Abstract
Purpose: To compare apparent diffusion coefficients (ADCs) of bone marrow on diffusion-weighted imaging (DWI) between two fat-suppression techniques, and to evaluate the association between bone-marrow ADCs and the proton density fat fraction (PDFF).
Methods: Seventy-seven patients underwent whole-body DWI with short-inversion time inversion-recovery (STIR) (DWISTIR) and/or STIR + selective water-excitation (spectral-spatial RF [SSRF]) (DWISTIR+SSRF). ADCs of lumbar vertebrae (L3 and L4) were compared between DWISTIR and DWISTIR+SSRF, and correlated with the PDFF.
Results: Lumbar ADCs obtained by DWISTIR and DWISTIR+SSRF were significantly correlated (L3: r = 0.90, P < 0.0001, L4: r = 0.90, P < 0.0001). Lumbar ADCs (× 10-6 mm2/s) obtained by DWISTIR were significantly lower than those by DWISTIR+SSRF (L3: 479 ± 137 and 490 ± 148, P < 0.05, L4: 456 ± 114 and 471 ± 118, P < 0.005). Residual fat signals were more clearly observed on DWISTIR than on DWISTIR+SSRF. The ADCs of L3 obtained by DWISTIR and DWISTIR+SSRF exhibited significant positive correlations with the PDFF (r = 0.51, P < 0.0001, and r = 0.45, P < 0.0001, respectively), and the ADCs of L4 obtained by DWISTIR and DWISTIR+SSRF exhibited significantly positive correlations with the PDFF (r = 0.40, P < 0.0005, and r = 0.40, P < 0.0005, respectively).
Conclusion: Irrespective of different fat-suppression methods, lumbar ADCs were positively correlated with the PDFF, being inconsistent with previous studies. Lumbar ADCs obtained by DWISTIR were significantly lower than those obtained by DWISTIR+SSRF, probably due to residual fat signals on DWISTIR. However, this difference (< 4%) did not explain the positive correlation between lumbar ADC and PDFF.
Keywords: bone marrow, apparent diffusion coefficient, fat suppression, fat fraction
Introduction
Diffusion-weighted imaging (DWI) is a quantitative functional MRI technique that reflects the restricted random movement of extra-cellular water protons that is in relation with cellular density. As with positron emission tomography with 2-[18F]-fluoro-2-deoxy-D-glucose ([18F]FDG PET), whole-body DWI with apparent diffusion coefficient (ADC) quantification is currently used for cancer staging and assessing treatment responses to diseases involving bone marrow.1–3 Whole-body bone marrows contain hematopoietic cells at birth (red marrow); these are gradually replaced by fat tissue (yellow marrow), and the physiological conversion is completed by age 25. Red marrow is replaced by yellow marrow proximally to the axial skeleton. We recently reported that proton density fat fraction (PDFF) of lumbar vertebrae increases with age,4 and the result was consistent with the previous report of Schmeel et al.5 Furthermore, we identified the factors influencing bone-marrow DWI signals in oncology patients without bone-marrow lesions. Age, hemoglobin (Hb), and the red cell distribution width (RDW) are the predominant predictors for bone-marrow DWI signals. A lower ADC and higher visibility of bone-marrow DWI were found to be associated with a younger age, anemia (lower Hb), and increased erythropoiesis (higher RDW).4 Thus, the ADCs of bone marrow were positively correlated with age and PDFF, which was inconsistent with previous reports demonstrating a negative correlation between bone-marrow ADC and age and the PDFF.6–10
A possible cause of the discrepancy with previous results was the different fat-suppression technique used for DWI in our recent study. Several vendor-specific fat-suppression techniques are available for 3T MR imaging systems,11 and some techniques can be combined with others to obtain more robust fat-suppression. A short inversion time inversion recovery (STIR) pre-pulse is most commonly used for fat suppression in whole-body DWI because the method is relatively insensitive to field inhomogeneities, and can be used near metal and over the large fields-of-view suitable for whole-body imaging.12 In our recent study, a spectral-spatial RF (SSRF) water excitation pre-pulse13 was used together with STIR to reduce the residual fat signals at the abdomen and pelvis, including the lumbar vertebrae and ilium.4 Differences in ADC measurements have been reported when different fat-suppression techniques were used in breast MR imaging,14,15 and it was likely that there were differences in ADC measurements of bone marrow rich in fat.16
The objectives of this study were: first, to investigate whether bone-marrow ADCs are significantly different between two fat-suppression techniques, STIR only and STIR + SSRF; and second, to evaluate the association between bone-marrow ADCs and the PDFF.
