Abstract
We investigated attachment processes of hydrophobic and hydrophilic particles (diameter = 1 μm) to mature biofilms grown on clay marbles in a sequencing batch biofilm reactor. During a treatment cycle with filtered wastewater containing different fluorescent beads, the progression of particle density in various biofilm compartments (carrier biofilm, basic biofilm layer, biofilm flocs, and sessile ciliates) was determined by flow cytometry, confocal laser scanning microscopy and automated image analysis. Particles were almost completely removed from wastewater by typical processes of particle retention: up to 58% of particles attached to clay marbles, up to 15% were associated with suspended flocs, and up to 10% were ingested by sessile ciliates. Ingestion of particles by ciliates was exceptionally high immediately after wastewater addition (1,200 particles grazer−1 h−1) and continued until approximately 14% of the water had been cleared by ciliate filter feeding. Most probably, ciliate bioturbation increases particle sorption to the basic biofilm. Backwashing of the reactor detached pieces of biofilm and thus released approximately 50% of the particles into rinsing water. Clay marbles in the upper part of the reactor were more efficiently abraded than in the lower part. No indications for selective attachment of the applied hydrophobic and hydrophilic beads were found. As a consequence of interception patterns, organisms at elevated biofilm structures are probably major profiteers of wastewater particles; among them, ciliates may be of major importance because of their highly active digestive food vacuoles.
Microbial activity in biofilms and physical processes such as adsorption are responsible for wastewater clearance in biofilters. These filters comprise mature biofilms with highly dynamic microbial populations, for which particulate material constitutes an important carbon resource (22, 28). Consequently, the interception of particles by the biofilm is essential for the performance of bioreactors (44). Since particles tend to accumulate hazardous pollutants at their surface, their removal from wastewater is all the more important (26). Processes of particle attachment and detachment are therefore of major interest in biofilm research (29, 35, 37, 43).
Recently, sequencing batch technology has been applied to biofilters (47). Wastewater is periodically loaded in a fixed bed reactor and circulated in a closed system with intermittent aeration. In contrast, other reactor types are operated in a continuous mode, in which wastewater permanently passes through the system. Particle retention is a fundamental factor in these technologies, since it determines biofilm function (35, 44). To achieve a better assessment of reactor types, particle retention should be specifically examined for each application.
Passive attachment of percolating particles in a porous matrix depends on physical mechanisms such as van der Waals forces or hydrophobic interactions (4, 32). Particle attachment to a mature biofilm is further influenced by biofilm texture, heterogeneity, surface charge, and the activity of organisms in various biofilm compartments (13, 35, 43). In mature biofilms, compartments can be divided into a surface layer, comprising metazoa, ciliates, ciliate stalks, flocculent filaments, and adjacent bacteria, and a basic layer, which is embedded in a polysaccharide matrix with channels and crevices (9, 10, 46). Each of these biofilm structures can be assumed to attract particles of different size, form, or hydrophobicity, thus leading to a specific pattern of particles in the biofilm.
For instance, sessile ciliates, which typically dominate the surface of mature biofilms, can accumulate particles of a certain size by grazing (11, 16). Moreover, they can spread particles from the bulk water to the biofilm through acceleration in grazing swirls, whereas their stalks might serve as a major attachment site (3, 40, 45). Until now, protozoan activity within biofilms has been investigated with the focus on particle detachment, i.e., bacterial grazing (14, 24, 31, 41). In mature biofilms, however, grazing activity of sessile ciliates can be assumed to enrich particulate organic resources within the biofilm. Information on the importance of this process is necessary, compared to passive attachment of particles.
Microscopical tools for the quantification of particle sorption by biofilms must provide rapid counts of a variety of particles. Flow cytometry and confocal laser scanning microscopy (CLSM) allow extremely fast enumeration of differently fluorescing particles if adequate software is available to analyze large data sets and perform accurate image analysis. In the present study, we have developed these techniques to evaluate the attachment of two kinds of fluorescing beads (hydrophilic and hydrophobic) to mature biofilms grown in a sequencing batch biofilm reactor.
MATERIALS AND METHODS
Reactor operation.
A bench-scale submerged biofilter (height = 63 cm; diameter = 19 cm) was routinely operated for the treatment of municipal wastewater. The reactor was sequentially charged with 17 liters of wastewater; an additional 5.5 liters remained in tubes or was associated with the biofilm during exchange. Sintered clay marbles with diameters of 4 to 8 mm were used as a support material for the biofilm. They comprised a total surface area of 11.5 m2 and an interstitial volume of 17 liters. In order to achieve elimination of organic pollution (dissolved and suspended solids) as well as nitrogen and phosphorus removal, the reactor was operated in the sequencing batch reactor mode (1, 47). For this purpose, a cycle consisting of different treatment phases was regularly applied, namely, a filling phase of 15 min and an unaerated recirculation phase of 3 h, followed by an aeration and recirculation phase of up to 24 h. Recirculation was accomplished by pumping 300 liters h−1 from top to bottom of the biofilter, resulting in an upwards flow velocity of 2.1 mm s−1. Aeration of 200 liters h−1 resulted in dissolved oxygen concentrations of approximately 6 mg of O2 liter−1. To avoid clogging, excess biomass was hydraulically removed by backwashing once a week with water and pressurized air. During backwashing 36 liters of water passed through the reactor bed within 150 s.
Microbead characteristics.
