Abstract
Purpose: The purpose of the current study was to clarify the blood flow pattern in the left atrium (LA), potentially causing the formation of thrombosis after left upper lobectomy (LUL). The blood flow in the LA was evaluated and compared between LUL patients with and without thrombosis. For the evaluation, we applied highly accelerated 4D flow MRI with dual-velocity encoding (VENC) scheme, which was expected to be able to capture slow flow components in the LA accurately.
Methods: Eight volunteers and 18 patients subjected to LUL underwent dual-VENC 4D Flow MRI. Eight patients had a history of thrombosis. We measured the blood flow velocity and stasis ratio (proportion in the volume that did not exceed 10 cm/s in any cardiac phase) in the LA and left superior pulmonary vein (LSPV) stump. For visual assessment, the presence of each collision of the blood flow from pulmonary veins and vortex flow in the LA were evaluated. Each acquired value was compared between healthy participants and LUL patients, and in LUL patients with and without thrombosis.
Results: In LUL patients, blood flow velocity near the inflow part of the left superior pulmonary vein (Lt Upp) and mean velocity in the LA were lower, and stasis ratio in the LA was higher compared with healthy volunteers (Lt Upp 9.10 ± 3.09 vs.13.23 ± 14.19 cm/s, mean velocity in the LA 9.81 ± 2.49 vs. 11.40 ± 1.15 cm/s, and stasis ratio 25.28 ± 18.64 vs. 4.71 ± 3.03%, P = 0.008, 0.037, and < 0.001). There was no significant difference in any quantification values between LUL patients with and without thrombosis. For visual assessment, the thrombus formation was associated with no collision pattern (62.5% vs. 10%, P = 0.019) and not with vortex flow pattern (50% vs. 30%, P = 0.751).
Conclusion: The net blood flow velocity was not associated with the thrombus formation. In contrast, a specific blood flow pattern, the absence of blood flow collision from pulmonary veins, correlates to the thrombus formation in the LA.
Keywords: left upper lobectomy, lung cancer, thrombosis, stroke, 4D flow MRI
Introduction
In patients undergoing lung cancer surgery, the incidence of stroke is approximately 1% and five times higher compared with patients who did not undergo surgery.1,2 In this cohort, the thrombus formation in the pulmonary vein stump occurs in 3.3%–3.6% of patients, among which the higher prevalence was observed in those who underwent left upper lobectomy (LUL) (13.5–17.9%).3,4 This thrombosis sometimes causes infarction in several organs.3–7 Despite the potential risk of this life-threatening complication, the post-surgery prevention for thrombosis in LUL patients is not well established because the risk stratification for this entity has not been achieved. One of the hypotheses to explain the higher risk of the thrombosis in this specific group is the existence of the longer pulmonary vein (PV) stump after LUL, which causes blood flow stasis, resulting in the formation of the embolic source.3,4,8 In addition to altered flow in the vein stump, the postoperative blood flow changes in the left atrium (LA) are supposed to further promote the formation of thrombosis.9,10 However, the latter hypothesis is not validated. There is only one case report that evaluated the blood flow in the LA after LUL.10
Phase-contrast MRI-based evaluation for the blood flow in the LA was first reported two decades ago.11,12 Thanks to the improvement of the scan scheme and post-processing software for time-resolved 3D phase-contrast (4D flow), the detailed blood flow in the LA has been actively investigated by using this modality in the latter half of the decade.13–18 In these studies, investigators tried to clarify the blood flow changes by atrial fibrillation (AF), which is familiar in relation to thrombosis and embolism. They revealed that the risk of thrombosis (e.g. CHA2DS2-VASc score) correlates to the velocity decrease and stasis of the blood flow in the LA.13,15,16 In the same context, one previous case report utilizing 4D flow MRI suggested that abnormal LA blood flow after LUL causes PV stump thrombosis, resulting in embolic cerebral infarction.10
The purpose of the current study was to clarify the blood flow pattern in the LA potentially causing the formation of thrombosis after LUL. The blood flow in the LA was evaluated and compared between LUL patients with and without thrombosis. For the evaluation, we applied highly accelerated 4D flow MRI with dual-velocity encoding (VENC) scheme, which was expected to be able to capture slow flow components in the LA accurately.
Materials and Methods
This study adhered to institutional ethics guidelines approved by the institutional review board, and all patients and volunteers provided informed written consent before the examinations.
Patients
The sample population in this study comprised 18 patients (11 men, 7 women; mean age: 73.3 years, range: 43–84 years) who underwent LUL at the authors’ hospital between January 2013 and October 2018. One patient was included from the previous case report.10 All 18 patients underwent 4D flow MRI between August 2018 and August 2019. Inclusion criterion was outpatients who had thrombosis during follow-up period, and patients without thrombosis were recruited so that the number of cases generally matched that of the thrombosis patients. Exclusion criteria were as follows: (1) patients having MRI scan contraindications, (2) patients having MRI contrast agent contraindications, and (3) patients who disagreed with the research agreements. Eight Patients (4 men, 4 women; mean age: 70.6 years, range: 43–84 years) had a history of thrombosis during follow-up. Out of eight patients, four patients had embolic cerebral infarction. We acquired clinical information from a retrospective review of medical records.
Volunteers
Eight participants (6 men, 2 women; mean age 31.3 years, range 29–36 years) with no special medical history were recruited from the authors’ institution.
Imaging techniques
We used Achieva 3.0T MRI (Philips Healthcare, Best, the Netherlands) with research scan program provided by a third party (FlowPatch; GyroTools, Zurich, Switzerland). To keep high SNR in the LA, we used a triphasic protocol with 0.1 mmol/kg gadobutrol injection (Gadovist; Bayer Yakuhin, Osaka, Japan) only in patients. First, we performed a bolus injection of half dose of contrast agent with saline flush to acquire time-resolved contrast-enhanced MR angiography, in which scan time was approximately 2 min. Following the scan, 4D flow MRI was started with a slow flow infusion of the remaining half dose of contrast agent and finally, by a slow flow saline flush injection.19 The detailed scan parameters were as follows: TR/TE ≈ 4.0/2.7 ms, flip angle (FA) = 13 degrees, voxel resolution = 1.7 * 1.7 * 2.0–3.0 mm, dual VENC acquisition = 50 and 150 cm/s, heart phase 24,k-t principle component analysis acceleration (acceleration factor = 8), scan time ≈ 12 min, and navigator-based respiratory gating (acceptable window = 12 mm [acceptance ratio ≈ 75%]).10,20 We applied dual-VENC acquisition to widen the dynamic range of velocity quantification and to capture slow flow in the LA accurately.