Materials and Methods
Patient population
We retrospectively reviewed the medical records of all tumor patients who underwent whole-body [18F]FDG PET/MRI for staging in our institute between June 2018 and December 2018. Patients were eligible for the study if they fulfilled the following criteria: (1) whole-body DWI with STIR (DWISTIR) from head to lower abdomen and with STIR + SSRF (DWISTIR+SSRF) from lower abdomen to mid-thigh were performed, and (2) ADC and PDFF maps were available. Patients were excluded if they had bone metastasis, hematological disorder (myeloma, leukemia, myelodysplastic syndrome, lymphoma, etc.) confirmed by diagnostic imaging and/or bone marrow biopsy, or a history of chemotherapy, radiotherapy, blood transfusion, or use of granulocyte colony-stimulating factor.
Seventy-seven patients (72 females, 5 males; mean age = 55.5 years; age range = 16 – 86 years) were identified. In detail, 71 patients had gynecological tumors, 3 had rectal cancer, and 3 had head and neck cancer. This retrospective study was approved by the ethics committee of our institute, and the requirement to obtain formal informed consent was waived.
Whole-body MRI
All patients underwent whole-body MRI using an integrated 3.0-T PET/MR scanner (Signa PET/MR; GE Healthcare, Waukesha, WI, USA). All MR sequences were acquired in the axial plane. An STIR pre-pulse was used for fat suppression from head to lower abdomen (from the 1st to the 4th bed positions) on whole-body DWI because of its insensitivity to magnetic field inhomogeneity, and an SSRF was used with STIR from lower abdomen to thigh (from the 4th to the 5th or 6th bed positions). The 4th bed position from vertex corresponded to lower abdomen including lumbar vertebrae (L3 and L4) to be assessed in this study. DWI was performed using a single-shot echoplanar imaging (EPI) sequence under free breathing (TR/TE/inversion time: 5000/59/250 ms for DWISTIR, and 5000/61/250 ms for DWISTIR+SSRF; b values: 0, 800 s/mm2; FOV 576 × 345 mm; matrix 128 × 128; slice thickness/overlap: 6/0 mm; 40 image/bed; imaging time: 2 min 30 sec for each DWI). PDFF measurements were performed using an iterative decomposition of water and fat with echo asymmetry, and a least-squares estimation quantitation sequence (IDEAL-IQ) (a quantitative chemical shift-based water–fat separation method with a multi-echo gradient echo;17 TR/TEs: 7.1/0.9 − 5.3 ms, 6 echoes; FOV 500 × 300 mm; matrix 256 × 192; slice thickness/overlap: 6/0 mm; 34 image/bed; imaging time: 20 sec). ADC and PDFF maps were generated and used in subsequent assessments.
