Abstract
Neurofibromatosis type 1 (NF1) is one of the most common genetic tumor predisposition syndrome, caused by mutations in the NF1. To date, few genotype-phenotype correlations have been discerned in NF1, due to a highly variable clinical presentation. We aimed to study the molecular spectrum of NF1 and genotype-phenotype correlations in a monocentric study cohort of 85 NF1 patients (20 relatives, 65 sporadic cases). Clinical data were collected at the time of the mutation analysis and reviewed for accuracy in this investigation. An internal phenotypic categorization was applied. The 94% of the patients enrolled showed a severe phenotype with at least one systemic complication and a wide range of associated malignancies. Spine deformities were the most common complications in this cohort. We also reported 66 different NF1 mutations, of which 7 are novel mutations. Correlation analysis identified a slight significant inverse correlation between age at diagnosis and delayed acquisition of psychomotor skills with residual multi-domain cognitive impairment. Odds ratio with 95% confidence interval showed a higher prevalence of learning disabilities in patients carrying frameshift mutations. Overall, our results aim to offer an interesting contribution to studies on the genotype–phenotype of NF1 and in genetic management and counselling.
Keywords: Neurofibromatosis type 1, monocentric study cohort, internal phenotypic categorization, NF1 mutational spectrum, novel and recurrent NF1 mutations, genotype-phenotype correlations
1. Introduction
Neurofibromatosis type 1 (NF1, OMIM #162200), formerly known as von Recklinghausen’s disease, is a complex tumor predisposition syndrome, inherited in autosomal dominant pattern with an estimated incidence of 1:2500–3000 live births [1,2]. The diagnosis of NF1 is based on clinical criteria established by the National Institutes of Health Consensus Development Conference in 1987 and recently updated [3,4]. Inclusion criteria consist on the presence of 6 or more café-au-lait macules (CALMs) over 5 mm in greatest diameter in pre-pubertal individuals and over 15 mm in greatest diameter in post-pubertal individuals, cutaneous or subcutaneous neurofibromas, plexiform neurofibromas (PNFs), axillary or inguinal freckling, optic gliomas, distinctive osseous lesions, two or more iris Lisch nodules and a first-degree relative affected by NF1 [4,5]. Two of these criteria are necessary for clinical diagnosis [3]. Despite NF1 is a completely penetrant disease in adulthood (close to 100%), many of the features are age-dependent with marked inter- and intra-familial variability and expressivity, and generally in this order: CALMs, axillary lentigines, Lisch nodules, and neurofibromas. About 97% of NF1 patients meet the NIH criteria by the age of 8 years and all do so by the age of 20 years [6]; only 50% of children with sporadic NF1 under the age of 2 years meet a single NIH criterion, which often leads to a delay in diagnosis [6]. CALMs are usually the initial clinical feature of NF1 and may occur at birth or in childhood [6]. Axillary lentigines, which appear in early childhood (usually between 3 and 5 years of age), are usually the second manifestation in NF1 children. Cutaneous neurofibromas usually occur in the prepubertal phase, but can develop at a much earlier age and the increase in size and number coincides with puberty and pregnancy. Plexiform neurofibromas, on the other hand, are typically congenital or very early; they can only be recognized as an enlargement of soft tissue or a patch of skin hyperpigmentation [6]. Tibial dysplasia occurs at birth; optic glioma develops in children under the age of 6 years [6].
Possible multisystemic complications including neurological, cardiovascular, gastrointestinal, endocrine, and orthopedic features and neoplastic conditions have been associated to NF1 [7,8].
Based on epidemiologic studies, cancer incidence in NF1 is approximately four-fold higher than in general population [5,9,10]. About 10% of NF1 patients develop malignant peripheral nerve sheath tumors (MPNSTs), usually arising from plexiform neurofibroma and this is the major cause of poor prognosis [11,12]. Pheochromocytoma, sarcoma, melanoma, breast cancer, leukemia, and gastrointestinal stromal tumors (GISTs) are also frequently associated to NF1 [13,14].
More recently, molecular genetic testing was added to the list of diagnostic criteria [2,4,15].
NF1 is caused by mutations in NF1 (17q11.2), which encodes neurofibromin, a large guanosine triphosphate GTPase-activating protein (GAP), which acts as a tumor suppressor by regulating RAS GTPase [16,17,18,19,20]. To date, more than 3000 different genetic mutations in the NF1 have been reported in the Human Gene Mutation Database (HGMD) [21,22]. It is estimated that approximately 50% of all NF1 cases are sporadic, caused by de novo variants usually linked to paternal gonadal mutations [21,23]. Single nucleotide variations (SNVs) and small deletions (20 bp or less) account for more than 70% of currently known mutations [21] and most of NF1 mutations lead to a synthesis of truncated and non-functional neurofibromin [24]. Although the great effort to finely define the correlation between the clinical phenotype and molecular genotype, so far few correlation studies have been recognized, due to high clinical variability, which is observed even within the same family.
NF1 whole-gene deletion, affecting approximately 4% of NF1 patients, causes severe form of the disease, characterized by cutaneous neurofibromas earlier in life, development of larger number of tumors, including MPNSTs, more frequent and more severe cognitive abnormalities, somatic overgrowth, large hands and feet, and dysmorphic facial features [25,26,27,28]. 3-bp deletion in NF1 exon 17 (c.2970_2972delAAT) has been associated with typical pigmentary features of NF1 without cutaneous or surface plexiform neurofibromas [29]. NF1 microdeletions were linked to more severe clinical characteristics and increased lifetime risk for MPNSTs [27,30]. Recently, evidence for a more severe phenotype with higher frequency of spinal and deep neurofibromas were reported in association to the presence of missense mutations located in codons 844–848 of the NF1 [30]. Missense mutations affecting residue p.Arg1809 in NF1 were associated to a mild phenotype characterized by multiple café au lait spots, learning disabilities, short stature, and pulmonic stenosis but absence of neurofibromas [31,32,33]. Three specific recurrent non-truncating NF1 hotspots (residues p.Met1149, p.Arg1276, and p.Lys1423) have been linked with Noonan-like features with specific phenotypes: p.Arg1276 and p.Lys1423 pathogenic missense variants were associated with a high prevalence of cardiovascular abnormalities, including pulmonic stenosis; additionally, p.Arg1276 had a high prevalence of symptomatic spinal neurofibromas; p.Met1149 positive cause a mild phenotype, characterized mainly by pigmentary manifestations [34]. In a recent NF1 single-center study, missense variants negatively correlated with neurofibromas while skeletal defects were linked to frameshift variants and whole gene deletions [35]. The c.3721C>T, p.R1241* variant was associated to structural brain alterations, whereas the c.6855C>A, p.Y2285* variant correlated with a higher prevalence of Lisch nodules and endocrinological disorders [35].
The aim of the present study is to delineate the mutational spectrum of NF1 mutations and to elucidate genotype-phenotype correlations through integrated mutational screening and clinical data collection in a monocentric cohort of selected NF1 patients. We analyzed the NF1 mutations for their type, pathogenicity, and distribution in the NF1 and in the domains of the neurofibromin. A genotype-phenotype correlation was performed dividing the clinical features of the NF1 disease into cardinal signs and complications, adopting an internal classification in five NF1 phenotypic groups. Our findings should provide a simple and effective strategy for early diagnosis and genetic counseling in the NF1 disease.
2. Materials and Methods
2.1. Patients
We retrospectively evaluated the clinical data from a cohort of 420 unselected output patients, referred to our Neurofibromatosis Center at 2nd Division of Neurology of the ‘Luigi Vanvitelli’ University Hospital between January 2013 to December 2020, with a clinical diagnosis or suspicion of NF1. The inclusion criteria consisted of the clinical and/or molecular diagnosis of NF1 during the first clinical evaluation or later during the follow-up (according to the established International Criteria [3,4]).
Our study was conducted on 85 NF1 adult patients [47 females (55%) and 38 males (45%)] matching inclusion criteria. Informed consent was obtained for all patients. NF1 patients were divided into two groups by familial history: familial cases (20 relatives, for a total of 9 NF1 families) and sporadic cases (65 unrelated probands). The clinical data were collected at the time of genetic analysis, updated during follow-up and re-examined for accuracy by clinicians co-authoring this manuscript at the time of this phenotype-genotype correlation study. At least five years follow-up is available for all patients. Clinical and demographic data of the enrolled patients were reviewed retrospectively: sex, age at evaluation, family history, clinical manifestations, as well as NF1 genetic results, interpretation of variant pathogenicity and mutation site in the NF1 protein domains were evaluated. With regard to the psycho-cognitive phenotype, we used anamnestic-clinical data, sharing previously obtained assessments with standard psychomotor and cognitive tests (e.g., Griffiths’ Scales of Infant Development, GMDS-ER and the Wechsler Scales of Intelligence) according to the patient’s age. To achieve a more stringent genotype/phenotype correlation, the clinical features were divided into cardinal signs and complications, adopting an internal phenotypic categorization into five specific clinical groups. In detail: Group 1 (G1) includes patients with classical NF1 features, presenting with two or more of the following features: 6 or more CALMs, axillary and inguinal freckling, two or more Lisch nodules and neurofibromas, without other manifestations of extra-cutaneous/ocular involvement; Group 2 (G2) encloses NF1 patients presenting with two or more phenotypic features of G1 plus involvement of skeletal apparatus (short stature, scoliosis, hyperkyphosis, bone dysplasia), central nervous system (epilepsy, intracranial vascular malformations, hamartomas/unidentified bright objects/UBOs) and mental system (intellectual disability, anxiety/depression/sleep disorders), vascular system and anomalies of internal organs (heart valve abnormalities and hypertension); Group 3 (G3) includes NF1 patients presenting with two or more phenotypic features of G1, plus multi-apparatus involvement and histological diagnosis of MPNST according to the American Joint Committee on Cancer Staging System for soft tissue sarcomas; Group 4 (G4) includes NF1 patients presenting with two or more phenotypic features of G1 group, plus multi-apparatus involvement and neoplasms of the central (optic glioma, pilocytic astrocytoma) and peripheral (ganglioneuroma, gangliocytoma) nervous systems; Group 5 (G5) includes NF1 patients presenting two or more clinical traits of G1, plus multi-apparatus involvement and neoplasms of variable grade of various organs and apparatus including GIST, endocrine system (pheochromocytomas, thyroid carcinoma), genitourinary system (ovarian, prostatic, testicular, bladder cancer), tumors of the blood series, breast cancer, cutaneous melanoma, ear cholesteatoma.
2.2. NF1 Mutation Analysis
Part of NF1 patients clinically evaluated in the present study was previously genetically characterized by RNA analysis and reported by Giugliano et al. [36]. For NF1 genetic investigation, all DNA was extracted using the Qiagen BioRobot DNA extraction kit (Qiagen Benelux B.V., Venlo, The Netherlands) according to the manufacturer’s instructions and quantified using Nanodrop spectral analysis (Thermo Fischer Scientific, Inc., Waltham, MA, USA). DNA fragmentation and degradation were evaluated by standard agarose gel electrophoresis (100 V, 30 min, 1.5% agarose gel in Tris-borate-EDTA buffer). DNA Library preparation and whole exon enrichment were performed employing Agilent All Exon V.6 kit (Agilent Technologies, Inc., Santa Clara, CA, USA). Library sequences were obtained using the HiSeq2500 Illumina Sequencer (125-bp paired end sequence mode). Bioinformatics analysis included the following: Next-generation sequencing (NGS) reads mapping to whole genomes using the Burrows-Wheeler Alignment tool with default parameters, polymerase chain reaction (PCR) duplicate removal using Picard (http://picard.sourceforge.net, accessed on 31 March 2022), single nucleotide polymorphisms and indel calling using the Genome Analysis Toolkit (GATK) UnifiedGenotyper, variant annotation using snpEff (http://snpeff.sourceforge.net, accessed on 31 March 2022) and false positive variant filtration using the GATK VariantFiltration module. Exome sequencing data and reads alignment analysis were checked for coverage depth and alignment quality employing Bedtools software package. NF1 variants (RefSeq: NM_000267.3) were filtered for allele frequency (gnomAD minor allele frequency < 1%), considering all potential modes of inheritance and prioritized according to phenotype overlap, gene function, conservation (phyloP) and in silico prediction scores (CADD, SIFT, PolyPhen2, MutationTaster and MutationAssessor). The classification was conducted in accordance with the guidelines from the American College of Medical Genetics and Genomics. In brief, variants were classified as follows: (i) pathogenic variants; (ii) likely pathogenic variants; (iii) variants of uncertain significance (VOUS) [37,38]. Sanger sequencing was performed to validate the presence of significant variants and to perform segregation analysis in related NF1 cases. CNV calling was performed by Varseq software. This algorithm uses changes in coverage depth relative to a collection of reference samples (30 or more reference samples recommended, having on average 100X across all regions, and derived from the same library prep methods) as evidence of CNV events. High-quality CNVs were then annotated and filtered against CNV and gene annotation tracks like OMIM, Orphanet, RefSeq Genes, ClinVar and ClinGen. Real Time PCR was conducted to validate the presence of copy number variations. Briefly, targeted SYBR green fluorescent amplification was performed using primer mapping within exons resulted duplicated or deleted. Threshold amplification cycles were normalized using a housekeeping gene and the resulting values were then compared with the amplification of control samples (ddCt method). Samples with negative CNV bioinformatic analysis were further analyzed by MLPA analysis (MRC-holland, probe set code P081 and P082) according to manufacture instructions. Fragment analysis was performed by Abi3130 automated sequencer equipped with 36 cm capillary array and data analysis was performed using Coffalyser software (MRC-Holland).