Regional and global flow quantification
The regional and global blood flow quantification was performed by using PGEM package of Pmod software (Ver 4.01; PMOD Technologies, Zurich Switzerland).
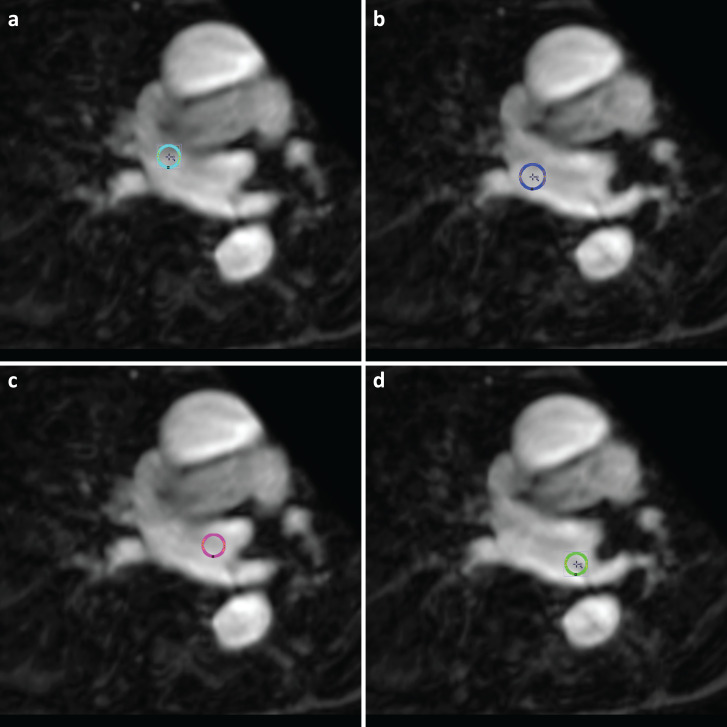
For regional flow evaluation, four voxels of interest (VOIs), 6 mm in diameter, were placed at representative regions corresponding to the inflow tract from each of the four pulmonary veins: right superior pulmonary vein (RSPV), right inferior pulmonary vein (RIPV), left superior pulmonary vein (LSPV), and left inferior pulmonary vein (LIPV). Each region was named as right upper region (Rt Upp), right lower region (Rt Low), left upper region (Lt Upp), and left lower region (Lt Low) (Fig. 1). This measurement was performed by two radiologists (M.N and T.S.) independently. In addition, repeated measurement was done to evaluate intra-operator reproducibility. We also segmented whole LSPV stump based on magnitude images. After the segmentation, we measured the average mean velocity (cm/s) and average maximum velocity (cm/s). Mean velocity represents the temporal average of the average velocity during cardiac cycle and maximum velocity represents the highest average velocity during one cardiac cycle. In LSPV stump, we calculated stasis which was defined as the proportion of voxels in the LA that did not exceed 10 cm/s during one cardiac cycle.13,16,17
Fig. 1.
Four VOIs were placed at the entry point from the pulmonary veins in the left atrium. Each colored circle represents the corresponding VOI (Light blue in a, Rt Upp; blue in b, Rt Low; red in c, Lt Upp; light green in d, Lt Low). Lt Low, left lower region; Lt Upp, left upper region; Rt Low, right lower region; Rt Upp, right upper region; VOI, voxel of interest.
For global flow evaluation, the segmentation of the LA was performed by one radiologist (M.N.) who had interpreted more than 100 cardiac MRI cases. Phase-contrast MR angiography and magnitude images were used for the segmentation. After the segmentation, we measured the LA volume (ml), average mean velocity in the LA (cm/s), average maximum velocity in the LA (cm/s), and stasis in the LA (%). Stasis (%) was calculated as the volume (%) of intra-left atrial VOI (voxel value) that is always less than 10 cm/s in one cardiac phase. This calculation formula is shown below.
Stasis (%) = VOI (mean voxel value) of intra-atrial volume that is always less than 10cm/s in one cardiac phase/VOI of left atrium (mean voxel value) × 100
Visual assessment
The visual assessment of flow pattern in the LA was performed by using GT Flow (Ver. 3.22; GyroTools). For visual assessment of blood flow, we evaluated its presence or absence, naming the blood flow collision and vortex flow in the LA. We focused on these flow patterns because the blood flow collision, followed by vortex flow, is the fundamental blood flow pattern in the LA.11,12,14,18,21 The blood flow was observed by overlaying in-plane flow vectors on magnitude images in multiplanar reconstruction view. We also generated 3D blood flow path lines in the LA from three pulmonary veins (LIPV, RSPV, and RIPV) by setting seed sources around the entry points of each pulmonary vein. First, the blood flow pattern in the LA was classified into two groups based on whether the blood flow collision was observed or not. Collision pattern was defined as the blood flow from the two or three pulmonary veins colliding with each other in the LA (Fig. 2 and Supplemental movies 1 and 2), while no collision pattern was defined as the blood flow from the three pulmonary veins passing each other (Fig. 3 and Supplemental movies 3 and 4). Second, the presence or absence of vortex was evaluated. In addition, vortex flow patterns were classified into standard pattern, counterclockwise vortex flow from the cranial side, or other types based on the previous study.18 All the blood flow patterns were independently evaluated by two radiologists (T.S. and M.N.) who had interpreted more than 100 cardiac MRI cases. When visual assessments did not match between them, the assessment was decided on through discussion.
