Abstract
The release of persistent per- and polyfluoroalkyl substances (PFAS) into the environment is a major concern for the United States Environmental Protection Agency (U.S. EPA). To complement its ongoing research efforts addressing PFAS contamination, the U.S. EPA’s Office of Research and Development (ORD) commissioned the PFAS Innovative Treatment Team (PITT) to provide new perspectives on treatment and disposal of high priority PFAS-containing wastes. During its six-month tenure, the team was charged with identifying and developing promising solutions to destroy PFAS. The PITT examined emerging technologies for PFAS waste treatment and selected four technologies for further investigation. These technologies included mechanochemical treatment, electrochemical oxidation, gasification and pyrolysis, and supercritical water oxidation. This paper highlights these four technologies and discusses their prospects and the development needed before potentially becoming available solutions to address PFAS-contaminated waste.
Innovative team approach
The presence of persistent per- and polyfluoroalkyl substances (PFAS) in the environment is one of the most pressing environmental issues of the 21st century (Lim 2019; Lindstrom, Strynar, and Libelo 2011; Pan et al. 2017; Sunderland et al. 2019). There is high interest in understanding and addressing PFAS contamination among industry, state and federal government, and internationally. In April of 2020, the United States Environmental Protection Agency (U.S. EPA) formed the PFAS Innovative Treatment Team (PITT) to explore innovative tools and methods for destroying all the carbon fluorine (C-F) bonds in PFAS-containing waste. This interdisciplinary team consisted of eleven scientists and engineers from the Office of Research and Development (ORD) and operated under the framework of a “Tiger Team” as used by The National Aeronautics and Space Administration (NASA) Dempsey et al. (1964) or “Skunk Works” teams as used by corporations Lockheed (2021). During the team’s six-month lifetime, it investigated numerous technologies to address PFAS contamination in various waste streams, with a goal of exploring several promising and understudied technologies. The team was provided with financial resources to initiate the study of different treatment technologies at the laboratory, pilot, or field scales. The work resulted in improved understanding and advancement of four innovative technologies to supplement ongoing EPA research into PFAS treatment technologies. These technologies are electrochemical oxidation (EO), supercritical water oxidation (SCWO), mechanochemical degradation (MCD), and gasification and pyrolysis. Efforts were also initiated to quantify PFAS destruction in traditional waste treatment processes such as conventional thermal incineration. Results from laboratory and field work initiated by or supported by the PITT are ongoing and will be presented in future publications.
In this paper, we provide an overview of PFAS waste disposal and destruction issues, a summary of the five PFAS-containing waste streams that were considered, a discussion of the four innovative technologies studied, and preliminary thoughts about the applicability of technologies to different waste streams. Technical results from laboratory and field tests of PFAS treatment by individual technologies will follow in future publications.
The PFAS challenge
The nature of the C-F bond makes PFAS difficult to destroy (Lemal 2004; O’Hagan 2008; Tsang, Burgess, and Babushok 1998). The small size and high electronegativity of fluorine creates the strongest bond in organic chemistry when bonded to carbon, which results in fairly inert molecules with very low surface energies (Lemal 2004; O’Hagan 2008). Molecules and polymers containing many C-F bonds are generally highly stable and unreactive. C-F bonds are very rare in nature Goldman (1969), but are abundant in several classes of industrially-produced chemicals, including PFAS. The PFAS class of compounds includes thousands of different substances (Lindstrom, Strynar, and Libelo 2011) which differ primarily by the number of C atoms in the alkyl chain and the functional groups typically attached to the end of the chain.
The first reported synthesis of a C-F bond was in 1862 Borodin, (1862). In 1890, Moissan described perfluorinated alkanes (Moissan 1890a, 1890b), a new class of fluorinated molecules that had been synthesized and isolated. He noted that the fluorinated compounds were particularly inert and stable; tetrafluoromethane could only be destroyed completely by heating it over sodium (Moissan 1890a, 1890b). Tetrafluoromethane and fluorinated alkyl molecules were first isolated in 192614, mass produced as precursors and refrigerants by the mid-1930’s (Daudt 1935; Okazoe 2009), and incorporated in greases by 1940 Gaylor (1940).
Research, development, and application of PFAS accelerated at the start of World War II, with strong links to the Manhattan Project. Liquid perfluorinated molecules and inert and formable fluoropolymers played a vital role in uranium enrichment, protective coatings, and other military applications Okazoe (2009). Many of these compounds were first developed in the late 1930s (IG Farbenindustrie, 1937, Plunkett 1941). Following World War II, companies involved in the war effort patented and developed commercial uses for the growing number of PFAS. Over the last 70 years, PFAS have been used in a variety of products. Products making extensive use of PFAS molecules include the following: protective non-stick and stain-resistant coatings Dinglasan-Panlilio and Mabury (2006), surfactants, fire-fighting agents (DeYoung 1994; Roth et al. 2020), temperature resistant products, food-contact materials (Schaider et al. 2017; Trier, Granby, and Christensen 2011), cosmetics Schultes et al. (2018), and more (Lemal 2004; Prevedouros et al. 2006). These synthetic compounds are used by industry, consumers, governments, and militaries around the world.
The characteristics that make PFAS useful in a wide variety of products also make them extremely difficult to destroy. The C-F bond is resistant to breakdown from ultraviolet light, biological processes, and heat. As a result, PFAS have become widespread persistent pollutants found around the world (Cicerone 1979; Cordner et al. 2019; Giesy and Kannan 2001; Lindstrom, Strynar, and Libelo 2011; Martin et al. 2002; McCord and Strynar 2019; Ravishankara et al. 1993; Stock et al. 2004). Some PFAS are detrimental to the environment (Ghisi, Vamerali, and Manzetti 2019; Ivy et al. 2012; Pan et al. 2017) and human health (Sunderland et al. 2019; Szilagyi, Avula, and Fry 2020). People can be exposed to PFAS from contaminated drinking water, fish, and food packaging (Sunderland et al. 2019; Trier, Granby, and Christensen 2011). Dermal, organ, and inhalation exposure to PFAS are all possible, especially for workers involved in making or processing PFAS and PFAS-containing materials where inhalation is the most likely route (Heydebreck et al. 2016). The best studied perfluoroalkyl acids are perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA). These long-chain PFAS can bioaccumulate over time to reach harmful levels and are linked to liver, immunological, developmental, reproductive, endocrine, cardiovascular, and cancer effects (Council 2020). With PFAS being in the blood of 98% of Americans, (Calafat et al. 2007) and PFAS’ associated adverse health effects (Eriksen et al. 2009; Grandjean et al. 2012; Lewis, Johns, and Meeker 2015; Lindstrom, Strynar, and Libelo 2011; Sunderland et al. 2019), it is important to find effective methods to reduce the amount of PFAS in the sources of our exposure.
PFAS are present in several high-volume waste streams. These include municipal solid waste, landfill leachate, wastewaters treated at wastewater treatment plants, sewage sludge and biosolids from sewage treatment, and both concentrated and diluted Aqueous Film Forming Foam (AFFF). Treating the PFAS in these wastes requires methods that ensure PFAS and other harmful chemicals do not continue to accumulate in people or the environment. It is relatively easy to remove the functional group from a PFAS molecule, and thus destroy the initial compound; however, this often results in the formation of a different PFAS or organofluorine compound (Krusic, Marchione, and Roe 2005; Krusic and Roe 2004). This can create misleading information about the ultimate destruction of PFAS. The destruction and removal efficiency (DRE) for a treatment method can be very high, but the C-F bonds may all still be intact Watanabe et al. (2018). C-F bonds can require temperatures over 1400°C to be destroyed thermally (Tsang, Burgess, and Babushok 1998) or highly reducing or oxidizing conditions for destruction at lower temperatures (Costello and McCarthy 1984; Lebeau and Damiens 1926; Moissan 1890a), so PFAS and organofluorine byproducts can be released into the environment from most treatment methods. Many of these partially destroyed PFAS molecules can react and be oxidized in the atmosphere or groundwater to form more harmful species, such as carboxylic acid functionalized PFAS (Ellis et al. 2004; Martin et al. 2006; Prevedouros et al. 2006; Stock et al. 2004). Ideal destruction methods would mineralize all the fluorine in the PFAS, breaking all the C-F bonds, to ensure that no organofluorine byproducts are produced.
The disposal and destruction of PFAS in contaminated sources is a complex area of research. PFAS are found throughout the world in most every matrix, making one universal destruction technique nearly impossible. Each of the main sources of PFAS needs to be carefully examined to determine the best approach for remediation or destruction.
PFAS waste streams
To properly address the PFAS waste management issues, it was first necessary to identify significant PFAS-containing waste streams and to understand both the nature of those wastes and the magnitude of the PFAS issue regarding those wastes. The five waste streams the PITT focused on were (1) landfill leachate, (2) biosolids, (3) contaminated soils, (4) granular activated carbon (GAC) or ion exchange resins used to remove PFAS from other media, and (5) AFFF, both concentrated and diluted. Each of these waste streams will be summarized in this section to provide a context for the PFAS destruction techniques explored in the following section.
Landfill leachate
In 2018 the U.S. disposed over 310 million metric tons of waste in nonhazardous waste landfills (U.S. EPA 2020), of which at least half was municipal solid waste (MSW). MSW contains many consumer products known to contain PFAS, including food contact paper (lined to-go containers), textiles (carpets and clothing), cleaning agents, and non-stick cookware, among others. Since PFAS are not considered hazardous wastes and are not accounted for accordingly, other commercial or industrial wastes deposited in landfills may also contain these compounds.
Modern sanitary landfills are lined with a plastic membrane overlying low-permeability soil, such as clay, which limits groundwater contamination but requires treating the collected leachate. Because of the direct leaching of PFAS-containing wastes, landfill leachate tends to have elevated PFAS levels compared to municipal wastewater alone (Huset et al. 2011; Masoner et al. 2020). Lang et al. (2017) estimated 61.1 million m3 of leachate were generated across the U.S. in 2013, comprising between 563 and 638 kg of PFAS.
Landfill leachate also tends to have higher suspended solids, chemical oxygen demand (COD), ammonia, salinity, and alkalinity than municipal wastewaters (Kjeldsen et al. 2002). The high organic matter loading makes leachate difficult to treat on its own, and any treatment of landfill leachate specifically for PFAS may require pre-treatment for co-contaminants (Wiszniowski et al. 2006). Many wastewater treatment plants do not contain the necessary treatment technologies (specially designed granular activated carbon, ion-exchange resins, reverse osmosis, and nanofiltration systems) to fully remove PFAS from treated water (Aro et al. 2021; Pan, Liu, and Ying 2016). As a result, the PFAS compounds have the potential to remain in the treated effluent.
