Abstract
Triple negative breast cancer (TNBC) is difficult to treat due to lack of druggable targets. We have found that treatment with the small molecule inhibitor KPT-9274 inhibits growth of TNBC cells and eventually leads to cell death. KPT-9274 is a dual specific inhibitor of PAK4 and Nicotinamide Phosphoribosyltransferase (NAMPT). The PAK4 protein kinase is often highly expressed in TNBC cells and has important roles in cell growth, survival, and migration. Previously we have found that inhibition of PAK4 leads to growth inhibition of TNBC cells both in vitro and in vivo. Likewise, NAMPT has been shown to be dysregulated in cancer due to its role in cell metabolism. In order to understand better how treating cells with KPT-9274 abrogates TNBC cell growth, we carried out an RNA sequencing of TNBC cells treated with KPT-9274. As a result, we identified Rictor as an important target that is inhibited in the KPT-9274 treated cells. Conversely, we found that Rictor is predicted to be activated when PAK4 is overexpressed in cells, which suggests a role for PAK4 in the regulation of Rictor. Rictor is a component of mTORC2, one of the complexes formed by the serine/threonine kinase mTOR. mTOR is important for the control of cell growth and metabolism. Our results suggest a new mechanism by which the KPT-9274 compound may block the growth of breast cancer cells, which is via inhibition of mTORC2 signaling. Consistent with this, sequencing analysis of PAK4 overexpressing cells indicates that PAK4 has a role in activation of the mTOR pathway.
Graphical Abstract

INTRODUCTION
Triple negative breast cancers (TNBC) are negative for hormone receptors and Her2. They are difficult to treat due to lack of specific druggable targets. General chemotherapy can eventually fail due to development of resistance, which is often due to metabolic reprogramming.5 The dual PAK4 and Nicotinamide Phosphoribosyltransferase (NAMPT) inhibitor, KPT-9274, a novel phase 1 anticancer drug, inhibits the growth of triple negative breast cancer cells both in vitro and in vivo.1 In addition to breast cancer, KPT-9274 was shown to inhibit the growth of several other tumor cells in vitro and in vivo including renal carcinomas, pancreatic ductal adenocarcinoma, multiple myeloma, esophageal cancer, and squamous cell carcinomas.1,6–9
While it was identified as a PAK4 inhibitor, KPT-9274 was found to be a dual specific inhibitor of PAK4 and the nicotinamide adenine dinucleotide (NAD) biosynthetic enzyme, Nicotinamide Phosphoribosyltransferase (NAMPT). The mechanism by which dual inhibition of PAK4 and NAMPT leads to the growth inhibition of cancer cells is still not completely defined. PAK4 is a serine/threonine kinase and belongs to the group B family of PAKs. It has important roles in signaling pathways that lead to cell survival and proliferation as well as regulation of cytoskeletal organization, anchorage independent growth, and migration,10 all of which are important for the oncogenic process. Increased PAK4 levels are associated with oncogenic transformation.11 PAK4 is upregulated in cancer usually via either increased mRNA expression or DNA amplification, rather than by genetic mutation. PAK4 mRNA levels are significantly increased in breast cancer compared with normal breast tissue.12–18 Studies from our lab and others have found high levels of PAK4 across all subtypes of breast cancer including ER+, Her2+, and triple negative and have implicated PAK4 in breast cancer tumorigenesis.12,14–17,19–22 Furthermore, in mammary epithelial cells PAK4 overexpression is sufficient to lead to oncogenic transformation in vivo while conversely, knockdown of PAK4 by siRNA reduces tumorigenesis in triple negative breast cancer cells.2
NAMPT also has important functions in cancer cell growth and survival due to its role in the production of the cofactor NAD. NAD can be generated by several mechanisms, including the salvage pathway via the rate-limiting enzyme NAMPT. NAD is important for cellular energy metabolism and many cellular processes that are necessary for cell survival, such as genomic stability, regulation of gene expression, and regulation of cell survival. In tumors, NAD is turned over rapidly but cancer cells usually depend on the salvage pathway (via NAMPT) to produce more NAD. NAMPT is thus often overproduced in cancer cells, and the inhibition of NAMPT has become an important strategy for cancer treatment.3
Here we have investigated the long-term effects that dual inhibition of PAK4/NAMPT has on gene expression in TNBC cells. The goal was to gain a better understanding of how PAK4 and NAMPT can lead to oncogenesis, and how their inhibition reduces cancer cell growth. We have found that dual inhibition of PAK4 and NAMPT with KPT-9274 leads to changes in gene expression that are consistent with Rictor inhibition. Rictor is a component of mTORC2, one of the two complexes formed by the serine/threonine kinase mTOR. Both mTOR complexes (mTORC1 and mTORC2) are known to play roles in control of cell growth and anabolic metabolism, although less is known on the regulation and functions of mTORC2 compared with mTORC1.23 mTORC2 is often highly upregulated in cancer, and upregulation of Rictor is associated with poor prognosis.24–30 When active, mTORC2 phosphorylates several substrates, but its canonical substrate is the cell survival protein AKT. Phosphorylation of AKT on Ser473 by Rictor is an important step in enhancing AKT activation and the subsequent increase in cell survival. Our results indicate a new mechanism by which KPT-9274 may inhibit the growth of TNBC breast cancer cells, via inhibition of mTORC2, and suggest that PAK4 and/or NAMPT may operate via regulation of Rictor.
MATERIALS AND METHODS
Reagents and Cell Culture.
KPT-9274 from Karyopharm Therapeutics Inc. (Newton, MA) was dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 10 mM. KPT-9274 was stored at −20° until it was used, and DMSO was stored at room temperature. SUM159 cells (from Asterland) were maintained in Ham’s F12 medium supplemented with 5% FBS.
RNA Sample Preparation and Collection.
Total RNA was collected from SUM159 cells treated with either DMSO (control) or KPT-9274 (300 nM for 4 days). Cells were treated at Day 0, and no additional drug was added throughout the 4 day period. Cells were harvested at Day 4. At first, the samples were homogenized using the Qiagen TissueLyser and extracted in the QIAsymphony using the QIAsymphony RNA kit. Following extraction, samples were quantified using the Trinean Dropsense96 and quality was assessed using the Caliper LabChip GX. The RNA samples were further processed by RUCDR, a Rutgers University based institution, that utilizes Next Generation Sequencing (NGS) platforms for gene profiling studies.
Library Preparation for Transcriptome Sequencing.
RNaseq libraries were prepared using Illumina RNA Library Prep Kit v2 according to manufacturer’s user guide with 400 ng of RNA as input. The libraries were then quantified using KAPA Library Quantification kit according to manufacturer’s user guide and pooled with barcodes. The pooled libraries were sequenced on NextSeq 550 system, using NextSeq 500/550 Mid Output v2 kit. The sequencing parameters used were 150 bp, single-end with 20 million reads per sample. Sequencing was run by RUCDR.
