroptosis — a form of inflammatory cell death. Although the outcome of inflammasome activation is largely conserved between different types of inflammasome, some inflammasomes have distinct expression patterns and specific activators. In atherosclerosis, a chronic inflammation of the arterial wall, the NLRP3 inflammasome — a sensor of various pathogen-associated or damage-associated patterns including cholesterol crystals — has received the most attention1. However, the AIM2 inflammasome, a sensor of cytosolic double-stranded DNA, has gained interest in the past 5 years in the context of vascular inflammation2,3.
The AIM2 inflammasome is constitutively expressed in the healthy vessel wall, but its expression increases with atherosclerosis burden. In mice, genetic deletion of Aim2 or inhibition of AIM2 using synthetic oligonucleotides reduces the levels of both IL-1β and IL-18 in atherosclerotic lesions and increases features of plaque stability (decreased necrotic core size and increased fibrous cap thickness)2. In clonal haematopoiesis, which is an important risk factor for atherosclerosis in ageing populations, AIM2 accelerates atherosclerosis development and promotes features of plaque destabilization, specifically in clonal haematopoiesis associated with the JAK2V617F (JAK2VF) variant3. Among common genetic variants that lead to clonal haematopoiesis, the JAK2VF variant, which increases JAK–STAT signalling, occurs at a younger age and is associated with the strongest risk of premature coronary heart disease. Mice with macrophage-specific expression of Jak2VF or with Jak2VF-driven clonal haematopoiesis had increased atherosclerosis with increased features of plaque instability, which was dependent on AIM2 inflammasome activation3. Inhibition of the AIM2 inflammasome product IL-1β reduced macrophage proliferation and necrotic core formation while increasing fibrous cap thickness, indicating that inhibition of IL-1β stabilized the atherosclerotic plaques3. These findings contrast with those observed in clonal haematopoiesis driven by TET2–/–, in which the NLRP3 inflammasome has a major role in increasing atherosclerosis in both mice and humans4. Together, these studies suggest that precise application of therapies that target IL-1β or specific inflammasomes according to clonal haematopoiesis status could reduce the risk of cardiovascular disease.
Stimuli that trigger AIM2 in atherosclerosis
The AIM2 inflammasome was initially identified as a sensor of cytoplasmic double-stranded DNA of host or pathogen origin (Fig. 1). AIM2 recognizes self-DNA after disruption of nuclear envelope integrity. In addition, AIM2 can sense DNA damage in the nucleus in the context of irradiation or cytostatic drug treatment. Host DNA can also be available outside the cell as a result of necrosis or neutrophil extracellular trap (NET) formation. Indeed, NETs induce IL-1β release from macrophages, a process that is connected to the aggravation of atherosclerosis in mice5. Mechanistic evidence indicates that AIM2 recognizes NET-DNA6. However, it is not clear how extracellular DNA is shuttled across the plasma membrane to be available for AIM2 sensing. In addition, histones in NET-DNA are heavily citrullinated, and the effect of this modification on DNA sensing requires further analysis.
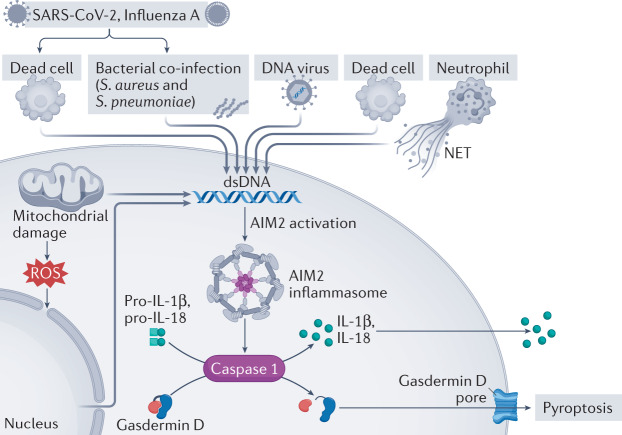
Fig. 1. The many ways to activate the AIM2 inflammasome.
The AIM2 inflammasome is activated by double-stranded DNA (dsDNA) derived from bacteria, DNA viruses or dead cells. Neutrophils release neutrophil extracellular traps (NETs) in the arterial lumen at sites of disturbed blood flow or in the atherosclerotic lesion. NET-DNA can interact with the AIM2 inflammasome. SARS-CoV-2 or influenza A virus can indirectly activate AIM2 by inducing cell death or by association with bacterial co-infection. Intracellularly, mitochondrial damage and nuclear dsDNA can activate AIM2 leading to the generation of IL-1β and IL-18 as well as the formation of gasdermin D pores. ROS, reactive oxygen species.
Epidemiological studies indicate a striking increase in the short-term risk of cardiovascular disease after bacterial or viral infection. As an example, acute respiratory tract infection with Staphylococcus aureus or Streptococcus pneumoniae transiently increases the risk of an acute coronary syndrome up to 20-fold. Given that both pathogens are sensed by the AIM2 inflammasome, one might speculate that AIM2 activation links bacterial infection with cardiovascular complications. In addition, the COVID-19 pandemic has epitomized the connection between viral infections and cardiovascular complications. This connection is not new because such a relationship was observed more than a century ago during the 1918 influenza pandemic. Neither SARS-CoV-2, which causes COVID-19, nor influenza A virus triggers AIM2 activation directly, but they might do so indirectly through accumulation of dead cells or bacterial co-infection7.