Biosolids
In addition to the treated wastewater, wastewater treatment plants must also safely manage the residual sludges produced during treatment processes. Influent wastewater streams generally contain PFAS in various concentrations, which are then detected in both the treated effluent water and in the biosolids and sludges (Aro et al. 2021; Chiavola et al. 2020; Dauchy et al. 2017; Gallen et al. 2018a; Loganathan et al. 2007a). In the U.S., solids and sludge are largely either incinerated (~16%), landfilled (~22%), or land applied (~52%) U.S. EPA (2019). For land application, time and temperature treatment of WWTP solids to form Class A or B biosolids is regulated by the EPA to reduce pathogens, vector attraction, and heavy metals before use as nutrients and soil conditioners (U.S. EPA 1994). Since 2001, a growing number of studies (Alder and van der Voet 2015; Chen et al. 2012; Gallen et al. 2018b; Loganathan et al. 2007b; Michigan Department of Environment, 2020; Navarro et al. 2016; Schultz et al. 2006; Sepulvado et al. 2011; Sinclair and Kannan 2006; Venkatesan and Halden 2013; Yoo et al. 2009; Yu et al. 2009) have shown PFAS in biosolids in the U.S. and internationally with typical results showing PFOA levels from <20 μg/kg to as much as 240 μg/kg dry wt. These surveys show a larger range in PFOS levels generally from <30 μg/kg to over 1000 μg/kg. The concentrations of individual PFAS in biosolids show a high spatial and temporal variability. As a result of several rare cases of land-applied biosolids containing high PFAS levels, some states in the U.S. (such as the state of Maine) limit the PFAS concentration of biosolids that are to be land applied. Addressing PFAS in biosolids in a way that allows beneficial reuse or resource recovery is a priority.
Contaminated soil
The heterogenous nature of soils creates complex interactions with PFAS. The soil’s organic content and surface charges are the major contributors to PFAS retention. Soils with high organic content can interact with and retain PFAS through hydrophobic interactions, while ions in the soil can interact with PFAS electrostatically. The length of the PFAS chain is the main factor in the PFAS partitioning into soil. As the tail gets longer, the PFAS is retained more due to the increasing size and greater hydrophobicity. A charged functional group can also increase retention (Mejia-Avendaño et al. 2020; Xiao et al. 2019).
The strong chemical structure of PFAS and their bonding with soil makes them challenging to eliminate from soil environments. Washington et al. calculated global soil loadings for eight PFAS compounds (Washington et al. 2019). The combined estimated load for the eight PFAS compounds ranged from 1500 to 9000 metric tons, with mean estimates of approximately 1000 metric tons for both PFOA and PFOS. These results indicate that soil has the potential to be a reservoir for PFAS. Soil concentrations reported for PFAS-contaminated sites are generally orders-of-magnitude greater than background levels (Brusseau, Anderson, and Guo 2020). Maximum reported PFOS concentrations for primary contaminated sites ranged upwards of several hundred mg/kg. For example, a maximum PFOS concentration of 373 mg/kg was measured at an AFFF source zone on a U.S. military installation (Brusseau, Anderson, and Guo 2020). PFAS soil concentrations for secondary-source contaminated sites (e.g., land application of municipal biosolids and use of contaminated ground water for irrigation) were about two orders of magnitude lower (Brusseau, Anderson, and Guo 2020).
Methods for PFAS removal from contaminated soils have been reviewed by Ross et al. (2018), Mahinroosta and Senevirathna (2020), and Bolan et al. (2021). Mature treatment technologies for soils include incineration, soil stabilization, excavation, and ex-situ thermal treatment Ross et al. (2018). Recent field tests focusing on sampling methods development have examined soil incineration of AFFF-contaminated soil as a secondary goal, cumulating in a proposed test method, OTM-45 (Merrill and Ryan 2021), and the Department of Defense’s (DoD’s) associated Strategic and Environmental Research and Development Program (SERDP) final report for Project ER19-1408 to be released on SERDP’s web site, www.SERDP.org. More experimental methods include soil washing, advanced oxidation or reduction processes, and ball milling (Bolan et al. 2021). Since the goal of the PITT was to identify technologies that would destroy PFAS completely, we focused on destruction methods versus stabilization or removal (e.g., washing or thermal desorption) methods.
Granular activated carbon/anion exchange resins
While ingestion of contaminated food has been identified as the primary PFOA exposure pathway for the general population, drinking water can become the primary exposure pathway in communities with contaminated water (Vestergren and Cousins 2009). To provide protection from long term exposure to PFOS and PFOA in drinking water, the U.S. EPA established drinking water health advisory concentration limits for PFOS and PFOA of 70 ng/L in 2016 (U.S. EPA 2016a, 2016b). To meet these limits, granular activated carbon (GAC) and anion exchange resins (AEX) have been utilized as treatment methods for separating PFAS from liquid streams, often at a fraction of the cost of other separation technologies such as nanofiltration and reverse osmosis (Dickenson and Higgins 2016). GAC and AEX are commonly used to separate PFAS from liquid streams during groundwater site remediation and manufacturing as well as drinking water treatment. GAC removes PFAS compounds from liquid by surface adsorption whereas AEX involves ion exchange onto a positively-charged surface. The surfaces of both materials become saturated over time and no longer able to remove PFAS. GAC can be regenerated but eventually loses its effectiveness and must be disposed. AEX resins used for treatment of PFAS may or may not be amenable to regeneration. Spent GAC and AEX are currently either landfilled or incinerated. The former may lead to PFAS ending up in the landfill leachate.
Aqueous film-forming foams
AFFF are water-based foams that are used to extinguish fires, generally those involving flammable liquids, and are commonly used with aircraft operations. They generally contain a mixture of hydrocarbon and fluorocarbon surfactants, such as fluorotelomers with zwitterionic, cationic, and anionic properties along with PFOA or PFOS (D’Agostino and Mabury 2014; Place and Field 2012). Fluorinated surfactants’ unique characteristics help the foams spread and isolate the fuel from the air, smothering the fire (Moody and Field 2000). The presence of fluorine on the molecules also makes PFAS effective free radical scavengers, which reduce the availability of hydroxyl radicals, the primary chemical component that serves to maintain combustion in conventional fires (Tsang, Burgess, and Babushok 1998).
There is a stockpile of existing AFFF materials, estimated at 9.9 million gallons (United Nations Environment Programme 2004), that now requires disposal to prevent environmental contamination. Most of these older formulations contain C8 PFAS foams and have been phased out in favor of C6 PFAS foams (Lim 2019), but still require disposal of the legacy stockpile. Alternate formulations that do not contain PFAS are being developed (Vergun 2019), but disposal of the legacy AFFF is problematic for both the military and civilian stockpiles.
According to a United Nations Environmental Program (UNEP) report United Nations Environment Programme (2004), quantities of AFFF are generally expressed as volume percentages of concentrate. In the foam industry, concentrates are typically referred to as “3%” or “6%” concentrate, depending on the mixture rate with water. For example, if the container of foam concentrate is labelled as 3%, then 97 volumes of water are mixed with 3 volumes of PFAS to make 100 volumes of foam solution for fire application. The high water concentration of AFFF, whether the concentrate or the diluted form, is problematic for multiple types of disposal methods.
On-going ORD research efforts, including projects initiated by the PITT, are underway to identify the temperature and time requirements for effective combustive destruction of AFFF. In addition to these combustion/incineration technologies, less developed, non-thermal approaches were considered by the PITT.
Potential, non-combustion treatment technologies
The PITT considered an array of different treatment technologies to deal with the five PFAS-containing waste streams discussed previously. The PITT specifically focused on promising technologies that were underfunded or understudied and that showed potential to advance with limited time and resources. The team considered various factors in its decision process, including those listed in Figure 1. The selection or dismissal of technologies was not an indication of the potential of those technologies, as the PITT passed over many promising technologies for a variety of different reasons unrelated to technology potential.
Figure 1.

Decision factors discussed about all technologies. Four technologies were selected based upon these factors.
The PITT elected to focus on four emerging technologies, including:
Mechanicochemical destruction (ball milling)
Electrochemical oxidation
Supercritical water oxidation (SCWO)
Pyrolysis/gasification
Each of these technologies has characteristics that generally align them with specific PFAS-contaminated materials. Research Briefs are available on the PITT’s web site, https://www.epa.gov/chemical-research/pfas-innovative-treatment-team-pitt, to explain each of the technologies, their benefits, and areas where more research is needed.
The factors related to treatment selection and process management are shown in Figure 2 for each of the four focus technologies. Selection of an applicable technology must consider how the waste characteristics and processing requirements match up with the capabilities of the technology. Factors related to engineering design considerations are indicated in Figure 2 for each of the four treatment technologies. The size or volume of the system is a function of the treatability goals and effectiveness, and whether the system is meant to be mobile or stationary. Consideration of energy consumption, capital costs, technology complexity, and lifecycle also are required considerations. In some cases, additional pretreatment unit operations such as de-watering or addition of co-mingling agents will either be required or aid in the effectiveness of the technology. Post-treatment unit operations may also be required such as when a byproduct of the process is expected. For example, PFAS breakdown may result in smaller C-number molecules that are volatile, requiring gas treatment to prevent emissions.
Figure 2.
