Abstract
Simple Summary
Cold atmospheric plasma (CAP) is generated in a rapid yet low-energy input streamer-discharge process at atmospheric pressure conditions. CAP is an ionized gas with a low ionization level and plenty of reactive species and radicals. These reactive components, and their near-room temperature nature, make CAP a powerful tool in medical applications, particularly cancer therapy. Here, we summarized the latest development and status of preclinical applications of CAP in cancer therapy, which may guide further clinical studies of CAP-based cancer therapy.
Abstract
CAP is an ionized gas generated under atmospheric pressure conditions. Due to its reactive chemical components and near-room temperature nature, CAP has promising applications in diverse branches of medicine, including microorganism sterilization, biofilm inactivation, wound healing, and cancer therapy. Currently, hundreds of in vitro demonstrations of CAP-based cancer treatments have been reported. However, preclinical studies, particularly in vivo studies, are pivotal to achieving a final clinical application. Here, we comprehensively introduced the research status of the preclinical usage of CAP in cancer treatment, by primarily focusing on the in vivo studies over the past decade. We summarized the primary research strategies in preclinical and clinical studies, including transdermal CAP treatment, post-surgical CAP treatment, CAP-activated solutions treatment, and sensitization treatment to drugs. Finally, the underlying mechanism was discussed based on the latest understanding.
Keywords: cold atmospheric plasma, cancer treatment, anti-tumor therapy, reactive species, non-invasive therapy, redox medicine, drug sensitization
1. CAP and Plasma Sources
CAP has been widely used in several branches of medicine, including wound healing, microorganism sterilization, biofilm inactivation, and cancer therapy [1,2,3]. CAP is an ionized gas composed of reactive compounds such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) [4,5], and is designed to work under atmospheric pressure at a near room temperature [6].
The physical foundation to generate CAP is briefly illustrated here. We take the atmospheric pressure plasma jet (APPJ) as an example, which has been more widely used in plasma medicine than any other sources [7]. Typical CAP generations usually rely on a specific ionization process, namely “positive streamer propagation,” as a kind of ionization wave [8,9]. The positive streamer propagation starts near the anode, where the seed electrons are attracted. During their movements, electrons collide with other particles. As the electric field near the anodes reaches an adequately high level, several electrons are accelerated sufficiently to ionize the particles, resulting in more electrons, namely an “avalanche,” while other electrons with lower velocities may only excite the particles. The ionization wavefront is where the “avalanche” occurs. The wavefront is the luminous region due to the photon emissions from the excited atoms or other particles, which is also known as the “plasma bullet” [8,9]. During the streamer propagation in the open air with a noble gas environment such as helium, hundreds of chemical reactions occur simultaneously, each with a unique but dynamic reaction rate [10]. These chemical reactions thus generate ROS/RNS. In many cases, CAP sources are powered with alternating current (AC) to ensure a continuous generation in the open air [11].
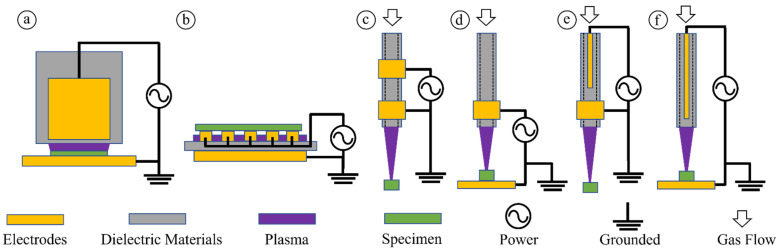
CAP sources, such as dielectric barrier discharge (DBD), APPJ, and plasma torch, are the foundation for plasma applications [12,13]. Six of the most commonly used CAP sources are shown in Figure 1. AC is a typical power supply for these CAP sources [14]. Type a and type b are DBD-style devices. DBD generates plasma between two electrodes powered by radiofrequency (RF) discharge voltage, usually around the kV scale. Multiple streamer propagations developed between the electrodes in each discharge cycle, and each streamer path is called a “filament” [15]. Although the horizontal spatial distribution of filaments is random and dynamic, the distribution is uniform when the two electrode surfaces are parallel, and the dielectric barrier material properties are uniform. Therefore, the plasma generated from DBD can cover a large area. On the other hand, APPJ (type c and type e) and plasma torch (type d and type f) are more focused on tools that can deliver ROS/RNS on a small size target more accurately as a single track of streamer propagation. An APPJ generator requires a hollow cathode to allow the streamer to pass through and reach the target below the cathode. However, the plasma torch is the standard model for streamer propagation between two electrodes. In addition to these typical CAP sources, some new sources have recently been developed and used in preclinical studies. One example is a non-invasive and non-thermally operated electrosurgical plasma source [16,17,18].
Figure 1.
Typical CAP sources used in plasma medicine. Type a. Volume DBD. Type b. Surface DBD. Type c. Two-ring electrodes APPJ. Type d. Plasma torch using ring electrode. Type e. One central electrode-one ring electrode APPJ. Type f. Plasma torch using a central electrode.
2. General Picture of In Vitro Studies
To date, the promising anti-cancer performance of CAP treatment in vitro has been extensively demonstrated in dozens of cancer types, including skin, breast, colorectal, brain, lung, cervical, head and neck cancer [3,7]. Plenty of reviews and articles have been published, most of them focused on in vitro studies and corresponding conclusions [19,20,21]. Several basic cellular responses have been repeatedly observed in the publications listed in Table 1. These basic cellular responses build the foundation for understanding the anti-cancer effect of CAP treatment in vitro and address some in vivo observations.
Table 1.
Basic cancer cellular responses of CAP treatment in vitro.
| Ref | Cancer Cellular Response | Years |
|---|---|---|
| [22] | Apoptosis | 2004 |
| [23] | Growth Inhibition | 2007 |
| [24] | Cytoskeletal Damage | 2009 |
| [25] | Selective Cell Death | 2010 |
| [26] | Cell Cycle Arrest | 2010 |
| [27] | Nuclear and DNA Damage | 2010 |
| [27] | Mitochondrial Damage | 2010 |
| [28] | Rise of Intracellular ROS | 2011 |
| [29] | Chemically-based Sensitization to Drugs | 2013 |
| [30] | Selective Rise of Intracellular ROS | 2013 |
| [31] | Senescence | 2013 |
| [32] | Immunogenic Cell Death | 2015 |
| [33] | Cell-based H2O2 Generation | 2017 |
| [34] | Autophagy-associated Cell Death | 2017 |
| [35] | Activation Phenomena | 2018 |
| [36] | Physically-triggered Necrosis | 2020 |
| [37] | Pyroptosis | 2020 |
| [38] | Physically-based Sensitization to Drugs | 2021 |
Together, some general conclusions can be summarized here. (1) Reactive species play a critical role in the liquid phase-based experimental setting [39]. Apoptosis, necrosis, and autophagy are the main cellular death approaches following CAP treatment with an adequately large dose [40]. (2) Physical factors, particularly electromagnetic effects from plasma, may exert a clear impact on cells, such as bacteria and mammalian cells [41,42]. (3) A noticeable rise in intracellular ROS is a pivotal cellular response to CAP treatment, which further triggers downstream cellular damage, including DNA damage, mitochondrial damage, cellular membrane damage, and cell death [1]. (4) Aqueous environment, such as a medium layer, plays a pivotal role in facilitating the transition of short-lived reactive species in the gas phase into long-lived reactive species in the liquid phase [43,44,45]. For in vitro studies, a medium layer is necessary for experimental design and is responsible for most observed cellular responses after CAP treatment, particularly for the cases involving CAP-treated solutions or media [40,46]. (5) CAP shows selective killing effect on cancer cell lines compared to their counterpart normal cell lines in many cases [47].
3. Direct CAP Treatment In Vivo
Like most medical studies, the conclusions obtained from in vitro studies cannot be easily used to directly predict the performance of in vivo studies. For example, the relatively dry skin barrier between plasma and targeted cancerous tissues or cells under the skin is quite different from the commonly accepted experimental conditions in vitro. The in vitro environment mainly involves a relatively thick medium layer to facilitate the transition of some short-lived reactive species in gas phase to long-lived reactive species in liquid phase. Moreover, both long-lived and short-lived reactive species will have complex reactions at this gas/liquid interface. For plasma medicine, in vivo studies play a cornerstone role before CAP can be used in clinical therapy [48,49,50]. More importantly, in vivo studies directly assess the CAP treatment’s safety on tissues and animals, such as carcinogenicity [51]. In this review, our preclinical studies’ discussion will be just limited to in vivo studies.
Compared to the abundant in vitro investigations, in vivo studies have gradually become the main approach to discovering novel tissue responses to CAP treatment. Animal models’ design directly determines the use of CAP in the in vivo studies. So far, three types of animal models have been widely used to demonstrate the anti-tumor efficacy of CAP treatment: subcutaneous model, intraperitoneal model, and orthotopic model [52]. To date, most of CAP’s anti-tumor capability was demonstrated by using subcutaneous models.
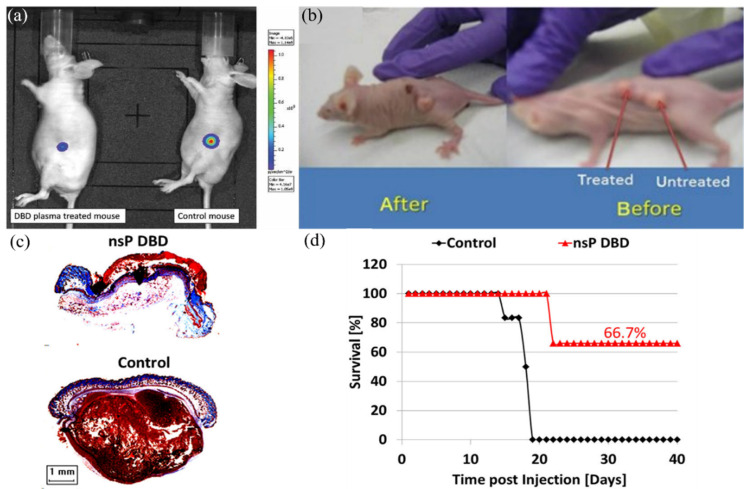
Subcutaneous models provided the earliest and the most apparent demonstration for the feasibility of using CAP as an anti-tumor modality. The earliest in vivo works were demonstrated by Marc Vandamme, et al. and Michael Keidar, et al. between 2010–2011 (Figure 2a,b). They used a glioblastoma U87MG xenograft mouse model and bladder xenograft tumor model to test a CAP treatment’s in vivo efficacy for just a few minutes, respectively. The two pioneering research articles demonstrated a drastic tumor volume reduction of more than 50% after floating electrode DBD treatment and APPJ treatment [49,53]. Correspondingly, the survival length of mice strongly increased by more than 60% in the two models [49,53]. These two works also first tested the safety of using CAP in animal studies. Results did not show any toxic side effects and potential physical damage from plasma.