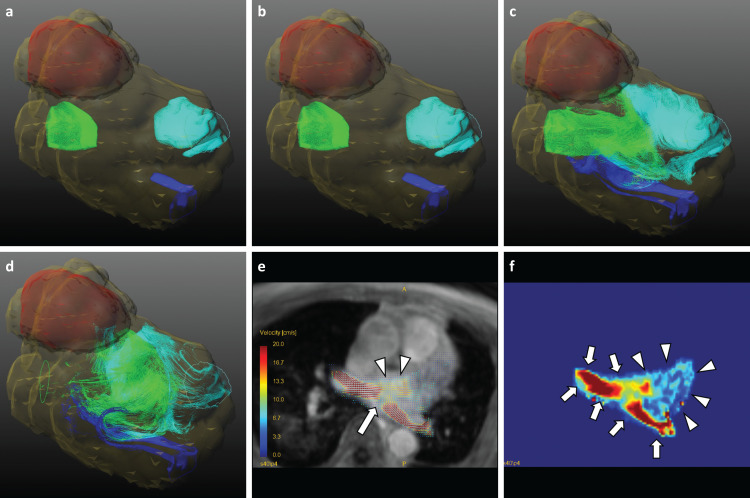
Fig. 2.
Collision pattern of blood flow. 77 years old man had never experienced any thrombosis after LUL. The left atrium and ventricle were segmented as yellow translucent VOIs. Each colored pathline represents blood flow from each pulmonary vein (Light blue, RSPV flow; blue, RIPV flow; light green, LIPV flow). The time order is from a to d. In this figure, the LIPV flow and RSPV flow collide with each other in c and flow to the mitral valve in d. The supplemental movie is referred (Supplemental movie 1, 2). The 2D overlay view shows clear collision of the flow from the right superior and left inferior pulmonary veins at the diastolic phase (arrow in e). The merged blood flow becomes the driving force behind the formation of the vortex flow in the left atrium (arrowheads in e). The velocity-weighted map more clearly depicts high velocity on and around inflow from the pulmonary vein (arrows in f), which gradually distributes to the rest areas of left atrium (arrowheads in f). LIPV, left inferior pulmonary vein; LUL, left upper lobectomy; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; VOIs, voxels of interest.
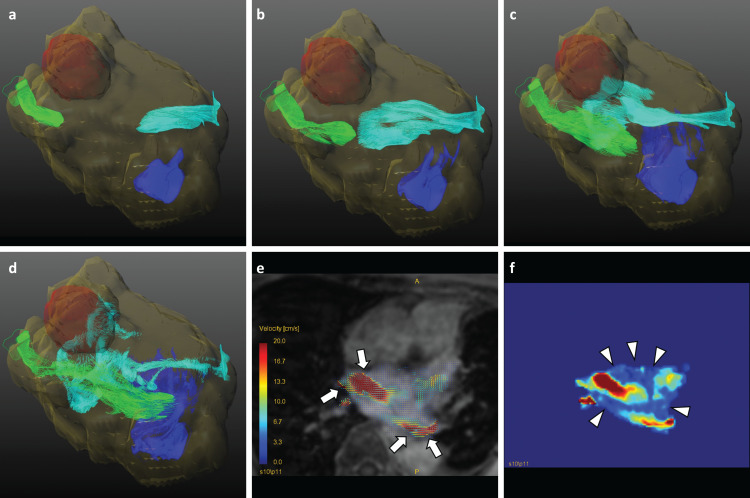
Fig. 3.
No collision pattern of blood flow. A 76 year-old woman had LSPV stump thrombus and cerebral infarction 4 days after LUL and anti-coagulant therapy started. Cerebral infarction was followed by MRI scan and LSPV stump thrombus was by contrast enhanced CT scan. LSPV thrombus disappeared when 4D flow MRI was performed. The left atrium and ventricle were segmented as yellow translucent VOIs. Each colored pathline represents the blood flow from each pulmonary vein (Light blue; RSPV flow, blue; RIPV flow, light green; LIPV flow). The time order is from a to d. In this figure, the flow does not collide and dissipate to the left atrium wall (Supplemental movies 3, 4). The 2D overlay view shows that the flows from the right superior and left inferior pulmonary veins pass each other at the diastolic phase (arrows in e). The velocity-weighted map acknowledges the slow flow areas where blood flow has been left out (arrowheads in f). Note that the velocity scale is the same as that in e and f. LIPV, left inferior pulmonary vein; LUL, left upper lobectomy; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; VOIs, voxels of interest.
Statistics
Intra-class correlation (ICC) was used to evaluate intra- and inter-operator reproducibility for regional flow evaluation. Continuous data were presented as mean ± standard deviation if the value was normal distributed and as median (1st quartile–3rd quartile) if the value was not. After checking the normal distribution with the Shapiro–Wilk Test, we performed an unpaired t-test in the values with normal distribution, and did the Mann–Whitney U test in the values without normal distribution. Nominal data were tested with the Chi-square test. We compared the blood flow velocity and stasis ratio between patients and volunteers, and patients with and without thrombosis. For the verification of visual assessments (presence or absence of collision or vortex flow in the LA), the agreements of the two radiologists were tested by using the kappa statistic. To compare the blood flow vortex and the blood flow collision in the LA between LUL patients with and without thrombosis, we used the chi-square test. For all analyses, P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 19.0.0 (IBM, Armonk, NY, USA).
Results
All the 18 LUL patients successfully underwent dual-VENC 4D flow MRI scan. The patients’ characteristics are described in Table 1.
Table 1.
