Abstract
Objective
To describe the trends of incidence and mortality of cervical cancer in different age groups and regions from 1990 to 2019.
Design
An international comparative study based on the Global Burden of Disease (GBD) study estimates.
Participants
Data were publicly available and individuals were not involved.
Methods
We collected detailed information on cervical cancer from the GBD study between 1990 and 2019. Average annual percentage changes (AAPCs) of age-standardised incidence and mortality rate (ASIR and ASMR) in cervical cancer, by age group and region, were calculated to quantify the temporal trends.
Results
Globally, the absolute numbers of incident cases and deaths were increasing, with the most cervical cancer cases and deaths being reported in China, India and Brazil. Although the ASIR and ASMR have declined overall from 1990 to 2019, an increasing or stable trend was also observed in East Asia and Southern sub-Saharan Africa. Particularly, we found that the age-specific AAPC of incidence showed an increasing trend in the age group of 15–49 years globally, and the high Sociodemographic Index region increased the most.
Conclusions
Cervical cancer remains a concerning disease that affects women all over the world, although the ASIR and ASMR are decreasing. Efforts to control the younger trend and to reduce the disparity between regions are imminent.
Keywords: epidemiology, health policy, public health
Strengths and limitations of this study.
This study is the first comprehensive overview of the patterns and temporal trends in the age-specific incidence and mortality of cervical cancer at the global, regional and national levels.
This study reminds the policymakers to pay more attention to the increasing incidence of cervical cancer in young women (15–49 years).
Although the Global Burden of Disease estimates are comprehensive and most refined, the quality of the data source needs to be further improved.
Introduction
Cervical cancer is the fourth most common cancer among women, which is caused by the formation of malignant cells in the tissues of the cervix.1 Persistent infection with certain types of human papillomavirus (HPV) could result in precancerous cervical lesions as well as invasive cervical cancer.2 Other associated factors include smoking, early age of sexual debut, oral hormonal contraception and multiple sexual partners.3 As one of the most preventable cancers, effective primary (HPV vaccination) and secondary prevention methods (screening and treatment of precancerous cervical lesions) will prevent or reduce the development of cervical cancer.4 Moreover, in 2020, WHO launched an ambitious call to all countries in the world to mobilise resources to accelerate the elimination of cervical cancer as a public health concern.5
Although global, regional and national efforts have been undertaken to eradicate cervical cancer, it remains a major public health problem facing the world, especially in low-income and middle-income countries (LMICs). In these countries, organised screening and HPV vaccination programmes are always inadequate due to the high cost of implementing and maintaining such programmes.6 In China, more cases of cervical cancer are diagnosed annually than in any other country, accounting for around 20% of all estimated cervical cancers diagnosed worldwide in 2018.7 Although the incidence trends have declined in urban areas, India alone accounts for one-quarter of the burden of cervical cancer in the world.8 However, high-quality cytology screening may not be implemented on a large scale due to the lack of required infrastructure. Furthermore, with an increased risk of cervical cancer in young women, we also need to arouse our vigilance.9
In order to provide information for policy development and to assess the effects of interventions, more knowledge on the trends of cervical cancer incidence and mortality in different age groups based on robust and multinational data will be necessary. In this study, we evaluated cervical cancer incidence, mortality and their geographical patterns and temporal trends based on incident cases and deaths, by age group for 204 countries and territories from 1990 to 2019. Additionally, the joinpoint regression analysis was used to detect changes in temporal trends among line segments in each Sociodemographic Index (SDI) region.
Methods
Study design
This is an international comparative study.
Patient and public involvement
Patients were not involved in this study.
Study data
The data for incidence and mortality of cervical cancer from 1990 to 2019 were collected from the Global Health Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool). The general methods used in the GBD study have been published previously.10 Briefly, the GBD estimation process is based on identifying multiple relevant data sources for cervical cancer including censuses, household surveys, civil registration and vital statistics, disease registries, disease notifications and other sources. In the GBD study 2019, the International Classification of Diseases (ICD) 9 (180-180.9, V10.41, V72.32) and ICD 10 (C53-C53.9, Z12.4 and Z85.41) were used to code the cervical cancer and then used a spatiotemporal Gaussian process regression and Cause of Death Ensemble models to estimate its incidence and mortality.10 Moreover, disease-related risk factors were also assessed in this database, including behavioural, environmental/occupational and metabolic risks.
Statistical analysis
The age-standardised incidence rate (ASIR) and age-standardised mortality rate (ASMR) of cervical cancer with 95% uncertainty interval (UI) and average annual percentage change (AAPC) were displayed according to hierarchical variables. First, we categorised 204 countries and territories into five regions (low, low-middle, middle, high-middle and high) according to the SDI used in GBD study. As a summary measurement that identifies which countries or other geographical areas sit on the spectrum of development, SDI is expressed on a scale of 0 to 1. Furthermore, all countries were divided into 21 areas based on geography, such as Central Asia (table 1). The ASIR, ASMR and AAPC were evaluated to quantify the incidence and mortality trends in cervical cancer. The ASR (per 100 000 population) is calculated as follows: , where is the age-specific rates (, where denotes the age class) and w is the number of population (or weight) () in the same subgroup. We fitted a logarithm of the ASR with year using generalised linear regression models, that is, , where , and . The AAPC was calculated as , and its 95% CI could also be obtained from this model.
Table 1.
