Abstract
In this study, we present images acquired by a fast-imaging method for the evaluation of endolymphatic hydrops after intravenous administration of a single dose of gadolinium-based contrast agent. We utilized the hybrid of reversed image of MR cisternography and a positive perilymph signal by heavily T2- weighted 3D-fluid attenuated inversion recovery-multiplied by T2 (HYDROPS2-Mi2) method combined with deep learning reconstruction denoising. The scan time for the fast protocol was approximately 5 mins, which is far shorter than previously reported scan times. The fast acquisition provides similar image quality and less motion artifacts compared to the longer method.
Keywords: magnetic resonance imaging, gadolinium, endolymphatic hydrops, artificial intelligence
Imaging of endolymphatic hydrops in Meniere’s disease with a single dose of intravenous gadolinium-based contrast agent (GBCA) is widely used.1–3 There is a report using a double dose of intravenous GBCA.4 However, considering the issue of gadolinium accumulation in the brain, it is difficult to justify this use of a double dose. In some countries, the use of more than a single dose may not be approved clinically.
To facilitate the imaging evaluation of endolymphatic hydrops, it is important to visualize the endolymph, perilymph, and surrounding bone separately, so that these three entities can be distinguished. The following methods have already been proposed for this purpose. First, the hybrid of reversed image of positive endolymph signal and native image of positive perilymph signal (HYDROPS) method, which subtracts a positive perilymph image (PPI) and a positive endolymph image (PEI)5 with different inversion times has been reported. Another study described the hybrid of reversed image of MR cisternography and positive perilymph signal by heavily T2-weighted 3D-fluid attenuated inversion recovery (FLAIR) (HYDROPS2) method. This method subtracts the MR cisternography (MRC) image from the heavily T2-weighted 3D-FLAIR image, which is a PPI.5 Finally, HYDROPS-multiplied by heavily T2-weighted MR cisternography (Mi2) and HYDROPS2-Mi2, which dramatically increase the contrast-to-noise ratio between the endolymph and perilymph by multiplying by the T2-weighted MRI cisternography, have also been reported.5,6 These methods use subtraction techniques, which can be susceptible to misregistration from body movement. As a non-subtraction method, an improved 3D-real inversion recovery (IR) imaging developed to diagnose endolymphatic hydrops with a single dose of intravenous GBCA has been reported.7
For all of the above, excellent images have already been reported with a single dose of intravenous GBCA; however, a long imaging time of more than 30 mins was initially required to generate the HYDROPS and HYDROPS-Mi2 images. Recently, the imaging time was reduced to approximately 10 mins in the improved 3D-real IR method7 and approximately 14 mins in the HYDROPS method.5 Currently, the shortest imaging protocol that can distinguish the perilymph, endolymph, and bone with a single dose of intravenous GBCA is approximately 8 mins for HYDROPS2 and HYDROPS2-Mi2.8 Longer imaging times may result in artifacts due to body movement and may decrease scan throughput. The evaluation of endolymphatic hydrops by MRI has been incorporated into the diagnostic criteria for Meniere’s disease.9 Considering that the clinical application of this imaging method will be applied more frequently in the future, a shorter scan time would be particularly useful.
Recently, it was reported that artificial intelligence image reconstruction can further increase the contrast-to-noise ratio of HYDROPS-Mi2 images and produce excellent images that differentiate between the endolymph, perilymph, and bone in 3D space.10 The total imaging time was reported to be approximately 20 mins. However, the extremely good contrast-to-noise ratio of these images seemed to indicate a possibility for further imaging time reduction.
Our group started to apply an imaging protocol that utilized artificial intelligence image reconstruction as described above to acquire images in approximately 5 mins, which distinguished the endolymph, perilymph, and surrounding bone. Initially, this method was meant to be a backup to the conventional method in case of body movement. The purpose of this paper is to present the images obtained in 5 mins with this new protocol and to compare to those obtained by the conventional method of 12 mins. Furthermore, we suggest that this imaging method could be adopted for the widespread use in clinical practice.