Sequencing Data Analysis.
Raw sequencing data was stored in FASTQ format. The reads were analyzed and mapped as described in ref 31. Briefly, the reads were mapped to a human reference genome (hg19) using Hisat2 software.32 Cufflinks31 was then used to assemble the transcript and calculate the fragment abundance, followed by use of Cuffdiff, a part of the Cufflinks package, to determine the differential gene expression pattern between the two conditions. Transcript abundance was expressed as FPKM (fragments per kilobase of transcript per million mapped fragments), and the log 2-fold change between the two conditions was indicated. We identified 5960 genes which showed a differential gene expression pattern between the two conditions with a log 2-fold change over 1 or under −1 (corresponding to a 2-fold increase or decrease), and 1656 had a log 2 fold change of over 2 or under −2) Differential expression was also analyzed independently in R33 using DEGSeq34,35 package on FPM (fragments per million reads)-normalized data with similar results. Statistical analysis and data visualization including heat maps, density plots, scatter plots, MA plots, and box plots were done in R.
Quantitative Real-Time Polymerase Chain Reaction (PCR).
RNA was reverse transcribed to cDNA using the One-Taq RT-PCR kit from New England Biolabs (Ipswich, MA). cDNA was then amplified using PrimeTime quantitative PCR (q-PCR) primers from Integrated DNA Technologies (Coralville, Iowa) and SYBR Green PCR Master Mix from Applied Biosystems using the ABI Prism 7000 Sequencing Detector (Applied Biosystems, Foster City, CA). Thermal cycling conditions were 1 cycle of 50° for 1 min, 1 cycle of 95° for 10 min, and 40 cycles of 95° for 15 s and 60° for 1 min. GAPDH was used as an internal control and the relative changes of gene expression were calculated by the following formula, fold change = 2^-ΔΔCt = 2−(ΔCt (test) − ΔCt(control) where ΔCt = Ct(PAK4) – Ct(GAPDH) and Ct = threshold cycle number.
Western Blot Analysis.
SUM159 cells were treated with either DMSO (control) or KPT-9274 (300 nM for 4 days). Cells were treated at Day 0, and no additional drug was added throughout the 4 day period. Cells were harvested at Days 0, 1, 2, 3, 4, and 5. All cells were approximately 70% confluent at the first day of treatment, and control cells were approximately 100% confluent by Day 5, while the concentration of cells was lower for the KPT-9274 treated cells. Cell lysates (25 μg) were resolved by SDS-PAGE and transferred PVDF membrane. The parts of the membranes corresponding in size to the bands of interested protein was excised and the membrane was blocked in TBS/T containing 0.1% Tween-20 (TBS/T) and 5% nonfat milk for 1 h. After washing with TBS/T, the membranes were incubated with the indicated antibodies and corresponding secondary antibodies/primary antibody in TBS/T containing 0.1% Tween-20 (TBS/T) and 5% BSA overnight. After washing three times with TBS/T, the membrane was probed with HRP conjugated secondary antibody for 1 h. The immunocomplexes were then visualized by Dura Supersignal from Thermofisher (Waltham, MA). Primary antibodies against Rictor, AKT, phospho S473 AKT, S6, and phospho S240/244-S6 were from Cell Signaling while α-Tubulin was from Santa Cruz. Primary antibodies were diluted into PBS/T containing 5% bovine serum albumin at 1:1000. Secondary antibody was diluted into PBS/T containing 5% nonfat dry milk at 1:5000. Images were visualized using SuperSignal ECL detection kit (ThermoFisher) and captured using Amersham Biosciences Imager 600 (GE Healthcare). Band intensity was quantitated using ImageJ software.36
IPA Analysis of Differentially Expressed Genes.
Significantly differentiated genes were further analyzed by using Ingenuity Pathway Analysis software (IPA, QIAGEN Redwood City, www.qiagen.com/ingenuity), which utilizes a gene pathway database resource for categorizing genes and analyzing their relationship with multiple cellular functions, diseases, and signaling pathways. The upstream analysis function was used to identify predicted upstream regulators that were most likely associated with the gene expression pattern observed in our data set. Genes that were upregulated or downregulated by at least a 4-fold change were analyzed.
RESULTS
Analysis of Differentially Expressed Genes in KPT-9274 vs DMSO Treated SUM159 cells.
Previous studies from our lab showed that treatment of SUM159 triple negative breast cancer cells with KPT-9274 causes growth inhibition both in vitro and in vivo.1 To study the gene expression changes induced by KPT-9274 in these cells, RNA samples were isolated from DMSO treated (control) SUM159 cells and from SUM159 cells treated with KPT-9274. RNA was sequenced using the Illumina NextSeq kit, as described in the Materials and Methods. This generated a list of 27 024 genes. Of these, 1656 genes showed a differential gene expression pattern between the two conditions, with a log 2-fold change greater than 2 or less than −2 (a fold change of 4 in either direction).
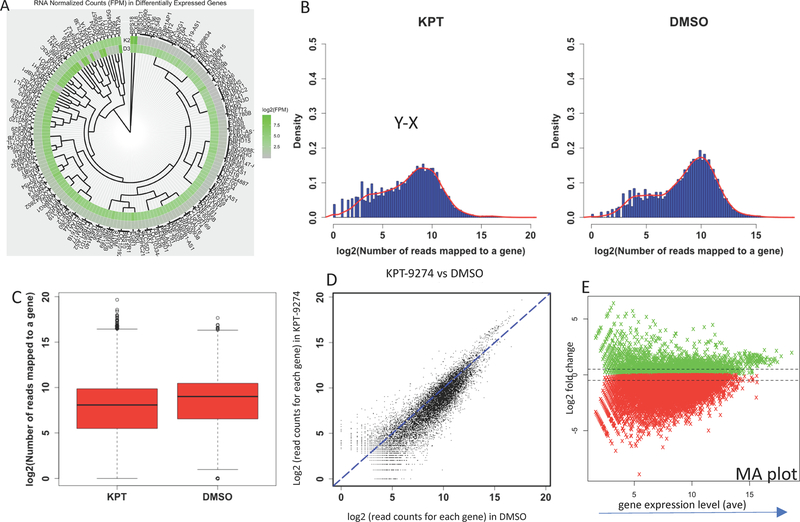
The normalized transcript levels of the top differentially expressed genes are shown in a heatmap showing (Figure 1A). The differences in the numbers of expressed genes in KPT-9274 vs DMSO treated cells are shown in a density plot (Figure 1B), and a box plot (Figure 1C) represents the expression levels of genes in treated vs control cells. A scatter plot and MA plot representing the results are shown in Figure 1D,E.
Figure 1.