The incident cases and age-standardised incidence rate of cervical cancer in 1990 and 2019, and change trends from 1990 to 2019
| Characteristics | 1990 | 2019 | 1990–2019 | ||
| Incident cases n×103 (95% UI) |
ASIR per 100 000 n (95% UI) |
Incident cases n×103 (95% UI) |
ASIR per 100 000 n (95% UI) |
AAPC n (95% CI) |
|
| Overall | 335.64 (300.35–393.89) | 7.64 (6.87–9.01) | 565.54 (481.52–636.43) | 6.81 (6.80–7.66) | −0.39 (−0.43 to 0.35) |
| Age | |||||
| 15–49 years | 160.77 (141.43–186.58) | 5.93 (5.21–6.88) | 256.9 (215.92–289.54) | 6.53 (5.49–7.36) | 0.28 (0.21 to 0.34) |
| 50–69 years | 131.88 (118.72–159.68) | 19.33 (17.40–23.41) | 232.42 (197.12–260.41) | 16.85 (14.29–18.88) | −0.42 (−0.51 to 0.34) |
| 70+ years | 42.99 (39.02–50.67) | 21.33 (19.36–25.14) | 76.22 (66.03–85.11) | 16.44 (14.24–18.36) | −0.94 (−0.98 to 0.91) |
| Sociodemographic Index | |||||
| Low | 41.5 (31.77–50.8) | 13.71 (6.65–16.94) | 78.82 (61.61–97.93) | 11.73 (6.25–14.54) | −0.42 (−0.51 to 0.33) |
| Low-middle | 66.22 (54.06–81.76) | 8.87 (6.30–11.06) | 125.96 (107.88–150.11) | 8.05 (6.92–9.63) | −0.6 (−0.64 to 0.57) |
| Middle | 92.18 (81.45–116.4) | 7.47 (6.61–9.47) | 183.34 (144.49–208.86) | 6.87 (6.42–7.80) | −0.22 (−0.27 to 0.17) |
| Middle-high | 75.8 (71.53–88.88) | 6.77 (6.38–7.96) | 113.12 (89.78–129.15) | 5.93 (6.71–6.78) | −1.09 (−1.19 to 1.01) |
| High | 59.69 (54.3–61.65) | 6.18 (6.60–6.38) | 63.86 (55.71–71.45) | 4.48 (6.89–5.02) | −0.34 (−0.45 to 0.23) |
| Region | |||||
| Central Asia | 5.27 (4.9, 5.63) | 10.19 (9.43–10.85) | 7.67 (6.65, 8.83) | 8.52 (7.44–9.77) | −0.43 (−0.54 to 0.32) |
| Central Europe | 15.39 (14.39–16.21) | 10.89 (10.16–11.47) | 2.3 (2.0–2.7) | 8.14 (6.70–9.51) | −1.16 (−1.30 to 1.03) |
| Eastern Europe | 22.82 (19.67–24.65) | 8.44 (7.29–9.14) | 23 (18.91–28.03) | 8.01 (6.50–9.82) | −0.19 (−0.34 to 0.05) |
| Australasia | 1.37 (1.15–1.47) | 6.07 (5.04–6.48) | 1.65 (1.27–2.11) | 4.23 (3.26–5.46) | −0.98 (−1.35 to 0.61) |
| High-income Asia Pacific | 12.47 (11.64–14.36) | 6.16 (5.73–7.10) | 15.06 (11.91–17.96) | 5.18 (4.02–6.22) | −0.65 (−0.79 to 0.50) |
| High-income North America | 17.53 (15.11–18.26) | 5.45 (4.67–5.67) | 21.85 (17.42–26.62) | 4.59 (3.65–5.61) | −0.38 (−0.43 to 0.33) |
| Southern Latin America | 6.48 (6.05–6.87) | 13.81 (12.90–14.62) | 9.84 (7.27–12.85) | 13 (9.54–17.07) | −0.4 (−0.54 to 0.27) |
| Western Europe | 28.6 (25.91–29.68) | 5.88 (5.23–6.11) | 27.17 (22.69–31.7) | 4.23 (3.51–4.95) | −1.07 (−1.16 to 0.98) |
| Andean Latin America | 4.1 (3.45–4.86) | 17.02 (14.39–20.21) | 9.1 (6.93–11.61) | 15.33 (11.7–19.49) | −0.5 (−0.62 to 0.38) |
| Caribbean | 4.12 (3.33–4.72) | 14.38 (11.66–16.35) | 6.86 (5.36–8.5) | 13.54 (10.55–16.81) | −0.24 (−0.29 to 0.18) |
| Central Latin America | 17.08 (15.8–17.85) | 16.66 (15.17–17.39) | 28.48 (23.11–35.03) | 11.35 (9.24–13.94) | −1.7 (−1.85 to 1.54) |
| Tropical Latin America | 14.12 (13.36–16.36) | 12.81 (12.06–14.77) | 23.74 (22.13–27.18) | 9.45 (8.82–10.81) | −1.27 (−1.37 to 1.17) |
| North Africa and Middle East | 7.03 (5.03–8.03) | 3.40 (2.42–3.88) | 14.63 (11.14–17.63) | 2.79 (2.14–3.33) | −0.69 (−0.77 to 0.6) |
| South Asia | 56.36 (44.21–68.59) | 7.61 (6.00–9.33) | 100.02 (80.11–124.77) | 6.18 (4.97–7.73) | −0.91 (−1.1 to 0.72) |
| East Asia | 45.26 (35.38–79.36) | 4.50 (3.55–7.78) | 115.38 (64.35–147.12) | 5.62 (3.15–7.16) | 1.34 (1.13 to 1.56) |
| Oceania | 0.57 (0.4–0.76) | 14.36 (10.38–19.33) | 1.33 (0.86–1.82) | 13.79 (9.28–18.56) | −0.04 (−0.11 to 0.03) |
| Southeast Asia | 0.41 (0.36–0.47) | 9.77 (7.46–12.33) | 0.95 (0.81–1.12) | 7.53 (6.12–9.92) | −1.07 (−1.18 to 0.97) |
| Central sub-Saharan Africa | 5.84 (3.95–7.83) | 19.73 (13.69–26.06) | 12.3 (8.23–16.88) | 17.12 (11.53–23.63) | −0.52 (−0.63 to 0.4) |
| Eastern sub-Saharan Africa | 19.08 (14.41– 23.81) | 19.42 (14.62–24.12) | 36.33 (25.76–48.45) | 16.42 (11.8–21.51) | −0.73 (−0.8 to 0.66) |
| Southern sub-Saharan Africa | 6.17 (4.68–7.53) | 17.93 (13.52–21.89) | 12.02 (9.74–14.44) | 18.08 (14.81–21.66) | 0.36 (0.11 to 0.61) |
| Western sub-Saharan Africa | 14.85 (11.66–18.64) | 13.79 (10.88–17.21) | 33.37 (26.14–42.54) | 13.31 (10.54–16.69) | −0.04 (−0.08 to 0.00) |
AAPC, average annual percentage change; ASIR, age standardised incidence rate; UI, uncertainty interval.
