Abstract
Coccidioidomycosis is a fungal infection caused by Coccidioides immitis and Coccidioides posadasii. The dimorphic fungi live in the soils of arid and semi-arid regions of the western United States, as well as parts of Mexico, Central America, and South America. Incidence of disease has risen consistently in recent years, and the geographic distribution of Coccidioides spp. appears to be expanding beyond previously known areas of endemicity. Climate factors are predicted to further extend the range of environments suitable for the growth and dispersal of Coccidioides species. Most infections are asymptomatic, though a small proportion result in severe or life-threatening forms of disease. Primary pulmonary coccidioidomycosis is commonly mistaken for community-acquired pneumonia, often leading to inappropriate antibacterial treatment and unnecessary healthcare costs. Diagnosis of coccidioidomycosis is challenging and often relies on clinician suspicion to pursue laboratory testing. Advancements in diagnostic tools and antifungal therapy developments seek to improve the early detection and effective management of infection. This review will highlight recent updates and summarize the current understanding of the epidemiology, diagnosis, and treatment of coccidioidomycosis.
Keywords: coccidioidomycosis, Coccidioides, Valley fever, endemic mycoses, fungal diseases
1. Introduction
Coccidioidomycosis, also known as Valley fever, is an infection caused by the inhalation of airborne arthroconidia from the soil-dwelling fungi, Coccidioides spp. Though often considered a rare disease, the environmental mycosis is a growing public health concern due to rising case counts and evidence of geographic expansion. Symptoms develop in approximately 40% of cases, frequently resembling other respiratory illnesses with signs such as cough, fever, shortness of breath, and fatigue [1]. Clinical findings may be indistinguishable from community-acquired pneumonia (CAP), which can lead to misdiagnosis and delays in appropriate antifungal treatment [2]. Although the infection is usually self-limiting, many patients require antifungal treatment to resolve illness, and a small subset of infections result in life-threatening severe pulmonary or disseminated disease [3,4]. Direct medical costs combined with potential productivity losses constitute a substantial economic burden [5,6,7,8].
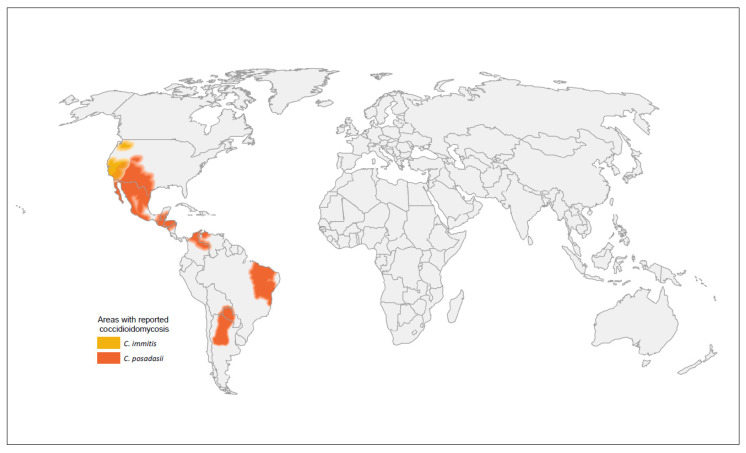
In the United States, coccidioidomycosis is known to be endemic in the Southwest, with southern Arizona and the San Joaquin Valley in California comprising hyperendemic zones [9]. Coccidioides immitis has been found as far north as Washington state [10,11]. Globally, a combination of case reports and skin test studies also established areas of endemicity in Mexico, parts of South America (Argentina, Bolivia, Brazil, Colombia, Paraguay, Venezuela), and parts of Central America (Guatemala, Honduras), though much remains unknown about coccidioidomycosis in these regions because of limited diagnostic capabilities and reporting [12,13]. The global geographic distribution of Coccidioides spp. is displayed in Figure 1. Reported cases of coccidioidomycosis increased steadily in recent years and likely underestimate the true burden of disease.
Figure 1.
Global geographic distribution of the Coccidioides species.
The development of more rapid diagnostics is key to early and accurate diagnosis. Despite improvements in test offerings and performance over time, challenges to coccidioidomycosis diagnosis persist. Results can be difficult to interpret, and sensitivity and specificity may vary based on immunosuppression status and stage of disease. These complexities are compounded by low clinician awareness, particularly outside of known endemic regions [14,15]. Continued developments in antifungal therapy aim to improve patient outcomes and address concerns of toxicities, though questions regarding early treatment and optimal management persist.
This review summarizes the current understanding of the epidemiology, diagnosis, and treatment and management of coccidioidomycosis.
2. Epidemiology
2.1. Increased Number of Reported Cases
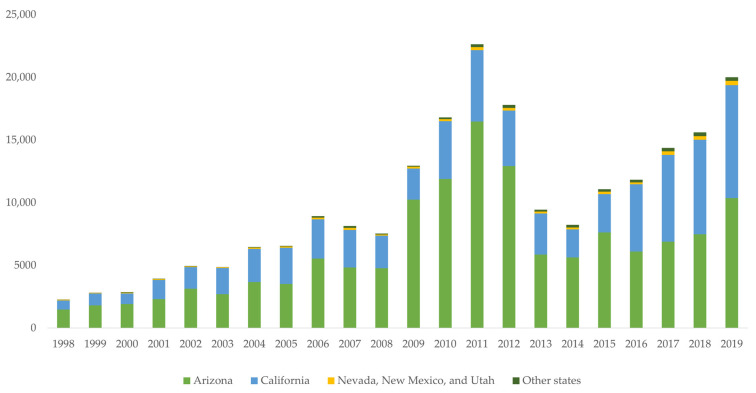
Coccidioidomycosis, caused by Coccidioides immitis and Coccidioides posadasii, is a nationally notifiable disease in the United States, though it is reportable only in 26 states and the District of Columbia [16]. Reportable status is designated by the state or jurisdiction and requires healthcare professionals and laboratories to notify public health departments of cases that meet the Council of State and Territorial Epidemiologists (CSTE) definition. For nationally notifiable diseases or conditions, states voluntarily submit case data to the US Centers for Disease Control and Prevention (CDC) for patients meeting CSTE criteria. The number of cases reported to the CDC rose considerably since 2014, as shown in Figure 2. Following a three-year decline from 2012–2014, case counts more than doubled from 8232 in 2014 to 20,003 in 2019 [17]. Arizona and California, which account for more than 95% of reported cases, showed similar trends in rates of disease. Incidence in Arizona grew from 84.4 cases per 100,000 population to 144.1 per 100,000 from 2014–2019, while California’s incidence more than tripled from 6.0 per 100,000 population to 22.5 per 100,000 population in the same timeframe [18,19]. Based on provisional counts, Arizona’s incidence increased to 161.1 per 100,000 population in 2020 and stayed relatively even at 159.8 per 100,000 population in 2021, while incidence in California dipped to 16.9 per 100,000 in 2020 before rising to 20.7 per 100,000 in 2021 [20,21].
Figure 2.
Coccidioidomycosis case counts submitted to the National Notifiable Diseases Surveillance System, 1998–2019. Case counts reported by individual states might differ slightly from those reported by the National Notifiable Diseases Surveillance System because of differences in the timing of reports or surveillance methods.
The cause of the concerning increase is likely multifaceted. Environmental factors favorable to the growth and subsequent dispersal of Coccidioides spp. may have contributed to a higher frequency of disease [22,23]. The causative fungus is known to live in arid and semi-arid regions, and statistical models demonstrated the influence of temperature and precipitation patterns on the proliferation of Coccidioides spp., though only a weak correlation was found in some highly endemic areas of California [22,24,25,26,27,28,29]. Periods of precipitation facilitate fungal growth in the environment, while ensuing periods of low precipitation and high temperature create ideal conditions to release fungal spores [28,29,30,31]. These effects may be amplified after droughts; incidence in California declined during the 2007–2009 and 2012–2015 droughts but increased markedly in the following two years [23]. Particulate matter of size less than 10 µm (PM10) is also thought to impact coccidioidomycosis incidence, and PM10 concentration rose by 12% in the Southwest and 29% in the West from 2010–2020 [30,32,33].
Population growth may have impacted the rising coccidioidomycosis case counts. Arizona continues to be one of the fastest-growing states, recording a 12% population increase from 2010 to 2020, driven primarily by a 16% increase in the highly-populated Maricopa County; California also experienced a 6% population increase during the same timeframe [34]. Many of the incoming residents are likely immunologically naïve to coccidioidomycosis. Across several endemic regions, desert land was converted to urban and suburban centers to accommodate population influx, resulting in substantial soil disturbance and potential exposure [35,36].
Advances in healthcare practices have expanded the at-risk population for coccidioidomycosis. Prolonged life spans have led to a growing population over 65 years of age [34]. This group has a higher prevalence of chronic disease and is more frequently diagnosed with Coccidioides spp. infection [37]. Developments in therapeutics and medical procedures extended survival for patients with weakened immune systems or previously fatal conditions. The number of stem cell transplants increased by 8% from 2015–2019, while solid organ transplantation grew by 45% from 2011–2021 [38,39]. Use of immunosuppressive agents has become more widespread with greater availability [40]. Transplants and immunosuppressants both represent known risk factors for developing severe coccidioidomycosis [41,42].