2.3. Statistical Analysis
Correlation analysis was performed using the Spearman rank correlation test and the point-biserial correlation coefficient for dichotomous variables. In detail, statistical analysis was applied to each of the clinical characteristics common to all NF1 patients in our cohort, to every clinical group (G1-G5) defined in the study, and to the NF1 mutation type. Odds ratio (OR) with 95% confidence interval (CI) was estimated to evaluate the prevalence of typical clinical symptoms in NF1 patients carrying different types of mutation. p values lower than 0.05 were considered significant.
3. Results
3.1. NF1 Molecular Findings
Molecular data of enrolled NF1 patients are listed in the Table 1, demographics and clinical features are reported in the Table S1.
Table 1.
Molecular characteristics of the NF1 cohort, according to clinical groups (G1–G5).
| ID | Clinical Group | Nucleotide Change |
Amino Acid Change |
Exon/ Intron |
Mutation Type | CS | Ref. | FH | ClinVar/ HGMD/ LOVD |
PD |
|---|---|---|---|---|---|---|---|---|---|---|
| NF_2 | G1 | c.3G>A | p.? | 1 | Start lost | P | [39,40] | Pat | LOVD: NF1_001130 |
- |
| NF_54 | G1 | c.1639G>T | p.Glu547* | 14 | Nonsense | P | [39] | - | LOVD: NF1_001185 |
CSRD |
| NF_68 | G1 | c. 6792C>A | p.Ala2253_Lys2286del | 46 | Splicing affected |
P | [41,42,43] | - | LOVD: NF1_000816 |
HLR |
| NF_83 | G1 | c.5673T>G | p.Ser1891Arg | 39 | Missense | - | - | - | LOVD: NF1_002964 |
HLR |
| NF_89 | G1 | c.(4661+1_4662-1)_(7258+1_7259-1)dup | - | - | Duplication | - | - | - | NR | Sec14-PH /HLR |
| NF_1 | G2 | c.(586+1_587-1)_(730+1_731-1)del | - | 6,7 | Deletion | P | [44,45] | Unk | - | - |
| NF_3 | G2 | c.3G>A | p.? | 1 | Start lost | P | [39,40] | Unk | LOVD: NF1_001130 |
- |
| NF_4 | G2 | c.3G>A | p.? | 1 | Start lost | P | [39,40] | Pat | LOVD: NF1_001130 |
- |
| NF_5 | G2 | c.1595T>G | p.Leu532Arg | 14 | Missense | P | [36,40] | Pat | LOVD: NF1_002498 | - |
| NF_8 | G2 | c.7884_7885del | p.(Phe2629Serfs*9) | - | Frameshift | - | - | - | NR | SBR |
| NF_10 | G2 | c.3826C>T | p.Arg1276* | 28 | Nonsense | P | [34,46] | Mat | ClinVar: variation ID 237556 |
GRD |
| NF_11 | G2 | c.3826C>T | p.Arg1276* | 28 | Nonsense | P | [34,46] | Unk | ClinVar: variation ID 237556 |
GRD |
| NF_13 | G2 | c.6892_6897del | p.Ala2300_Val2301del | - | Deletion | - | [36] | - | - | HLR |
| NF_20 | G2 | c.1783_1784del | p.Glu595fs | 16 | Frameshift | P | [39] | - | LOVD: NF1_001194 |
CSRD |
| NF_21 | G2 | c.(?_-383)_(*3522_?)del (whole NF1 deletion) |
- | - | Deletion | P | [27,47,48,49,50,51] | Mat | - | - |
| NF_25 | G2 | c.3496+1G>A | p.Tyr1106Leufs*28 | 26 | Frameshift | - | [36] | Mat | HGMD: CS072245 | TBD |
| NF_28 | G2 | c.1A>G | p.? | 1 | Missense | P | [52,53,54] | Pat | LOVD: NF1_000140 |
- |
| NF_30 | G2 | c.3728T>C | p.Leu1243Pro | 28 | Missense | LP | [36] | Pat | LOVD: NF1_001544 |
GRD |
| NF_38 | G2 | c.4768C>T | p.Arg1590Trp | 36 | Missense | VOUS | [36,55,56,57,58,59] | - | HGMD: CM971051 | Sec14-PH |
| NF_40 | G2 | c.1466A>G | p.Tyr489Cys | 13 | Splicing affected |
P | [41,54,60,61,62,63] | Pat | LOVD: NF1_000063 |
- |
| NF_43 | G2 | c.2352G>C | p.Trp784Cys | 20 | Missense | P | [36,64,65] | Pat | LOVD: NF1_001853 | CSRD |
| NF_45 | G2 | c.2307dup | p.Thr770Hisfs*6 | 19 | Frameshift | - | [36] | Pat | - | CSRD |
| NF_46 | G2 | c.2307dup | p.Thr770Hisfs*6 | 19 | Frameshift | - | [36] | Pat | - | CSRD |
| NF_47 | G2 | c.2307dup | p.Thr770Hisfs*6 | 19 | Frameshift | - | [36] | Pat | - | CSRD |
| NF_48 | G2 | c.1A>G | p.? | 1 | Missense | P | [52,53,54] | - | LOVD: NF1_000140 |
- |
| NF_50 | G2 | c.3497_3974del | - | - | Deletion | - | [36,54,66,67] | - | - | GRD |
| NF_51 | G2 | c.4381dup | p.Ile1461Asnfs*4 | 34 | Frameshift | P | [68] | Pat | LOVD: NF1_001553 |
GRD |
| NF_53 | G2 | c.1378dup | p.Ile460Asnfs*10 | 12 | Frameshift | - | [36] | - | - | - |
| NF_56 | G2 | c.2409+1G> Ac.2375T>A |
p.? p.Leu792His |
20i | Splicing affected Missense |
P LP |
[62,69] - |
- | LOVD: NF1_000203 ClinVar: variation ID 665425 |
- CSRD |
| NF_57 | G2 | c.1381C>T | p.Arg461* | 12 | Nonsense | P | [42,53,61,70,71,72] | - | LOVD: NF1_000056 |
- |
| NF_59 | G2 | c.4270-2A>G | p.Ile1424_Gln1426del | 32i | Splicing affected |
P | [73,74] | Mat | LOVD: NF1_000479 |
GRD |
| NF_61 | G2 | 4367+2T>C | p.? | - | Splicing affected |
LP | - | Pat | ClinVar: variation ID 527560 |
- |
| NF_62 | G2 | c.1260+1G>A | p.Ser421fs | 11i | Splicing affected |
P | [75,76] | Pat | LOVD: NF1_000036 |
- |
| NF_63 | G2 | c.4733C>A | p.(Ser1578Tyr) | - | Missense | - | - | Unk | NR | Ses14-PH |
| NF_66 | G2 | c.2665 A>G | p.Thr889Ala | - | Missense | VOUS | - | - | ClinVar: variation ID 527580 |
CSRD |
| NF_71 | G2 | c.2409+1G>C | p.? | 20i | Splicing affected |
P | [69,77] | Pat | LOVD: NF1_000204 |
- |
| NF_77 | G2 | c.2326G>A | p.Ala776_Gln803del | - | Splicing affected |
- | [36] | - | - | CSRD |
| NF_80 | G2 | c.4278G>C | p. Gln1426His | 33 | Missense | P | [36,78] | Pat | ClinVar: variation ID 233115 |
GRD |
| NF_86 | G2 | c.4923G>A | p.Trp1641* | - | Nonsense | P | [52] | Pat | LOVD: NF1_001303 |
Sec14-PH |
| NF_90 | G2 | c.(4661+1_4662-1)_(7258+1_7259-1)dup | - | - | Duplication | - | - | Mat | NR | Sec14-PH /HLR |
| NF_94 | G2 | c.2252-3T>G | P.? | - | Splicing affected |
P | [79] | - | ClinVar: variation ID 374022 |
- |
| NF_96 | G2 | c.(?_-383)_(*3522_?)del (whole NF1 deletion) |
- | - | - | P | [27,47,48,49,50,51] | - | - | - |
| NF_100 | G2 | c.4537C>T | p.Arg1513* | 35 | Nonsense | P | [42,63,80,81,82,83,84] | - | LOVD: NF1_000521 |
GRD |
| NF_102 | G2 | c.1381C>T | p.Arg461* | 12 | Nonsense | P | [41,42,53,61,70,71,72] | Pat | LOVD: NF1_000056 |
- |
| NF_103 | G2 | c.2251 G>C | p.Asp668Glufs*9 | - | Frameshift | - | [36] | + | - | CSRD |
| NF_18 | G3 | c.3326T>G | p.Leu1109* | 26 | Nonsense | - | [36] | - | - | TBD |
| NF_52 | G3 | c.4309G>T | p.(Glu1437*) | - | Nonsense | - | - | - | NR | GRD |
| NF_127 | G3 | c.2540T>G | p.Leu847Arg | 21 | Missense | P | [24,30,38,53,61,65,70,85,86,87,88] | - | ClinVar: variation ID 573019 |
CSRD |
| NF_6 | G4 | c.479+5G>A | p.Leu94fs | 4i | Frameshift | P | [55,89] | Mat | ClinVar: variation ID 237521 |
- |
| NF_17 | G4 | c.6364+4A>G | p.Val2029Lysfs*7 | 41i | Splicing affected |
- | [36] | - | HGMD: CS941517 | HLR |
| NF_26 | G4 | c.1246C>T | p.Arg416* | 11 | Nonsense | P | [70,90] | - | LOVD: NF1_000034 |
- |
| NF_35 | G4 | c.4269+2T>C | p.? | 32i | Splicing affected |
P | [36] | Pat | - | - |
| NF_36 | G4 | c.6335T>C | p.Leu2112Pro | 42 | Missense | P | [36] | - | LOVD: NF1_000756 | HLR |
| NF_37 | G4 | c.(?_-383)_(*3522_?)del (whole NF1 deletion) |
- | - | Deletion | P | [27,47,48,49,50,51] | - | - | - |
| NF_41 | G4 | c.1499_1501delinsAAA | p.Ile500_His501delinsLysAsn | 13 | INDEL | - | [36] | - | - | - |
| NF_60 | G4 | c.1756_1759del | p.Thr586Valfs*18 | 16 | Frameshift | P | [42,62,91,92,93,94,95,96] | Pat | LOVD: NF1_000113 |
CSRD |
| NF_65 | G4 | c.(?_-383)_(*3522_?)del (whole NF1 deletion) |
- | - | Deletion | P | [27,47,48,49,50,51] | Mat | - | - |
| NF_67 | G4 | c.6084+1G>A | p.? | - | Splicing affected |
P | [38,39,43,70,71,97] | Pat | ClinVar: variation ID 404489 |
- |
| NF_70 | G4 | c.2409+1G>C | p.? | 20i | Splicing affected |
P | [69,77] | Pat | LOVD: NF1_000204 |
- |
| NF_72 | G4 | c.7884_7885del | p.(Phe2629Serfs*9) | - | Frameshift | - | - | Pat | NR | SBR |
| NF_76 | G4 | c.1845+1_1845+5del | p.Ala548_Lys615del | 16 | Splicing affected |
P | [68,70] | - | LOVD: NF1_001511 |
CSRD |
| NF_81 | G4 | c.5819del | p.(Lys1940Serfs*18) | - | Frameshift | - | - | Unk | NR | HLR |
| NF_82 | G4 | c.8051-1 G>C | p.? | - | Splicing affected |
- | [36] | - | - | - |
| NF_85 | G4 | c.6621dup | p.(Trp2208Valfs*13) | - | Frameshift | - | - | - | NR | HLR |
| NF_99 | G4 | c.2851G>T | p.Val951Phe | 22 | Missense | LP | [68] | Mat | LOVD: NF1_001526 |
- |
| NF_115 | G4 | c.2446C>T | p.Arg816* | 21 | Nonsense | P | [56,70,98] | - | LOVD: NF1_000214 |
CSRD |
| NF_15 | G5 | c.2619dup | p.Lys874* | 21 | Nonsense | P | [41,60] | - | ClinVar: variation ID 404563 |
CSRD |
| NF_19 | G5 | c.3826C>T | p.Arg1276* | 28 | Nonsense | P | [34,46] | Mat | LOVD: NF1_000403 |
GRD |
| NF_22 | G5 | c.4982_4983del | p.Cys1661* | 37 | Nonsense | P | - | - | LOVD: NF1_000602 |
Sec14-PH |
| NF_23 | G5 | c.3496+1G>A | p.Tyr1106Leufs*28 | 26 | Frameshift | - | [36] | Unk | HGMD: CS072245 | TBD |
| NF_29 | G5 | c.1595T>G | p.Leu532Arg | 14 | Missense | P | [36,40] | Pat | LOVD: NF1_002498 | - |