Second, we examined the changes in incidence and mortality trends of cervical cancer using the Joinpoint Regression Program (V.4.5.0.1; NCI, Bethesda, Maryland, USA) and applied to the log rates globally and by SDI regions. We determined the number of joinpoints using the permutation test with the default maximum number of three. In describing the change, the terms ‘increase’ or ‘decrease’ were used when the annual percentage change (APC) was statistically significant; otherwise, the term ‘stable’ was used. All analyses were performed using the R statistical software (V.3.6.1). A p value of less than 0.05 was considered statistically significant.
Results
Geographical variation of incidence and change trends in cervical cancer
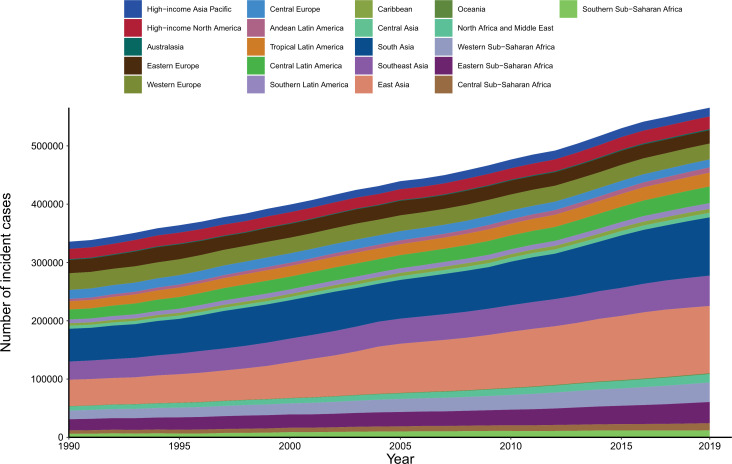
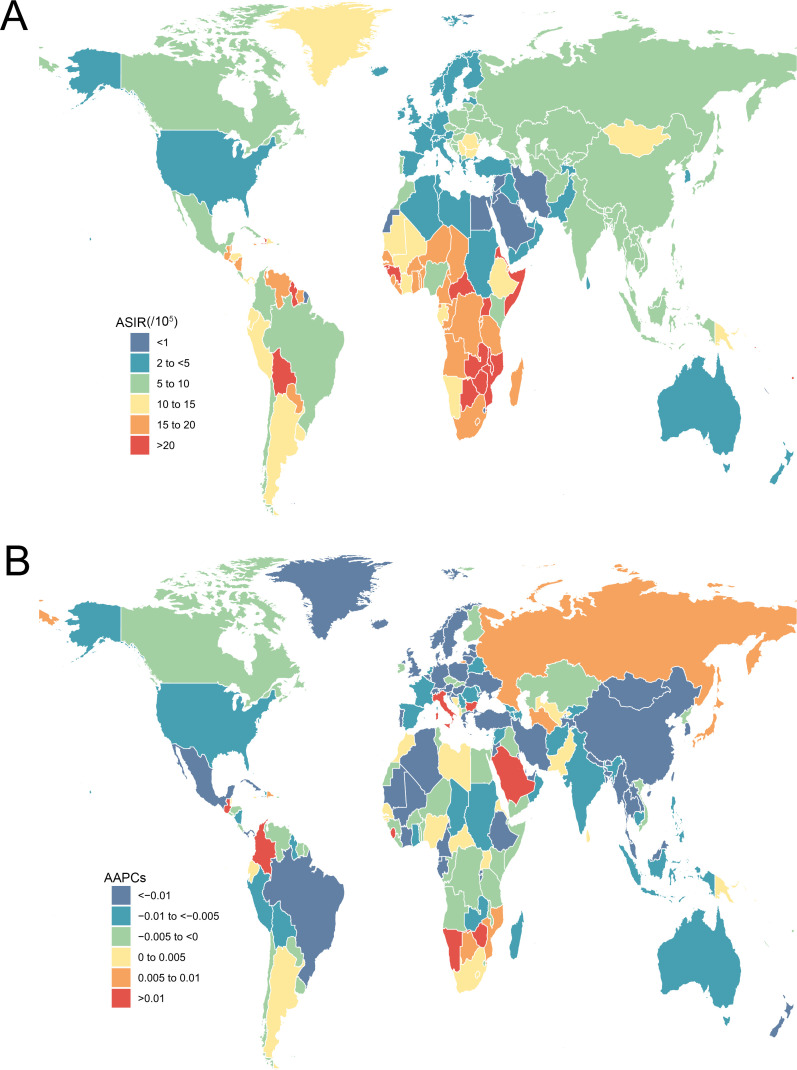
A total of 565 541 incident cases of cervical cancer were reported in 2019 all over the world, with 19.4% of new cases in China (109 759), followed by India (84 981) and Brazil (22 650). The majority of increases in the absolute number of incident cases came from East Asia, South Asia and Southeast Asia (figure 1). The ASIR of cervical cancer varied greatly worldwide in 2019. The highest ASIR was observed in Kiribati (60.37 per 100 000 person-years), followed by Palau (30.91) and Lesotho (29.55) (figure 2A). Globally, the ASIR of cervical cancer decreased from 7.64 (95% UI 6.87 to 9.01) in 1990 to 6.81 (95% UI 6.80 to 7.66) in 2019, with an AAPC of −0.39 (−0.4, –0.35). The AAPC of cervical cancer from 1990 to 2019 differed substantially between the GBD regions, with Central Latin America (−1.70 to –1.85 to −1.54), Tropical Latin America (−1.27 to –1.37 to −1.17) and Central Europe (−1.16 to –1.30 to −1.03) showing the largest decreases. By contrast, East Asia (1.34, 1.13 to 1.56) and Southern sub-Saharan Africa (0.36, 0.11 to 0.61) showed increasing trends during this period (table 1). The largest decrease in ASIR of cervical cancer was observed in Maldives (−3.80 to −4.03 to −3.57), followed by Singapore (−3.73 to –3.95 to −3.52) and Taiwan (China) (−3.39 to –3.73 to −3.05). Meanwhile, the greatest increase in ASIR was observed in Lesotho (3.47, 2.95 to 4.00), followed by Italy (1.91, 1.60 to 2.23) and Colombia (1.62, 1.37 to 1.87) (figure 2B). From 1990 to 2019, no unified pattern of ASIRs at the SDI level was found among the 21 GBD world areas. The estimated relationship between SDI and ASIR of cervical cancer, shown as blue line in online supplemental figure S1, was a gradual decline as SDI increases, with a relatively slow decline at the middle level of SDI.