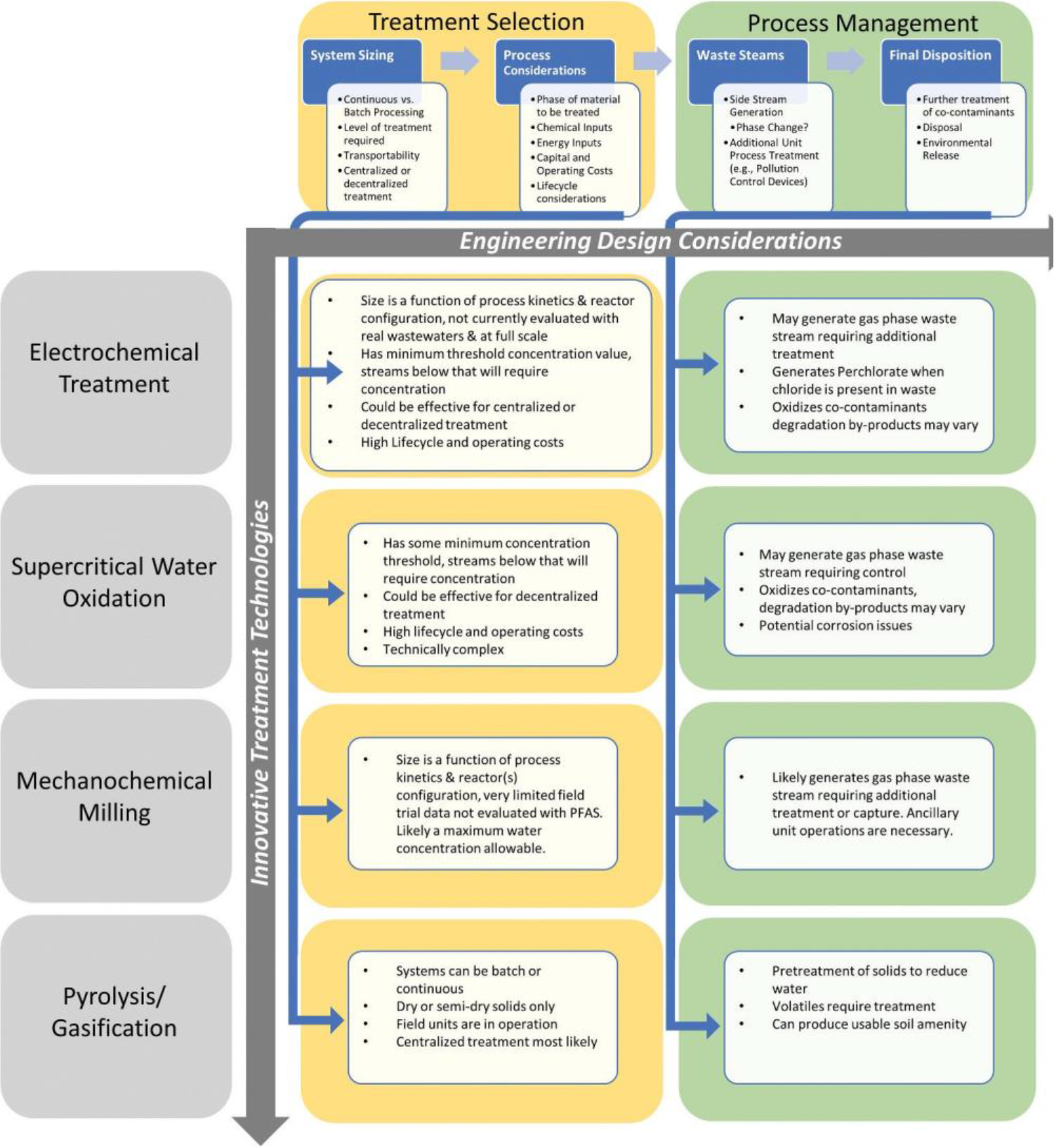
Innovative treatment technology considerations.
Waste-specific characteristics, such as the physical form of the PFAS waste source, are a major determinant of technology suitability. Figure 3 defines the technology selection in terms of the waste factors, handling characteristics, volume, co-contaminants, site locations, and concentrations. The PFAS phase, for example liquid leachate or soil contaminant, is a major factor in technology selection.
Figure 3.
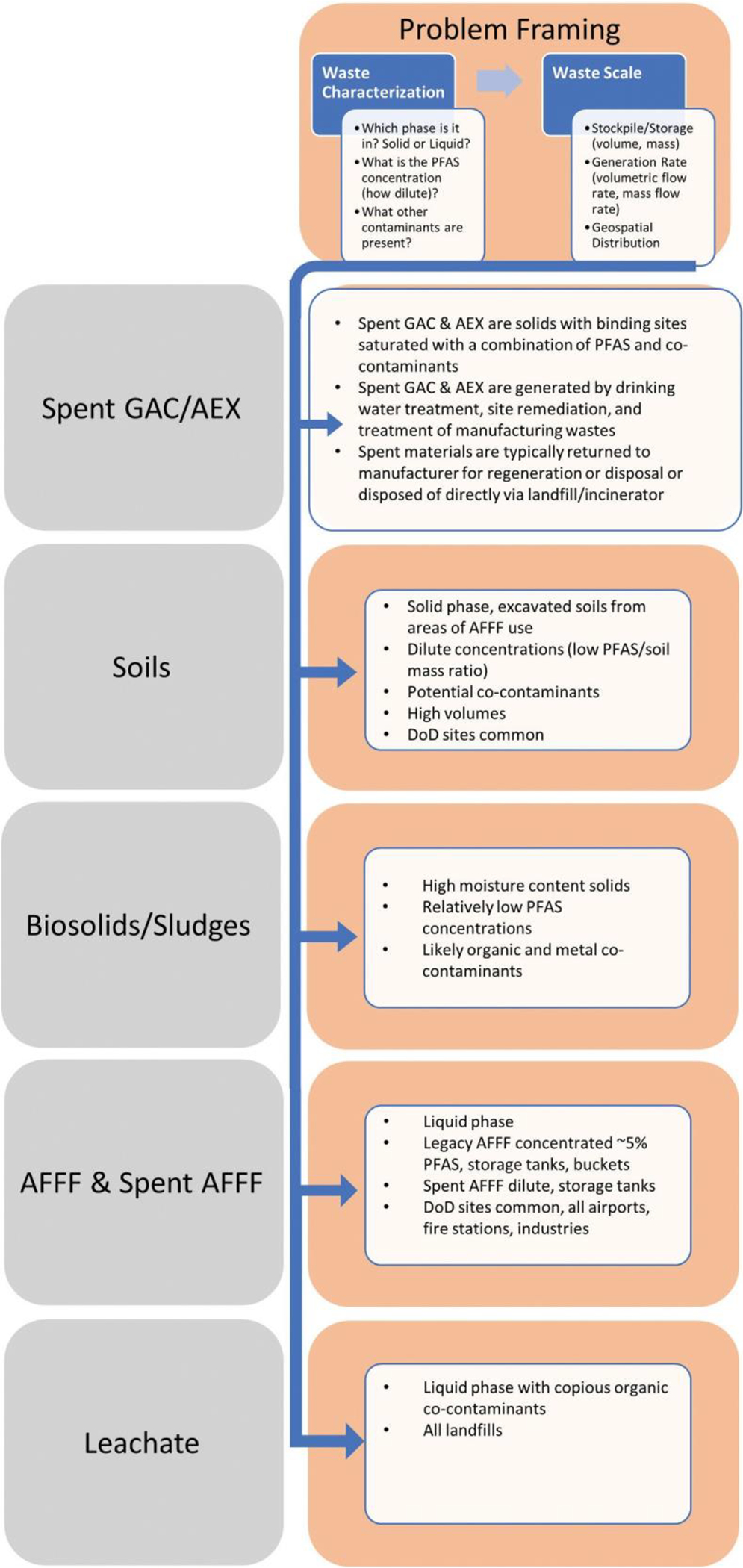
Characteristics of PFAS contaminated matrices.
Mechanochemical milling
Treatment of solid or semi-solid matrices in high energy ball mills has been proposed as a means of treating PFAS-containing soils and dewatered sludges. Mechanochemical degradation (MCD) has been undertaken on organic-contaminated media (Cagnetta et al. 2016) often with silica or alkaline earth elements to react with fluorine and encourage reactivity. Reactive radicals, electrons, heat, and even plasma in localized areas of the crystalline structures are created by the impact of stainless steel milling balls in the rotating vessel (Nakayama 2010). Effective destruction (> 99%) of PFAS molecules, including PFOS and PFOA, have been demonstrated in laboratory scale ball mills using pure PFAS compounds and different co-milling reagents (including calcium oxide, potassium hydroxide, lanthanum oxide, sodium persulfate, and sand) (Bolan et al. 2021; Cagnetta et al. 2016, 2017; Lu et al. 2017; Wang et al. 2019; Zhang et al. 2013). Mechanochemical treatments have been effective in soil remediation for chlorinated chemicals (Bolan et al. 2021); however, the treatment of PFAS-containing wastes at a commercially relevant scale (~3 tons per hour) using mechanochemical methods requires additional study. In particular, the effectiveness of MCD at destroying PFAS in soil matrixes (with or without co-milling reagents) remains to be proven.
MCD could be developed in a portable (trailer-based) system or set up on-site for extensive remediation projects, as some commercial companies (e.g., Environmental Decontamination Limited) have done successfully in the past (Bulley 2020). In addition, MCD is unique in that it treats materials in the solid phase without combustion, as compared with most other non-combustion techniques that remove the PFAS from the solid phase into the liquid (e.g., soil washing) or gas (e.g., thermal desorption) phases for treatment. Despite this, it is possible that gaseous PFAS compounds could be released during the MCD treatment, especially in open systems, so a full MCD treatment system must address potential release of volatile PFAS during treatment.
MCD is most amenable to highly contaminated soils, requiring a pre-treatment drying step to remove moisture from the soils, followed by addition of one or more co-milling reagents to react with the PFAS molecules. The resulting soils would be much finer than the initial soils and would lose much of their microstructure. This may affect the fertility of the treated soils. However, mixing treated soils with untreated soils (such as clean soil from off-site) could allow partial recycling of site soils without the potential mechanical and biological issues associated with the milled soils. Another potential issue is the impact of any co-milling reagents on the resulting treated soils. For this reason, non-reactive reagents (such as silicon dioxide or other metal oxides) are preferable to highly reactive (e.g., oxidizing, such as persulfate, or reducing, such as metal hydrides) or pH-modifying (such as acids or hydroxides) reagents. PFAS impacted soils have recently been remediated with and without co-milling reagents in a benchtop ball-mill and showed over 99% destruction of the PFAS with limited byproducts that the authors could analytical resolve (Turner et al. 2021). Further studies are needed to evaluate the gas phase emissions and to better determine any PFAS byproducts.
It is possible that MCD could be used to treat other solid waste streams containing high PFAS concentrations, such as dried GAC. This is especially true with the addition of co-milling reagents to add reactivity and change the mechanical properties of the milled solid. Experiments are required to determine the feasibility of such approaches. More information on MCD can be found in the U.S. EPA’s Mechanochemical Degradation Research Brief (Shields and Whitehill 2021).