| NF_31 | G5 | c.7686delG | p.Ile2563Phefs*40 | 53 | Frameshift | P | [36,99] | Pat | LOVD: NF1_002529 | CTD |
| NF_32 | G5 | c.7686delG | p.Ile2563Phefs*40 | 53 | Frameshift | P | [36,99] | Pat | LOVD: NF1_002529 | CTD |
| NF_39 | G5 | c.1009G>T | p.Glu337* | - | Nonsense | - | - | Pat | ClinVar: 439994 |
- |
| NF_42 | G5 | c.3502-3519del | p.Gly1169-Leu1173del | - | - | - | [36] | Mat | - | TBD |
| NF_55 | G5 | c.4537C>T | p.Arg1513* | 35 | Nonsense | P | [41,42,63,80,81,82,83,84] | - | LOVD: NF1_000521 |
GRD |
| NF_73 | G5 | c.4923G>A | p.Trp1641* | 37 | Nonsense | P | [52] | Unk | LOVD: NF1_001303 |
Sec14-PH |
| NF_78 | G5 | c.2329T>C | p.Trp777Arg | 20 | Missense | P | [1,39,100] | Mat | LOVD: NF1:000186 |
CSRD |
| NF_79 | G5 | c.3916C>T c.1975C>T |
p.Arg1306* p.Arg659Trp |
29 17 |
Nonsense Missense |
P VOUS |
[41,54,67,70,91,101,102][98] | - | LOVD: NF1_000416 LOVD: NF1_002592 |
GRD CSRD |
| NF_87 | G5 | c.7259C>A | p.Ala2420Asp | 50 | Missense | - | - | - | LOVD: NF1_000867 |
HLR |
| NF_92 | G5 | c.4278G>C | p.Gln1426His | 33 | Missense | P | [36,78] | Pat | ClinVar: Variation ID 233115 |
GRD |
| NF_97 | G5 | c.4515-2A>G | p.? | 34i | Splicing affected |
P | - | Mat | LOVD: NF1_000518 |
- |
| NF_98 | G5 | c.7089dup | p.Asn2364* | 48 | Nonsense | - | - | - | LOVD: NF1_001359 |
HLR |
| NF_101 | G5 | c.1329delT | p.Phe443Leufs*29 | - | Frameshift | - | [36,103,104] | - | - | - |
| NF_105 | G5 | c.7532C>T | p.(Ala2532Val) | - | Missense | - | - | Mat | NR | CTD |
Each NF1 patient was assigned a specific code (NF_ followed by an Arabic number) corresponding to the ‘unique patient identifier’ of the clinical center. Group 1 (G1) includes patients with classical NF1 phenotype, including six or more café-au-lait spots (CALMs), axillary and inguinal freckling, two or more Lisch nodules and neurofibromas, without other manifestations of extra-cutaneous/ocular involvement; Group 2 (G2) encloses NF1 patients presenting the phenotypic features of G1 plus involvement of skeletal apparatus, central nervous system (epilepsy, intracranial vascular malformations, hamartomas/UBOs), and mental system (intellectual disability, anxiety/depression/sleep disorders), vascular system and anomalies of internal organs; Group 3 (G3) includes NF1 patients presenting the phenotypic features of G1 plus multi-apparatus involvement and histological diagnosis of MPNST; Group 4 (G4) includes NF1 patients presenting the phenotypic features of group G1 plus multi-apparatus involvement and neoplasms of the central and peripheral nervous systems; Group 5 (G5) includes NF1 patients presenting the clinical traits of G1 plus multi-apparatus involvement and neoplasms of variable grade of various organs and apparatus. Clinical Significance (CS): P, pathogenic; LP, Likely pathogenic; VOUS, variant of uncertain clinical significance. Family History (FH): Mat, Maternal; Pat, Paternal; Unk, unknown, “-”, sporadic case. Publicly available databases of variants annotated on the disease: ClinVar (www.ncbi.nlm.nih.gov/clinvar, accessed on 31 March 2022), Human Genome Variation Database (HGMD; www.hgmd.cf.ac.uk, accessed on 31 March 2022) and Leiden Open Variation Database (LOVD; databases.lovd.nl/shared/genes). Protein Domain (PD): CSRD, cysteine/serine-rich domain; GRD, GAP-related domain; TBD, tubulin-binding domain; HLR, HEAT-like regions repeat; NLS, nuclear localization signal; Sec14-PH, Sec14 homologous domain and Pleckstrin Homology domain; CTD, C-terminal domain; SBR, Syndecan-Binding Region.
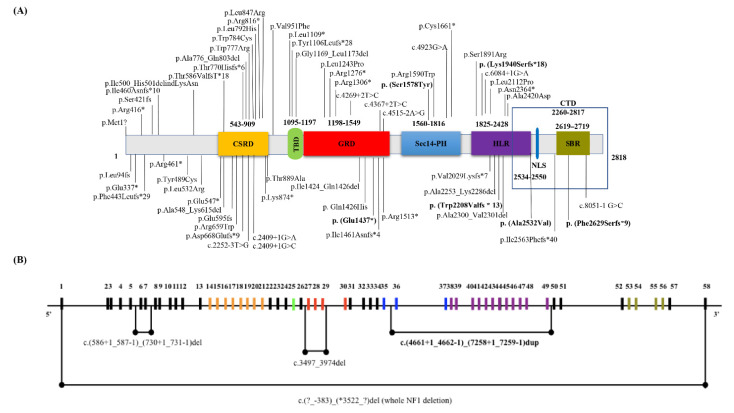
We present a total of 66 NF1 mutations, including missense, nonsense, start loss, frameshift, splicing, as well as indel mutations. The average depth, depth of the identified variants and minimum and maximum depth obtained by NGS analysis in each sample have been reported in the Supplementary Table S2 [37,38,105,106].
About 59 mutations (89%) have been previously described elsewhere and 7 are novel mutations (11%). The NF1 novel mutations are listed below: c.7884_7885del, p.(Phe2629Serfs*9) (NF_8, NF_72); c.4309G>T, p.(Glu1437*) (NF_52); c.4733C>A, p.(Ser1578Tyr) (NF_63); c.5819del, p.(Lys1940Serfs*18) (NF_81); c.6621dup, p.(Trp2208Valfs*13) (NF_85); c.7532C>T, p.(Ala2532Val) (NF_105); c.(4661+1_4662-1)_(7258+1_7259-1)dup (NF_89 and NF_90 related patients). The distribution of all NF1 mutations identified in our cohort is represented in Figure 1A. By in silico analysis 35 NF1 variants were predicted as pathogenic (53%), 4 as likely pathogenic (6%), 27 as VOUS (41%). 45 mutations were single base substitutions (68%), including 16 missense (36%), 11 nonsense (24%), 14 splicing (31%), 3 frameshift (7%), 1 start loss (2%). About 10 small base pairs deletions (15%) and 6 duplications (9%) as well as 1 indel mutations were identified. Whole-gene deletions and multi-exons NF1 duplications/deletions were detected: c.(4661+1_4662-1)_(7258+1_7259-1)dup, c.(586+1_587-1)_(730+1_731-1)del, and c.3497_3974del (Figure 1B).
Figure 1.
(A) Distribution of the identified mutations in the NF1 gene. Details of the 66 NF1 genetic mutations identified in our NF1 Italian cohort. The position of genetic variations detected in the NF1 from each NF1 patient is shown and their distribution in the NF1 domains is reported. Vertical lines show variant position. NF1 novel variants are shown in bold. Neurofibromin domains: CSRD, cysteine/serine-rich domain (543–909 residues); GRD, GAP related domain (1198–1549 residues); TBD, tubulin-binding domain (1095–1197 residues); HLR, HEAT-like repeat regions (1825–2428 residues); NLS, nuclear localization signal (2534–2550 residues); Sec14-PH, Sec14-homologous domain (1560–1816 residues) and Pleckstrin Homology domain (1716–1816 residues); CTD, C-terminal domain (2260–2817 residues); SBR, Syndecan-Binding Region (2619–2719 residues). (B) Map of the NF1 region indicating the large duplications and deletions sequences identified in our NF1 cohort. The black horizontal line represents the entire NF1 gene, while the vertical lines indicate the NF1 exons. Different colors were used for NF1 exons according to the relative functional domains as shown in (A).
Of the 85 NF1 patients of our cohort, 65 were sporadic and 20 had a positive family history. Results of NF1 segregation analysis in the relatives are the follow: c.3G>A, p.? in NF_2, 3, 4; c.1595T>G, p.Leu532Arg in NF_5, 29; c.3826C>T, p.Arg1276* in NF_10, 11; c.3496+1G>A, p.Tyr1106Leufs*28 in NF_23, 25; c.7686delG, p.Ile2563Phefs*40 in NF_31, 32; c.2307dup, p.Thr770Hisfs*6 in NF_45, 46, 47; c.2409+1G>C, p.? in NF_70, 71; c.4278G>C, p.Gln1426His in NF_80, 92; c.(4661+1_4662-1)_(7258+1_7259-1)dup in NF_89, NF_90. Six different NF1 mutations were shared in unrelated patients: c.1A>G, p.? in NF_28 and NF_48; c.7884_7885del, p.Phe2629Serfs*9 in NF_72 and NF_8; c.4537C>T, p.Arg1513* was common in NF_55 and NF_100; c.1381C>T, p.Arg461* in NF_57 and NF_102; c.4923G>A, p.Trp1641* in NF_73 and NF_26 patients. NF_19 shared the c.3826C>T, p.Arg1276* mutation with NF_10, 11 familial cases. Interestingly, two unrelated patients (NF_56, NF_79) carried two variants in the NF1.
3.2. Genotype-Phenotype Correlation Study
Genetic and clinical results of all 85 NF1 patients enrolled in this study are summarized in Table 1 and in the Supplementary Table S1, respectively. In detail, the 6% showed a mild phenotype and belong to the clinical group G1, with typical NF1 pigmentary manifestations, including CALMs and freckling as well as cutaneous and subcutaneous neurofibromas. The 94% of remaining patients presented at least one systemic complication, often in associations with other tumors, showing a more severe phenotype (G2, G3, G4, G5 clinical groups). Spine deformities, including scoliosis, kyphosis, and joint spondylosis, were the most common complications in our cohort (64/85 patients, 75%). The 83% of patients showed spinal complications. The most common mutations, associated to this clinical trait, were frameshift (15/64 patients, 23.4%) and nonsense (14/64 patients, 23%) variants, potentially resulting in truncated or absent neurofibromin. About 7% of NF1 patients (6/85 patients) presented bone abnormalities (including short stature, osteoporosis, osteopenia, non-ossifying and ossifying fibromas and foot deformities), mainly associated to mutations resulting in a truncated neurofibromin (66%).