Figure 2.
Some representative anti-tumor demonstrations use subcutaneous models. (a) U87 xenografted nude mice mouse was irradiated (6 min) by a DBD device for 5 consecutive days. Bioluminescence imaging (BLI) was used to quantify tumor growth and size. Reprinted with permission from Ref. [53]. 2010, Wiley. (b) Typical image of mice with three subcutaneous Bladder xenografted tumors before and 24 h after APPJ treatment. [49]. (c) nsp DBD treatment strongly eradiated melanoma tumors in mice. Trichrome staining of DBD treated tumor (top) and control tumor (bottom). (d) Survival for DBD treated tumors (red) and untreated tumors in control (black) as a function of time post-injection. [48].
Due to the subcutaneous nature of melanomas, it has become one of the more promising candidates for CAP-based cancer therapy. Many studies have been performed on melanoma models [7]. As shown in Figure 2c, a nanosecond pulsed DBD (nsP DBD) completely eradicated the xenografted melanoma tumor in mice after direct treatment on the skin above the melanoma. Histology of an nsP DBD treated tumor showed a typical red skin staining without tumor tissue below the epithelium. Correspondingly, the survival rate of mice increased from 0% to 66.7% 20–40 days succeeding the nsp DBD treatment (Figure 2d) [48].
Similar trends have been repeatedly observed in a series of following studies. Table 2 lists representative in vivo anti-tumor demonstrations (2010–2018) on subcutaneous xenograft tumors in mice. In the subcutaneous model, CAP treatment was mainly carried out by treating the skin above tumorous tissues. In such a setting, the effective factor, either chemical or physical factors in CAP, must penetrate the skin barrier and further trigger biological pathways to inhibit tumorous growth, therefore providing CAP treatment as a potential non-invasive anti-tumor modality. Among these studies, some general trends have been observed. First, a treatment just above the skin could strongly inhibit the growth of tumors and significantly extend the life length of mice [53]. Second, a fractionated, multi-time consecutive treatment may generate a much better therapeutic effect than a single but long treatment, which may be due to the long-term anti-tumor effect of CAP treatment [54]. A multi-time consecutive treatment may consecutively trigger these long-term anti-tumor effects, such as an immune response.
Table 2.
Representative in vivo demonstrations on subcutaneous xenografted tumor models (2010–2018).
| Ref | Years | Tumor Types | Tumor Size | Survival Rate | Tumor Diagnostics |
|---|---|---|---|---|---|
| [53] | 2010 | Glioblastoma | Decreased | N/A | Bioluminescence imaging |
| [49] | 2010 | Bladder cancer | Decreased | Increased | Tissue size measurement |
| [54] | 2011 | Glioblastoma | Decreased | Increased | Bioluminescence imaging |
| [55] | 2012 | Pancreatic carcinoma | Decreased | N/A | Bioluminescence imaging |
| [56] | 2012 | Glioblastoma | Decreased | N/A | Bioluminescence imaging |
| [57] | 2013 | Neuroblastoma | Decreased | Increased | Tissue size measurement |
| [58] | 2014 | Melanoma | Decreased | N/A | Tissue size measurement |
| [59] | 2014 | Head and neck cancer | Decreased | N/A | Tissue size measurement |
| [48] | 2015 | Melanoma | Decreased | Increased | Tissue size measurement |
| [60] | 2015 | Endometrioid adenocarcinoma | Decreased | N/A | Tissue size measurement |
| [61] | 2016 | Glioblastoma | Decreased | N/A | Tissue size measurement |
| [62] | 2016 | Breast cancer | Decreased | N/A | Tissue size measurement |
| [50] | 2017 | Melanoma | Decreased | N/A | Bioluminescence imaging |
| [63] | 2018 | Colorectal tumor | Decreased | N/A | Tissue size measurement |
4. CAP-Activated Solutions (PAS) and In Vivo Application
Direct CAP treatment is based on the touch of bulk plasma with or near a target. In contrast, indirect treatment is based on the CAP-activated (treated, stimulated) solutions to affect the growth of cancer cells or tissues. Over the past decade, CAP-activated solutions (PAS) have shown promising applications in cancer treatment in vitro and in vivo [46]. Once PAS is made, it can be stored for days or weeks and used in cases without a CAP source [64,65]. PAS can be employed to inhibit the growth of tumorous tissue by subcutaneous or intraperitoneal injection in mice [66]. Moreover, PAS can be used in the lavage of patients suffering from peritoneal carcinomatosis adjuvant to standard chemotherapy [67,68]. Typically, PAS is made by treating biologically adaptable solutions such as a medium or phosphate-buffered saline (PBS) using APPJ or DBD above a solution’s surface [69,70,71]. PAS can also be made by underwater discharge in solutions [72]. PAS can be synergistically used to enhance the therapeutic effects of chemotherapy drugs and other chemicals [73,74,75]. PAS selectively kills colon, lung, cervical, bladder, melanoma, and breast cancer cells in vitro [76,77,78,79,80]. Preliminary studies on immuno-deficient nude mice by oral lavage treatment of CAP-activated water did not find lethal effects and acute toxicity [81,82]. Furthermore, the mice had no significant changes, including body weight, survival status, organ coefficient, function, and tissue structure of heart, liver, spleen, lung, and kidney [81,82].
Several in vivo studies present the potential use of PAS in cancer therapy. Several representative studies were introduced here. Fumi Utsumi et al. first achieved an evident anti-tumor efficacy in mice by injecting CAP-activated Ringer’s lactate into subcutaneous tumors grown from the xenografted chemical-resistant ovarian cancer cells [83]. Compared with PBS, Ringer’s lactate solution is closer to the translation solution, which could be used as a clinical modality [84,85,86]. Ringer’s lactate solution contains sodium chloride, potassium chloride, calcium chloride, sodium lactate, and sodium bicarbonate. PAS can also be used for intraperitoneally xenografted tumor models. Shigeomi Takeda et al. demonstrated that PAS effectively decreased the peritoneal metastatic nodules by 60% in mice without causing adverse events [67]. Similar anti-tumor performance by injection of PAS in vivo have been observed in other subsequent studies [80,87].
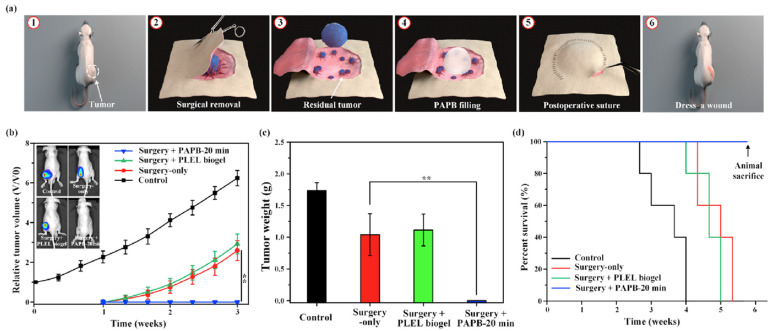
Recently, a novel strategy to use PAS has been demonstrated. Post-surgical residual tumor tissues or cells are the primary cause of relapse and progression of cancer post-surgery. A fillable plasma-activated biogel was made on a thermosensitive biogel, (poly-dl-lactide)-(poly-ethylene-glycol)-(poly-dl-lactide), PLEL, with the aid of PAS for local post-surgical removal of tumors in mice models [88]. Preliminary in vivo data demonstrated that the plasma-activated PLEL biogel (PAPB) entirely eliminated residual in situ tumor tissue recurrence after a removal surgery without showing evident systemic toxicity (Figure 3). More attractively, PAPB allowed a slow release of ROS. Altogether, this study provided a solid foundation to use PAS in local post-operative cancer treatments [88].
Figure 3.
Anti-tumor performance of a plasma-activated PLEL biogel (PAPB) on residual tumor after surgical removal. (a) Schematic illustration of post-operative PAPB treatment. (b) Whole-body bioluminescence imaging and tumor growth in mice: control, surgical removal-only, surgical removal + PLEL (thermosensitive biogel), and surgical removal + PAPB treatment. (c) Weight of excised tumors. (d) Survival of tumor-bearing mice (n = 5, ** p < 0.01). Reprinted with permission from Ref. [88]. 2021, Elsevier Ltd.
5. Abscopal Effect
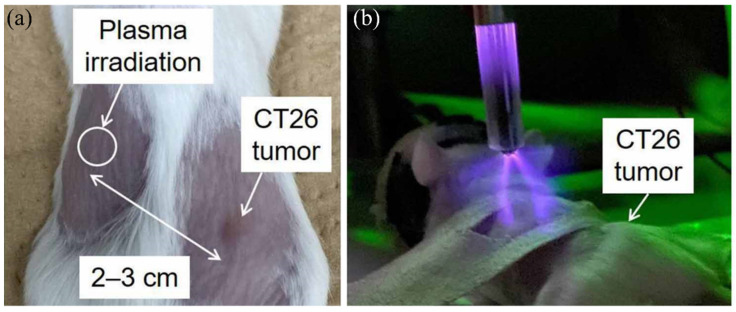
An anti-tumor abscopal effect was rarely observed in plasma medicine and radiotherapy. To date, only two examples were reported in 2017 and 2022, providing a novel approach to using CAP in cancer therapy. Compared to previous studies, these two studies demonstrated that the tumor growth at a non-treated site on a mouse could be suppressed by a CAP treatment on another nearby tumor site or even by a CAP treatment on the health tissue site on the same mouse (Figure 4). Moreover, these surprising phenomena appeared just one day post the treatment [89]. Particularly, when CAP treatment was performed on the skin above the healthy tissue of the left limb, the abscopal effect was only significant for the mice with small tumors of the right limb [90]. Fundamentally, the abscopal effect triggered by a CAP treatment on the skin above normal tissue was comparable to the anti-tumor efficacy of a direct CAP treatment on the skin above tumorous tissue.