In the present clinical protocol, all patients were imaged using a 32-channel head coil on a clinical 3T MR scanner (Vantage Centurian; Canon Medical Systems, Tochigi, Japan) 4 hours after an intravenous injection of a single dose (0.1 mmol/kg) of GBCA, gadobutrol (Gadovist; Bayer Yakuhin, Osaka, Japan). MR imaging data for the generation of HYDROPS-Mi2 were acquired in 20 mins under the previously reported imaging conditions.10 From these data, a conventional HYDROPS2-Mi2 was created using only the PPI and MRC. A new clinical MR imaging protocol for endolymphatic hydrops evaluation in our hospital includes data acquisition for the generation of a 20-min HYDROPS-Mi2 (448 seconds PPI, 448 seconds PEI, and 287 seconds MRC), as well as a 4-min PPI and a 1-min MRC for a backup in case of body movement. We created a shortened imaging time version of HYDROPS2-Mi2 (sHYDROPS2-Mi2) from the 5-min data and compared it to the conventional HYDROPS2-Mi2 (cHYDROPS2-Mi2) in terms of distinguishing endolymphatic hydrops and the presence of misregistration artifacts. Deep learning reconstruction (DLR) (Advanced Intelligent Clear-IQ Engine [AiCE]; Canon Medical Systems) was applied for all MR images.10
The reduction in imaging time from the conventional method was mainly achieved by decreasing the number of slices. Other parameters were also slightly altered. The detailed imaging parameters are described in Table 1.
Table 1.
Pulse sequence parameters
| Sequence name | Type | TR (ms) | Echo time (ms) | Inversion time (ms) | Flip angle (degree) | Section thickness/reconstruction step (mm) | Pixel size (mm) | Number of slices | Echo spacing (ms) | Echo train length | FOV (mm) | Matrix size | Number of excitations | Scan time (min) | SPEEDER (phase direction x slice direction) | Scan option | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional protocol | Conventional HYDROPS2-Mi2 (12 mins) | MR cisternography | FASE3D | 3500 | 299 | NA | 90–160 constant | 2.0/1.0 | 0.5 x 0.5 | 224 | 6.5 | 158 | 180 x 200 | 352 x 384 | 1 | 4min 47s | 2 x 1.4 | Flip back pulse at the end of echo train |
| Positive perilymph image (heavily T2-weighted 3D-FLAIR) | FASE3D-MPV with inversion pulse | 16000 | 546 | 2850 | 90–170-constant 160 | 2.0/1.0 | 0.5 x 0.5 | 224 | 6.5 | 258 | 180 x 200 | 352 x 384 | 1 | 7min 28s | 2 x 1.5 | Frequency selective fat suppression pre-pulse | ||
| Positive endolymph image | FASE3D-MPV with inversion pulse | 16000 | 546 | 2400 | 90–170-constant 160 | 2.0/1.0 | 0.5 x 0.5 | 224 | 6.5 | 258 | 180 x 200 | 352 x 384 | 1 | 7min 28s | 2 x 1.5 | Frequency selective fat suppression pre-pulse | ||
| Short protocol | Short HYDROPS2-Mi2 (5 mins) | MR cisternography | FASE3D | 3000 | 299 | NA | 90–160 constant | 2.0/1.0 | 0.5 x 0.5 | 60 | 6.5 | 158 | 180 x 200 | 352 x 384 | 1 | 1min 06s | 2 x 1.0 | Flip back pulse at the end of echo train, slice partial Fourier of 70%, phase over sampling of 20% |
| Positive perilymph image (heavily T2-weighted 3D-FLAIR) | FASE3D-MPV with inversion pulse | 16000 | 546 | 2850 | 90–170-constant 160 | 2.0/1.0 | 0.5 x 0.5 | 60 | 6.5 | 258 | 180 x 200 | 352 x 384 | 1 | 4min 00s | 2 x 1.0 | Frequency selective fat suppression pre-pulse, slice partial Fourier of 75% |
SPEEDER (Canon Medical Systems, Tochigi, Japan): Parallel imaging technique in the image domain. 3D slab is set in an axial orientation. FASE3D-MPV: equivalent to the variable flip angle 3D turbo spin echo technique such as SPACE (sampling perfection with application optimized contrast using different flip angle evolution). FASE, fast advanced spin echo; FLAIR, fluid attenuated inversion recovery; HYDROPS2, hybrid of reversed image of MR cisternography and positive perilymph signal by heavily T2-weighted 3D-FLAIR; HYDROPS2-Mi2, HYDROPS2 multiplied by heavily T2-weighted MR cisternography; MPV, multiplanar voxel.