Additional factors that may have contributed to the increase in coccidioidomycosis case counts include changed or improved reporting practices, increased laboratory testing, or heightened awareness. Patients in Arizona who heard of the disease before seeking healthcare were diagnosed earlier than patients who were unaware of coccidioidomycosis and were also more likely to request testing [43]. However, surveys of representative samples showed low Valley fever awareness, even in regions of known endemicity [44,45]. In California, only 25.0% of respondents living in high-incidence areas knew that Coccidioides spp. (termed ‘Valley fever fungus’ in the survey) existed in their area of residence, and just 3.5% of respondents with a risk factor for severe coccidioidomycosis knew that they were at an elevated risk for severe infection [45]. Tailored messaging to vulnerable populations may increase the knowledge of disease and consequently influence healthcare-seeking behavior and testing practices.
2.2. Geographic Expansion of Coccidioides Species
The initial geographic distribution of coccidioidomycosis was established in the 1940s and 1950s through extensive coccidioidin skin tests to assess prevalence [9]. Although many cases reported outside of the traditional areas of endemicity are attributed to travel in endemic regions or reactivation of a latent infection, several outbreaks in California and Utah have indicated that the geographic range is extending northward [46,47,48]. Whole-genome sequencing confirmed the local acquisition of coccidioidomycosis in 2010 in Washington. A clinical isolate from the patient and soil isolates retrieved from the suspected point of exposure were found to be identical, providing the first evidence of Coccidioides endemicity in the state [10,49].
Reasons for the expansion beyond traditionally recognized areas are not certain, though several have been theorized. Climate factors are known to influence environmental dynamics, and it is hypothesized they may create suitable conditions for Coccidioides spp. habitation in areas that did not previously support fungal growth. A climate niche model used disease incidence data and climate projections to predict that, by the year 2100, the area of coccidioidomycosis endemicity will more than double and cases will increase by 50%, based on global warming scenarios [24]. Although evidence demonstrated the potential influence of climate change on the future geographic distribution of Coccidioides spp., it is unclear whether climate contributed to the current observed expansion. Climate change may additionally trigger an increase in severe weather events, such as wildfires, which have been linked to infection and cause substantial disruption and dispersal of soil [50,51]. Dust storms (haboobs) were previously thought to drive increases in coccidioidomycosis incidence, but recent studies found no significant association [52,53,54]. Air sampling studies in Arizona even suggest that dust storms may diminish the concentration of arthroconidia in the air [54].
Some research also suggests that rodents may contribute to the geographic distribution by serving as a reservoir for coccidioidomycosis, though conflicting results called this theory into question. The hypothesis, first proposed in the 1940s, was largely discarded following contradictory results from large-scale soil sampling [55,56]. However, several studies since found a correlation between the amount of Coccidioides spp. recovered from soil obtained from rodent burrows compared with surrounding topsoil [57,58,59]. Contemporary genomic analysis also spurred a resurgence of the theory, as the findings indicated a higher proportion of animal versus plant tissue-associated genes [60]. Notably, recent systematic soil sampling in Washington did not yield any association between rodent burrows and the presence of Coccidioides [49]. The rodent hypothesis offers a conceivable explanation for the fungus’ patchy distribution in endemic regions, but more research is needed to better understand the role of rodents in the Coccidioides life cycle. Continued surveillance will be essential to monitor trends in case counts and geographic expansion.
2.3. Risk Factors
Coccidioidomycosis can affect anyone who is exposed to the causative fungus, but certain groups may be at higher risk of infection or severe disease. People with weakened immune systems have demonstrated greater susceptibility to disease. Elevated risk exists for severe coccidioidomycosis among people living with HIV/AIDS, particularly those with low CD4 counts, though incidence among this population decreased with the advent of antiretroviral therapy [61,62,63]. Transplant recipients and patients receiving immunosuppressive medications such as corticosteroids, chemotherapy, or tumor necrosis factor inhibitors represent additional at-risk groups owing to cellular immunodeficiencies [64,65].
Reactivation of a latent infection is also a concern among transplant recipients. Transplant centers in endemic areas may administer antifungal prophylaxis to prevent recurrence of coccidioidomycosis, often up to one-year post-transplantation [4]. Both universal and targeted programs aimed at patients with positive serologic results or a history of Coccidioides spp. infection demonstrated encouraging results [66,67,68,69]. However, no consensus exists regarding drug type or duration [69,70]. Further research is needed to define optimal prophylaxis strategies among certain patient groups.
Pregnancy is an established risk factor for coccidioidomycosis. Evidence suggests a correlation between the severity of coccidioidomycosis and the length of gestation, with the most severe disease occurring in the late stages of pregnancy and the immediate post-partum period. Illness in pregnant women is further complicated by treatment challenges because azole antifungals are known teratogens [71,72]. Epidemiologic studies showed that people of African American or Filipino descent are at a heightened risk for infection, particularly severe or disseminated disease, though reasons are unknown [73,74,75].
Occupational hazards have been documented for workers exposed to dust from soil disturbance. Jobs involving digging, excavation, or soil disruption in endemic areas are considered to pose the greatest risk of coccidioidomycosis; common examples include construction, archaeology, agriculture, firefighting, and mining, gas, or oil extraction [76]. Although the aforementioned activities are more commonly associated with Coccidioides spp. exposure, cases related to minimal soil disturbance have been reported, such as an outbreak among cast and crew on an outdoor television set, as well as extremely high rates among employees and inmates at state prisons in California [77,78].
Researchers have long postulated that males are at an elevated risk of Coccidioides spp. infection based on the observed differences in disease incidence by sex [79,80,81]. Some claim that the disparity is likely because of the disproportionate representation of males in occupations or recreational activities associated with dust or soil exposure. Recent data furthered the assertion of sex as an independent risk factor through a survey of human patients, veterinary patients, and nonhuman primates [82]. Results showed significantly higher rates of infection, severe disease, and greater average maximum serum complement fixation (CF) titers among human males compared with females. Significant differences in incidence emerged at age 19 and remained until age 80. Additionally, nonhuman primate and unaltered canine males exhibited a significantly increased risk for coccidioidomycosis compared with their female counterparts, though no significant sex differences were observed between castrated male dogs and female dogs [82]. Findings from this study suggest that biological mechanisms may contribute to the reported differences in Coccidioides spp. infection between males and females.
2.4. Coccidioidomycosis and COVID-19
Several reports described a co-infection of Coccidioides spp. and severe acute respiratory syndrome novel coronavirus (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19) [83,84,85,86,87,88,89,90]. Common risk factors include older age, diabetes, immunosuppression, African American or Latino heritage, and smoking [91]. The interaction between the two infections remains largely unclear. Underlying respiratory illness and chronic lung disease are associated with severe COVID-19, suggesting that patients with chronic pulmonary coccidioidomycosis may be at an elevated risk of severe COVID-19 [92,93,94]. SARS-CoV-2 infection may also increase the risk of coccidioidomycosis reactivation because of immune dysregulation within the host, though at present only one case report is indicative of reactivation [90,95,96].
The COVID-19 pandemic may have lengthened delays in diagnosis as a result of similar symptoms between SARS-CoV-2 and Coccidioides spp. infection. A survey of infectious disease physicians indicated that testing practices did not change because of COVID-19, though primary care doctors were not among the respondents to this survey [97]. It is also unknown whether health-seeking behavior or access to healthcare during the pandemic affected coccidioidomycosis case counts. Further research is needed to understand the relationship between coccidioidomycosis and COVID-19.
3. Diagnosis
3.1. Diagnostic Challenges
Healthcare providers are faced with a variety of challenges in relation to coccidioidomycosis diagnosis, though they generally fall into two categories: (1) technical efficiency of laboratory testing and (2) provider knowledge and behavior. Nonspecific symptoms resemble other respiratory illnesses, and laboratory test results may be difficult to interpret or logistically challenging. A delayed or missed diagnosis can consequently lead to adverse patient outcomes in the absence of proper antifungal treatment. Presenting symptoms are often mistaken for bacterial or viral pneumonia, yet current Infectious Disease Society of America (IDSA) diagnostic guidelines for CAP do not recommend testing for Coccidioides spp. infection, despite evidence that the fungal disease accounts for up to one third of CAP etiologies in some endemic areas [98,99].
According to recent enhanced surveillance, 70% of patients had another condition diagnosed before being tested for coccidioidomycosis, and the median duration from seeking healthcare to diagnosis was 38 days [14]. Another study found that 70% of patients received antibiotics in the three months before their first positive coccidioidal test, and those patients were prescribed a median of three antibiotic courses [100]. Implications of inappropriate antibacterial treatment can include drug resistance and unnecessary costs.
A variety of laboratory diagnostic tests are available to detect Coccidioides spp. infection, but testing rates remain low. Performance measures and considerations for the various laboratory tests are described in Table 1. A survey of healthcare providers revealed that only 3.7% reported “frequently” testing CAP patients for coccidioidomycosis, and 15.0% tested “sometimes”. Even in Arizona and California, just 32.4% and 7.4% of providers, respectively, reported frequent testing [15]. Just over 60% of healthcare providers surveyed in Arizona were confident in their ability to diagnose Coccidioides infection, and just under 60% claimed to be knowledgeable about laboratory tests used for detecting coccidioidal antibodies [101].
Table 1.
