Abstract
This study evaluated HCV treatment initiation among people who inject drugs (PWID) following an intervention of campaign days involving peer connection, point-of-care HCV RNA testing, and linkage to nursing support. ETHOS Engage is an observational cohort study of PWID attending 25 drug treatment clinics and needle and syringe programs in Australia (May 2018–September 2019). Point-of-care results were provided to the nurse, facilitating confirmatory testing and treatment. The study aimed to evaluate treatment uptake and factors associated with treatment at 24 months post-enrolment. There were 317 people with current HCV infection and eligible for treatment (median age 43, 65% male, 15% homeless, 69% receiving opioid agonist treatment, 70% injected in last month). Overall, 15% (47/317), 27% (85/317), 38% (120/317), and 49% (155/317) of people with current HCV infection had initiated treatment at 3-, 6-, 12-, and 24-months following testing, respectively. Homelessness (adjusted hazard ratio (aHR): 0.40; 95% confidence interval: 0.23, 0.71) and incarceration in the past 12 months (vs. never, aHR:0.46; 0.28, 0.76) were associated with decreased treatment initiation in the 24 months post-enrolment. This testing campaign intervention facilitated HCV treatment uptake among PWID. Further interventions are needed to achieve HCV elimination among people experiencing homelessness or incarceration.
Keywords: direct-acting antiviral era, Hepatitis C virus elimination, Hepatitis C virus infection, Hepatitis C virus treatment, people who inject drugs
1. Introduction
As part of the Global Health Sector Strategy on Viral Hepatitis 2016–21, the World Health Organisation aims to eliminate hepatitis C (HCV) as a major public health threat by 2030 [1]. Reaching the target of treating 80% of eligible people diagnosed with chronic HCV requires global and targeted efforts to improve access to testing and treatment services [1]. Globally, an estimated 6.1 million people who inject drugs were living with HCV in 2019 [2]. The criminalisation of people who use drugs increases the stigma around drug use and HCV [3], which may dissuade people from initiating HCV treatment [4]. People who inject drugs may receive suboptimal care due to multiple barriers including stigma, housing, criminalisation, and burdensome treatment pathways [5]. Novel interventions are needed to ensure PWID can access HCV testing and treatment [1]. Australia is uniquely placed to achieve HCV elimination given that highly curative direct-acting antiviral (DAA) therapy has been available since March 2016, regardless of virus acquisition and with no restrictions based on drug and alcohol use [6]. The advent of unrestricted access to DAAs is promising, yet recent work demonstrates that inequities in treatment uptake among PWID persist [7,8].
Complex HCV diagnostic pathways require multiple visits and can be difficult to navigate for people seeking HCV care [9]. Embedding HCV testing and treatment within services regularly used by PWID can increase access to testing and opportunities to initiate treatment [10]. Campaign days, where staff are deployed to screen large numbers of people as part of an event, have been used globally and show promise in improving testing and linkage to care [11,12]. Studies have evaluated HCV treatment uptake among PWID [7,8], but there is a need for research evaluating novel models of care in the DAA era [13]. Rapid diagnosis and treatment of HCV can reduce onward transmission and prevent progression of liver disease [14]. Understanding factors associated with delayed treatment uptake can highlight sub-populations of people who inject drugs who face greater barriers to care and can also facilitate the design of interventions to achieve HCV elimination.
The ETHOS Engage Study recruited a national cohort of people who inject drugs from opioid agonist treatment and needle and syringe programs during an era of ongoing provision of unrestricted HCV DAA treatment. At baseline, 24% of participants had current HCV infection and current infection was associated with homelessness, recent incarceration, and daily drug injection [7]. The study also found that 66% of people with previous chronic or current HCV had ever been treated [7].
This study extends the previously published observational study [7], to longitudinally evaluate the interventional component of ETHOS Engage which integrated peer connection, point-of-care HCV RNA testing, and nurse-led linkage to care delivered through screening and linkage to care campaign days. All the components were integrated on the campaign day to provide engagement with testing and linkage to care for HCV within a service providing opioid agonist treatment or NSP (as its primary function). The primary aim of this study was to evaluate treatment uptake among people diagnosed with current HCV infection following the intervention. The secondary aims were to evaluate factors associated with treatment uptake and report the estimated proportion of people with previous chronic or current HCV who initiated treatment in the 24 months following the intervention.
2. Materials and Methods
2.1. Study Design, Setting, and Participants
The ETHOS Engage Study is an observational cohort study [15]. An intervention is embedded within the study, aiming to enhance HCV screening, diagnosis, and linkage to care. Participants were recruited between May 2018–September 2019 from drug treatment clinics (n = 21) and needle and syringe programs (NSPs) (n = 4) in four Australian States: New South Wales (n = 17), Queensland (n = 4), South Australia (n = 2), and Western Australia (n = 2).
Inclusion criteria were informed consent, ≥18 years of age, history of injecting drug use, and either injecting drug use in the previous six months or current opioid agonist treatment (OAT). Pregnant women were excluded given that FibroScan® (Echosens, Paris, France) was contraindicated at time of study protocol approval. The study protocol was approved by the Human Research Ethics Committees at St Vincent’s Hospital, Sydney and the Aboriginal Health and Medical Research Council (HREC Ref: HREC/17/SVH/113).
2.2. Procedures
ETHOS Engage campaign days were advertised using posters (Supplementary Figure S1), cards distributed with injecting equipment, and by word of mouth. Recruitment spanned one to five days at each site and included a team of university staff, peer workers specialised in either HCV or injecting drug use, and clinic personnel. The campaign days were embedded within primary operation of drug treatment clinics and NSPs, without appointments, allowing for opportunistic engagement of participants attending the site for standard services.
The interventional component of ETHOS Engage consisted of multiple stages. People attending the clinic were approached by peer workers who informed them about the study, providing transparent information about the implications of study participation. On each campaign day, one peer was working on-site to initiate the recruitment of study participants and to provide education about point-of-care HCV testing and treatment. The peer worker offered the opportunity for people to discuss any HCV-related concerns before agreeing to participate in the study. If the person was eligible to participate and gave informed consent, a 100 µL finger-stick capillary whole-blood was collected to test for HCV RNA using the point-of-care Xpert HCV Viral Load Fingerstick Assay (Cepheid, Sunnyvale, CA, USA; lower limit of quantification 100 IU/mL, upper limit of quantification 108 log10 IU/mL; 100% sensitivity, 100% specificity) [9]. Baseline data were collected by participants using a self-administered computer tablet-based questionnaire. This data included demographics, behavioural risk, and HCV history (testing, infection status, and treatment). Liver fibrosis stage was assessed using transient elastography (FibroScan®, Echosens, Paris, France) with a lower and upper detection limit of 2.5 and 75 kPa, respectively. Participants then underwent a brief consultation with clinical staff. Participation was compensated with a shopping voucher (AUD$30) for their time and effort in participating.