Characteristics of patients with LUL
| Patients with thrombosis (n = 8) | Patients without thrombosis (n = 10) | P value | |
|---|---|---|---|
| Age, mean age (range) years old | 70.6 (43–84) | 75.5 (71–79) | 0.308 |
| Sex, n (%) Male/Female | 4 (50.0)/4 (50.0) | 7 (70.0)/3 (30.0) | 0.387 |
| Comorbidity, n (%) | |||
| Thrombosis | 8 (100.0) | 0 (0) | N/A |
| Cerebral infarction | 4 (50.0) | ||
| Thrombi in LSPV stump | 4 (50.0) | ||
| Arrhythmia | 1 (12.5) | 2 (25.0) | 0.521 |
| Preoperative anti-coagulate drug | 1 (12.5) | 2 (25.0) | 0.521 |
| Congestive Heart Failure | 0 (0) | 0 (0) | N/A |
| Cardiovascular Disease | 1 (12.5) | 0 (0) | N/A |
| Hypertension | 6 (75.0) | 8 (66.7) | 0.690 |
| Diabetes Mellitus | 0 (0) | 3 (25.0) | N/A |
| Dyslipidemia | 2 (25.0) | 2 (16.7) | 0.648 |
| LA volume (ml) | 73.29 ± 18.09 | 99.83 ± 62.13 | 0.224 |
| The duration between the operation and the onset of thrombosis, mean duration (range), days | |||
| Cerebral infarction | 92.2 (4–196) | ||
| Thrombi in LSPV stump | 100.50 (80–113) | ||
| Follow-up duration, mean duration (range), days | |||
| 725.6 (440–1064) | 1278.6 (404–2532) | 0.0642 | |
| The interval between operation and 4D Flow MRI scan, (range), days | |||
| 352.2 (122–667) | 745.8 (90–2066) | 0.121 | |
LA, left atrium; LUL, left upper lobectomy; LSPV, left superior pulmonary vein; N/A, not applicable.
Thrombi were observed in LSPV stump among seven of eight patients with thrombosis with CT scan. The maximum diameter of thrombosis in each patient was 10 × 5 mm, 5 × 4 mm, 23 × 17 mm, 3 × 4 mm, 9 × 3 mm, 12 × 9 mm, and 4 × 3 mm. The other one patient with thrombosis had cerebral infarction and thrombus was detected with echocardiography.
One of patients with thrombosis had a history of atrial fibrillation and percutaneous coronary intervention (PCI), and had been taking anticoagulant and antiplatelet drug before the surgery. Heparinisation had been performed from 5 days to 6 hours before the surgery, and these medications resumed from 2 days after the surgery. Two of patients without thrombosis had a history of atrial fibrillation and had been taking anticoagulant drug before the surgery. Heparinisation had been performed as the same protocol above.
Blood flow velocity in LSPV stump and Stasis (%) of patients with thrombosis were not normally distributed (P = 0.00743 and P = 0.0164 with Shapiro–Wilk Test).
Regional flow quantification (Table 2)
Table 2.
Regional velocity quantification
| LUL patients (n = 18) | Volunteer participants (n = 8) | P value | |
|---|---|---|---|
| Rt Upp (cm/s) | 14.19 ± 4.84 | 12.32 ± 3.05 | 0.248 |
| Rt Low (cm/s) | 14.79 ± 5.92 | 12.90 ± 2.53 | 0.265 |
| Lt Upp (cm/s) | 8.83 ± 3.22 | 13.47 ± 3.24 | 0.008 |
| Lt Low(cm/s) | 10.76 ± 4.47 | 13.34 ± 3.50 | 0.099 |
| Patients with thrombosis (n = 8) | Patients without thrombosis (n = 10) | P value | |
| Rt Upp (cm/s) | 13.66 ± 3.54 | 14.61 ± 5.84 | 0.675 |
| Rt Low (cm/s) | 14.57 ± 5.65 | 14.96 ± 6.42 | 0.894 |
| Lt Upp (cm/s) | 9.30 ± 2.94 | 9.55 ± 2.37 | 0.586 |
| Lt Low (cm/s) | 12.14 ± 3.68 | 9.66 ± 4.91 | 0.239 |
| LSPV stump (cm/s)* | 5.21 (3.91–6.55) | 6.31 (4.18–6.52) | 0.514 |
Continuous data were presented as mean ± standard deviation if the value was normally distributed and median (1st quartile–3rd quartile) if the value was not. All values were normally distributed except blood flow velocity in LSPV stump of patients. LSPV, left superior pulmonary vein; Lt Low, left lower region; Lt Upp, left upper region; LUL, left upper lobectomy; Rt Low, right lower region; Rt Upp, right upper region.
The ICC between the first versus second measurement was 0.970 (0.948–0.983) and the ICC between two operators was 0.949 (0.912–0.970).
There was a significant difference in the average blood flow velocity only in the Lt Upp between LUL patients and volunteer participants (Lt Upp 8.83 ± 3.22 vs. 13.34 ± 3.50 cm/s, P = 0.008; Lt Low 10.76 ± 4.47 vs. 13.47 ± 3.24 cm/s, P = 0.099; Rt Upp 14.19 ± 4.84 vs. 12.32 ± 3.05 cm/s, P = 0.248; Rt Low 14.79 ± 5.92 vs. 12.90 ± 2.53 cm/s, P = 0.265).
There was no significant difference in the average blood flow velocity at any region between patients with and without thrombosis (Lt Upp 9.30 ± 2.94 vs. 8.46 ± 3.53 cm/s, P = 0.586; Lt Low 12.14 ± 3.68 vs. 9.66 ± 4.91 cm/s, P = 0.239; Rt Upp 13.66 ± 3.54 vs. 14.61 ± 5.84 cm/s, P = 0.675; Rt Low 14.57 ± 5.65 vs. 14.96 ± 6.42 cm/s, P = 0.894; LSPV stump 5.21[3.91–6.55] vs. 6.31[4.18–6.52] cm/s, P = 0.514). Either, there was no significant difference in LSPV stump stasis or maximum velocity between LUL patients with and without thrombosis (stasis 61.58 ± 29.88 vs. 56.12 ± 28.57%, P = 0.700; maximum velocity 24.97 ± 10.83 vs. 21.69 ± 8.24 cm/s, P = 0.490).
Global flow quantification (Table 3)
Table 3.
Global velocity quantification
| LUL patients (n = 18) | Volunteer participants (n = 8) | P value | |
|---|---|---|---|
| Stasis (%) | 25.28 ± 18.64 | 4.71 ± 3.03 | < 0.001 |
| Mean velocity (cm/s) | 9.81 ± 2.49 | 11.40 ± 1.15 | 0.037 |
| Maximum velocity (cm/s) | 12.12 ± 3.12 | 21.97 ± 4.47 | < 0.001 |
| Patients with thrombosis (n = 8) | Patients without thrombosis (n = 10) | P value | |
| Stasis (%)* | 27.65 (7.49–37.87) | 16.79 (12.84–38.77) | 0.746 |
| Mean velocity (cm/s) | 10.15 ± 2.77 | 9.55 ± 2.37 | 0.637 |
| Maximum velocity (cm/s) | 12.23 ± 3.45 | 12.03 ± 3.01 | 0.637 |
Continuous data were presented as mean ± standard deviation if the value was normally distributed and median (1st quartile–3rd quartile) if the value was not. All values were normally distributed except stasis in patients. LUL, left upper lobectomy.