Electrochemical treatment
Electrochemical oxidation (EO) has been used to oxidize pollutants by means of passing an electrical current through a solution. The electronegativity and electron affinity of fluorine allows the C-F bond to be broken and the fluorine atoms reduced when a high overpotential, > 3 V, is applied to a solution (Niu et al. 2016). The general destruction mechanism involves a step-wise removal of CF2 groups and the synthesis of shorter carboxylic acid PFAS until all the carbons have been defluorinated (Le et al. 2019; Niu et al. 2016). PFAS, including PFOA and PFOS, have been destroyed using several different types of electrodes in a search to improve efficiencies and allow for process scale up. Materials such as boron-doped diamond (Liao and Farrell 2009; Soriano, Gorri, and Urtiaga 2017), titanium suboxides (Le et al. 2019; Wang et al. 2020), and tin and lead oxides (Niu et al. 2016) have been used to successfully degrade PFAS in aqueous solutions. The process has been demonstrated on PFAS at the bench and pilot scale (Liang et al. 2018; Nzeribe et al. 2019). The technology has advantages of being able to operate at ambient temperature and pressure. EO is diffusion limited and the remediation tends to slow as the concentration of PFAS decreases, so innovative electrode and reactor designs are needed to allow for scale-up. Other issues involve the buildup of minerals on the anode, possible formation of perchlorate and other inorganic by-products, and the generation of volatile by-products which need to be treated. Investigations into the use of an electric field to transport and destroy PFAS in soils have been started and show promise (Skinn 2019) as have tests on landfill leachate (Pierpaoli et al. 2021) and AFFF contaminated groundwater (Schaefer et al. 2019). Further information on this relatively low-energy technology is available in the U.S. EPA’s Electrochemical Oxidation Research Brief (Krause, Magnuson, and Crone 2021).
Supercritical Water Oxidation (SCWO)
Above 374°C and 22.11 MPa, water reaches its supercritical state in which it behaves as a solvent for organics and enhances chemical oxidation reactions in the presence of an oxidizing agent such as O2, air, or hydrogen peroxide. SCWO has been demonstrated to treat halogenated waste materials including polychlorinated biphenyls (Abeln et al., 2001; Kim et al. 2010b). SCWO is able to quickly treat not only water based waste streams, but also sludges (Svanström et al. 2004) and slurries (Kim et al. 2010a); providing a possible way to treat most any pumpable matrix. Although SCWO requires initial energy input to reach the required temperature and pressure, the resulting reactions often produce a significant amount of heat themselves, which can be harvested to keep driving the process. The concentration of oxidizable organic molecules in the material being processed must be controlled and moderated, either by diluting the influent stream or adding additional fuels (such as alcohols). During the oxidation process, SCWO produces many acidic species, such as sulfuric acid (from sulfur-containing species) and hydrochloric acid (from chlorine-containing species). This results in a drop in pH during the process, which can cause significant corrosion of the reactor if not addressed. This can be addressed to some extent by adding alkalinity to the influent solution. The increased alkalinity of the modified influent solution may aid in the destruction of PFAS, as alkaline solutions show greater destruction and mineralization of PFOS at sub-critical conditions than acidic or neutral solutions (Wu et al. 2019). An additional challenge of SCWO is the precipitation of salts. The three major product streams from a SCWO reactor are the effluent, any evolved gases, and the salts that precipitate out from the reaction. A thorough evaluation of PFAS destruction using SCWO must address all of these product streams. Secondary air treatment technology might be necessary if gaseous PFAS compounds are evolved as a result of the SCWO process. There are different manifestations of SCWO based on different process designs. The PITT explored four different manifestations of SCWO technology to evaluate the potential for PFAS destruction.
Technical challenges being addressed when creating commercial products (374water 2020; Battelle Memorial Institute 2019; General Atomics, industrial Supercritical Water Oxidation (iSCWO) 2021; Jama et al. 2020) for the widespread implementation of SCWO include the buildup of corrosive gases during the oxidation reaction, the precipitation of salts, and the high energy requirements. More information is available in the U.S. EPA’s Research Brief on SCWO (Sahle-Damesessie and Krause 2021).
Pyrolysis/gasification
Pyrolysis decomposes solid or semi-solid materials at temperatures typically in the 300°C to 1000°C range in an oxygen-free environment. Gasification is similar to pyrolysis but operates at temperatures typically in the 800°C to 1650°C range with substoichiometric quantities of oxygen in a partial combustion process to provide additional energy to the process (Fytili and Zabaniotou 2008; Patel et al. 2020; Winchell et al. 2020). Pyrolysis can form a useful char (Boni et al. 2021) product whereas gasification typically forms low carbon ash. Both processes can generate a hydrogen-rich synthetic gas (syngas) depending on operating conditions. The high temperatures and residence times achieved by the combination of pyrolysis or gasification, followed directly by combustion of the hydrogen-rich syngas stream in a thermal oxidizer could potentially destroy PFAS by breaking apart the chemicals into inert or less recalcitrant constituents, although this remains a subject for further research. Compared to traditional incineration, pyrolysis and gasification require much lower air flows than incineration, which reduces the size and capital expense of air pollution control equipment. More information is available in the U.S. EPA’s Research Brief on Pyrolysis and Gasification of Biosolids (Acheson et al. 2021).
Waste source and treatment technology applicability
Some of the basic factors and characteristics involved in matching PFAS waste sources with these four innovative technologies are illustrated in Figure 2 for the technology focus and Figure 3 for the waste focus. These illustrations are meant to serve as a general guide to technology selection, management of the processes, and issues to consider PFAS waste characteristics.
A crosswalk between the technology factors for these four treatment methods and the PFAS waste characteristics, coupled with a literature review on the level of technology development, is presented in Figure 4. These factors are synthesized into an assessment of Technology Readiness Level (TRL, see Figure 4 Key in Figure 5) by the PITT to understand both the waste type applicability and state of development of each technology. These assessments were made by the PITT based on available information and the team’s best judgement and should be considered a starting point for discussion and technology development prioritization.
Figure 4.
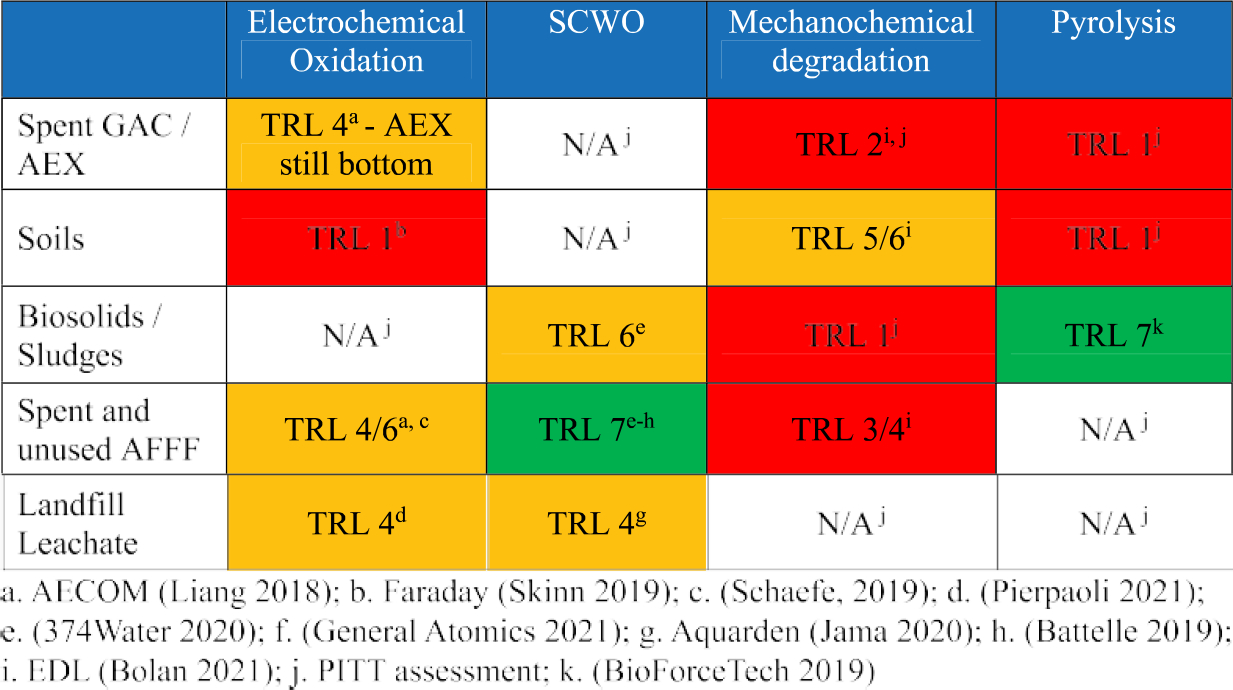
Crosswalk between PFAS waste and innovative Technology Readiness Level (TRL). Colors correspond to TRL categories described in Figure 5. No color indicates the non-applicability (N/A) of the technology to the waste stream.
Figure 5.
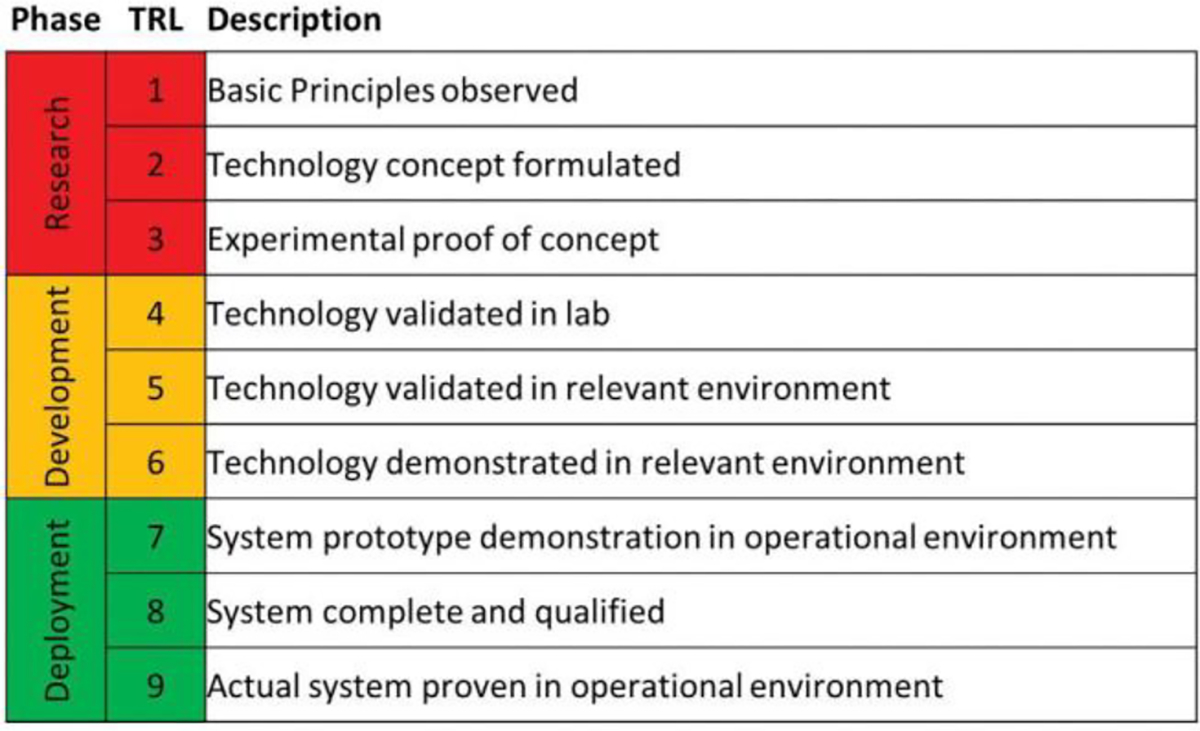
Key for Figure 4 technology readiness levels. Note that technologies were rated for PFAS destruction and may not capture their full capacity or overall readiness-level for other treatment purposes. Source: https://www.twi-global.com/technical-knowledge/faqs/technology-readiness-levels.