The HYDROPS2-Mi2 image was created using the following formula11
where k was determined prior to the start of this study by measuring the cochlear signal so that the perilymph signal in the PPI was halved after the subtraction. k was set to 0.08 for both the cHYDROPS2-Mi2 and sHYDROPS2-Mi2 images.
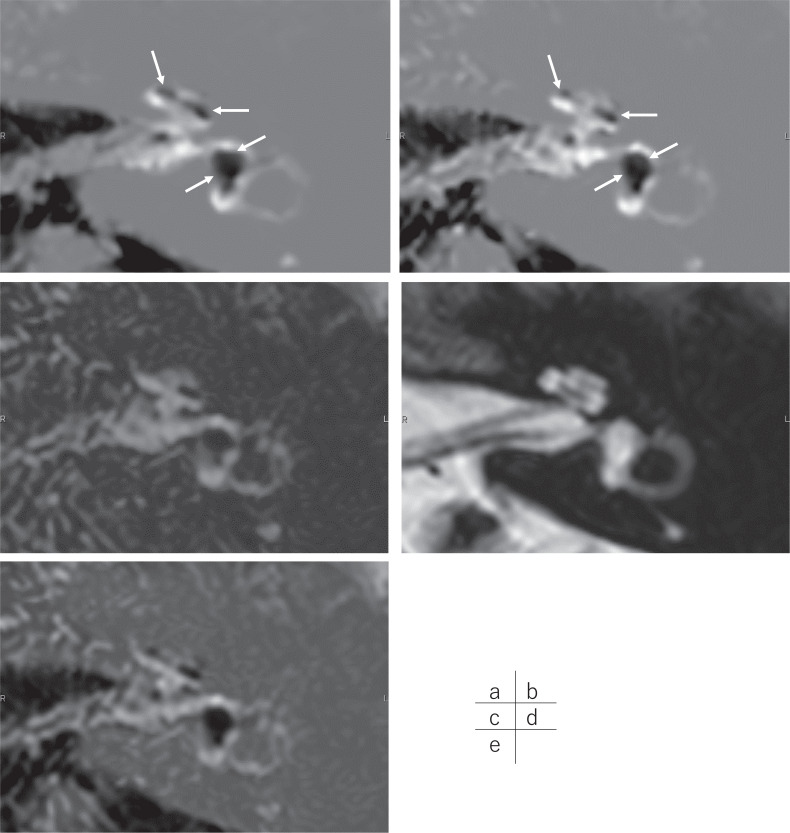
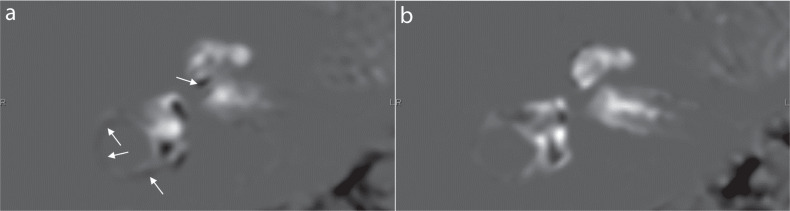
The clinical protocol for the present study included six patients with suspected endolymphatic hydrops. The retrospective study was approved by the ethics committee of our hospital and the requirement for written informed consent was waived. The grading of endolymphatic hydrops was done by an experienced radiologist using the Nakashima grading scale.12 For both imaging methods, the grade was consistent for both the cochlea and the vestibule in the ears of all the patients (Fig. 1). In one patient, there was misregistration due to motion with the conventional method, but there was no obvious movement with the 5-min protocol (Fig. 2).