There were significant differences in the LA volume, stasis, mean velocity, and maximum velocity between LUL patients and volunteer participants (volume 88.04 ± 48.54 vs. 38.01 ± 8.50 ml, P < 0.001; stasis 25.28 ± 18.64 vs. 4.71 ± 3.03%, P < 0.001; mean velocity 9.81 ± 2.49 vs. 11.40 ± 1.15 cm/s, P = 0.037; maximum velocity 12.12 ± 3.12 vs. 21.97 ± 4.47 cm/s, P < 0.001).
There was no significant difference in the LA volume, stasis, mean velocity, or maximum velocity between LUL patients with and without thrombosis [volume 73.29 ± 18.09 vs. 99.83 ± 62.13 ml, P = 0.224; Stasis (%) 27.65 (7.49–37.87) vs. 16.79 (12.84–38.77) %, P = 0.736; mean velocity 10.15 ± 2.77 vs. 9.55 ± 2.37 cm/s, P = 0.637; maximum velocity 12.23 ± 3.45 vs. 12.03 ± 3.01 cm/s, P = 0.899].
Visual assessment (Table 4)
Table 4.
Blood flow pattern in the left atrium between patients with LUL with and without thrombosis
| Patients with thrombosis (n = 8) | Patients without thrombosis (n = 10) | P value | |
|---|---|---|---|
| Collision pattern | 3 | 9 | |
| No collision pattern | 5 | 1 | |
| 0.0188 | |||
| vortex | 4 | 5 | |
| counterclockwise* | 4 | 3 | |
| other# | 0 | 2 | |
| no vortex | 4 | 5 | |
| 0.751 | |||
| Volunteer participants (n = 8) | P value | ||
| Collision pattern | 8 | ||
| No collision pattern | 0 | ||
| N/A | |||
| vortex | 8 | ||
| counterclockwise* | 7 | ||
| other# | 1 | ||
| no vortex | 0 | ||
| N/A |
* Counterclockwise from the cranial side. # Counterclockwise from the right side. LUL, left upper lobectomy.
In each visual evaluation of the blood flow collision and vortex flow, one assessment was disagreed upon. For these patients, final classifications were decided after the elaborate discussion between the two radiologists. The inter-observer agreement was high, both in the evaluation of the blood flow collision and vortex flow (kappa value = 0.879 and 0.888).
Vortex formation was observed in all the eight volunteer participants and no collision pattern was not observed in volunteer participants.
Five out of eight patients with thrombosis were classified into no collision pattern. On the other hand, only one out of ten patients without thrombosis was classified into no collision pattern. No collision pattern had a significantly higher proportion in patients with thrombosis (P = 0.0188). The sensitivity, specificity, positive predictive value, and negative predictive value of this imaging sign was 62.5%, 90%, 83.3%, and 75.0%. The vortex flow was observed in four out of eight patients with thrombosis, all of whom had counterclockwise vortex flow. In the patients without thrombosis, three out of ten patients were classified into counterclockwise vortex, two into clockwise vortex, and five into no vortex. There was no significant difference on the presence or absence of blood flow vortex in the LA between the patients with and without thrombosis (P = 0.635).
Disscussion
In the current study, 4D flow MRI clarified several blood flow characteristics in LUL patients. The regional blood flow velocity at LSPV inflow part was significantly lower in LUL patients compared with that of healthy participants but was not different between the patients with and without thrombosis. Either, flow parameter in the LSPV stump was not different between the patients with and without thrombosis. In the global blood flow quantification in the LA, average velocity, maximum velocity, and flow stasis were not associated with thrombosis in the patients with LUL. In the visual assessment of the flow pattern, no collision pattern was frequently observed in LUL patients with thrombosis (62.5%). This pattern was rarely observed in the patients without thrombosis (10%). Therefore, this imaging sign had high specificity (90%).
Regarding the flow analysis, the strength in the current study was that a dual-VENC scheme (VENC, 50 and 150 cm/s) was applied.22 Despite the scan prolongation where one k-space segment becomes 4TR to 7TR, we were able to minimize the scan time penalty by combining a highly accelerated k-t principle component analysis (PCA) technique (acceleration factor = 8).23 Though the direct comparison to the conventional 4D flow MRI was not performed in the current study itself, the accuracy of quantification and visual analysis was expected to be improved.
Cardiac MRI (CMR) with contrast injection has equivalent diagnostic accuracy for the detection of LAA thrombus compared to the gold standard, transesophageal echocardiography.24 Therefore, contrast-enhanced CMR including 4D flow MRI may be utilized as one-stop shopping imaging modality for the patients with the risk of LAA thrombus though further prospective study is needed.
The flow quantification of LA
For the quantification of the blood flow characteristics in the current study, the regional and global blood flow measurements in the LA including LSPV stump were performed. We measured the inflow tract from LSPV, where the VOI with sufficient volume could be placed. The blood flow in this region was expected to represent the blood flow velocity in the LSPV stump. In addition to the regional blood flow quantification, we performed global blood flow quantification in the LA. Before starting the study, we had assumed that the blood flow in the LA was more important than regional blood flow with regards to thrombus formation. This assumption was based on the previous studies evaluating the patients with AF.13,15–17 In these studies, global LA blood flow velocity was associated with the risk of thrombosis. On the other hand, the blood flow velocity in the LA appendage (LAA) was constant regardless of disease conditions though most of the AF thrombi occur within the LAA.16 The low spatial resolution of 4D flow MRI may cause the quantification error due to the partial volume effect or the blur from the respiratory and cardiac motion. One can assume that the blood flow in the LA represents and/or influences blood flow in the LAA.25 This context was expected to be the same as LSPV stump thrombosis after LUL.