Spent GAC/AEX are solids that may be applicable to treatment in MCD or pyrolysis/gasification systems but are not applicable to liquid-based waste treatment technologies such as SCWO or EO. To our knowledge, no PFAS-laden GAC/AEX tests with MCD and pyrolysis/gasification have been undertaken. The crushing and grinding of the MCD systems seem an appropriate test for the solid GAC media. The elevated temperatures in pyrolysis/gasification systems may be an issue for the polymer-based AEX resins, causing melting and fouling of internal systems.
Like GAC/AEX, PFAS-contaminated soils could be treated in an MCD or pyrolysis/gasification unit. Both systems are ideally suited for treatment of solid materials. MCD has been used to treat soils contaminated with polychlorinated biphenyls, achieving over 99% mass reduction of soil contamination (Bulley 2020). We could not find literature references to pyrolysis/gasification treatment of PFAS-laden soils. However, the material handling characteristics and treatment operations are similar to those used in thermal decontamination of PFAS-contaminated soils (Bolan et al. 2021; Crownover et al. 2019; Sörengård et al. 2020). Therefore, although the release and treatment of volatiles requires additional study, the treatment of PFAS-contaminated soils using pyrolysis or gasification is technically feasible.
Biosolids/sludges are likely candidates for treatment in MCD, pyrolysis/gasification, and SCWO systems. Sufficiently dewatered sludge, or wet sludge dried by addition of co-milling agents, would be applicable to treatment in an MCD system. No such tests with PFAS have been identified in the literature. Preliminary studies on sludge treatment with SCWO have shown strong reductions in PFOS and PFOA levels in the processed effluent (374water 2020). PFAS testing on a biosolids pyrolysis system (Bioforcetech Corporation 2019) was repeated in a test commissioned by the PITT confirming high levels of degradation of the target PFAS compounds analyzed in the feed. Results on the latter are expected to be published in 2021.
A common source of stockpiled, legacy AFFF concentrate has been designated for upcoming SCWO, EO, and laboratory-scale MCD testing by the PITT with four SCWO providers. This legacy AFFF is comprised of 8-carbon PFAS, acids, and zwitterions as well as nonfluorinated surfactants and stabilizing chemicals (Material Safety Data Sheet FC-203CF 1996). Preliminary results from these tests show up to 99% degradation of the initial (“target”) PFAS compounds in the feed. However, in all cases, the potential non-target byproducts have yet to be analyzed.
PITT efforts
A variety of mechanisms have been put into place to test AFFF destruction using SCWO with four commercial companies. Where feasible, a common AFFF source has been used for these tests. These studies have achieved preliminary results; complete studies on emissions and byproducts are underway. Hydrothermal oxidation, the lower pressure, lower temperature variant of SCWO, is undergoing both laboratory scale tests at EPA and pilotscale commercial tests (as a SCWO pretreatment step). EO of dilute AFFF is undergoing testing in a laboratory scale reactor, accompanied by collection and analysis of the off-gases for volatile fluorinated organics. Laboratory scale studies of MCD of AFFF-laden soil are being conducted at EPA and a commercial laboratory. Scale-up plans and emission sampling tests have been proposed to extend this effort. Sludge gasification studies have been conducted at a field unit. The destruction of inlet PFAS is being assessed as well as the potential for fluorinated compound emissions. Preliminary results from all four technology/waste tests are expected to be available by 2022.
Summary
Four non-combustion technologies were highlighted for their potential to treat PFAS-laden waste streams: electrochemical oxidation, mechanochemical degradation, supercritical water oxidation, and pyrolysis/gasification. Considerations regarding technology factors and waste characteristics were cross-walked to aid in selection of treatment options. Finally, the technology applicability and readiness level were presented based upon existing, published information. Refinement of technology selection will be dependent on additional data; currently, only extremely limited testing results are available. Programs put into place by the PITT to examine these technologies are expected to yield published results by the end of 2022.
Implications:
This paper examines four novel, non-combustion technologies or applications for the treatment of persistent per- and polyfluoroalkyl substances (PFAS) wastes. These technologies are introduced to the reader along with their current state of development and areas for further development. This information will be useful for developers, policy makers, and facility managers that are facing increasing issues with disposal of PFAS wastes.
Acknowledgment
Funding for this work was provided by the U.S. EPA Office of Research and Development. The authors claim no competing interests. The views expressed in this paper are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection agency.
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 U.S.C. 105, no copyright protection is available for such works under U.S. Law.
Biographies
About the authors
Chelsea Berg is a biologist with EPA’s Office of Research and Development, Center for Environmental Measurement and Modeling in Research Triangle Park, NC, currently serving in a research support capacity. Her research areas include translational science, solutions-driven research, health impact assessments, and ecosystem services.
Brian Crone is an environmental engineer with EPA’s Office of Research and Development, Center for Environmental Solutions and Emergency Response in Cincinnati, OH. His research focuses on membrane bioreactor systems.
Brian Gullett is a Scientific and Technical Professional (ST level), Senior Research Engineer with the U.S. Environmental Protection Agency’s Office of Research and Development (ORD), located in Research Triangle Park, North Carolina. His research has investigated formation mechanisms of chlorinated dioxins and furans in combustion systems, as well as other organohalogen compounds. His current work involves development of emission samplers for use on unmanned aircraft systems to characterize hazardous pollutants from open air combustion and detonation processes.
Mark Higuchi is a supervisory toxicologist with EPA’s Office of Research and Development, Center for Public Health and Environmental Assessment in Research Triangle Park, NC. His research focuses on inhalation toxicology.
Max J. Krause is a research environmental engineer with EPA’s Office of Research and Development, Center for Environmental Solutions and Emergency Response in Cincinnati, OH. His research focuses on PFAS, landfills, and waste management topics.
Paul M. Lemieux is a senior research engineer with EPA’s Office of Research and Development, Center for Environmental Solutions and Emergency Response in Research Triangle Park, NC. His research focuses on waste management and development of decision support tools to help decision makers deal with wide-area CBRN responses.
Todd Martin is a research chemist with EPA’s Office of Research and Development, Center for Computational Toxicology and Exposure in Cincinnati, OH. His research focuses on computational toxicology, development of computer software applications, and quantitative structure activity relationship (QSAR) models. He is the lead developer of TEST (Toxicity Estimation Software Tool) which allows users to easily estimate several toxicity and physical property endpoints from molecular structure.
Erin P. Shields is a physical scientist with EPA’s Office of Research and Development, Center for Environmental Measurement and Modeling in Research Triangle Park, NC. He currently researches methods to evaluate PFAS destruction techniques and characterize their emissions.
Ed Struble is a management and program analyst with EPA’s Office of Research and Development, Center for Environmental Measurement and Modeling in Research Triangle Park, NC.
Eben Thoma is a research physical scientist with EPA’s Office of Research and Development, Center for Environmental Measurement and Modeling in Research Triangle Park, NC. His research focuses on characterization of difficult to measure air pollution sources and their impacts using next generation measurement approaches.