Figure 1.
Absolute number of incident cases of cervical cancer for 21 Global Burden of Disease regions, from 1990 to 2019.
Figure 2.
The ASIR in 2019 (A) and AAPC from 1990 to 2019 (B) of cervical cancer for all age groups in 195 countries and territories. AAPC, average annual percentage change; ASIR, age-standardised incidence rate.
bmjopen-2021-055470supp001.pdf (1.5MB, pdf)
Geographical variation of mortality and change trends in cervical cancer
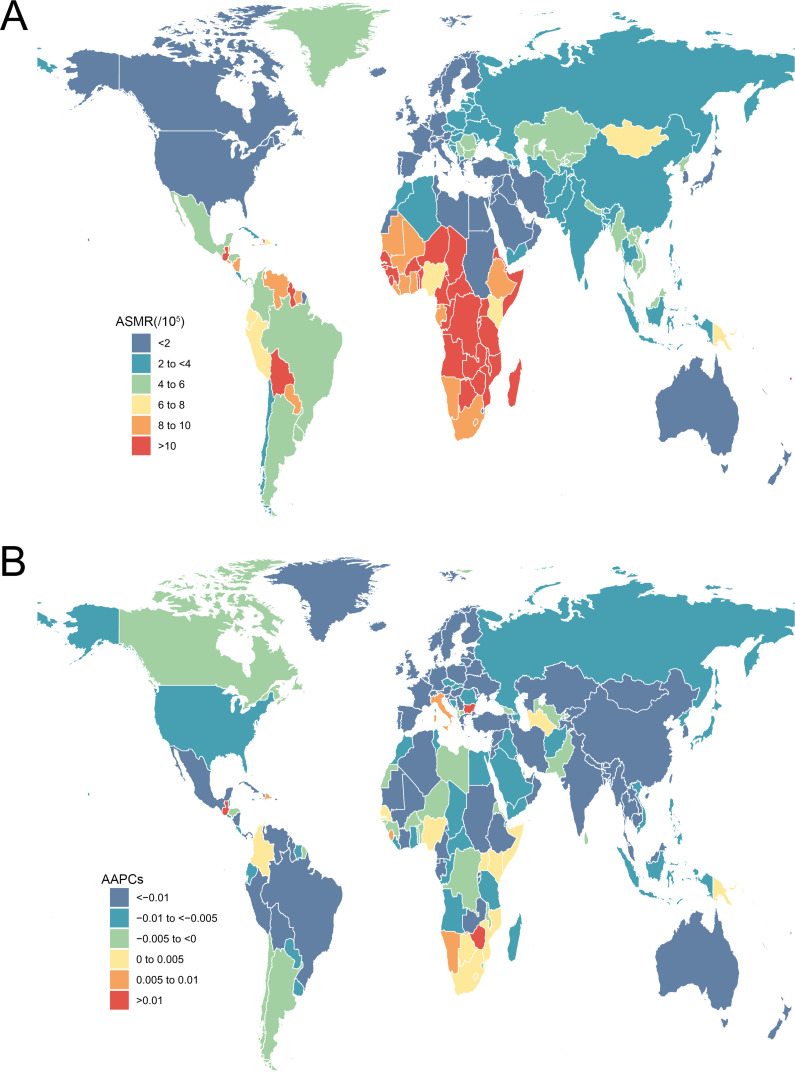
In the same way, a total of 280 479 death cases of cervical cancer were reported in 2019 all over the world, with 19.1% of new cases in China (53 441), followed by India (45 446) and Brazil (11 074). The majority of increases in the absolute number of deaths came from East Asia, South Asia and Western sub-Saharan Africa (online supplemental figure S2). The ASMR of cervical cancer was significantly heterogeneous worldwide in 2019. The highest ASMR was observed in Kiribati (39.95 per 100 000 person-years), followed by Lesotho (21.10) and Guinea (18.27) (figure 3A). Globally, the ASMR of cervical cancer decreased from 4.46 (95% UI 4.00 to 5.31) in 1990 to 3.40 (95% UI 2.90 to 3.81) in 2019, with an AAPC of −0.96 (–1.01, –0.92) (table 2). In all GBD regions, there was a decrease in AAPC of cervical cancer from 1990 to 2019, with Central Latin America (−2.52 to –2.67 to −2.37), Tropical Latin America (−1.96 to –2.05 to −1.87) and Western Europe (−1.82 to –1.92 to −1.73) showing the largest decreases. The largest decrease in ASMR of cervical cancer was observed in Maldives (−4.37, −4.65 to −4.09), followed by Singapore (−4.30, −4.51 to −4.08) and Taiwan (China) (−3.83, −4.16 to −3.51). Meanwhile, the greatest increase in ASMR was observed in Lesotho (3.32, 2.80 to 3.84), followed by Zimbabwe (1.80, 1.30 to 2.29) and Bulgaria (1.17, 0.89 to 1.44) (figure 3B). Similarly, the downtrend relationship was also found between SDI and ASMR.
Figure 3.