Figure 4.
Anti-tumor abscopal effects in mice induced by a CAP treatment on normal tissue. (a) CAP irradiation site and murine colorectal carcinoma CT26 tumor on day 6. (b) CAP treatment on a normal tissue site can inhibit the nearby tumor’s growth [90].
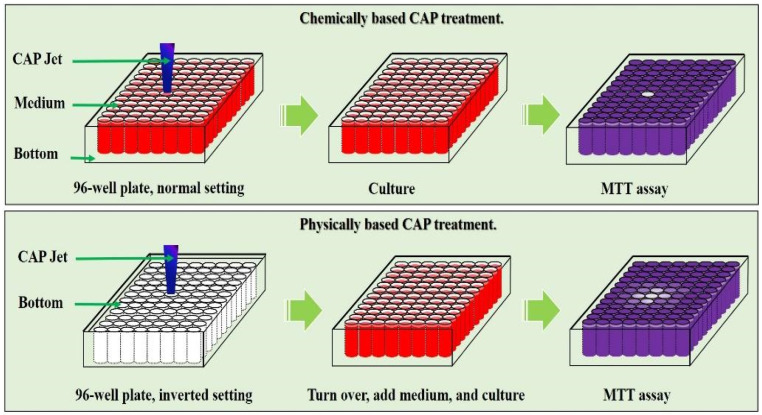
Due to the very limited data from these two studies, the underlying mechanism is entirely unknown. The authors explained that the innate immune response in vivo might trigger such a rapid abscopal effect after observing the production of inflammatory cytokines (IFN -γ) from splenocytes post CAP treatment [89]. Here, based on recent discoveries of the physically based CAP treatment, we proposed that physical factors in CAP may explain these abscopal effects. Physical factors in CAP, likely mainly electromagnetic (EM) effect, can affect the target with an area much larger than the plasma size or the contacting area between plasma and target. As shown in Figure 5, physically based treatment can affect an area much larger than chemically based treatment because the EM effect can penetrate the dielectric physical barrier between every well on a 96-well plate [41]. In our recent study, an APPJ’s tip was less than 1 mm; however, the EM effect generated from APPJ could affect an area with a diameter of at least 2 cm [91]. As shown in Figure 4a, the abscopal effect affects the tumor at a distance of 2–3 cm. The physical effect of CAP treatment is much more complex than its chemical effect in terms of spatial distributions and anisotropic effect, which may cause a series of novel biological effects.
Figure 5.
Physical factors in CAP can affect a much bigger area than the plasma contacting area on samples. A representative illustration of chemical factors and physical factors in CAP and their killing effect on cancer cells in vitro [41].
6. Sensitization of Tumor to Drugs
The primary rationale for using CAP in cancer treatment is to achieve a direct killing effect on tumor or cancer cells in vitro and in vivo. Recently, a novel rationale for using CAP has caught attention, which is focusing on using CAP to sensitize cancer cells or tissues to the existing chemotherapy, particularly the cytotoxicity of drugs. Unlike previous studies focusing on the chemically based sensitization of cancer cells to some drugs, this new approach focused on using the physical factors, mainly EM effect or EM emission from CAP, to affect tumors in depth rather than just to affect the subcutaneous models. So far, it is still unclear the effective EM frequency range to cause these EM effects. Thus, the current studies focused on demonstrating the sensitization capability of CAP treatment on brain cancer models such as glioblastoma in mice brains. The underlying mechanism is entirely unknown at the current stage [92].
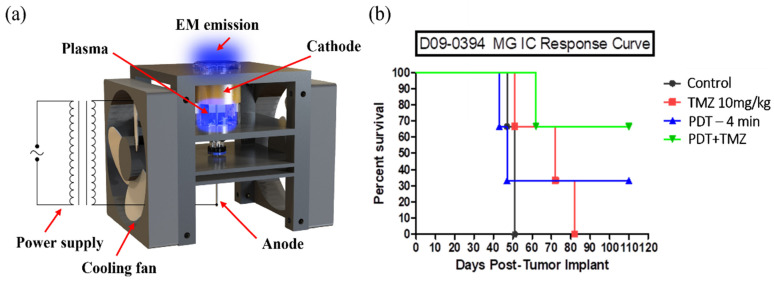
Glioblastoma (GBM) is one of the most aggressive brain cancers. GBM is also highly resistant to treatment [93,94]. Temozolomide (TMZ) is a widely used FDA-approved alkylating chemotherapy agent, particularly for high-grade malignant GBM treatment [94]. Recently, two studies demonstrated that just a CAP treatment during a mouse’s brain neurosurgery could achieve a noticeable enhanced therapeutic efficacy of TMZ. First, a single APPJ treatment on the mouse’s head could sensitize brain tumors in the skull to the cytotoxicity of GBM [95]. Another example was using a helium radial cold plasma discharge tube (PDT) as a tunable EM emission source, which only allowed the EM effect of CAP to affect targets because all chemical factors have been blocked in PDT (Figure 6a). PDT selectively increased the cytotoxicity of TMZ on two glioblastoma cell lines A172 and U87MG, compared to the standard astrocyte cell line hTERT/E6/E7 to some extent in vitro [96]. More attractively, preliminary in vivo studies demonstrated a drastically improved mean survival day of patient-derived xenografted glioblastoma mice models by 100% compared to the control group (Figure 6b). PDT was independent of continuous gas supply; thus, it has the potential to be a portable and small CAP source. Together, these two studies demonstrated that the EM effect in CAP could penetrate the skin and the skull, providing an unprecedented vision for further CAP-based cancer therapy.
Figure 6.
Sensitization of brain cancer cells to temozolomide (TMZ) by a cold plasma discharge tube (PDT). (a) Basic structure of PDT source. (b) Mouse survival rate curve. Reprinted with permission from Ref. [96]. 2022, American Chemical Society.
7. Clinical Anti-Tumor Trials
The clinical use of CAP as an anti-tumor modality is an ultimate goal in plasma medicine. Unfortunately, CAP’s clinical tests in cancer therapy are still quite rare. A few examples were illustrated here, which provide critical clues to guide the use of CAP in therapeutic advances. In 2015, a private company, US Medical Innovation (USMI), carried out a clinical trial on stage IV metastatic colon cancer at Baton Rouge General Medical Center in Baton Rouge, Louisiana, USA. CAP treatment was performed on the post-surgical tissue to kill potential residual cancer cells after a removal surgery, and no relapse and progression of cancer occurred in patients. The trial also tested the safety of CAP treatment [97]. In Germany, CAP treatment was performed on 12 patients with advanced squamous cell carcinoma of the head and neck [98]. CAP was used to decontaminate infected cancer ulcerations in this trial. It is found that CAP treatment generated positive effects in patients, including a decreased request for pain medication and a reduction of typical fetid odor and microbial load [98]. In some cases, superficial partial remission of tumor and even wound healing of the infected ulcerations have been observed [98].
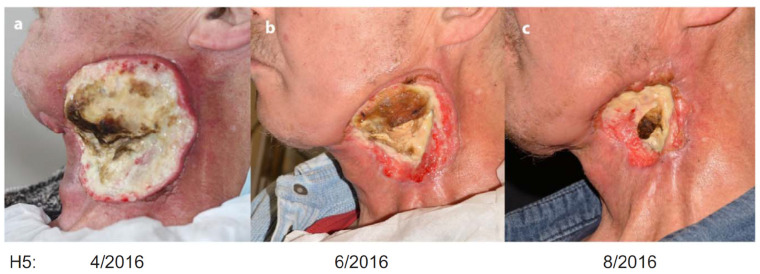
In 2017, USMI used Canady Helios Cold Plasma and Hybrid Plasma Scalpels in the clinical liver resection to remove and selectively kill liver tumor cells [97]. In the same year, there was another impactful treatment performed by Metelmann et al. [99]. Their trial enrolled six patients with local advanced (pT4) squamous cell carcinoma of the oropharynx with open infected ulcerations. Six patients were treated by an APPJ in a cycle of three single applications within a week, each followed by an intermittence of another week [99]. As shown in Figure 7, CAP treatment noticeably improved the therapeutic effect of this locally advanced head and neck cancer. CAP treatment not only improved patients’ social functions, but also caused a reduction in odor and pain medication requirements [99]. In addition, partial remission in two patients has been observed, and the incisional biopsies found a moderate level of apoptotic tumor cells and a desmoplastic reaction in the connective tissue [99]. These clinical trials also strongly suggested that CAP treatment’s widely observed wound healing capability will play a critical supporting role in cancer therapy [100]. In May 2022, USMI presented the results of a two-year follow up phase I clinical trial of Canady Helios Cold Atmospheric Plasma (CHCP) treatment for patients with advanced stage IV metastatic and recurrent solid tumors at the Biannual Conference of the Israeli Society of Surgical Oncology. Twenty patients were recruited from U.S. and Israel. Patients received intra-operative CHCP treatment at the operative site after removing the tumor. The primary endpoint was safety [101]. Together, the existing clinical trial suggests that CAP treatment can be a powerful supplemental tool to improve the current surgery and chemotherapy efficacy.
Figure 7.
The clinical effect of CAP treatment on a patient (H5) with locally advanced head and neck cancer. Reprinted with permission from Ref. [99]. 2017, Elsevier GmbH. The patient’s therapeutic effect was recorded in April/2016 (a), June/2016 (b), and August/2016 (c), respectively.