Fluorescent beads (diameter = 1 μm) were added to 17 liters of membrane-filtered (0.45-μm pore size) municipal wastewater, which was pumped to the reactor at the beginning of a sequencing batch cycle. Two types of beads were evenly added to a final particle concentration of 3.23 × 107 beads per ml: green fluorescing hydrophobic beads (latex [maximum excitation and emission wavelengths, 458 and 540 nm, respectively]; catalog no. 17154; Polysciences, Eppelheim, Germany) and red fluorescing hydrophilic beads (carboxylated polystyrene [maximum excitation and emission wavelengths, 625 and 645 nm, respectively]; catalog no. F8816; Molecular Probes, Leiden, The Netherlands). Different hydrophobicity was demonstrated by shaking of an ultrasonically treated aqueous suspension of beads in n-hexadecane or n-octane (modified after the method of Rosenberg et al. [38]), which removed more latex beads from the aqueous phase (69 or 97%, respectively) than carboxylated polystyrene beads (47 or 76%, respectively).
Sampling.
Before sampling, water circulation was stopped and the reactor was turned cautiously into a horizontal position. To avoid disturbance by movement of clay marbles, the bed was fixed by a holed plate, fastened at the top. At each sampling time (0, 20, 45, 75, 210, 330, and 450 min and 24 h after reactor loading) two samples were taken from lateral ports at the lower region (height = 16 cm) and at the upper region (height = 52 cm) of the biofilter. Twenty clay marbles and 9 ml of interstitial water per sample were collected using tweezers or syringes, respectively. Each marble was softly panned in 40 ml of water so that loose biofilm flocs adjacent to the marble surface were detached and quantitatively separated. Bulk water from the reactor supernatant liquid was drawn from the reactor recirculation tube. Two final samples were taken subsequent to reactor washing after 24.1 h. All samples were immediately fixed with buffered paraformaldehyde (4.4% [wt/vol] in [per liter] 7.60 g of NaCl, 1.25 g of Na2HPO4, and 0.41 g of NaH2PO4; pH 7.2), stored at 4°C, and analyzed within 6 weeks.
Microbead detection and quantification.
Bead densities were determined from the bulk biofilm at clay marbles (carrier biofilm) and its basic layer (basic biofilm), from sessile ciliates of the biofilm surface layer, and from separated biofilm flocs. In addition, beads were quantified in the biofilters' supernatant and pore water.
Flow cytometry was applied to determine the density of fluorescing particles attached to 10 clay marbles, associated with the flocs separated from these marbles, and in bulk water from the biofilter supernatant and pore water. In all samples, particles were detached and suspended by shaking (1 min) and strong sonication in a Branson (Dietzenbach, Germany) Sonifier (model B12; resonator tip, 0.5 in.; 450 W, 40% power; amplitude, ∼75 μm; time, 2 min), adequately diluted, and quantitatively introduced into a FACS Star Plus cytometer (Becton Dickinson, Paramus, N.J.) for 3 to 10 min at a rate of 500 to 1,200 particles per s. Green and red fluorescence excitation was performed by two lasers (argon, 488 nm; helium-neon, 633 nm) in a single focus. Using a long-path filter (<515 nm) combined with a band-pass blocking filter centered around 633 nm (Kaiser Optical Systems, Ann Arbor, Mich.), fluorescence of both bead types could be determined without interference by the HeNe laser. Both bead types were much brighter than the background and could be monitored on one fluorescence channel. For a clear discrimination of red fluorescing beads, about 30% of the emission light was directed by a mirror to a second fluorescence channel equipped with a long-path filter (>645 nm; Schott, Mainz, Germany). Data were analyzed by DAS 4.4 software (2).
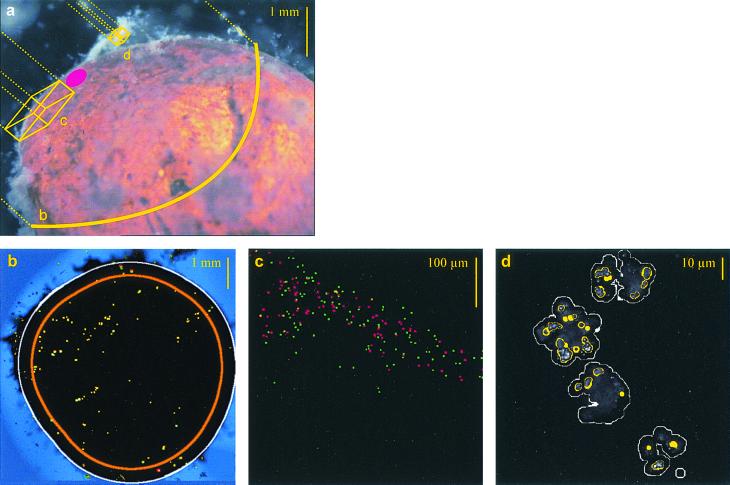
Particle density in the basic biofilm layer and in food vacuoles of sessile ciliates was estimated by automated analysis of CLSM images. Image acquisition was performed with a Zeiss (Jena, Germany) 510 confocal laser scanning microscope. An argon laser with a 488-nm wavelength activated the fluorescence of green beads (band-pass fluorescence filter, 505 to 550 nm), light from a helium-neon laser with 543 nm excited the red beads (long-path fluorescence filter, 560 nm). To take an image, a clay marble was placed in a water-filled plastic dish with a bottom coverslip (Matek, Ashland, Mass.) and observed around its point of support with an inversion microscope (Fig. 1a). Images from the marble surface were recorded at various microscopical resolutions to quantify beads in the basic biofilm, the number of sessile ciliate grazers, and their ingested beads. Three image analysis programs were therefore created with Zeiss KS400 software.
FIG. 1.