The ASMR in 2019 (A) and AAPC from 1990 to 2019 (B) of cervical cancer for all age groups in 195 countries and territories. AAPC, average annual percentage change; ASMR, age-standardised mortality rate.
Table 2.
The death cases and age-standardised mortality rate of cervical cancer in 1990 and 2019, and change trends from 1990 to 2019
| Characteristics | 1990 | 2019 | 1990–2019 | ||
| Death cases n×103 (95% UI) |
ASMR per 100 000 n (95% UI) |
Death cases n×103 (95% UI) |
ASMR per 100 000 n (95% UI) |
AAPC n (95% CI) |
|
| Overall | 184.53 (164.84–218.94) | 4.46 (4.00–5.31) | 280.48 (238.86–313.93) | 3.40 (2.90–3.81) | −0.96 (−1.01 to 0.92) |
| Age | |||||
| 15–49 years | 58.08 (50.01–68.38) | 2.14 (1.84–2.52) | 76.55 (64.14–86.52) | 1.95 (1.63–2.20) | −0.42 (−0.50 to 1.40) |
| 50–69 years | 84.63 (75.6–102.94) | 12.41 (1.08–15.09) | 130.78 (109.46–146.34) | 9.48 (1.93–10.61) | −0.93 (−1.02 to 2.94) |
| 70+ years | 41.81 (37.72–49.83) | 20.75 (1.71–24.73) | 73.14 (62.92–81.76) | 15.78 (1.57–17.63) | −0.97 (−1.01 to 2.95) |
| Sociodemographic Index | |||||
| Low | 26.08 (20.23–32.11) | 9.48 (7.42–11.7) | 45.54 (35.8–56.26) | 7.66 (6.06–9.39) | −0.88 (−0.96 to 0.81) |
| Low-middle | 39.21 (32.46–50.05) | 5.80 (4.81–7.45) | 66.68 (57.27–81.24) | 4.58 (3.94–5.61) | −0.8 (−0.83 to 0.76) |
| Middle | 52.53 (46.63–65.12) | 4.79 (4.28–5.93) | 90.1 (71.33–103.2) | 3.54 (2.83–4.06) | −0.98 (−1.04 to 0.92) |
| Middle-high | 41.35 (38.69–48.4) | 3.84 (3.59–4.49) | 51.77 (41.66–57.87) | 2.60 (2.10–2.91) | −1.75 (−1.85 to 1.65) |
| High | 25.22 (23.28–26.19) | 2.51 (2.33–2.61) | 26.17 (22.82–28.15) | 1.52 (1.35–1.63) | −1.72 (−1.79 to 1.65) |
| Region | |||||
| Central Asia | 2.72 (2.48–2.89) | 5.63 (5.09–5.97) | 3.42 (3–3.93) | 4.19 (3.71–4.81) | −0.86 (−0.98 to 0.73) |
| Central Europe | 8 (7.6–8.53) | 5.57 (5.30–5.94) | 6.88 (5.82–7.99) | 3.59 (3.02–4.17) | −1.65 (−1.77 to 1.53) |
| Eastern Europe | 12.94 (10.95–13.86) | 4.73 (4.00–5.07) | 10.04 (8.47–11.91) | 3.19 (2.67–3.79) | −1.64 (−1.79 to 1.49) |
| Australasia | 0.45 (0.39–0.48) | 2.00 (1.71–2.10) | 0.52 (0.45–0.58) | 1.14 (0.99–1.27) | −1.66 (−2.03 to 1.29) |
| High-income Asia Pacific | 4.64 (4.35–5.32) | 2.33 (2.17–2.68) | 5.6 (4.58–6.22) | 1.42 (1.17–1.56) | −0.81 (−0.95 to 0.68) |
| High-income North America | 6.74 (5.97–7.04) | 2.02 (1.78–2.11) | 8.8 (7.47–9.34) | 1.58 (1.34–1.67) | −1.38 (−1.44 to 1.33) |
| Southern Latin America | 3.07 (2.89–3.31) | 6.68 (6.27–7.24) | 4.18 (3.55–4.6) | 5.23 (4.43–5.74) | −1.01 (−1.13 to 0.89) |
| Western Europe | 13.13 (12.04–13.61) | 2.44 (2.23–2.52) | 11.75 (10.27–12.69) | 1.42 (1.27–1.52) | −1.82 (−1.92 to 1.73) |
| Andean Latin America | 2.33 (1.96–2.76) | 10.43 (8.81–12.30) | 4.28 (3.32–5.38) | 7.47 (5.81–9.38) | −1.29 (−1.4 to 1.17) |
| Caribbean | 2.23 (1.75–2.55) | 8.17 (6.50–9.32) | 3.47 (2.72–4.26) | 6.77 (5.30–8.33) | −0.62 (−0.69 to 0.55) |
| Central Latin America | 9.59 (8.7–10.01) | 10.54 (9.41–11.03) | 13.83 (11.53–16.8) | 5.71 (4.78–6.92) | −2.52 (−2.67 to 2.37) |
| Tropical Latin America | 7.68 (7.21–8.88) | 7.82 (7.27–9.11) | 11.58 (10.71–13.66) | 4.71 (4.35–5.56) | −1.96 (−2.05 to 1.87) |
| North Africa and Middle East | 3.97 (2.82–4.53) | 2.17 (1.54–2.48) | 7 (5.44–8.31) | 1.54 (1.21–1.81) | −1.16 (−1.26 to 1.06) |
| South Asia | 33.34 (26.22–39.95) | 4.98 (3.94–6.00) | 53.3 (42.87–69.95) | 3.54 (2.86–4.64) | −1.39 (−1.56 to 1.22) |
| East Asia | 28.4 (22.32–46.14) | 3.12 (2.47–5.01) | 55.96 (33.19–71.36) | 2.68 (1.61–3.40) | −0.05 (−0.28 to 0.18) |
| Oceania | 0.3 (0.22–0.42) | 8.92 (6.59–12.4) | 0.67 (0.45–0.91) | 8.08 (5.68–10.92) | −0.19 (−0.28 to 0.1) |
| Southeast Asia | 16.71 (12.57–21.47) | 5.85 (4.44–7.72) | 25.13 (20.52–34.98) | 3.94 (3.24–5.54) | −1.49 (−1.57 to 1.4) |
| Central sub-Saharan Africa | 3.72 (2.61–4.84) | 13.94 (9.97–18.18) | 7.3 (4.91–10.06) | 11.79 (7.89–16.57) | −0.6 (−0.74 to 0.45) |
| Eastern sub-Saharan Africa | 11.94 (9.08–14.92) | 13.46 (10.19–16.98) | 21.11 (15.48–27.86) | 11.02 (7.91–14.41) | −0.8 (−0.85 to 0.74) |
| Southern sub-Saharan Africa | 3.24 (2.48–4.07) | 10.61 (8.06–13.5) | 6.56 (5.39–7.75) | 11.11 (9.10–13.04) | 0.58 (0.28 to 0.88) |
| Western sub-Saharan Africa | 9.38 (7.56–12.31) | 9.62 (7.8–12.56) | 19.09 (15.04–24.01) | 8.81 (7.00–10.98) | −0.21 (−0.24 to 0.17) |
ASMR, age standardised mortality rate; UI, uncertainty interval; AAPC, average annual percentage change.