Andrew Whitehill is an analytical chemist and Physical Scientist with EPA’s Office of Research and Development, Environmental Measurement and Modeling in Research Triangle Park, NC. Dr. Whitehill’s research includes mechanochemical destruction of PFAS and measurement of air toxics and volatile organic compounds in ambient air.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 374water. 2020. Supercritical Water Oxidation (SCWO) destruction of PFAS contaminated municipal sludge. Accessed 2021. https://www.bluetechforum.com/wp-content/uploads/1.-374Water-BlueTech-Case-Study-Rev1.pdf
- Abeln J, Kluth M, Petrich G, and Schmieder H. 2001. Supercritical water oxidation (SCWO): A process for the treatment of industrial waste effluents. High Press Res 20 (1– 6):537–47. doi: 10.1080/08957950108206202. [DOI] [Google Scholar]
- Acheson C, Mills M, Krause M, and Thoma E. 2021. Potential PFAS destruction technology: pyrolysis and gasification. In U.S. EPA Research Briefs. https://www.epa.gov/sites/production/files/2021-01/documents/pitt_research_brief_pyrolysis_final_jan_27_2021_508.pdf [Google Scholar]
- Alder AC, and van der Voet J. 2015. Occurrence and point source characterization of perfluoroalkyl acids in sewage sludge. Chemosphere 129:62–73. doi: 10.1016/j.chemosphere.2014.07.045. [DOI] [PubMed] [Google Scholar]
- Aro R, Eriksson U, Kärrman A, Chen F, Wang T, and Yeung LW. 2021. Fluorine mass balance analysis of effluent and sludge from Nordic Countries. ACS ES&T Water 1 (9):2087–96. doi: 10.1021/acsestwater.1c00168. [DOI] [Google Scholar]
- Battelle Memorial Institute. 2019. PFAS Annihilator™ destruction. Technology. Accessed 2021. https://www.battelle.org/government-offerings/energy-environment/environmental-services/pfas-assessment-mitigation/pfas-annihilator-destruction-technology [Google Scholar]
- Bioforcetech Corporation. 2019. Eliminating PFAS from biosolids is no longer a mystery. Accessed 2021. https://medium.com/nature-is-awesome-bioforcetech/eliminating-pfas-from-biosolids-is-no-longer-a-mystery-f56b94d7bfb
- Bolan N, Sarkar B, Yan Y, Li Q, Wijesekara H, Kannan K, Tsang DCW, Schauerte M, Bosch J, Noll H, et al. 2021. Remediation of poly- and perfluoroalkyl substances (PFAS) contaminated soils - To mobilize or to immobilize or to degrade? J. Hazard. Mater. 401:123892. doi: 10.1016/j.jhazmat.2020.123892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni M, Marzeddu S, Tatti F, Raboni M, Mancini G, Luciano A, and Viotti P. 2021. Experimental and numerical study of biochar fixed bed column for the adsorption of arsenic from aqueous solutions. Water 13 (7):7. doi: 10.3390/w13070915. [DOI] [Google Scholar]
- Borodin A 1862. Made for use in the history of fluorides and the preparation of benzoyl fluoride. Compt. rend. 55:553–56. [Google Scholar]
- Brusseau ML, Anderson RH, and Guo B. 2020. PFAS concentrations in soils: Background levels versus contaminated sites. Science of The Total Environment 740:140017. doi: 10.1016/j.scitotenv.2020.140017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulley M, Black B. 2020. Mechano-Chemical Remediation (MCD) of pop’s contaminated soil. In WasteMINZ, ed. E. D. Limited, EDL, Greenmount, Ackland, NZ. https://www.wasteminz.org.nz/wp-content/uploads/Mike-Bulley.pdf. [Google Scholar]
- Cagnetta G, Huang J, Wang B, Deng S, and Yu G. 2016. A comprehensive kinetic model for mechanochemical destruction of persistent organic pollutants. Chem. Eng. J. 291:30–38. doi: 10.1016/j.cej.2016.01.079. [DOI] [Google Scholar]
- Cagnetta G, Zhang Q, Huang J, Lu M, Wang B, Wang Y, Deng S, and Yu G. 2017. Mechanochemical destruction of perfluorinated pollutants and mechanosynthesis of lanthanum oxyfluoride: A waste-to-materials process. Chem. Eng. J. 316:1078–90. doi: 10.1016/j.cej.2017.02.050. [DOI] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, and Needham LL. 2007. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 115 (11):1596–602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang C, Han J, Yu Y, and Zhang P. 2012. PFOS and PFOA in influents, effluents, and biosolids of Chinese wastewater treatment plants and effluent-receiving marine environments. Environ. Pollut. 170:26–31. doi: 10.1016/j.envpol.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Chiavola A, Di Marcantonio C, Boni MR, Biagioli S, Frugis A, and Cecchini G. 2020. Experimental investigation on the perfluorooctanoic and perfluorooctane sulfonic acids fate and behaviour in the activated sludge reactor. Process Safety and Environmental Protection 134:406–15. doi: 10.1016/j.psep.2019.11.003. [DOI] [Google Scholar]
- Cicerone RJ 1979. Atmospheric carbon tetrafluoride: a nearly inert gas. Science 206 (4414):59–61. doi: 10.1126/science.206.4414.59. [DOI] [PubMed] [Google Scholar]
- Cordner A, De La Rosa VY, Schaider LA, Rudel RA, Richter L, and Brown P. 2019. Guideline levels for PFOA and PFOS in drinking water: the role of scientific uncertainty, risk assessment decisions, and social factors. J Expo Sci Environ Epidemiol 29 (2):157–71. doi: 10.1038/s41370-018-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello CA, and McCarthy TJ. 1984. Surface modification of poly(tetrafluoroethylene) with benzoin dianion. Macromolecules 17 (12):2940–42. doi: 10.1021/ma00142a094. [DOI] [Google Scholar]
- Council ITR 2020. Human and ecological health effects of select PFAS. Accessed August 28, 2020. https://pfas-1.itrcweb.org/7-human-and-ecological-health-effects-of-select-pfas/
- Crownover E, Oberle D, Kluger M, and Heron G. 2019. Perfluoroalkyl and polyfluoroalkyl substances thermal desorption evaluation. Remediation Journal 29 (4):77–81. doi: 10.1002/rem.21623. [DOI] [Google Scholar]
- D’Agostino LA, and Mabury SA. 2014. Identification of novel fluorinated surfactants in aqueous film forming foams and commercial surfactant concentrates. Environ. Sci. Technol. 48 (1):121–29. doi: 10.1021/es403729e. [DOI] [PubMed] [Google Scholar]
- Dauchy X, Boiteux V, Bach C, Colin A, Hemard J, Rosin C, and Munoz JF. 2017. Mass flows and fate of per- and polyfluoroalkyl substances (PFASs) in the wastewater treatment plant of a fluorochemical manufacturing facility. Sci. Total Environ. 576:549–58. doi: 10.1016/j.scitotenv.2016.10.130. [DOI] [PubMed] [Google Scholar]
- Daudt HWY 1935. Mortimer alexander organic fluorine compound. US2005706. [Google Scholar]
- Dempsey JR, Davis WA, Crossfield AS, and Williams WC. 1964. Program management in design and development. In Third Annual Aerospace Reliability and Maintainability Conference Proceedings, Washington, DC, Society of Automotive Engineers, pp. 7–8. [Google Scholar]
- DeYoung DJ 1994. National Security and the U.S. Naval Research Laboratory. Seventy years of science for the navy and the nation (1923–1993). Washington, DC: Naval Research Laboratory. [Google Scholar]
- Dickenson E, and Higgins C. 2016. Treatment mitigation strategies for Poly-and Perfluoroalkyl substances. Water research foundation web report, 4322. [Google Scholar]
- Dinglasan-Panlilio MJA, and Mabury SA. 2006. Significant residual fluorinated alcohols present in various fluorinated materials. Environ. Sci. Technol. 40 (5):1447–53. doi: 10.1021/es051619. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Martin JW, De Silva AO, Mabury SA, Hurley MD, Sulbaek Andersen MP, and Wallington TJ. 2004. Degradation of fluorotelomer alcohols: A likely atmospheric source of perfluorinated carboxylic acids. Environ. Sci. Technol. 38 (12):3316–21. doi: 10.1021/es049860w. [DOI] [PubMed] [Google Scholar]
- Eriksen KT, Sørensen M, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, and Raaschou-Nielsen O. 2009. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general danish population. JNCI: Journal of the National Cancer Institute 101 (8):605–09. doi: 10.1093/jnci/djp041. [DOI] [PubMed] [Google Scholar]
- Fytili D, and Zabaniotou A. 2008. Utilization of sewage sludge in EU application of old and new methods—A review. Renewable and Sustainable Energy Reviews 12 (1):116–40. doi: 10.1016/j.rser.2006.05.014. [DOI] [Google Scholar]
- Gallen C, Eaglesham G, Drage D, Nguyen TH, and Mueller JF. 2018b. A mass estimate of perfluoroalkyl substance (PFAS) release from Australian wastewater treatment plants. Chemosphere 208:975–83. doi: 10.1016/j.chemosphere.2018.06.024. [DOI] [PubMed] [Google Scholar]
- Gallen C, Eaglesham G, Drage D, Nguyen TH, and Mueller J. 2018a. A mass estimate of perfluoroalkyl substance (PFAS) release from Australian wastewater treatment plants. Chemosphere 208:975–83. doi: 10.1016/j.chemosphere.2018.06.024. [DOI] [PubMed] [Google Scholar]
- Gaylor PJ 1940. Fluorinated compound and method of producing the same. US2186916. [Google Scholar]
- General Atomics, industrial Supercritical Water Oxidation (iSCWO). 2021. Accessed 2021. https://www.ga.com/hazardous-waste-destruction
- Ghisi R, Vamerali T, and Manzetti S. 2019. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res. 169:326–41. doi: 10.1016/j.envres.2018.10.023. [DOI] [PubMed] [Google Scholar]
- Giesy JP, and Kannan K. 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 35 (7):1339–42. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Goldman P 1969. The carbon-fluorine bond in compounds of biological interest. Science 164 (3884):1123–30. doi: 10.1126/science.164.3884.1123. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, and Heilmann C. 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307 (4):391–97. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydebreck F, Tang J, Xie Z, and Ebinghaus R. 2016. Emissions of per- and polyfluoroalkyl substances in a textile manufacturing plant in China and their relevance for workers’ exposure. Environ. Sci. Technol. 50 (19):10386–96. doi: 10.1021/acs.est.6b03213. [DOI] [PubMed] [Google Scholar]
- Huset CA, Barlaz MA, Barofsky DF, and Field JA. 2011. Quantitative determination of fluorochemicals in municipal landfill leachates. Chemosphere 82 (10):1380–86. doi: 10.1016/j.chemosphere.2010.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IG Farbenindustrie AG. 1937. Manufacture of polymerisation products. U.K. Patent, GB465520A.