8. Mechanism Discussion
Due to the complex nature of CAP and tissues, the anti-tumor mechanism of CAP in vivo is an open question. Reactive species, particularly ROS, have been widely regarded as the main factors in CAP to cause cellular damage and ultimate cell death in vitro [5,102]. The strong rise of ROS in the tumor tissue after CAP treatment has also been observed [103]. This cellular response may be due to the transdermal diffusion of reactive species [104]. Because of the complexity of mammalian skin, the transdermal diffusion process was mainly studied using skin substitutes such as agarose gels [105]. For example, it is found that H2O2 and NO2− could be slowly (30 min) transported through an agarose gel with a thickness of 1.5 mm [106]. Furthermore, the transportation of reactive species through an agarose gel is highly dependent on gel thickness [107]. A 10-mm thick gap of gas between the agarose gel and CAP could inhibit reactive species’ transmembrane diffusion [107]. Recently, via using contact- and marker-independent Raman microscopy, it was found that the APPJ can penetrate the basal cell layer of a cervical epithelium sample with a depth of roughly 270 μm [108].
The physical pathways in the skin necessary for the transdermal diffusion of reactive species are still unknown. Possible pathway candidates may include hair follicles, microneedles, electroporation, or other transcellular and intracellular routes [109]. Recently, the transdermal transportation of reactive species across animal skin, such as mouse and pig skin, have been studied. An air CAP source was used to treat mouse skin with a thickness of 0.75 mm, and the authors did not observe the formation of H2O2 and NO2−/NO3− in the deionized water underneath the skin [110]. Nevertheless, the authors did observe the transdermal diffusion of NO2−/NO3− in the CAP-activated deionized water across the mouse skin [110]. The transdermal diffusion of RONS across pig skin has been observed under specific operational conditions, such as discharge frequency [111,112]. A recent study found that a 300-ns 50-kV/cm pulsed electric field increased the transdermal diffusion of RONS across a pig skin model [113]. Altogether, these preliminary investigations suggest that the transdermal diffusion of reactive species underneath a millimeter-level thick tissue-mimic film is possible after CAP treatment.
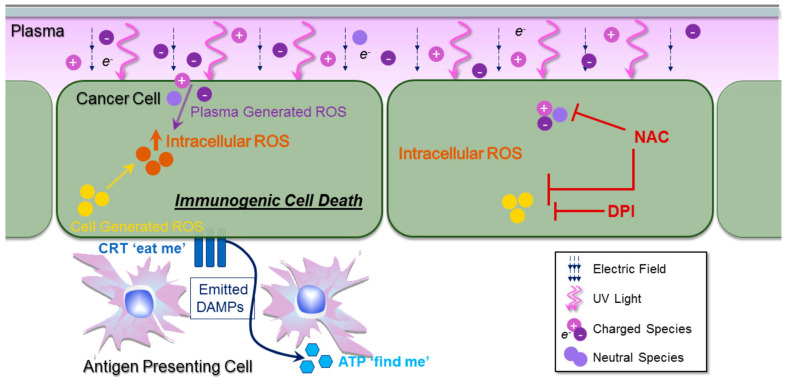
How do the CAP-originated or secondary reactive species affect subcutaneous tumor tissues? The widely observed subcutaneous anti-tumor effect may affect the tissues by using the direct killing effect from these RONS or due to the activation of an immune response triggered by CAP treatment. The development of CAP in combination with cancer immunotherapeutics has received growing attention recently. One rationale is that CAP may activate the immune system to attack tumorous tissue by reactive species or other factors [114,115]. These immune responses have been named as immunogenic cell death (ICD) [32,63,89,114,116,117,118]. One early representative observation found that macrophages could be activated in vitro by nsp DBD [116]. Some studies found that CAP could trigger cancer cells to emit signals known as damage-associated molecular patterns (DAMP), which may attract and stimulate local immune cells [119]. As shown in Figure 8, DAMP include at least two types of signals: a “find me” signal such as ATP and an “eat me” signal such as ecto-CRT [117]. Some studies observed increasing exposure of CRT on a cancer cells’ surface [117,120]. Moreover, specific expression of molecular pattern signals ATP has been observed and were believed to further trigger immunogenic attack on cancer cells [117].
Figure 8.
A CAP-based immunogenic cell death (ICD) in vivo model. Both chemical and physical factors in CAP may affect cellular functions. ROS, RNS, or other chemical factors may mainly contribute to eliciting ICD. CAP triggers the DAMP signals, such as ecto-CRT and ATP secretion. ATP acts as a “find me” DAMP signal to recruit immune cells, and surface-exposed CRT serves as an “eat me” signal. Diphenyleneiodonium (DPI) could inhibit cellular ROS production. NAC could scavenge ROS from different sources [117].
CAP causes ICD far beyond these approaches. For example, the injection of the CAP-treated CT26 colorectal cancer cells in mice caused a noticeable growth inhibition in the tumor compared to injecting the CT26 cells without CAP treatment [120]. In other words, the CAP-treated cancer cells can be used as a whole-cell vaccine to elicit protective immunity in at least CT26 colorectal tumor mouse models [63]. Similar research strategies have been reported recently in other studies [121]. Furthermore, the synergistic use of CAP with vaccination enhanced the cancer-specific T-cell responses as well [63,120]. In short, immunogenic cell death may be a core process to understand in vivo anti-tumor performance of a CAP treatment.
Other factors in CAP may also trigger an immune response. For example, a strong (micromolar level) cell-based H2O2 generation has been observed during and following a CAP treatment using APPJ in vitro [33,122,123]. H2O2 is a second messenger to activate lymphocyte [124,125]. Micromole levels of H2O2 could rapidly activate the transcription factor NF-κB and early gene expression of interleukin-2 (IL-2) [124]. Short-lived ROS such as superoxide or single oxygen may activate cancerous cells to generate H2O2 [33]. Tumorous tissues may generate plenty of H2O2 after CAP treatment in vivo, and the CAP-originated H2O2 does not directly touch these tissues, which explains the strong rise of subcutaneous ROS in the CAP-treated mice [103]. If similar phenomena also occur in vivo, the CAP-affected tissue may become a target for the immune system’s attack.
Nevertheless, the conclusion may not simply be used to explain many observations in vivo. Physical factors in CAP may play critical roles when CAP source is used to directly treat tumor tissues or even just using CAP source to affect the subcutaneous tissue with skin as the barrier [126]. For in vitro studies, a liquid layer always covers the cells due to the experimental setting. As a result, physical factors, thermal, UV, or other EM effects, have been entirely or largely blocked by the liquid layer [40]. Thus, physical factors’ effect has not been observed until the recent direct demonstration of a strong anti-cancer effect using physically based CAP treatment. Physical factors, likely mainly the EM effect generated in CAP, have several novel features compared with chemical factors such as ROS and RNS in CAP. First, physical factors in CAP can penetrate the dielectric barrier (~1 mm) and air gap (~8 mm) to affect cells, which is consistent with the widely observed non-invasive nature of the transdermal capability of CAP treatment on the skin above tumor tissues [36,91]. Second, physical factors in CAP cause strong necrosis [36,41,127]. Necrosis will trigger inflammation and other immune responses in vivo, which may explain some immune responses in many in vivo studies.
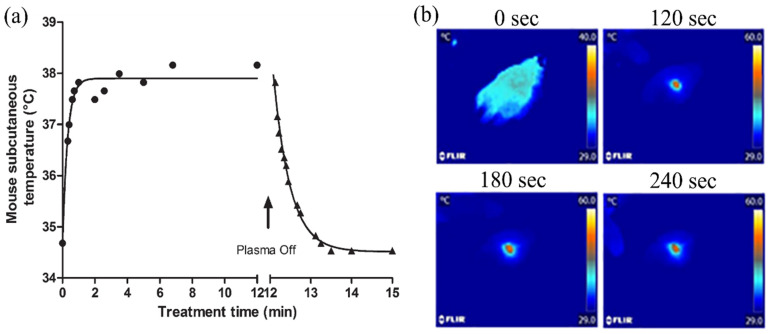
Furthermore, the thermal effect of CAP treatment cannot be simply ignored. For example, in the earliest in vivo demonstration (Figure 9a), the subcutaneous temperature of a mouse after the treatment was close to the mouse’s body temperature [53]. However, in another example (Figure 9b), the highest temperature in the treated mouse’s skin was at least 50 °C after 4 min of treatment [50]. Clearly, the heating effect in this case and other similar cases must be considered when the anti-tumor mechanism is analyzed. Thus, the temperature data or the thermal effect in CAP treatment must be provided or be considered in future in vivo studies. The naming of “CAP” cannot naturally guarantee the plasma is actually “cold” or “nonthermal”. For example, some so-called “self-organized” patterns of plasma have a temperature of hundreds °C [128]. Strictly speaking, these “hot” CAP sources cannot be regarded as cold plasma sources.
Figure 9.
Thermal effect of CAP treatment on the mouse. (a) Evolution of mouse subcutaneous temperature during and after DBD treatment. Reprinted with permission from Ref. [53]. 2010, Wiley. (b) Temperature distribution infrared imaging on the APPJ-treated (4 min) mouse by a thermal visor [50].
9. Conclusions
Altogether, it is promising that CAP will play a unique role as a novel, self-adaptive anti-tumor weapon. In a clinic, however, CAP cannot be used to quickly remove a tumor tissue, which may be the largest limitation of its anti-tumor performance compared to surgical approaches. To date, in clinical trials, CAP has been used either by treating the potential residual tumor tissues post-surgical removal or by directly treating tumor sites to improve other therapeutic modalities’ efficacy. Currently, CAP is more like a surgery-assistant tool in cancer therapy.
Further clinical applications may be beyond this vision. Over the past decade, three strategies to use CAP have been proposed and demonstrated in preclinical studies. First, the direct killing effect of CAP on tumors by either the non-invasive transdermal diffusion of reactive species or by the immunogenic cell death of cancer cells after CAP treatment. This strategy is suitable for subcutaneous tumors, such as melanoma and neck and head cancer. Second, for the tumors in depth, such as intraperitoneally tumor models, PAS can be injected into deep tissue to inhibit tumor growth. Lastly, a novel strategy is the sensitization of cancer cells to the cytotoxicity of chemotherapeutic drugs, either by reactive species or by physical factors such as EM emission from CAP sources. Generally, these biological responses of CAP may be not only due to chemical factors such as reactive species but also physical factors such as EM and thermal effects.