Joinpoint regression analysis of incidence and mortality trends in cervical cancer
We applied joinpoint regression analysis to assess temporal trend changes with APC for incidence and mortality of cervical cancer from 1990 to 2019. The results showed that the APC of incidence was almost decreased or stable globally except for the low-middle SDI region, where the APC increased during 2010–2019. Correspondingly, the APC of mortality was declining in all SDI regions (online supplemental figure S3).
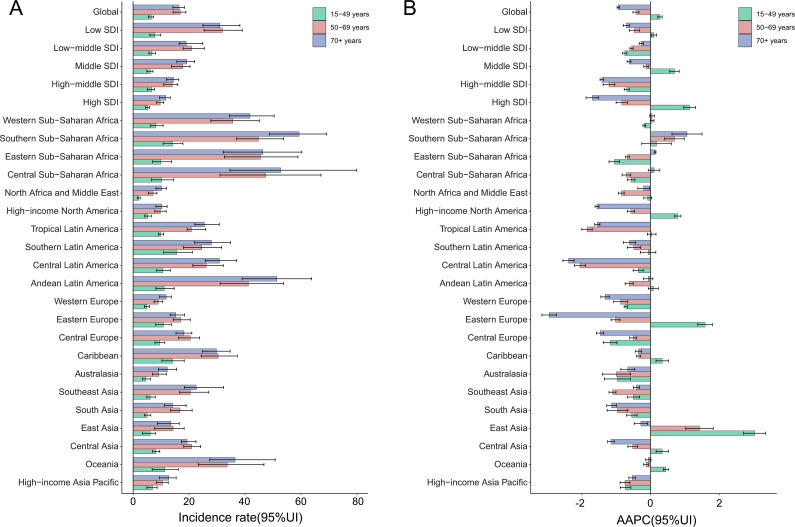
Trends in incidence and mortality of cervical cancer stratified by age group
We stratified the population according to age, divided into the following age groups: 15–49 years, 50–69 years and 70+ years. The results showed that the incidence and mortality rate of the 70+ years age group were the highest in most regions, and the low SDI region has the highest incidence and mortality in all age groups. The age-specific incidence decreased in all SDI regions at the age groups of 50–69 and 70+ years. While at the age group of 15–49 years, the increasing trends of incidence were found in high-middle SDI and middle SDI regions. The results of age-specific AAPC of incidence showed an increasing trend in the age group of 15–49 years globally, and the high SDI region increased the most. As for geographical areas, AAPC increased in 10 areas at the age group of 15–49 years, and East Asia was the highest (figure 4A, B). Concerning age-specific AAPC of mortality of cervical cancer, it decreased in all SDI regions at all age groups. Although the AAPC of mortality decreased globally at all age groups, the mortality in East Asia, Eastern Europe and Central Asia at the age group of 15–49 years still presented an increasing trend. Especially, the increase was also found in Southern sub-Saharan Africa at the age groups of 50–69 years and 70+ years (online supplemental figure S4 A, B).
Figure 4.
The incidence rates in 2019 (A) and AAPC from 1990 to 2019 (B) of cervical cancer in different age groups at global, regional and national levels. Error bars indicate 95% uncertainty intervals. AAPC, average annual percentage change; SDI, Sociodemographic Index.
Age-specific numbers and rates of incident cases and deaths from cervical cancer
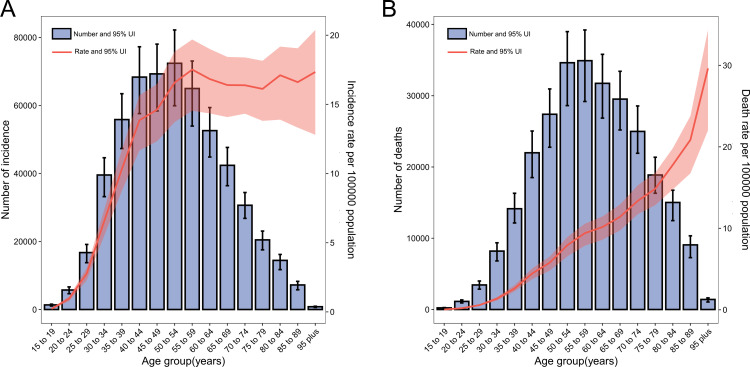
In 2019, the number of cases and deaths followed a normal distribution and peaked at ages 50–54 years and 55–59 years, respectively (figure 5A, B). The rates of incidence increased with increasing age group up to the ages of 55–59 years, after which the incidence started to be stable. While the rates of mortality increased with increasing age group up to the oldest age group (≥95 years).
Figure 5.
Global numbers and rates of incident cases (A) and (B) deaths from cervical cancer by age, 2019. Error bars indicate the 95% uncertainty interval for incident cases. Shading indicates the 95% uncertainty interval for the incidence/mortality rate.