Quantitative image assessment
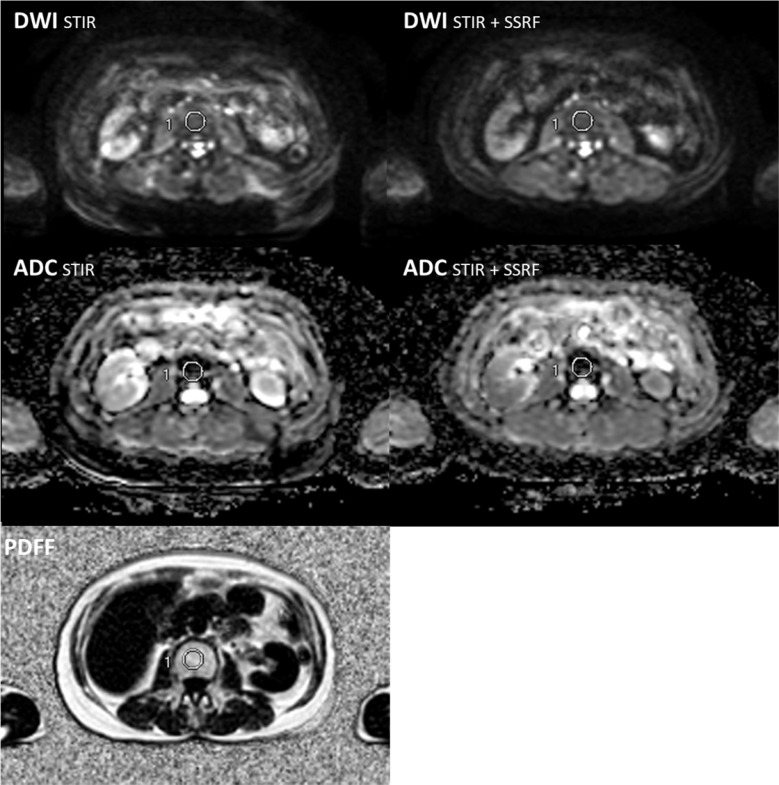
MR images were transferred to the GE workstation (AW 4.6; GE Healthcare) and evaluated using matched spatial registration. Three circular ROIs with a fixed diameter of 20 mm were placed on lumbar vertebrae (L3 and L4, separately) in the same coordinate positions on DWI, ADC, and PDFF maps (Fig. 1).4 To reduce the bias in the ROI selection process, the number of ROIs placed on a vertebra was tripled in a different patient group from that in our previous study4 and L5 was excluded from the evaluation due to inaccurate measurements of ADC and PDFF on spondylotic changes. The ADC and PDFF were measured and averaged at L3 and L4 by the agreement of experienced radiologists (TT and HO with 20 and 30 years of experience, respectively). ADCs of L3 and L4 were compared between DWISTIR and DWISTIR+SSRF using Wilcoxon’s signed rank test due to the non-normal distribution of ADCs. Regression analyses were performed using Spearman’s correlation coefficient. All statistical analyses were performed using SPSS statistics version 22 (IBM, Armonk, NY, USA). P < 0.05 was considered to be significant.
Fig. 1.
ROI placement on L4 in the same coordinate positions on DWI, ADC, and PDFF maps. ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; PDFF, proton density fat fraction; SSRF, spectral-spatial RF; STIR, short inversion time inversion recovery.
Results
Correlation and comparison of lumbar ADCs
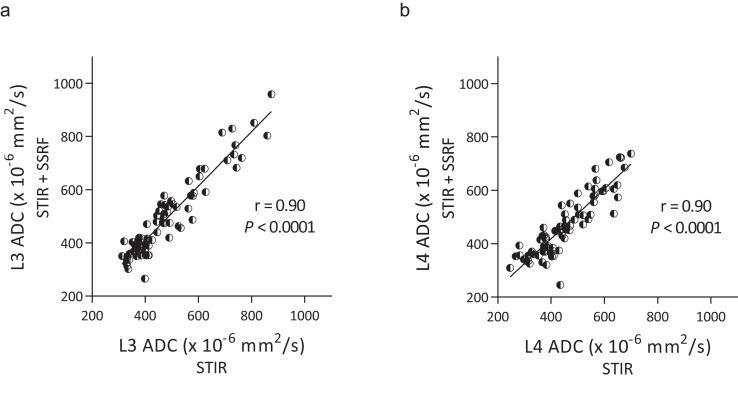
Bone-marrow ADCs of L3 obtained by DWISTIR and DWISTIR+SSRF were significantly correlated (r = 0.90, P < 0.0001) (Fig. 2a), and the ADCs of L4 obtained by DWISTIR and DWISTIR+SSRF were significantly correlated (r = 0.90, P < 0.0001) (Fig. 2b).
Fig. 2.
Correlation of lumbar ADCs, L3 (a) and L4 (b), between DWISTIR and DWISTIR+SSRF. Regression lines are shown with Spearman’s correlation coefficients r and associated P-values. ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; SSRF, spectral-spatial RF; STIR, short inversion time inversion recovery.