Abbreviations
| AC | Alternating Current |
| APPJ | Atmospheric Pressure Plasma Jet |
| ATP | Adenosine Triphosphate |
| CAP | Cold Atmospheric Plasma |
| CHCP | Canady Helios Cold Atmospheric Plasma |
| CRT | Calreticulin |
| DAMP | Damage-Associated Molecular Patterns |
| DBD | Dielectric Barrier Discharge |
| DPI | Diphenylenyleneiodonium |
| EM | Electromagnetic |
| ICD | Immunogenic Cell Death |
| IFN | Inflammatory Cytokines |
| IL | Interleukin |
| nsP | Nanosecond Pulsed |
| PAS | CAP-Activated Solutions |
| PAPB | PLEL Biogel |
| PBS | Phosphate-Buffered Saline |
| PDT | Cold Plasma Discharge Tube |
| PLEL | (Poly-DL-Lactide)-(Poly-Ethylene-Glycol)-(Poly-DL-Lactide) |
| RF | Radiofrequency |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| TMZ | Temozolomide |
| USMI | US Medical Innovation |
Author Contributions
Conceptualization, R.L., D.Y. and M.K.; writing, R.L., D.Y., L.L. and M.K.; supervision, D.Y. and M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Science Foundation grant, grant number 1747760.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Von Woedtke T., Schmidt A., Bekeschus S., Wende K., Weltmann K.D. Plasma medicine: A field of applied redox biology. In Vivo. 2019;33:1011–1026. doi: 10.21873/invivo.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fridman G., Friedman G., Gutsol A., Shekhter A.B., Vasilets V.N., Fridman A. Applied plasma medicine. Plasma Processes Polym. 2008;5:503–533. doi: 10.1002/ppap.200700154. [DOI] [Google Scholar]
- 3.Keidar M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015;24:033001. doi: 10.1088/0963-0252/24/3/033001. [DOI] [Google Scholar]
- 4.Keidar M. A prospectus on innovations in the plasma treatment of cancer. Phys. Plasmas. 2018;25:083504. doi: 10.1063/1.5034355. [DOI] [Google Scholar]
- 5.Graves D.B. Reactive species from cold atmospheric plasma: Implications for cancer therapy. Plasma Processes Polym. 2014;11:1120–1127. doi: 10.1002/ppap.201400068. [DOI] [Google Scholar]
- 6.Tendero C., Tixier C., Tristant P., Desmaison J., Leprince P. Atmospheric pressure plasmas: A review. Spectrochim. Acta B At. Spectrosc. 2006;61:2–30. doi: 10.1016/j.sab.2005.10.003. [DOI] [Google Scholar]
- 7.Yan D., Sherman J.H., Keidar M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget. 2017;8:15977. doi: 10.18632/oncotarget.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X., Naidis G.V., Laroussi M., Ostrikov K. Guided ionization waves: Theory and experiments. Phys. Rep. 2014;540:123–166. doi: 10.1016/j.physrep.2014.02.006. [DOI] [Google Scholar]
- 9.Lu X.P., Ostrikov K.K. Guided ionization waves: The physics of repeatability. Appl. Phys. Rev. 2018;5:031102. doi: 10.1063/1.5031445. [DOI] [Google Scholar]
- 10.Lin L., Yan D., Lee T., Keidar M. Self-adaptive plasma chemistry and intelligent plasma medicine. Adv. Intell. Syst. 2022;4:2100112. doi: 10.1002/aisy.202100112. [DOI] [Google Scholar]
- 11.Keidar M. A map of control for cold atmospheric plasma jets: From physical mechanisms to optimizations. Appl. Phys. Rev. 2021;8.1:011306. [Google Scholar]
- 12.Kogelschatz U. Atmospheric-pressure plasma technology. Plasma Phys. Control. Fusion. 2004;46:B63–B75. doi: 10.1088/0741-3335/46/12B/006. [DOI] [Google Scholar]
- 13.Park G.Y., Park S.J., Choi M.Y., Koo I.G., Byun J.H., Hong J.W., Sim J.Y., Collins G.J., Lee J.K. Atmospheric-pressure plasma sources for biomedical applications. Plasma Sources Sci. Technol. 2012;21:043001. doi: 10.1088/0963-0252/21/4/043001. [DOI] [Google Scholar]
- 14.Yan D., Lin L., Xu W., Nourmohammadi N. Cold plasma-based control of the activation of pancreatic adenocarcinoma cells. J. Phys. D Appl. Phys. 2019;52.44:445202. doi: 10.1088/1361-6463/ab36d4. [DOI] [Google Scholar]
- 15.Laroussi M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Processes Polym. 2005;2:391–400. doi: 10.1002/ppap.200400078. [DOI] [Google Scholar]
- 16.Feil L., Koch A., Utz R., Ackermann M., Barz J., Stope M., Krämer B., Wallwiener D., Brucker S.Y., Weiss M. Cancer-selective treatment of cancerous and non-cancerous human cervical cell models by a non-thermally operated electrosurgical argon plasma device. Cancers. 2020;12:1037. doi: 10.3390/cancers12041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenzel T., Berrio D.A.C., Reisenauer C., Layland S., Koch A., Wallwiener D., Brucker S.Y., Schenke-Layland K., Brauchle E.M., Weiss M. Trans-mucosal efficacy of non-thermal plasma treatment on cervical cancer tissue and human cervix uteri by a next generation electrosurgical argon plasma device. Cancers. 2020;12:267. doi: 10.3390/cancers12020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzi J., Stope M.B., Henes M., Koch A., Wenzel T., Holl M., Layland S.L., Neis F., Bösmüller H., Ruoff F. Noninvasive physical plasma as innovative and tissue-preserving therapy for women positive for cervical intraepithelial neoplasia. Cancers. 2022;14:1933. doi: 10.3390/cancers14081933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan D., Talbot A., Nourmohammadi N., Sherman J.H., Cheng X., Keidar M. Toward understanding the selective anticancer capacity of cold atmospheric plasma—A model based on aquaporins. Biointerphases. 2015;10:040801. doi: 10.1116/1.4938020. [DOI] [PubMed] [Google Scholar]
- 20.Hirst A.M., Frame F.M., Arya M., Maitland N.J., O’Connell D. Low temperature plasmas as emerging cancer therapeutics: The state of play and thoughts for the future. Tumor Biol. 2016;37:7021–7031. doi: 10.1007/s13277-016-4911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laroussi M., Lu X., Keidar M. Perspective: The Physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. J. Appl. Phys. 2017;122:020901. doi: 10.1063/1.4993710. [DOI] [Google Scholar]
- 22.Kieft I.E., Dvinskikh N.A., Broers J.L.v., Slaaf D.W., Stoffels E. Effect of plasma needle on cultured cells. Proc. SPIE. 2004;5483:247–251. [Google Scholar]
- 23.Fridman G., Shereshevsky A., Jost M.M., Brooks A.D., Fridman A., Gutsol A., Vasilets V., Friedman G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem. Plasma Process. 2007;27:163–176. doi: 10.1007/s11090-007-9048-4. [DOI] [Google Scholar]
- 24.Lee H.J., Shon C.H., Kim Y.S., Kim S., Kim G.C., Kong M.G. Degradation of adhesion molecules of g361 melanoma cells by a non-thermal atmospheric pressure microplasma. New J. Phys. 2009;11:115026. doi: 10.1088/1367-2630/11/11/115026. [DOI] [Google Scholar]
- 25.Georgescu N., Lupu A.R. Tumoral and normal cells treatment with high-voltage pulsed cold atmospheric plasma jets. IEEE Trans. Plasma Sci. 2010;38.8:1949–1955. doi: 10.1109/TPS.2010.2041075. [DOI] [Google Scholar]
- 26.Kim C.-H., Bahn J.H., Lee S.-H., Kim G.-Y., Jun S.-I., Lee K., Baek S.J. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J. Biotechnol. 2010;150:530–538. doi: 10.1016/j.jbiotec.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Kim G.J., Kim W., Kim K.T., Lee J.K. DNA damage and mitochondria dysfunction in cell apoptosis induced by nonthermal air plasma. Appl. Phys. Lett. 2010;96:021502. doi: 10.1063/1.3292206. [DOI] [Google Scholar]
- 28.Ahn H.J., Kim K.I., Kim G., Moon E., Yang S.S., Lee J.S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE. 2011;6:e28154. doi: 10.1371/journal.pone.0028154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köritzer J., Boxhammer V., Schäfer A., Shimizu T., Klämpfl T.G., Li Y.F., Welz C., Schwenk-Zieger S., Morfill G.E., Zimmermann J.L., et al. Restoration of sensitivity in chemo-resistant glioma cells by cold atmospheric plasma. PLoS ONE. 2013;8:e64498. doi: 10.1371/journal.pone.0064498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ja Kim S., Min Joh H., Chung T.H. Production of intracellular reactive oxygen species and change of cell viability induced by atmospheric pressure plasma in normal and cancer cells. Appl. Phys. Lett. 2013;103:153705. doi: 10.1063/1.4824986. [DOI] [Google Scholar]
- 31.Arndt S., Wacker E., Li Y.F., Shimizu T., Thomas H.M., Morfill G.E., Karrer S., Zimmermann J.L., Bosserhoff A.K. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp. Dermatol. 2013;22:284–289. doi: 10.1111/exd.12127. [DOI] [PubMed] [Google Scholar]
- 32.Lin A., Truong B., Pappas A., Kirifides L., Oubarri A., Chen S., Lin S., Dobrynin D., Fridman G., Fridman A., et al. Uniform nanosecond pulsed dielectric barrier discharge plasma enhances anti-tumor effects by induction of immunogenic cell death in tumors and stimulation of macrophages. Plasma Processes Polym. 2015;12:1392–1399. doi: 10.1002/ppap.201500139. [DOI] [Google Scholar]