Discussion
This article provides the first comprehensive overview of the patterns and temporal trends in the age-specific incidence and mortality of cervical cancer at the global, regional and national levels. In general, cervical cancer is still an important cause of incidence and mortality (although vary widely with geographical location) in females, and the absolute number of incident cases and deaths are increasing around the world. East Asia and Southern sub-Saharan Africa contribute the largest number of incident cases and deaths from cervical cancer globally. Although the ASIR and ASMR have declined overall, an increasing or stable trend is also observed in these two regions. Particularly, the incidence and mortality of cervical cancer appear to be rising among younger women in certain regions.
With the development of the economy and the popularisation of screening, the overall incidence and mortality of cervical cancer in the world have shown a downward trend, which is consistent with the results of previous studies.11 In addition, our findings also demonstrated that the incidence of cervical cancer in LMICs is much higher than that observed in high-income countries. This discrepancy is largely attributed to insufficient appropriately trained healthcare personnel, limited medical facilities, inequality of access and fragile healthcare systems, and lack of sanitation in LMICs.12–14 Preventive HPV vaccination and effective screening are the main strategies to prevent cervical cancer. Currently, 124 countries have included HPV vaccines in their immunisation programmes, which have shown to be beneficial and cost-effective in preventing cervical cancer and reducing the HPV infection rate.15 In high-income countries such as Australia, the incidence and mortality of cervical cancer in women are expected to be reduced to a very low level due to HPV vaccination. Simultaneously, HPV-based screening has also been shown to be more efficacious at detecting cervical precancers than the original cytological methods.16 17 Unfortunately, the introduction of vaccine and screening programmes in LMICs has been restricted by cost, cultural challenges and difficulties in reaching the target population.6 18 To accelerate the elimination of cervical cancer, effective cancer control programmers, infrastructure development and further government investment need to be established in LMICs.
Notably, on the basis of the joinpoint regression analysis results, a decreasing trend of incidence and mortality over the period occurred in all SDI regions except in the low-middle SDI region, which showed an increasing incidence trend in the past 10 years. Similar results have been reported in many low-middle SDI countries, like Swaziland, Zimbabwe and Sudan.19–21 More specific researches are needed to explore the possible reasons for this phenomenon.
Our GBD 2019 estimates are relatively consistent with the latest GLOBOCAN report in 2018 (570 000 incident cases and 311 000 deaths), although our results were slightly lower than theirs, which could be due to the different data source and estimate methods.3 In line with previous studies, the higher ASIR and ASMR of cervical cancer always occur in East Asia and Southern sub-Saharan Africa over the period of 1990 to 2019. According to the data from WHO, 80% of cervical cancer incidence and 90% of deaths in the world are in LMICs.22 In China, the incidence and mortality of cervical cancer in rural areas are significantly higher than those in cities, and economically backward areas are significantly higher than those in economically developed areas. Furthermore, there has been a continuous upward trend in the past 20 years.23 From 2000 to 2014, the average annual growth rate of cervical cancer incidence in China reached 10.5%, and the age of onset showed a younger trend. Fortunately, China has made remarkable achievements in cervical cancer prevention and management.24 On the contrary, the situation of cervical cancer in sub-Saharan Africa remains severe, which could be explained by the inadequate resources and trained manpower, lack of awareness of cervical cancer, unorganised cervical screening and HPV vaccination programmes. Another important reason is that the increased number of HIV infections is due to the higher risk of HPV infection in HIV-infected women.25 Notably, the coverage and quality of the data in sub-Saharan Africa are not enough (most counties are no vital registration or verbal autopsy data), so the trend of cervical cancer incidence and mortality in this region has yet to be further verified by more reliable data.
Interestingly, the incidence of cervical cancer in young women (15–49 years old) is increasing globally, especially in areas with high SDI. This may be caused by a wide range of factors, such as increasing HPV exposure, higher screening participation rates, earlier sexual debut and history of a high number of sexual partners, etc. According to several reports from Japan, the rate of having sexual intercourse among high school students (16–18 years old) increased from 9% in 1981 to 24% in 2011. Similarly, the sexual activity rate of college students (aged 18 years old) also increased from 19% to 47%.17 Due to changes in sexual concepts, including early age at first intercourse and multiple sexual partners, the incidence of cervical cancer in China has tended to be younger overall in the past 20 years. In addition, environmental pollution, endocrine and other factors have also exerted strong effects on the risk of cervical cancer in young people.26 Under the severe situation of the increasing risk of young-onset cervical cancer, it is necessary to advance the time of HPV-based screening programmes in high-risk populations for early intervention and treatment.
Limitations
Several limitations of our findings should be noted. First, the robustness of the GBD data depends on the quality of the data source. Although different data sources are used to produce cancer estimates, relevant data are unavailable or sparse in some countries. Taking the aforementioned into consideration, integrating multiple health data sources could give a more accurate and complete picture of the incidence and mortality trends of cervical cancer. Second, estimates of recent cervical cancer data relied on past trends and covariates because there is a lag in data availability. However, the GBD estimates are updated each year with improvements in the modelling strategy, and the incompatible data or unexpected results will be further confirmed.
Conclusion
Cervical cancer remains a concerning disease that affects more than half a million women every year all over the world. Although most cervical cancer burden can be avoided by HPV-based vaccination and screening, efforts must be focused on the disparity between regions and countries. In addition, the younger trend of cervical cancer also deserves people’s attention. More recently, WHO released the ‘Global Strategy for Accelerating the Elimination of Cervical Cancer and the Interim Goals for 2030’, jointly committed by 194 countries. Vaccination, screening and treatment are the important steps in achieving the triple intervention goals. By providing annually updated estimates of cervical cancer at the regional and national levels, future iterations of GBD will be useful for defining the epidemiological patterns and monitoring the success of prevention strategies.