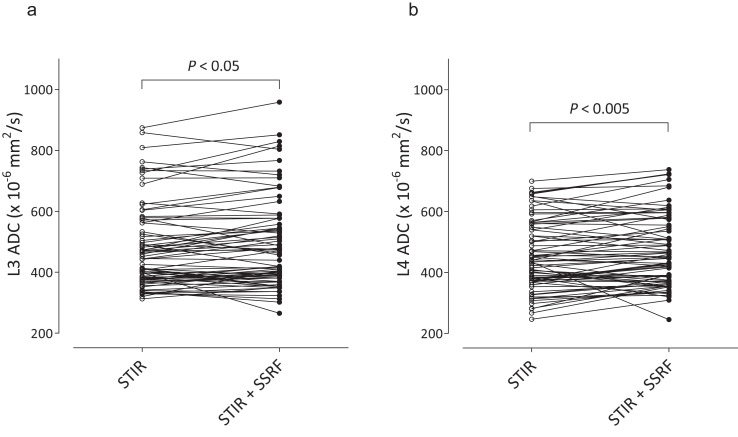
The ADC of L3 (× 10-6 mm2/s) obtained by DWISTIR was significantly lower than that obtained by DWISTIR+SSRF (479 ± 137 and 490 ± 148, P < 0.05) (Fig. 3a), and the ADC of L4 obtained by DWISTIR was significantly lower than that obtained by DWISTIR+SSRF (456 ± 114 and 471 ± 118, P < 0.005) (Fig. 3b). The mean differences in ADCs were 10.9 (2.3%) for L3 and 14.8 (3.2%) for L4.
Fig. 3.
Comparison of lumbar ADCs, L3 (a) and L4 (b), between DWISTIR and DWISTIR+SSRF. Graphs show individual pairs of data with connecting lines. The significance of differences was assessed by Wilcoxon’s signed rank test. ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; SSRF, spectral-spatial RF; STIR, short inversion time inversion recovery.
The correlations and comparisons of ADCs using all ROI measurements (n = 231) were shown in supplemental Figs. 1 and 2.
Correlation between lumbar ADC and the PDFF
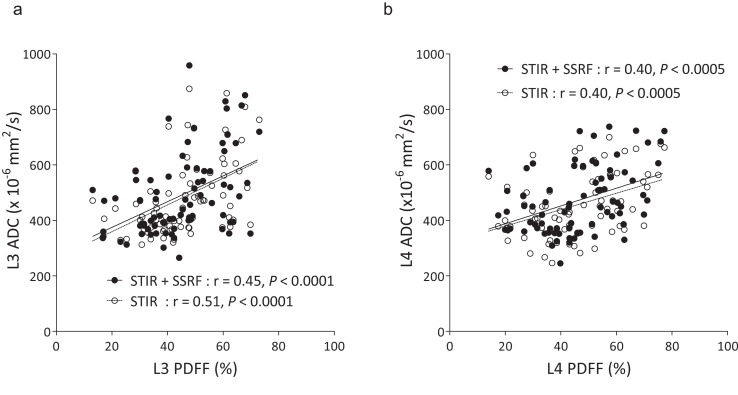
The ADCs of L3 obtained by DWISTIR and DWISTIR+SSRF exhibited significantly moderate positive correlations with the PDFF (r = 0.51, P < 0.0001, and r = 0.45, P < 0.0001, respectively) (Fig. 4a), and the ADCs of L4 obtained by DWISTIR and DWISTIR+SSRF exhibited significantly moderate positive correlations with the PDFF (r = 0.40, P < 0.0005, and r = 0.40, P < 0.0005, respectively) (Fig. 4b).
Fig. 4.
Correlation between lumbar PDFF and ADC in L3 (a) and L4 (b). Regression lines are separately shown with Spearman’s correlation coefficients r and associated P-values. ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; SSRF, spectral-spatial RF; STIR, short inversion time inversion recovery.
