Abstract
Pseudomonas putida DOT-T1E was isolated as a toluene-tolerant strain. We show that it is also able to grow on high concentrations (up to 17 g/liter [123 mM]) of p-hydroxybenzoate (4HBA). Tolerance to this aromatic carboxylic acid (up to 30 g/liter [217 mM]) is improved by preexposing the cells to low 4HBA concentrations; the adaptation process is caused by the substrate itself rather than by products resulting from its metabolism. The mechanisms of 4HBA tolerance seem to involve increased rigidity of the cell membrane as a result of a decrease in the cis/trans ratio of unsaturated fatty acids. In addition, energy-dependent efflux systems seem to operate in the exclusion of 4HBA from the cell membranes.
Bacteria tolerant to organic solvents have been isolated from polluted and unpolluted environments (2, 7, 14, 30, 35). These microorganisms are of interest for the bioremediation of sites that are heavily polluted with these compounds, for the biotransformation of low-solubility chemicals in water, and for the construction of robust biosensor strains for the in situ detection of pollutants (12, 13, 34). Most of the bacteria that have been isolated so far as being tolerant to p-xylene, styrene, and toluene belong to the fluorescent group of Pseudomonas (2, 14, 30, 35). The molecular basis for solvent tolerance involves changes in the lipid composition of the membrane, including isomerization of cis unsaturated fatty acids to trans, the biosynthesis of saturated lipids versus unsaturated ones (11, 16, 19, 28, 31, 35), and a series of energy-dependent efflux pumps (3, 15, 18–20, 23, 24, 29, 31, 34).
Transformation of toluene into p-hydroxybenzoate (4HBA) is of potential use to the chemical industry for the synthesis of parabens, liquid crystal polymers, and other polymers used in the biosynthesis of plastics and other materials. This biotransformation can be achieved through the toluene mono-oxygenase pathway, in which toluene is first oxidized to p-cresol and the lateral alkyl chain is successively oxidized to yield 4HBA (36). The blockage of 4HBA hydroxylase by disruption of its gene (pobA) allows the synthesis and accumulation of 4HBA from toluene (5). However, this production system uses the Pseudomonas mendocina KR1 strain and may be limited by the toxicity of the solvent and product. This prompted us to analyze 4HBA tolerance in the toluene-tolerant Pseudomonas putida strain DOT-T1E, which thrives in the presence of supersaturating concentrations of toluene (30). In the present study we show that this strain exhibits a high level of tolerance to 4HBA, withstanding concentrations of this hydroxylated aromatic acid up to 30 g/liter and growing at concentrations of 4HBA up to 17 g/liter. This is about two- to threefold higher than the concentration that allows the growth of P. mendocina KR1, a 4HBA-producing strain (A. Ben-Bassat, M. Cattermole, A. A. Gatenby, K. J. Gibson, M. I. Ramos-González, J. L. Ramos, and S. Sariaslani, June 2000, patent application BC1018US NA) or strains able to use 4HBA as the sole carbon source (i.e., Pseudomonas aeruginosa, Acinetobacter sp., and Rhizobium sp.) (4, 8, 26, 27).
The effect of the adaptive response on the tolerance to 4HBA in P. putida DOT-T1E.
Tolerance was measured as the ability of a culture to grow on double diffusion plates containing a linear gradient of 4HBA. With this technique we observed that P. putida DOT-T1E tolerated 15 to 17 g of 4HBA/liter, whereas P. mendocina tolerated about 8 to 10 g/liter on glucose-supplemented M9 minimal medium (1) agar plates.