In the patients after LUL, the mean blood flow velocity in the LA was 9.82 cm/s which is lower than AF patients in the previous reports (10–13 cm/s).16,21 In the stasis analysis, the blood flow velocity did not exceed 10 cm/s in one-fourth regions in the LA during any cardiac phase in the LUL patients, while only less than 5% regions in volunteers. It must be noted that the previous studies used slightly different methodology and/or velocity threshold based on single-VENC 4D flow MRI in which slow flow within velocity noise might be overestimated.13,15–17,21 The decrease in the total blood flow volume from pulmonary veins after thrombectomy leads to the higher prevalence of flow stasis in LUL cohort.21,26 Unexpectedly, any quantified value did not discriminate the patients with thrombosis from those without. Further metrics that were associated with flow alteration, such as vortex and energy loss quantification, may be needed.13,21,27
The flow visualization of LA
Several studies evaluating cardiac flow dynamics have pointed out that a flow collision from the pulmonary veins followed by vortex flow formation is one of the standard flow patterns in the LA.11–14,18,21 The study conducted by Kilner et al. showed that inflows with a relatively higher volume from the left side contributed a counterclockwise vortex.12 The significance of the vortex flow has been considered as below. First, the rotational flow avoids energy dissipation by limiting flow separation and instability.11,12 Second, the vortex flow with higher velocities along the arterial wall may have washing and rinse effects resulting in the prevention of thrombosis.11,21 One previous report separately quantifying the blood flow profile in the center and peripheral (near wall) regions of the LA supported this hypothesis.17 The study revealed that the blood flow velocity in the peripheral regions was preserved compared to the center regions in the healthy volunteers, which flow pattern is an opposite phenomenon in the conduit tube, such as the aorta. Interestingly, the same study also reported that a lower blood flow velocity in the peripheral regions was observed in the patients with persistent AF, who had higher risk of acquiring thrombosis.
In the current study, the absence of blood flow collision from pulmonary veins was highly associated with the thrombosis after LUL. This is because the collision is the initial driving force of vortex flow. After lung resection, especially in upper lobe lobectomy, residual lung inflates to compensatory values. Therefore, deformation of lung should be shown even without lobular torsion.28 Lung deformation can cause pulmonary vein angle rotation, leading to changes in blood inflow pattern in the LA. This torsion might cause the change of the collision pattern.
In contrast to the blood flow collision, the vortex formation itself was not associated with thrombosis after LUL. It might be derived from the limitation in terms of visual inspection. To maintain the inter-observer reproducibility, we assigned the presence of the vortex when there existed any small vortex, some of which did not have enough contribution for washing the LA. It has been already mentioned that the various vortex flow patterns exist in the LA depending on age, gender, and disease conditions.13,14,21 Further studies quantifying vorticity may be needed.13,21 Among the nine patients in the vortex flow group, two did not have the normal vortex pattern. This can relate to blood flow changes in the LA after LUL.
Previous investigations of the thrombosis after lobectomy especially in LUL
The postoperative thrombosis after LUL is a well-known complication.29–31 In this decade, its relationship with cerebral or other infarctions in other organs has been originally reported by several Japanese researchers.3–5,7 In their studies, LUL had a higher risk compared with other lobectomies. Following these studies, the same disease entity was reported in other countries.1,6,32 This relationship was not discussed until recently. Two reasons can be assumed. First, postoperative follow-up was generally done with unenhanced CT by which thrombosis at the pulmonary vein stump cannot be detected. Second, if the embolism at the brain or other organs occurs, clinicians assumed it as cancer-related because the cancer itself has a high risk for developing thrombosis.33 This assumption was disproved by the large nationwide study.1 They recruited 20624 subjects who underwent pneumonectomy and lobectomy. In the study, a detailed comparison between lung cancer with and without surgery, or lung cancer with pneumonectomy or lobectomy and with wedge resection, was performed to exclude the bias from other risk factors, such as cancer itself, higher burden of comorbidity, and life-style. Their result confirmed that pneumonectomy or lobectomy was independently associated with the development of stroke regardless of atrial fibrillation.
The current hypothesis for the thrombosis formation after LUL
In this decade, the underlying mechanism for the higher prevalence of thrombosis after LUL than other lobectomies has been discussed. Several studies mentioned that the LSPV stump is longer than other PV stumps, resulting in the flow stasis in this stump.3,6,7,34,35 Ohtaka et al. measured the length of each pulmonary vein stump in 38 patients after lobectomy.3 They showed LSPV stump was significantly longer than other PV stumps (median, 1.71 cm of LSPV stump vs. 0.50, 0.56, and 0.54 cm of right inferior, right superior, and left inferior PV stump, respectively). One previous study evaluated whether shortening the LSPV stump could prevent the thrombosis formation.35 They ligated the LSPV at the pericardial reflection, which was a more proximal region of LSPV, resulting in shortened LSPV stump and halving the length compared with conventional surgery. Though the number of recruited patients was small (n = 8 with proximal ligation), the prevalence of the thrombosis was reduced.
In addition to the evaluation of the morphological characteristics of the LSPV stump, its blood flow characteristics were also evaluated in another study. Ohtaka et al. measured blood flow velocity in the stump by using intraoperative ultrasonography.8 They revealed that the blood flow was significantly slower in the LSPV stump than the right superior or right inferior pulmonary vein stump (P value, 0.048 or 0.015), but not than the left inferior PV stump (10.4 cm/s in the LSPV stump vs. 15.4, 20.9, and 12.9 cm/s in the right superior, right inferior, and left inferior PV stump, respectively). This study showed the tendency of decreased blood flow velocity in LSPV but with wider overlap among PV stumps. From the results, one can assume that the decreased blood flow velocity in the PV stump is not the sole cause associated with thrombosis.