At the time of the study, the Australian Therapeutics Goods Administration had not yet approved the Xpert HCV Viral Load Fingerstick assay and so HCV RNA test results could not be provided to participants in the same visit. Results were returned to clinics or programs after in-house quality assurance checks. Clinic or service staff were asked to facilitate confirmatory HCV testing via venous blood draw (not necessarily on the same day as point-of-care testing), communicate results from confirmatory HCV testing, and facilitate treatment initiation. Clinics employed nurse-led linkage to care but this was not standardised and so strategies employed by the clinics were heterogenous.
Follow-up data were collected at the site level. Participants were not asked to attend for follow-up visits as part of the study and data were collected during routine clinic visits. A standardised online case report form was completed by clinic nurses based on a medical record review at 12- and 24-months post-enrolment. This was completed for each participant who had HCV infection at the time of enrolment, including data on HCV treatment initiation and loss to follow-up.
2.3. Outcomes
The primary outcome was HCV treatment initiation following diagnosis of current HCV infection in ETHOS Engage (detected with an HCV RNA assay). Treatment initiation was assessed at 3-, 6-, 12-, and 24-months following detection of current HCV infection. The secondary outcome was time to initiation of HCV treatment within the 24-month period following diagnosis with HCV infection in ETHOS Engage.
2.4. Statistical Analysis
Demographic and behavioural factors hypothesised to be associated with HCV treatment initiation were determined using previously published results from ETHOS Engage [7,8] and included: (i) age at survey; (ii) gender (male, female, other); (iii) Aboriginal or Torres Strait Islander; (iv) homelessness (when asked where they had spent the majority of nights in the past six months, participants responded no usual residence/shelter/squat); (v) currently receiving OAT (no/yes); (vi) incarceration history (never/more than 12 months ago/in last 12 months); (vii) injection drug use within the last month (no/yes); (viii) hazardous alcohol consumption (defined by AUDIT-C [16]); (ix) liver fibrosis stage (liver stiffness measurement <7.0 kpa no significant fibrosis [F0/F1] or ≥7.0 kpa significant fibrosis [≥F2]) [17]. Participants with no FibroScan score or invalid results were classified as “Unknown”, and (x) if ever diagnosed with HCV self-report at enrolment (no/yes, never treated/yes, ever treated).
People who had initiated HCV treatment in the 12 weeks prior to enrolment, with no further treatment initiation recorded post-enrolment, were not considered “at-risk” and were excluded from treatment uptake analyses. Observation time for treatment initiation commenced on date of ETHOS Engage enrolment (i.e., date of HCV RNA test) and ended on date of HCV treatment prescription, date of death, date reported by clinic site as lost to follow-up, or 24 months post enrolment in ETHOS Engage, whichever occurred first. Participants who were reported as lost to follow-up who had missing information on date of loss to follow-up, were censored at the time of the reporting. The cumulative proportion of participants who initiated treatment at each timepoint was reported along with 95% confidence intervals, using the total sample as the denominator. Kaplan Meier estimates were used to report cumulative probability of HCV treatment initiation over time, with 95% confidence intervals. Cox regression models were used to identify factors associated with time to HCV treatment initiation, giving crude and adjusted hazard ratios (crude HR and adjusted HR). Variables with p value < 0.1 in the univariate Cox regression models were retained in the multivariate model.
Evidence was based on the self-reported history of HCV treatment among participants with either previous (self-reported history of HCV treatment) or current HCV infection (in participants who have been treatment eligible) [8]). All analyses were conducted using Stata 14.0 (StataCorp, College Station, TX, USA).
3. Results
3.1. Sample Characteristics
Overall, 1443 participants were recruited between May 2018 and September 2019, of whom 1388 had a HCV RNA point-of-care test result (Supplementary Figure S2). Among people with a HCV RNA test result, 24% (n = 331) had current HCV infection. People reporting HCV treatment in the 12 weeks prior to enrolment who were RNA positive and had no post-enrolment treatment initiation were excluded (4%, n = 14), leaving 317 people with current HCV infection eligible for treatment (Supplementary Table S1). Compared to people without current HCV infection, people with current HCV infection had a higher proportion of homelessness (15% vs. 9%, p = 0.002), incarceration > 12 months before enrolment (61% vs. 48%, p < 0.001), to have injected drugs in the last month (70% vs. 61%, p = 0.006), and to have advanced liver disease (FibroScan; liver stiffness measurement > 7.0 kpa; 36% vs. 19%, p < 0.001) (Supplementary Table S1).
3.2. Prevalence and Characteristics of HCV Treatment Initiation at Three Months Post-Enrolment
Of 317 people with current HCV infection eligible for treatment, 15% (n = 47, 95% confidence interval (CI): 11–19%) had initiated treatment at three months. At three months post-enrolment, treatment initiation was lower among people who were homeless (4% vs. 17%, p = 0.002), people incarcerated in last 12 months (vs. more than 12 months ago or no incarceration history; 8% vs. 16% and 19%, p = 0.165), people who injected drugs in the last month (14% vs. 16%, p = 0.792), and people who had never previously been diagnosed with HCV (8% vs. 15% amongst those diagnosed but never treated and 21% among those previously treated, p = 0.150) (Table 1). Overall, 15% (47/317, 95% CI: 11–19%), 27% (85/317, 95% CI 22–32%), 38% (120/317, 95% 32–43%), and 49% (155/317, 95% CI 43–55%) of people with current HCV infection had initiated treatment at 3-, 6-, 12-, and 24-months following testing, respectively.
Table 1.
Baseline characteristics of people with HCV infection, by treatment initiation status three months following HCV diagnosis in ETHOS Engage (n = 317).
| Characteristic | Current HCV Infection | No Treatment Initiation within Three Months or Lost to Follow-Up | Initiated Treatment Three Months Post Diagnosis | ||
|---|---|---|---|---|---|
| n (col%) | n (% of Current HCV Infection) | n (% of Current HCV Infection) | p Value | ||
| Total (N) | 317 (100%) | 270 (85%) | 47 (15%) | ||
| Age at enrolment | <45 | 184 (58%) | 159 (86%) | 25 (14%) | |
| ≥45 | 133 (42%) | 111 (83%) | 22 (17%) | 0.465 | |
| Gender | Male | 205 (65%) | 177 (86%) | 28 (14%) | |
| Female | 110 (35%) | 91 (83%) | 19 (17%) | ||
| Other | 2 (1%) | 2 (100%) | 0 (0%) | 0.580 | |
| Aboriginal or Torres Strait Islander | No | 241 (76%) | 204 (85%) | 37 (15%) | |
| Yes | 76 (24%) | 66 (87%) | 10 (13%) | 0.639 | |
| Homeless | No | 268 (85%) | 223 (83%) | 45 (17%) | |
| Yes | 49 (15%) | 47 (96%) | 2 (4%) | 0.021 | |
| Currently receiving OAT | No | 98 (31%) | 80 (82%) | 18 (18%) | |
| Yes | 219 (69%) | 190 (87%) | 29 (13%) | 0.235 | |
| Incarceration history | Never | 73 (23%) | 59 (81%) | 14 (19%) | |
| More than 12 months ago | 172 (54%) | 145 (84%) | 27 (16%) | ||
| In last 12 months | 72 (23%) | 66 (92%) | 6 (8%) | 0.165 | |
| Recency of injecting | More than a month ago | 96 (30%) | 81 (84%) | 15 (16%) | |
| Within last month | 221 (70%) | 189 (86%) | 32 (14%) | 0.792 | |
| Hazardous alcohol consumption † | No | 188 (59%) | 160 (85%) | 28 (15%) | |
| Yes | 127 (40%) | 108 (85%) | 19 (15%) | 0.839 | |
| Fibrosis -Fibroscan result (kpa) | <7.0 | 184 (58%) | 154 (84%) | 30 (16%) | |
| >7.0 | 115 (36%) | 101 (88%) | 14 (12%) | ||
| Unknown | 18 (6%) | 15 (83%) | 3 (17%) | 0.604 | |
| No | 61 (19%) | 56 (92%) | 5 (8%) | ||
| Diagnosed with HCV prior to study | Yes, never treated | 204 (64%) | 173 (85%) | 31 (15%) | |
| Yes, ever treated | 52 (16%) | 41 (79%) | 11 (21%) | 0.150 | |
† Excluding people who did not identify as men or women (n = 2). Acronyms–OAT: opioid agonist treatment, HCV: hepatitis C virus. p value based on chi-square test of differences.