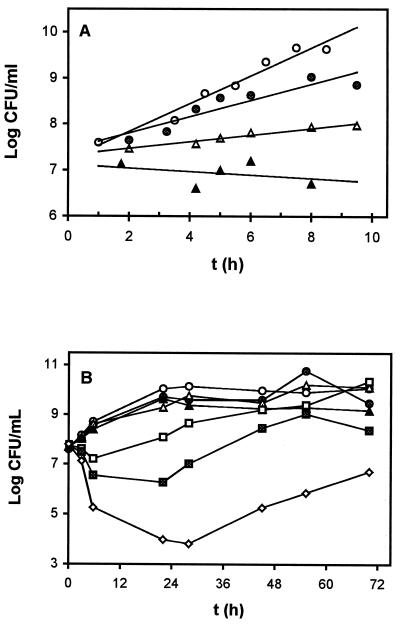
Tolerance was also tested in liquid medium. To this end P. putida DOT-T1E cells were grown on glucose-supplemented M9 minimal medium with and without 4HBA (5 g/liter) and then transferred to culture media with increasing 4HBA concentrations (0 to 24 g/liter). For glucose-grown cells, transfer to a medium with glucose and 4HBA at 0, 6, and 12 g/liter resulted in their immediate growth, with doubling times of 85, 100, and 244 min, respectively (Fig. 1A). A higher concentration of 4HBA (18 g/liter) hampered cell growth (Fig. 1A) and eventually led to a decrease in viable cells as determined by counting CFU per milliliter in Luria-Bertani (LB) medium. After 24 h cells became unrecoverable on LB agar plates (not shown). (LB medium was used for counting because colonies of a reasonable size could be counted after 18 to 24 h of incubation at 30°C.)
FIG. 1.
Effect of 4HBA on the growth of P. putida DOT-T1E. (A) Glucose-supplemented M9 minimal medium was inoculated with DOT-T1E and incubated overnight at 30°C with shaking. Thirty milliliters of the same medium containing 0 (○), 6 (●), 12 (▵), or 18 (▴) g of 4HBA/liter was inoculated with 0.3 ml of the overnight culture. (B) Bacterial cells were pregrown with glucose (0.5%, wt/vol) and 5 g of 4HBA/liter. Aliquots of this culture were used to inoculate glucose-supplemented M9 minimal medium with 0 (○), 6 (●), 12 (▵), 18 (▴), 24 (□), 30 (▪), or 36 (◊) g of 4HBA/liter. Cell growth was followed by counting CFU per milliliter on LB agar plates supplemented with rifampin (10 μg/ml). Each experiment was repeated at least three times. Results of a typical experiment from a single culture are presented. Duplicate measurements of CFU in a single experiment yielded an average standard deviation of 2 to 5%.
P. putida DOT-T1E cells pregrown on 5 g/liter 4HBA tolerated up to 24 g of 4HBA/liter (Fig. 1B), although the higher the concentration tested was, the longer the lag phase was before growth resumed (Fig. 1B). Higher 4HBA concentrations led to an initial decrease in cell viability, which was about 1 and 4 log units at concentrations of 4HBA of 30 and 36 g/liter, respectively, after 24 h of exposure, although growth resumed afterwards (Fig. 1B). We therefore suggest that preexposure of DOT-T1E cells to 4HBA has a beneficial effect on the tolerance to high concentrations of this compound.
The adaptive response of P. putida DOT-T1E to 4HBA was relatively fast: after as little as 1 to 2 h of incubation in the presence of 3 g of 4HBA/liter, cells were able to withstand an 18-g/liter shock without inhibition of viability, in contrast to cells that had not been preadapted to this compound (not shown). If 4HBA-preadapted cells are transferred to nonselective LB medium, reexposure of these cells to 4HBA results in growth inhibition (not shown). This supports the notion of the physiological adaptation of P. putida DOT-T1E cells to high 4HBA concentrations, rather than the appearance of mutant cells exhibiting greater tolerance to 4HBA than the original wild-type strain.