Performance and considerations for coccidioidomycosis laboratory diagnostic tests.
| Test | Sensitivity | Specificity ‡ | Considerations |
|---|---|---|---|
| Serology | |||
| Antibody | Antibody production may lag behind symptom onset. Sensitivity is often lower in immunosuppressed patients. |
||
| EIA IgG or IgM [103,104,105] | 59–88% | 68–96% | Rapid performance time within hours. Often used as a screening test, later confirmed by ID or CF. IgM only may lead to more false positives than IgG only. |
| EIA IgG [103,104,105] | 47–87% | 89–97% | |
| EIA IgM [103,104,105] | 22–61% | 70–99% | |
| ID § [103,118] | 60–91% | 99–100% | Results may take several days to receive. Some specialized training is required. Methods are not standardized across laboratories. |
| CF § [103,108,109,118] | 65–98% | 80–98% | Titers may offer prognostic value of disease progression. Measurement of IgG only. Highly specialized training is required. Methods are not standardized across laboratories. |
| LFA § [117,118] | 31–99% | 92–98% | Rapid 1-h performance time. |
| Antigen | |||
| Urine and serum [113] | 57% | 99% | May detect Coccidioides in the early stages of the disease [112]. May be preferred to antibody tests for immunocompromised patients. Substantial cross-reactivity with other dimorphic fungi. |
| Urine [111,113] | 37–71% | 99% | |
| Serum [119] | 73% | 100% | |
| Microscopy and culture | |||
| Culture [114] | 23–93% | High | Considered the gold standard of coccidioidomycosis diagnosis. Biosafety level 3 lab needed for safe isolation of Coccidioides. Culture growth may take up to a week. Sensitivity is heavily dependent on specimen quality. |
| Histopathology [114] | 23–84% | High | |
| Cytology [114] | 15–75% | High | |
| Additional laboratory methods | |||
| PCR [115,116] | 56–75% | 99–100% | Rapid 4-h performance time. Site of specimen collection may influence results. |
| (1→3) β-d-glucan [117] | 44% | 91% | Lower sensitivity among patients with acute pulmonary coccidioidomycosis. Values correlate poorly with CF titers. Test cannot detect specific pathogens. |
Abbreviations: CF, complement fixation; EIA, enzyme immunoassay; ID, immunodiffusion; IgG, immunoglobulin G; IgM, immunoglobulin M; PCR, polymerase chain reaction. ‡ Specificity is based on published results; estimates may not be directly comparable, as different control populations were used in some cases. § Sensitivity and specificity ranges include testing from outbreak investigations.
A recent assessment of practice patterns in an Arizona healthcare system showed that 73% of coccidioidomycosis diagnoses were made during hospital admission. Nearly half of those patients had at least one prior healthcare encounter related to their symptoms, but coccidioidal serology was only obtained for 29% of them during those visits [102]. Their subsequent hospitalizations suggest that retesting may have been beneficial to counteract any false negatives in the early stages of the disease.
3.2. Serology
Serologic antibody testing is the most frequently used diagnostic tool for coccidioidomycosis. Antibody development may trail illness onset by several weeks; serial testing is therefore recommended, should symptoms persist following an initial negative test result. Consideration of other laboratory tests may be warranted for immunosuppressed patients, as sensitivities for coccidioidal antibody tests are generally lower among this population.
Enzyme immunoassay (EIA) tests are widely available and offer rapid results, detecting coccidioidal antibodies within hours. EIA testing of both immunoglobulin (Ig) M and IgG levels can be highly sensitive (59–88%) and specific (68–90%), though results are variable [103,104,105]. Results for IgG EIA alone are generally preferred to those for IgM alone, as IgM EIA tests are known to show false positives and should be interpreted with caution; IgG EIA test sensitivities range from 47–87% with specificities of 90–96%, while IgM EIA test sensitivities range from 22–61% with specificities of 70–99% [104,105,106]. A lateral flow assay (LFA) has been developed to offer a fast and simple alternative for antibody detection, but initial data have shown markedly lower sensitivity compared to EIA [107].
Alternative serologic tests for antibody detection include immunodiffusion (ID) and complement fixation (CF), which are less sensitive than EIA but more specific and commonly serve as confirmatory tests [103]. ID tests measure IgG or IgM but can take several days to return results, limiting their utility for quick diagnosis. Quantitative CF results are valuable measures to assess disease severity and progression; higher CF titers may indicate dissemination, and increasing titers are associated with clinical deterioration. However, CF tests measure only IgG antibodies, and cross-reactivity with other dimorphic fungi may influence results [108,109,110]. All coccidioidomycosis antibody tests involve specialty equipment, and CF testing also requires a high level of specialized training. Methods for ID and CF vary across laboratories, leading to a considerable variability of results. Standardization is needed to increase the reliability and comparability of these diagnostic tools.
3.3. Antigen Detection
Antigen testing may inform the detection of Coccidioides in early stages of disease, particularly among immunocompromised patients [111,112]. A commercially-available test for serum, urine, or cerebrospinal fluid (CSF) samples has demonstrated high specificity. Sensitivity is moderate among immunocompromised populations but drops considerably among immunocompetent patients, and CSF antigen testing is sensitive only in patients with coccidioidal meningitis. Cross-reactivity with blastomycosis and histoplasmosis may complicate diagnosis in the absence of additional testing [109,111,113].
3.4. Microscopy and Culture
Identification of Coccidioides in clinical specimens by culture remains the gold standard for coccidioidomycosis diagnosis, though biosafety concerns and potential challenges visualizing the spherules may limit the use of this method. Additionally, culture growth may take up to a week, and sensitivity is dependent on specimen quality.
Histopathology and cytopathology represent other traditional methods used to identify the organism in clinical specimens. Sensitivities of histopathology (23–84%) and cytopathology (15–75%) also vary greatly [114].
3.5. Additional Laboratory Diagnostic Methods
Encouraging results from polymerase chain reaction (PCR) testing demonstrate the potential value of molecular methods as a diagnostic tool for coccidioidomycosis. At present, PCR tests are not commonly used directly on clinical specimens, and the site of specimen collection may impact test performance [115,116]. A study to evaluate performance of the serum (1→3) β-d-glucan (BG) assay showed sensitivity (43.9%) and specificity (91.1%) comparable to BG testing for other invasive mycoses, such as aspergillosis and candidiasis. However, sensitivity was lower among patients with acute pulmonary coccidioidomycosis (19.1%), specificity was determined against healthy controls, and BG values correlated poorly with serum coccidioidal CF titers [117]. The utility of BG testing as a clinical diagnostic tool is limited by its inability to detect specific pathogens.
4. Treatment
Management of coccidioidomycosis often depends on the severity of disease and clinical history of the patient. Acute pulmonary coccidioidomycosis in immunocompetent hosts typically resolves without antifungal intervention and results in life-long immunity [3]. Regular assessment to monitor symptoms and radiological results may prove sufficient for these patients. Some physicians advise empiric antifungal therapy to shorten symptom duration and prevent dissemination, though there are no data from prospective randomized clinical trials to support the efficacy of early treatment on these outcomes [120].
Antifungal treatment for primary pulmonary coccidioidomycosis is recommended for immunosuppressed hosts or patients with particularly devastating forms of disease. IDSA guidelines outline symptoms that may warrant the initiation of therapy; these symptoms include substantial weight loss, persistent intense night sweats, infiltrates involving more than half of 1 lung or portions of both lungs, prominent or persistent hilar adenopathy, CF titers exceeding 1:16, inability to work, or symptoms that persist for more than 2 months. Additional considerations for early treatment include a history of diabetes, frailty because of old age, comorbidities, or African American or Filipino ancestry, though the IDSA guidelines note that ethnicity and diabetes status should only modestly influence management decisions [4].
4.1. Azoles
The advent of azole therapy significantly influenced the antifungal treatment of coccidioidomycosis. Ketoconazole became the first azole used against Coccidioides in 1981, though it is no longer recommended because of concerns of adverse effects and apparently superior efficacy of other drugs [121,122]. Fluconazole is the most commonly prescribed antifungal agent for coccidioidomycosis, available in oral and intravenous formulations. Its low cost, tolerability, and penetration into most body sites make it an appealing option for drug administration [4,123]. Side effects may include alopecia, dry skin, chapped lips, and arthropathy, and effects may become more pronounced with higher dosage [124]. Fluconazole in vitro minimum inhibitory concentrations (MICs) were found to be significantly higher than those of other triazoles in limited reports, though this has not been correlated with patient outcomes [125].
Itraconazole is also commonly used to treat coccidioidomycosis and is available as an oral solution or a capsule. Findings from a randomized double-blind trial showed that itraconazole had a higher efficacy (70% response rate) compared to fluconazole (37% response rate) for patients with skeletal forms of disease, though absorption can be challenging. Relapse rates were also lower for patients treated with itraconazole (18%) as opposed to fluconazole (28%) [126]. Hypertension, hypokalemia, sodium retention, and decreased myocardial contractility constitute reported adverse effects from itraconazole therapy, and consequently the drug is not recommended for patients at risk of heart failure [127,128,129].
Voriconazole may be prescribed for patients who are intolerant or unresponsive to fluconazole or itraconazole. It can be administered intravenously or through oral formulation and has extensive distribution throughout the body, including CSF penetration. Although voriconazole has exhibited efficacy in cases of coccidioidal meningitis, concerns of drug-drug interactions and toxicities may limit its use [130,131,132]. Voriconazole has been associated with visual impairments, altered mental status, and harmful cutaneous effects, including photodermatitis, melanoma, and squamous cell carcinoma [133,134,135].