3.3. Factors Associated with Time to HCV Treatment Initiation
For people diagnosed with current HCV infection, the median follow-up was 365 days (IQR: 171–395 days). For people who initiated HCV treatment at 24 months post-enrolment (n = 155), the median time between testing and treatment initiation was 169 days (IQR: 73–335 days) or around six months.
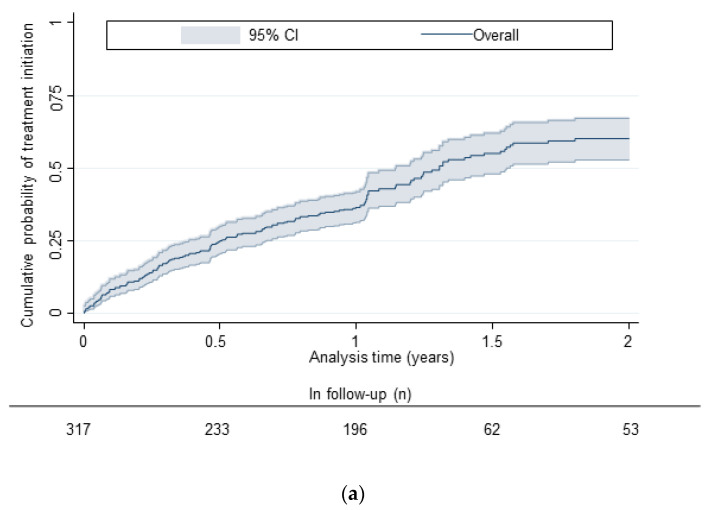
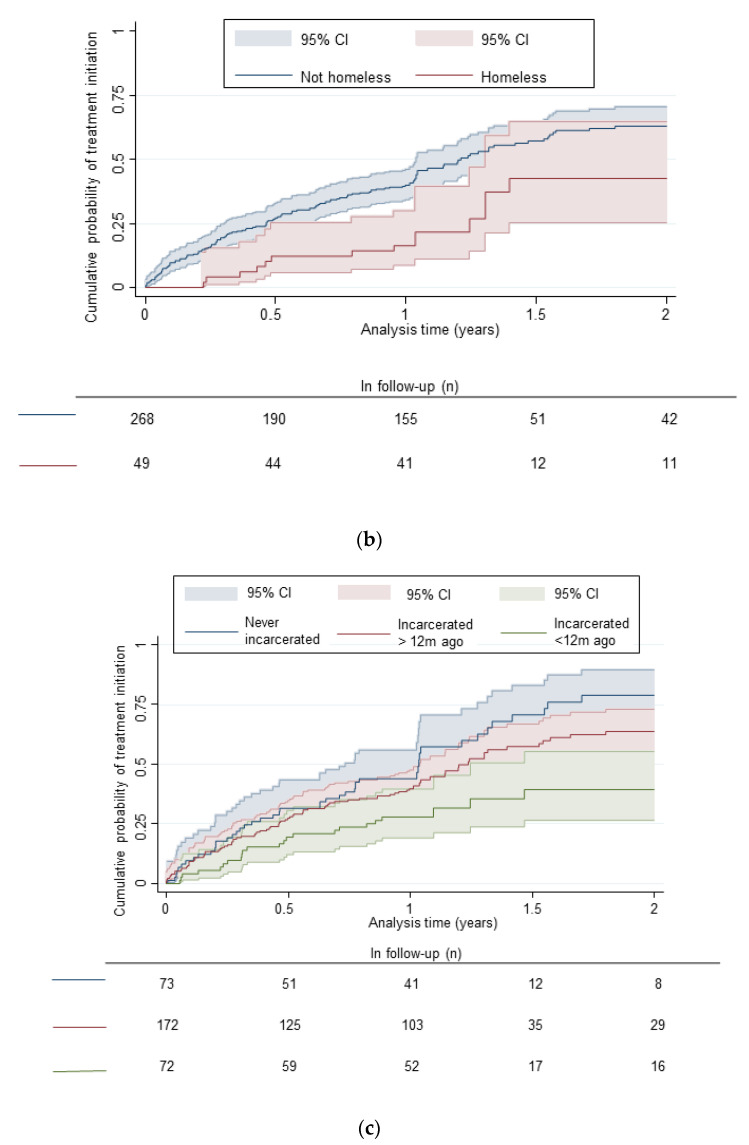
In the Kaplan Meier estimates, among all those diagnosed with current HCV infection, the cumulative probabilities of HCV treatment initiation were 15% (95% CI: 12%–20%), 27% (95% CI: 22%–32%), 38% (95% CI: 33%–44%), and 62% (95% CI: 55%–69%) at 3-, 6-, 12-, and 24-months following testing, respectively (Figure 1, Supplementary Table S2). Cumulative probability of treatment initiation by 24 months post-enrolment was lower in people with a history of incarceration (vs. no history of incarceration) and people who were homeless (vs. not) (Figure 1, Supplementary Table S2).
Figure 1.
(a) Kaplan-Meier curves depicting estimated time (years) to DAA treatment initiation among people diagnosed with current HCV infection in ETHOS Engage overall. (b) Kaplan-Meier curves depicting estimated time (years) to DAA treatment initiation among people diagnosed with current HCV infection in ETHOS Engage by homelessness. (c) Kaplan-Meier curves depicting estimated time (years) to DAA treatment initiation among people diagnosed with current HCV infection in ETHOS Engage by history of incarceration.