Statistical analysis of differentially expressed genes from DMSO (control) or KPT-9274 treated genes. (A) Heat map of the top 160 differentially expressed genes. K2, KPT-9274 treated cells; D3, DMSO treated cells. (B) Density plot representing the expression levels for all genes in KPT-9274 treated cells and DMSO (control) treated cells. (C) Box plot illustrating expression levels for all genes in KPT treated cells and DMSO treated cells. (D) Scatter plot. Each dot represents expression level of one gene. The level in KPT treated cells (y-axis) is compared with DMSO treated cells (x-axis). (E) Expression levels of genes expressed in KPT or DMSO treated cells, expressed as an MA plot.
Identification of Rictor as the Top Inhibited Regulatory Protein in Response to KPT-9274 Treatment.
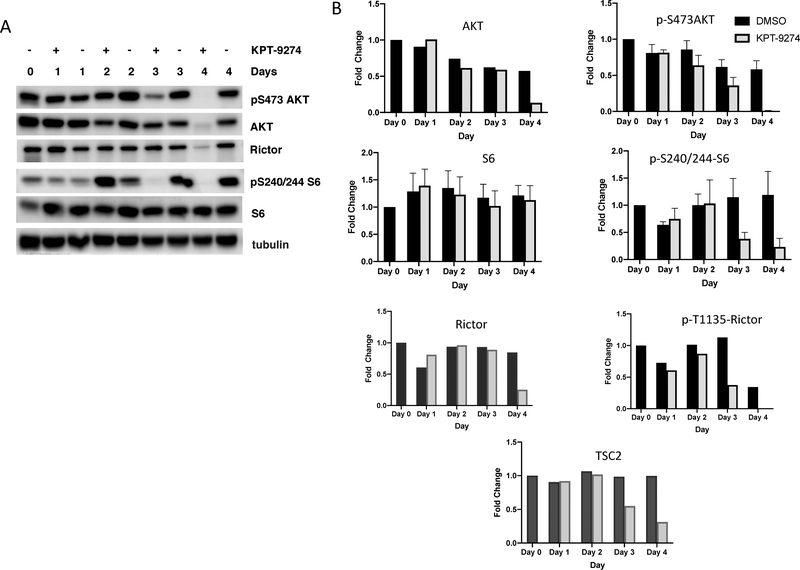
The RNA sequencing results were analyzed by using Ingenuity Pathway Analysis (IPA) software to determine which upstream regulators are most likely to account for the differential expression pattern that resulted in response to drug treatment. This analysis predicted Rictor as the top regulatory protein to be inactivated accounting for the gene expression pattern that was observed (Table 1). Rictor makes up an important component of mTORC2, which is one of the two complexes formed by the mTOR protein kinase.4 mTORC2 is composed of several proteins, including mTOR, Rictor, SIN1 and mLST8. mTORC2 is also regulated by TSC2,37 which interestingly is also predicted to be downregulated according to IPA analysis (Table 1). To validate Rictor inhibition in the KPT-9274 treated cells, we carried out Western blot analysis of lysates from KPT-9274 treated and untreated SUM159 cells. Our results indicate that the phosphorylation of S473 on AKT, considered to be a hallmark of mTORC2 activation, was reduced after 3 days of drug treatment (Figure 2A,B). Phosphorylation of the AKT substrate GSK3β was also reduced (data not shown). Rictor levels also became reduced in KPT-9274 treated cells but only at the 4 day time-point (Figure 2A and B). These results are consistent with an inhibition of Rictor/mTORC2 activity by Day 3 after drug treatment, followed by a reduction in Rictor levels by 4 days of treatment. Interestingly, the phosphorylated form of Rictor (phospho T1135) became reduced even earlier, at Day 3 (see quantitation on Figure 2B), raising the possibility that PAK4leads either directly or indirectly to Rictor phosphorylation. TSC2 protein, which is part of the mTORC2 complex, was also downregulated in response to KPT-9274 treatment (see Figure 2B). Moreover, phosphorylation of the mTORC1 effector, ribosomal S6 protein (S240/244-S6), which is involved in translation initiation, was nearly abolished by Day 3 of drug treatment, suggesting that mTORCl also becomes inhibited in response to prolonged drug treatment.
Table 1.
Top Upstream Regulators That Are Predicted to Be Inhibited in KPT-9274 Treated Cells, As Assessed by IPA Analysis of RNA Sequencing Dataa
| upstream regulator | molecule type | predicted activation state | activation z-score | p-value of overlap |
|---|---|---|---|---|
| RICTOR | other | inhibited | −9.058 | 1.01 × 10−22 |
| KDM5A | transcription regulator | inhibited | −5.364 | 0.0000107 |
| ESR1 | ligand-dependent nuclear receptor | inhibited | −4.433 | 5.29 × 10−11 |
| Alpha catenin | group | inhibited | −3.852 | 0.0432 |
| INSIG1 | other | inhibited | −3.643 | 0.0457 |
| DUSP1 | phosphatase | inhibited | −3.32 | 0.000114 |
| PTPN6 | phosphatase | inhibited | −3.31 | 0.107 |
| VCAN | other | inhibited | −3.302 | 0.00626 |
| IgG | complex | inhibited | −3.225 | 0.0000627 |
| HOXA10 | transcription regulator | inhibited | −3.023 | 0.139 |
| ZFP36 | transcription regulator | inhibited | −2.999 | 0.00786 |
| CD3 | complex | inhibited | −2.982 | 0.00367 |
| TSC2 | other | inhibited | −2.973 | 0.00035 |
| JAG2 | growth factor | inhibited | −2.913 | 0.193 |
| APOE | transporter | inhibited | −2.884 | 0.00373 |
Rictor is predicted to be the most strongly inhibited regulator.
Figure 2.
mTORC2 is inhibited in response to KPT-9274 treatment. mTORC2, which is made up of several proteins including Rictor and mTOR, is inhibited upon KPT-9274 treatment, as illustrated by Western blot analysis. SUM159 cells were either left untreated (0 time point) or treated with DMSO (−) or KPT-9274 (+) for 1, 2, 3, or 4 days. Lysate was separated by SDS-PAGE and analyzed by Western blots probed with the indicated antibodies. (A) Western blot analysis of Rictor, the mTORC2 substrate AKT, and the mTORC1 substrate S6 kinase are shown. (B) Quantitation of multiple Western blots that were probed with AKT, phospho-AKT, S6, phospho-S6, Rictor, phospho-Rictor, and TSC2 are shown. Phospho-AKT, S6, and phospho-S6 were done a total of four times and averaged.
Identification of Rictor as the Top Activated Regulatory Protein in Response to PAK4 Overexpression.