Posaconazole is an alternate antifungal option for refractory cases of coccidioidomycosis. Originally available only as an oral suspension, the development of an intravenous formulation and delayed response oral tablet markedly improved absorption [136]. Posaconazole penetrates most body sites, with the exception of CSF, and is generally considered to be effective, even demonstrating superior performance to other triazoles in murine models [130,137,138,139,140,141]. Side effects are commonly gastrointestinal in nature, including nausea, vomiting, and diarrhea. Hypokalemia, hypertension, and peripheral edema are also reported to be associated with Posaconazole treatment [98].
Most recently, isavuconazole has been made available in oral and intravenous formulations. It is widely distributed throughout the body, yet although isavuconazole has proven to be effective against other mycoses, data for coccidioidomycosis patients in a clinical setting are limited [142,143,144]. Gastrointestinal side effects similar to those of Posaconazole have been observed.
4.2. Polyenes
Prior to the introduction of triazole antifungal therapy, amphotericin B served as the primary agent for coccidioidomycosis treatment. Multiple formulations are available intravenously: amphotericin B deoxycholate (AmBd), liposomal amphotericin B (L-AMB), amphotericin B colloidal dispersion (ABCD), and amphotericin B lipid complex (ABLC). All forms are associated with nephrotoxicity, though the frequency of adverse events is generally lower in the lipid variations [145,146]. Use of amphotericin B is now primarily limited to cases that are intolerant or resistant to triazoles. However, intrathecal AmBd may still be administered in patients with coccidioidal meningitis or in their first trimester of pregnancy to avoid potential teratogenic effects of triazoles [4,147].
4.3. Treatment Duration and Follow-Up
Duration of coccidioidomycosis treatment varies based on disease type and progression. Therapy for uncomplicated acute pulmonary infection is commonly discontinued after 3–6 months, whereas patients with severe or chronic forms of disease may require life-long treatment. Antifungal regimens should be routinely assessed for possible adverse effects, drug-drug interactions, and therapeutic drug monitoring if needed. Cessation of treatment is generally prompted by diminished symptoms, improvements of imaging results, and declining CF titers.
Regardless of treatment status, clinical follow-up is essential to evaluate the resolution of signs and symptoms and to identify possible relapse or dissemination. Assessments often incorporate a combination of serologic testing, radiological examination, and patient interview to monitor the course of infection. Follow-up is recommended for at least one year once the patient shows signs of improvement.
5. Conclusions
Our understanding of coccidioidomycosis has no doubt deepened in recent years, but much remains to be learned. Incidence of disease is rising, and the geographic range of Coccidioides spp. is growing. Continued and expanded surveillance is needed in the United States and across Central and South America to monitor trends and identify potential new areas of endemicity to inform public health efforts. Increased clinician awareness and knowledge of suitable diagnostic methods are essential to improve early detection and avoid inappropriate treatment and unnecessary medical costs. Furthermore, the development of new and more rapid diagnostic tools, as well as antifungal therapies that target Coccidioides spp., is necessary to advance the diagnosis and subsequent resolution of disease. Insights into the epidemiology, diagnosis, and treatment of coccidioidomycosis can be used to guide future prevention and management strategies to minimize morbidity and mortality from this important disease.
Acknowledgments
The authors wish to acknowledge Brendan Jackson, Mitsuru Toda, Kaitlin Benedict, Dallas Smith, Ana Litvintseva, and Mark Lindsley for their support of this work.
Author Contributions
S.L.W. and T.C. have made substantial contributions to this review. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer
The findings and conclusions of this paper are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC).
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith C.E., Whiting E.G., Baker E.E., Rosenberger H.G., Beard R.R., Saito M.T. The Use of Coccidioidin1, 2. Am. Rev. Tuberc. 1948;57:330–360. doi: 10.1164/art.1948.57.4.330. [DOI] [PubMed] [Google Scholar]
- 2.Donovan F.M., Wightman P., Zong Y., Gabe L., Majeed A., Ynosencio T., Bedrick E.J., Galgiani J.N. Delays in Coccidioidomycosis Diagnosis and Associated Healthcare Utilization, Tucson, Arizona, USA. Emerg. Infect. Dis. 2019;25:1745–1747. doi: 10.3201/eid2509.190023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith C.E., Beard R.R. Varieties of Coccidioidal Infection in Relation to the Epidemiology and Control of the Diseases. Am. J. Public Health Nations Health. 1946;36:1394–1402. doi: 10.2105/AJPH.36.12.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galgiani J.N., Ampel N.M., Blair J.E., Catanzaro A., Geertsma F., Hoover S.E., Johnson R.H., Kusne S., Lisse J., MacDonald J.D., et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2016;63:e112–e146. doi: 10.1093/cid/ciw538. [DOI] [PubMed] [Google Scholar]
- 5.Benedict K., Jackson B.R., Chiller T., Beer K.D. Estimation of direct healthcare costs of fungal diseases in the United States. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019;68:1791–1797. doi: 10.1093/cid/ciy776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedict K., Whitham H.K., Jackson B.R. Economic Burden of Fungal Diseases in the United States. Open Forum Infect. Dis. 2022;9:ofac097. doi: 10.1093/ofid/ofac097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson L., Ting J., Lin H., MacLean M., Peterson M.W., Stockamp N., Libke R., Brown P. The Rise of Valley Fever: Prevalence and Cost Burden of Coccidioidomycosis Infection in California. Int. J. Environ. Res. Public Health. 2019;16:1113. doi: 10.3390/ijerph16071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grizzle A.J., Wilson L., Nix D.E., Galgiani J.N. Clinical and Economic Burden of Valley Fever in Arizona: An Incidence-Based Cost-of-Illness Analysis. Open Forum Infect. Dis. 2020;8:ofaa623. doi: 10.1093/ofid/ofaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards P.Q., Palmer C.E. Prevalence of Sensitivity to Coccidioidin, With Special Reference to Specific and Nonspecific Reactions to Coccidioidin and to Histoplasmin. Dis. Chest. 1957;31:35–60. doi: 10.1378/chest.31.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Marsden-Haug N., Goldoft M., Ralston C., Limaye A.P., Chua J., Hill H., Jecha L., Thompson G.R., Chiller T. Coccidioidomycosis Acquired in Washington State. Clin. Infect. Dis. 2013;56:847–850. doi: 10.1093/cid/cis1028. [DOI] [PubMed] [Google Scholar]
- 11.Benedict K., Thompson G.R., Deresinski S., Chiller T. Mycotic Infections Acquired outside Areas of Known Endemicity, United States. Emerg. Infect. Dis. 2015;21:1935–1941. doi: 10.3201/eid2111.141950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laniado-Laborín R., Arathoon E.G., Canteros C., Muñiz-Salazar R., Rendon A. Coccidioidomycosis in Latin America. Med. Mycol. 2019;57((Suppl. 1)):S46–S55. doi: 10.1093/mmy/myy037. [DOI] [PubMed] [Google Scholar]
- 13.Ashraf N., Kubat R.C., Poplin V., Adenis A.A., Denning D.W., Wright L., McCotter O., Schwartz I.S., Jackson B.R., Chiller T., et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia. 2020;185:843–865. doi: 10.1007/s11046-020-00431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedict K., Ireland M., Weinberg M.P., Gruninger R.J., Weigand J., Chen L., Perez-Lockett K., Bledsoe C., Denny L., Cibulskas K., et al. Enhanced Surveillance for Coccidioidomycosis, 14 US States, 2016. Emerg. Infect. Dis. 2018;24:1444–1452. doi: 10.3201/eid2408.171595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedict K., Li Y., Molinari N.A.M., Jackson B.R. Health Care Providers’ Testing Practices for Coccidioidomycosis and Histoplasmosis in Patients With Community-Acquired Pneumonia—United States, 2020. Open Forum Infect. Dis. 2021;8:ofab020. doi: 10.1093/ofid/ofab020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reportable Fungal Diseases by State Fungal Diseases CDC. [(accessed on 1 April 2022)]; Published 19 November 2021. Available online: https://www.cdc.gov/fungal/fungal-disease-reporting-table.html.
- 17.Valley Fever Statistics Coccidioidomycosis Types of Fungal Diseases Fungal CDC. [(accessed on 26 March 2022)]; Published 15 October 2021. Available online: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html.
- 18.Epidemiologic Summary of Valley Fever (Coccidioidomycosis) in California, 2019. California Department of Public Health; Sacramento, CA, USA: 2019. [(accessed on 12 April 2022)]. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/CocciEpiSummary2019.pdf. [Google Scholar]
- 19.Valley Fever 2019 Annual Report; Arizona Department of Health Services. [(accessed on 12 April 2022)]; Available online: https://www.azdhs.gov/documents/preparedness/epidemiology-disease-control/valley-fever/reports/valley-fever-2019.pdf.
- 20.Coccidioidomycosis in California Provisional Monthly Report, January–February 2022; California Department of Public Health. [(accessed on 12 April 2022)]; Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/CocciinCAProvisionalMonthlyReport.pdf.
- 21.Year-to-Date Communicable Disease Summary; Arizona Department of Health Services: 2022. [(accessed on 12 April 2022)]; Available online: https://www.azdhs.gov/documents/preparedness/epidemiology-disease-control/disease-data-statistics-reports/data-statistics-archive/2021/2021-ytd-communicable-disease-summary.pdf.