In the 50% of NF1 cases showing bone anomalies the NF1 presented clearly pathogenic variants. 33% of patients manifested both spine and bone deformities (28/85 patients), showing a most severe skeletal phenotype. Nonsense NF1 mutations were the most common type of variants (34%) associated to this clinical combination (spine and bone alterations). Interestingly, skeletal complications were more common in females than males in our case series (spine deformities: 56% females; bone deformities: 67% females; both phenotypes: 64% females). About 21% of NF1 patients manifested spinal neurofibromas that were strongly associated with pain and scoliosis, with a high prevalence in males (67%).
In this study, cardiovascular anomalies associated to NF1 mutations (24/85 patients, 28%) included hypertension, aortic and mitral valves insufficiency, Moyamoya disease (MMS), aortic stenosis, and coarctation of the aorta. This phenotype was predominantly evident in patients carrying missense (20%) or nonsense (21%) NF1 mutations.
UBOs were one of the most frequent brain lesions associated to NF1 [46]. In our cohort 18% of patients (15/85 patients) were affected by UBOs with a higher prevalence in females (12 females, 80%). This clinical condition was associated to different types of variants: missense (27%), nonsense (27%), frameshift (20%), and splicing (7%) mutations.
Less common NF1 complications including obesity (5%, 4/85 patients) and hypercholesterolemia (3/85, 4%) were present in our patients.
PNFs affected 13% of NF1 patients (11/85 patients), 82% of which also presented cutaneous, subcutaneous, or spinal neurofibromas. About 55% (6/11) of these patients showed a more severe clinical phenotype associated to other malignancies (clinical groups G3, G4, G5).
3.3. NF1-Associated Malignancies and Other Complications
Malignancies and other non-neoplastic complications may worsen prognosis of NF1 patients.
Nervous system malignancies affected 21% (18/85) of our case series, all grouped in G4. Optic glioma and astrocytoma were the most common for this specific clinical condition (10/85 patients, 12%). About 80% of NF1 patients showing optic glioma or astrocytoma were female, carrying mainly frameshift mutations in the NF1 gene (4/8, 50%). Different types of organ tumors were diagnosed in 19 individuals (19/85 patients, 22%) belonging to G5 clinical group, including GIST (10%), breast (16%), and prostate (5%) cancers, as well as seminoma (5%) and lymphoma (5%). In two cases other malignancies such as ganglioneuroma (NF_37) and optic glioma (NF_65) were associated to NF1. Moreover, three patients of G5 clinical group showed malignant pheochromocytoma/paragangliomas associated to NF1 and carried three different nonsense mutations (p.Arg1276*, p.Trp1641*, and p.Asn2364*) in the NF1, distributed in different protein domains (Figure 1A). Interestingly, the p.Arg1276* mutation in the GAP-related domain (GRD) was already reported in one patient with NF1 and pheochromocytoma [77]. Furthermore, the same mutation was recently associated to cardiovascular abnormalities in NF1 disease [34]. Moreover, in our cohort the p.Arg1276* mutation was identified in the related NF_10 and NF_11 patients affected by cardiovascular abnormalities.
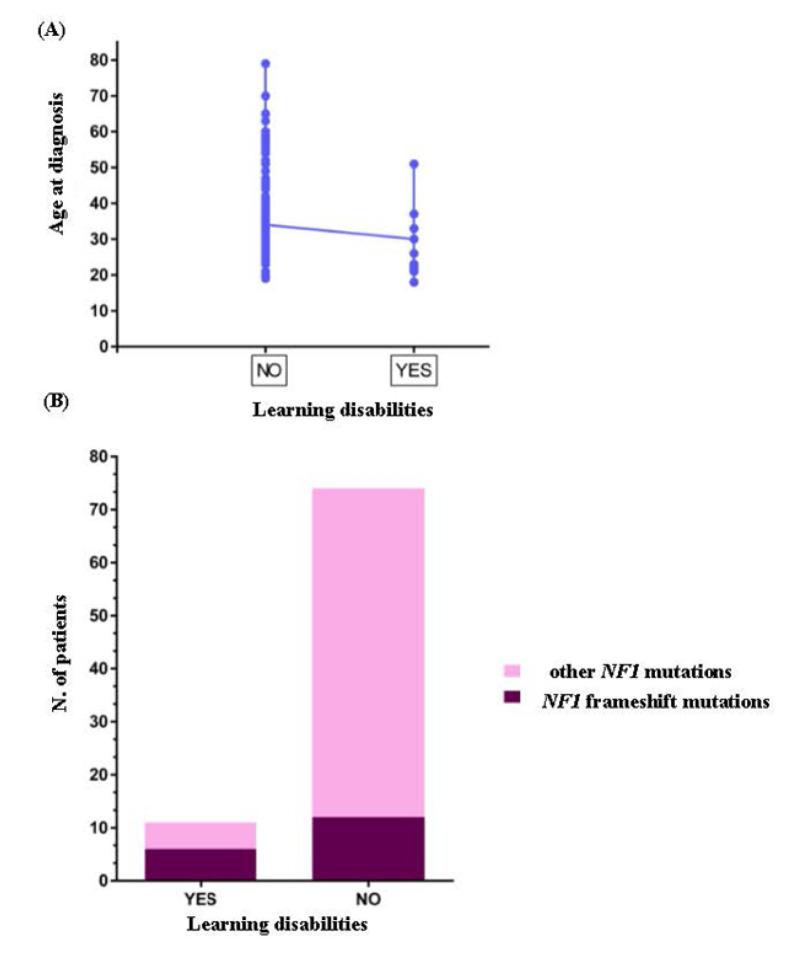
The clinical picture of G5 group was worsened to several systemic complications including spine (79%, 15/19 patients), bone (42%, 8/19 patients) deformities and cardiovascular defects (32%, 7/19 patients). Eight patients carried whole NF1 or large intergenic deletions/duplications associated to different systemic complications, including spine deformities (88%, 7/8 patients), cardiovascular (38%, 3/8 patients), and learning (25%, 2/8 patients) defects. In our cohort five different NF1 mutations were shared in unrelated patients. c.1A>G, p.? pathogenic mutation was common to NF_28 and NF_48 patients, both female with a similar age, belonging to the G2 clinical group. NF_72 and NF_8 carried the novel mutation c.7884_7885del, p.(Phe2629Serfs*9) located in the syndecan-binding region (SBR) of NF1. Both patients disclosed a severe clinical picture, which in the case of NF_8 the spinal form of NF1 was associated to learning disabilities and obesity, while in NF_72 the disease phenotype was complicated by 25-OH vitamin D deficiency as well as optic glioma and astrocytoma. NF_57 and NF_102 carried the pathogenic mutation c.1381C>T, p.Arg461* and disclosed a clinical phenotype mainly complicated by spine and bone deformities. The nonsense mutation c.4537C>T, p.Arg1513* was shared by NF_55 and NF_100 patients, while the c.3826C>T, p.Arg1276* mutation was shared by NF_19 and two familial cases (NF_10 and NF_11). Both mutations were linked to a complex and severe NF1 phenotype with multisystemic complications including neurological, cardiovascular, endocrine, skeletal, and neoplastic features. Interestingly, in our cohort two unrelated patients (NF_56, NF_79) showed two different variants in the NF1 gene. In detail, NF_56 carried the c.2409+1G>A, p.? pathogenetic splice site mutation and the c.2375T>A, p.Leu792His likely pathogenetic variant, while NF_79 presented the pathogenic mutation c.3916C>T, p.Arg1306*, and c.1975C>T, p.Arg659Trp classified as VOUS. Unfortunately, for these two patients segregation analysis was not performed as parents or other family members were not available, therefore we cannot disclose the cis or trans location of these mutations. Of interest, both patients disclosed skeletal anomalies as common complications, in association to cardiovascular and gastric disorders in patient NF_56 and tubular adenoma in patient NF_79. Moreover, in our case series a slight but significant inverse correlation between delayed acquisition of psychomotor skills with residual multi-domain cognitive impairment and age at the time of diagnosis (r = −0.35, p < 0.001) was observed (Figure 2A). Odds ratio analysis revealed that the probability of having learning disabilities was higher in patients carrying frameshift mutations (OR 6.2, CIs 1.626 to 23.64, p < 0.01) (Figure 2B). No other significant correlation between sex, age at diagnosis, clinical groups, single clinical features, and type of mutation was found.
Figure 2.
(A) Correlation analysis in the NF1 patients. Graph showing an inverse correlation between the presence of learning disabilities and age at diagnosis. (B) Odds ratio analysis. Odds ratio with 95% CI revealed a higher probability to have learning disabilities in NF1 patients carrying frameshift mutation in the NF1 gene.
4. Discussion
Diagnosis of NF1 is usually based on clinical findings according to NIH diagnostic criteria, nevertheless, owing to the extreme variability in clinical expression and age dependency of most clinical manifestations, molecular testing could represent a simple and effective strategy for early and differential diagnosis. Due to the high spectrum of genotypic and phenotypic heterogeneity few genotype–phenotype correlations have been previously reported [29,30,31,33,34,71]. In this study, we analyzed mutational spectrum of 85 clinically well-characterized familial/sporadic NF1 patients and statistically evaluated prevalence of specific clinical features associated to NF1 in the different NF1 mutation subtypes. For genotype–phenotype correlation study we have adopted a detailed internal clinical dissection, dividing the NF1 patients in five clinical groups according to disease phenotype. As this is a monocentric study, we reduced the potential bias in clinical evaluation between different centers. NF1 is characterized by complete age-dependent penetrance. The careful selection of the NF1 patients and the availability of the long follow-up, of at least 5 years for each patient, allowed us to have a full clinical view of the NF1 disease and to get insight the possible genotype–phenotype correlations of the NF1 mutations identified in our investigation.
Our findings showed that the 6% of study cohort disclosed a relatively mild phenotype with typical NF1 pigmentary manifestations (clinical group G1) and the remaining 94% disclosed a more severe phenotype, presented at least one systemic complication (clinical groups G2, G3, G4, G5), and of these the 47% were affected by other malignancies. In this study, we reported 66 different NF1 mutations, 7 of which were novel mutations. Mutations were distributed along the entire NF1, however exons 16 and 21, at least in our cohort of patients, appeared to be more mutation rich.
NF1 is a multisystem disorder associated to several clinical complications, including skeletal abnormalities. The spine deformities were manifested in the 75% of our case series and scoliosis was the most common musculoskeletal manifestation (50/85 patients), in line with past research [107,108]. In the 33% of patients, the skeletal phenotype was worsened by the concomitant expression of both spine and bone deformities. In our cohort, patients carrying deletion of the entire NF1 gene showed spine abnormalities, and the most common mutations associated to this clinical trait resulted in truncated neurofibromin. NF_40 patient, showing kyphoscoliosis, carried the same pathogenic mutation c.1466A>G, p.Tyr489Cys previously identified in four NF1 patients with scoliosis [42]. The 21% of NF1 patients manifested spinal neurofibromas that were strongly associated with pain and scoliosis. Learning disabilities, including visual perception, language, executive functions, attention, and motor skills, represent one of the most common and challenging complications of NF1 [109]. Previous research reported the association of facial dysmorphism and learning deficits with NF1 microdeletions [27].
Interestingly, our results demonstrated a higher frequency of cognitive disorder, including delayed acquisition of psychomotor skills and residual multi-domain cognitive impairment, in patients carrying NF1 frameshift mutations (OR 6.2, CIs 1.626 to 23.64, p < 0.01). To support these preliminary findings further investigations are needed. The involvement of the cardiovascular system in NF1 is strongly supported by several studies [110,111,112]. Cardiovascular disorders were detected in 28% of our cohort. Two NF1 patients, NF_10 affected by hypertension while NF_11 showed mitral and aortic insufficiency, carried the nonsense mutation, c.3826C>T, p.Arg1276* that was previously described to be correlated with a high prevalence of cardiovascular abnormalities [34].