KPT-9274 is an inhibitor of both PAK4 and NAMPT, and it is therefore not yet clear which of these targets are most likely to be responsible for Rictor inhibition in response to KPT-9274. To determine whether PAK4 has an important role in regulating Rictor, we analyzed RNA sequencing studies that we previously carried out on mouse mammary epithelial cells (iMMECs) that overexpress PAK4, compared with wild-type iMMECs.38 iMMECs are a model of normal mammary epithelial cells, but when they are stably transfected with PAK4 they become transformed and develop into tumors in mice.12 Interestingly, when RNA sequencing results from this study were analyzed by using IPA software Rictor was identified as the top activated regulatory protein (see Table 2). These results are consistent, therefore, with a connection between PAK4 expression, regulation of Rictor, mTORC2 signaling, and mammary carcinogenesis.
Table 2.
Rictor Is Identified as the Top Positive Upstream Regulator in iMMEC+Pak4 Cells by IPA Analysis
| upstream regulator | molecule type | predicted activation state | activation z-score | p-value of overlap |
|---|---|---|---|---|
| RICTOR | other | activated | 5.186 | 8.96 × 10−18 |
| Interferon alpha | group | activated | 4.125 | 1.08 × 10−08 |
| IRF7 | transcription regulator | activated | 4.025 | 1.55 × 10−11 |
| IRF3 | transcription regulator | activated | 3.776 | 1.62 × 10−09 |
| Ifnar | group | activated | 3.679 | 1.84 × 10−12 |
| IFNB1 | cytokine | activated | 3.648 | 8.72 × 10−09 |
| CD 437 | chemical drug | activated | 3.606 | 0.000000939 |
| STAT1 | transcription regulator | activated | 3.323 | 0.000858 |
| IFNL1 | cytokine | activated | 3.162 | 1.69 × 10−08 |
| ST1926 | chemical drug | activated | 3.162 | 0.0000217 |
Validation of Differentially Regulated Target Genes.
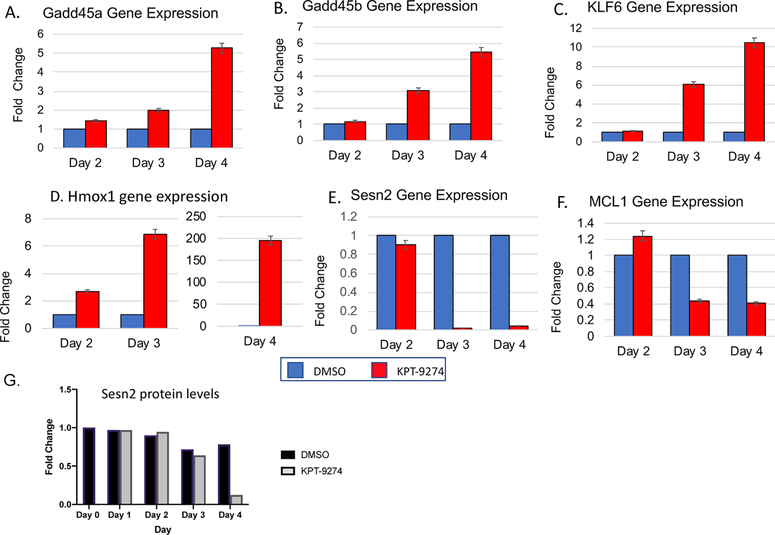
RNA sequencing results predict that mTORC2 is inactivated in the KPT-9274 treated cells. When active, mTORC2 is known to phosphorylate AKT at Ser473 and to enhance its activation. Activation of AKT leads in turn to regulation of various signaling pathways that regulate cell fate and usually promote cell survival.39 One way that AKT operates is via inhibition of Foxo transcription factors, leading consequently to altered regulation of Foxo regulated target genes. We identified a number of genes within our RNA sequencing data set that are consistent with downregulation of Rictor, and subsequent decreased AKT activation, allowing for active Foxo transcription factors. We have analyzed six of these further by q-PCR to verify their regulation by KPT-9274. The RNA sequencing results for the genes we analyzed are illustrated in Table 3, which indicates their changes in RNA levels in response to KPT-9274 treatment (log 2-fold change). q-PCR validation of the gene expression levels is shown in Figure 3A–F. The top four upregulated genes were GADD45a, GADD45b, KLF6, and HMOX1. The upregulation of these genes in response to KPT-9274, was consistent with findings that they are upregulated by Foxo transcription factors.39–44 The second set of genes were downregulated in response to KPT-9274 treatment. This set included MCL1 and Sestrin2, which are regulated by several mechanisms, but whose expression levels are downregulated when Foxo is active.45,46 In all six examples, the gene expression pattern observed after q-PCR followed the same trend that was observed in the RNA sequencing study. Sestrin2 was also analyzed at the protein level, as indicated in Figure 3G. Protein levels also decreased in response to KPT-9274 treatment, although the decrease in protein occurred more slowly (becoming evident only at day 4), compared with the RNA levels.
Table 3.
RNA Sequencing Results Showing the Differentially Expressed Genes That Were Subsequently Validated by q-PCR
| gene | sample-1 | sample-2 | value-1 | value-2 | log 2-fold change |
|---|---|---|---|---|---|
| GADD45A | DMSO | KPT-9274 | 105.471 | 783.886 | 2.88019 |
| GADD45B | DMSO | KPT-9274 | 28.6863 | 341.809 | 3.57476 |
| KLF6 | DMSO | KPT-9274 | 20.3208 | 97.2923 | 2.25937 |
| HMOX1 | DMSO | KPT-9274 | 95.117 | 500.126 | 2.39452 |
| MCL1 | DMSO | KPT-9274 | 79.238 | 21.5971 | −1.87535 |
| SESN2 | DMSO | KPT-9274 | 18.2631 | 0.5446889 | −5.06735 |
Figure 3.
(A–F) q-PCR analysis of differentially expressed genes (GADD45a, GADD45b, KLF6, HMOX1, SESN2, and MCL1) is consistent with RNA-sequencing results. q-PCR was carried out at the indicated time points after exposure to either DMSO (control) or KPT-9274 for 2, 3, or 4 days. Experiments were done either 2 or 3 times for each condition and done in triplicate. Fold differences relative to DMSO treated (control) cells which were set to 1 (blue bars) are shown for each time point. The HMOX1 results are shown on two separate graphs because of the large change seen on day 4. (G) SESN2 protein expression is verified by Western blot analysis and quantified.
DISCUSSION
KPT-9274 is a dual specific inhibitor of both PAK4 and NAMPT. PAK4 and NAMPT play key roles in cell survival and both are often mis-regulated in cancer.3,11 They are therefore promising drug targets for cancer. Treatment of triple negative breast cancer cells with KPT-9274 strongly reduces cancer cell growth both in vitro and in vivo.1,11 PAK4 is often found to be highly expressed in cancer cells and tumors, and its overexpression is sufficient to cause transformation in mammary epithelial and other cens.12,47 Conversely, inhibition of PAK4 can block growth of triple negative breast cancer cells in vitro and in vivo.1,2 While regulation of cell survival as well as cytoskeletal organization play important roles in PAK4 mediated transformation,11 the exact mechanism by which PAK4 promotes cell growth and oncogenesis are not entirely understood.