- 22.Gorris M.E., Cat L.A., Zender C.S., Treseder K.K., Randerson J.T. Coccidioidomycosis Dynamics in Relation to Climate in the Southwestern United States. GeoHealth. 2018;2:6–24. doi: 10.1002/2017GH000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Head J.R., Sondermeyer-Cooksey G., Heaney A.K., Yu A.T., Jones I., Bhattachan A., Campo S., Wagner R., Mgbara W., Phillips S., et al. Influence of Meteorological Factors and Drought on Coccidioidomycosis Incidence in California, 2000–2020. Epidemiology. 2022 doi: 10.1101/2022.02.03.22270412. [DOI] [Google Scholar]
- 24.Gorris M.E., Treseder K.K., Zender C.S., Randerson J.T. Expansion of Coccidioidomycosis Endemic Regions in the United States in Response to Climate Change. GeoHealth. 2019;3:308–327. doi: 10.1029/2019GH000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baptista-Rosas R.C., Hinojosa A., Riquelme M. Ecological Niche Modeling of Coccidioides spp. in Western North American Deserts. Ann. N. Y. Acad. Sci. 2007;1111:35–46. doi: 10.1196/annals.1406.003. [DOI] [PubMed] [Google Scholar]
- 26.Kolivras K.N., Comrie A.C. Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int. J. Biometeorol. 2003;47:87–101. doi: 10.1007/s00484-002-0155-x. [DOI] [PubMed] [Google Scholar]
- 27.Tamerius J.D., Comrie A.C. Coccidioidomycosis Incidence in Arizona Predicted by Seasonal Precipitation. PLoS ONE. 2011;6:e21009. doi: 10.1371/journal.pone.0021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talamantes J., Behseta S., Zender C.S. Fluctuations in Climate and Incidence of Coccidioidomycosis in Kern County, California. Ann. N. Y. Acad. Sci. 2007;1111:73–82. doi: 10.1196/annals.1406.028. [DOI] [PubMed] [Google Scholar]
- 29.Zender C.S., Talamantes J. Climate controls on valley fever incidence in Kern County, California. Int. J. Biometeorol. 2006;50:174–182. doi: 10.1007/s00484-005-0007-6. [DOI] [PubMed] [Google Scholar]
- 30.Comrie A.C. Climate Factors Influencing Coccidioidomycosis Seasonality and Outbreaks. Environ. Health Perspect. 2005;113:688–692. doi: 10.1289/ehp.7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park B.J., Sigel K., Vaz V., Komatsu K., McRill C., Phelan M., Colman T., Comrie A.C., Warnock D.W., Galgiani J.N., et al. An Epidemic of Coccidioidomycosis in Arizona Associated with Climatic Changes, 1998–2001. J. Infect. Dis. 2005;191:1981–1987. doi: 10.1086/430092. [DOI] [PubMed] [Google Scholar]
- 32.US EPA O. Particulate Matter (PM10) Trends. [(accessed on 1 April 2022)]; Published 19 July 2016. Available online: https://www.epa.gov/air-trends/particulate-matter-pm10-trends.
- 33.Kollath D.R., Mihaljevic J.R., Barker B.M. PM10 and Other Climatic Variables Are Important Predictors of Seasonal Variability of Coccidioidomycosis in Arizona. Microbiol. Spectr. 2022;10:e0148321. doi: 10.1128/spectrum.01483-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Census Bureau QuickFacts. [(accessed on 26 March 2022)]; Available online: https://www.census.gov/quickfacts/fact/table/maricopacountyarizona,CA,AZ,US/AGE775220.
- 35.Colson A.J., Vredenburgh L., Guevara R.E., Rangel N.P., Kloock C.T., Lauer A. Large-Scale Land Development, Fugitive Dust, and Increased Coccidioidomycosis Incidence in the Antelope Valley of California, 1999–2014. Mycopathologia. 2017;182:439–458. doi: 10.1007/s11046-016-0105-5. [DOI] [PubMed] [Google Scholar]
- 36.Guevara R.E., Motala T., Terashita D. The Changing Epidemiology of Coccidioidomycosis in Los Angeles (LA) County, California, 1973–2011. PLoS ONE. 2015;10:e0136753. doi: 10.1371/journal.pone.0136753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leake J.A.D., Mosley D.G., England B., Graham J.V., Plikaytis B.D., Ampel N.M., Perkins B.A., Hajjeh R.A. Risk Factors for Acute Symptomatic Coccidioidomycosis among Elderly Persons in Arizona, 1996–1997. J. Infect. Dis. 2000;181:1435–1440. doi: 10.1086/315400. [DOI] [PubMed] [Google Scholar]
- 38.Center for International Blood and Marrow Transplant Research Transplant Activity Report Covering 2009–2013. [(accessed on 26 March 2022)]; Available online: https://bloodstemcell.hrsa.gov/data/donation-and-transplantation-statistics/transplant-activity-report.
- 39.Organ Procurement and Transplantation Network: View Data Reports. [(accessed on 26 March 2022)]; Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/
- 40.Casadevall A. Fungal Diseases in the 21st Century: The Near and Far Horizons. Pathog. Immun. 2018;3:183–196. doi: 10.20411/pai.v3i2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown J., Benedict K., Park B.J., Thompson G.R. Coccidioidomycosis: Epidemiology. Clin. Epidemiol. 2013;5:185–197. doi: 10.2147/CLEP.S34434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odio C.D., Marciano B.E., Galgiani J.N., Holland S.M. Risk Factors for Disseminated Coccidioidomycosis, United States. Emerg. Infect. Dis. 2017;23:308–311. doi: 10.3201/eid2302.160505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsang C.A., Anderson S.M., Imholte S.B., Erhart L.M., Chen S., Park B.J., Christ C., Komatsu K.K., Chiller T., Sunenshine R.H. Enhanced Surveillance of Coccidioidomycosis, Arizona, USA, 2007–2008. Emerg. Infect. Dis. 2010;16:1738–1744. doi: 10.3201/eid1611.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedict K., Molinari N.A.M., Jackson B.R. Public Awareness of Invasive Fungal Diseases—United States, 2019. Morb. Mortal. Wkly. Rep. 2020;69:1343–1346. doi: 10.15585/mmwr.mm6938a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurd-Kundeti G., Sondermeyer Cooksey G.L., Jain S., Vugia D.J. Valley Fever (Coccidioidomycosis) Awareness—California, 2016–2017. Morb. Mortal. Wkly. Rep. 2020;69:1512–1516. doi: 10.15585/mmwr.mm6942a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner S.B., Pappagianis D. Coccidioidomycosis in Northern California—An Outbreak among Archeology Students near Red Bluff. Calif. Med. 1973;119:16–20. [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen L.R., Marshall S.L., Barton C., Hajjeh R.A., Lindsley M.D., Warnock D.W., Panackal A.A., Shaffer J.B., Haddad M.B., Fisher F.S., et al. Coccidioidomycosis among Workers at an Archeological Site, Northeastern Utah. Emerg. Infect. Dis. 2004;10:637–642. doi: 10.3201/eid1004.030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Werner S.B., Pappagianis D., Heindl I., Mickel A. An Epidemic of Coccidioidomycosis among Archeology Students in Northern California. N. Engl. J. Med. 1972;286:507–512. doi: 10.1056/NEJM197203092861003. [DOI] [PubMed] [Google Scholar]
- 49.Factors Influencing Distribution of Coccidioides Immitis in Soil, Washington State, 2016-PubMed. [(accessed on 23 March 2022)]; doi: 10.1128/mSphere.00598-21. Available online: https://pubmed.ncbi.nlm.nih.gov/34730378/ [DOI] [PMC free article] [PubMed]
- 50.Kobziar L.N., Thompson G.R. Wildfire smoke, a potential infectious agent. Science. 2020;370:1408–1410. doi: 10.1126/science.abe8116. [DOI] [PubMed] [Google Scholar]
- 51.Laws R.L., Jain S., Cooksey G.S., Mohle-Boetani J., McNary J., Wilken J., Harrison R., Leistikow B., Vugia D.J., Windham G.C., et al. Coccidioidomycosis outbreak among inmate wildland firefighters: California, 2017. Am. J. Ind. Med. 2021;64:266–273. doi: 10.1002/ajim.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong D.Q., Wang J.X.L., Gill T.E., Lei H., Wang B. Intensified dust storm activity and Valley fever infection in the southwestern United States. Geophys. Res. Lett. 2017;44:4304–4312. doi: 10.1002/2017GL073524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Comrie A.C. No Consistent Link Between Dust Storms and Valley Fever (Coccidioidomycosis) GeoHealth. 2021;5:e2021GH000504. doi: 10.1029/2021GH000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gade L., McCotter O.Z., Bowers J.R., Waddell V., Brady S., Carvajal J.A., Sunenshine R., Komatsu K.K., Engelthaler D.M., Chiller T., et al. The detection of Coccidioides from ambient air in Phoenix, Arizona: Evidence of uneven distribution and seasonality. Med. Mycol. 2020;58:552–559. doi: 10.1093/mmy/myz093. [DOI] [PubMed] [Google Scholar]