(a) Clay marble, partially masked by biofilm flocs, with investigated biofilm zones schematized. The red dot marks the area of support at a glass slide during microscopical observation with an inverse CLSM; dotted lines depict the line of sight. Yellow letters correspond to exemplary images below: (b) ciliate grazers at a defined marble area (inner circle) discernible by their fluorescing food vacuoles (two-dimensional image); (c) basic biofilm (single layer of a three-dimensional CLSM image with beads fluorescing in the green and red channel); (d) ciliate grazers (white) with densely packed beads in food vacuoles (yellow circles) (single layer of a three-dimensional CLSM image). Printed images were scaled down and modified by a photo editing program (Adobe Photoshop) to illustrate the quality of the originals.
Fluorescent beads in the basic biofilm were imaged with a 20× objective featuring a numerical aperture (NA) of 0.5 (Fig. 1c). From three to six marbles per sample, a biofilm area of 0.46 by 0.46 mm2 was documented, which was embedded in a viscous matrix and free from flocculent structures or ciliates. Biofilm depth was displayed by 25 to 75 images stacked at an optical distance of 2 μm. The three-dimensional, smallest units of an image (voxels) had a horizontal density of 512 by 512, so that each voxel had a volume of 1.62 μm3. The brightness of beads surpassed background fluorescence of the biofilm by far. The volumes of red and green fluorescing beads were determined by automated image analysis using a constant threshold. Summarized bead volumes from each CLSM image were averaged for every sample, resulting in a general mean coefficient of variation (CV) of 0.57. For evaluation of bead numbers, the volume values were converted by division through the screen volume of a single bead, i.e., the volume of a bead measured by CLSM. The screen volumes (11.0 μm3 for green beads and 12.5 μm3 for red beads; n > 100) had been determined separately.
The number of grazing ciliates was discernible by their fluorescing food vacuoles and estimated on two-dimensional surface images of six to seven clay marbles per sample (Fig. 1b). The objective lens was 1.25× (NA, 0.035) for the sampling times 15 to 210 min due to a documented area of about 30 mm2. No single beads were discernible at this resolution, but food vacuoles from neighboring grazers were sometimes merged to one object, which led to an underestimation of grazer numbers. A circular area of the marble surface was defined using automated image analysis, and fluorescing objects with diameters of more than 10 μm were counted as ciliate grazers. The threshold was automatically adapted to regional variations of the background by a dynamic filter, which improved the segmentation of grazers (KS400, manual for imaging system release 3.0; Carl Zeiss Vision, Munich, Germany]). Direct microscopic controls at higher resolutions verified that more than 90% of the counted objects were sessile ciliates. However, high bead densities covered the biofilm 5 h after bead exposure and caused a disturbing background fluorescence. Therefore, grazers were identified with a greater objective (4×; NA, 0.1) in subsequent samples, taking into account a 10-fold-smaller imaged area. The overall CV per sample was 0.46 for the smaller and 0.73 for the greater objective.
Particle volumes of red and green fluorescing beads in ciliate food vacuoles were determined from three-dimensional images taken with a 63× objective (NA, 1.2, in water). We recorded the particle content of 8 to 28 grazers per sample, from at least three marbles. The horizontal image size was 0.084 mm2, with a z stack of 30 to 100 images at an optical distance of 0.5 μm. Thus, the voxel volume was 0.042 μm3. The volumes of red and green, mostly agglomerated beads in each ciliate were measured by automated image analysis and averaged for each sample, with a mean CV of 0.71 per sample.
To discriminate between loosely distributed beads (optically singular) and densely packed beads (i.e., optically clustered) in a ciliate, the following basic steps were performed by automated image analysis (Fig. 1d). To define every single ciliate, fluorescing objects were strongly diluted until the beads within each ciliate were merged together. Contours of the resulting volume arbitrarily defined the ciliates' bodies. Closely arranged ciliates, which occasionally had been merged too, were manually divided. From the original image, objects were radially smoothed and eliminated if <100 voxels, i.e., singular beads, were excluded (KS400 manual). The residual clustered beads were enclosed and defined as food vacuole content. Image superimposition of ciliate and vacuole contours revealed beads inside the food vacuoles of each ciliate. Since these beads were packed in clusters, their volumes were converted to numbers by subtraction of pore space (40% at closely cubic package according to Busch et al. [6]) and subsequent division through the geometric volume of a single bead (0.51 μm3). The volumes of single beads outside of the vacuoles were converted by division through the screen volume of a single bead. This volume (3.4 μm3 for green beads and 4.1 μm3 for red beads) was derived from separate measurements of single beads (n > 90).
Calculation of ciliate clearance.
The clearance of wastewater by ciliate feeding was estimated from water volumes represented by ingested beads. Assuming equal concentration (c) of beads in bulk water, one ingested bead was equivalent to a certain volume (Vb = 1/c) of cleared water that increased with diminishing bead concentration. For each sampling interval (i = 1, 2, …8), the cleared water volume was calculated from the water volume represented by one bead in the middle of the interval [(Vbi = 2/(ci − 1 + ci)], multiplied by the newly ingested beads per grazer at the end of the interval (ΔGi), and the extrapolated number of grazers in the reactor (Ni). The total volume (Vcl) of cleared water can therefore be determined as follows:
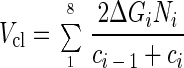 |
RESULTS
Attachment to biofilm compartments.