- 33.Yan D., Cui H., Zhu W., Talbot A., Zhang L.G., Sherman J.H., Keidar M. The strong cell-based hydrogen peroxide generation triggered by cold atmospheric plasma. Sci. Rep. 2017;7:10831. doi: 10.1038/s41598-017-11480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi L., Ito F., Wang Y., Okazaki Y., Tanaka H., Mizuno M., Hori M., Hirayama T., Nagasawa H., Richardson D.R., et al. Non-thermal plasma induces a stress response in mesothelioma cells resulting in increased endocytosis, lysosome biogenesis and autophagy. Free Radic. Biol. Med. 2017;108:904–917. doi: 10.1016/j.freeradbiomed.2017.04.368. [DOI] [PubMed] [Google Scholar]
- 35.Yan D., Xu W., Yao X., Lin L., Sherman J.H., Keidar M. The cell activation phenomena in the cold atmospheric plasma cancer treatment. Sci. Rep. 2018;8:15418. doi: 10.1038/s41598-018-33914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan D., Wang Q., Adhikari M., Malyavko A., Lin L., Zolotukhin D.B., Yao X., Kirschner M., Sherman J.H., Keidar M. A physically triggered cell death via transbarrier cold atmospheric plasma cancer treatment. ACS Appl. Mater. Interfaces. 2020;12:34548–34563. doi: 10.1021/acsami.0c06500. [DOI] [PubMed] [Google Scholar]
- 37.Yang X., Chen G., Yu K.N., Yang M., Peng S., Ma J., Qin F., Cao W., Cui S., Nie L., et al. Cold atmospheric plasma induces GSDME-dependent pyroptotic signaling pathway via ROS generation in tumor cells. Cell Death Dis. 2020;11:295. doi: 10.1038/s41419-020-2459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao X., Lin L., Soni V., Gjika E., Sherman J.H., Yan D., Keidar M. Sensitization of glioblastoma cells to temozolomide by a helium gas discharge tube. Phys. Plasmas. 2020;27:114502. doi: 10.1063/5.0017913. [DOI] [Google Scholar]
- 39.Yan D., Talbot A., Nourmohammadi N., Cheng X., Canady J., Sherman J., Keidar M. Principles of using cold atmospheric plasma stimulated media for cancer treatment. Sci. Rep. 2015;5:18339. doi: 10.1038/srep18339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan D., Malyavko A., Wang Q., Ostrikov K.K., Sherman J.H., Keidar M. Multi-modal biological destruction by cold atmospheric plasma: Capability and mechanism. Biomedicines. 2021;9:1259. doi: 10.3390/biomedicines9091259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q., Malyavko A., Yan D., Lamanna O.K., Hsieh M.H., Sherman J., Keidar M. A Comparative study of cold atmospheric plasma treatment, chemical versus physical strategy. J. Phys. D Appl. Phys. 2020;54:095207. doi: 10.1088/1361-6463/abc6d5. [DOI] [Google Scholar]
- 42.Dezest M., Chavatte L., Bourdens M., Quinton D., Camus M., Garrigues L., Descargues P., Arbault S., Burlet-Schiltz O., Casteilla L. Mechanistic insights into the impact of cold atmospheric pressure plasma on human epithelial cell lines. Sci. Rep. 2017;7.1:41163. doi: 10.1038/srep41163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorbanev Y., O’Connell D., Chechik V. Non-thermal plasma in contact with water: The origin of species. J. Chem. 2016;22:3496–3505. doi: 10.1002/chem.201503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou R., Zhou R., Wang P., Xian Y., Mai-prochnow A. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020;53:303001. doi: 10.1088/1361-6463/ab81cf. [DOI] [Google Scholar]
- 45.Brisset J.L., Pawlat J. Chemical effects of air plasma species on aqueous solutes in direct and delayed exposure modes: Discharge, post-discharge and plasma activated water. Plasma Chem. Plasma Process. 2016;36:355–381. doi: 10.1007/s11090-015-9653-6. [DOI] [Google Scholar]
- 46.Tanaka H., Bekeschus S., Yan D., Hori M., Keidar M., Laroussi M. Plasma-Treated Solutions (PTS) in cancer therapy. Cancers. 2021;13:1737. doi: 10.3390/cancers13071737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan D., Horkowitz A., Wang Q., Keidar M. On the selective killing of cold atmospheric plasma cancer treatment: Status and beyond. Plasma Processes Polym. 2021;18:202100020. doi: 10.1002/ppap.202100020. [DOI] [Google Scholar]
- 48.Chernets N., Kurpad D.S., Alexeev V., Rodrigues D.B., Freeman T.A. Reaction chemistry generated by nanosecond pulsed dielectric barrier discharge treatment is responsible for the tumor eradication in the B16 melanoma mouse model. Plasma Processes Polym. 2015;12:1400–1409. doi: 10.1002/ppap.201500140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keidar M., Walk R., Shashurin A., Srinivasan P., Sandler A., Dasgupta S., Ravi R., Guerrero-Preston R., Trink B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer. 2011;105:1295–1301. doi: 10.1038/bjc.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binenbaum Y., Ben-David G., Gil Z., Slutsker Y.Z., Ryzhkov M.A., Felsteiner J., Krasik Y.E., Cohen J.T. Cold atmospheric plasma, created at the tip of an elongated flexible capillary using low electric current, can slow the progression of melanoma. PLoS ONE. 2017;12:e0169457. doi: 10.1371/journal.pone.0169457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boehm D., Heslin C., Cullen P.J., Bourke P. Cytotoxic and mutagenic potential of solutions exposed to cold atmospheric plasma. Sci. Rep. 2016;6:21464. doi: 10.1038/srep21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan D., Malyavko A., Wang Q., Lin L., Sherman J.H. Applied sciences cold atmospheric plasma cancer treatment, a critical review. Appl. Sci. 2021;11:7757. doi: 10.3390/app11167757. [DOI] [Google Scholar]
- 53.Vandamme M., Robert E., Pesnel S., Barbosa E., Dozias S., Sobilo J., Lerondel S., le Pape A., Pouvesle J.M. Antitumor effect of plasma treatment on U87 glioma xenografts: Preliminary results. Plasma Processes Polym. 2010;7:264–273. doi: 10.1002/ppap.200900080. [DOI] [Google Scholar]
- 54.Vandamme M., Robert E., Dozias S., Sobilo J., Lerondel S., le Pape A., Pouvesle J.-M. Response of human glioma U87 xenografted on mice to non thermal plasma treatment. Plasma Med. 2011;1:27–43. doi: 10.1615/PlasmaMed.v1.i1.30. [DOI] [Google Scholar]
- 55.Brullé L., Vandamme M., Riès D., Martel E., Robert E., Lerondel S., Trichet V., Richard S., Pouvesle J.M., le Pape A. Effects of a non thermal plasma treatment alone or in combination with gemcitabine in a MIA PaCa2-luc orthotopic pancreatic carcinoma model. PLoS ONE. 2012;7:e52653. doi: 10.1371/journal.pone.0052653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vandamme M., Robert E., Lerondel S., Sarron V., Ries D., Dozias S., Sobilo J., Gosset D., Kieda C., Legrain B., et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer. 2012;130:2185–2194. doi: 10.1002/ijc.26252. [DOI] [PubMed] [Google Scholar]
- 57.Walk R.M., Snyder J.A., Srinivasan P., Kirsch J., Diaz S.O., Blanco F.C., Shashurin A., Keidar M., Sandler A.D. Cold atmospheric plasma for the ablative treatment of neuroblastoma. J. Pediatr. Surg. 2013;48:67–73. doi: 10.1016/j.jpedsurg.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 58.Yajima I., Iida M., Kumasaka M.Y., Omata Y., Ohgami N., Chang J., Ichihara S., Hori M., Kato M. Non-equilibrium atmospheric pressure plasmas modulate cell cycle-related gene expressions in melanocytic tumors of RET-transgenic mice. Exp. Dermatol. 2014;23:424–425. doi: 10.1111/exd.12415. [DOI] [PubMed] [Google Scholar]
- 59.Kang S.U., Cho J.-H., Chang J.W., Shin Y.S., Kim K.I., Park J.K., Yang S.S., Lee J.-S., Moon E., Lee K., et al. Nonthermal plasma induces head and neck cancer cell death: The potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014;5:e1056. doi: 10.1038/cddis.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ikeda J.I., Tsuruta Y., Nojima S., Sakakita H., Hori M., Ikehara Y. Anti-cancer effects of nonequilibrium atmospheric pressure plasma on cancer-initiating cells in human endometrioid adenocarcinoma cells. Plasma Processes Polym. 2015;12:1370–1376. doi: 10.1002/ppap.201500097. [DOI] [Google Scholar]
- 61.Kaushik N.K., Kaushik N., Yoo K.C., Uddin N., Kim J.S., Lee S.J., Choi E.H. Low doses of PEG-coated gold nanoparticles sensitize solid tumors to cold plasma by blocking the PI3K/AKT-driven signaling axis to suppress cellular transformation by inhibiting growth and EMT. Biomaterials. 2016;87:118–130. doi: 10.1016/j.biomaterials.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 62.Mirpour S., Piroozmand S., Soleimani N., Jalali Faharani N., Ghomi H., Fotovat Eskandari H., Sharifi A.M., Mirpour S., Eftekhari M., Nikkhah M. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci. Rep. 2016;6:29048. doi: 10.1038/srep29048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin A.G., Xiang B., Merlino D.J., Baybutt T.R., Sahu J., Fridman A., Snook A.E., Miller V. Non-thermal plasma induces immunogenic cell death in vivo in murine CT26 colorectal tumors. Oncoimmunology. 2018;7:e1484978. doi: 10.1080/2162402X.2018.1484978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan D., Nourmohammadi N., Bian K., Murad F., Sherman J.H., Keidar M. Stabilizing the cold plasma-stimulated medium by regulating medium’s composition. Sci. Rep. 2016;6:26016. doi: 10.1038/srep26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka H., Mizuno M., Ishikawa K., Nakamura K., Kajiyama H., Kano H., Kikkawa F., Hori M. Plasma-activated medium selectively kills glioblastoma brain tumor cells by down-regulating a survival signaling molecule, AKT kinase. Plasma Med. 2011;1:265–277. doi: 10.1615/PlasmaMed.2012006275. [DOI] [Google Scholar]