- 55.Emmons C.W. Coccidioidomycosis in Wild Rodents. A Method of Determining the Extent of Endemic Areas. Public Health Rep. (1896–1970) 1943;58:1–5. doi: 10.2307/4584326. [DOI] [Google Scholar]
- 56.Health ASD of Proceedings of Symposium on Coccidioidomycosis: Held at Phoenix, Ariz.-Feb. 11–13, 1957. U.S. Department of Health, Education, and Welfare Public Health Service, Bureau of State Services, Communicable Disease Center; Washington, DC, USA: 1957. [Google Scholar]
- 57.Kollath D.R., Miller K.J., Barker B.M. The mysterious desert dwellers: Coccidioides immitis and Coccidioides posadasii, causative fungal agents of coccidioidomycosis. Virulence. 2019;10:222–233. doi: 10.1080/21505594.2019.1589363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soil Ecology of Coccidioides Immitis at Amerindian Middens in California Applied Microbiology. [(accessed on 23 March 2022)]. Available online: https://journals.asm.org/doi/abs/10.1128/am.27.2.379-388.1974. [DOI] [PMC free article] [PubMed]
- 59.Cordeiro R.A., Brilhante R.S.N., Rocha M.F.G., Fechine M.A.B., Camara L.M.C., Camargo Z.P., Sidrim J.J.C. Phenotypic characterization and ecological features of Coccidioides spp. from Northeast Brazil. Med. Mycol. 2006;44:631–639. doi: 10.1080/13693780600876546. [DOI] [PubMed] [Google Scholar]
- 60.Sharpton T.J., Stajich J.E., Rounsley S.D., Gardner M.J., Wortman J.R., Jordar V.S., Maiti R., Kodira C.D., Neafsey D.E., Zeng Q., et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009;19:1722–1731. doi: 10.1101/gr.087551.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ampel N.M., Dols C.L., Galgiani J.N. Coccidioidomycosis during human immunodeficiency virus infection: Results of a prospective study in a coccidioidal endemic area. Am. J. Med. 1993;94:235–240. doi: 10.1016/0002-9343(93)90054-S. [DOI] [PubMed] [Google Scholar]
- 62.Woods C.W., McRill C., Plikaytis B.D., Rosenstein N.E., Mosley D., Boyd D., England B., Perkins B.A., Ampel N.M., Hajjeh R.A. Coccidioidomycosis in Human Immunodeficiency Virus–Infected Persons in Arizona, 1994–1997: Incidence, Risk Factors, and Prevention. J. Infect. Dis. 2000;181:1428–1434. doi: 10.1086/315401. [DOI] [PubMed] [Google Scholar]
- 63.Masannat F.Y., Ampel N.M. Coccidioidomycosis in Patients with HIV-1 Infection in the Era of Potent Antiretroviral Therapy. Clin. Infect. Dis. 2010;50:1–7. doi: 10.1086/648719. [DOI] [PubMed] [Google Scholar]
- 64.Blair J.E., Logan J.L. Coccidioidomycosis in Solid Organ Transplantation. Clin. Infect. Dis. 2001;33:1536–1544. doi: 10.1086/323463. [DOI] [PubMed] [Google Scholar]
- 65.Bergstrom L., Yocum D.E., Ampel N.M., Villanueva I., Lisse J., Gluck O., Tesser J., Posever J.P., Miller M., Araujo J., et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor α antagonists. Arthritis Rheum. 2004;50:1959–1966. doi: 10.1002/art.20454. [DOI] [PubMed] [Google Scholar]
- 66.Blair J.E., Douglas D.D., Mulligan D.C. Early results of targeted prophylaxis for coccidioidomycosis in patients undergoing orthotopic liver transplantation within an endemic area. Transpl. Infect. Dis. 2003;5:3–8. doi: 10.1034/j.1399-3062.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 67.Kahn A., Carey E.J., Blair J.E. Universal fungal prophylaxis and risk of coccidioidomycosis in liver transplant recipients living in an endemic area. Liver Transpl. 2015;21:353–361. doi: 10.1002/lt.24055. [DOI] [PubMed] [Google Scholar]
- 68.Keckich D.W., Blair J.E., Vikram H.R., Seville M.T., Kusne S. Reactivation of Coccidioidomycosis Despite Antifungal Prophylaxis in Solid Organ Transplant Recipients. Transplantation. 2011;92:88–93. doi: 10.1097/TP.0b013e31821c1df6. [DOI] [PubMed] [Google Scholar]
- 69.Truong C.N., Nailor M.D., Walia R., Cherrier L., Nasar A., Goodlet K.J. Universal lifelong fungal prophylaxis and risk of coccidioidomycosis in lung transplant recipients living in an endemic area. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021;74:ciab752. doi: 10.1093/ofid/ofab466.1174. [DOI] [PubMed] [Google Scholar]
- 70.Al-Obaidi M.M., Nematollahi S., Nix D.E., Zangeneh T.T. Remarks on the universal lifelong coccidioidomycosis prophylaxis in lung transplant recipients. Clin. Infect. Dis. 2021;74:ciab878. doi: 10.1093/cid/ciab878. [DOI] [PubMed] [Google Scholar]
- 71.Bercovitch R.S., Catanzaro A., Schwartz B.S., Pappagianis D., Watts D.H., Ampel N.M. Coccidioidomycosis During Pregnancy: A Review and Recommendations for Management. Clin. Infect. Dis. 2011;53:363–368. doi: 10.1093/cid/cir410. [DOI] [PubMed] [Google Scholar]
- 72.Crum N.F., Ballon-Landa G. Coccidioidomycosis in Pregnancy: Case Report and Review of the Literature. Am. J. Med. 2006;119:e11–e993. doi: 10.1016/j.amjmed.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 73.Crum N.F., Lederman E.R., Stafford C.M., Parrish J.S., Wallace M.R. Coccidioidomycosis: A Descriptive Survey of a Reemerging Disease. Clinical Characteristics and Current Controversies. Medicine. 2004;83:149–175. doi: 10.1097/01.md.0000126762.91040.fd. [DOI] [PubMed] [Google Scholar]
- 74.Sondermeyer Cooksey G.L., Nguyen A., Vugia D., Jain S. Regional Analysis of Coccidioidomycosis Incidence—California, 2000–2018. Morb. Mortal. Wkly. Rep. 2020;69:1817–1821. doi: 10.15585/mmwr.mm6948a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang D.C., Anderson S., Wannemuehler K., Engelthaler D.M., Erhart L., Sunenshine R.H., Burwell L.A., Park B.J. Testing for Coccidioidomycosis among Patients with Community-Acquired Pneumonia. Emerg. Infect. Dis. 2008;14:1053–1059. doi: 10.3201/eid1407.070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valley Fever (Coccidioidomycosis), Jobs at Risk NIOSH CDC. [(accessed on 26 March 2022)]; Published 11 March 2022. Available online: https://www.cdc.gov/niosh/topics/valleyfever/risk.html.
- 77.de Perio M.A., Niemeier R.T., Burr G.A. Coccidioides Exposure and Coccidioidomycosis among Prison Employees, California, United States. Emerg. Infect. Dis. 2015;21:1031–1033. doi: 10.3201/eid2106.141201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilken J.A., Marquez P., Terashita D., McNary J., Windham G., Materna B. Coccidioidomycosis Among Cast and Crew Members at an Outdoor Television Filming Event—California, 2012. MMWR Morb. Mortal Wkly Rep. 2014;63:20. [PMC free article] [PubMed] [Google Scholar]
- 79.Durry E., Pappagianis D., Werner S.B., Hutwagner L., Sun R.K., Maurer M., McNeil M.M., Pinner R.W. Coccidioidomycosis in Tulare County, California, 1991: Reemergence of an endemic disease. J. Med. Vet. Mycol. 1997;35:321–326. doi: 10.1080/02681219780001361. [DOI] [PubMed] [Google Scholar]
- 80.Sondermeyer G., Lee L., Gilliss D., Tabnak F., Vugia D. Coccidioidomycosis-associated Hospitalizations, California, USA, 2000–2011. Emerg. Infect. Dis. 2013;19:1590–1597. doi: 10.3201/eid1910.130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Einstein H.E., Johnson R.H. Coccidioidomycosis: New Aspects of Epidemiology and Therapy. Clin. Infect. Dis. 1993;16:349–356. doi: 10.1093/clind/16.3.349. [DOI] [PubMed] [Google Scholar]
- 82.McHardy I., Reagan K.L., Sebastian J.F., Barker B., Bays D.J., Dandekar S., Cohen S.H., Jennings K.E., Sykes J., Thompson G.R. Sex Differences in Susceptibility to Coccidioidomycosis. Open Forum Infect. Dis. 2022;9:ofab543. doi: 10.1093/ofid/ofab543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shah A.S., Heidari A., Civelli V.F., Sharma R., Clark C.S., Munoz A.D., Ragland A.S., Johnson R.H. The Coincidence of 2 Epidemics, Coccidioidomycosis and SARS-CoV-2: A Case Report. J. Investig. Med. High Impact Case Rep. 2020;8:2324709620930540. doi: 10.1177/2324709620930540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang C.C., Senining R., Kim J., Goyal R. An Acute Pulmonary Coccidioidomycosis Coinfection in a Patient Presenting With Multifocal Pneumonia With COVID-19. J. Investig. Med. High Impact Case Rep. 2020;8:2324709620972244. doi: 10.1177/2324709620972244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen J.C., Wong D., Rabi S., Worswick S., DeClerck B., Gibb J. All That Coughs Is Not COVID-19: A Delayed Diagnosis of Disseminated Coccidioidomycosis Following Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Open Forum Infect. Dis. 2021;8:ofab246. doi: 10.1093/ofid/ofab246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nielsen M.C., Reynoso D., Ren P. The Brief Case: A Fatal Case of SARS-CoV-2 Coinfection with Coccidioides in Texas—Another Challenge We Face. Burnham, C.A.D., Ed. J. Clin. Microbiol. 2021;59:e00163-21. doi: 10.1128/JCM.00163-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ko J., Lee M.M. TP98. TP098 FUNGUS AMONG-US-RARE FUNGAL CASE REPORTS. American Thoracic Society; New York, NY, USA: 2021. A Case of Disseminated Coccidioidomycosis in a Patient with a Prolonged Course of COVID-19 Pneumonia; p. A3997. American Thoracic Society International Conference Abstracts. [DOI] [Google Scholar]
