Abstract
Objective
A new adult-onset autoinflammatory syndrome has been described, named VEXAS (Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic). We aimed to compare the clinical characteristics, the laboratory features and the outcomes between idiopathic-relapsing polychondritis (I-RP) and VEXAS-relapsing polychondritis (VEXAS-RP).
Methods
Patients from French retrospective multicentre cohort of RP were separated into two groups: a VEXAS-RP and an I-RP.
Results
Compared with patients with I-RP (n=40), patients with VEXAS-RP (n=55) were men (96% vs 30%, p<0.001) and were older at diagnosis (66 vs 44 years, p<0.001). They had a greater prevalence of fever (60% vs 10%, p<0.001), of skin lesions (82% vs 20%, p<0.001), of ocular involvement (57% vs 28%, p=0.01), of pulmonary infiltrates (46% vs 0%, p<0.001), of heart involvement (11% vs 0%, p=0.0336) and with higher median C-reactive protein levels (64 mg/L vs 10 mg/L, p<0.001). Seventy-five per cent of the patients with VEXAS-RP had myelodysplastic syndrome (MDS) versus none in I-RP group. The glucocorticoids use, and the number of steroid sparing agents were similar in both groups, but patients with VEXAS-RP had more frequent refractory disease (remission obtained in 27% vs 90%, p<0001). VEXAS-RP was associated with higher risk of death: six patients (11%) died in the VEXAS-RP group after a median follow-up of 37 months and none in the I-RP group after a median follow-up of 92 months (p<0.05).
Conclusion
We report the largest cohort of VEXAS-RP, characterised by high prevalence of male sex, fever, skin lesion, ocular involvement, pulmonary infiltration, heart involvement, older age and MDS association.
Keywords: Relapsing Polychondritis, VEXAS syndrome, Somatic Mutations inUBA1
WHAT IS ALREADY KNOWN ON THIS TOPIC
Relapsing polychondritis (RP) could be a part of VEXAS (Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic) phenotype and UBA1 mutation should be done to exclude VEXAS syndrome.
WHAT THIS STUDY ADDS
VEXAS-RP phenotype have specific features, such as frequent fever, skin lesions and pulmonary infiltrates, and frequent relapse and steroid dependence which distinguish from idiopathic-RP.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
UBA1 related pathways should be interesting mechanisms to understand and could trigger pathophysiological studies to understand the inflammatory pathways.
Introduction
Relapsing polychondritis (RP) is a rare systemic inflammatory disease, with a recent estimated prevalence of 0.71 per million population per year.1 The disease affects the cartilaginous structures of the ears, nose and tracheobronchial tree,2–6 but also joints, skin, eyes, inner ear and cardiovascular system.3 7–12 The most widely used classification criteria are those by Michet et al.3 The exact cause of RP is still unknown and the pathogenesis remains unclear including genetic susceptibility, especially association with the human leucocyte antigen allele DR4,4 13 immunisation against cartilaginous components14–20 and release of cytokines and chemokines.21–24 An association with other rheumatic and autoimmune diseases and myelodysplastic syndromes (MDS) is well-documented.2–5 9 25–28 Three clinical phenotypes with differing prognoses were identified in a large case series of 142 French patients.29 A first group with haematological disorders, especially MDS, had the worst prognosis, a second group with laryngotracheal or bronchial involvement experienced more infections and intensive care unit admissions and the third cluster consisted of patients with mild disease.
Recently, a new adult-onset autoinflammatory syndrome has been described, named VEXAS (Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic) and characterised by somatic mutation (at methionine-41) of the UBA1 gene which is located on the X chromosome.30 UBA1 gene encodes for the major E1 enzyme that initiates protein ubiquitination in the cell cytoplasm. VEXAS syndrome occurs in late adulthood, mostly in men but could also be present in woman with X acquired monosomy.31 32 In the princeps publication, main clinical features were recurrent fever, neutrophilic dermatoses and skin vasculitis, pulmonary involvement, ear and nose chondritis, venous thrombosis, cytopenia especially macrocytic anaemia, associated with vacuoles in myeloid and erythroid precursor cells and dysplastic bone marrow.30 More recently joint involvement,33 34 ocular, lymph node enlargement, gastrointestinal and peripheral nervous system involvements35 had expanded the clinical phenotype of VEXAS syndrome. Sixty per cent of initial 25 patients met the diagnostic criteria of RP30 and an additional study showed that 7.6% of patients with RP had UBA1 mutations (VEXAS-RP).36 In the second study, 13 patients with VEXAS-RP and 85 patients with idiopathic-RP (I-RP) were included and patients with VEXAS-RP were men, ≥45 years at disease onset and commonly had fever, ear chondritis, skin involvement, venous thrombosis and pulmonary infiltrates.36 Mortality seems to be greater in VEXAS-RP than I-RP,36 37 but large case series comparing VEXAS-RP and I-RP are still lacking.