Metabolism of 4HBA in P. putida DOT-T1E proceeds via protocatechuate, which is further metabolized via the β-ketoadipate pathway (10). Other compounds such as ferulic, vanillic, shikimic, and quinic acids are also metabolized through the β-ketoadipate pathway (10, 25). P. putida DOT-T1E failed to use ferulic and vanillic acid as carbon sources, but 10 mM quinic and shikimic acids were used as the sole carbon sources for growth. To determine whether 4HBA tolerance was promoted by itself or by a product resulting from its metabolism, we investigated the ability of quinate and shikimate to induce tolerance to a 4HBA shock. P. putida DOT-T1E cells pregrown on either of these compounds as the sole C source were subjected to 4HBA shocks, and growth and viability were determined. Quinate- and shikimate-grown cells did not tolerate sudden shocks with 18-g/liter 4HBA. These results suggested that the response to 4HBA was a consequence of 4HBA itself and not of a product resulting from its metabolism. A P. putida DOT-T1E pobA mutant was constructed in our laboratory (J. L. Ramos, A. Ben-Bassat, P. Godoy, M. I. Ramos-González, and E. Duque, September 2000, patent application UBC1030US NA). This strain grows on protocatechuate but not on 4HBA, although it did grow in the presence of low 4HBA concentrations with glucose as the C source. We exposed protocatechuate- and glucose-4HBA-grown cells to an 18-g/liter 4HBA shock. We found that the pobA mutant strain tolerated a sudden 4HBA shock if preexposed to a low 4HBA concentration but not when grown on protocatechuate. These results further support the argument that 4HBA per se, rather than metabolites of its assimilation, acts as an inducer of 4HBA tolerance.
Does inhibition of 4HBA uptake via the main 4HBA transporter system have a beneficial effect on 4HBA tolerance?
p-Hydroxybenzoic acid, an apolar molecule, can passively diffuse into the cell, whereas the entry of charged polar 4HBA is an active transport process mediated by the product of the pcaK gene (9). In Acinetobacter sp. all pca structural genes are grouped within a single operon (21). In P. putida the pcaK gene is part of the pcaRKF cluster (9), but each gene is transcribed monocistronically (M. Alaminos and J. L. Ramos, unpublished results). The pcaRKF gene cluster of P. putida DOT-T1E was recovered from a genome library of the strain after colony screening hybridization with pcaR as a probe. A knockout of the pcaK gene was generated in vitro by inserting a cassette containing an Ω interposon that encoded kanamycin resistance. The mutant allele was subcloned in pKNG101 (17) and used to create a pcaK mutant via double homologous recombination, exactly as described before, to obtain other mutants of P. putida (22, 23, 32). The PcaK-deficient mutant did not exhibit chemotaxis to 4HBA, and growth at alkaline pHs with 4HBA as the sole carbon source was significantly retarded with respect to that of the wild type (generation time was 88 and 69 min at pH 7.0 and 120 and 68 min at pH 8.1 for the mutant and the wild-type strain, respectively), in accordance with previous findings in other P. putida strains (9). We then tested 4HBA tolerance of mutant cells on double diffusion plates with a 0- to 25-g/liter linear gradient of this compound at pH 7.0 and pH 8.1. The results obtained were similar to those obtained for the wild-type strain, although the 4HBA tolerance of both strains was slightly enhanced at pH 8.1. It then follows that the PcaK transporter does not play a major role in the 4HBA-adaptative tolerance to 4HBA.
Mechanisms of tolerance to 4HBA.
As mentioned above, cis-to-trans isomerization of phospholipids and extrusion of solvents are key players in toluene tolerance in a number of P. putida strains. We have now tested changes in the phospholipid composition in P. putida DOT-T1E grown with glucose and with different 4HBA concentrations in the culture medium (0, 3, 6, 12, and 15 g/liter). The higher the concentration of 4HBA was, the greater the changes in fatty acid composition were. The most significant changes were (i) the transformation of 9-cis-hexadecenoic acid (C16:1,9 cis) to the corresponding trans isomer instead of it being channeled to render cis-9,10-methylene hexadecanoic acid (C17:cyclopropane) (6) and (ii) isomerization of cis-vaccenic acid (C18:1,11 cis) to the trans isomer (Table 1). As a result, the cis/trans and saturated/unsaturated fatty acid ratios decreased (Table 1). These results suggest that the cell membranes become more rigid in response to this toxic compound in the culture medium.