After wastewater loading, the particle concentration in wastewater declined exponentially within 7.5 h, almost to 1% of the initial concentration (Fig. 2). This was apparent from an increasing transparency of the supernatant. Particle density in the biofilters' pore water was always slightly higher, preferably in the lower part of the reactor (Table 1). The overall amount of floc-associated particles was already high after 20 min (7% of total), with a clear majority in the lower part of the reactor. It doubled within the next 7 h but declined to 2% of loaded particles in the following 17 h. The particle concentration at the carrier biofilm (including ciliates, ciliate stalks, flocculent structures, and basic biofilm) increased rapidly to a level of ca. 37,000 particles mm−2 after 5.5 h. This density was generally greater in the upper segment of the reactor, with the maximum difference (46%) to the lower segment reached after 24 h. Particle density at the basic biofilm layer paralleled that of the carrier biofilm at a level of 30 to 50% and remained relatively stable after 45 min at up to 10,700 particles mm−2. More than 90% of loaded particles could be detected in the first hour of the experiment, only 40% could be detected after 24 h, but 74% could again be detected after reactor backwashing.
FIG. 2.
Percentage of particles (diameter = 1 μm) recovered from several compartments of a sequencing batch biofilm reactor after addition of particle-containing wastewater. The reactor was back washed 24 h after wastewater charge. Symbols: ●, bulk wastewater; ○, total biofilm including flocs; ▵, biofilm on carrier material; □, basic biofilm layer; ×, sessile ciliate grazers.
TABLE 1.
Mean particle concentrations and percentages of hydrophobic and hydrophilic particles in wastewater and biofilm compartments of a sequencing batch biofilm reactor
| Compartment | Time (h) after wastewater charge
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.33 | 0.75 | 1.25 | 3.50 | 5.50 | 7.50 | 24.00 | 24.10a | |
| Supernatantb concnf | 51,171 | 35,821 | 26,186 | 10,383 | 3,998 | 803 | 20 | 18,291 |
| % Hydrophobic particlesc | 51 | 51 | 50 | 50 | 54 | 45 | 49 | 50 |
| Pore waterb concn | 53,412 | 35,393 | 30,703 | 11,703 | 5,231 | 2,831 | 1,495 | 1,602 |
| % Upper segment of the reactorc | 47 | 46 | 48 | 46 | 42 | 47 | 33 | 61 |
| % Hydrophobic particles | 51 | 50 | 52 | 51 | 54 | 50 | 49 | 52 |
| Biofilm flocd concn | 4,451 | 8,545 | 6,840 | 5,684 | 7,577 | 9,444 | 1,525 | 5,932 |
| % Upper segment of the reactor | 26 | 15 | 53 | 52 | 41 | 18 | 90 | 4 |
| % Hydrophobic particles | 50 | 50 | 50 | 49 | 49 | 50 | 52 | 51 |
| Carrier biofilm concn | 5,902 | 15,914 | 23,155 | 30,906 | 37,025 | 36,994 | 24,138 | 11,860 |
| % Upper segment of the reactor | 57 | 57 | 68 | 66 | 54 | 52 | 73 | 40 |
| % Hydrophobic particles | 51 | 52 | 51 | 49 | 48 | 50 | 50 | 51 |
| Basic biofilm layer concn ± CVe | 2,290 ± 0.59 | 8,696 ± 0.27 | 8,072 ± 1.07 | 10,359 ± 0.42 | 8,716 ± 0.80 | 10,289 ± 0.44 | 10,700 ± 0.39 | 1,707 ± 0.52 |
| % Upper segment of the reactor | 55 | 18 | 47 | 53 | 55 | 55 | 70 | 18 |
| % Hydrophobic particles | 52 | 57 | 57 | 51 | 57 | 58 | 50 | 57 |
| Sessile ciliate concn | 361 | 1,040 | 878 | 5,062 | 689 | 273 | 0 | 0 |
| % Upper segment of the reactor | 40 | 52 | 51 | 69 | 24 | 21 | ||
| % Hydrophobic particles | 52 | 50 | 57 | 48 | 51 | 59 | ||
| Grazers/mm2 ± CVe | 0.9 ± 0.34 | 1.6 ± 0.52 | 1.7 ± 0.43 | 5.1 ± 0.66 | 2.7 ± 0.53 | 1.6 ± 0.58 | 0 | 0 |
| No. of ingested particles/grazer ± CVe | 399 ± 0.93 | 678 ± 0.90 | 532 ± 0.57 | 958 ± 0.62 | 239 ± 0.70 | 141 ± 0.55 | 0 | 0 |
| No. of ingested particles/grazer/h | 1,209 | 904 | 426 | 274 | 43 | 19 | 0 | 0 |
| % Densely packed particles | 91 | 95 | 95 | 95 | 82 | 74 | ||
| Interval (nl) of cleared water/grazer | 13 | 13 | 0 | 45 | 0 | 0 | 0 | 0 |
| % Intermediately cleared water | 1 | 1 | 0 | 12 | 0 | 0 | ||
After reactor backwashing; rinsing water is included in the supernatant compartment.
Converted from volumetric concentrations for better comparability with other compartments.
Percentage contribution of this particle fraction to a density of 100% in the entire compartment.
Easily detached from carrier biofilm.
Coefficient of variation resulting from 6 to 14 analyzed images.
Mean particle concentration per square millimeter of biofilter.
Reactor backwashing.
Backwashing detached pieces of biofilm, so that particle densities at the marble biofilm and its basic layer substantially declined. Consequently, 45% of loaded particles were released into bulk water, mainly into the outflowing rinsing fraction. The majority of attached particles switched from the upper to the lower segment of the reactor. In parallel, floc-associated particles increased about fourfold in number and accumulated almost exclusively in the lower region.
Ciliate grazing.