After adjusting for age and Aboriginal and Torres Strait Islander identification, homelessness (adjusted HR 0.40, 95% CI 0.23–0.71) and incarceration in the 12 months prior to enrolment (vs. no history of incarceration, adjusted HR 0.46, 95% CI 0.28–0.76) were associated with longer time to HCV treatment initiation (Table 2).
Table 2.
Cox regression–factors associated with treatment initiation at 24 months post-diagnosis (n = 317).
| Characteristic | Person-Years Observation | Incidence Rate | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95%CI) | |
|---|---|---|---|---|---|
| Age at enrolment | Year | 1.30 (0.95–1.78) | 1.00 (0.98–1.01) | ||
| Gender | Male | 199 | 0.48 | Ref | |
| Female | 104 | 0.54 | 1.12 (0.80–1.55) | ||
| Transgender | 1 | 2.00 | 2.88 (0.71–11.73) | ||
| Aboriginal or Torres Strait Islander | No | 226 | 0.55 | Ref | Ref |
| Yes | 79 | 0.37 | 0.67 (0.45–1.00) | 0.66 (0.44–0.99) | |
| Homeless | No | 247 | 0.57 | Ref | Ref |
| Yes | 57 | 0.23 | 0.41 (0.23–0.72) | 0.40 (0.23–0.71) | |
| Currently receiving OAT | No | 94 | 0.41 | Ref | |
| Yes | 211 | 0.54 | 1.29 (0.90–1.86) | ||
| Incarceration history | Never | 64 | 0.70 | Ref | Ref |
| More than 12 months ago | 164 | 0.52 | 0.77 (0.54–1.10) | 0.83 (0.58–1.19) | |
| In last 12 months | 77 | 0.30 | 0.44 (0.27–0.73) | 0.46 (0.28–0.76) | |
| Recency of injecting | More than a month ago | 98 | 0.58 | Ref | |
| Within last month | 206 | 0.47 | 0.84 (0.61–1.17) | ||
| Hazardous alcohol consumption † | No | 183 | 0.49 | Ref | |
| Yes | 120 | 0.52 | 1.07 (0.78–1.48) | ||
| Fibrosis-Fibroscan result (kpa) | <7.0 | 171 | 0.54 | Ref | |
| >7.0 | 116 | 0.46 | 0.84 (0.60–1.17) | ||
| Unknown | 18 | 0.44 | 0.82 (0.40–1.69) | ||
† Excluding people who did not identify as men or women (n = 2). Acronyms–OAT: opioid agonist treatment, HCV: hepatitis C virus, CI: confidence interval.
3.4. Progress towards Elimination Targets
Among everyone with a valid HCV RNA result at enrolment, 57% (788/1388) had evidence of previous or current HCV infection. Of those, 66% (520/788) reported having ever initiated HCV treatment. Adding people who initiated treatment in the 24 months post-enrolment, a total of 83% (652/788) of those ever eligible initiated HCV treatment.
4. Discussion
An intervention consisting of screening campaign days with peer connection, point-of-care HCV RNA testing, and nurse-led linkage to care, resulted in 15% of people with current HCV infection at enrolment initiating treatment within three months (27% at six months and 49% at 24 months). When combined with pre-intervention treatment, a total of 83% of participants with previous or current chronic HCV infection initiated HCV treatment, advancing progress to WHO HCV elimination targets [1]. The analysis of treatment uptake in the 24 months post-enrolment reveals disparities with lower treatment uptake observed among people who are homeless or recently incarcerated. These findings are consistent with previous analyses of factors associated with treatment uptake from ETHOS Engage [7,8], highlighting the need for tailored support to be made available in services which are frequently used by these populations.
Fingerstick point-of-care HCV RNA testing identified 317 people with current HCV infection who were eligible for treatment, with a cumulative probability of 27% initiating HCV treatment within six months of enrolment. This is comparable with data from New South Wales, Australia, where treatment uptake among people with drug dependence was estimated at 27% in the six months following diagnosis [18]. This suggests the HCV testing campaigns in ETHOS Engage facilitated an increased reach in HCV testing, by providing testing for people who would not have received it had this study not been performed. As such, it is not surprising that the proportion with treatment uptake was similar to standard of care given most people had to come back for multiple visits to either have confirmatory testing or treatment. The higher treatment uptake in the period immediately following diagnosis indicates the utility of running testing campaigns at frequent intervals, either annually or biannually. Using the fingerstick HCV RNA assay in the ETHOS Engage study allowed testing to take place on-site without venepuncture. The lack of Australian Therapeutics Goods Administration approval at the time of the study prevented results from being communicated on the same day, and the requirements to assess genotypes before initiating treatment obliged people to later have venepuncture for confirmatory testing. Studies providing HCV RNA point-of-care testing and same-day results to people who inject drugs have reported higher proportions of people initiating treatment in a needle and syringe program (74%) [19], medically supervised injecting sites (89%) [20], mobile outreach models (74%) [21], and prison (93%) [22]. This demonstrates the importance of offering single-visit testing and treatment to considerably increase HCV treatment uptake among populations of people who inject drugs. In the second recruitment wave of ETHOS Engage (2019–2021), the Xpert HCV Viral Load Fingerstick assay had been approved by the Therapeutics Goods Administration, so the results of point-of-care HCV RNA testing could be provided directly to participants and should lead to increased treatment uptake. The need for confirmatory testing via venepuncture will no longer be required, which may increase treatment uptake given a preference for finger-stick testing among people who inject drugs [23]. Follow-ups to assess treatment initiation after enrolment in the second recruitment wave of ETHOS Engage is ongoing.
Homelessness was associated with reduced HCV treatment uptake, consistent with previous studies [7,8]. Among people who were homeless, the cumulative probability of HCV treatment uptake was 42% at 24 months, compared to 62% overall. There are multiple mechanisms by which homelessness might impact treatment uptake including providers considering homelessness a sign of “unmanageability” [5], and people considering treatment initiation as less urgent than other, competing priorities [24,25]. Although opioid agonist treatment services and NSPs do not require people to be housed to access services, people experiencing homelessness may still face increased structural barriers to access [26]. Stable housing reduces the risk of HCV transmission [27] and removes stressors to allow people to prioritise health and wellbeing [28]. Some interventions have attempted to mitigate the effects of homelessness with regards to treatment uptake, by providing flexible appointments [26] or mobile testing and treatment [29]. A mobile unit employing a same day ‘test and treat’ model for a cohort in which the majority were experiencing homelessness, reported 77% initiating treatment [30]. Although there is weak evidence for the effectiveness of financial incentives to broadly increase HCV treatment uptake [13], high willingness to partake in such studies [31] indicates the need for further investigation. Less investigated are the additional system level barriers to providing incentives for treatment, such as provider or clinic reluctance.