TABLE 1.
Lipid composition of P. putida DOT-T1E growing in the absence and in the presence of 4HBAa
| Fatty acid | % of total lipid with 4HBA concn (g/liter) of:
|
|||
|---|---|---|---|---|
| 0 | 6 | 12 | 15 | |
| C14:0 | 0.95 | 1.15 | 0.47 | 0.36 |
| C15:0 | 0.12 | 0.04 | 0.06 | 0.05 |
| C16:1,9cis | 9.8 | 15.2 | 12.2 | 14.8 |
| C16:1,9trans | 1.4 | 8.7 | 25.1 | 23.5 |
| C16:0 | 42.8 | 42.7 | 40.9 | 39.9 |
| C17:cyclopropane | 26.7 | 16.1 | 4.5 | 1.6 |
| C17:0 | 0.16 | 0.08 | 0.07 | 0.07 |
| C18:1,11cis vaccenic | 17.0 | 14.0 | 13.1 | 14.6 |
| C18:1,11trans vaccenic | 0.11 | 0.80 | 4.9 | 4.0 |
| C18:0 | 0.92 | 1.1 | 1.1 | 2.2 |
| saturated to unsat | 1.6 | 1.2 | 0.7 | 0.73 |
| cis to trans | 17.8 | 3.1 | 0.84 | 1.1 |
One hundred milliliters of glucose-supplemented M9 minimal medium with the indicated concentration of 4HBA was inoculated with 1 ml of an overnight culture cultivated with glucose. After incubation for 24 h, 30 ml was harvested and washed with glucose-free M9 minimal medium. Lipids were extracted, transesterified, separated by gas chromatography, and identified by mass spectrometry as previously described (37). Experiments were repeated three times, and each sample was analyzed in duplicate. Standard deviations were less than 10% of the average given value.
We analyzed the role of cis-to-trans isomerization on 4HBA tolerance with the DOT-T1E cti-lacking derivative, which was unable to carry out the cis-to-trans isomerization (16). This mutant was more sensitive to toluene than the wild type at temperatures above 37°C; however, its resistance to toluene was comparable to the wild type's at 30°C (16). The cti mutant tolerated the same amount of 4HBA as the wild type on double diffusion plates with a 0- to 25-g/liter linear gradient of this compound at 30 and 37°C. This finding is not surprising because the cis-to-trans isomerization is considered a short-term defense mechanism (minutes after exposure to the offensive agent), and 4HBA's negative effect required a long exposure to the compound as shown in Fig. 1. We, therefore, suggest that the cis-to-trans isomerization of phospholipid fatty acids is part of a general unspecific defense mechanism that operates in response to 4HBA, as it is also the case with other organic compounds (6, 11, 16, 34) and heavy metals (P. Godoy and J. L. Ramos, unpublished data).