NF1 and Cancer Implication
Individuals affected by NF1 present a higher risk to develop specific malignancies compared to the general population [113]. A wide range of benign and malignant tumors in both central and peripheral nervous systems, as well as other organ malignancies, has been described in association to NF1 [113].
In this study, nervous system malignancies and organ tumors affected respectively 21% and 22% of our cohort. PNFs represent an uncommon variant (5–15%) of NF1 [114]. According to scientific literature, in our series PNFs represented a rare clinical condition (13%; 11/85 patients). MPNSTs are the most common malignant tumors associated with NF1 condition, usually arising from PNFs, and represent the major cause of poor prognosis [11,115]. In our study, we described three NF1 patients, belonging to G3 clinical group, affected by MPNST. NF_18 presented lumbar spinal MPNST associated to c.3326T>G, p.Leu1109* mutation, located in the tubulin-binding domain (TBD) of NF1 protein. Recently, this mutation has been described in one NF1 patient showing mild phenotype, without MPNST [36]. NF_52 carried the novel c.4309G>T, p.(Glu1437*) mutation in the GRD and manifested MPNST of the left thigh, in combination to GIST. NF_127 was affected by a rare condition of intracranial MPNST linked to c.2540T>G, p.Leu847Arg missense mutation in the cysteine and serine-rich domain (CSRD). It was interesting that this mutation was described in nine NF1 patients with a very high number of neurofibromas, including two individuals with metastasized MPNSTs [30]. Optic glioma (OPG) and astrocytoma were the most NF1-associated nervous system malignancies. Recent evidence has suggested that the specific genotype may be the main determinant of the development of OPG, with the risk being higher in patients harboring NF1 mutations in the 5’ tertile (exon 1–21) and in the CSRD domain (residues 543–909), whereas mutations in the HEAT-like repeat regions (HLR, 1825–2428) were negatively associated with OPG [116,117,118]. We described ten NF1 patients showing OPG, two of which presented OPG and astrocytoma. In agreement to recent evidence, four patients (NF_26, 60, 76, 115) carried mutations in the 5’ tertile and in CSRD domain. Nevertheless, further investigation of larger case series of patients will be needed to confirm our preliminary evidence. Other malignancies detected in our case series include GIST, breast and prostate cancers, seminoma, lymphoma and malignant pheochromocytoma. In our cohort three different nonsense mutations c.3826C>T, p.Arg1276* in NF_19; c.4923G>A, p.Trp1641* in NF_73; c.7089dup, p.Asn2364* in NF_98 were associated to malignant pheochromocytoma.
It is intriguing that the p.Arg1276* mutation was associated to cancer development (our study and ref. [77]) and cardiovascular abnormalities (our study in NF_10, 11, and ref. [34]); possibly a different genetic background (e.g., variants in NF1-related genes) or non-genetic factors (e.g., environment, exposure to chemicals and hazardous substances, etc.) may explain the diverse clinical manifestations.
The NF_92 patient, carrying the c.4278G>C, p.Gln1426His missense mutation, showed GIST, lymphoma associated to malar facial PNF as well as spine deformities and aortic and mitral valves insufficiency. This mutation was previously reported associated to NF1 with pulmonary stenosis [78] and in two NF1 patients with mild phenotype [36]. Moreover, in our study five different NF1 mutations were shared in unrelated patients, showing different clinical manifestations.
Furthermore, findings of segregation analysis in the 20 familial cases of our case series confirmed the variability in clinical expression of NF1 within relatives carrying the same NF1 mutations. A typical case is the NF_29 patient, that, although sharing the same missense mutation c.1595T>G, p.Leu532Arg with the sibling NF_5, had a more severe phenotype worsened by epilepsy syndrome and seminoma (NF_5, G2; NF_29, G5).
On the other hand, the influence of variants in other genes on NF1 phenotype cannot be excluded. Several studies showed that different mutations of NF1 and related genetic modifiers might contribute together to clinical features in NF1, including tumor development, making the scenario more complex [119]. It will be very important to perform this analysis in our cohort of patients in the future.
Finally, a limitation of the NF1 cohort study we present may be related to the small size of each patient group (G1-G5). However, given the considerable clinical heterogeneity of NF1 patients, the inclusion of phenotypically homogeneous NF1 individuals in each group allowed a more appropriate genotype–phenotype analysis and more reliable conclusions. Undoubtedly, the observation of a larger cohort of NF1 patients will allow further considerations of clinical-genetic utility.
5. Conclusions
The cohort of NF1 patients presented here provides the opportunity to expand the spectrum of NF1 mutations and confirms that NF1 is a highly phenotypically heterogeneous disease with a wide range of effects on the CNS and consequent impairment of cognitive and educational functions. Indeed, some NF1 patients present with an uncomplicated picture, which is the most common case, but others may develop serious manifestations, such as tumors of different organs and apparatuses and of varying malignancy, which may complicate the clinical picture.
Studies looking at specific cognitive domains associated with NF1 have come to very different conclusions. In our cohort, we found a higher prevalence of learning disabilities in patients with NF1 frameshift mutations. Findings from several studies involving very young NF1 children confirm that developmental delays and subsequent academic difficulties and learning disabilities represent a major psychosocial burden during the life of NF1 patients. In addition, our study particularly highlights that cognitive aspects, such as the delay in the acquisition of psychomotor skills, unlike other symptoms, can be a warning sign, allowing for earlier diagnosis and a more effective therapeutic and rehabilitation approach to reduce residual cognitive impairment in multiple domains and the psychosocial impact of the disease. Hence, the perception of disease severity correlates with medical, behavioral, and cognitive severity scores, and the spectre of death from cancer or other complications has profound emotional, psychological, and social effects on all NF1 patients and their families. Therefore, for adequate genotype–phenotype analysis in NF1, better clinical management and appropriate genetic counseling we advocate a longitudinal, patient-centered model of care, with age-dependent monitoring of clinical manifestations aimed at early diagnosis and symptomatic treatment of emerging complications.
Overall, our study aims to contribute to a better definition of the genotype–phenotype correlation and may improve the management and genetic counseling of NF1 patients.
Acknowledgments
The authors would like to express their sincere thanks to all patients and family members, when involved, who donated samples and time for the advancement in clinical research and knowledge. Special thanks to Antonia Auletta for the help with the graphical abstract.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13071130/s1, Table S1: Demographics and phenotypic features according to clinical groups (G1-G5) in the NF1 cohort; Table S2: Sequencing techniques performed and NGS details.
Author Contributions
Conceptualization of the study, F.N., M.A.B.M.; writing—original draft preparation and figure preparation, F.N., M.D.; mutation analysis, G.P., C.S., D.C. and A.P.; statistical analysis, C.T.; methodology and data curation, G.F., M.T.G., S.P., P.D.B. and S.S. All authors contributed to the overall interpretation of the clinical and genetic results and to the manuscript revision. All authors have approved the manuscript and agree with its submission to Genes journal, Special Issue “Feature Papers in Human Genomics and Genetic Diseases”. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the 1964 Helsinki Declaration on Human Rights and its later amendments or comparable ethical standards in parallel with National and European legislation for Biomedical Research, and approved by the Medical Research Ethics Committee of the University of Campania “Luigi Vanvitelli”—University Hospital “Luigi Vanvitelli”, Naples Italy (Protocol Number 311, 20 May 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Due to the sensitive nature of the data, information created during and/or analysed during the current study is available from corresponding authors on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by several scientific projects. F.N. is financed by the Italian Ministry of Health—Starting Grant-Ricerca Finalizzata 2018 (Project code: SG-2018-12367471; “Novel and non-invasive diagnostic and prognostic biomarkers for Neurofibromatosis type 1 and related cancers: expression profiling of serum circulating miRNAs and patho-physiological implications”). M.A.B.M. and S.S. thank the Regione Campania (RIS 3—POR FESR 2007/2013—Obiettivo 2.1, DIP. 54-DG 91 n. 403, 15/10/2015), Inter-University Center for Research in Neurosciences and University of Campania “Luigi Vanvitelli” (project V:ALERE 2019 Id343-TRANSITION “Nutri-epigenetics and physical activity: a natural help for Neurofibromatosis type 1”). M.A.B.M. is financed by Italian Ministry of Economic Development (MiSE)—Fund for Sustainable Development—Call “HORIZON2020” PON I&C 2014–2020, FOR.TUNA project, code No. F/050347/01_03/X32. S.P. thanks the POR Campania FESR 2014–2020 “SATIN” grant.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williams V.C., Lucas J., Babcock M.A., Gutmann D.H., Korf B., Maria B.L. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123:124–133. doi: 10.1542/peds.2007-3204. [DOI] [PubMed] [Google Scholar]
- 2.Friedman J.M. Neurofibromatosis 1. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Mirzaa G., Amemiya A., editors. GeneReviews. University of Washington; Seattle, WA, USA: 2019. pp. 1993–2021. [Google Scholar]
- 3.Stumpf D., Alksne J., Annegers J., Brown S., Conneally P., Housman D. Neurofibromatosis: Conference statement: National Institutes of Health Consensus Development Conference. Arch. Neurol. 1988;45:575–578. [PubMed] [Google Scholar]
- 4.Legius E., Messiaen L., Wolkenstein P., Pancza P., Avery R.A., Berman Y., Blakeley J., Babovic-Vuksanovic D., Cunha K.S., Ferner R., et al. International Consensus Group on Neurofibromatosis Diagnostic Criteria (I-NF-DC), Huson SM, Evans DG, Plotkin SR. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: An international consensus recommendation. Genet Med. 2021;23:1506–1513. doi: 10.1038/s41436-021-01170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miraglia E., Moliterni E., Iacovino C., Roberti V., Laghi A., Moramarco A., Giustini S. Cutaneous manifestations in neurofibromatosis type 1. Clin. Ter. 2020;171:e371–e377. doi: 10.7417/CT.2020.2242. [DOI] [PubMed] [Google Scholar]
- 6.DeBella K., Szudek J., Friedman J.M. Use of the National Institutes of Health criteria for the diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000;105:608–614. doi: 10.1542/peds.105.3.608. [DOI] [PubMed] [Google Scholar]