History of incarceration was associated with reduced HCV treatment initiation. Other Australian studies found, in adjusted analyses, recent incarceration was not associated with treatment uptake [7,8,18] or was associated with increased treatment uptake [32]. Availability of HCV testing and treatment in Australian prisons and implementation of initiatives for treatment scale up in prison [33] may be improving treatment initiation among incarcerated people. In the current study, lower treatment uptake amongst people who have been incarcerated may be due to missing information on follow-up in this population, possibly due to reincarceration. Nevertheless, subgroups of people with recent history of incarceration may have poorer treatment uptake, such as people serving sentences that are shorter than the duration of treatment. Housing, mental health, social support, and economic hardship have been identified as structural factors which impact health service access upon release from prison [34] and likely influence HCV treatment initiation. Despite access to HCV care in Australian prisons, criminalisation and imprisonment is damaging to health and wellbeing. Criminalisation exacerbates the stigma experienced by people who inject drugs [3], which diminishes access to healthcare such as HCV treatment [4]. There is a need for more research to understand the period post-release to ensure people are supported to initiate or complete HCV treatment. There is evidence that patient navigation and transitional care coordination can mitigate structural barriers to initiating treatment for people being released from prison [35]. Decarceration as a public health strategy has had increased attention during the COVID-19 pandemic [36] and could improve outcomes for other infectious diseases including HCV.
This study has limitations. At the time of study, the test was not approved for diagnosis and so results were not provided to participants on the day of test, therefore not realising the full potential of HCV RNA point-of-care testing. Nevertheless, the incorporation of peers and the relatively quick return of results to the clinic represented an improvement upon current standard of care. Several of the variables in the Cox regression model are time varying by nature (e.g., homelessness, OAT, recent injecting drug use) but, because of the design of the study as an observational cohort, they are included in the model as fixed variables. In Wave 2, ETHOS Engage recruited a similar population which suggests minimal change in these variables over time [8]. There was no control group in this study, making it difficult to assess what outcomes would have been in the absence of this intervention. The study follow-up only collected data on treatment initiation, not cure or reinfection, which would likely have an impact on the achievement of the WHO target to treat 80% of persons diagnosed with chronic HCV infection. Recruitment mainly took place within opioid agonist treatment settings, potentially biasing our cohort to people who were already engaged with services and under-representing the population of PWID other than opioids. Clinics had autonomy in how to manage patient follow-up and care which may have impacted results. Services which require registration of patients (i.e., to provide OAT) may have had improved treatment uptake compared to low threshold service providers such as NSPs. Given the importance of embedding HCV care in services which are frequently used by people who inject drugs, same day ‘test and treat’ models are likely to be particularly important to reduce loss to follow-up in low threshold services like NSPs. The analysis made no distinction between people lost to follow-up and people who had moved services or been imprisoned, who may remain engaged with care and have higher treatment uptake. Finally, the ETHOS Engage survey did not account for mental health comorbidities and inpatient hospitalisation, factors that have been associated with lower treatment uptake [33,37].
These results have implications for public health. This screening and linkage to care campaign day intervention brings low threshold testing and treatment to services utilised by PWID, improving accessibility and reducing opportunities for stigma [38]. Peer workers are integral to the ETHOS model, connecting with people at risk of HCV with information to make informed decisions on healthcare [39]. Peer workers allow participants to discuss HCV testing and treatment without impacting the therapeutic relationship with OAT providers [40]. This study supports findings that integrated care can be effective at increasing treatment uptake [13]. In addition, venepuncture is a barrier to testing for people who inject drugs, recognised amongst both practitioners [41] and patients [25]. The decentralisation of diagnostics and utilisation of innovative diagnostic technology (including point-of-care and dried blood spot testing) is acceptable among people who inject drugs [23], has been shown to increase testing [13], and will be key in reducing drop-off along the HCV care cascade. Event-based models of testing and treatment, or the use of campaign days, have been successful in engaging large numbers of people at risk of HCV [42]. Further research is needed to evaluate effectiveness and cost-effectiveness of these interventions, particularly with availability of novel diagnostic approaches that facilitate single-visit test and treat strategies. Traditional models of care and wider structural barriers may prevent people who are homeless or have a history of incarceration from initiating treatment compared with others. Criminalisation of drug possession [43] and lack of appropriate housing [44] are detrimental to health and access to health services, likely slowing progress to HCV elimination.
5. Conclusions
HCV screening and linkage to care campaign days, including peer connection, point-of-care HCV RNA testing, and nurse-led linkage to care in drug treatment clinics, were facilitated by HCV treatment initiation in people who inject drugs. Although the proportion of treatment initiation was similar to population-based estimates, the campaign day model may have improved the reach of testing for people who would otherwise not have tested and treated for HCV. Building upon the 66% of eligible ETHOS Engage participants who had ever received HCV treatment at enrolment, 24 months post-intervention, that proportion rose to 83%. Importantly, this result surpasses WHO elimination goals in this population. Offering same-day results to facilitate linkage to care could be an important strategy to improve the impact of a similar intervention. Further studies are needed to evaluate the effectiveness and cost-effectiveness of HCV screening and linkage to care campaign days. Homelessness and incarceration history are associated with lower treatment uptake—highlighting the crucial message that people who inject drugs require tailored support in accessing and succeeding in HCV treatment. Public health responses that address housing, decarceration, and decriminalisation of drugs will be critical in reducing barriers to care and advancing progress towards HCV elimination.