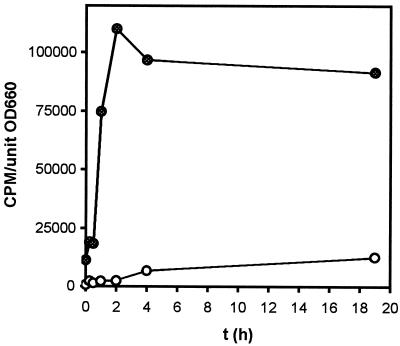
We also tested whether an exclusion system for 4HBA was operative in P. putida DOT-T1E. To prevent the metabolism of 4HBA we used DOT-T1E mutants which do not utilize 4HBA as the sole carbon source, namely, the DOT-T1E pobA mutant and DOT-T1E PhoA5. The former is a mutant with a knocked-out pobA gene (J. L. Ramos et al., patent application), and the latter is a mutant that carries a mini-Tn5-phoA transposon in the exbD gene, which forms part of the exbBD tonB operon (J. L. Ramos et al., patent application). We showed before that neither the exbD mutant nor the pobA mutant metabolize 4HBA, although they grow on protocatechuate as the sole C source. We studied the accumulation of [14C]4HBA by these cells in the mid-log growth phase in M9 minimal medium with glucose plus 5 mM 4HBA. The accumulation of radioactivity was assumed to be due to the persistence of [14C]4HBA in the membranes since 4HBA was not assimilated by the mutant cells. The PhoA-5 mutant cells accumulated up to 75,000 cpm of label per unit of cell density at 660 nm during the first 45 min and then reached a plateau of around 100,000 cpm per unit of cell density at 660 nm (Fig. 2). In contrast, in the DOT-T1E-pobA mutant, 14C accumulation was negligible in the short term and reached a maximum of 10% with respect to the level found in the PhoA-5 strain after 19 h (Fig. 2). In double diffusion plates with a 4HBA gradient, the DOT-T1E-pobA mutant tolerated concentrations of this aromatic carboxylic acid as high as those tolerated by the wild type (15 to 17 g/liter), whereas the DOT-T1E PhoA5 mutant did not tolerate concentrations higher than 6 g of 4HBA/liter. We suggest that a putative efflux system for 4HBA may be active in the PobA mutant and inactive in the exbD mutant.
FIG. 2.
Accumulation of [14C]4HBA in P. putida cells. Strains used were the DOT-T1E pobA-lacking derivative (○) or DOT-T1E PhoA5 (●). Mid-log-phase cells grown on 0.5% (wt/vol) glucose and 5 mM 4HBA-supplemented M9 minimal medium were washed in M9 buffer and concentrated in 2 ml of 0.3% (wt/vol) glucose-supplemented M9 minimal medium at a final turbidity of 2.5 at 660 nm. The cells were incubated for 10 min at 30°C under shaking. One and a half microcuries of [14C]4HBA (specific activity, 11.5 mCi/mmol) was added to the cells in 2 ml of the same medium. Aliquots of 400 μl were filtered through a Millipore type HA 0.45-μm-pore size filter at the indicated times. The membranes were washed with 5 mM 4HBA-supplemented M9 buffer. The counts per minute was measured with a scintillation counter (Packard Radiometer). The assays were performed at 30°C with shaking. Each experiment was repeated at least twice. Results of a typical experiment from a single culture are presented.
Toluene in P. putida DOT-T1E involves three efflux pumps, called TtgABC, TtgDEF, and TtgGHI (33). Mutants in the TtgABC and TtgGHI pumps are hypersensitive to toluene. Mutants in the TtgABC and TtgDEF efflux pumps are slightly more sensitive to 4HBA than the wild type and than a mutant in the TtgGHI efflux pump, and this effect was more evident at pH 8.1. The wild type and the mutant TtgGHI tolerated 4HBA up to 17 g/liter, whereas the mutants in the TtgABC and TtgDEF efflux pumps tolerated only up to 12 to 13 g/liter. Although the two above-mentioned efflux pumps may be involved in 4HBA extrusion, other extrusion systems may also remove 4HBA from the cell membranes. However, conclusive evidence that other efflux pumps extrude 4HBA requires that strains bearing mutations in the genes that encode for other proteins involved in the removal of 4HBA be isolated.
Acknowledgments
This study was supported by a grant from DuPont de Nemours.
We thank M. J. Campos for technical assistance and Karen Shashok and Carmen Lorente for checking the use of English in the manuscript.