- 7.Upadhyaya M. Neurofibromatosis type 1: Diagnosis and recent advances. Expert Opin. Med. Diagn. 2010;4:307–322. doi: 10.1517/17530059.2010.494660. [DOI] [PubMed] [Google Scholar]
- 8.Jett K., Friedman J.M. Clinical and genetic aspects of neurofibromatosis 1. Genet. Med. 2010;12:1–11. doi: 10.1097/GIM.0b013e3181bf15e3. [DOI] [PubMed] [Google Scholar]
- 9.Patil S., Chamberlain R.S. Neoplasms associated with germline and somatic NF1 gene mutations. Oncologist. 2012;17:101–116. doi: 10.1634/theoncologist.2010-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiuru M., Busam K.J. The NF1 gene in tumor syndromes and melanoma. Lab. Investig. 2017;97:146–157. doi: 10.1038/labinvest.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich R.E., Kluwe L., Fünsterer C., Mautner V.F. Malignant peripheral nerve sheath tumors (MPNST) in neurofibromatosis type 1 (NF1): Diagnostic findings on magnetic resonance images and mutation analysis of the NF1 gene. Anticancer Res. 2005;25:1699–1702. [PubMed] [Google Scholar]
- 12.Yohay K. Neurofibromatosis type 1 and associated malignancies. Curr. Neurol Neurosci. 2009;9:247–253. doi: 10.1007/s11910-009-0036-3. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum T., Wimmerm K. Neurofibromatosis type 1 (NF1) and associated tumors. Klin. Padiatr. 2014;226:309–315. doi: 10.1055/s-0034-1382021. [DOI] [PubMed] [Google Scholar]
- 14.Arif A.A., Kim P.T.W., Melck A., Churg A., Schwartz Z., Stuart H.C. Pancreatic Gastrinoma, Gastrointestinal Stromal Tumor (GIST), Pheochromocytoma, and Hürthle Cell Neoplasm in a Patient with Neurofibromatosis Type 1: A Case Report and Literature Review. Am. J. Case Rep. 2021;22:e927761. doi: 10.12659/AJCR.927761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huson S.M. The neurofibromatosis: Classification, Clinical Features and Genetic Counselling. In: Kaufmann D., editor. Neurofibromatoses. Volume 16. Karger Publishers; Basel, Switzerland: 2008. [DOI] [Google Scholar]
- 16.Upadhyaya M., Spurlock G., Monem B., Thomas N., Friedrich R.E., Kluwe L., Mautner V. Germline and somatic NF1 gene mutations in plexiform neurofibromas. Hum. Mutat. 2008;29:E103–E111. doi: 10.1002/humu.20793. [DOI] [PubMed] [Google Scholar]
- 17.Milla P.C., Rosales L.J.M., Montiel L.J., Garrido A.L.D., Linares S.C., Tamajón C.S., Fernández T.C., González S.P., Freire F.S., Lopez B.C., et al. Neurofibromatosis type I: Mutation spectrum of NF1 in spanish patients. Ann. Hum. Genet. 2018;82:425–436. doi: 10.1111/ahg.12272. [DOI] [PubMed] [Google Scholar]
- 18.Bollag G., McCormick F., Clark R. Characterization of full-length neurofibromin: Tubulin inhibits Ras GAP activity. EMBO J. 1993;12:1923–1927. doi: 10.1002/j.1460-2075.1993.tb05841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legius E., Marchuk D.A., Collins F.S., Glover T.W. Somatic deletion of the neurofibromatosis type 1 gene in a neurofibrosarcoma supports a tumour suppressor gene hypothesis. Nat. Genet. 1993;3:122–126. doi: 10.1038/ng0293-122. [DOI] [PubMed] [Google Scholar]
- 20.Denayer E., De Ravel T., Legius E. Clinical and molecular aspects of RAS related disorders. J. Med. Genet. 2008;45:695–703. doi: 10.1136/jmg.2007.055772. [DOI] [PubMed] [Google Scholar]
- 21.Mao B., Chen S., Chen X., Yu X., Zhai X., Yang T., Li L., Wang Z., Zhao X., Zhang X. Clinical characteristics and spectrum of NF1 mutations in 12 unrelated Chinese families with neurofibromatosis type 1. BMC Med. Genet. 2018;19:101. doi: 10.1186/s12881-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legius E., Brems H. Genetic basis of neurofibromatosis type 1 and related conditions, including mosaicism. Childs Nerv. Syst. 2020;36:2285–2295. doi: 10.1007/s00381-020-04771-8. [DOI] [PubMed] [Google Scholar]
- 23.Rahbari R., Wuster A., Lindsay S.J., Hardwick R.J., Alexandrov L.B., Turki S.A., Dominiczak A., Morris A., Porteous D., Smith B., et al. Timing, rates and spectra of human germline mutation. Nat. Genet. 2016;48:126–133. doi: 10.1038/ng.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esposito T., Piluso G., Saracino D., Uccello R., Schettino C., Dato C., Capaldo G., Giugliano T., Varriale B., Paolisso G., et al. A novel diagnostic method to detect truncated neurofibromin in neurofibromatosis 1. J. Neurochem. 2015;135:1123–1128. doi: 10.1111/jnc.13396. [DOI] [PubMed] [Google Scholar]
- 25.De Luca A., Bottillo I., Dasdia M.C., Morella A., Lanari V., Bernardini L., Divona L., Giustini S., Sinibaldi L., Novelli A., et al. Deletions of NF1 gene and exons detected by multiplex ligation-dependent probe amplification. J. Med. Genet. 2007;44:800–808. doi: 10.1136/jmg.2007.053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mautner V.F., Kluwe L., Friedrich R.E., Roehl A.C., Bammert S., Högel J., Spöri H., Cooper D.N., Kehrer-Sawatzki H. Clinical characterisation of 29 neurofibromatosis type-1 patients with molecularly ascertained 1.4 Mb type-1 NF1 deletions. J. Med. Genet. 2010;47:623–630. doi: 10.1136/jmg.2009.075937. [DOI] [PubMed] [Google Scholar]
- 27.Pasmant E., Sabbagh A., Spurlock G., Laurendeau I., Grillo E., Hamel M.J., Martin L., Barbarot S., Leheup B., Rodriguez D., et al. NF1 microdeletions in neurofibromatosis type 1: From genotype to phenotype. Hum. Mutat. 2010;31:E1506–E1518. doi: 10.1002/humu.21271. [DOI] [PubMed] [Google Scholar]
- 28.Kehrer-Sawatzki H., Vogt J., Mußotter T., Kluwe L., Cooper D.N., Mautner V.F. Dissecting the clinical phenotype associated with mosaic type-2 NF1 microdeletions. Neurogenetics. 2012;13:229–236. doi: 10.1007/s10048-012-0332-y. [DOI] [PubMed] [Google Scholar]
- 29.Upadhyaya M., Huson S.M., Davies M., Thomas N., Chuzhanova N., Giovannini S., Evans D.G., Howard E., Kerr B., Griffiths S., et al. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970-2972 delAAT): Evidence of a clinically significant NF1 genotype-phenotype correlation. Am. J. Hum. Genet. 2007;80:140–151. doi: 10.1086/510781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koczkowska M., Chen Y., Callens T., Gomes A., Sharp A., Johnson S., Hsiao M.C., Chen Z., Balasubramanian M., Barnett C.P., et al. Genotype-Phenotype Correlation in NF1: Evidence for a More Severe Phenotype Associated with Missense Mutations Affecting NF1 Codons 844-848. Am. J. Hum. Genet. 2018;102:69–87. doi: 10.1016/j.ajhg.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinna V., Lanari V., Daniele P., Consoli F., Agolini E., Margiotti K., Bottillo I., Torrente I., Bruselles A., Fusilli C., et al. p.Arg1809Cys substitution in neurofibromin is associated with a distinctive NF1 phenotype without neurofibromas. Eur. J. Hum. Genet. 2015;23:1068–1071. doi: 10.1038/ejhg.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rojnueangnit K., Xie J., Gomes A., Sharp A., Callens T., Chen Y., Liu Y., Cochran M., Abbott M.A., Atkin J., et al. High Incidence of Noonan Syndrome Features Including Short Stature and Pulmonic Stenosis in Patients carrying NF1 Missense Mutations Affecting p.Arg1809: Genotype-Phenotype Correlation. Hum. Mutat. 2015;36:1052–1063. doi: 10.1002/humu.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santoro C., Maietta A., Giugliano T., Melis D., Perrotta S., Nigro V., Piluso G. Arg(1809) substitution in neurofibromin: Further evidence of a genotype-phenotype correlation in neurofibromatosis type 1. Eur. J. Hum. Genet. 2015;23:1460–1461. doi: 10.1038/ejhg.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koczkowska M., Callens T., Chen Y., Gomes A., Hicks A.D., Sharp A., Johns E., Uhas K.A., Armstrong L., Bosanko K.A., et al. Clinical spectrum of individuals with pathogenic NF1 missense variants affecting p.Met1149, p.Arg1276, and p.Lys1423: Genotype-phenotype study in neurofibromatosis type 1. Hum. Mutat. 2020;41:299–315. doi: 10.1002/humu.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scala M., Schiavetti I., Madia F., Chelleri C., Piccolo G., Accogli A., Riva A., Salpietro V., Bocciardi R., Morcaldi G., et al. Genotype-Phenotype Correlations in Neurofibromatosis Type 1: A Single-Center Cohort Study. Cancers. 2021;13:1879. doi: 10.3390/cancers13081879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giugliano T., Santoro C., Torella A., Del Vecchio Blanco F., Grandone A., Onore M.E., Melone M., Straccia G., Melis D., Piccolo V., et al. Clinical and Genetic Findings in Children with Neurofibromatosis Type 1, Legius Syndrome, and Other Related Neurocutaneous Disorders. Genes. 2019;10:580. doi: 10.3390/genes10080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nykamp K., Anderson M., Powers M., Garcia J., Herrera B., Ho Y.Y., Kobayashi Y., Patil N., Thusberg J., Westbrook M., et al. Sherloc: A comprehensive refinement of the ACMG-AMP variant classification criteria. Genet. Med. 2017;19:1105–1117. doi: 10.1038/gim.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabbagh A., Pasmant E., Imbard A., Luscan A., Soares M., Blanché H., Laurendeau I., Ferkal S., Vidaud M., Pinson S., et al. NF1 molecular characterization and neurofibromatosis type I genotype-phenotype correlation: The French experience. Hum. Mutat. 2013;34:1510–1518. doi: 10.1002/humu.22392. [DOI] [PubMed] [Google Scholar]
- 40.Mattocks C., Baralle D., Tarpey P., French-Constant C., Bobrow M., Whittaker J. Automated comparative sequence analysis identifies mutations in 89% of NF1 patients and confirms a mutation cluster in exons 11-17 distinct from the GAP related domain. J. Med. Genet. 2004;41:e48. doi: 10.1136/jmg.2003.011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frayling I.M., Mautner V.F., Van Minkelen R., Kallionpaa R.A., Aktaş S., Baralle D., Ben-Shachar S., Callaway A., Cox H., Eccles D.M., et al. Breast cancer risk in neurofibromatosis type 1 is a function of the type of NF1 gene mutation: A new genotype-phenotype correlation. J. Med. Genet. 2019;56:209–219. doi: 10.1136/jmedgenet-2018-105599. [DOI] [PubMed] [Google Scholar]
- 42.Ars E., Kruyer H., Morell M., Pros E., Serra E., Ravella A., Estivill X., Lázaro C. Recurrent mutations in the NF1 gene are common among neurofibromatosis type 1 patients. J. Med. Genet. 2003;40:e82. doi: 10.1136/jmg.40.6.e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson P.N., Böddrich A., Peters H., Tinschert S., Buske A., Kaufmann D., Nürnberg P. Two recurrent nonsense mutations and a 4 bp deletion in a quasi-symmetric element in exon 37 of the NF1 gene. Hum. Genet. 1995;96:95–98. doi: 10.1007/BF00214193. [DOI] [PubMed] [Google Scholar]
- 44.Ponti G., Losi L., Martorana D., Priola M., Boni E., Pollio A., Neri T.M., Seidenari S. Clinico-pathological and biomolecular findings in Italian patients with multiple cutaneous neurofibromas. Hered. Cancer Clin. Pract. 2011;9:6. doi: 10.1186/1897-4287-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsiao M.C., Piotrowski A., Callens T., Fu C., Wimmer K., Claes K.B., Messiaen L. Decoding NF1 Intragenic Copy-Number Variations. Am. J. Hum. Genet. 2015;97:238–249. doi: 10.1016/j.ajhg.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heim R.A., Kam-Morgan L.N., Binnie C.G., Corns D.D., Cayouette M.C., Farber R.A., Aylsworth A.S., Silverman L.M., Luce M.C. Distribution of 13 truncating mutations in the neurofibromatosis 1 gene. Hum. Mol. Genet. 1995;4:975–981. doi: 10.1093/hmg/4.6.975. [DOI] [PubMed] [Google Scholar]