Based on a French multicentre VEXAS cohort, we aimed to compare clinical characteristics, laboratory features and outcomes between I-RP and VEXAS-RP.
Methods
Study design and patients
A retrospective multicentre study was conducted in France between December 2019 and June 2021. Patients with RP met Michet’s criteria which require the presence of proven inflammation in at least two of three of the auricular, nasal or laryngotracheal cartilages, or proven inflammation in one cartilage associated to two other signs, including ocular inflammation, hearing loss, vestibular dysfunction or seronegative inflammatory arthritis.3 Diagnosis of MDS was made according to WHO criteria and classified within International Prognostic Scoring System (IPSS) and Revised International Prognostic Scoring System (IPSS-R) prognostic categories.38
Patients with RP were classified as VEXAS-RP (RP with the presence of UBA1 mutation), and I-RP (RP without UBA1 mutation or without VEXAS syndrome according to Ferrada’s algorithm based on three clinical variables : male sex, mean corpuscular volume >100 fL and platelet count ).36 Even some patients from I-RP could not have the UBA1 screening, the absence of any cytopenia and macrocytosis, the absence of underlying MDS, the male sex make extremely improbable the VEXAS syndrome. Even age less than 50 years could also help to exclude VEXAS syndrome, some few recent cases described patients with VEXAS nearby 50 years.
Data collection
Data were collected by clinicians belonging to the French file for rare autoinflammatory/autoimmune diseases (FAI2R), French group of MDS (GFM), French VEXAS and/or French group for immunohaematological disorders (MINHEMON).
Clinical parameters included fever, ear, nose, and large airway chondritis, ocular, joint, skin, heart, auditory or vestibular, central nervous system and kidney involvements, venous thrombosis, MDS at diagnosis of first symptoms and the follow-up. Laboratory data were performed at the time of MDS/VEXAS diagnosis and if available at the time of relapse and remission, and included haemoglobin, lymphocytes, neutrophils, platelets, C-reactive protein (CRP) levels, creatininaemia, immunological screening (antinuclear antibodies, antineutrophil cytoplasmic antibodies, rheumatoid factor). Analysis of bone marrow, karyotype and additional somatic mutations by Next-Generation Sequencing (NGS) were recorded when available. Treatments, especially the use of glucocorticoids, conventional disease-modifying antirheumatic drugs (DMARDs), targeted biological drugs and azacytidine were recorded during the follow-up. Death was collected for each patient. Remission was defined as the absence of symptoms with a daily dose of corticosteroids <10 mg for more than 3 months. The definition of relapse is a serious damage which need to change treatment or increase the daily dose of corticosteroids >0.5 mg/kg.
UBA1 mutation genetic screening
Sixty-two patients underwent the genetic testing: 55 from VEXAS-RP group and 7 from I-RP group.
Genomic DNA extracted from bone marrow or blood samples were analysed by Sanger sequencing or next generation sequencing to detect mutations of UBA1.
Some case reports from this study have been previously reported in the French VEXAS cohort.35
Statistical analysis
Data are expressed as medians with IQR and numbers with frequencies. Qualitative variables and quantitative variables were compared using Fisher’s exact and Kruskal-Wallis tests, respectively. Cumulative incidence curves of remission, relapse and death were generated using Kaplan-Meier and compared using the log rank-test. We used Cox model to obtain HR. Proportional hazards assumptions were checked using Schoenfeld residuals. Regarding remission and death analysis, we considered the date of the relapsing chondritis diagnosis as the inclusion date and the date of last hospital contact or the outcome occurrence date as the last follow-up date. Regarding the relapse subanalysis, the first remission date was considered as the start date of the analyses. Multiple imputation with chained equations (MICE) to handle missing data were used using the R ‘MICE’ package (V.3.5.0).39 Estimates were pooled across 10 imputed data sets. Two-sided testing was used, with p<0.05 considered statistically significant. All analyses were performed using R software V.3.6.0 for Mac (Foundation for Statistical Computing, Vienna, Austria).