- 88.Mathew J., Cherukuri S.V., Dihowm F. SARS-CoV-2 with concurrent coccidioidomycosis complicated by refractory pneumothorax in a Hispanic male: A case report and literature review. World J. Respirol. 2021;11:1–11. doi: 10.5320/wjr.v11.i1.1. [DOI] [Google Scholar]
- 89.Patel B., Jarrett B., Bixby B. Diagnostic Error and Cognitive Bias in the Era of COVID-19: Don’t Forget About Endemic Diseases. Chest. 2020;158:A541–A542. doi: 10.1016/j.chest.2020.08.512. [DOI] [Google Scholar]
- 90.Krauth D.S., Jamros C.M., Rivard S.C., Olson N.H., Maves R.C. Accelerated Progression of Disseminated Coccidioidomycosis Following SARS-CoV-2 Infection: A Case Report. Mil. Med. 2021;186:usab132. doi: 10.1093/milmed/usab132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heaney A.K., Head J.R., Broen K., Click K., Taylor J., Balmes J.R., Zelner J., Remais J.V. Coccidioidomycosis and COVID-19 Co-Infection, United States, 2020. Emerg. Infect. Dis. 2021;27:1266–1273. doi: 10.3201/eid2705.204661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: Findings of 487 cases outside Wuhan. Crit. Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.CDC COVID-19 Response Team Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019—United States, 12 February–28 March 2020. Morb. Mortal. Wkly. Rep. 2020;69 doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020;71:ciaa248. doi: 10.2139/ssrn.3541136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beaman L., Benjamini E., Pappagianis D. Activation of macrophages by lymphokines: Enhancement of phagosome-lysosome fusion and killing of Coccidioides immitis. Infect. Immun. 1983;39:1201–1207. doi: 10.1128/iai.39.3.1201-1207.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benedict K., Williams S., Beekmann S.E., Polgreen P.M., Jackson B.R., Toda M. Testing Practices for Fungal Respiratory Infections and SARS-CoV-2 among Infectious Disease Specialists, United States. J. Fungi. 2021;7:605. doi: 10.3390/jof7080605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olson G., Davis A.M. Diagnosis and Treatment of Adults With Community-Acquired Pneumonia. JAMA. 2020;323:885. doi: 10.1001/jama.2019.21118. [DOI] [PubMed] [Google Scholar]
- 99.Valdivia L., Nix D., Wright M., Lindberg E., Fagan T., Lieberman D., Stoffer T., Ampel N.M., Galgiani J.N. Coccidioidomycosis as a Common Cause of Community-acquired Pneumonia. Emerg. Infect. Dis. 2006;12:958–962. doi: 10.3201/eid1206.060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chi G.C., Benedict K., Beer K.D., Jackson B.R., McCotter O., Xie F., Lawrence J.M., Tartof S.Y. Antibiotic and antifungal treatment among persons with confirmed coccidioidomycosis—Southern California, 2011. Med. Mycol. 2020;58:411–413. doi: 10.1093/mmy/myz073. [DOI] [PubMed] [Google Scholar]
- 101.Chen S., Erhart L.M., Anderson S., Komatsu K., Park B., Chiller T., Sunenshine R. Coccidioidomycosis: Knowledge, attitudes, and practices among healthcare providers—Arizona, 2007. Med. Mycol. 2011;49:649–656. doi: 10.3109/13693786.2010.547995. [DOI] [PubMed] [Google Scholar]
- 102.Pu J., Donovan F.M., Ellingson K., Leroy G., Stone J., Bedrick E., Galgiani J.N. Clinician Practice Patterns That Result in the Diagnosis of Coccidioidomycosis Before or During Hospitalization. Clin. Infect. Dis. 2021;73:e1587–e1593. doi: 10.1093/cid/ciaa739. [DOI] [PubMed] [Google Scholar]
- 103.Malo J., Holbrook E., Zangeneh T., Strawter C., Oren E., Robey I., Erickson H., Chahal R., Durkin M., Thompson C., et al. Enhanced Antibody Detection and Diagnosis of Coccidioidomycosis with the MiraVista IgG and IgM Detection Enzyme Immunoassay. J. Clin. Microbiol. 2017;55:893–901. doi: 10.1128/JCM.01880-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malo J., Holbrook E., Zangeneh T., Strawter C., Oren E., Robey I., Erickson H., Carranza-Chahal R., Durkin M., Thompson C., et al. Comparison of three anti-coccidioides antibody enzyme immunoassay kits for the diagnosis of coccidioidomycosis. Med. Mycol. 2020;58:774–778. doi: 10.1093/mmy/myz125. [DOI] [PubMed] [Google Scholar]
- 105.Grys T.E., Brighton A., Chang Y.H., Liesman R., Bolster LaSalle C., Blair J.E. Comparison of two FDA-cleared EIA assays for the detection of Coccidioides antibodies against a composite clinical standard. Med. Mycol. 2019;57:595–600. doi: 10.1093/mmy/myy094. [DOI] [PubMed] [Google Scholar]
- 106.Kuberski T., Herrig J., Pappagianis D. False-Positive IgM Serology in Coccidioidomycosis. J. Clin. Microbiol. 2010;48:2047–2049. doi: 10.1128/JCM.01843-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Donovan F.M., Ramadan F.A., Khan S.A., Bhaskara A., Lainhart W.D., Narang A.T., Mosier J.M., Ellingson K.D., Bedrick E.J., Saubolle M.A., et al. Comparison of a Novel Rapid Lateral Flow Assay to Enzyme Immunoassay Results for Early Diagnosis of Coccidioidomycosis. Clin. Infect. Dis. 2021;73:e2746–e2753. doi: 10.1093/cid/ciaa1205. [DOI] [PubMed] [Google Scholar]
- 108.Huppert M. Serology of coccidioidomycosis. Mycopathol. Mycol. Appl. 1970;41:107–113. doi: 10.1007/BF02051487. [DOI] [PubMed] [Google Scholar]
- 109.Blair J.E., Coakley B., Santelli A.C., Hentz J.G., Wengenack N.L. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia. 2006;162:317–324. doi: 10.1007/s11046-006-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mendoza N., Blair J.E. The Utility of Diagnostic Testing for Active Coccidioidomycosis in Solid Organ Transplant Recipients. Am. J. Transplant. 2013;13:1034–1039. doi: 10.1111/ajt.12144. [DOI] [PubMed] [Google Scholar]
- 111.Durkin M., Connolly P., Kuberski T., Myers R., Kubak B.M., Bruckner D., Pegues D., Wheat L.J. Diagnosis of Coccidioidomycosis with Use of the Coccidioides Antigen Enzyme Immunoassay. Clin. Infect. Dis. 2008;47:e69–e73. doi: 10.1086/592073. [DOI] [PubMed] [Google Scholar]
- 112.Galgiani J.N., Grace G.M., Lundergan L.L. New Serologic Tests for Early Detection of Coccidioidomycosis. J. Infect. Dis. 1991;163:671–674. doi: 10.1093/infdis/163.3.671. [DOI] [PubMed] [Google Scholar]
- 113.Kassis C., Durkin M., Holbrook E., Myers R., Wheat L. Advances in Diagnosis of Progressive Pulmonary and Disseminated Coccidioidomycosis. Clin. Infect. Dis. 2021;72:968–975. doi: 10.1093/cid/ciaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wheat L.J., Knox K.S., Hage C.A. Approach to the Diagnosis of Histoplasmosis, Blastomycosis and Coccidioidomycosis. Curr Curr. Treat. Options Infect. Dis. 2014;6:337–351. doi: 10.1007/s40506-014-0020-6. [DOI] [Google Scholar]
- 115.Dizon D., Mitchell M., Dizon B., Libke R., Peterson M.W. The utility of real-time polymerase chain reaction in detecting Coccidioides immitis among clinical specimens in the Central California San Joaquin Valley. Med. Mycol. 2019;57:688–693. doi: 10.1093/mmy/myy111. [DOI] [PubMed] [Google Scholar]
- 116.Vucicevic D., Blair J.E., Binnicker M.J., McCullough A.E., Kusne S., Vikram H.R., Parish J.M., Wengenack N.L. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia. 2010;170:345–351. doi: 10.1007/s11046-010-9327-0. [DOI] [PubMed] [Google Scholar]