Fig. 1.
A male patient in his 50s. Images were obtained at 4 hours after intravenous administration of a single dose of gadolinium-based contrast agent. (a) The conventional version of the HYDROPS2-Mi2 image generated from the heavily T2-weighted 3D-FLAIR PPI obtained with 448 seconds scan time and heavily T2-weighted MRC obtained with 287 seconds scan time. Total scan time was 12 mins and 15 seconds. Significant endolymphatic hydrops in the cochlea and the vestibule are clearly visible as the black area (arrows). (b) The shortened scan time version of the HYDROPS2-Mi2 image generated from the PPI obtained over 240 seconds and MRC obtained over 66 seconds. The total scan time was 5 mins and 6 seconds. Significant endolymphatic hydrops in the cochlea and the vestibule are clearly visible as the black areas (arrows). This shorter scan time HYDROPS2-Mi2 image depicts significant endolymphatic hydrops as clearly as the longer scan time image (a). Background noise is slightly more noticeable in the shorter scan time image (b) than in the longer scan time image (a). (c) The source PPI image obtained in 240 seconds to generate a shorter scan time version of the HYDROPS2-Mi2 image (b). (d) The source MRC in 66 seconds to generate a shorter scan time version of the HYDROPS2-Mi2 image (b). (e) The shorter scan time HYDROPS2 image generated by the subtraction of the magnitude adjusted MRC image (i.e., (d)*0.08) from the source PPI image (c). By multiplying (d) onto (e), the final image of the shorter scan time version HYDROPS2-Mi2 image (b) was generated. FLAIR, fluid attenuated inversion recovery; HYDROPS2, hybrid of reversed image of MR cisternography and positive perilymph signal by heavily T2-weighted 3D-FLAIR; HYDROPS2-Mi2, HYDROPS2 multiplied by heavily T2-weighted MR cisternography; MRC, MR cisternography; PPI, positive perilymph image.
Fig. 2.
A female patient in her 40s. Images were obtained at 4 hours after an intravenous administration of a single dose of gadolinium-based contrast agent. There is no endolymphatic hydrops in her right cochlea and vestibule. (a) The conventional version of the HYDROPS2-Mi2 image generated from the PPI and MRC data obtained over 448 seconds and 287 seconds, respectively. The total scan time was 12 mins and 15 seconds. Black misregistration artifacts (arrows) can be seen in the lateral semicircular canal and scala tympani in the basal turn of the cochlea. Anatomically, there is no endolymphatic space in the lower side of the scala tympani of the basal turn of the cochlea. These misregistration artifacts are caused by the movement between the PPI and MR cisternography scans. (b) A shortened scan time version of the HYDROPS2-Mi2 image generated from the PPI and MRC data obtained over 240 seconds and 66 seconds, respectively. The total scan time was 5 mins and 6 seconds. No apparent misregistration artifact can be seen. HYDROPS2, hybrid of reversed image of MR cisternography and positive perilymph signal by heavily T2-weighted 3D-fluid attenuated inversion recovery; HYDROPS2-Mi2, HYDROPS2 multiplied by heavily T2-weighted MR cisternography; MRC, MR cisternography; PPI, positive perilymph image.
With the fast method proposed here, the imaging can be performed in approximately 5 mins, allowing the evaluation of endolymphatic hydrops in an even greater number of patients. Furthermore, even in patients who have undergone contrast-enhanced MR for other purposes, it will also be easier to evaluate endolymphatic hydrops, if necessary, by waiting for 4 hours after the intravenous GBCA injection. In the patient with motion, the body movement seemed to occur during the silent period of the scan interval between the PPI (448 seconds) and the MRC (287 seconds). In the future, it is expected that the MRC and the PPI will be performed as a single continuous sequence to further reduce the possibility of movement between the sequences. It is also expected that the post-processing of the images will be automated to allow a wider distribution of this method.