Acknowledgments
The authors thank all participants who took part in the ETHOS Engage study. The authors first give special acknowledgement to the following peer workers and organizations who helped invaluably with participant recruitment: The NSW Users and AIDS Association (NUAA): Sara Adey, Rodd Hinton, Melanie Joyce; Youth Link Needle and Syringe Program, Cairns: Astrid Carthew; Hepatitis South Australia: Lisa Carter, Carol Holly; Harm Reduction Western Australia: Lyn Murphy. We also thank the contributions of members of the ETHOS Engage Study Group: Protocol Steering Committee: Jason Grebely (Kirby Institute, University of New South Wales [UNSW] Sydney), Gregory J. Dore (Kirby Institute, UNSW Sydney), David Silk (Kirby Institute, UNSW Sydney), Nicky Bath (LGBTI Health Programming and Development), Carla Treloar (Centre for Social Research in Health, UNSW Sydney), Andrew Milat (Centre for Epidemiology and Evidence, NSW Health), Adrian Dunlop (Hunter New England Local Health District), Janaki Amin (Macquarie University, Kirby Institute, UNSW Sydney), Jo Holden (Population Health Strategy and Performance, NSW Health), Charles Henderson (NUAA), Kyle Leadbeatter (Hepatitis NSW), Emma Day (Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine [ASHM]), Nikitah Habraken (ASHM), Louisa Degenhardt (National Drug and Alcohol Research Centre, UNSW Sydney), Clarke Scott (Centre for Addiction Medicine, Nepean Blue Mountains Local Health District), and Phillip Read (Kirketon Road Centre). Coordinating Centre (The Kirby Institute, UNSW Sydney): Jason Grebely (Co-Principal Investigator), Gregory J. Dore (Co-Principal Investigator), Maryam Alavi, David Silk (Clinical Project Coordinator), Heather Valerio (PhD candidate), Shane Tillakeratne (Data Manager), Pip Marks (Clinical Trials Manager), Indika Jayasinghe (Laboratory Coordinator), Hannah Reid, Valerie Gleeson, Jodi Van Dyk, Gerard Estivill Mercade, Alison D. Marshall, Stephanie Obeid, Samira Hosseini Hooshyar, Beth Catlett, Andrey Verich (campaign day implementation). Site Principal Investigators: Nadine Ezard (Rankin Court), David Reid (Illawarra Shoalhaven Local Health District), Carla Gorton (Cairns Sexual Health Service), Jeremy Hayllar (Metro North Hospital and Health Service, Brisbane), Thao Lam (Western Sydney Local Health District), Adrian Dunlop (Hunter New England Local Health District), Prasun Datta (Nepean Blue Mountains Local Health District), Alex Wade (Mid North Coast Local Health District), Sally Spruce (Mid North Coast Local Health District), Vicky Cock (Drug and Alcohol Services, South Australia), Mark Cornwell (Northern NSW Local Health District), Michael Christmas (Next Step, Perth), Craig Connelly (Next Step, Perth), Angela Cooper (Townsville Hospital and Health Services), Mark Montebello (Northern Sydney Local Health District). Site Coordinators: Robert Cherry (Rankin Court), Julie Dyer (Rankin Court), Shikha Arawal (Rankin Court), Nadine Horasak (Youth Link Needle and Syringe Program, Cairns), Rhondda Lewis (Cairns Sexual Health Service), Kathy Clark (Youth Link Needle and Syringe Program, Cairns), Daniel Morris (Youth Link Needle and Syringe Program, Cairns), Kathy Donohue (Youth Link Needle and Syringe Program, Cairns), Kathy Griffiths (Biala), Jason Dalla Lana (Biala), Sue Shin (Biala), Connie Graf (Illawarra Shoalhaven Local Health District), Adele Hampson (Illawarra Shoalhaven Local Health District), Carina Burns (Southwest Sydney Local Health District), Ravina Raidu (Southwest Sydney Local Health District), Kylie Stolzenhein (Southwest Sydney Local Health District), Wanda Brabender (Southwest Sydney Local Health District), Nargis Abram (Nepean Blue Mountains Local Health District), Rick Turner (Nepean Blue Mountains Local Health District), Stuart Larter (Nepean Blue Mountains Local Health District), Fiona Goodberg (Nepean Blue Mountains Local Health District), Jennifer Luksza (Western Sydney Local Health District), Michelle Hall (Hunter New England Local Health District), Susan Hazelwood (Hunter New England Local Health District), Krista Zohrab (Mid North Coast Local Health District), Belinda McClurg (Mid North Coast Local Health District), Kate Salisbury (Northern NSW Local Health District), Julie Markham (Queensland Injectors Health Network, Townsville), Jacky Talmet (Drug and Alcohol Services, South Australia), Sandy Dunn (Drug and Alcohol Services, South Australia), Fionnualh Smyth (Western Sydney Local Health District), Lisa Snell (Western Sydney Local Health District), Elizabeth Laing (Next Step, Perth) Martin Clark (Next Step, Perth), Justin Dorigo (Next Step, Perth), Brent Fergusson (Townsville Hospital and Health Service), Bonny Puszka (Northern Sydney Local Health District), Gai Duncan (Northern Sydney Local Health District), Fiona Baker (Northern Short Local Health District), and Jayde Walsh (Northern Sydney Local Health District).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14071555/s1, Figure S1: Promotional material to advertise ETHOS Engage study prior to recruitment; Figure S2: ETHOS Engage participant flowchart, current HCV status and treatment uptake at 24 months post-enrolment (n = 1388); Table S1: Characteristics of study population according to current HCV infection (n = 1374); Table S2: Cumulative probability of treatment initiation by 12 months and 24 months following HCV diagnosis.
Author Contributions
Conceptualization, J.G., G.J.D., C.T., A.D.M., A.D., A.M. and J.A.; methodology, A.C., J.G., H.V., G.J.D. and B.H.; formal analysis, A.C.; writing—original draft preparation, A.C.; writing—review and editing, J.G, M.A., B.H., M.M. (Marianne Martinello), A.M., C.M., B.P., C.H., P.R., P.M., L.D., J.H., D.R.; C.G.; T.L., M.C., A.W., M.M. (Mark Montebello); supervision, J.G.; project administration, D.S. and P.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study protocol was approved by the Human Research Ethics Committees at St Vincent’s Hospital, Sydney and the Aboriginal Health and Medical Research Council (HREC Ref: HREC/17/SVH/113).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
J.G. reports grants from Camurus, grants from Cepheid, grants from Hologic, grants from Indivior, grants from Merck, during the conduct of the study; grants and personal fees from Abbvie, grants and personal fees from Gilead Sciences, grants and personal fees from Merck, grants and personal fees from Cepheid, outside the submitted work. G.J.D. reports grants from Gilead, Abbvie, and Merck. L.D. has received investigator-initiated untied educational grants for studies of opioid medications in Australia from Indivior, Mundipharma and Seqirus. All remaining authors have no potential conflicts to declare. Cepheid and Merck, Sharp, and Dohme Corporation were not involved in the study design, methodology, and writing of this manuscript. The opinions expressed in the paper are those of the authors and do not necessarily represent those of Cepheid or Merck Sharp, and Dohme Corporation. The views expressed in this manuscript do not necessarily represent the position of the Australian Government.