NAMPT also plays an important role in cancer, partly because of the unique metabolic needs of cancer cells.48 One important property of cancer cells is enhanced glycolysis, often including aerobic glycolysis, with the outcome being the production of metabolites that are necessary for cell proliferation. High levels of NAD are associated with increased glycolysis and other metabolic pathways in cancer cells. NAMPT, which has a key role in the production of NAD, is often elevated in cancer cells. Likewise, NAMPT has been shown to have key roles in the regulation of cell growth and cell survival. NAMPT, like PAK4, is thus often considered to be a good target for cancer therapy.
Here we report RNA sequencing results that indicate that treatment of SUM159 breast cancer cells with KPT-9274 leads to transcriptome changes that are consistent with inhibition of Rictor, an important component of mTORC2. Likewise, KPT-9274 also leads to changes that are consistent with downregulation of TSC2 (Tuberous Sclerosis Complex 2; see Table 1), which has been shown to be required for activation of mTORC2.37 KPT-9274 is a dual specific inhibitor of both PAK4 and NAMPT,1,6,49 and both pathways may have roles in the inhibition of mTORC2 and subsequent inhibition of cancer cell growth. However, it is notable that overexpression of PAK4 leads to the opposite effect of KPT-9274 in that it leads to a gene expression pattern that is consistent with the activation of mTORC2. Taken together, these results strongly suggest a link between PAK4 and mTORC2 activity (see Table 2).
mTORC2 is one of the two complexes which contain the serine/threonine kinase mTOR. This complex also contains Rictor, SIN1, and mLST8. The other complex formed by mTOR is mTORC1, which consists of mTOR, Raptor, and mLST8.50,51 Both mTORC1 and mTORC2 integrate environmental signals and in turn play roles in cell survival, proliferation, and metabolism. Overall, mTORC1 has been characterized more fully than mTORC2 but improper regulation of both of these complexes have been linked to uncontrolled cell growth.
mTOR, as part of mTORC1, was originally identified to be a target for the immunosuppressant rapamycin. mTORC1 responds to the presence of nutrients, particularly amino acids, and to growth factors and hormones such as insulin. mTORC1 in turn promotes anabolic metabolism and cell growth.51 mTOR inhibitors are currently undergoing clinical trials for cancer therapy but early results have so far been modest due to incomplete inhibition of mTOR by rapamycin analogues and dose-limiting toxicities from the use of mTOR ATP-competitive inhibitors.52 Hence, there is a need to further understand the functions and regulation of the two mTOR complexes. In contrast to mTORC1, less is known about mTORC2. It has been shown to be regulated by growth factors and by ribosomal association.4 mTORC2 augments mTORC1 activity to promote anabolic processes when nutrients are sufficient. Recently, mTORC2 was also shown to respond to intracellular metabolite levels to reprogram metabolism when nutrients become limiting.53,54 How mTORC2 is activated both by growth factors and by nutrient limitation remains to be elucidated. In highly proliferating cancer cells that require abundant nutrients for rapid growth and proliferation, mTORC2 is often found to be highly active. Rictor, the major component of mTORC2, is often upregulated in tumors and its increased expression is associated with poor prognosis.26–30 The gene for Rictor was found to be amplified in breast cancer, including triple negative breast cancer that was resistant to therapy.55 It is also sometimes amplified in other cancers such as lung cancer,56,57 gastric cancer,58 and melanoma.59 The Rictor gene is often overexpressed in cancers such as gliomas, colorectal, and some Her2+ breast cancers.60–62 In contrast, downregulation of Rictor expression has been shown to delay or suppress tumor growth and progression.60,61 Regulation of mTORC2 is complex but our results suggest that tuberous sclerosis protein complex (TSC1/TSC2), which is a tumor suppressor,63 could be involved. While TSC2 is thought to have a negative role in mTORC1 activation, our results show that decreased TSC2 activity and levels (see Table 1 and Figure 2B) correlate with decreased Rictor/mTORC2. Although the mechanisms involved remain unclear, these results are consistent with the TSC complex as a positive regulator of mTORC2. Previous studies have also shown that the TSC complex is required for activation of mTORC2.37,64
Here we show that KPT-9274 can downregulate Rictor. How this occurs is unclear at the moment. However, since the decrease in AKT-Ser473 phosphorylation and mTORC1 activation (which both occurred after 3 days of drug treatment) preceded the decrease in Rictor levels (which occurred after 4 days of drug treatment), it is possible that metabolic changes that lead to severe nutrient stress could affect Rictor as well as AKT at the protein level. We have previously observed attenuated AKT levels and Ser473 phosphorylation accompanied by abolished mTORC1 activation during extended glutamine starvation.53 Hence, prolonged KPT-9274 treatment likely affects intracellular metabolite levels crucial for cell survival. This would be consistent with the role for KPT-9274 in inhibition of NAMPT, which affects NAD levels. Likewise, PAK4 may play a role in mTORC2 activation either by phosphorylation, or by regulation of gene expression. Given that mTORC2 plays a role in actin cytoskeleton reorganization and cell migration/metastasis,65,66 whether PAK4 could mediate these mTOR functions remain to be elucidated.
A major function of mTORC2 is to promote celi survival. This is achieved in large part via phosphorylation of its canonical substrate AKT, which is a well-known cell survival protein, often improperly activated in cancer.39 mTORC2 enhances AKT activation by phosphorylating it on Serine 473, an allosteric regulatory site. Increased phosphorylation of AKT at Ser473 is often seen in highly proliferating cells, such as cancer cells. Once activated, AKT sets in motion other pathways in the cell that can encourage cell survival and growth. One important way that it operates is by phosphorylating and thereby inhibiting the activities of transcription factors in the Foxo family, which in turn leads to altered expression of Foxo target genes.39 In mTORC-disrupted cells wherein AKT-Ser473 phosphorylation is absent, the phosphorylation of FoxO1/3a but not other known AKT substrates such as GSK3 was specifically diminished.67 Hence, phosphorylation of AKT-Ser473 by mTORC2 is likely critical to repress FoxO1/3a activity. Our RNA sequencing results are consistent with the regulation of AKT and subsequent regulation of Foxo target genes. For example, four of the genes that were upregulated in response to KPT-9274 include GADD45a, GADD45b, KLF6, and HMOX1. All of these genes are upregulated by Foxo transcription factors,39–44 which would be consistent with inhibition of Rictor, and subsequent inhibition of AKT, allowing for activation of Foxo transcription factors. In contrast, two genes that were downregulated by KPT-9274, MCL1, and SESN2, are also downregulated by Foxo transcription factors45,46 (Figure 4).