Another mechanism of thrombus formation is the physiological response to tissue injury after surgery. One case study reported that a thrombus with platelet aggregation and leukocyte recruitment was observed in a patient who developed cerebral embolism 27 hours after lung lobectomy.36 This pathological finding suggested endothelial injury at the pulmonary vein stump caused by surgery. In line with the suggestion, one previous study showed that the operative time weakly correlated to the thrombosis generation.3 However, this correlation was not generally observed in other studies.4,6,7
One of the common risk factors of the thrombosis generation in the LA is AF, for which left lobectomy might be a risk factor.37 However, the previous studies considering AF proved that the effect is minimum.1,3–5,7 The large cohort study revealed that the incidence rate ratio (ICC) of thrombosis after lobectomy or pneumonectomy was higher, regardless of the presence of AF (4.66 without AF and 5.43 with AF).1
The relevance of the factors described above in the LA thrombosis is still not clear. Further study evaluating blood flow in the individuals at the timing of both pre- and post-operation and/or the pathological assessment of thrombectomy specimens may be desirable.38 In the future, the blood flow assessment just after surgery and/or the computational fluid dynamic simulation before lung surgery would be desirable to support the decision-making regarding the indication of post-operative administration of anti-coagulation drugs.26,39
Limitations
In this study, only LUL patients were included, and the results could not represent the whole-lung dissection in patients of lung cancer. The aim of the study was not the comprehensive evaluation among patients with all kinds of lobectomies, but rather focusing on evaluating blood flow characteristics associated with thrombosis after LUL. Furthermore, the small number of patients could be a limitation in this study. Nevertheless, the specific blood flow pattern was observed in LUL patients with thrombosis group. The segmentation of the LA was performed by a single observer, which might impair the reliability of the analysis. However, high inter-observer reproducibility has been already confirmed in the previous studies.15,17 The background of volunteers does not match the patients. In addition, 4D flow MRI without contrast injection among volunteer group may hamper the accurate detection of blood flow. SNR of 4D flow MRI without contrast injection decreases to two thirds compared with that with contrast injection. However, even without contrast injection, SNR was maintained at above 40.40 In that situation, the difference of standard deviation of velocity between with and without contrast injection is estimated as lower than 1 cm/s even under 150cm/s single-VENC acquisition. In addition, the main evaluation of this preliminary study was to compare the LUL patients with and without thrombosis to clarify the specific blood flow characteristics associated with thrombosis after LUL.
Conclusion
4D flow MRI revealed the decrease in the blood flow velocity with higher prevalence of the stasis in the LA after LUL. In these patients, the net blood flow velocity was not associated with the thrombus formation. In contrast, a specific blood flow pattern, the absence of blood flow collision from pulmonary veins, correlates to the thrombus formation in the LA.
Acknowledgments
This work was supported by JSPS KAKENHI (Grant Number 17K18160 and 19K17151).
Footnotes
Conflicts of Interest
Makoto Obara is an employee of Philips Electronics Japan. Olgierd Leonowicz is a contractor of PMOD Technologies LlC. Only non-Philips or non-Pmod Inc employees had control of the inclusion of data and information. The other authors declare no conflicts of interest.
Supplementary Materials: Supplemental movies 1 and 2
These movies were recorded from cranial side and dorsal (posterior) side. The left atrium was segmented as yellow translucent VOI and LSPV stump as red translucent VOI. Each colored pathline represents blood flow from each pulmonary vein (Light blue, RSPV flow; blue, RIPV flow; light green, LIPV flow). In these movies, the LIPV flow and RSPV flow collide with each other. The key images are referred to Fig. 3.
Supplemental movies 3 and 4
These movies were recorded from cranial side and dorsal (posterior) side. The left atrium was segmented as yellow translucent VOI and LSPV stump as red translucent VOI. Each colored pathline represents the blood flow from each pulmonary vein (Light blue; RSPV flow, blue; RIPV flow, light green; LIPV flow). In this movie, the flow does not collide and dissipate to the left atrium wall. The key images are referred to Fig. 4.
References
- 1.Riddersholm S, Tayal B, Kragholm Ket al. Incidence of stroke after pneumonectomy and lobectomy. Stroke 2019; 50:1052–1059. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto K, Sato S, Okumura Met al. Frequency of cerebral infarction after pulmonary resection: a multicenter, retrospective study in Japan. Surg Today 2018; 48:571–572. [DOI] [PubMed] [Google Scholar]
- 3.Ohtaka K, Hida Y, Kagaet al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann Thorac Surg 2013; 95:1924–1928. [DOI] [PubMed] [Google Scholar]
- 4.Ohtaka K, Hida Y, Kaga Ket al. Left upper lobectomy can be a risk factor for thrombosis in the pulmonary vein stump. J Cardiothorac Surg 2014; 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hattori A, Takamochi K, Kitamura Yet al. Risk factor analysis of cerebral infarction and clinicopathological characteristics of left upper pulmonary vein stump thrombus after lobectomy. Gen Thorac Cardiovasc Surg 2019; 67:247–253. [DOI] [PubMed] [Google Scholar]
- 6.Xie N, Meng X, Wu Cet al. Both left upper lobectomy and left pneumonectomy are risk factors for postoperative stroke. Sci Rep 2019; 9:10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto T, Suzuki H, Nagato Ket al. Is left upper lobectomy for lung cancer a risk factor for cerebral infarction? Surg Today 2016; 46:780–784. [DOI] [PubMed] [Google Scholar]