Funding Statement
The ETHOS Engage study is funded by a National Health and Medical Research Council (NHMRC) Partnership project grant (grant number 1103165), including funding from New South Wales Health and Cepheid. This study was also supported in part by a research grant from Investigator-Initiated Studies Program of Merck, Sharp and Dohme Corporation. The Kirby Institute is funded by the Australian Government Department of Health and Ageing. L.D. is supported by a US National Institute of Health (NIH), National Institute on Drug Abuse (NIDA) grant (R01DA1104470). G.J.D. is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (2008276). J.G. is supported by an NHMRC Investigator Grant (1176131). The contents of the published material are solely the responsibility of the individual authors and do not reflect the views of NHMRC.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . Global Health Sector Strategy on Viral Hepatitis 2016-2021: Towards Ending Viral Hepatitis. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 2.Grebely J., Larney S., Peacock A., Colledge S., Leung J., Hickman M., Vickerman P., Blach S., Cunningham E.B., Dumchev K., et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction. 2019;114:150–166. doi: 10.1111/add.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS-Joint United Nations Programme on HIV/AIDS . Health, Rights and Drugs-Harm Reduction, Decriminalization and Zero Discrimination for People Who Use Drugs. UNAIDS; Geneva, Switzerland: 2019. [Google Scholar]
- 4.Hopwood M., Treloar C., Bryant J. Hepatitis C and injecting-related discrimination in New South Wales, Australia. Drugs Educ. Prev. Policy. 2006;13:61–75. doi: 10.1080/09687630500481150. [DOI] [Google Scholar]
- 5.Harris M., Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: A review mapping the role of social factors. Harm Reduct. J. 2013;10:1–11. doi: 10.1186/1477-7517-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajarizadeh B., Grebely J., Matthews G., Martinello M., Dore G.J. Uptake of direct-acting antiviral treatment for chronic hepatitis C in Australia. J. Viral Hepat. 2017;25:640–648. doi: 10.1111/jvh.12852. [DOI] [PubMed] [Google Scholar]
- 7.Valerio H., Alavi M., Silk D., Treloar C., Martinello M., Milat A., Dunlop A., Holden J., Henderson C., Amin J., et al. Progress Towards Elimination of Hepatitis C Infection Among People Who Inject Drugs in Australia: The ETHOS Engage Study. Clin. Infect. Dis. 2020;73:e69–e78. doi: 10.1093/cid/ciaa571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valerio H., Alavi M., Conway A., Silk D., Treloar C., Martinello M., Milat A., Dunlop A., Murray C., Henderson C., et al. Declining Prevalence of Current HCV Infection and Increased Treatment Uptake Among People Who Inject Drugs: The ETHOS Engage Study. Int. J. Drug Policy. 2022;105:103706. doi: 10.1016/j.drugpo.2022.103706. [DOI] [PubMed] [Google Scholar]
- 9.Bajis S., Dore G.J., Hajarizadeh B., Cunningham E.B., Maher L., Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: A systematic review. Int. J. Drug Policy. 2017;47:34–46. doi: 10.1016/j.drugpo.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Oru E., Trickey A., Shirali R., Kanters S., Easterbrook P. Decentralisation, integration, and task-shifting in hepatitis C virus infection testing and treatment: A global systematic review and meta-analysis. Lancet Glob. Health. 2021;9:e431–e445. doi: 10.1016/S2214-109X(20)30505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajis S., Grebely J., Hajarizadeh B., Applegate T., Marshall A.D., Ellen Harrod M., Byrne J., Bath N., Read P., Edwards M., et al. Hepatitis C virus testing, liver disease assessment and treatment uptake among people who inject drugs pre- and post-universal access to direct-acting antiviral treatment in Australia: The LiveRLife study. J. Viral Hepat. 2020;27:281–293. doi: 10.1111/jvh.13233. [DOI] [PubMed] [Google Scholar]
- 12.Gasbarrini N., Dubravić D., Combs L., Dišković A., Ankiersztejn-Bartczak M., Colaiaco F., Wawer I., Wysocki P., Rosińska M., Marzec-Boguslawska A., et al. Increasing integrated testing in community settings through interventions for change, including the Spring European Testing Week. BMC Infect. Dis. 2021;21:1–9. doi: 10.1186/s12879-021-06555-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham E., Wheeler A., Hajarizadeh B., French C., Roche R., Marshall A., Fontaine G., Conway A., Valencia B.M., Bajis S., et al. Interventions to enhance testing and linkage to treatment for hepatitis C infection: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021;7:426–445. doi: 10.1016/S2468-1253(21)00471-4. [DOI] [PubMed] [Google Scholar]
- 14.EASL EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Valerio H., Alavi M., Law M., Tillakeratne S., Amin J., Janjua N.Z., Krajden M., George J., Matthews G.V., Hajarizadeh B., et al. High hepatitis C treatment uptake among people with recent drug dependence in New South Wales, Australia. J. Hepatol. 2021;74:293–302. doi: 10.1016/j.jhep.2020.08.038. [DOI] [PubMed] [Google Scholar]
- 16.Bradley K.A., McDonell M.B., Bush K., Kivlahan D.R., Diehr P., Fihn S.D. The AUDIT Alcohol Consumption Questions. Alcohol. Clin. Exp. Res. 1998;22:1842. doi: 10.1097/00000374-199811000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Mauss S., Pol S., Buti M., Duffell E., Gore C., Lazarus J.V., Logtenberg-van der Grient H.L., Lundgren J., Mozalevskis A., Raben D., et al. Late presentation of chronic viral hepatitis for medical care: A consensus definition. BMC Med. 2017;15:92. doi: 10.1186/s12916-017-0856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yousafzai M.T., Alavi M., Valerio H., Hajarizadeh B., Grebely J., Dore G.J. Time to hepatitis C RNA testing and treatment in the era of direct-acting antiviral therapy among people with hepatitis C in New South Wales, Australia. Viruses. 2022;14:1496. doi: 10.3390/v14071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grebely J., Gilliver R., Mcnaughton T., Henderson C., Hadlow B., Molloy K., Tillakeratne S., Pepolim L., Harrod M.E., Dore G.J., et al. Point-of-care HCV RNA testing, linkage to nursing care, and peer-supported engagement and delivery to enhance HCV treatment among people with recent injecting drug use at a community-led needle and syringe program: The TEMPO pilot study; Proceedings of the International Conference on Hepatitis Care in Substance Users; Virtual conference. 13–15 October 2021. [Google Scholar]
- 20.Macisaac M.B., Whitton B., Anderson J., Hornung M., Elmore K., Pemberton D., Penn M., Hellard M., Stoove M., Wilson D., et al. Rapid point of care HCV testing allows high throughout HCV screening and rapid treatment uptake among PWID attending a medically supervised injecting room; Proceedings of the International Conference on Hepatitis Care in Substance Users; Virtual conference. 13–15 October 2021. [Google Scholar]
- 21.O’Loan J., Young M., Mooney M., O’Flynn M. Same day delivery! HCV point of care testing in South East Queensland marginalised communities simplifies diagnosis and ensures rapid access to treatment; Proceedings of the International Conference on Hepatitis Care in Substance Users; Virtual conference. 13–15 October 2021. [Google Scholar]
- 22.Ralton L., McCartney E.M., Ferguson C., Dawe J., Richmond J., Tse E., Wigg A., Cock V., Rees T., Sha D. Prompt-point of care testing for hepatitis C in the priority settings of mental health, prisons and drug & alcohol facilities; Proceedings of the International Conference on Hepatitis Care in Substance Users; Virtual conference. 13–15 October 2021. [Google Scholar]