In conclusion, it was suggested that imaging at 4 hours after the intravenous administration of a single dose of GBCA for the evaluation of endolymphatic hydrops can be sufficiently acquired in approximately 5 mins. Shortening the imaging time is expected to promote even a wider use of MRI for the evaluation of endolymphatic hydrops and to reduce the possibility of image degradation due to body movement.
Footnotes
Conflicts of Interest
Toshiaki Taoka and Rintaro Ito are professors in the Department of Innovative Biomedical Visualization (iBMV), which is financially supported by CANON MEDICAL SYSTEMS CORPORATION. Mayuko Sakai is an employee of CANON MEDICAL SYSTEMS CORPORATION. All other authors declare that they have no conflicts of interest regarding this manuscript.
References
- 1.Naganawa S, Yamazaki M, Kawai H, et al. Visualization of endolymphatic hydrops in Ménière’s disease with single-dose intravenous gadolinium-based contrast media using heavily T(2)-weighted 3D-FLAIR. Magn Reson Med Sci 2010; 9:237–242. [DOI] [PubMed] [Google Scholar]
- 2.Attyé A, Barma M, Schmerber S, et al. The vestibular aqueduct sign: magnetic resonance imaging can detect abnormalities in both ears of patients with unilateral Meniere’s disease. J Neuroradiol 2020; 47:174–179. [DOI] [PubMed] [Google Scholar]
- 3.Bier G, Bongers MN, Schabel C, et al. In vivo assessment of an endolymphatic hydrops gradient along the cochlea in patients with Menière’s disease by magnetic resonance imaging-a pilot study. Otol Neurotol 2018; 39:e1091–e1099. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Hui L, Zhang B, et al. The correlation between endolymphatic hydrops and clinical features of Meniere disease. Laryngoscope 2021; 131:E144–E150. [DOI] [PubMed] [Google Scholar]
- 5.Naganawa S, Nakashima T. Visualization of endolymphatic hydrops with MR imaging in patients with Ménière’s disease and related pathologies: current status of its methods and clinical significance. Jpn J Radiol 2014; 32:191–204. [DOI] [PubMed] [Google Scholar]
- 6.Naganawa S, Suzuki K, Yamazaki M, et al. Time course for measuring endolymphatic size in healthy volunteers following intravenous administration of gadoteridol. Magn Reson Med Sci 2014; 13:73–80. [DOI] [PubMed] [Google Scholar]
- 7.Naganawa S, Kawai H, Taoka T, et al. Improved 3D-real inversion recovery: a robust imaging technique for endolymphatic hydrops after intravenous administration of gadolinium. Magn Reson Med Sci 2019; 18:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naganawa S, Ohashi T, Kanou M, et al. Volume quantification of endolymph after intravenous administration of a single dose of gadolinium contrast agent: comparison of 18- versus 8-minute imaging protocols. Magn Reson Med Sci 2015; 14:257–262. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki S, Shojaku H, Murofushi T, et al. Committee for clinical practice guidelines of Japan society for equilibrium research. Diagnostic and therapeutic strategies for Meniere’s disease of the Japan society for equilibrium research. Auris Nasus Larynx 2021; 48:15–22. [DOI] [PubMed] [Google Scholar]
- 10.Naganawa S, Nakamichi R, Ichikawa K, et al. MR imaging of endolymphatic hydrops: utility of iHYDROPS-Mi2 combined with deep learning reconstruction denoising. Magn Reson Med Sci 2020. August 21. doi: 10.2463/mrms.mp.2020-0082. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naganawa S, Yamazaki M, Kawai H, et al. Imaging of Ménière’s disease by subtraction of MR cisternography from positive perilymph image. Magn Reson Med Sci 2012; 11:303–309. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima T, Naganawa S, Pyykko I, et al. Grading of endolymphatic hydrops using magnetic resonance imaging. Acta Otolaryngol 2009; 129(sup560):5–8. [DOI] [PubMed] [Google Scholar]