Figure 4.
Model for the effect of PAK4 inhibition by KPT-9274 on mTORC2 signaling. According to the model, prolonged KPT-9274 treatment of TNBC cells inhibits PAK4 and NAMPT, leading to decreased Rictor expression and mTORC2 activity. The decrease in mTORC2 activity downregulates AKT, which in turn derepresses Foxo, thereby increasing expression of Foxo-target genes with growth inhibitory functions while decreasing those with growth-promoting functions. Six of these target genes which were regulated as predicted by KPT-9274 are shown here.
The six genes described above which have been validated in this study give us a clue about the mechanism by which KPT-9274 inhibits cancer cell growth. The genes that were upregulated all have growth inhibitory functions. Growth Arrest and DNA Damage Inducible Proteins Gadd45a and Gadd45b are strongly upregulated in response to KPT-9274. The GADD45 proteins are involved in regulation of many cellular functions including DNA repair, cell cycle control, senescence, and various responses to stress. They are proapoptotic, and can be considered to be tumor suppressors. They are essential for the function of many chemotherapeutic drugs. KLF6 (Kruppel-like zinc finger transcription factor) is also upregulated by KPT-9274. It is often considered to be a tumor suppressor and frequently found to be inactivated in cancer via Loss of Heterozygosity (LOH). HMOX1 (a Heme Oxygenase) is an essential enzyme in heme catabolism. It inhibits epithelial mesenchymal transition (EMT) in breast cancer and also inhibits migration and invasion.68 Thus, upregulation of GADD45a and GADD45b and upregulation of both KLF6 and HMOX1 are all consistent with inhibition of cancer cell growth by KPT-9274. Their modes of regulation are also consistent with inhibition of mTORC2 and subsequent decrease in AKT activity and hence the presence of active Foxo transcription factors (Figure 4).
In contrast to the upregulated genes, MCL1 and Sesn2 were downregulated by KPT-9274 and both have growth promoting activities. MCL1 is an antiapoptotic protein of the Bcl family, and it is considered to be a good target for some cancer drugs. Its downregulation by KPT-9274 supports a mechanism by which the drug can promote apoptosis, via inactivation of AKT, and a subsequent increase in MCL1. Sesn2 is also downregulated by KPT-9274. It is a member of the Sestrin family of stress-inducible proteins. The expression of these genes is known to increase in response to AKT activation and therefore their inhibition is consistent with inhibition of mTORC2 and subsequent inactive AKT. The relationship between the sestrins and the mTOR proteins are complex, and different types of functions for the Sestrins have been reported under different conditions. The Sestrins may act both downstream and upstream to mTOR complexes. While they block mTORC1,69,70 they may actually activate mTORC2,71 thus making Sesn2 downregulation consistent with inhibition of mTORC2. The expression of the Sestrins are regulated by many stresses, and Sesn2, along with AKT, has a protective role in cells, where it protects cells against various stresses including oxidative and genotoxic stresses.45,72 Loss of Sesn2 has been shown to sensitize cells to apoptosis, and it has been shown to be one of the targets of the tumor suppressor p53.72 Importantly, low expression of Sesn2 in tumors can predict better responses to therapies that target cancer cell metabolism.45
Our data described above suggest that treatment of SUM159 cells with KPT-9274 leads to inhibition of mTORC2 activity and subsequently impacts downstream pathways that are involved in the regulation of cell growth and survival (see Figure 4). Upon treatment of cells with KPT-9274, inhibition of mTORC2 results in decreased AKT activation and eventually diminished AKT expression. Downregulation of AKT in turn derepresses the expression of genes involved in increasing cell death while decreasing cell growth. This is mediated by alteration in the phosphorylation and activity of Foxo transcription factors, and subsequent altered regulation of Foxo target genes. The target genes validated in this study all have an important role in regulating cell growth, differentiation, proliferation, and survival. It will be important to determine the mechanism by which KPT-9274 may impact Rictor and mTORC2 activity. KPT-9274 is a dual specific inhibitor of PAK4 and NAMPT. Results from our studies with PAK4 overexpressing iMMECs (see Table 2) strongly support the idea that PAK4 plays a key role in the regulation of mTORC2. PAK4 is known to be a regulator of the actin cytoskeleton, and KPT-9274 treatment can lead to a decrease in phosphorylation of cofilin, an important actin binding protein.1 PAK4 is also thought lead to the regulation of target proteins that are involved in cell survival pathways.1 However, the downstream transcription targets of Pak4 that are directly involved in oncogenesis are still mostly elusive. Here, we have made the novel finding that one way PAK4 may operate is by activation of Rictor and hence of mTORC2. Rictor is known to be regulated by phosphorylation,73 and it will be interesting to determine whether PAK4 regulates Rictor at the level of phosphorylation, acetylation, cellular localization, gene expression, or other mechanisms. While this potential connection between PAK4 and Rictor is compelling, we can not rule out the idea that NAMPT may also play an important role in mTORC2 activation. This may be due to its effects on NAD and subsequent changes in metabolites that in turn may impact cell survival in part via mTORC2 regulation. Our findings suggest that KPT-9274 could be an effective drug to target cancers with increased Rictor expression and/or mTORC2 activity.
ACKNOWLEDGMENTS
We acknowledge support from NIH (GM079176) and New Jersey Commission for Cancer Research Bridge Grant (DFHS18CRF008) (E.J.), and New Jersey Health Foundation (NJHF-PC-31-18) (A.M.).
Footnotes
The authors declare no competing financial interest.
Contributor Information
Emma Cordover, Rutgers, The State University of New Jersey, Piscataway, New Jersey.
Janet Wei, Rutgers-Robert Wood Johnson Medical School, Piscataway, New Jersey.
Chadni Patel, Rutgers-Robert Wood Johnson Medical School, Piscataway, New Jersey.
Naing Lin Shan, Rutgers, The State University of New Jersey, Piscataway, New Jersey.
John Gionco, Rutgers, The State University of New Jersey, Piscataway, New Jersey.
Davit Sargsyan, Rutgers, The State University of New Jersey, Piscataway, New Jersey.
Renyi Wu, Rutgers, The State University of New Jersey, Piscataway, New Jersey.
Li Cai, Rutgers, The State University of New Jersey, Piscataway, New Jersey.
Ah-Ng Kong, Rutgers, The State University of New Jersey, Piscataway, New Jersey.
Estela Jacinto, Rutgers-Robert Wood Johnson Medical School, Piscataway, New Jersey.
Audrey Minden, Rutgers, The State University of New Jersey, Piscataway, New Jersey.