- 23.Bajis S., Maher L., Treloar C., Hajarizadeh B., Lamoury F.M.J., Mowat Y., Hajarizadeh B., Martinello M., Adey S., Read P., et al. Acceptability and preferences of point-of-care finger-stick whole-blood and venepuncture hepatitis C virus testing among people who inject drugs in Australia. Int. J. Drug Policy. 2018;61:23–30. doi: 10.1016/j.drugpo.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Bajis S., Grebely J., Cooper L., Smith J., Owen G., Chudleigh A., Hajarizadeh B., Martinello M., Adey S., Read P., et al. Hepatitis C virus testing, liver disease assessment and direct-acting antiviral treatment uptake and outcomes in a service for people who are homeless in Sydney, Australia: The LiveRLife homelessness study. J. Viral Hepat. 2019;26:969–979. doi: 10.1111/jvh.13112. [DOI] [PubMed] [Google Scholar]
- 25.Madden A., Hopwood M., Neale J., Treloar C. Beyond interferon side effects: What residual barriers exist to DAA hepatitis C treatment for people who inject drugs? PLoS ONE. 2018;13:1–10. doi: 10.1371/journal.pone.0207226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paisi M., Crombag N., Burns L., Bogaerts A., Withers L., Bates L., Crowley D., Witton R., Shawe J. Barriers and facilitators to hepatitis C screening and treatment for people with lived experience of homelessness: A mixed-methods systematic review. Health Expect. 2021;25:48–60. doi: 10.1111/hex.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone J., Artenie A., Hickman M., Martin N.K., Degenhardt L., Fraser H., Vickerman P. The contribution of unstable housing to HIV and hepatitis C virus transmission among people who inject drugs globally, regionally, and at country level: A modelling study. Lancet Public Health. 2022;7:e136–e145. doi: 10.1016/S2468-2667(21)00258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolfe S., Garnham L., Godwin J., Anderson I., Seaman P., Donaldson C. Housing as a social determinant of health and wellbeing: Developing an empirically-informed realist theoretical framework. BMC Public Health. 2020;20:1–19. doi: 10.1186/s12889-020-09224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valencia J., Lazarus J.V., Ceballos F.C., Troya J., Cuevas G., Resino S., Torres-Macho J., Ryan P. Differences in the hepatitis C virus cascade of care and time to initiation of therapy among vulnerable subpopulations using a mobile unit as point-of-care. Liver Int. 2021;42:309–319. doi: 10.1111/liv.15095. [DOI] [PubMed] [Google Scholar]
- 30.Ryan P., Valencia J., Cuevas G., Torres-Macho J., Troya J., Pueyo Á., Muñoz-Gómez M.J., Muñoz-Rivas N., Vázquez-Morón S., Martinez I., et al. Detection of active hepatitis C in a single visit and linkage to care among marginalized people using a mobile unit in Madrid, Spain. Int. J. Drug Policy. 2021;96:103424. doi: 10.1016/j.drugpo.2021.103424. [DOI] [PubMed] [Google Scholar]
- 31.Marshall A.D., Conway A., Cunningham E.B., Valerio H., Silk D., Alavi M., Wade A., Lam T., Zohrab K., Dunlop A., et al. Willingness of people who inject drugs to participate in a randomised controlled trial involving financial incentives to initiate hepatitis C treatment. Drug Alcohol Depend. 2022;235:109438. doi: 10.1016/j.drugalcdep.2022.109438. [DOI] [PubMed] [Google Scholar]
- 32.Valerio H., Alavi M., Law M., McManus H., Tillakeratne S., Bajis S., Martinello M., Matthews G.V., Amin J., Janjua N.Z., et al. Opportunities to Enhance Linkage to Hepatitis C Care Among Hospitalized People With Recent Drug Dependence in New South Wales, Australia: A Population-based Linkage Study. Clin. Infect. Dis. 2021;73:2037–2044. doi: 10.1093/cid/ciab526. [DOI] [PubMed] [Google Scholar]
- 33.Hajarizadeh B., Grebely J., Byrne M., Marks P., Amin J., McManus H., Butler T., Cunningham E., Vickerman P., Martin N., et al. Evaluation of hepatitis C treatment-as-prevention within Australian prisons (SToP-C): A prospective cohort study. Lancet Gastroenterol. Hepatol. 2021;6:533–546. doi: 10.1016/S2468-1253(21)00077-7. [DOI] [PubMed] [Google Scholar]
- 34.Treloar C., Schroeder S., Lafferty L., Marshall A., Drysdale K., Higgs P., Baldry E., Stoove M., Dietze P. Structural competency in the post-prison period for people who inject drugs: A qualitative case study. Int. J. Drug Policy. 2021;95:103261. doi: 10.1016/j.drugpo.2021.103261. [DOI] [PubMed] [Google Scholar]
- 35.Akiyama M.J., Columbus D., MacDonald R., Jordan A.O., Schwartz J., Litwin A.H., Eckhardt B., Carmody E. Linkage to hepatitis C care after incar-ceration in jail: A prospective, single arm clinical trial. BMC Infect. Dis. 2019;19:1–10. doi: 10.1186/s12879-019-4344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franco-Paredes C., Ghandnoosh N., Latif H., Krsak M., Henao-Martinez A.F., Robins M., Barahona L.V., Poeschla E.M. Decarceration and community re-entry in the COVID-19 era. Lancet Infect. Dis. 2021;21:e11–e16. doi: 10.1016/S1473-3099(20)30730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain M.K., Thamer M., Therapondos G., Shiffman M.L., Kshirsagar O., Clark C., Wong R.J. Has Access to Hepatitis C Virus Therapy Changed for Patients With Mental Health or Substance Use Disorders in the Direct-Acting-Antiviral Period? Hepatology. 2019;69:51–63. doi: 10.1002/hep.30171. [DOI] [PubMed] [Google Scholar]
- 38.Harris M., Rhodes T., Martin A. Taming systems to create enabling environments for HCV treatment: Negotiating trust in the drug and alcohol setting. Soc. Sci. Med. 2013;83:19–26. doi: 10.1016/j.socscimed.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 39.Henderson C., Madden A., Kelsall J. Beyond the willing & the waiting—The role of peer-based approaches in hepatitis C diagnosis & treatment. Int. J. Drug Policy. 2017;50:111–115. doi: 10.1016/j.drugpo.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Treloar C., Rance J., Bath N., Everingham H., Micallef M., Day C., Hazelwood S., Grebely J., Dore G.J. Evaluation of two community-controlled peer support services for assessment and treatment of hepatitis C virus infection in opioid substitution treatment clinics: The ETHOS study, Australia. Int. J. Drug Policy. 2015;26:992–998. doi: 10.1016/j.drugpo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Marshall A.D., Grebely J., Dore G.J., Treloar C. Barriers and facilitators to engaging in hepatitis C management and DAA therapy among general practitioners and drug and alcohol specialists—The practitioner experience. Drug Alcohol Depend. 2020;206:107705. doi: 10.1016/j.drugalcdep.2019.107705. [DOI] [PubMed] [Google Scholar]
- 42.O’Flynn M., Young M., White S., Grimstrup D., Mooney M., O’Loan J. HCV Blitzing in Corrections with Just a Fingerstick; Proceedings of the International Network on Health and Hepatitis in Substance Users Conference; Virtual conference. 13–15 October 2021. [Google Scholar]
- 43.DeBeck K., Cheng T., Montaner J.S., Beyrer C., Elliott R., Sherman S., Wood E., Baral S. HIV and the criminalisation of drug use among people who inject drugs: A systematic review. Lancet HIV. 2017;4:e357–e374. doi: 10.1016/S2352-3018(17)30073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aldridge R.W., Story A., Hwang S.W., Nordentoft M., Luchenski S.A., Hartwell G., Tweed E.J., Lewer D., Katikireddi S.V., Hayward A.C. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: A systematic review and meta-analysis. Lancet. 2018;391:241–250. doi: 10.1016/S0140-6736(17)31869-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.