- 117.Thompson G.R., Bays D.J., Johnson S.M., Cohen S.H., Pappagianis D., Finkelman M.A. Serum (1→3)-β-d-Glucan Measurement in Coccidioidomycosis. J. Clin. Microbiol. 2012;50:3060–3062. doi: 10.1128/JCM.00631-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caceres D.H., Chiller T., Lindsley M.D. Immunodiagnostic Assays for the Investigation of Fungal Outbreaks. Mycopathologia. 2020;185:867–880. doi: 10.1007/s11046-020-00452-x. [DOI] [PubMed] [Google Scholar]
- 119.Durkin M., Estok L., Hospenthal D., Crum-Cianflone N., Swartzentruber S., Hackett E., Wheat L.J. Detection of Coccidioides Antigenemia following Dissociation of Immune Complexes. Clin. Vaccine Immunol. 2009;16:1453–1456. doi: 10.1128/CVI.00227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Messina J.A., Maziarz E.K., Galgiani J., Truong J.T., Htoo A.K., Heidari A., Johnson R.H., Narang A.T., Donovan F.M., Ewell M., et al. A randomized, double-blind, placebo-controlled clinical trial of fluconazole as early empiric treatment of coccidioidomycosis pneumonia (Valley Fever) in adults presenting with community-acquired pneumonia in endemic areas (FLEET-Valley Fever) Contemp. Clin. Trials. Commun. 2021;24:100851. doi: 10.1016/j.conctc.2021.100851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sugar A.M., Alsip S.G., Galgiani J.N., Graybill J.R., Dismukes W.E., Cloud G.A., Craven P.C., Stevens D.A. Pharmacology and toxicity of high-dose ketoconazole. Antimicrob. Agents Chemother. 1987;31:1874–1878. doi: 10.1128/AAC.31.12.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pont A., Graybill J.R., Craven P.C., Galgiani J.N., Dismukes W.E., Reitz R.E., Stevens D.A. High-Dose Ketoconazole Therapy and Adrenal and Testicular Function in Humans. Arch. Intern. Med. 1984;144:2150–2153. doi: 10.1001/archinte.1984.04400020052007. [DOI] [PubMed] [Google Scholar]
- 123.Arndt C.A.S., Walsh T.J., McCully C.L., Balis F.M., Pizzo P.A., Poplack D.G. Fluconazole Penetration into Cerebrospinal Fluid: Implications for Treating Fungal Infections of the Central Nervous System. J. Infect. Dis. 1988;157:178–180. doi: 10.1093/infdis/157.1.178. [DOI] [PubMed] [Google Scholar]
- 124.Brewer A.C., Huber J.T., Girardo M.E., Kosiorek H.E., Burns M.W., Stewart T.D., Blair J.E. Cutaneous effects associated with fluconazole in patients treated for coccidioidomycosis. Int. J. Dermatol. 2019;58:250–253. doi: 10.1111/ijd.14238. [DOI] [PubMed] [Google Scholar]
- 125.Thompson G.R., Barker B.M., Wiederhold N.P. Large-Scale Evaluation of In Vitro Amphotericin B, Triazole, and Echinocandin Activity against Coccidioides Species from U.S. Institutions. Antimicrob. Agents Chemother. 2017;61:e02634-16. doi: 10.1128/AAC.02634-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galgiani J.N. Comparison of Oral Fluconazole and Itraconazole for Progressive, Nonmeningeal Coccidioidomycosis: A Randomized, Double-Blind Trial. Ann. Intern. Med. 2000;133:676. doi: 10.7326/0003-4819-133-9-200011070-00009. [DOI] [PubMed] [Google Scholar]
- 127.Hoffmann W.J., McHardy I., Thompson G.R., III Itraconazole induced hypertension and hypokalemia: Mechanistic evaluation. Mycoses. 2018;61:337–339. doi: 10.1111/myc.12749. [DOI] [PubMed] [Google Scholar]
- 128.Lestner J.M., Roberts S.A., Moore C.B., Howard S.J., Denning D.W., Hope W.W. Toxicodynamics of Itraconazole: Implications for Therapeutic Drug Monitoring. Clin. Infect. Dis. 2009;49:928–930. doi: 10.1086/605499. [DOI] [PubMed] [Google Scholar]
- 129.Sharkey P.K., Rinaldi M.G., Dunn J.F., Hardin T.C., Fetchick R.J., Graybill J.R. High-dose itraconazole in the treatment of severe mycoses. Antimicrob. Agents Chemother. 1991;35:707–713. doi: 10.1128/AAC.35.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim M.M., Vikram H.R., Kusne S., Seville M.T., Blair J.E. Treatment of Refractory Coccidioidomycosis With Voriconazole or Posaconazole. Clin. Infect. Dis. 2011;53:1060–1066. doi: 10.1093/cid/cir642. [DOI] [PubMed] [Google Scholar]
- 131.Cortez K.J., Walsh T.J., Bennett J.E. Successful Treatment of Coccidioidal Meningitis with Voriconazole. Clin. Infect. Dis. 2003;36:1619–1622. doi: 10.1086/375235. [DOI] [PubMed] [Google Scholar]
- 132.Freifeld A., Proia L., Andes D., Baddour L.M., Blair J., Spellberg B., Arnold S., Lentnek A., Wheat L.J. Voriconazole Use for Endemic Fungal Infections. Antimicrob. Agents Chemother. 2009;53:1648–1651. doi: 10.1128/AAC.01148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Epaulard O., Villier C., Ravaud P., Chosidow O., Blanche S., Mamzer-Bruneel M., Thiebaut A., Leccia M., Lortholary O. A Multistep Voriconazole-Related Phototoxic Pathway May Lead to Skin Carcinoma: Results From a French Nationwide Study. Clin. Infect. Dis. 2013;57:e182–e188. doi: 10.1093/cid/cit600. [DOI] [PubMed] [Google Scholar]
- 134.Haylett A.K., Felton S., Denning D.W., Rhodes L.E. Voriconazole-induced photosensitivity: Photobiological assessment of a case series of 12 patients. Br. J. Dermatol. 2013;168:179–185. doi: 10.1111/j.1365-2133.2012.11196.x. [DOI] [PubMed] [Google Scholar]
- 135.Williams K., Mansh M., Chin-Hong P., Singer J., Arron S.T. Voriconazole-Associated Cutaneous Malignancy: A Literature Review on Photocarcinogenesis in Organ Transplant Recipients. Clin. Infect. Dis. 2014;58:997–1002. doi: 10.1093/cid/cit940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pham A.N., Bubalo J.S., Lewis J.S., II Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses. 2016;59:226–233. doi: 10.1111/myc.12452. [DOI] [PubMed] [Google Scholar]
- 137.Anstead G.M., Corcoran G., Lewis J., Berg D., Graybill J.R. Refractory Coccidioidomycosis Treated with Posaconazole. Clin. Infect. Dis. 2005;40:1770–1776. doi: 10.1086/430303. [DOI] [PubMed] [Google Scholar]
- 138.Catanzaro A., Cloud G.A., Stevens D.A., Levine B.E., Williams B.L., Johnson R.H., Rendon A., Mirels L.F., Lutz J.E., Holloway M., et al. Safety, Tolerance, and Efficacy of Posaconazole Therapy in Patients with Nonmeningeal Disseminated or Chronic Pulmonary Coccidioidomycosis. Clin. Infect. Dis. 2007;45:562–568. doi: 10.1086/519937. [DOI] [PubMed] [Google Scholar]
- 139.Ruping M.J.G.T., Albermann N., Ebinger F., Burckhardt I., Beisel C., Muller C., Vehreschild J.J., Kochanek M., Fatkenheuer G., Bangard C., et al. Posaconazole concentrations in the central nervous system. J. Antimicrob. Chemother. 2008;62:1468–1470. doi: 10.1093/jac/dkn409. [DOI] [PubMed] [Google Scholar]
- 140.Schein R., Homans J., Larsen R.A., Neely M. Posaconazole for Chronic Refractory Coccidioidal Meningitis. Clin. Infect. Dis. 2011;53:1252–1254. doi: 10.1093/cid/cir734. [DOI] [PubMed] [Google Scholar]
- 141.González G.M., Tijerina R., Najvar L.K., Bocanegra R., Rinaldi M., Loebenberg D., Graybill J.R. In Vitro and In Vivo Activities of Posaconazole against Coccidioides immitis. Antimicrob. Agents Chemother. 2002;46:1352–1356. doi: 10.1128/AAC.46.5.1352-1356.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Donnelley M.A., Zhu E.S., Thompson G.R. Isavuconazole in the treatment of invasive aspergillosis and mucormycosis infections. Infect. Drug Resist. 2016;9:79–86. doi: 10.2147/IDR.S81416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thompson G.R., Rendon A., Ribeiro dos Santos R., Queiroz-Telles F., Ostrosky-Zeichner L., Azie N., Maher R., Lee M., Kovanda L., Engelhardt M., et al. Isavuconazole Treatment of Cryptococcosis and Dimorphic Mycoses. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016;63:356–362. doi: 10.1093/cid/ciw305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial Elsevier Enhanced Reader. Lancet. 2015;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 145.Hamill R.J. Amphotericin B Formulations: A Comparative Review of Efficacy and Toxicity. Drugs. 2013;73:919–934. doi: 10.1007/s40265-013-0069-4. [DOI] [PubMed] [Google Scholar]
- 146.Saravolatz L.D., Ostrosky-Zeichner L., Marr K.A., Rex J.H., Cohen S.H. Amphotericin B: Time for a New “Gold Standard”. Clin. Infect. Dis. 2003;37:415–425. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- 147.Stevens D.A., Shatsky S.A. Intrathecal amphotericin in the management of coccidioidal meningitis. Semin. Respir. Infect. 2001;16:263–269. doi: 10.1053/srin.2001.29298. [DOI] [PubMed] [Google Scholar]