- 8.Ohtaka K, Takahashi Y, Uemura Set al. Blood stasis may cause thrombosis in the left superior pulmonary vein stump after left upper lobectomy. J Cardiothorac Surg 2014; 9:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichimura H, Ozawa Y, Nishina Het al. Thrombus formation in the pulmonary vein stump after left upper lobectomy: a report of four cases. Ann Thorac Cardiovasc Surg 2014; 20 Suppl:613–616. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M, Takegahara K, Inoue Tet al. 4D flow MR imaging reveals a decrease of left atrial blood flow in a patient with cardioembolic cerebral infarction after pulmonary left upper lobectomy. Magn Reson Med Sci 2020; 19:290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fyrenius A, Wigström L, Ebbers Tet al. Three dimensional flow in the human left atrium. Heart 2001; 86:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilner PJ, Yang GZ, Wilkes AJet al. Asymmetric redirection of flow through the heart. Nature 2000; 404:759–761. [DOI] [PubMed] [Google Scholar]
- 13.Cibis M, Lindahl TL, Ebbers Tet al. Left atrial 4D blood flow dynamics and hemostasis following electrical cardioversion of atrial fibrillation. Front Physiol 2017; 8:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Föll D, Taeger S, Bode Cet al. Age, gender, blood pressure, and ventricular geometry influence normal 3D blood flow characteristics in the left heart. Eur Heart J Cardiovasc Imaging 2013; 14:366–373. [DOI] [PubMed] [Google Scholar]
- 15.Lee DC, Markl M, Ng Jet al. Three-dimensional left atrial blood flow characteristics in patients with atrial fibrillation assessed by 4D flow CMR. Eur Heart J Cardiovasc Imaging 2016; 17:1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markl M, Lee DC, Furiasse Net al. Left Atrial and Left Atrial Appendage 4D Blood Flow Dynamics in Atrial Fibrillation. Circ Cardiovasc Imaging 2016; 9:e004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markl M, Lee DC, Ng Jet al. Left atrial 4-Dimensional flow magnetic resonance imaging: stasis and velocity mapping in patients with atrial fibrillation. Invest Radiol 2016; 51:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suwa K, Saitoh T, Takehara Yet al. Characteristics of intra-left atrial flow dynamics and factors affecting formation of the vortex flow – analysis with phase-resolved 3-dimensional cine phase contrast magnetic resonance imaging. Circ J 2015; 79:144–152. [DOI] [PubMed] [Google Scholar]
- 19.Azarine A, Garçon P, Stansal Aet al. Four-dimensional flow MRI: principles and cardiovascular applications. Radiographics 2019; 39:632–648. [DOI] [PubMed] [Google Scholar]
- 20.Iwata K, Matsuda J, Imori Yet al. Four-dimensional flow magnetic resonance imaging reveals the reduction in turbulent kinetic energy after percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy. Eur Heart J 2020; 41:1454. [DOI] [PubMed] [Google Scholar]
- 21.Garcia J, Sheitt H, Bristow MSet al. Left atrial vortex size and velocity distributions by 4D flow MRI in patients with paroxysmal atrial fibrillation: Associations with age and CHA2 DS2 -VASc risk score. J Magn Reson Imaging 2020; 51:871–884. [DOI] [PubMed] [Google Scholar]
- 22.Knobloch V, Binter C, Gülan Uet al. Mapping mean and fluctuating velocities by Bayesian multipoint MR velocity encoding-validation against 3D particle tracking velocimetry. Magn Reson Med 2014; 71:1405–1415. [DOI] [PubMed] [Google Scholar]
- 23.Giese D, Schaeffter T, Kozerke S. Highly undersampled phase-contrast flow measurements using compartment-based k-t principal component analysis. Magn Reson Med 2013; 69:434–443. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Zhang H, Zhu Det al. Cardiac MRI for detecting left atrial/left atrial appendage thrombus in patients with atrial fibrillation: Meta-analysis and systematic review. Herz 2019; 44:390–397. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Assa E, Manning WJ. When Virchow meets Da Vinci: correlating thrombogenesis with intracardiac flow dynamics. Circ Cardiovasc Imaging 2016; 9:e005438. [DOI] [PubMed] [Google Scholar]
- 26.Lantz J, Gupta V, Henriksson Let al. Impact of pulmonary venous inflow on cardiac flow simulations: comparison with in vivo 4D flow MRI. Ann Biomed Eng 2019; 47:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker AJ, van Ooij P, Bandi Ket al. Viscous energy loss in the presence of abnormal aortic flow. Magn Reson Med 2014; 72:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai J, Xie D, Wang Het al. Predictors of survival in lung torsion: a systematic review and pooled analysis. J Thorac Cardiovasc Surg 2016; 152:737–745.e3. [DOI] [PubMed] [Google Scholar]
- 29.Hovaguimian H, Morris JF, Gately HLet al. Pulmonary vein thrombosis following bilobectomy. Chest 1991; 99:1515–1516. [DOI] [PubMed] [Google Scholar]
- 30.Porres DV, Morenza OP, Pallisa Eet al. Learning from the pulmonary veins. Radiographics 2013; 33:999–1022. [DOI] [PubMed] [Google Scholar]
- 31.Venter CP, Dannheimer IP. Pulmonary venous thrombosis complicating lobectomy. S Afr Med J 1973; 47:2339–2342. [PubMed] [Google Scholar]
- 32.Moon MH, Beck KS, Moon YKet al. Incidence and clinical features of the incidentally found vascular stump thrombus during routine follow up after oncologic lung surgery. PLoS One 2017; 12:e0185140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navi BB, Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol 2018; 83:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassani C, Saremi F. Comprehensive cross-sectional imaging of the pulmonary veins. Radiographics 2017; 37:1928–1954. [DOI] [PubMed] [Google Scholar]
- 35.Nakano T, Kaneda H, Kawaura Tet al. Ligating the pulmonary vein at the pericardial reflection is useful for preventing thrombus formation in the pulmonary vein stump after left upper lobectomy. Gen Thorac Cardiovasc Surg 2019; 67:450–456. [DOI] [PubMed] [Google Scholar]
- 36.Usui G, Takayama Y, Hashimoto Het al. Cerebral embolism caused by thrombus in the pulmonary vein stump after left lower lobectomy: A case report and literature review. Intern Med 2019; 58:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin Y, Hida Y, Kaga Ket al. Left lobectomy might be a risk factor for atrial fibrillation following pulmonary lobectomy. Eur J Cardiothorac Surg 2014; 45:247–250. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto H, Usui G, Tsugeno Yet al. Cerebral thromboembolism after lobectomy for lung cancer: pathological diagnosis and mechanism of thrombus formation. Cancers (Basel) 2019; 11:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lantz J, Gupta V, Henriksson Let al. Intracardiac flow at 4D CT: comparison with 4D flow MRI. Radiology 2018; 289:51–58. [DOI] [PubMed] [Google Scholar]
- 40.Bock J, Frydrychowicz A, Stalder AFet al. 4D phase contrast MRI at 3 T: effect of standard and blood-pool contrast agents on SNR, PC-MRA, and blood flow visualization. Magn Reson Med 2010; 63:330–338. [DOI] [PubMed] [Google Scholar]