- 47.Kehrer-Sawatzki H., Kluwe L., Sandig C., Kohn M., Wimmer K., Krammer U., Peyrl A., Jenne D.E., Hansmann I., Mautner V.F. High frequency of mosaicism among patients with neurofibromatosis type 1 (NF1) with microdeletions caused by somatic recombination of the JJAZ1 gene. Am. J. Hum. Genet. 2004;75:410–423. doi: 10.1086/423624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantripragada K.K., Thuresson A.C., Piotrowski A., Díaz de Ståhl T., Menzel U., Grigelionis G., Ferner R.E., Griffiths S., Bolund L., Mautner V., et al. Identification of novel deletion breakpoints bordered by segmental duplications in the NF1 locus using high resolution array-CGH. J. Med. Genet. 2006;43:28–38. doi: 10.1136/jmg.2005.033795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogt J., Mussotter T., Bengesser K., Claes K., Högel J., Chuzhanova N., Fu C., van den Ende J., Mautner V.F., Cooper D.N., et al. Identification of recurrent type-2 NF1 microdeletions reveals a mitotic nonallelic homologous recombination hotspot underlying a human genomic disorder. Hum. Mutat. 2012;33:1599–1609. doi: 10.1002/humu.22171. [DOI] [PubMed] [Google Scholar]
- 50.Roehl A.C., Mussotter T., Cooper D.N., Kluwe L., Wimmer K., Högel J., Zetzmann M., Vogt J., Mautner V.F., Kehrer-Sawatzki H. Tissue-specific differences in the proportion of mosaic large NF1 deletions are suggestive of a selective growth advantage of hematopoietic del(+/−) stem cells. Hum. Mutat. 2012;33:541–550. doi: 10.1002/humu.22013. [DOI] [PubMed] [Google Scholar]
- 51.Summerer A., Mautner V.F., Upadhyaya M., Claes K.B.M., Högel J., Cooper D.N., Messiaen L., Kehrer-Sawatzki H. Extreme clustering of type-1 NF1 deletion breakpoints co-locating with G-quadruplex forming sequences. Hum. Genet. 2018;137:511–520. doi: 10.1007/s00439-018-1904-1. [DOI] [PubMed] [Google Scholar]
- 52.Brinckmann A., Mischung C., Bässmann I., Kühnisch J., Schuelke M., Tinschert S., Nürnberg P. Detection of novel NF1 mutations and rapid mutation prescreening with Pyrosequencing. Electrophoresis. 2007;28:4295–4301. doi: 10.1002/elps.200700118. [DOI] [PubMed] [Google Scholar]
- 53.Ko J.M., Sohn Y.B., Jeong S.Y., Kim H.J., Messiaen L.M. Mutation spectrum of NF1 and clinical characteristics in 78 Korean patients with neurofibromatosis type 1. Pediatr. Neurol. 2013;48:447–453. doi: 10.1016/j.pediatrneurol.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Tsipi M., Poulou M., Fylaktou I., Kosma K., Tsoutsou E., Pons M.R., Kokkinou E., Kitsiou-Tzeli S., Fryssira H., Tzetis M. Phenotypic expression of a spectrum of Neurofibromatosis Type 1 (NF1) mutations identified through NGS and MLPA. J. Neurol. Sci. 2018;395:95–105. doi: 10.1016/j.jns.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Evans D.G., Bowers N., Burkitt-Wright E., Miles E., Garg S., Scott-Kitching V., Penman-Splitt M., Dobbie A., Howard E., Ealing J., et al. Comprehensive RNA Analysis of the NF1 Gene in Classically Affected NF1 Affected Individuals Meeting NIH Criteria has High Sensitivity and Mutation Negative Testing is Reassuring in Isolated Cases with Pigmentary Features Only. EBioMedicine. 2016;7:212–220. doi: 10.1016/j.ebiom.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Upadhyaya M., Maynard J., Osborn M., Harper P.S. Six novel mutations in the the neurofibromatosis type 1 (NF1) gene. Hum. Mutat. 1997;10:248–250. doi: 10.1002/(SICI)1098-1004(1997)10:3<248::AID-HUMU14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 57.Welti S., Kühn S., D’Angelo I., Brügger B., Kaufmann D., Scheffzek K. Structural and biochemical consequences of NF1 associated nontruncating mutations in the Sec14-PH module of neurofibromin. Hum. Mutat. 2011;32:191–197. doi: 10.1002/humu.21405. [DOI] [PubMed] [Google Scholar]
- 58.Stella A., Lastella P., Loconte D.C., Bukvic N., Varvara D., Patruno M., Bagnulo R., Lovaglio R., Bartolomeo N., Serio G., et al. Accurate Classification of NF1 Gene Variants in 84 Italian Patients with Neurofibromatosis Type 1. Genes. 2018;9:216. doi: 10.3390/genes9040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrea C., Vaessen S., Bulk S., Harvengt J., Misson J.P. Phenotype-Genotype Correlation in Children with Neurofibromatosis Type 1. Neuropediatrics. 2018;49:180–184. doi: 10.1055/s-0037-1620239. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J., Tong H., Fu X., Zhang Y., Liu J., Cheng R., Liang J., Peng J., Sun Z., Liu H., et al. Molecular Characterization of NF1 and Neurofibromatosis Type 1 Genotype-Phenotype Correlations in a Chinese Population. Sci. Rep. 2015;5:11291. doi: 10.1038/srep11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Messiaen L.M., Callens T., Mortier G., Beysen D., Vandenbroucke I., Van Roy N., Speleman F., Paepe A.D. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum. Mutat. 2000;15:541–555. doi: 10.1002/1098-1004(200006)15:6<541::AID-HUMU6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 62.Laycock-van Spyk S., Thomas N., Cooper D.N., Upadhyaya M. Neurofibromatosis type 1-associated tumours: Their somatic mutational spectrum and pathogenesis. Hum. Genomics. 2011;5:623–690. doi: 10.1186/1479-7364-5-6-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao R., Yu T., Xu Y., Yu L., Wang J., Wang X., Wang J., Shen Y. Clinical Presentation and Novel Pathogenic Variants among 68 Chinese Neurofibromatosis 1 Children. Genes. 2019;10:847. doi: 10.3390/genes10110847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griffiths S., Thompson P., Frayling I., Upadhyaya M. Molecular diagnosis of neurofibromatosis type 1: 2 years experience. Fam. Cancer. 2007;6:21–34. doi: 10.1007/s10689-006-9001-3. [DOI] [PubMed] [Google Scholar]
- 65.Han D., Spengler B.A., Ross R.A. Increased wild-type N-ras activation by neurofibromin down-regulation increases human neuroblastoma stem cell malignancy. Genes Cancer. 2011;2:1034–1043. doi: 10.1177/1947601912443127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bottillo I., Torrente I., Lanari V., Pinna V., Giustini S., Divona L., De Luca A., Dallapiccola B. Germline mosaicism in neurofibromatosis type 1 due to a paternally derived multi-exon deletion. Am. J. Med. Genet. A. 2010;152A:1467–1473. doi: 10.1002/ajmg.a.33386. [DOI] [PubMed] [Google Scholar]
- 67.Imbard A., Pasmant E., Sabbagh A., Luscan A., Soares M., Goussard P., Blanché H., Laurendeau I., Ferkal S., Vidaud M., et al. NF1 single and multi-exons copy number variations in neurofibromatosis type 1. J. Hum. Genet. 2015;60:221–224. doi: 10.1038/jhg.2015.6. [DOI] [PubMed] [Google Scholar]
- 68.Pasmant E., Parfait B., Luscan A., Goussard P., Briand-Suleau A., Laurendeau I., Fouveaut C., Leroy C., Montadert A., Wolkenstein P., et al. Neurofibromatosis type 1 molecular diagnosis: What can NGS do for you when you have a large gene with loss of function mutations? Eur. J. Hum. Genet. 2015;23:596–601. doi: 10.1038/ejhg.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee M.J., Su Y.N., You H.L., Chiou S.C., Lin L.C., Yang C.C., Lee W.C., Hwu W.L., Hsieh F.J., Stephenson D.A., et al. Identification of forty-five novel and twenty-three known NF1 mutations in Chinese patients with neurofibromatosis type 1. Hum. Mutat. 2006;27:832. doi: 10.1002/humu.9446. [DOI] [PubMed] [Google Scholar]
- 70.Fahsold R., Hoffmeyer S., Mischung C., Gille C., Ehlers C., Kücükceylan N., Abdel-Nour M., Gewies A., Peters H., Kaufmann D., et al. Minor lesion mutational spectrum of the entire NF1 gene does not explain its high mutability but points to a functional domain upstream of the GAP-related domain. Am. J. Hum. Genet. 2000;66:790–818. doi: 10.1086/302809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Luca A., Schirinzi A., Buccino A., Bottillo I., Sinibaldi L., Torrente I., Ciavarella A., Dottorini T., Porciello R., Giustini S., et al. Novel and recurrent mutations in the NF1 gene in Italian patients with neurofibromatosis type 1. Hum. Mutat. 2004;23:629. doi: 10.1002/humu.9245. [DOI] [PubMed] [Google Scholar]
- 72.Stewart H., Bowker C., Edees S., Smalley S., Crocker M., Mechan D., Forrester N., Spurlock G., Upadhyaya M. Congenital disseminated neurofibromatosis type 1: A clinical and molecular case report. Am. J. Med. Genet. A. 2008;146A:1444–1452. doi: 10.1002/ajmg.a.32305. [DOI] [PubMed] [Google Scholar]
- 73.Raygada M., Arthur D.C., Wayne A.S., Rennert O.M., Toretsky J.A., Stratakis C.A. Juvenile xanthogranuloma in a child with previously unsuspected neurofibromatosis type 1 and juvenile myelomonocytic leukemia. Pediatr. Blood Cancer. 2010;54:173–175. doi: 10.1002/pbc.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jang M.A., Kim Y.E., Kim S.K., Lee M.K., Kim J.W., Ki C.S. Identification and characterization of NF1 splicing mutations in Korean patients with neurofibromatosis type 1. J. Hum. Genet. 2016;61:705–709. doi: 10.1038/jhg.2016.33. [DOI] [PubMed] [Google Scholar]
- 75.Eisenbarth I., Beyer K., Krone W., Assum G. Toward a survey of somatic mutation of the NF1 gene in benign neurofibromas of patients with neurofibromatosis type 1. Am. J. Hum. Genet. 2000;66:393–401. doi: 10.1086/302747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maertens O., Brems H., Vandesompele J., De Raedt T., Heyns I., Rosenbaum T., De Schepper S., De Paepe A., Mortier G., Janssens S., et al. Comprehensive NF1 screening on cultured Schwann cells from neurofibromas. Hum. Mutat. 2006;27:1030–1040. doi: 10.1002/humu.20389. [DOI] [PubMed] [Google Scholar]
- 77.Bausch B., Borozdin W., Mautner V.F., Hoffmann M.M., Boehm D., Robledo M., Cascon A., Harenberg T., Schiavi F., Pawlu C., et al. Germline NF1 mutational spectra and loss-of-heterozygosity analyses in patients with pheochromocytoma and neurofibromatosis type 1. J. Clin. Endocrinol. Metab. 2007;92:2784–2792. doi: 10.1210/jc.2006-2833. [DOI] [PubMed] [Google Scholar]
- 78.Ben-Shachar S., Constantini S., Hallevi H., Sach E.K., Upadhyaya M., Evans G.D., Huson S.M. Increased rate of missense/in-frame mutations in individuals with NF1-related pulmonary stenosis: A novel genotype-phenotype correlation. Eur. J. Hum. Genet. 2013;21:535–539. doi: 10.1038/ejhg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wimmer K., Schamschula E., Wernstedt A., Traunfellner P., Amberger A., Zschocke J., Kroisel P., Chen Y., Callens T., Messiaen L. AG-exclusion zone revisited: Lessons to learn from 91 intronic NF1 3’ splice site mutations outside the canonical AG-dinucleotides. Hum. Mutat. 2020;41:1145–1156. doi: 10.1002/humu.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Side L., Taylor B., Cayouette M., Conner E., Thompson P., Luce M., Shannon K. Homozygous inactivation of the NF1 gene in bone marrow cells from children with neurofibromatosis type 1 and malignant myeloid disorders. N. Engl. J. Med. 1997;336:1713–1720. doi: 10.1056/NEJM199706123362404. [DOI] [PubMed] [Google Scholar]
- 81.Calì F., Chiavetta V., Ruggeri G., Piccione M., Selicorni A., Palazzo D., Bonsignore M., Cereda A., Elia M., Failla P., et al. Mutation spectrum of NF1 gene in Italian patients with neurofibromatosis type 1 using Ion Torrent PGM™ platform. Eur. J. Med. Genet. 2017;60:93–99. doi: 10.1016/j.ejmg.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Marwaha A., Malach J., Shugar A., Hedges S., Weinstein M., Parkin P.C., Pope E., Lara-Corrales I., Kannu P. Genotype-phenotype data from a case series of patients with mosaic neurofibromatosis type 1. Br. J. Dermatol. 2018;179:1216–1217. doi: 10.1111/bjd.16929. [DOI] [PubMed] [Google Scholar]