- 66.Yan D., Sherman J.H., Keidar M. The application of the cold atmospheric plasma-activated solutions in cancer treatment. Anti-Cancer Agents Med. Chem. 2018;18:769–775. doi: 10.2174/1871520617666170731115233. [DOI] [PubMed] [Google Scholar]
- 67.Takeda S., Yamada S., Hattori N., Nakamura K., Tanaka H., Kajiyama H., Kanda M., Kobayashi D., Tanaka C., Fujii T., et al. Intraperitoneal administration of plasma-activated medium: Proposal of a novel treatment option for peritoneal metastasis from gastric cancer. Ann. Surg. Oncol. 2017;24:1188–1194. doi: 10.1245/s10434-016-5759-1. [DOI] [PubMed] [Google Scholar]
- 68.Harley J.C., Suchowerska N., Mckenzie D.R. Cancer treatment with gas plasma and with gas plasma-activated liquid: Positives, potentials and problems of clinical translation. Biophys. Rev. 2020;12:989–1006. doi: 10.1007/s12551-020-00743-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan D., Sherman J.H., Cheng X., Ratovitski E., Canady J., Keidar M. Controlling plasma stimulated media in cancer treatment application. Appl. Phys. Lett. 2014;105:224101. doi: 10.1063/1.4902875. [DOI] [Google Scholar]
- 70.Girard P.-M., Arbabian A., Fleury M., Bauville G., Puech V., Dutreix M., Sousa J.S. Synergistic effect of H2O2 and NO2 in cell death induced by cold atmospheric He plasma. Sci. Rep. 2016;6:29098. doi: 10.1038/srep29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan D., Cui H., Zhu W., Nourmohammadi N., Milberg J., Zhang L.G., Sherman J.H., Keidar M. The specific vulnerabilities of cancer cells to the cold atmospheric plasma-stimulated solutions. Sci. Rep. 2017;7:4479. doi: 10.1038/s41598-017-04770-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar N., Park J.H., Jeon S.N., Park B.S., Choi E.H., Attri P. The Action of microsecond-pulsed plasma-activated media on the inactivation of human lung cancer cells. J. Phys. D Appl. Phys. 2016;49:115401. doi: 10.1088/0022-3727/49/11/115401. [DOI] [Google Scholar]
- 73.Chen C.Y., Cheng Y.C., Cheng Y.J. Synergistic effects of plasma-activated medium and chemotherapeutic drugs in cancer treatment. J. Phys. D Appl. Phys. 2018;51:13LT01. doi: 10.1088/1361-6463/aaafc4. [DOI] [Google Scholar]
- 74.Yan D., Nourmohammadi N., Talbot A., Sherman J.H., Keidar M. The strong anti-glioblastoma capacity of the plasma-stimulated lysine-rich medium. J. Phys. D Appl. Phys. 2016;49:274001. doi: 10.1088/0022-3727/49/27/274001. [DOI] [Google Scholar]
- 75.Kaushik N., Lee S.J., Choi T.G., Baik K.Y., Uhm H.S., Kim C.H., Kaushik N.K., Choi E.H. Non-thermal plasma with 2-deoxy-D-glucose synergistically induces cell death by targeting glycolysis in blood cancer cells. Sci. Rep. 2015;5:8726. doi: 10.1038/srep08726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adachi T., Nonomura S., Horiba M., Hirayama T., Kamiya T., Nagasawa H., Hara H. Iron stimulates plasma-activated medium-induced A549 cell injury. Sci. Rep. 2016;6:20928. doi: 10.1038/srep20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen N.H., Park H.J., Yang S.S., Choi K.S., Lee J.S. Anti-cancer efficacy of nonthermal plasma dissolved in a liquid, liquid plasma in heterogeneous cancer cells. Sci. Rep. 2016;6:29020. doi: 10.1038/srep29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saito K., Asai T., Fujiwara K., Sahara J., Koguchi H., Fukuda N., Suzuki-Karasaki M., Soma M., Suzuki-Karasaki Y. Tumor-selective mitochondrial network collapse induced by atmospheric gas plasma-activated medium. Oncotarget. 2016;7:19910. doi: 10.18632/oncotarget.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang P., Zhou R., Thomas P., Zhao L., Zhou R., Mandal S., Jolly M.K., Richard D.J., Rehm B.H.A., Ostrikov K., et al. Epithelial-to-Mesenchymal transition enhances cancer cell sensitivity to cytotoxic effects of zcold atmospheric plasmas in breast and bladder cancer systems. Cancers. 2021;13:2889. doi: 10.3390/cancers13122889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liedtke K.R., Bekeschus S., Kaeding A., Hackbarth C., Kuehn J.-P., Heidecke C.-D., von Bernstorff W., von Woedtke T., Partecke L.I. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Sci. Rep. 2017;7:8319. doi: 10.1038/s41598-017-08560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu D., Cui Q., Xu Y., Wang B., Tian M., Li Q., Liu Z., Liu D., Chen H., Kong M.G. Systemic study on the safety of immuno- deficient nude mice treated by atmospheric plasma-activated water. Plasma Sci. Technol. 2018;20:44003. doi: 10.1088/2058-6272/aa9842. [DOI] [Google Scholar]
- 82.Nastasa V., Pasca A.S., Malancus R.N., Bostanaru A.C., Ailincai L.I., Ursu E.L., Vasiliu A.L., Minea B., Hnatiuc E., Mares M. Toxicity assessment of long-term exposure to non-thermal plasma activated water in mice. Int. J. Mol. Sci. 2021;22:11534. doi: 10.3390/ijms222111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Utsumi F., Kajiyama H., Nakamura K., Tanaka H., Mizuno M., Ishikawa K., Kondo H., Kano H., Hori M., Kikkawa F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE. 2013;8:e81576. doi: 10.1371/journal.pone.0081576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liedtke K.R., Freund E., Hermes M., Oswald S., Heidecke C.D., Partecke L.I., Bekeschus S. Gas plasma-conditioned ringer’s lactate enhances the cytotoxic activity of cisplatin and gemcitabine in pancreatic cancer in vitro and in ovo. Cancers. 2020;12:123. doi: 10.3390/cancers12010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang L., Zheng H., Lyu Q., Hayashi S., Sato K., Sekido Y. Redox biology lysosomal nitric oxide determines transition from autophagy to ferroptosis after exposure to plasma-activated ringer’s lactate. Redox Biol. 2021;43:101989. doi: 10.1016/j.redox.2021.101989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishikawa K., Hosoi Y., Tanaka H., Jiang L., Toyokuni S., Nakamura K., Kajiyama H., Kikkawa F., Mizuno M., Hori M. Non-thermal plasma–activated lactate solution kills U251SP glioblastoma cells in an innate reductive manner with altered metabolism. Arch. Biochem. Biophys. 2020;688:108414. doi: 10.1016/j.abb.2020.108414. [DOI] [PubMed] [Google Scholar]
- 87.Rouven K., Freund E., Hackbarth C., Heidecke C. A myeloid and lymphoid infiltrate in murine pancreatic tumors exposed to plasma-treated medium. Clin. Plasma Med. 2020;11:10–17. [Google Scholar]
- 88.Zhang H., Xu S., Zhang J., Wang Z., Liu D., Guo L., Cheng C., Cheng Y., Xu D., Kong M.G., et al. Plasma-activated thermosensitive biogel as an exogenous ROS carrier for post-surgical treatment of cancer. Biomaterials. 2021;276:121057. doi: 10.1016/j.biomaterials.2021.121057. [DOI] [PubMed] [Google Scholar]
- 89.Mizuno K., Yonetamari K., Shirakawa Y., Akiyama T., Ono R. Anti-tumor immune response induced by nanosecond pulsed streamer discharge in mice. J. Phys. D: Appl. Phys. 2017;50:12LT01. doi: 10.1088/1361-6463/aa5dbb. [DOI] [Google Scholar]
- 90.Jinno R., Komuro A., Yanai H., Ono R. Antitumor abscopal effects in mice induced by normal tissue irradiation using pulsed streamer discharge plasma. J. Phys. D Appl. Phys. 2022;55:17LT01. doi: 10.1088/1361-6463/ac4c23. [DOI] [Google Scholar]
- 91.Yan D., Wang Q., Yao X., Malyavko A. Anti-melanoma capability of contactless cold atmospheric plasma treatment. Int. J. Mol. Sci. 2021;22:11728. doi: 10.3390/ijms222111728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mitra S., Nguyen L.N., Akter M., Park G., Choi E.H., Kaushik N.K. Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation. Cancers. 2019;11:1030. doi: 10.3390/cancers11071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eramo A., Ricci-Vitiani L., Zeuner A., Pallini R., Lotti F., Sette G., Pilozzi E., Larocca L.M., Peschle C., de Maria R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 94.Lee S.Y. Temozolomide Resistance in Glioblastoma Multiforme. Genes Dis. 2016;3:198–210. doi: 10.1016/j.gendis.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soni V., Adhikari M., Simonyan H., Lin L., Sherman J.H., Young C.N., Keidar M. In vitro and in vivo enhancement of temozolomide effect in human glioblastoma by non-invasive application of cold atmospheric plasma. Cancers. 2021;13:4485. doi: 10.3390/cancers13174485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao X., Yan D., Lin L., Sherman J.H., Peters K.B., Keir S.T., Keidar M. Cold plasma discharge tube enhances antitumoral efficacy of temozolomide. ACS Appl. Bio Mater. 2022;5.4:1610–1623. doi: 10.1021/acsabm.2c00018. [DOI] [PubMed] [Google Scholar]