“the AIM2 inflammasome might be an important target for precision medicine”
In mice with Jak2VF-accelerated atherosclerosis, increased macrophage proliferation and increased glycolytic flux with higher mitochondrial production of reactive oxygen species (ROS) seem to promote AIM2 inflammasome activation3. Increased ROS production and oxidative DNA damage occur upstream of AIM2 inflammasome activation, because these changes were further increased in Jak2VF-expressing macrophages that were deficient in caspase 1–caspase 11 or gasdermin D3. Increased ROS levels and oxidative DNA damage have been implicated in activation of both the NLRP3 inflammasome8 and the AIM2 inflammasome9, and activation of both inflammasomes was increased in human and mouse JAK2VF macrophages. However, experiments using Jak2VFNlrp3−/− mice and Jak2VFAim2−/− mice showed that, in vivo, the AIM2 inflammasome has a more predominant role than the NLRP3 inflammasome in promoting atherosclerosis3. This specificity was explained by increased levels of AIM2 in plaque macrophages and bone marrow-derived macrophages in Jak2VF mice, whereas NLRP3 levels were reduced3. Type 1 interferons induce Aim2 expression, and increased Aim2 mRNA in mouse Jak2VF macrophages was dependent on interferon-γ (IFNγ)3, which is induced downstream of JAK–STAT signalling in Jak2VF cells. Given that macrophage proliferation and glycolytic flux are commonly increased in atherosclerosis and that IFNγ levels increase in standard mouse models of atherosclerosis, similar mechanisms are likely to underlie a broader role of AIM2 inflammasome activation in atherosclerosis than previously appreciated.
AIM2 as a target for precision medicine
Given the importance of the AIM2 inflammasome in patients at high risk of cardiovascular complications (such as patients with acute infection or clonal haematopoiesis), the AIM2 inflammasome might be an important target for precision medicine. However, how to inhibit AIM2 or its downstream effects is unclear. In a study in mice, administration of synthetic oligonucleotides targeted to AIM2 reduced atherosclerotic plaque destabilization2. However, these oligonucleotides might have off-target effects because they potentially also dampen Toll-like receptor 9 activation, as this receptor also recognizes DNA motifs. A subsequent study reported a compound that inhibits both NLRP3 and AIM2 in vitro10, but further studies are needed to test the in vivo specificity and efficacy of this drug. Drugs that interfere with the downstream inflammasome pathway, including caspase 1 (VX765), IL-1β (canakinumab) and IL-1 receptor (anakinra), have shown encouraging results in animal models and, to some extent, in clinical studies. Nevertheless, the strategy of direct inference with the AIM2 inflammasome is surprisingly in its infancy, and data from preclinical studies support the further development of AIM2-targeted medicines.
“data from preclinical studies support the further development of AIM2-targeted medicines”
In conclusion, while the CANTOS trial on canakinumab highlighted the potential therapeutic value of suppressing IL-1β, more precise approaches to anti-inflammatory treatment are needed. Preclinical studies have uncovered a role of AIM2 activation in promoting atherosclerosis. AIM2 might be a more appropriate therapeutic target in specific groups of patients. Challenges include the development of safe and specific AIM2 inhibitors and the identification of appropriate target populations.
Acknowledgements
O.S. receives funding from Deutsche Forschungsgemeinschaft (CRC TRR332 TP A2 & Z1, CRC914 TP B8, CRC1009 TP A13, CRC1123 TP A6 and OR465/1-1), Else Kröner Fresenius Stiftung, IZKF at the Münster Medical Faculty, Novo Nordisk and Leducq Foundation. A.R.T. receives funding from NIH grants HL107653 and HL155431 and the Leducq Foundation.
Competing interests
O.S. holds patents on neutralization of histones and interference with chemokines in inflammation; receives funding from Novo Nordisk to study the role of histones in inflammation and to study chronopharmacological intervention strategies in atherosclerosis; and is on the scientific advisory board of ResoTher. A.R.T. is on the scientific advisory board of Tensixteen Bio and Beren Pharmaceuticals and is a consultant for Commonwealth Serum Laboratories.
References
- 1.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulin N, et al. Double-strand DNA sensing Aim2 inflammasome regulates atherosclerotic plaque vulnerability. Circulation. 2018;138:321–323. doi: 10.1161/CIRCULATIONAHA.117.033098. [DOI] [PubMed] [Google Scholar]
- 3.Fidler TP, et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature. 2021;592:296–301. doi: 10.1038/s41586-021-03341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuster JJ, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antiochos B, et al. The DNA sensors AIM2 and IFI16 are SLE autoantigens that bind neutrophil extracellular traps. eLife. 2022;11:e72103. doi: 10.7554/eLife.72103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vora SM, Lieberman J, Wu H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021;21:694–703. doi: 10.1038/s41577-021-00588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong Z, et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018;560:198–203. doi: 10.1038/s41586-018-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B, et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science. 2016;354:765–768. doi: 10.1126/science.aaf7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao Y, et al. Discovery of a novel and potent inhibitor with differential species-specific effects against NLRP3 and AIM2 inflammasome-dependent pyroptosis. Eur. J. Med. Chem. 2022;232:114194. doi: 10.1016/j.ejmech.2022.114194. [DOI] [PubMed] [Google Scholar]