Results
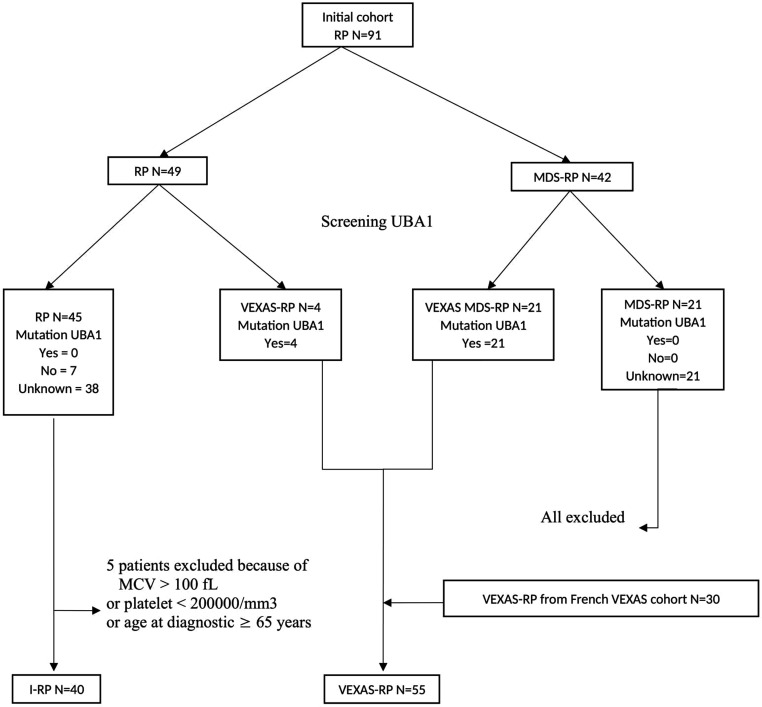
The initial cohort contained two groups: RP with 49 patients and MDS-RP with 42 patients (figure 1). When VEXAS syndrome have been described, the genomic DNA was analysed, when available, to detect the somatic mutations of UBA1. We screened for the UBA1 mutations 21 patients from the group MDS-RP, all had confirmed mutations in UBA1, and 11 patients from the group RP, with 4 of them had confirmed mutations in UBA1 gene. Thus, two new groups were constituted based on UBA1 mutational status, RP with 45 patients and VEXAS-RP with 25 patients; and patients from the group MDS-RP who did not undergo genetic testing were all excluded. In order to constitute a I-RP group without VEXAS syndrome, five patients were excluded because of MCV >100 fL or platelet count or age ≥65 years at disease onset. In the I-RP group, the absence of UBA1 somatic mutations on genetic sequencing was documented in seven participants (17.5%). For the other 33 patients, we considered them negative according to the algorithm developed by Ferrada et al.36 In addition to patients recruited within our cohort, patients with UBA1 mutations identified in the French VEXAS cohort who met diagnostic criteria for RP (30 patients) were also included in the group VEXAS-RP of this study. In the VEXAS-RP group, the presence of UBA1 somatic mutations on genetic sequencing was documented in all the participants.
Figure 1.
Flowchart. I-RP, idiopathic-RP; MCV, mean corpuscular volume; MDS, myelodysplastic syndromes; RP, relapsing polychondritis.
Finally, two groups were analysed: I-RP with 40 patients and VEXAS-RP with 55 patients.
Clinical characteristics of patients with VEXAS-RP compared with I-RP
Clinical presentation and laboratory findings
Compared with patients with I-RP (n=40), patients with VEXAS-RP (n=55) were mostly men (96% vs 30%, p<0.001) and older at diagnosis (66 vs 44 years, p<0.001), (table 1). Two women from RP-VEXAS have acquired monosomy X related to MDS. They had a greater prevalence of fever (60% vs 10%, p<0.001), skin lesions (82% vs 20%, p<0.001), ocular involvement (57% vs 28%, p=0.01), pulmonary infiltrates (46% vs 0%, p<0.001) and heart involvement (11% vs 0%, p=0.0336). MDS was common in patients with VEXAS-RP (75%), whereas no patient with I-RP had MDS (p<0.001). Peripheral joint involvement was frequent in both groups (67% vs 68%, p=1) and no significant difference was noted in the prevalence of costochondritis (12% vs 25%, p=0.339), of large airway chondritis (25% vs 45%, p=0.064) and of venous thrombosis (26% vs 20%, p=0.635). Patients with I-RP had a higher prevalence of nose chondritis (70% vs 47%, p=0.047), without any difference of ear chondritis (90% vs 94%, p=0.698). In patients with VEXAS-RP, median CRP levels were higher (69 mg/L vs 10 mg/L, p<0.001) whereas haemoglobin, platelets and neutrophils levels were significantly lower (table 1).
Table 1.