REFERENCES
- 1.Abril M A, Michán C, Timmis K T, Ramos J L. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J Bacteriol. 1989;171:6782–6790. doi: 10.1128/jb.171.12.6782-6790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aono R, Ito M, Inoue A, Horikoshi K. Isolation of novel toluene-tolerant strain of Pseudomonas aeruginosa. Biosci Biotechnol Biochem. 1992;56:145–146. [Google Scholar]
- 3.Aono R, Tsukagoshi N, Yamamoto M. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance level of Escherichia coli K-12. J Bacteriol. 1998;180:938–944. doi: 10.1128/jb.180.4.938-944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canovas J L, Stanier R Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. Eur J Biochem. 1967;1:289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- 5.Chen, K. K., and R. L. Gerlak. June 1998. Production of p-hydroxybenzoate using pobA Pseudomonas strains which have a toluene degradation pathway and do not use p-hydroxybenzoate. Patent WO-9856920.
- 6.Cronan J E, Jr, William D N, Batchelor J G. Studies on the biosynthesis of cyclopropane fatty acids in Escherichai coli. Biochim Biophys Acta. 1974;348:63–75. doi: 10.1016/0005-2760(74)90093-9. [DOI] [PubMed] [Google Scholar]
- 7.Cruden D L, Wolfram J H, Rogers R D, Gibson D T. Physiological properties of a Pseudomonas strain which grows with p-xylene in a two-phase (organic-aqueous) medium. Appl Environ Microbiol. 1992;58:2723–2729. doi: 10.1128/aem.58.9.2723-2729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golovleva L A, Golovlev E L, Panchak N V, Ganbarov K G. Variability in the enzyme properties of Pseudomonas aeruginosa strain 2x oxidizing p-xylene. Biol Bull Acad Sci USSR. 1979;6:459–463. [PubMed] [Google Scholar]
- 9.Harwood C S, Nichols N N, Kim M-K, Ditty J L, Parales R E. Identification of the pcaKRF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J Bacteriol. 1994;176:6479–6488. doi: 10.1128/jb.176.21.6479-6488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harwood C S, Parales R E. The β-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 11.Heipieper H J, Weber F J, Sikkema J, Keweloh H, de Bont J A M. Mechanisms behind resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994;12:409–415. [Google Scholar]
- 12.Huertas M J, Duque E, Marqués S, Ramos J L. Survival in soil of different toluene-degrading Pseudomonas strains after solvent shock. Appl Environ Microbiol. 1998;64:38–42. doi: 10.1128/aem.64.1.38-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huertas M J, Duque E, Rosselló-Mora R, Mosqueda G, Godoy P, Christensen B, Molin S, Ramos J L. Tolerance to organic solvents by soil bacteria. Environ Sci Technol. 2000;34:3395–3400. [Google Scholar]
- 14.Inoue A, Horikoshi K. A Pseudomonas thrives in high concentrations of toluene. Nature. 1989;388:264–266. [Google Scholar]
- 15.Isken S, de Bont J A M. Active efflux of toluene in a solvent-resistant bacterium. J Bacteriol. 1996;178:6056–6058. doi: 10.1128/jb.178.20.6056-6058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junker F, Ramos J L. Involvement of the cis/trans isomerase Cti in solvent resistance of Pseudomonas putida DOT-T1E. J Bacteriol. 1999;181:5693–5700. doi: 10.1128/jb.181.18.5693-5700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaniga K, Delar I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene in Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 18.Kieboom J, Dennis J J, de Bont J A M, Zylstra G J. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem. 1997;273:85–91. doi: 10.1074/jbc.273.1.85. [DOI] [PubMed] [Google Scholar]