Representative images
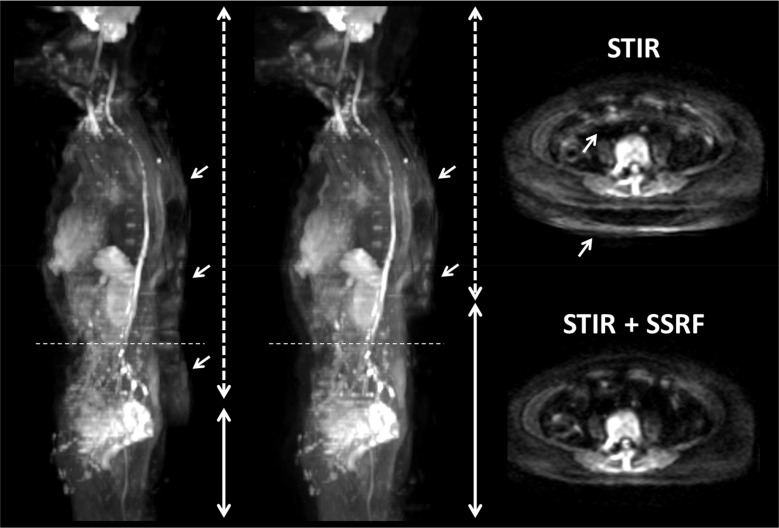
Figure 5 shows maximum intensity projection (MIP) and transaxial DW images of a 54-year-old woman with endometrial cancer. Dotted and solid bidirectional arrows on MIP images represent the ranges of fat-suppression methods: STIR and STIR + SSRF, respectively. Horizontal dotted lines on MIP images represent the slice plane of the transaxial DW image. Residual fat signals were more clearly observed on MIP and transaxial DW images with STIR than STIR + SSRF, leading to ghosting artifacts (arrows). The ADC of L4 on DWISTIR (422 × 10-6 mm2/s) was significantly lower than that on DWISTIR+SSRF (446 × 10-6 mm2/s).
Fig. 5.
MIP and transaxial DW images of a 54-year-old woman with endometrial cancer. Dotted and solid bidirectional arrows on MIP images represent the ranges of fat suppression methods: STIR and STIR + SSRF, respectively. Horizontal dotted lines on MIP images represent the slice plane of the transaxial DW image. Residual fat signals were more clearly observed on MIP and transaxial DW images with STIR than STIR + SSRF, leading to ghosting artifacts (arrows). DW, diffusion-weighted; MIP, maximum intensity projection; SSRF, spectral-spatial RF; STIR, short inversion time inversion recovery.
Discussion
The present study demonstrated that the bone-marrow ADCs are well correlated, but the significant differences exist between two fat-suppression techniques. DWISTIR exhibited higher residual fat signals and lower lumbar ADCs than DWISTIR+SSRF. The results were consistent with a previous study of breast DWI at 3 T by Nogueira et al.15 They evaluated the influence of the fat-suppression technique on breast ADC measurements between DWI with STIR and spectral adiabatic inversion recovery (SPAIR). In their study, fat-suppression uniformity was better for DWI with STIR, and the ADCs of breast lesions and normal tissue were lower for DWI with SPAIR, exhibiting higher residual fat signals. Since lipids have been reported to have low ADC values,14 a lower ADC will be measured as found with STIR than STIR + SSRF due to unsuppressed fat signals in our study. Their previous study was different from the present study in some aspects: breast MRI vs. whole-body MRI, STIR and SPAIR vs. STIR and STIR + SSRF. However, bone marrow is also a tissue rich in fat and the combined fat-suppression (STIR + SSRF) exhibited more clearly adequate fat-suppressing effects than STIR-only (Fig. 5). The residual fat signals may have caused the significant decrease in lumbar ADC measurements.16 The difference in ADCs between DWISTIR and DWISTIR+SSRF was 2.3% for L3 and 3.2% for L4. Although the differences in lumbar ADCs between STIR and STIR + SSRF were not large (less than 4%), the same fat-suppression technique should be used for assessing ADCs of the bone-marrow lesions over time, e.g. in the assessment of response to treatment in patients with multiple myeloma or bone-marrow involvement of lymphoma.3,18–20
This study was motivated by our recent work showing ADC had a moderate positive correlation with PDFF,4 which was not consistent with previous studies,6–10 and it was hypothesized that the fat-suppression method might explain the differences. To help ensure accurate results, we evaluated a different patient population and the total number of ROIs placed on L3 and L4 separately was three times that placed in our recent study. However, irrespective of different fat-suppression methods, lumbar ADCs were positively correlated with the PDFF (Fig. 4), being inconsistent with the previous studies. It is suggested that STIR does not provide sufficient fat suppression, supported by the different ADCs found with the two methods, but this difference does not explain the positive correlation between lumbar ADC and PDFF. Dieckmeyer et al. reported the confounding effects of residual fat on ADC measurements of the vertebral bone-marrow water component.21 They noted a strong underestimation of ADC increasing with the PDFF, which means that the true diffusion rate of water molecules in lumbar vertebrae may be much higher than expected in the elderly. The changes with age in osseous trabeculation may have affected our results in the present study. Hematopoietic marrow is confined within the spaces defined by the trabeculae and supported by reticulum cells and fat cells. If these trabeculae with supporting reticulum/fat cells cause lower water diffusion, the trabecular bone loss can increase the bone-marrow ADC in the elderly. Further research is required to assess the effects of the trabecular bone loss on bone-marrow ADCs using bone biopsy samples and quantitative image analyses.