- 83.Chai P., Luo Y., Zhou C., Wang Y., Fan X., Jia R. Clinical characteristics and mutation Spectrum of NF1 in 12 Chinese families with orbital/periorbital plexiform Neurofibromatosis type 1. BMC Med. Genet. 2019;20:158. doi: 10.1186/s12881-019-0877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu G., Zheng Y., Liu Y., Yan A., Hu Z., Yang Y., Xiang S., Li L., Chen W., Peng Y., et al. Identification and characterization of NF1 and non-NF1 congenital pseudarthrosis of the tibia based on germline NF1 variants: Genetic and clinical analysis of 75 patients. Orphanet. J. Rare Dis. 2019;14:221. doi: 10.1186/s13023-019-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Luca A., Buccino A., Gianni D., Mangino M., Giustini S., Richetta A., Divona L., Calvieri S., Mingarelli R., Dallapiccola B., et al. NF1 gene analysis based on DHPLC. Hum. Mutat. 2003;21:171–172. doi: 10.1002/humu.9111. [DOI] [PubMed] [Google Scholar]
- 86.Maruoka R., Takenouchi T., Torii C., Shimizu A., Misu K., Higasa K., Matsuda F., Ota A., Tanito K., Kuramochi A., et al. The use of next-generation sequencing in molecular diagnosis of neurofibromatosis type 1: A validation study. Genet. Test. Mol. Biomarkers. 2014;18:722–735. doi: 10.1089/gtmb.2014.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ben-Salem S., Al-Shamsi A.M., Ali B.R., Al-Gazali L. The mutational spectrum of the NF1 gene in neurofibromatosis type I patients from UAE. Childs Nerv. Syst. 2014;30:1183–1189. doi: 10.1007/s00381-013-2352-9. [DOI] [PubMed] [Google Scholar]
- 88.Cunha K.S., Oliveira N.S., Fausto A.K., De Souza C.C., Gros A., Bandres T., Idrissi Y., Merlio J.P., de Moura Neto R.S., Silva R., et al. Hybridization Capture-Based Next-Generation Sequencing to Evaluate Coding Sequence and Deep Intronic Mutations in the NF1 Gene. Genes. 2016;7:133. doi: 10.3390/genes7120133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nemethova M., Bolcekova A., Ilencikova D., Durovcikova D., Hlinkova K., Hlavata A., Kovacs L., Kadasi L., Zatkova A. Thirty-nine novel neurofibromatosis 1 (NF1) gene mutations identified in Slovak patients. Ann. Hum. Genet. 2013;77:364–379. doi: 10.1111/ahg.12026. [DOI] [PubMed] [Google Scholar]
- 90.Osborn M.J., Upadhyaya M. Evaluation of the protein truncation test and mutation detection in the NF1 gene: Mutational analysis of 15 known and 40 unknown mutations. Hum. Genet. 1999;105:327–332. doi: 10.1007/s004399900135. [DOI] [PubMed] [Google Scholar]
- 91.Park V.M., Pivnick E.K. Neurofibromatosis type 1 (NF1): A protein truncation assay yielding identification of mutations in 73% of patients. J. Med. Genet. 1998;35:813–820. doi: 10.1136/jmg.35.10.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wimmer K., Roca X., Beiglböck H., Callens T., Etzler J., Rao A.R., Krainer A.R., Fonatsch C., Messiaen L. Extensive in silico analysis of NF1 splicing defects uncovers determinants for splicing outcome upon 5’ splice-site disruption. Hum. Mutat. 2007;28:599–612. doi: 10.1002/humu.20493. [DOI] [PubMed] [Google Scholar]
- 93.Anastasaki C., Woo A.S., Messiaen L.M., Gutmann D.H. Elucidating the impact of neurofibromatosis-1 germline mutations on neurofibromin function and dopamine-based learning. Hum. Mol. Genet. 2015;24:3518–3528. doi: 10.1093/hmg/ddv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yap P., Super L., Qin J., Burgess T., Prodanovic Z., Edwards C., Thomas R., Carpenter K., Tan T.Y. Congenital Retroperitoneal Teratoma in Neurofibromatosis Type 1. Pediatr. Blood Cancer. 2016;63:706–708. doi: 10.1002/pbc.25812. [DOI] [PubMed] [Google Scholar]
- 95.Pemov A., Li H., Patidar R., Hansen N.F., Sindiri S., Hartley S.W., Wei J.S., Elkahloun A., Chandrasekharappa S.C., Boland J.F., et al. The primacy of NF1 loss as the driver of tumorigenesis in neurofibromatosis type 1-associated plexiform neurofibromas. Oncogene. 2017;36:3168–3177. doi: 10.1038/onc.2016.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Corsello G., Antona V., Serra G., Zara F., Giambrone C., Lagalla L., Piccione M., Piro E. Clinical and molecular characterization of 112 single-center patients with Neurofibromatosis type 1. Ital. J. Pediatr. 2018;44:45. doi: 10.1186/s13052-018-0483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bianco G., Greco G., Antonelli M., Casali S., Castagnini C. An histologically atypical NF-type 1 patient with a new pathogenic mutation. Neurol. Sci. 2012;33:1483–1485. doi: 10.1007/s10072-011-0897-5. [DOI] [PubMed] [Google Scholar]
- 98.Momozawa Y., Iwasaki Y., Parsons M.T., Kamatani Y., Takahashi A., Tamura C., Katagiri T., Yoshida T., Nakamura S., Sugano K., et al. Germline pathogenic variants of 11 breast cancer genes in 7051 Japanese patients and 11,241 controls. Nat. Commun. 2018;9:4083. doi: 10.1038/s41467-018-06581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Micaglio E., Monasky M.M., Ciconte G., Vicedomini G., Conti M., Mecarocci V., Giannelli L., Giordano F., Pollina A., Saviano M., et al. SCN5A Nonsense Mutation and NF1 Frameshift Mutation in a Family with Brugada Syndrome and Neurofibromatosis. Front. Genet. 2019;10:50. doi: 10.3389/fgene.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cai Y., Fan Z., Liu Q., Li J., Du J., Shen Y., Wang S. Two novel mutations of the NF1 gene in Chinese Han families with type 1 neurofibromatosis. J. Dermatol. Sci. 2005;39:125–127. doi: 10.1016/j.jdermsci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 101.Anastasaki C., Morris S.M., Gao F., Gutmann D.H. Children with 5’-end NF1 gene mutations are more likely to have glioma. Neurol. Genet. 2017;3:e192. doi: 10.1212/NXG.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen L., Xue F., Xu J., He J., Fu W., Zhang Z., Kang Q. Five novel NF1 gene pathogenic variants in 10 different Chinese families with neurofibromatosis type 1. Mol. Genet. Genom. Med. 2019;7:9. doi: 10.1002/mgg3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santoro C., Di Rocco F., Kossorotoff M., Zerah M., Boddaert N., Calmon R., Vidaud D., Cirillo M., Cinalli G., Mirone G., et al. Moyamoya syndrome in children with neurofibromatosis type 1: Italian-French experience. Am. J. Med. Genet. A. 2017;173:1521–1530. doi: 10.1002/ajmg.a.38212. [DOI] [PubMed] [Google Scholar]
- 104.Santoro C., Giugliano T., Kraemer M., Torella A., Schwitalla J.C., Cirillo M., Melis D., Berlit P., Nigro V., Perrotta S., et al. Whole exome sequencing identifies MRVI1 as a susceptibility gene for moyamoya syndrome in neurofibromatosis type 1. PLoS ONE. 2018;13:e0200446. doi: 10.1371/journal.pone.0200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rehm H.L., Bale S.J., Bayrak-Toydemir P., Berg J.S., Brown K.K., Deignan J.L., Friez M.J., Funke B.H., Hegde M.R., Lyon E. Working Group of the American College of Medical Genetics and Genomics Laboratory Quality Assurance Commitee. ACMG clinical laboratory standards for next-generation sequencing. Genet. Med. 2013;15:733–747. doi: 10.1038/gim.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Green R.C., Berg J.S., Grody W.W., Kalia S.S., Korf B.R., Martin C.L., McGuire A.L., Nussbaum R.L., O’Daniel J.M., Ormond K.E., et al. American College of Medical Genetics and Genomics. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet. Med. 2013;15:565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsirikos A.I., Saifuddin A., Noordeen M.H. Spinal deformity in neurofibromatosis type-1: Diagnosis and treatment. Eur. Spine J. 2005;14:427–439. doi: 10.1007/s00586-004-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shah S., George K.J. The association of spinal deformity with dural ectasia in neurofibromatosis type 1. Br. J. Neurosurg. 2019;33:620–623. doi: 10.1080/02688697.2019.1673313. [DOI] [PubMed] [Google Scholar]
- 109.Hyman S.L., Shores A., North K.N. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- 110.Lin A.E., Birch P.H., Korf B.R., Tenconi R., Niimura M., Poyhonen M., Armfield Uhas K., Sigorini M., Virdis R., Romano C., et al. Cardiovascular malformations and other cardiovascular abnormalities in neurofibromatosis 1. Am. J. Med. Genet. 2000;95:108–117. doi: 10.1002/1096-8628(20001113)95:2<108::AID-AJMG4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 111.Lama G., Graziano L., Calabrese E., Grassia C., Rambaldi P.F., Cioce F., Tedesco M.A., Di Salvo G., Esposito-Salsano M. Blood pressure and cardiovascular involvement in children with neurofibromatosis type1. Pediatr. Nephrol. 2004;19:413–418. doi: 10.1007/s00467-003-1397-5. [DOI] [PubMed] [Google Scholar]
- 112.İncecik F., Hergüner Ö.M., Alınç Erdem S., Altunbaşak Ş. Neurofibromatosis type 1 and cardiac manifestations. Turk. Kardiyol. Dern Ars. 2015;43:714–716. doi: 10.5543/tkda.2015.27557. [DOI] [PubMed] [Google Scholar]
- 113.Uusitalo E., Rantanen M., Kallionpää R.A., Pöyhönen M., Leppävirta J., Ylä-Outinen H., Riccardi V.M., Pukkala E., Pitkäniemi J., Peltonen S., et al. Distinctive Cancer Associations in Patients with Neurofibromatosis Type 1. J. Clin. Oncol. 2016;34:1978–1986. doi: 10.1200/JCO.2015.65.3576. [DOI] [PubMed] [Google Scholar]
- 114.Poswal P., Bhutani N., Arora S., Kumar R. Plexiform neurofibroma with neurofibromatosis type I/ von Recklinghausen’s disease: A rare case report. Ann. Med. Surg. 2020;57:346–350. doi: 10.1016/j.amsu.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCaughan J.A., Holloway S.M., Davidson R., Lam W.W. Further evidence of the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J. Med. Genet. 2007;44:463–466. doi: 10.1136/jmg.2006.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu M., Xiong H., Han Y., Li C., Mai S., Huang Z., Ai X., Guo Z., Zeng F., Guo Q. Identification of Mutation Regions on NF1 Responsible for High- and Low-Risk Development of Optic Pathway Glioma in Neurofibromatosis Type I. Front. Genet. 2018;9:270. doi: 10.3389/fgene.2018.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anastasaki C., Gao F., Gutmann D.H. Commentary: Identification of Mutation Regions on NF1 Responsible for High- and Low-Risk Development of Optic Pathway Glioma in Neurofibromatosis Type I. Front. Genet. 2019;10:115. doi: 10.3389/fgene.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Melloni G., Eoli M., Cesaretti C., Bianchessi D., Ibba M.C., Esposito S., Scuvera G., Morcaldi G., Micheli R., Piozzi E., et al. Risk of Optic Pathway Glioma in Neurofibromatosis Type 1: No Evidence of Genotype-Phenotype Correlations in A Large Independent Cohort. Cancers. 2019;11:1838. doi: 10.3390/cancers11121838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang W., Wei C.J., Cui X.W., Li Y.H., Gu Y.H., Gu B., Li Q.F., Wang Z.C. Impacts of NF1 Gene Mutations and Genetic Modifiers in Neurofibromatosis Type 1. Front. Neurol. 2021;12:704639. doi: 10.3389/fneur.2021.704639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the sensitive nature of the data, information created during and/or analysed during the current study is available from corresponding authors on reasonable request.