Clinical characteristics of patients with VEXAS-RP compared with I-RP (n=95)
| I-RP | VEXAS-RP | P value | |
| N | 40 | 55 | |
| Male gender (%) | 12 (30) | 53 (96) | <0.001 |
| Age at diagnosis of RP (median (IQR)) (years) | 44(38, 52) | 66(61, 72) | <0.001 |
| Fever (%) | 4 (10) | 33 (60) | <0.001 |
| Chondritis (%) | 40 (100) | 52 (98) | 1 |
| Ear chondritis (%) | 36 (90) | 50 (94) | 0.698 |
| Nasal chondritis (%) | 28 (70) | 25 (47) | 0.047 |
| Ocular involvement (%) | 11 (28) | 30 (57) | 0.01 |
| Uveitis (%) | 2 (5) | 9 (17) | 0.148 |
| Scleritis (%) | 4 (10) | 7 (13) | 0.881 |
| Episcleritis (%) | 6 (15) | 15 (28) | 0.205 |
| Retinal vasculitis (%) | 1 (3) | 2 (4) | 1 |
| Peripheral joint involvement (%) | 27 (68) | 36 (67) | 1 |
| Costochondritis (%) | 10 (25) | 3 (12) | 0.339 |
| Skin lesions (%) | 8 (20) | 44 (82) | <0.001 |
| Large airway chondritis (%) | 18 (45) | 13 (25) | 0.064 |
| Pulmonary infiltrates (%) | 0 (0) | 13 (46) | <0.001 |
| Heart involvement (%) | 0 (0) | 6 (11) | 0.0336 |
| Myocarditis (%) | 0 (0) | 3 (6) | 0.349 |
| Pericarditis (%) | 0 (0) | 3 (6) | 0.349 |
| Mitral or aortic valvular disease (%) | 2 (5) | 0 (0) | 0.691 |
| Arterial involvement | |||
| Arterial thrombosis (%) | 0 (0) | 1 (4) | 0.811 |
| Aortitis (%) | 2 (5) | 3 (6) | 1 |
| Venous thrombosis (%) | 8 (20) | 14 (26) | 0.635 |
| MDS (%) | 0 (0) | 41 (75) | <0.001 |
| Renal failure (%) | 0 (0) | 4 (7) | 0.22 |
| Central nervous system involvement (%) | 1 (3) | 3 (6) | 0.835 |
| Vestibular dysfunction (%) | 3 (8) | 2 (8) | 1 |
| Deafness sensorineural (%) | 6 (15) | 4 (16) | 1 |
| Laboratory data | |||
| Haemoglobin (median (IQR)) (g/L) | 137(130, 140) | 103(90, 120) | <0.001 |
| Platelets (median (IQR)) ( ) | 257(209, 303) | 163(115, 236) | <0.001 |
| Neutrophils (median (IQR)) (G/L) | 4(3, 5) | 2.7(2, 4) | 0.018 |
| C-reactive protein (median (IQR)) (mg/L) | 10(2, 23) | 69(30, 107) | <0.001 |
| Treatment data | |||
| Systemic glucocorticoids (%) | 35 (88) | 51 (94) | 0.413 |
| Glucocorticoid-dependency (%) | 24 (62) | 37 (71) | 0.459 |
| Number of immunosuppressive medications (median (IQR)) | 2(1, 3) | 1(1, 3) | 0.85 |
| Remission (%) | 36 (90) | 15 (27) | <0.001 |
| Time to remission (median (IQR)) (months) | 19(11, 49) | 30(12, 69) | 0.387 |
| Duration of remission (median (IQR)) (months) | 24(4, 76) | 7(3, 21) | 0.067 |
| Relapse (%) | 14 (39) | 7 (50) | 0.692 |
| Death (%) | 0 (0) | 6 (11) | 0.083 |
| Duration of follow-up (median (IQR)) (months) | 92(37, 160) | 37(15, 76) | 0.001 |
I-RP, idiopathic RP; MDS, myelodysplastic syndromes; RP, relapsing polychondritis.
Clinical and laboratory features, treatment responses and overall survival were not different between the MDS VEXAS-RP and non-MDS VEXAS-RP groups.
Therapeutic management and outcome
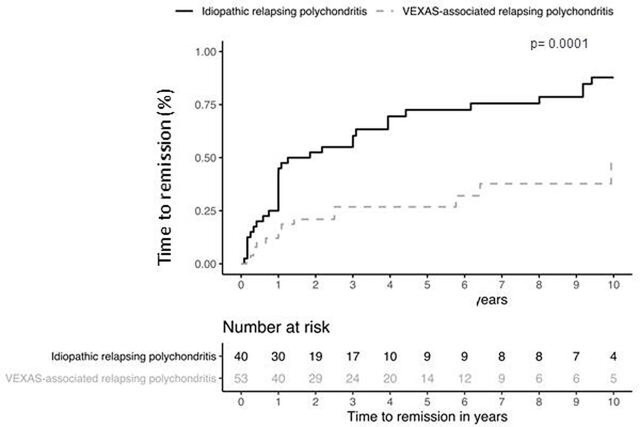
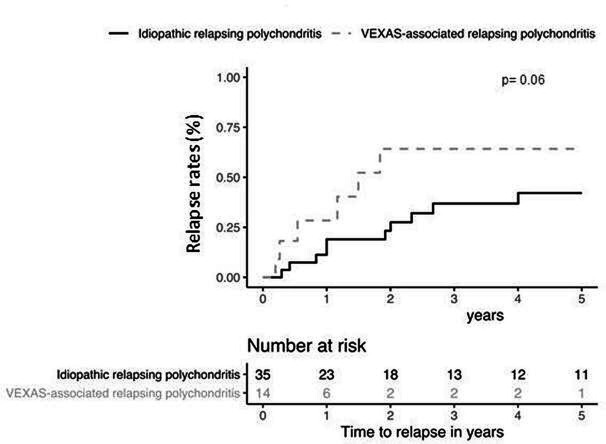
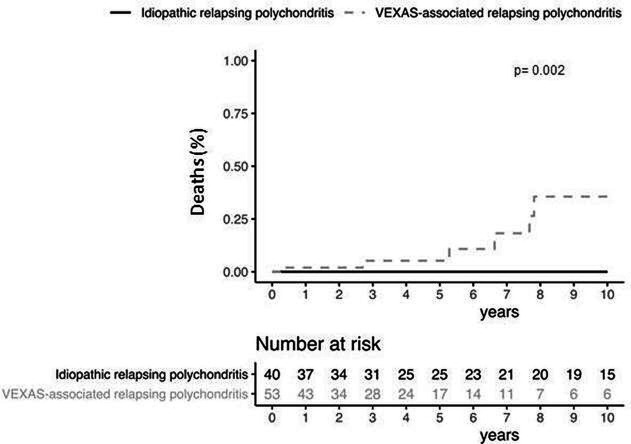
There were no differences between the two groups in term of glucocorticoid use and daily median prednisone dose, of number of combined DMARDs and of biological drugs. The rate of glucocorticoid dependency was similar into the two groups (71% vs 62%, p=0.459), as were the frequencies of DMARDs and biological drugs. Seventeen of patients with VEXAS-RP (31%) had been treated by azacitidine. All of them had underlying MDS and experienced MDS haematological complete response (n=; 73%), partial response (n=; 13%) and none in 13%. Azacitidine was given for steroid dependent or refractory autoinflammatory symptoms in 10/17 patients (%) and among them 5 had a complete clinical and laboratory response and the 5 remaining cases at least 50% improvement of clinical and laboratory CRP parameters. The number of patients who achieved remission under treatment was lower in VEXAS-RP group (27% vs 90%, p<0001). Besides, patients with VEXAS-RP have time to remission which was significantly longer than those with I-RP (14% vs 43% at 1 year, 27% vs 73% at 5 years and 48% vs 88% at 10 years, p=0.0001) (figure 2). Patients with VEXAS-RP tended to have a higher risk of relapse (28% vs 19% at 1 year, 64% vs 37% at 5 years and 64% vs 42% at 10 years, p=0.06) (figure 3). No significant factor among age, disease duration, different clinical features, CRP levels and number of immunosuppressive therapies, have been associated with the remission and the risk of relapse in multivariate analysis (data not shown). Six patients (11%) died in the VEXAS-RP group after a median follow-up of 37 months and none in the I-RP group after a median follow-up of 92 months. VEXAS-RP was associated with a higher risk of death (2% vs 0% at 1 year, 5% vs 0% at 5 years and 36% vs 0% at 10 years, p=0.002) (figure 4).
Figure 2.
Time to remission survival between idiopathic and VEXAS-associated relapsing polychondritis.
Figure 3.
Relapse rates between idiopathic and VEXAS-associated relapsing polychondritis.
Figure 4.
Deaths rates between idiopathic and VEXAS-associated relapsing polychondritis.
Discussion
In this study, we highlight several important features of VEXAS-RP, such as characterised by the fever, skin lesions and pulmonary impairment, and higher rates of steroid dependence and relapse rates. Since the somatic mutations of UBA1 causing the VEXAS syndrome had been descripted by Beck et al in October 2020,30 13 and 9 cases of VEXAS-RP were reported, respectively, by Ferrada et al 36 and Tsuchida et al.37 In the publication by Ferrada et al, main clinical features of VEXAS-RP included fever (100%), ear (100%) and nose (92%) chondritis, skin involvement (85%), pulmonary infiltrates (77%) and venous thrombosis (62%), and the disease was restricted to men ≥45 years at disease onset.36 We report here the largest cohort of VEXAS-RP, which was compared with 40 patients with I-RP. Like the previous studies, our patients with VEXAS-RP were almost all men (96%) and older than patients with I-RP at disease onset (66 vs 44 years, and 62 years in Ferrada’s cohort). The two VEXAS-RP who were women had both acquired monosomy X. We confirmed the higher prevalence of fever (60%), of skin lesion (82%) and of pulmonary infiltrates (46%) in VEXAS-RP group whereas large airway chondritis seemed to be more frequent in I-RP group. In the present study, we add other clinical key features into the spectrum of VEXAS-RP, such as the highest prevalence of ocular and heart involvement. Previous study by Ferrada et al included 13 patients with VEXAS-RP and no patient with VEXAS-RP had chondritis of the large airways or parasternal joint in the Ferrada’s cohort whereas it was possible in our patients without difference between the two groups for the parasternal joint chondritis. Another new data are the highest prevalence of nose chondritis in patients with I-RP. In the original description of VEXAS syndrome by Beck et al, venous thrombosis seemed to be a frequent clinical feature and Ferrada reported that it was more prevalent in VEXAS-RP than in I-RP (62% vs 5%), but the prevalence of venous thrombosis was quite similar in our paper about 25% in each group. Dion et al shows that kidney involvement in RP which was described in up to 22% of patients in previous studies,4 40 most frequently consistent with lesions of necrotising glomerulonephritis.29 In our study we had only 4 cases of kidney involvement, all in VEXAS-RP group, and in the French VEXAS cohort of 116 patients, 11 cases of kidney involvement were reported usually without glomerulonephritis.35 In the three clinical phenotypes of RP described by Dion et al, the group with haematological disorders, mostly MDS, had the worst prognosis.29 We observed a high prevalence of MDS in our patients with VEXAS-RP (75% vs 23% in Ferrada’s cohort). In our study, there was no impact of the MDS in the patients with VEXAS-RP in term of treatment, response rates and overall survival suggesting that the poor prognosis is the consequence of the VEXAS syndrome and not of the MDS. These data would have to be confirmed by comparing in patients with RP and MDS those with and without somatic mutations in UBA1.
The treatment in RP is not yet codified, mostly based on case reports and few case series. It usually includes glucocorticoids, conventional DMARDs and biological targeted drugs.1 41 42 No difference in treatment management was shown between VEXAS-RP and I-RP especially in glucocorticoids use and dose, and number of steroid sparing agents, except the use of azacytidine in MDS-related VEXAS. The patients with MDS-VEXAS management is still need to be described, and some recent small studies reported the potential benefit of JAK inhibitors in patients with VEXAS syndrome43 and of azacitidine. Azacitidine was effective in 5 out of 11 patients with VEXAS with MDS in a French series44 and 2 out of 3 patients in a Dutch series.45 Among patients with VEXAS with RP, our case series confirmed that azacitidine could be effective to control autoinflammatory symptoms even in low-risk MDS.
Our study has some limitations, mainly the lack of UBA1 status for 33 patients in the I-RP group, related to the retrospective and multicentre design of a recently described disease. This study was designed to exclude MDS from the I-RP, so it is difficult to compare VEXAS-RP and I-RP with respect to the presence or absence of MDS.
Conclusion
We report the largest cohort of VEXAS-RP, which is characterised by high prevalence of male sex, fever, skin lesion, ocular involvement, pulmonary infiltration, heart involvement, older age and MDS association and a low risk of nose chondritis compared with the I-RP. Patients with VEXAS-RP have a worse prognosis with more refractory disease and higher mortality. Despite some important differences in I-RP from VEXAS-RP, the UBA1-related pathways should be interesting mechanisms to understand even in not mutated UBA molecules and could trigger further pathophysiological studies to understand the inflammatory pathways implicated in various RP diseases.
Footnotes
Contributors: Study design and conception: M-YK and AM. Data analysis and paper writing: M-YK, AFG, SG-L, BT, DS, JS, MlB, CDM, GD, MG-V, JSA, AM, LB, VG, JG, OK, AD, MD, BS, JS, PM-G, SA, SP, MR-S, VJ, PH, OF, AM. AM is guarantor for this paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was conducted in compliance with the Good Clinical Practices protocol and Declaration of Helsinki principles and received approval from the Cochin Hospital Institutional Review Board (CLEP Decision N°: AAA-2021–08040).