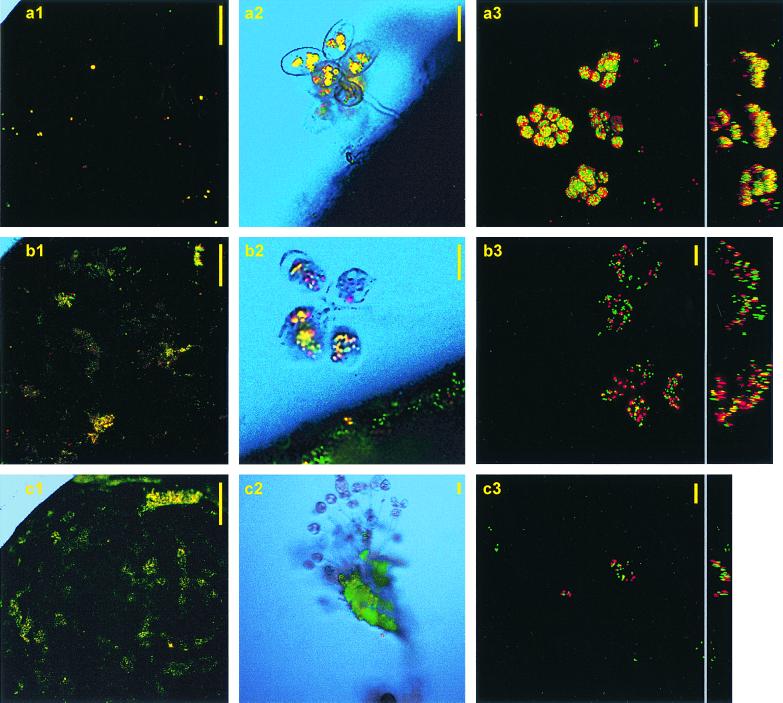
The sessile ciliate community in the bioreactor was dominated by the peritrich ciliate genus Epistylis, which made up to more than 99% of the sessile community and about 87% of the total ciliate community (J. Fried, personal communication). These ciliates reacted to wastewater addition with immediate grazing activity, exerting maximum grazing rates within the first 20 min (ca. 1,200 particles h−1; Fig. 3a2 and a3). The maximum number of ingested particles per grazer (ca. 1,000 particles) was reached after 3.5 h, concurring with the maximum number of grazers (5.1 grazers mm−2; Table 1). Their individual clearance rate for this interval was 20 nl ciliate−1 h−1, corresponding with filtration of 12% of wastewater by the ciliate community. Altogether, about 14% of wastewater was filtrated through ciliate feeding. After 3.5 h, the number of grazers as well as the ingested particles decreased rapidly. Vacuoles with conglomerated particles started to dilate, so that higher amounts of loosely distributed particles in ciliate cells were measured (Fig. 3b2 and b3). Until 24 h, ciliates still populated the surface biofilm but contained no fluorescing particles (Fig. 3c2). Instead, ciliate stalks and other large flocculent structures of the biofilm were densely covered by particles (Fig. 3c1). Neither for ciliates nor for the basic biofilm did we find indications for sites or periods of selective attachment of the applied hydrophilic and hydrophobic particles.
FIG. 3.
Hydrophilic (red) and hydrophobic (green) fluorescing particles in sessile ciliates of a mature biofilm from a sequenced batch biofilm reactor that was charged with particle-containing wastewater at 15 min (a), 7.5 h (b) and 24 h (c) after wastewater addition. Columns show increasing grazer densities (a1 and b1) and particle accumulation at elevated biofilm structures (c1); sessile ciliate grazer colonies with compact (a2), dilated (b2), and empty (c2) vacuoles; horizontal and lateral projections of three-dimensional CLSM images of ciliates, and immediately after prey acquisition (a3), during vacuole processing (b3), and during particle egestion (c3). Bars in columns 1 to 3 represent 1 mm, 25 μm, and 10 μm, respectively. Printed images were scaled down and modified by a photo editing program to illustrate the quality of the originals.
DISCUSSION
Particle retention in bioreactors.
It was found that particle attachment to a mature biofilm is triggered by three different processes: passive interception by biofilm flocs, sorption of particles to the basic biofilm, and grazing activity of sessile ciliates at the surface biofilm. All processes contribute considerably to rapid removal of particles from wastewater in a biofilter operated in the sequencing batch mode.
At the end of the treatment cycle almost total clearance of 1-μm-diameter particles was accomplished. Okabe et al. (35) exposed particles of the same diameter in a rotating disk reactor and found that one-third of particles remained in bulk water. Studies on a rotating annular reactor or an airlift suspension reactor also showed lower adsorption efficiencies of biofilms in the diurnal time frame (13, 43, 44). Reactors in these studies worked in a continuous mode, which entails lower and more constant particle concentrations than the batch mode. Biofilms in batch reactors seem to have a higher sorption capacity and thus might have a higher ability to cope with shock loading conditions. The high clearance efficiency of particles in the sequencing batch reactor can be explained by lower porosity of the filter bed and frequent recirculation of wastewater, which contribute to a high contact frequency of carriers and particles (23, 32).
Adsorption to biofilm compartments.
In the biofilter, the adsorption of particles was different for each biofilm compartment. Immediately after addition of wastewater to the reactor, biofilm flocs intercepted substantial amounts of particles, since they had primary and ambient contact with the fluid-transported particles. The loosely attached flocs intercepted particles preferentially in the lower part of the reactor, whereas in the upper part, particles adsorbed more efficiently to clay marbles. Presumably, flocs masked the biofilm carriers in the lower segment (Fig. 1a), so that the underlying carrier biofilm had less contact with particles. Floc-associated particles declined after several hours, as flocs seemed to assemble more closely with the carrier biofilm and integrate into the surface layer.