REFERENCES
- (1).Rane C, Senapedis W, Baloglu E, Landesman Y, Crochiere M, Das-Gupta S, and Minden A (2017) A novel orally bioavailable compound KPT-9274 inhibits PAK4, and blocks triple negative breast cancer tumor growth. Sci. Rep. 7, 42555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Wong LE, Chen N, Karantza V, and Minden A (2013) The Pak4 protein kinase is required for oncogenic transformation of MDA-MB-231 breast cancer cells. Oncogenesis 2, e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Sampath D, Zabka TS, Misner DL, O’Brien T, and Dragovich PS (2015) Inhibition of nicotinamide phosphoribosyl-transferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol. Ther. 151, 16–31. [DOI] [PubMed] [Google Scholar]
- (4).Xie J, Wang X, and Proud CG (2018) Who does TORC2 talk to? Biochem. J. 475 (10), 1721–1738. [DOI] [PubMed] [Google Scholar]
- (5).Gandhi N, and Das GM (2019) Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications. Cells 8 (2), 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Aboukameel A, Muqbil I, Senapedis W, Baloglu E, Landesman Y, Shacham S, Kauffman M, Philip PA, Mohammad RM, and Azmi AS (2017) Novel p21-Activated Kinase 4 (PAK4) Allosteric Modulators Overcome Drug Resistance and Stemness in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 16 (1), 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Abu Aboud A, Chen CH, Senapedis W, Baloglu E, Argueta C, and Weiss R H. (2016) Dual and specific inhibition of NAMPT and PAK4 by KPT-9274 decreases kidney cancer growth. Mol. Cancer Ther. 15 (9), 2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Fulciniti M, Martinez-Lopez J, Senapedis W, Oliva S, Lakshmi Bandi R, Amodio N, Xu Y, Szalat R, Gulla A, Samur MK, Roccaro A, Linares M, Cea M, Baloglu E, Argueta C, Landesman Y, Shacham S, Liu S, Schenone M, Wu SL, Karger B, Prabhala R, Anderson KC, and Munshi NC (2017) Functional role and therapeutic targeting of p21-activated kinase 4 in multiple myeloma. Blood 129 (16), 2233–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Jiang YY, Lin DC, Mayakonda A, Hazawa M, Ding LW, Chen WW, Xu L, Chen Y, Xiao JF, Senapedis W, Baloglu E, Kanojia D, Shang L, Xu X, Yang H, Tyner JW, Wang MR, and Koeffler HP (2017) Targeted super-enhancer associated oncogenes in oesophageal squamous cell carcinoma. Gut 66, 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, Belisle B, and Minden A (1998) PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 17, 6527–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Rane CK, and Minden A (2019) P21 activated kinase signaling in cancer. Semin. Cancer Biol 54, 40. [DOI] [PubMed] [Google Scholar]
- (12).Liu Y, Chen N, Cui X, Zheng X, Deng L, Price S, Karantza V, and Minden A (2010) The protein kinase Pak4 disrupts mammary acinar architecture and promotes mammary tumorigenesis. Oncogene 29 (44), 5883–5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, Jallal B, and Smeal T (2002) Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J. Biol. Chem. 277 (1), 550–558. [DOI] [PubMed] [Google Scholar]
- (14).Zhuang T, Zhu J, Li Z, Lorent J, Zhao C, Dahlman-Wright K, and Stromblad S (2015) p21-activated kinase group II small compound inhibitor GNE-2861 perturbs estrogen receptor alpha signaling and restores tamoxifen-sensitivity in breast cancer cells. Oncotarget 6 (41), 43853–43868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Bi Y, Tian M, Le J, Wang L, Liu X, Qu J, and Hao M (2016) Study on the expression of PAK4 and P54 protein in breast cancer. World. World J Surg Onc 14 (1), 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Dart AE, Box GM, Court W, Gale ME, Brown JP, Pinder SE, Eccles SA, and Wells CM (2015) PAK4 promotes kinase-independent stabilization of RhoU to modulate cell adhesion. J. Cell Biol. 211 (4), 863–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).He LF, Xu HW, Chen M, Xian ZR, Wen XF, Chen MN, Du CW, Huang WH, Wu JD, and Zhang GJ (2017) Activated-PAK4 predicts worse prognosis in breast cancer and promotes tumorigenesis through activation of PI3K/AKT signaling. Oncotarget 8 (11), 17573–17585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Tang Z, Li C, Kang B, Gao G, Li C, and Zhang Z (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45 (W1), W98–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Minden A (2012) The pak4 protein kinase in breast cancer. ISRN Oncol 2012, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Liu Y, Xiao H, Tian Y, Nekrasova T, Hao X, Lee HJ, Suh N, Yang CS, and Minden A (2008) The Pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Mol. Cancer Res. 6 (7), 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Santiago-Gomez A, Kedward T, Simoes BM, Dragoni I, NicAmhlaoibh R, Trivier E, Sabin V, Gee JM, Sims AH, Howell SJ, and Clarke RB (2019) PAK4 regulates stemness and progression in endocrine resistant ER-positive metastatic breast cancer. Cancer Lett. 458, 66–75. [DOI] [PubMed] [Google Scholar]
- (22).Wang F, Gao Y, Tang L, Ning K, Geng N, Zhang H, Li Y, Li Y, Liu F, and Li F (2019) A novel PAK4-CEBPB-CLDN4 axis involving in breast cancer cell migration and invasion. Biochem. Biophys. Res. Commun. 511 (2), 404–408. [DOI] [PubMed] [Google Scholar]
- (23).Lynch T, Moloughney J, and Jacinto E (2016) The mTOR complexes in cancer cell metabolism. PI3K-mTOR in cancer and cancer therap. ed Jones B., De P,Dey N. Springer, 29–63. [Google Scholar]
- (24).Jebali A, and Dumaz N (2018) The role of RICTOR downstream of receptor tyrosine kinase in cancers. Mol. Cancer 17 (1), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Masui K, Cavenee WK, and Mischel PS (2014) mTORC2 in the center of cancer metabolic reprogramming. Trends Endocrinol. Metab. 25 (7), 364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Oneyama C, Kito Y, Asai R, Ikeda J, Yoshida T, Okuzaki D, Kokuda R, Kakumoto K, Takayama K, Inoue S, Morii E, and Okada M (2013) MiR-424/503-mediated Rictor upregulation promotes tumor progression. PLoS One 8 (11), e80300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Verreault M, Weppler SA, Stegeman A, Warburton C, Strutt D, Masin D, and Bally MB (2013) Combined RNAi-mediated suppression of Rictor and EGFR resulted in complete tumor regression in an orthotopic glioblastoma tumor model. PLoS One 8 (3), e59597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Benavides-Serrato A, Lee J, Holmes B, Landon KA, Bashir T, Jung ME, Lichtenstein A, and Gera J (2017) Specific blockade of Rictor-mTOR association inhibits mTORC2 activity and is cytotoxic in glioblastoma. PLoS One 12 (4), e0176599. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (29).Bian YH, Xu J, Zhao WY, Zhang ZZ, Tu L, Cao H, and Zhang ZG (2017) Targeting mTORC2 component rictor inhibits cell proliferation and promotes apoptosis in gastric cancer. Am. J. Transl Res. 9 (9), 4317–4330. [PMC free article] [PubMed] [Google Scholar]