Our study had three limitations. First, the TE of DWISTIR+SSRF (61 ms) was slightly longer than that of DWISTIR (59 ms), and the difference of 2 ms may have affected the results. Ogura et al. previously evaluated the effects of TE differences on ADC measurements using agarose phantoms.22 Since they found no significant difference in ADC between TEs of 50 ms and 100 ms, the small TE difference (2 ms) between DWISTIR and DWISTIR+SSRF did not seem to be influential in this study. Second, vertebral ADCs were not compared to those in other organs such as liver, because whole-body DWI was performed with free breathing12 and motion artefacts affected the ADC measurements on the other organs. Third, the gender difference was not evaluated because the study population mainly consisted of women (with gynecological tumors). Especially in middle-aged women, perimenopausal status and/or pathophysiological responses (red marrow reconversion) to anemia might influence bone-marrow ADCs.4 These results require further validation with functional and molecular imaging of bone marrow, and a larger number of male patients are required to evaluate the gender difference in future studies.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This retrospective study was approved by the ethics committee of the Faculty of Medical Sciences, University of Fukui (No. 20170206).
Conclusion
Irrespective of different fat-suppression methods [STIR vs. STIR + SSRF (water excitation)], lumbar ADCs were positively correlated with the PDFF, being inconsistent with previous studies. Bone-marrow ADCs obtained by DWISTIR were significantly lower than those obtained by DWISTIR+SSRF, probably due to inadequate fat-suppressing effects of STIR, which may have affected the differences in ADC measurements. However, this difference (< 4%) does not explain the positive correlation between lumbar ADC and PDFF. Further research is required to assess the effects of the trabecular bone loss on bone-marrow ADCs using bone biopsy samples and quantitative image analyses.
Acknowledgments
This study was partly funded by a Grant-in-Aid for scientific research from the Japan Society for the Promotion of Science (19K08170) and Takeda Science Foundation.