Firmly attached flocculent structures of the carrier biofilm, mostly formed by sessile ciliate colonies, protruded beyond the basic biofilm up to a millimeter and obviously intercepted great amounts of particles through oscillation in the streaming liquid (42). They account for the difference between particle densities in the carrier biofilm and its basic layer compartment. However, the observed pattern of reduced particle attachment to indentations might be modified at higher flow velocities, which can lead to more intense percolation of biofilm crevices (12). Neither biofilm compartment showed selective attachment of the applied hydrophobic and hydrophilic beads. Nevertheless, selective sorption of more different particles could indicate specific features of biofilm surfaces (5, 48).
Reactor backwashing.
After 1 day of operation, flocs and particles were transported to the upper region of the biofilter, where particle densities increased. In this reactor segment, backwashing led to most efficient abrasion of the biofilm, because of more frequent collisions of clay marbles. Numerous flocs with sorbed particles were detached and accumulated in the lower reactor segment. The different efficiency of backwashing might lead to an unfavorable, inhomogeneous biomass distribution. A complete fluidization of the filter bed during washing should therefore be ensured (33).
Until backwashing, the total amount of detected particles continuously decreased. Nondetected particles might have been trapped in unsampled pockets of the reactor system (e.g., by wall effects). Alternatively, the detection of fluorescent beads in the biofilm could have been ineffective for bead clusters, which increased with proceeding particle adsorption.
CLSM and image analysis.
Automated analysis of CLSM images provides a detailed and fast spatial exploration of biofilms (27, 30). However, clustered particles, as they typically occur in mature biofilms, have to be enumerated by estimate conversions of object volumes. This would demand a precise optical screening of single particles. We found that the volume of a single bead determined by CLSM was several times greater than its geometric volume, which can be explained by the limited vertical resolution in the CLSM technique and the extreme brightness of beads (20). To avoid substantial underestimation, we therefore preferred a geometric conversion of bead clusters in food vacuoles. Another limitation was a shading effect within densely filled food vacuoles. Beads from the upper part of vacuoles blocked light from underlying beads, so that the vacuoles were discerned as hemispheres. Despite these restrictions, automated CLSM image analysis allowed more-precise and unbiased quantification of selective ingestion and vacuole dilation. Alternative direct microscopic quantification of ingested beads would involve more-laborious and inaccurate estimates of grazer density, vacuole numbers, and vacuole diameters (14).
Grazing rates and particle processing by sessile ciliates.
Grazing rates and particle release by epilithic sessile ciliates were quantitatively investigated for the first time in this study. Sessile grazers intercepted the newly available batch of particulate resources with very high ingestion rates (1,200 particles ciliate−1 h−1 within 20 min), which are in the upper range compared to other ciliate feeding types (7, 15, 21, 25, 36). Carrias et al. (8) report even higher grazing rates for epibiotic peritriches on a pelagic diatom, but they employed very small particles (0.5 μm), which occupy less space in food vacuoles. The present data are the first in situ grazing rates of sessile ciliates in a wastewater treatment system, a system where they generally dominate the surface layer of mature biofilms (11, 19, 36).
Grazing behavior was obviously pulsed by wastewater addition to the bioreactor. The maximum rate of ingestion occurred immediately after particle addition, when suspended particles were most available. Because of their high initial grazing rates, we assume that many ciliates were satiated after 3 to 4 h and then started vacuole processing, which would fit with cyclic regeneration of prey reported for other ciliate species (34, 39). Possibly, vacuole processing was powered by recurring oxygen availability. Still unsatiated grazers probably tried to compensate for the decreasing availability of suspended particles with higher clearance rates, until particle concentration fell below the economical limit. In this situation, the energetic costs for clearance activity exceeded the potential nutritive gain of ingested particles. The period before ingestion peak (at 3.5 h) was therefore the most intense in wastewater filtering by grazers, especially since they had also reached their maximum number. The subsequent start of vacuole processing can thus be explained by satiation or by low availability of particles. The cyclic regeneration of ingested material typical for ciliates was finally synchronized by the sequencing batch operation.
Importance of ciliate grazing activity.
Compared to other biofilm compartments, the interception of particles by ciliates was relatively low. Particle ingestion by sessile ciliates might reach a magnitude of 10% of the loaded batch. Considering particle degradation, ciliates may have a major impact because of their extremely active digestive food vacuoles (17, 18, 34). Ingested wastewater particles are probably most efficiently degraded, and released digestion products will trigger microbial activity in the bioreactor (36).
About 14% of wastewater was cleared through ciliate filter feeding, and even more was moved along the ciliates' bodies. Water swirls that are generated by grazing activity can reach an extension of 400 μm (3, 16, 40). This substantial bioturbation probably affects the liquid boundary layer at the biofilm surface and weakens its barrier effect for dissolved nutrients (49).
Conclusions.
Small particles can accumulate very rapidly in a mature biofilm due to protozoan grazing activity, passive interception by flocculent structures, and sorption to the basic biofilm. In batch biofilm reactors, the retention of particles by these processes is highly effective. Thus, if wastewater particles are nutritious, they are obviously an essential energy resource for the biofilm community. A major profit can then be assumed for sessile ciliates, bacteria at ciliate stalks, and bacteria at other elevated biofilm structures. Future experiments and models should address the versatile function of ciliates in mature biofilms and the nutritional value of wastewater particles in bioreactors.
ACKNOWLEDGMENTS
We are grateful to Johannes Fried for determination of ciliates and to Eberhard Morgenroth and Stefan Wuertz for helpful comments on the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft through its Research Center (SFB411) on Fundamental Studies of Aerobic Biological Wastewater Treatment, Munich, Germany.