Supplementary Material
Footnotes
Contributors: MY and HX designed the study. MY and JD collected the data. MY performed statistical analysis. All authors conducted results interpretation. MY drafted this manuscript. HX, HM, HL and FX revised the manuscript. HX had primary responsibility for final content. All authors have read and approved the final manuscript. HX is the guarantor of the manuscript.
Funding: This study was supported by grants from the National Natural Science Foundation of China (81201604).
Map disclaimer: The depiction of boundaries on the map(s) in this article does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. The map(s) are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Data are available in the Global Health Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. The Lancet 2019;393:169–82. 10.1016/S0140-6736(18)32470-X [DOI] [PubMed] [Google Scholar]
- 2.Small W, Bacon MA, Bajaj A, et al. Cervical cancer: a global health crisis. Cancer 2017;123:2404–12. 10.1002/cncr.30667 [DOI] [PubMed] [Google Scholar]
- 3.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020;8:e191–203. 10.1016/S2214-109X(19)30482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Xu H, Zhang L, et al. Cervical cancer: epidemiology, risk factors and screening. Chin J Cancer Res 2020;32:720–8. 10.21147/j.issn.1000-9604.2020.06.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das M. Who launches strategy to accelerate elimination of cervical cancer. Lancet Oncol 2021;22:20–1. 10.1016/S1470-2045(20)30729-4 [DOI] [PubMed] [Google Scholar]
- 6.Pilleron S, Cabasag CJ, Ferlay J, et al. Cervical cancer burden in Latin America and the Caribbean: where are we? International Journal of Cancer 2020;147:1638–48. 10.1002/ijc.32956 [DOI] [PubMed] [Google Scholar]
- 7.Xia C, Hu S, Xu X, et al. Projections up to 2100 and a budget optimisation strategy towards cervical cancer elimination in China: a modelling study. The Lancet Public Health 2019;4:e462–72. 10.1016/S2468-2667(19)30162-8 [DOI] [PubMed] [Google Scholar]
- 8.Sankaranarayanan R, Basu P, Kaur P, et al. Current status of human papillomavirus vaccination in India’s cervical cancer prevention efforts. Lancet Oncol 2019;20:e637–44. 10.1016/S1470-2045(19)30531-5 [DOI] [PubMed] [Google Scholar]
- 9.Wojtyla C, Janik-Koncewicz K, La Vecchia C. Cervical cancer mortality in young adult European women. Eur J Cancer 2020;126:56–64. 10.1016/j.ejca.2019.11.018 [DOI] [PubMed] [Google Scholar]
- 10.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. The Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vu M, Yu J, Awolude OA, et al. Cervical cancer worldwide. Curr Probl Cancer 2018;42:457–65. 10.1016/j.currproblcancer.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Cervical cancer: unequal progress. Lancet 2019;393:104. 10.1016/S0140-6736(19)30003-0 [DOI] [PubMed] [Google Scholar]
- 13.Canfell K, Kim JJ, Brisson M, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. The Lancet 2020;395:591–603. 10.1016/S0140-6736(20)30157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vale DB, Teixeira JC, Bragança JF, et al. Elimination of cervical cancer in low- and middle-income countries: inequality of access and fragile healthcare systems. Int J Gynaecol Obstet 2021;152:7–11. 10.1002/ijgo.13458 [DOI] [PubMed] [Google Scholar]
- 15.Simms KT, Hanley SJB, Smith MA, et al. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. The Lancet Public Health 2020;5:e223–34. 10.1016/S2468-2667(20)30010-4 [DOI] [PubMed] [Google Scholar]
- 16.Kramer J. Eradicating cervical cancer: lessons learned from Rwanda and Australia. Int J Gynaecol Obstet 2021;154:270–6. 10.1002/ijgo.13601 [DOI] [PubMed] [Google Scholar]
- 17.Sauvaget C, Nishino Y, Konno R, et al. Challenges in breast and cervical cancer control in Japan. Lancet Oncol 2016;17:e305–12. 10.1016/S1470-2045(16)30121-8 [DOI] [PubMed] [Google Scholar]
- 18.Lopez MS, Baker ES, Maza M, et al. Cervical cancer prevention and treatment in Latin America. J Surg Oncol 2017;115:615–8. 10.1002/jso.24544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mapanga W, Girdler-Brown B, Singh E. Knowledge, attitudes and practices of young people in Zimbabwe on cervical cancer and HPV, current screening methods and vaccination. BMC Cancer 2019;19:845. 10.1186/s12885-019-6060-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginindza TG, Sartorius B. Projected cervical cancer incidence in Swaziland using three methods and local survey estimates. BMC Cancer 2018;18:639. 10.1186/s12885-018-4540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elhasan LME, Bansal D, Osman OF, et al. Prevalence of human papillomavirus type 16 in Sudanese women diagnosed with cervical carcinoma. J Cancer Res Ther 2019;15:1316–20. 10.4103/jcrt.JCRT_656_18 [DOI] [PubMed] [Google Scholar]
- 22.Xue P, Ng MTA, Qiao Y. The challenges of colposcopy for cervical cancer screening in LMICs and solutions by artificial intelligence. BMC Med 2020;18:169. 10.1186/s12916-020-01613-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma L, Wang Y, Gao X, et al. Economic evaluation of cervical cancer screening strategies in urban China. Chin J Cancer Res 2019;31:974–83. 10.21147/j.issn.1000-9604.2019.06.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Zheng R, Li X, et al. Trends of incidence rate and age at diagnosis for cervical cancer in China, from 2000 to 2014. Chin J Cancer Res 2017;29:477–86. 10.21147/j.issn.1000-9604.2017.06.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngune I, Kalembo F, Loessl B, et al. Biopsychosocial risk factors and knowledge of cervical cancer among young women: a case study from Kenya to inform HPV prevention in sub-Saharan Africa. PLoS One 2020;15:e0237745. 10.1371/journal.pone.0237745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu T, Song Y, Chen R, et al. Solid fuel use for heating and risks of breast and cervical cancer mortality in China. Environ Res 2020;186:109578. 10.1016/j.envres.2020.109578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055470supp001.pdf (1.5MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Data are available in the Global Health Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool).