Abstract
This cohort study evaluates an electronic medical record–based tool for diagnosis of primary hyperparathyroidism in patients with chronic hypercalcemia.
Primary hyperparathyroidism (PHPT), the predominant cause of chronic hypercalcemia, is diagnosed by elevated serum calcium levels in the presence of elevated or inappropriately normal parathyroid hormone (PTH) levels.1 Complications of untreated PHPT include osteoporosis, fragility fractures, and nephrolithiasis.2 Definitive treatment is achieved with surgical parathyroidectomy.3 Studies have reported that PHPT is underdiagnosed and undertreated and have recommended development of clinical decision support tools to aid in PHPT management.4 This cohort study evaluated the association of an electronic medical record (EMR)–based tool with frequency of serum PTH level measurement among patients with chronic hypercalcemia and described their subsequent treatment in a large academic health system.
Methods
The EMR-based tool (eFigure in the Supplement) identified outpatients with chronic hypercalcemia from the EMR of UCLA Health and triggered an alert prompting physicians to order a PTH level measurement. This study followed the STROBE reporting guideline and was approved by the UCLA institutional review board, which waived informed consent because data were deidentified.
Patients were identified from May to November 2020 and were followed up for 16 months. The alert algorithm was triggered in adults (aged ≥18 years) with 2 instances of hypercalcemia (total calcium level >10.4 mg/dL [to convert to mmol/L, multiply by 0.25]) in a 6-month interval and absent PTH level measurement in the following year. The tool encoded logic to exclude patients with secondary hyperparathyroidism, tertiary hyperparathyroidism, and nonparathyroid causes of hypercalcemia (eTables 1 and 2 in the Supplement). Baseline sequelae of PHPT were evaluated using ICD-9 and ICD-10 codes (eTable 3 in the Supplement). Patients with confirmed hypercalcemia and an elevated or inappropriately normal serum PTH level (>11 pg/mL [to convert to ng/L, multiply by 1]) were diagnosed with PHPT based on biochemical findings. During follow-up, we examined the frequencies of bone mineral density assessment by dual energy x-ray absorptiometry, kidney ultrasonography, parathyroidectomy, and initiation of pharmacologic therapy for osteopenia or osteoporosis (eMethods in the Supplement). Two-sided P < .05 was considered significant. We analyzed data using R (version 3.6.2).
Results
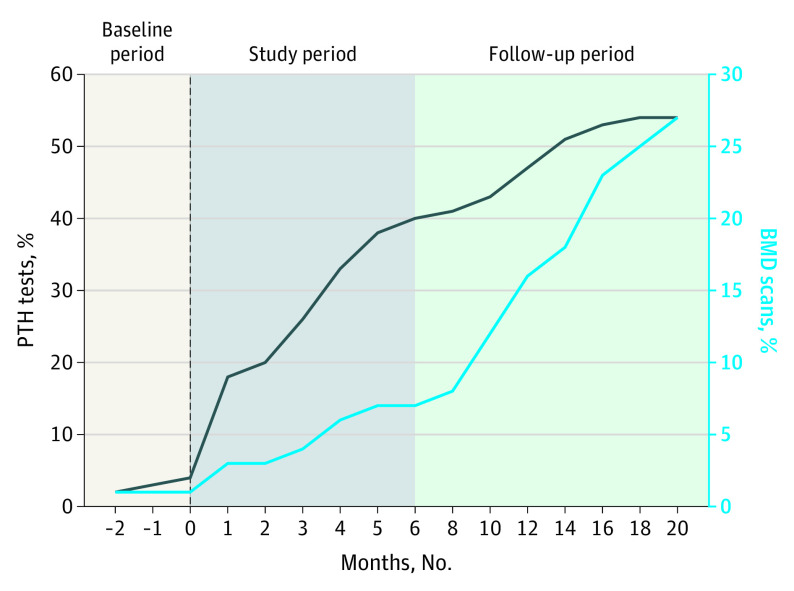
The population included 395 patients with chronic hypercalcemia (Table). During the baseline period, 9 patients (7%) who would have met alert criteria subsequently had their serum PTH measured. This rate increased to 45% (n = 31) during the patient identification period (Figure) and 54% (n = 215) during 6-month follow-up; 213 (99%) were diagnosed with PHPT, but 13 (6%) had low to normal PTH levels (11-20 ng/L). Physicians’ responses to the tool were as follows: 182 patients (46%) had PTH ordered, 87 (22%) alerts were dismissed, 59 (15%) were deferred to another practitioner, and 67 (17%) were deemed inappropriate for the encounter.
Table. Patient Demographic and Clinical Characteristics.
| Characteristic | Patients with confirmed hypercalcemiaa | ||
|---|---|---|---|
| With PTH level measured (n = 215)b | Without PTH level measured (n = 180) | P value | |
| Age, median (IQR), y | 72 (59-78) | 69 (58-77) | .09 |
| Body mass index, median (IQR)c | 26.4 (23.28-30.95) | 27.03 (23.68-31.28) | .49 |
| Sex | |||
| Female | 138 (64.2) | 123 (68) | .52 |
| Male | 74 (34.4) | 57 (32) | |
| Declined | 1 (0.5) | 0 | |
| Other | 2 (0.9) | 0 | |
| Raced | |||
| Alaska Native | 1 (0.5) | 1 (0.6) | .60 |
| Asian | 17 (7.9) | 11 (6) | |
| Black/African American | 36 (16.7) | 26 (14) | |
| White | 136 (63.3) | 114 (63) | |
| Declined | 10 (4.7) | 6 (3) | |
| Othere | 13 (6) | 21 (12) | |
| Unknown | 2 (0.9) | 1 (0.6) | |
| Ethnicityd | |||
| Hispanic or Latino | 14 (6.5) | 11 (6) | .47 |
| Non-Hispanic or non-Latino | 185 (86) | 161 (89) | |
| Declined | 14 (6.5) | 7 (4) | |
| Unknown | 2 (0.9) | 1 (0.6) | |
| Baseline laboratory values, median (IQR) | |||
| Total calcium level, mg/dL | 10.65 (10.55-10.8) | 10.65 (10.55-10.75) | .41 |
| eGFR level, mL/min/1.73 m2 | 69 (52-84) | 76 (61-90) | .004 |
| Vitamin D (25-hydroxyvitamin D) level, ng/mL | 35.5 (26.75-45.25) | 34 (24.75-45) | .49 |
| Baseline comorbidities | |||
| Osteoporosis or osteopenia | 60 (27.9) | 43 (24) | .42 |
| Nephrolithiasis | 16 (7.4) | 19 (11) | .29 |
| Any fracture | 33 (15.3) | 32 (18) | .59 |
| Male hypogonadism | 9 (4.2) | 4 (2) | .40 |
| Hyperthyroidism | 3 (1.4) | 4 (2) | .71 |
Abbreviations: eGFR, estimated glomerular filtration rate; PHPT, primary hyperparathyroidism; PTH, parathyroid hormone.
SI conversion factors: To convert PTH level to ng/L, multiply by 1; total calcium to mmol/L, multiply by 0.25; eGFR to mL/s/m2, multiply by 0.0167; and vitamin D (25-hydroxyvitamin D) to nmol/L, multiply by 2.496.
Data are presented as number (percentage) of patients unless otherwise indicated. Confirmed hypercalcemia was defined as 2 instances of an elevated serum total calcium concentration (>10.4 mg/dL, our institution’s upper limit of the 95% laboratory reference range) in a 6-month interval.
Of these patients, 213 were found to have PHPT, as evidenced by an elevated or inappropriately normal PTH level (>11 pg/mL).
Calculated as weight in kilograms divided by height in meters squared.
Race and ethnicity were self-reported and were provided for comprehensive demographic data to analyze whether certain races and ethnicities were evaluated differently for PHPT.
Other was not specified further in the electronic medical record database.
Figure. Cumulative Frequency of Parathyroid Hormone (PTH) Level and Bone Mineral Density (BMD) Assessments Among Patients With Hypercalcemia After Implementation of an Electronic Medical Record–Based Tool.
Patient identification occurred during the study period. Vertical dashed line represents the time of alert activation.
At 16 months’ follow-up, 79 patients (37%) with PTH assessment had a bone mineral density scan ordered vs 22 of 180 (12%) without PTH assessment (P < .001). Thirty-six patients (17%) with PTH assessment were given new diagnoses of osteopenia or osteoporosis vs 7 (4%) without PTH assessment (P < .001). Parathyroidectomy was performed in 21 patients (10%) with PTH assessment vs 0 without PTH assessment (P < .001). Kidney ultrasonography was ordered for 34 patients (16%) with PTH assessment vs 18 (10%) without PTH assessment (P = .10).
Discussion
The EMR-based intervention was associated with a 6-fold increase in serum PTH assessment among outpatients with chronic hypercalcemia, nearly all of whom had PHPT. After PTH assessment, the frequency of appropriate downstream diagnostic and therapeutic interventions increased in the absence of further prompting. The EMR-based tool extracted abnormal laboratory results from a patient’s EMR and nudged physicians toward appropriate diagnostic action, while still preserving their decision-making autonomy.5 The tool underwent prelaunch refinements to minimize disruptions to physician workflow and reduce cognitive strain.
Limitations include reliance on diagnosis and procedure codes for data extraction, examination of total serum rather than albumin-adjusted or ionized calcium levels, and inclusion of patients with low to normal PTH levels. This study suggests a role for implementation science methods as a solution to PHPT underdiagnosis and undertreatment.
eTable 1. CPT/ICD-9 Codes for Nonparathyroid Causes of Hypercalcemia
eTable 2. CPT Codes for Calcium-Rich Metal Implantation in Orthopedic Surgery Patients
eTable 3. ICD9 and ICD10 Codes for Clinical Sequelae of Primary Hyperparathyroidism
eFigure. EMR-Based Tool Prompting PTH Assessment in Patients with Hypercalcemia
eMethods. Subjects and Methods (Expanded)
References
- 1.Yeh MW, Ituarte PHG, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122-1129. doi: 10.1210/jc.2012-4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3561-3569. doi: 10.1210/jc.2014-1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh MW, Zhou H, Adams AL, et al. The relationship of parathyroidectomy and bisphosphonates with fracture risk in primary hyperparathyroidism: an observational study. Ann Intern Med. 2016;164(11):715-723. doi: 10.7326/M15-1232 [DOI] [PubMed] [Google Scholar]
- 4.Alore EA, Suliburk JW, Ramsey DJ, et al. Diagnosis and management of primary hyperparathyroidism across the Veterans Affairs health care system. JAMA Intern Med. 2019;179(9):1220-1227. doi: 10.1001/jamainternmed.2019.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Yale University Press; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. CPT/ICD-9 Codes for Nonparathyroid Causes of Hypercalcemia
eTable 2. CPT Codes for Calcium-Rich Metal Implantation in Orthopedic Surgery Patients
eTable 3. ICD9 and ICD10 Codes for Clinical Sequelae of Primary Hyperparathyroidism
eFigure. EMR-Based Tool Prompting PTH Assessment in Patients with Hypercalcemia
eMethods. Subjects and Methods (Expanded)