- Ivy DJ, Rigby M, Baasandorj M, Burkholder JB, and Prinn RG. 2012. Global emission estimates and radiative impact of C4F10, C5F12, C6F14, C7F16 and C8F18. Atmos. Chem. Phys. 12 (16):7635–45. doi: 10.5194/acp-12-7635-2012. [DOI] [Google Scholar]
- Jama Y, Lindholst S, Andreasen RR, Andersen HR, Kokkoli A, Svendsen T, Cai Z, and Kragelund C. 2020. PFAS removal from percolate by super critical water oxidation (SCWO). In 14th DWF Water Research Conference, University of Copenhagen, Frederiksberg, Vol. Abstracts, pp. 48. [Google Scholar]
- Kim K, Son SH, Kim K, Han JH, Han KD, and Do SH. 2010a. Treatment of radioactive ionic exchange resins by super- and sub-critical water oxidation (SCWO). Nucl. Eng. Des. 240 (10):3654–59. doi: 10.1016/j.nucengdes.2010.06.018. [DOI] [Google Scholar]
- Kim K, Son SH, Kim K, Kim K, and Kim Y-C. 2010b. Environmental effects of supercritical water oxidation (SCWO) process for treating transformer oil contaminated with polychlorinated biphenyls (PCBs). Chem. Eng. J. 165 (1):170–74. doi: 10.1016/j.cej.2010.09.012. [DOI] [Google Scholar]
- Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, and Christensen TH. 2002. Present and long-term composition of MSW landfill leachate: A review. Crit Rev Environ Sci Technol 32 (4):297–336. doi: 10.1080/10643380290813462. [DOI] [Google Scholar]
- Krause M, Magnuson M, and Crone B 2021. Potential PFAS destruction technology: Electrochemical oxidation. In U.S. EPA. https://www.epa.gov/sites/production/files/2021-01/documents/pitt_research_brief_electrochemical_oxidation_final_jan_25_2021_508.pdf [Google Scholar]
- Krusic PJ, Marchione AA, and Roe DC. 2005. Gas-phase NMR studies of the thermolysis of perfluorooctanoic acid. J. Fluorine Chem. 126 (11–12):1510–16. doi: 10.1016/j.jfluchem.2005.08.016. [DOI] [Google Scholar]
- Krusic PJ, and Roe DC. 2004. Gas-phase NMR technique for studying the thermolysis of materials: Thermal decomposition of ammonium perfluorooctanoate. Anal. Chem. 76 (13):3800–03. doi: 10.1021/ac049667k. [DOI] [PubMed] [Google Scholar]
- Lang JR, Allred BM, Field JA, Levis JW, and Barlaz MA. 2017. National estimate of Per- and Polyfluoroalkyl Substance (PFAS) release to U.S. municipal landfill leachate. Environ. Sci. Technol. 51 (4):2197–205. doi: 10.1021/acs.est.6b05005. [DOI] [PubMed] [Google Scholar]
- Le TXH, Haflich H, Shah AD, and Chaplin BP. 2019. Energy-efficient electrochemical oxidation of perfluoroalkyl substances using a Ti4O7 reactive electrochemical membrane anode. Environ. Sci. Tech. Let. 6 (8):504–10. doi: 10.1021/acs.estlett.9b00397. [DOI] [Google Scholar]
- Lebeau P, and Damiens A. 1926. Carbon tetrafluoride. Compt. rend. 182:1340–42. [Google Scholar]
- Lemal DM 2004. Perspective on fluorocarbon chemistry. J. Org. Chem. 69 (1):1–11. doi: 10.1021/jo0302556. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Johns LE, and Meeker JD. 2015. Serum biomarkers of exposure to perfluoroalkyl substances in relation to serum testosterone and measures of thyroid function among adults and adolescents from NHANES 2011–2012. Int J Environ Res Public Health 12 (6):6098–114. doi: 10.3390/ijerph120606098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Pierce RD, Lin H, Chiang S-YD, and Huang QJ. 2018. Electrochemical oxidation of PFOA and PFOS in concentrated waste streams. Remediation Journal 28 (2):127–34. doi: 10.1002/rem.21554. [DOI] [Google Scholar]
- Liao Z, and Farrell J. 2009. Electrochemical oxidation of perfluorobutane sulfonate using boron-doped diamond film electrodes. J. Appl. Electrochem. 39 (10):1993–99. doi: 10.1007/s10800-009-9909-z. [DOI] [Google Scholar]
- Lim X 2019. Tainted water: the scientists tracing thousands of fluorinated chemicals in our environment. Nature 566 (7742):26–29. doi: 10.1038/d41586-019-00441-1. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, and Libelo EL. 2011. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 45 (19):7954–61. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Lockheed M, and Skunk W. 2021. Accessed 2021. https://www.lockheedmartin.com/en-us/who-we-are/business-areas/aeronautics/skunkworks.html
- Loganathan BG, Sajwan KS, Sinclair E, Kumar KS, and Kannan K. 2007a. Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia. Water Res. 41 (20):4611–20. doi: 10.1016/j.watres.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Loganathan BG, Sajwan KS, Sinclair E, Senthil Kumar K, and Kannan K. 2007b. Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia. Water Res. 41 (20):4611–20. doi: 10.1016/j.watres.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Lu M, Cagnetta G, Zhang K, Huang J, and Yu G. 2017. Mechanochemical mineralization of “very persistent” fluorocarbon surfactants 6:2 fluorotelomer sulfonate (6:2FTS) as an example. Sci Rep 7 (1):17180. doi: 10.1038/s41598-017-17515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahinroosta R, and Senevirathna L. 2020. A review of the emerging treatment technologies for PFAS contaminated soils. J. Environ. Manage. 255:109896. doi: 10.1016/j.jenvman.2019.109896. [DOI] [PubMed] [Google Scholar]
- Martin JW, Ellis DA, Mabury SA, Hurley MD, and Wallington TJ. 2006. Atmospheric chemistry of Perfluoroalkanesulfonamides: Kinetic and product studies of the OH Radical and Cl Atom Initiated Oxidation of N-Ethyl Perfluorobutanesulfonamide. Environ. Sci. Technol. 40 (3):864–72. doi: 10.1021/es051362f. [DOI] [PubMed] [Google Scholar]
- Martin JW, Muir DCG, Moody CA, Ellis DA, Kwan WC, Solomon KR, and Mabury SA. 2002. Collection of airborne fluorinated organics and analysis by gas chromatography/chemical ionization mass spectrometry. Anal. Chem. 74 (3):584–90. doi: 10.1021/ac015630d. [DOI] [PubMed] [Google Scholar]
- Masoner JR, Kolpin DW, Cozzarelli IM, Smalling KL, Bolyard SC, Field JA, Furlong ET, Gray JL, Lozinski D, Reinhart D, et al. 2020. Landfill leachate contributes per-/poly-fluoroalkyl substances (PFAS) and pharmaceuticals to municipal wastewater. Environmental Science: Water Research & Technology 6 (5):1300–11. doi: 10.1039/D0EW00045K. [DOI] [Google Scholar]
- Material safety data sheet FC-203CF, Light water brand aqueous film forming foam. In Minnesota Mining & MFG Co. 1996 [Google Scholar]
- McCord J, and Strynar M. 2019. Identification of Per- and Polyfluoroalkyl substances in the cape fear river by high resolution mass spectrometry and nontargeted screening. Environ. Sci. Technol 53 (9):4717–27. doi: 10.1021/acs.est.8b06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia-Avendaño S, Zhi Y, Yan B, and Liu J. 2020. Sorption of polyfluoroalkyl surfactants on surface soils: Effect of molecular structures, soil properties, and solution chemistry. Environ. Sci. Technol. 54 (3):1513–21. doi: 10.1021/acs.est.9b04989. [DOI] [PubMed] [Google Scholar]
- Merrill R, and Ryan J. 2021. Other test method 45 (OTM-45) measurement of selected Per- and Polyfluorinated Alkyl substances from stationary sources. Washington, DC: U.S. Environmental Protection Agency. https://www.epa.gov/sites/production/files/2021-01/documents/otm_45_semivolatile_pfas_1-13-21.pdf [Google Scholar]
- Michigan Department of Environment, Great Lakes, and Energy, Summary Report. 2020. Initiatives to evaluate the presence of PFAS in municipal wastewater and associated residuals.
- Moissan H 1890a. Carbon tetrafluoride. Compt. rend. 110:951–54. [Google Scholar]
- Moissan H 1890b. Action of fluorine on different forms of carbon. Compt. rend. 110:276–79. [Google Scholar]
- Moody CA, and Field JA. 2000. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol. 34 (18):3864–70. doi: 10.1021/es991359u. [DOI] [Google Scholar]
- Nakayama K 2010. Triboplasma Generation and Triboluminescence: Influence of stationary sliding partner. Tibol Lett 37 (2):215–28. doi: 10.1007/s11249-009-9516-5. [DOI] [Google Scholar]
- Navarro I, de La Torre A, Sanz P, Pro J, Carbonell G, and Martínez MDLÁ. 2016. Bioaccumulation of emerging organic compounds (perfluoroalkyl substances and halogenated flame retardants) by earthworm in biosolid amended soils. Environ. Res. 149:32–39. doi: 10.1016/j.envres.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Niu J, Li Y, Shang E, Xu Z, and Liu J. 2016. Electrochemical oxidation of perfluorinated compounds in water. Chemosphere 146:526–38. doi: 10.1016/j.chemosphere.2015.11.115. [DOI] [PubMed] [Google Scholar]
- Nzeribe BN, Crimi M, Mededovic Thagard S, and Holsen TM. 2019. Physico-chemical processes for the treatment of Per- And Polyfluoroalkyl Substances (PFAS): A review. Crit Rev Environ Sci Technol 49 (10):866–915. doi: 10.1080/10643389.2018.1542916. [DOI] [Google Scholar]
- O’Hagan D 2008. Understanding organofluorine chemistry. An introduction to the C-F bond. Chem. Soc. Rev. 37 (2):308–19. doi: 10.1039/b711844a. [DOI] [PubMed] [Google Scholar]
- Okazoe T 2009. Overview on the history of organofluorine chemistry from the viewpoint of material industry. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci 85 (8):276–89. doi: 10.2183/pjab.85.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C-G, Liu Y-S, and Ying -G-G. 2016. Perfluoroalkyl substances (PFASs) in wastewater treatment plants and drinking water treatment plants: Removal efficiency and exposure risk. Water Res. 106:562–70. doi: 10.1016/j.watres.2016.10.045. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang H, Cui Q, Sheng N, Yeung LWY, Guo Y, Sun Y, and Dai J. 2017. First report on the occurrence and bioaccumulation of hexafluoropropylene oxide trimer acid: An emerging concern. Environ. Sci. Technol. 51 (17):9553–60. doi: 10.1021/acs.est.7b02259. [DOI] [PubMed] [Google Scholar]
- Patel S, Kundu S, Halder P, Ratnnayake N, Marzbali MH, Aktar S, Selezneva E, Paz-Ferreiro J, Surapaneni A, de Figueiredo CC, et al. 2020. A critical literature review on biosolids to biochar: an alternative biosolids management option. Reviews in Environmental Science and Bio/Technology 19 (4):807–41. doi: 10.1007/s11157-020-09553-x. [DOI] [Google Scholar]
- Pierpaoli M, Szopińska M, Wilk BK, Sobaszek M, Łuczkiewicz A, Bogdanowicz R, and Fudala-Książek S. 2021. Electrochemical oxidation of PFOA and PFOS in landfill leachates at low and highly boron-doped diamond electrodes. J. Hazard. Mater. 403:123606. doi: 10.1016/j.jhazmat.2020.123606. [DOI] [PubMed] [Google Scholar]
- Place BJ, and Field JA. 2012. Identification of novel fluorochemicals in aqueous film-forming foams used by the US military. Environ. Sci. Technol. 46 (13):7120–27. doi: 10.1021/es301465n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett RJ 1941. Tetrafluoroethylene polymers US2230654.