References
- 1. Hazra N, Dregan A, Charlton J, et al. Incidence and mortality of relapsing polychondritis in the UK: a population-based cohort study. Rheumatology 2015;54:kev240–7. 10.1093/rheumatology/kev240 [DOI] [PubMed] [Google Scholar]
- 2. McAdam LP, O'Hanlan MA, Bluestone R, et al. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine 1976;55:193–215. [PubMed] [Google Scholar]
- 3. Michet CJ, McKenna CH, Luthra HS, et al. Relapsing polychondritis. survival and predictive role of early disease manifestations. Ann Intern Med 1986;104:74–8. 10.7326/0003-4819-104-1-74 [DOI] [PubMed] [Google Scholar]
- 4. Zeuner M, Straub RH, Rauh G, et al. Relapsing polychondritis: clinical and immunogenetic analysis of 62 patients. J Rheumatol 1997;24:96–101. [PubMed] [Google Scholar]
- 5. Kent PD, Michet CJ, Luthra HS. Relapsing polychondritis. Curr Opin Rheumatol 2004;16:56–61. 10.1097/00002281-200401000-00011 [DOI] [PubMed] [Google Scholar]
- 6. Rafeq S, Trentham D, Ernst A. Pulmonary manifestations of relapsing polychondritis. Clin Chest Med 2010;31:513–8. 10.1016/j.ccm.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 7. O'Hanlan M, McAdam LP, Bluestone R, et al. The arthropathy of relapsing polychrondritis. Arthritis Rheum 1976;19:191–4. 10.1002/art.1780190210 [DOI] [PubMed] [Google Scholar]
- 8. Balsa A, Expinosa A, Cuesta M, et al. Joint symptoms in relapsing polychondritis. Clin Exp Rheumatol 1995;13:425–30. [PubMed] [Google Scholar]
- 9. Francès C, el Rassi R, Laporte JL, et al. Dermatologic manifestations of relapsing polychondritis. A study of 200 cases at a single center. Medicine 2001;80:173–9. 10.1097/00005792-200105000-00003 [DOI] [PubMed] [Google Scholar]
- 10. Isaak BL, Liesegang TJ, Michet CJ. Ocular and systemic findings in relapsing polychondritis. Ophthalmology 1986;93:681–9. 10.1016/S0161-6420(86)33695-9 [DOI] [PubMed] [Google Scholar]
- 11. Cody DT, Sones DA. Relapsing polychondritis: audiovestibular manifestations. Laryngoscope 1971;81:1208–22. 10.1288/00005537-197108000-00004 [DOI] [PubMed] [Google Scholar]
- 12. Del Rosso A, Petix NR, Pratesi M, et al. Cardiovascular involvement in relapsing polychondritis. Semin Arthritis Rheum 1997;26:840–4. 10.1016/S0049-0172(97)80028-5 [DOI] [PubMed] [Google Scholar]
- 13. Terao C, Yoshifuji H, Yamano Y, et al. Genotyping of relapsing polychondritis identified novel susceptibility HLA alleles and distinct genetic characteristics from other rheumatic diseases. Rheumatology 2016;55:1686–92. 10.1093/rheumatology/kew233 [DOI] [PubMed] [Google Scholar]
- 14. Foidart JM, Abe S, Martin GR, et al. Antibodies to type II collagen in relapsing polychondritis. N Engl J Med 1978;299:1203–7. 10.1056/NEJM197811302992202 [DOI] [PubMed] [Google Scholar]
- 15. Ebringer R, Rook G, Swana GT, et al. Autoantibodies to cartilage and type II collagen in relapsing polychondritis and other rheumatic diseases. Ann Rheum Dis 1981;40:473–9. 10.1136/ard.40.5.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herman JH, Dennis MV. Immunopathologic studies in relapsing polychondritis. J Clin Invest 1973;52:549–58. 10.1172/JCI107215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansson AS, Heinegård D, Holmdahl R. A new animal model for relapsing polychondritis, induced by cartilage matrix protein (matrilin-1). J Clin Invest 1999;104:589–98. 10.1172/JCI5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hansson AS, Heinegård D, Piette JC, et al. The occurrence of autoantibodies to matrilin 1 reflects a tissue-specific response to cartilage of the respiratory tract in patients with relapsing polychondritis. Arthritis Rheum 2001;44:2402–12. [DOI] [PubMed] [Google Scholar]
- 19. Hansson A-S, Johannesson M, Svensson L, et al. Relapsing polychondritis, induced in mice with matrilin 1, is an antibody- and complement-dependent disease. Am J Pathol 2004;164:959–66. 10.1016/S0002-9440(10)63183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buckner JH, Van Landeghen M, Kwok WW, et al. Identification of type II collagen peptide 261-273-specific T cell clones in a patient with relapsing polychondritis. Arthritis Rheum 2002;46:238–44. [DOI] [PubMed] [Google Scholar]
- 21. Herman JH, Greenblatt D, Khosla RC, et al. Cytokine modulation of chondrocyte proteinase release. Arthritis Rheum 1984;27:79–91. 10.1002/art.1780270113 [DOI] [PubMed] [Google Scholar]
- 22. Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 1986;322:547–9. 10.1038/322547a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dingle JT, Page Thomas DP, King B, et al. In vivo studies of articular tissue damage mediated by catabolin/interleukin 1. Ann Rheum Dis 1987;46:527–33. 10.1136/ard.46.7.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stabler T, Piette J-C, Chevalier X, et al. Serum cytokine profiles in relapsing polychondritis suggest monocyte/macrophage activation. Arthritis Rheum 2004;50:3663–7. 10.1002/art.20613 [DOI] [PubMed] [Google Scholar]