- 97.Canady J., Gordon S., Zhuang T., Wigh S., Rowe W., Shashurin A., Chiu D., Jones S., Wiley K., Cohen E., et al. Cold atmospheric plasma (cap) combined with chemo-radiation and cytoreductive surgery: The first clinical experience for stage iv metastatic colon cancer. In: Metelmann H.R., Von Woedtke T., Weltmann K.D., editors. Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application. Springer-Nature; Cham, Switzerland: 2018. pp. 163–183. [Google Scholar]
- 98.Metelmann H.R., Nedrelow D.S., Seebauer C., Schuster M., von Woedtke T., Weltmann K.-D., Kindler S., Metelmann P.H., Finkelstein S.E., von Hoff D.D., et al. Head and neck cancer treatment and physical plasma. Clin. Plasma Med. 2015;3:17–23. doi: 10.1016/j.cpme.2015.02.001. [DOI] [Google Scholar]
- 99.Metelmann H.R., Seebauer C., Miller V., Fridman A., Bauer G., Graves D.B., Pouvesle J.M., Rutkowski R., Schuster M., Bekeschus S., et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018;9:6–13. doi: 10.1016/j.cpme.2017.09.001. [DOI] [Google Scholar]
- 100.Bekeschus S., von Woedtke T., Emmert S., Schmidt A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms: Mechanisms of gas plasma-assisted wound healing. Redox Biol. 2021;46:102116. doi: 10.1016/j.redox.2021.102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Canady J. Phase I Clinical Trial of Canady Helios Cold Atmospheric Plasma (CHCP) Treatment for Patients with Advanced Stage IV Metastatic and Recurrent Solid Tumors: A Novel Potential 4th Treatment Arm for Cancer; Proceedings of the Bi-annual Conference of the Israeli Society of Surgical Oncology (ISSO); Haifa, Israel. 13 May 2022. [Google Scholar]
- 102.Graves D.B. Oxy-nitroso shielding burst model of cold atmospheric plasma therapeutics. Clin. Plasma Med. 2014;2:38–49. doi: 10.1016/j.cpme.2014.11.001. [DOI] [Google Scholar]
- 103.Szili E.J., Oh J.S., Fukuhara H., Bhatia R., Gaur N., Nguyen C.K., Hong S.H., Ito S., Ogawa K., Kawada C., et al. Modelling the helium plasma jet delivery of reactive species into a 3D cancer tumour. Plasma Sources Sci. Technol. 2017;27:014001. doi: 10.1088/1361-6595/aa9b3b. [DOI] [Google Scholar]
- 104.Szili E.J., Hong S., Oh J., Gaur N., Short R.D. Tracking the penetration of plasma reactive species in tissue models. Trends Biotechnol. 2018;36:594–602. doi: 10.1016/j.tibtech.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 105.Oh J.S., Szili E.J., Gaur N., Hong S.H., Furuta H., Kurita H., Mizuno A., Hatta A., Short R.D. How to assess the plasma delivery of RONS into tissue fluid and tissue. J. Phys. D Appl. Phys. 2016;49:304005. doi: 10.1088/0022-3727/49/30/304005. [DOI] [Google Scholar]
- 106.Oh J.S., Szili E.J., Ito S., Hong S.H., Gaur N., Furuta H., Short R.D., Hatta A. Slow molecular transport of plasma generated reactive oxygen and nitrogen species. Plasma Med. 2015;5:2–4. doi: 10.1615/PlasmaMed.2016015740. [DOI] [Google Scholar]
- 107.Szili E.J., Oh J.S., Hong S.H., Hatta A., Short R.D. Probing the transport of plasma-generated RONS in an agarose target as surrogate for real tissue: Dependency on time, distance and material composition. J. Phys. D Appl. Phys. 2015;48:202001. doi: 10.1088/0022-3727/48/20/202001. [DOI] [Google Scholar]
- 108.Wenzel T., Carvajal Berrio D.A., Daum R., Reisenauer C., Weltmann K.D., Wallwiener D., Brucker S.Y., Schenke-Layland K., Brauchle E.M., Weiss M. Molecular Effects and tissue penetration depth of physical plasma in human mucosa analyzed by contact- and marker-independent raman microspectroscopy. ACS Appl. Mater. Interfaces. 2019;11:42885. doi: 10.1021/acsami.9b13221. [DOI] [PubMed] [Google Scholar]
- 109.Lu X., Keidar M., Laroussi M., Choi E., Szili E.J., Ostrikov K. Transcutaneous plasma stress: From soft-matter models to living tissues. Mater. Sci. Eng. R Rep. 2019;138:36–59. doi: 10.1016/j.mser.2019.04.002. [DOI] [Google Scholar]
- 110.Kimura K., Tsuchida A., Kimura K., Tsuchida A. Drying dissipative patterns of the colloidal crystals of silica spheres in an DC-electric field. Colloids Surf. B Biointerfaces. 2007;56:201–209. doi: 10.1016/j.colsurfb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 111.Omran A.V., Busco G., Ridou L., Dozias S., Grillon C., Pouvesle J.-M., Robert E. Cold atmospheric single plasma jet for RONS delivery on large biological surfaces-IOPscience cold atmospheric single plasma jet for RONS delivery on large biological surfaces. Plasma Sources Sci. Technol. 2020;29:105002. doi: 10.1088/1361-6595/abaffd. [DOI] [Google Scholar]
- 112.Ki S.H., Masur K., Baik K.Y., Choi E.H. UV absorption spectroscopy for the diffusion of plasma-generated reactive species through a skin model. Appl. Sci. 2021;11:7958. doi: 10.3390/app11177958. [DOI] [Google Scholar]
- 113.Jiang C., Oshin E.A., Guo S., Scott M., Li X., Mangiamele C., Heller R. Synergistic effects of an atmospheric-pressure plasma jet and pulsed electric field on cells and skin. IEEE Trans. Plasma Sci. 2021;49:3317–3324. doi: 10.1109/TPS.2021.3113260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller V., Lin A., Fridman A. Why target immune cells for plasma treatment of cancer. Plasma Chem. Plasma Process. 2016;36:259–268. doi: 10.1007/s11090-015-9676-z. [DOI] [Google Scholar]
- 115.Cheng F., Yan D., Chen J., Wang Z., Horkowitz A., Keidar M., Sotomayor E.M. Enhancing innate and adaptive immune systems by cold atmospheric plasma (CAP) and its antitumor immunity. arXiv. 2022;2201:12737. [Google Scholar]
- 116.Miller V., Lin A., Fridman G., Dobrynin D., Fridman A. Plasma stimulation of migration of macrophages. Plasma Processes Polym. 2014;11:1193–1197. doi: 10.1002/ppap.201400168. [DOI] [Google Scholar]
- 117.Lin A., Truong B., Patel S., Kaushik N., Choi E.H., Fridman G., Fridman A., Miller V. Nanosecond-pulsed Dbd plasma-generated reactive oxygen species trigger immunogenic cell death in A549 lung carcinoma cells through intracellular oxidative stress. Int. J. Mol. Sci. 2017;18:966. doi: 10.3390/ijms18050966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin A., Gorbanev Y., Cos P., Smits E., Bogaerts A. Plasma elicits immunogenic death in melanoma cells regulation of antigen-presenting machinery in melanoma after plasma treatment. Clin. Plasma Med. 2018;9:9. doi: 10.1016/j.cpme.2017.12.013. [DOI] [Google Scholar]
- 119.Khalili M., Daniels L., Lin A., Krebs F.C., Snook A.E., Bekeschus S., Bowne W.B., Miller V. Non-thermal plasma-induced immunogenic cell death in cancer. J. Phys. D Appl. Phys. 2019;52:423001. doi: 10.1088/1361-6463/ab31c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bekeschus S., Mueller A., Miller V., Gaipl U., Weltmann K. Physical plasma elicits immunogenic cancer cell death and mitochondrial singlet oxygen. IEEE Trans. Radiat. Plasma Med. Sci. 2018;2:138–146. doi: 10.1109/TRPMS.2017.2766027. [DOI] [Google Scholar]
- 121.Mohamed H., Esposito R.A., Kutzler M.A., Wigdahl B., Krebs F.C., Miller V. Nonthermal plasma as part of a novel strategy for vaccination. Plasma Processes Polym. 2020;17:2000051. doi: 10.1002/ppap.202000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nasri Z., Bruno G., Bekeschus S., Weltmann K.D., von Woedtke T., Wende K. Development of an electrochemical sensor for in-situ monitoring of reactive species produced by cold physical plasma. Sens. Actuators B Chem. 2021;326:129007. doi: 10.1016/j.snb.2020.129007. [DOI] [Google Scholar]
- 123.Yan D., Lin L., Xu W., Nourmohammadi N., Jonathan H., Keidar M. Universality of micromolar-level cell-based hydrogen peroxide generation during direct cold atmospheric plasma treatment. Plasma Med. 2018;8:335–343. doi: 10.1615/PlasmaMed.2018028781. [DOI] [Google Scholar]
- 124.Los M., Dröge W., Stricker K., Baeuerle P.A., Schulze-Osthoff K. Hydrogen peroxide as a potent activator of t-lymphocyte functions. Eur. J. Immunol. 1995;25:159–165. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- 125.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 126.Graves D.B. Lessons from tesla for plasma medicine. IEEE Trans. Radiat. Plasma Med. Sci. 2018;2:594–607. doi: 10.1109/TRPMS.2018.2866373. [DOI] [Google Scholar]
- 127.Yan D., Wang Q., Malyavko A., Zolotukhin D.B., Adhikari M., Sherman J.H., Keidar M. The anti-glioblastoma effect of cold atmospheric plasma treatment: Physical pathway v.s. chemical pathway. Sci. Rep. 2020;10:11788. doi: 10.1038/s41598-020-68585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Z., Xu R.G., Chen P., Wang Q. Potential agricultural and biomedical applications of cold atmospheric plasma-activated liquids with self-organized patterns formed at the interface. IEEE Trans. Plasma Sci. 2020;48:3455–3471. doi: 10.1109/TPS.2020.3019995. [DOI] [Google Scholar]