Footnotes
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- 1.Lin C, Luciani A, Itti E, et al. Whole-body diffusion-weighted magnetic resonance imaging with apparent diffusion coefficient mapping for staging patients with diffuse large B-cell lymphoma. Eur Radiol 2010; 20:2027–2038. [DOI] [PubMed] [Google Scholar]
- 2.Messiou C, Giles S, Collins DJ, et al. Assessing response of myeloma bone disease with diffusion-weighted MRI. Br J Radiol 2012; 85:e1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giles SL, Messiou C, Collins DJ, et al. Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma. Radiology 2014; 271:785–794. [DOI] [PubMed] [Google Scholar]
- 4.Tsujikawa T, Oikawa H, Tasaki T, et al. Whole-body bone marrow DWI correlates with age, anemia, and hematopoietic activity. Eur J Radiol 2019; 118:223–230. [DOI] [PubMed] [Google Scholar]
- 5.Schmeel FC, Luetkens JA, Wagenhäuser PJ, et al. Proton density fat fraction (PDFF) MRI for differentiation of benign and malignant vertebral lesions. Eur Radiol 2018; 28:2397–2405. [DOI] [PubMed] [Google Scholar]
- 6.Lavdas I, Rockall AG, Castelli F, et al. Apparent diffusion coefficient of normal abdominal organs and bone marrow from whole-body DWI at 1.5 T: The effect of sex and age. AJR Am J Roentgenol 2015; 205:242–250. [DOI] [PubMed] [Google Scholar]
- 7.Cui FZ, Cui JL, Wang SL, et al. Signal characteristics of normal adult bone marrow in whole-body diffusion-weighted imaging. Acta Radiol 2016; 57:1230–1237. [DOI] [PubMed] [Google Scholar]
- 8.Chen YY, Wu CL, Shen SH. High signal in bone marrow on diffusion-weighted imaging of female pelvis: correlation with anemia and fibroid-associated symptoms. J Magn Reson Imaging 2018; 48:1024–1033. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann J, Krstin N, Schoennagel BP, et al. Age-related distribution of vertebral bone-marrow diffusivity. Eur J Radiol 2012; 81:4046–4049. [DOI] [PubMed] [Google Scholar]
- 10.Schraml C, Schmid M, Gatidis S, et al. Multiparametric analysis of bone marrow in cancer patients using simultaneous PET/MR imaging: Correlation of fat fraction, diffusivity, metabolic activity, and anthropometric data. J Magn Reson Imaging 2015; 42:1048–1056. [DOI] [PubMed] [Google Scholar]
- 11.Del Grande F, Santini F, Herzka DA, et al. Fat-suppression techniques for 3-T MR imaging of the musculoskeletal system. Radiographics 2014; 34:217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahara T, Imai Y, Yamashita T, et al. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 2004; 22:275–282. [PubMed] [Google Scholar]
- 13.Meyer CH, Pauly JM, Macovski A, et al. Simultaneous spatial and spectral selective excitation. Magn Reson Med 1990; 15:287–304. [DOI] [PubMed] [Google Scholar]
- 14.Partridge SC, Singer L, Sun R, et al. Diffusion-weighted MRI: influence of intravoxel fat signal and breast density on breast tumor conspicuity and apparent diffusion coefficient measurements. Magn Reson Imaging 2011; 29:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogueira L, Brandão S, Nunes RG, et al. Breast DWI at 3 T: influence of the fat-suppression technique on image quality and diagnostic performance. Clin Radiol 2015; 70:286–294. [DOI] [PubMed] [Google Scholar]
- 16.Ababneh ZQ, Beloeil H, Berde CB, et al. In vivo lipid diffusion coefficient measurements in rat bone marrow. Magn Reson Imaging 2009; 27:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H, Shimakawa A, Hines CD, et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med 2011; 66:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horger M, Weisel K, Horger W, et al. Whole-body diffusion-weighted MRI with apparent diffusion coefficient mapping for early response monitoring in multiple myeloma: preliminary results. AJR Am J Roentgenol 2011; 196:W790–795. [DOI] [PubMed] [Google Scholar]
- 19.Sun M, Cheng J, Zhang Y, et al. Application value of diffusion weighted whole body imaging with background body signal suppression in monitoring the response to treatment of bone marrow involvement in lymphoma. J Magn Reson Imaging 2016; 44:1522–1529. [DOI] [PubMed] [Google Scholar]
- 20.Littooij AS, Kwee TC, de Keizer B, et al. Whole-body MRI-DWI for assessment of residual disease after completion of therapy in lymphoma: A prospective multicenter study. J Magn Reson Imaging 2015; 42:1646–1655. [DOI] [PubMed] [Google Scholar]
- 21.Dieckmeyer M, Ruschke S, Eggers H, et al. ADC quantification of the vertebral bone marrow water component: removing the confounding effect of residual fat. Magn Reson Med 2017; 78:1432–1441. [DOI] [PubMed] [Google Scholar]
- 22.Ogura A, Hayakawa K, Miyati T, et al. Imaging parameter effects in apparent diffusion coefficient determination of magnetic resonance imaging. Eur J Radiol 2011; 77:185–188. [DOI] [PubMed] [Google Scholar]