REFERENCES
- 1.Arnz P, Arnold E, Wilderer P A. Enhanced biological phosphorus removal in a semi full-scale SBBR. Water Sci Technol. 2000;43:167–174. [PubMed] [Google Scholar]
- 2.Beisker W. A new combined integral-light and slit-scan data analysis system (DAS) for flow cytometry. Comput Methods Programs Biomed. 1994;42:15–26. doi: 10.1016/0169-2607(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 3.Blake J R, Otto S R. Filter feeding, chaotic filtration, and a blinking stokeslet. Theor Comp Fluid Dyn. 1998;10:23–36. [Google Scholar]
- 4.Bouwer E J. Theoretical investigation of particle deposition in biofilm systems. Water Res. 1987;21:1489–1498. [Google Scholar]
- 5.Briandet R, Herry J-M, Bellon-Fontaine M-N. Determination of the van der Waals, electron donor and electron acceptor surface tension components of static Gram positive microbial biofilms. Colloid Surface B Biointerfaces. 2001;21:299–310. doi: 10.1016/s0927-7765(00)00213-7. [DOI] [PubMed] [Google Scholar]
- 6.Busch K F, Luckner L, Thiemer K. Geohydraulik. Lehrbuch der Hydrogeologie. Berlin, Germany: Gebrüder Bornträger; 1993. [Google Scholar]
- 7.Capriulo G M, editor. Ecology of marine protozoa. New York, N.Y: Oxford University Press; 1990. pp. 186–259. [Google Scholar]
- 8.Carrias J, Amblard C, Bourdier G. Protistan bacterivory in an oligomesotrophic lake: importance of attached ciliates and flagellates. Microb Ecol. 1996;31:249–268. doi: 10.1007/BF00171570. [DOI] [PubMed] [Google Scholar]
- 9.Characklis W G, Wilderer P A. Structure and function of biofilms. In: Characklis W G, Wilderer P A, editors. Structure and function of biofilms. Bath, Great Britain: Bath Press; 1988. pp. 5–18. [Google Scholar]
- 10.Costerton J W, Lewandowski Z, de Beer D, Caldwell D, James G. Biofilms, the customized microniche. J Bacteriol. 1994;176:2137–2142. doi: 10.1128/jb.176.8.2137-2142.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curds C R. The ecology and role of protozoa in aerobic sewage treatment processes. Annu Rev Microbiol. 1982;36:27–46. doi: 10.1146/annurev.mi.36.100182.000331. [DOI] [PubMed] [Google Scholar]
- 12.de Beer D, Stoodley P. Relation between the structure of an aerobic biofilm and transport phenomena. Water Sci Technol. 1995;32:11–18. [Google Scholar]
- 13.Drury W J, Characklis W G, Stewart P S. Interactions of 1 μm latex particles with Pseudomonas aeruginosa biofilms. Water Res. 1993;27:1119–1126. doi: 10.1002/bit.260420115. [DOI] [PubMed] [Google Scholar]
- 14.Eisenmann H, Harms H, Meckenstock R, Meyer E I, Zehnder A J B. Grazing of Tetrahymena sp. on adhered bacteria in percolated columns monitored by in situ hybridization with fluorescent oligonucleotide probes. Appl Environ Microbiol. 1998;64:1264–1269. doi: 10.1128/aem.64.4.1264-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein S S, Shiaris M P. Rates of microbenthic and meiobenthic bacterivory in a temperate muddy tidal flat community. Appl Environ Microbiol. 1992;58:2426–2431. doi: 10.1128/aem.58.8.2426-2431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenchel T. Protozoan filter feeding. In: Corliss J O, Patterson D J, editors. Progress in protistology. Bristol, Great Britain: Biopress Ltd.; 1986. pp. 65–113. [Google Scholar]
- 17.Fok A, Lee Y, Allen R D. The correlation of digestive vacuole pH and size with the digestive cycle in Paramecium caudatum. J Protozool. 1982;29:409–414. [Google Scholar]
- 18.Fok A K, Shokley B U. Processing of digestive vacuoles in Tetrahymena and the effects of dichloroisoproterenol. J Protozool. 1985;32:6–9. doi: 10.1111/j.1550-7408.1985.tb03004.x. [DOI] [PubMed] [Google Scholar]
- 19.Fried J, Mayr G, Berger H, Traunspurger W, Psenner R, Lemmer H. Monitoring protozoa and metazoa biofilm communities for assessing wastewater quality impact and reactor up-scaling effects. Water Sci Technol. 2000;41:309–316. [Google Scholar]
- 20.Hell S, Lehtonen E, Stelzer E H K. Confocal fluorescence microscopy: wave optics considerations and applications to cell biology. In: Kriete A, editor. Visualization in biomedical microscopies. New York, N.Y: VCH Publishers; 1992. pp. 145–160. [Google Scholar]
- 21.Hoffman R L, Atlas R M. Measurement of the effects of cadmium stress on protozoan grazing of bacteria (bacterivory) in activated sludge by fluorescence microscopy. Appl Environ Microbiol. 1987;53:2440–2444. doi: 10.1128/aem.53.10.2440-2444.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janning K-F, Le-Tallec X, Harremoës P. Hydrolysis of organic wastewater particles in laboratory scale and pilot scale biofilm reactors under anoxic and aerobic conditions. Water Sci Technol. 1998;38:179–188. [Google Scholar]
- 23.Kaballo H-P, Zhao Y, Wilderer P A. Elimination of p-chlorophenol in biofilm reactors: a comparative study of continuous flow and sequenced batch operation. Water Sci Technol. 1995;31:51–60. [Google Scholar]