- 25. Diebold L, Rauh G, Jäger K, et al. Bone marrow pathology in relapsing polychondritis: high frequency of myelodysplastic syndromes. Br J Haematol 1995;89:820–30. 10.1111/j.1365-2141.1995.tb08420.x [DOI] [PubMed] [Google Scholar]
- 26. Hebbar M, Brouillard M, Wattel E, et al. Association of myelodysplastic syndrome and relapsing polychondritis: further evidence. Leukemia 1995;9:731–3. [PubMed] [Google Scholar]
- 27. Letko E, Zafirakis P, Baltatzis S, et al. Relapsing polychondritis: a clinical review. Semin Arthritis Rheum 2002;31:384–95. 10.1053/sarh.2002.32586 [DOI] [PubMed] [Google Scholar]
- 28. Horváth A, Páll N, Molnár K, et al. A nationwide study of the epidemiology of relapsing polychondritis. Clin Epidemiol 2016;8:211–30. 10.2147/CLEP.S91439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dion J, Costedoat-Chalumeau N, Sène D, et al. Relapsing polychondritis can be characterized by three different clinical phenotypes: analysis of a recent series of 142 patients. Arthritis Rheumatol 2016;68:2992–3001. 10.1002/art.39790 [DOI] [PubMed] [Google Scholar]
- 30. Beck DB, Ferrada MA, Sikora KA, et al. Somatic Mutations in UBA1 and Severe Adult-Onset Autoinflammatory Disease. N Engl J Med 2020;383:2628–38. 10.1056/NEJMoa2026834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arlet J-B, Terrier B, Kosmider O. Mutant UBA1 and severe adult-onset autoinflammatory disease. N Engl J Med 2021;384:2163. 10.1056/NEJMc2102124 [DOI] [PubMed] [Google Scholar]
- 32. Barba T, Jamilloux Y, Durel C-A, et al. VEXAS syndrome in a woman. Rheumatology 2021;60:keab392:e402–3. 10.1093/rheumatology/keab392 [DOI] [PubMed] [Google Scholar]
- 33. Lacombe V, Kosmider O, Prévost M, et al. Severe joint involvement in VEXAS syndrome: a case report. Ann Intern Med 2021;174:1025–7. 10.7326/L21-0023 [DOI] [PubMed] [Google Scholar]
- 34. Magnol M, Couvaras L, Degboé Y. VEXAS syndrome in a patient with previous spondyloarthritis with favorable response to intravenous immunoglobulin anti-IL17 therapy. Rheumatology 2021;10. 10.1093/rheumatology/keab211 [DOI] [PubMed] [Google Scholar]
- 35. Georgin-Lavialle S, Terrier B, Guedon AF, et al. Further characterization of clinical and laboratory features in VEXAS syndrome: large-scale analysis of a multicentre case series of 116 French patients. Br J Dermatol 2022;186:564–74. 10.1111/bjd.20805 [DOI] [PubMed] [Google Scholar]
- 36. Ferrada MA, Sikora KA, Luo Y, et al. Somatic mutations in UBA1 define a distinct subset of relapsing polychondritis patients with VEXAS. Arthritis Rheumatol 2021;73:1886–95. 10.1002/art.41743 [DOI] [PubMed] [Google Scholar]
- 37. Tsuchida N, Kunishita Y, Uchiyama Y. Pathogenic UBA1 variants associated with VEXAS syndrome in Japanese patients with relapsing polychondritis. Ann Rheum Dis 2021;1061. [Epub ahead of print: 31 Mar 2021]. 10.1136/annrheumdis-2021-220089 [DOI] [PubMed] [Google Scholar]
- 38. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the world Health organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 39. Zhang Z. Multiple imputation with multivariate imputation by chained equation (mice) package. Ann Transl Med 2016;4:7. 10.3978/j.issn.2305-5839.2015.12.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang-Miller A, Okamura M, Torres VE, et al. Renal involvement in relapsing polychondritis. Medicine 1987;66:202–17. 10.1097/00005792-198705000-00004 [DOI] [PubMed] [Google Scholar]
- 41. Rednic S, Damian L, Talarico R, et al. Relapsing polychondritis: state of the art on clinical practice guidelines. RMD Open 2018;4:e000788. 10.1136/rmdopen-2018-000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moulis G, Pugnet G, Costedoat-Chalumeau N, et al. Efficacy and safety of biologics in relapsing polychondritis: a French national multicentre study. Ann Rheum Dis 2018;77:annrheumdis-2017-212705–8. 10.1136/annrheumdis-2017-212705 [DOI] [PubMed] [Google Scholar]
- 43. Bourbon E, Heiblig M, Gerfaud Valentin M, et al. Therapeutic options in VEXAS syndrome: insights from a retrospective series. Blood 2021;137:3682–4. 10.1182/blood.2020010177 [DOI] [PubMed] [Google Scholar]
- 44. Comont T, Heiblig M, Rivière E, et al. Azacitidine for patients with vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic syndrome (VEXAS) and myelodysplastic syndrome: data from the French VEXAS registry. Br J Haematol 2022;196:969–74. 10.1111/bjh.17893 [DOI] [PubMed] [Google Scholar]
- 45. Raaijmakers MHGP, Hermans M, Aalbers A, et al. Azacytidine treatment for VEXAS syndrome. Hemasphere 2021;5:e661. 10.1097/HS9.0000000000000661 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available in a public, open access repository.