- 19.Kieboom J, Dennis J J, Zylstra G J, de Bont J A M. Active efflux of organic solvents by Pseudomonas putida S12 is induced by solvents. J Bacteriol. 1998;180:6769–6772. doi: 10.1128/jb.180.24.6769-6772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K, Lee S, Lee K, Lim D. Isolation and characterization of toluene-sensitive mutants from toluene-resistant bacterium Pseudomonas putida GM73. J Bacteriol. 1998;180:3692–3696. doi: 10.1128/jb.180.14.3692-3696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowalchuk G A, Hartnett G B, Benson A, Houghton J E, Ngai K L, Ornston L N. Contrasting patterns of evolutionary divergence within the Acinetobacter calcoaceticus pca operon. Gene. 1994;146:23–30. doi: 10.1016/0378-1119(94)90829-x. [DOI] [PubMed] [Google Scholar]
- 22.Llamas M A, Ramos J L, Rodríguez-Herva J J. Mutations in each of the tol genes Pseudomonas putida reveal that they are critical for maintenance of outer membrane stability. J Bacteriol. 2000;182:4764–4772. doi: 10.1128/jb.182.17.4764-4772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosqueda G, Ramos J L. A set of genes encoding a second toluene efflux system in Pseudomonas putida DOT-T1E is linked to the tod genes for toluene metabolism. J Bacteriol. 2000;182:937–943. doi: 10.1128/jb.182.4.937-943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ornston L N, Stanier R Y. The conversion of catechol and procatechuate to β-ketoadipate by Pseudomonas putida J. Biol Chem. 1966;241:3776–3786. [PubMed] [Google Scholar]
- 26.Parke D. Supraoperonic clustering of pca genes for catabolism of the phenolic compound protocatechuate, in Agrobacterium tumefaciens. J Bacteriol. 1995;177:3808–3817. doi: 10.1128/jb.177.13.3808-3817.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parke D, Rynne F, Glenn A. Regulation of phenolic catabolism in Rhizobium leguminosarum biovar trifolii. J Bacteriol. 1991;173:5546–5550. doi: 10.1128/jb.173.17.5546-5550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinkart H C, White D C. Phospholipid biosynthesis and solvent tolerance in Pseudomonas putida strains. J Bacteriol. 1997;179:4219–4226. doi: 10.1128/jb.179.13.4219-4226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos J L, Duque E, Godoy P, Segura A. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J Bacteriol. 1998;180:3323–3329. doi: 10.1128/jb.180.13.3323-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos J L, Duque E, Huertas M J, Haïdour A. Isolation and expansion of the catabolic potential of a Pseudomonas putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J Bacteriol. 1995;177:3911–3916. doi: 10.1128/jb.177.14.3911-3916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos J L, Duque E, Rodríguez-Herva J J, Godoy P, Haïdour A, Reyes F, Fernández-Barrero A. Mechanisms for solvent tolerance in bacteria. J Biol Chem. 1997;272:3887–3890. doi: 10.1074/jbc.272.7.3887. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Herva J J, Ramos J L. Characterization of an OprL null mutant of Pseudomonas putida. J Bacteriol. 1996;178:5836–5840. doi: 10.1128/jb.178.19.5836-5840.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rojas A, Duque E, Mosqueda G, Golden G, Hurtado A, Ramos J L, Segura A. Three efflux pumps are required to provide efficient tolerance to toluene in Pseudomonas putida DOT-T1E. J Bacteriol. 2001;183:3967–3973. doi: 10.1128/JB.183.13.3967-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segura A, Duque E, Mosqueda G, Ramos J L, Junker F. Multiple responses of gram-negative bacteria to organic solvents. Environ Microbiol. 1999;1:191–198. doi: 10.1046/j.1462-2920.1999.00033.x. [DOI] [PubMed] [Google Scholar]
- 35.Weber F J, Ooijkaas L P, Schemen R M W, Hartmans S, de Bont J A M. Adaptation of Pseudomonas putida S12 to high concentrations of styrene and other organic solvents. Appl Environ Microbiol. 1993;59:3502–3504. doi: 10.1128/aem.59.10.3502-3504.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright A, Olsen R H. Self-mobilization and organization of the genes encoding the toluene metabolic pathway of Pseudomonas mendocina KR1. Appl Environ Microbiol. 1994;60:235–242. doi: 10.1128/aem.60.1.235-242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]