- 24.Kalmbach S, Manz W, Szewczyk U. Dynamics of biofilm formation in drinking water: phylogenetic affiliation and metabolic potential of single cells assessed by formazan reduction and in situ hybridization. FEMS Microbiol Ecol. 1997;22:265–279. [Google Scholar]
- 25.Kemp P F. The fate of benthic bacterial production. Rev Aquat Sci. 1990;2:109–124. [Google Scholar]
- 26.Kern U, Li C-C, Westrich B. Assessment of sediment contamination from pollutant discharge in surface waters. Water Sci Technol. 1998;37:1–8. [Google Scholar]
- 27.Kuehn M, Hausner M, Bungartz H-J, Wagner M, Wilderer P A, Wuertz S. Automated confocal laser scanning microscopy and semiautomated image processing for analysis of biofilms. Appl Environ Microbiol. 1998;64:4115–4127. doi: 10.1128/aem.64.11.4115-4127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen T E, Harremoës P. Degradation mechanisms of colloidal organic matter in biofilm reactors. Water Res. 1994;28:1443–1452. [Google Scholar]
- 29.Lawler D F. Particle size distribution in treatment processes: theory and practice. Water Sci Technol. 1997;36:15–23. [Google Scholar]
- 30.Lawrence J R, Neu T R. Confocal laser scanning microscopy for analysis of microbial biofilms. Methods Enzymol. 1999;310:131–144. doi: 10.1016/s0076-6879(99)10011-9. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence J R, Snyder R A. Feeding behaviour and grazing impacts of a Euplotes sp. on attached bacteria. Can J Microbiol. 1998;44:623–629. [Google Scholar]
- 32.Martin R E, Bouwer E J, Hanna L M. Application of clean-bed filtration theory to bacterial deposition in porous media. Environ Sci Technol. 1992;26:1053–1058. [Google Scholar]
- 33.Morgenroth E, Wilderer P A. Controlled biomass removal: the key parameter to achieve enhanced biological removal in biofilm systems. Water Sci Technol. 1999;39:33–40. [Google Scholar]
- 34.Nisbet B. Nutrition and feeding strategies in protozoa. London, Great Britain: Croom Helm; 1984. [Google Scholar]
- 35.Okabe S, Yasuda T, Watanabe Y. Uptake and release of inert fluorescence particles by mixed population biofilms. Biotechnol Bioeng. 1997;53:459–469. doi: 10.1002/(SICI)1097-0290(19970305)53:5<459::AID-BIT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 36.Ratsak C H, Maarsen K A, Kooijman S A L M. Effects of protozoa on carbon mineralization in activated sludge. Water Res. 1996;30:1–12. [Google Scholar]
- 37.Reichert P, Wanner O. Movement of solids in biofilms: significance of liquid phase transport. Water Sci Technol. 1997;36:321–328. [Google Scholar]
- 38.Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 39.Sherr B F, Sherr E B, Rassoulzadegan F. Rates of digestion of bacteria by marine phagotrophic protozoa: temperature dependence. Appl Environ Microbiol. 1988;54:1091–1095. doi: 10.1128/aem.54.5.1091-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sleigh M A, Barlow D. Collection of food by Vorticella. Trans Am Microsc Soc. 1976;95:482–486. [Google Scholar]
- 41.Starink M, Krylova I N, Gilissen M J, Bak R P M, Cappenberg T E. Rates of benthic protozoan grazing on free and attached sediment bacteria measured with fluorescently stained sediment. Appl Environ Microbiol. 1994;60:2259–2264. doi: 10.1128/aem.60.7.2259-2264.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoodley P, Boyle J D, de Beer D, Lappin-Scott H M. Evolving perspectives of biofilm structure. Biofouling. 1999;14:75–90. [Google Scholar]
- 43.Tijhuis L, van Benthum W A J, van Loosdrecht M C M, Heijnen J J. Solids retention time in spherical biofilms in a biofilm airlift suspension reactor. Biotechnol Bioeng. 1994;44:867–879. doi: 10.1002/bit.260440802. [DOI] [PubMed] [Google Scholar]
- 44.van Benthum W A J, van Loosdrecht M C M, Tijhuis L, Heijnen J J. Solids retention time in heterotrophic and nitrifying biofilms in a biofilm airlift suspension reactor. Water Sci Technol. 1995;32:53–60. [Google Scholar]
- 45.van Leeuwenhoek A. Further microscopical observations on the animalcules found upon duckweed. Phil Trans R Soc. 1713;28:160. [Google Scholar]
- 46.Walker J T, Mackerness C W, Rogers J, Keevil C W. Heterogeneous mosaic biofilm: a haven for waterborne pathogens. In: Lappin-Scott H M, Costerton J W, editors. Microbial biofilms. Cambridge, Great Britain: Cambridge University Press; 1995. pp. 196–204. [Google Scholar]
- 47.Wilderer P A. Sequencing batch biofilm technology. In: Ladisch M R, Bose A, editors. Harnessing bio/technology for the 21st century. Boston, Mass: American Chemical Society; 1992. pp. 475–479. [Google Scholar]
- 48.Woolfaardt G M, Lawrence J R, Robarts R D, Caldwell D E. In situ characterization of biofilm exopolymers involved in the accumulation of chlorinated organics. Microb Ecol. 1998;35:213–223. doi: 10.1007/s002489900077. [DOI] [PubMed] [Google Scholar]
- 49.Zhang T C, Bishop P L. Experimental determination of the dissolved oxygen boundary layer and mass transfer resistance near the fluid-biofilm interface. Water Sci Technol. 1994;30:47–58. [Google Scholar]