- Prevedouros K, Cousins IT, Buck RC, and Korzeniowski SH. 2006. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 40 (1):32–44. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Ravishankara AR, Solomon S, Turnipseed AA, and Warren RF. 1993. Atmospheric lifetimes of long-lived halogenated species. Science 259 (5092):194–99. doi: 10.1126/science.259.5092.194. [DOI] [PubMed] [Google Scholar]
- Ross I, McDonough J, Miles J, Storch P, Thelakkat Kochunarayanan P, Kalve E, Hurst J, Dasgupta SS, and Burdick J. 2018. A review of emerging technologies for remediation of PFASs. Remediation Journal 28 (2):101–26. doi: 10.1002/rem.21553. [DOI] [Google Scholar]
- Roth J, Abusallout I, Hill T, Holton C, Thapa U, and Hanigan D. 2020. Release of volatile Per- and Polyfluoroalkyl substances from aqueous film-forming foam. Environ. Sci. Tech. Let. 7 (3):164–70. doi: 10.1021/acs.estlett.0c00052. [DOI] [Google Scholar]
- Sahle-Damesessie E, and Krause M. 2021. Potential PFAS destruction technology: supercritical water oxidation. In U.S. EPA Research Briefs. https://www.epa.gov/sites/production/files/2021-01/documents/pitt_research_brief_scwo_final_jan_25_2021_508.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer CE, Andaya C, Maizel A, and Higgins CP. 2019. Assessing continued electrochemical treatment of groundwater impacted by aqueous film-forming foams. Journal of Environmental Engineering 145 (12):06019007. doi: 10.1061/(asce)ee.1943-7870.0001605. [DOI] [Google Scholar]
- Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, Lunderberg DM, Lang JR, and Peaslee GF. 2017. Fluorinated compounds in U.S. fast food packaging. Environ. Sci. Tech. Let. 4 (3):105–11. doi: 10.1021/acs.estlett.6b00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultes L, Vestergren R, Volkova K, Westberg E, Jacobson T, and Benskin JP. 2018. Per- and polyfluoroalkyl substances and fluorine mass balance in cosmetic products from the Swedish market: Implications for environmental emissions and human exposure. Environ Sci Process Impacts 20 (12):1680–90. doi: 10.1039/C8EM00368H. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Higgins CP, Huset CA, Luthy RG, Barofsky DF, and Field JA. 2006. Fluorochemical mass flows in a municipal wastewater treatment facility. Environ. Sci. Technol. 40 (23):7350–57. doi: 10.1021/es061025m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulvado JG, Blaine AC, Hundal LS, and Higgins CP. 2011. Occurrence and fate of perfluorochemicals in soil following the land application of municipal biosolids. Environ. Sci. Technol. 45 (19):8106–12. doi: 10.1021/es103903d. [DOI] [PubMed] [Google Scholar]
- Shields E, and Whitehill A. 2021. Potential PFAS destruction technology: mechanochemical degradation. In U.S. EPA. https://www.epa.gov/sites/production/files/2021-01/documents/pitt_research_brief_mechanochemical_final_jan_25_2020_508.pdf [Google Scholar]
- Sinclair E, and Kannan K. 2006. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ. Sci. Technol. 40 (5):1408–14. doi: 10.1021/es051798v. [DOI] [PubMed] [Google Scholar]
- Skinn B 2019. EPA SBIR 68HERD19C0023. Electrochemical Extraction and Remediation of PFAS in Soils. Accessed 2021. https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.abstractDetail/abstract/10972/report/0 [Google Scholar]
- Sörengård M, Lindh AS, Ahrens L, and DeWitt JC. 2020. Thermal desorption as a high removal remediation technique for soils contaminated with per- and polyfluoroalkyl substances (PFASs). PLOS ONE 15 (6): e0234476. doi: 10.1371/journal.pone.0234476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano Á, Gorri D, and Urtiaga A. 2017. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Res. 112:147–56. doi: 10.1016/j.watres.2017.01.043. [DOI] [PubMed] [Google Scholar]
- Stock NL, Lau FK, Ellis DA, Martin JW, Muir DCG, and Mabury SA. 2004. polyfluorinated telomer alcohols and sulfonamides in the north american troposphere. Environ. Sci. Technol. 38 (4):991–96. doi: 10.1021/es034644t. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, and Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29 (2):131–47. doi: 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanström M, Fröling M, Modell M, Peters WA, and Tester J. 2004. Environmental assessment of supercritical water oxidation of sewage sludge. Resources, Conservation and Recycling 41 (4):321–38. doi: 10.1016/j.resconrec.2003.12.002. [DOI] [Google Scholar]
- Szilagyi JT, Avula V, and Fry RC. 2020. Perfluoroalkyl Substances (PFAS) and their effects on the placenta, pregnancy, and child development: a potential mechanistic role for Placental Peroxisome Proliferator-Activated Receptors (PPARs). Curr. Environ. Health Rep 7 (3):222–30. Ahead of Print. doi: 10.1007/s40572-020-00279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier X, Granby K, and Christensen JH. 2011. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res Int 18 (7):1108–20. doi: 10.1007/s11356-010-0439-3. [DOI] [PubMed] [Google Scholar]
- Tsang W, Burgess DR, and Babushok V. 1998. On the incinerability of highly fluorinated organic compounds. Combust. Sci. Technol. 139 (1):385–402. doi: 10.1080/00102209808952095. [DOI] [Google Scholar]
- Turner LP, Kueper BH, Jaansalu KM, Patch DJ, Battye N, El-Sharnouby O, Mumford KG, and Weber KP. 2021. Mechanochemical remediation of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) amended sand and aqueous film-forming foam (AFFF) impacted soil by planetary ball milling. Sci. Total Environ. 765:142722. doi: 10.1016/j.scitotenv.2020.142722. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. 2019. Facility search – Enforcement and compliance data. Accessed 2019.
- U.S. EPA. 2020. Basic information about landfills. Accessed 2021. https://www.epa.gov/landfills/basic-informationabout-landfills
- U.S. EPA, Office of Water 1994. A plain english guide to the EPA part 503 biosolids rule. Accessed 2020. https://www.epa.gov/sites/production/files/2018-12/documents/plain-englishguide-part503-biosolids-rule.pdf
- United Nations Environment Programme. 2004. Estimated quantities of Aqueous Film Forming Foam (AFFF) in the United States. Accessed August 7, 2020. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPSPOPRC13FU-SUBM-PFOA-FFFC-2-20180112.En.pdf
- U.S. EPA. 2016a. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). United States Environmental Protection Agency, Office of Water EPA; 822-R-16–00. [Google Scholar]
- U.S. EPA. 2016b. Drinking water health advisory for perfluorooctane sulfonate (PFOS), United States Environmental Protection Agency, Office of Water; 822-R-16–004. [Google Scholar]
- Venkatesan AK, and Halden RU. 2013. National inventory of perfluoroalkyl substances in archived U.S. biosolids from the 2001 EPA national sewage sludge survey. J. Hazard. Mater. 252–253:413–18. doi: 10.1016/j.jhazmat.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergun D 2019. U.S. department of defense. Naval Research Lab Chemists Search for PFAS-Free Firefighting Foam. Accessed 2021. https://www.defense.gov/Explore/News/Article/Article/2017249/naval-research-lab-chemists-search-for-pfas-free-firefighting-foam/
- Vestergren R, and Cousins IT. 2009. Tracking the pathways of human exposure to Perfluorocarboxylates. Environ. Sci. Technol. 43 (15):5565–75. doi: 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- Wang N, Lv H, Zhou Y, Zhu L, Hu Y, Majima T, and Tang H. 2019. Complete defluorination and mineralization of perfluorooctanoic acid by a mechanochemical method using alumina and persulfate. Environ. Sci. Technol. 53 (14):8302–13. doi: 10.1021/acs.est.9b00486. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pierce RD, Shi H, Li C, and Huang Q. 2020. Electrochemical degradation of perfluoroalkyl acids by titanium suboxide anodes. Environmental Science: Water Research & Technology 6 (1):144–52. doi: 10.1039/C9EW00759H. [DOI] [Google Scholar]
- Washington JW, Rankin K, Libelo EL, Lynch DG, and Cyterski M. 2019. Determining global background soil PFAS loads and the fluorotelomer-based polymer degradation rates that can account for these loads. Sci. Total Environ. 651 (Pt 2):2444–49. doi: 10.1016/j.scitotenv.2018.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Takata M, Takemine S, and Yamamoto K. 2018. Thermal mineralization behavior of PFOA, PFHxA, and PFOS during reactivation of granular activated carbon (GAC) in nitrogen atmosphere. Environ Sci Pollut Res Int 25 (8):7200–05. doi: 10.1007/s11356-015-5353-2. [DOI] [PubMed] [Google Scholar]
- Winchell LJ, Ross JJ, Wells MJM, Fonoll X, Norton JW, and Bell KY. 2020. Per- and polyfluoroalkyl substances thermal destruction at water resource recovery facilities: A state of the science review. Water environment research: a research publication of the Water Environment Federation. doi: 10.1002/wer.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiszniowski J, Robert D, Surmacz-Gorska J, Miksch K, and Weber JV. 2006. Landfill leachate treatment methods: A review. Environ Chem Lett 4 (1):51–61. doi: 10.1007/s10311-005-0016-z. [DOI] [Google Scholar]
- Wu B, Hao S, Choi Y, Higgins CP, Deeb R, and Strathmann TJ. 2019. Rapid destruction and defluorination of perfluorooctanesulfonate by alkaline hydrothermal reaction. Environ. Sci. Tech. Let. 6 (10):630–36. doi: 10.1021/acs.estlett.9b00506. [DOI] [Google Scholar]
- Xiao F, Jin B, Golovko SA, Golovko MY, and Xing B. 2019. Sorption and desorption mechanisms of cationic and zwitterionic Per- and Polyfluoroalkyl substances in natural soils: Thermodynamics and Hysteresis. Environ. Sci. Technol. 53 (20):11818–27. doi: 10.1021/acs.est.9b05379. [DOI] [PubMed] [Google Scholar]
- Yoo H, Washington JW, Jenkins TM, and Laurence Libelo E. 2009. Analysis of perfluorinated chemicals in sludge: Method development and initial results. J. Chromatogr. A 1216 (45):7831–39. doi: 10.1016/j.chroma.2009.09.051. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu J, Tanaka S, and Fujii S. 2009. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in sewage treatment plants. Water Res. 43 (9):2399–408. doi: 10.1016/j.watres.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Zhang K, Huang J, Yu G, Zhang Q, Deng S, and Wang B. 2013. Destruction of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) by Ball Milling. Environ. Sci. Technol. 47 (12):6471–77. doi: 10.1021/es400346n. [DOI] [PubMed] [Google Scholar]