- (30).Wen SY, Li CH, Zhang YL, Bian YH, Ma L, Ge QL, Teng YC, and Zhang ZG (2014) Rictor is an independent prognostic factor for endometrial carcinoma. Int. J. Clin Exp Pathol 7 (5), 2068–78. [PMC free article] [PubMed] [Google Scholar]
- (31).Trapnell C, Roberts G, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, and Pachter L (2012) Differential gene and transcription expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kim D, Langmead B, and Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12 (4), 357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Team RC. R: A language and environment for statistical computing; http://www.R-project.org. R. Foundation for Statstical Computing: Vienna, Austria, 2019. [Google Scholar]
- (34).Wang L, and Wang X DEGseq: Identify differentially expressed genes from RNA-seq data. R package, version 1.40.0; 2019. https://bioconductor.org/packages/release/bioc/html/DEGseq.html. [DOI] [PubMed] [Google Scholar]
- (35).Wang L, Feng Z, Wang X, Wang X, and Zhang X (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26 (1), 136–8. [DOI] [PubMed] [Google Scholar]
- (36).Schneider CA, Rasband w. S., and Eliceira KW. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Huang J, Dibble CC, Matsuzaki M, and Manning BD (2008) The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol. Cell. Biol. 28 (12), 4104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Rane CK, Patel M, Cai L, Senapedis W, Baloglu E, and Minden A (2018) Decrypting the PAK4 transcriptome profile in mammary tumor forming cells using Next Generation Sequencing. Genomics 110, 248. [DOI] [PubMed] [Google Scholar]
- (39).Manning BD, and Toker A (2017) AKT/PKB Signaling: Navigating the Network. Cell 169 (3), 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Carter M, and Brunet A (2007) FOXO transcription factors. Curr. Biol. 17 (4), R113–R114. [DOI] [PubMed] [Google Scholar]
- (41).Huang H, and Tindall D (2007) Dynamic FoxO transcription factors. J. Cell Sci. 120 (15), 2479–2487. [DOI] [PubMed] [Google Scholar]
- (42).Ponugoti B, Dong G, and Graves D (2012) Role of Forkhead Transcription Factors in Diabetes-Induced Oxidative Stress. Exp. Diabetes Res. 2012, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Sangodkar J, Dhawan N, Melville H, Singh VJ, Yuan E, Rana H, Izadmehr S, Farrington C, Mazhar S, Katz S, Albano T, Arnovitz P, Okrent R, Ohlmeyer M, Galsky M, Burstein B, Zhang D, Politi K, DiFeo A, and Narla G (2012) Targeting the FOXO1/KLF6 axis regulates EGFR signaling and treatment response. J. Clin. Invest. 122 (7), 2637–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zhang X, Tang N, Hadden TJ, and Rishi AK (2011) Akt, FoxO and the regulation of apoptosis. Biochim. Biophys. Acta, Mol. Cell Res. 1813, 1978–1986. [DOI] [PubMed] [Google Scholar]
- (45).Ben-Sahra I, Dirat B, Laurent K, Puissant A, Auberger P, Budanov A, Tanti JF, and Bost F (2013) Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death. Cell Death Differ. 20 (4), 611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Lin C-L, Chen C-M, Lin C-L, Cheng C-W, Lee C-H, and Hsieh Y-H (2017) Norcantharidin induces mitochondrial dependent apoptosis through Mcl-1 inhibition in human prostate cancer cells. Biochim. Biophys. Acta, Mol. Cell Res. 1864, 1867–1876. [DOI] [PubMed] [Google Scholar]
- (47).Liu Y, Xiao H, Tian Y, Nekrasova T, Hao X, Lee HJ, Suh N, Yang CS, and Minden A (2008) The Pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Mol. Cancer Res. 6 (7), 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Yaku K, Okabe K, Hikosaka K, and Nakagawa T (2018) NAD Metabolism in Cancer Therapeutics. Front. Oncol. 8, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Abu Aboud O, Chen CH, Senapedis W, Baloglu E, Argueta C, and Weiss RH (2016) Dual and Specific Inhibition of NAMPT and PAK4 By KPT-9274 Decreases Kidney Cancer Growth. Mol. Cancer Ther. 15 (9), 2119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Shimobayashi M, and Hall MN (2014) Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 15 (3), 155–62. [DOI] [PubMed] [Google Scholar]
- (51).Saxton RA, and Sabatini DM (2017) mTOR Signaling in Growth, Metabolism, and Disease. Cell 169 (2), 361–371. [DOI] [PubMed] [Google Scholar]
- (52).Magaway C, Kim E, and Jacinto E (2019) Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells 8 (12), 1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Moloughney JG, Kim PK, Vega-Cotto NM, Wu CC, Zhang S, Adlam M, Lynch T, Chou PC, Rabinowitz JD, Werlen G, and Jacinto E (2016) mTORC2 Responds to Glutamine Catabolite Levels to Modulate the Hexosamine Biosynthesis Enzyme GFAT1. Mol. Cell 63 (5), 811–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Moloughney JG, Vega-Cotto NM, Liu S, Patel C, Kim PK, Wu CC, Albaciete D, Magaway C, Chang A, Rajput S, Su X, Werlen G, and Jacinto E (2018) mTORC2 modulates the amplitude and duration of GFAT1 Ser-243 phosphorylation to maintain flux through the hexosamine pathway during starvation. J. Biol. Chem. 293 (42), 16464–16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).El Shamieh S, Saleh F, Moussa S, Kattan J, and Farhat F (2018) RICTOR gene amplification is correlated with metastasis and therapeutic resistance in triple-negative breast cancer. Pharmacogenomics 19 (9), 757–760. [DOI] [PubMed] [Google Scholar]
- (56).Sakre N, Wildey G, Behtaj M, Kresak A, Yang M, Fu P, and Dowlati A (2017) RICTOR amplification identifies a subgroup in small cell lung cancer and predicts response to drugs targeting mTOR. Oncotarget 8 (4), 5992–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Cheng H, Zou Y, Ross JS, Wang K, Liu X, Halmos B, Ali SM, Liu H, Verma A, Montagna C, Chachoua A, Goel S, Schwartz EL, Zhu C, Shan J, Yu Y, Gritsman K, Yelensky R, Lipson D, Otto G, Hawryluk M, Stephens PJ, Miller VA, Piperdi B, and Perez-Soler R (2015) RICTOR Amplification Defines a Novel Subset of Patients with Lung Cancer Who May Benefit from Treatment with mTORC1/2 Inhibitors. Cancer Discovery 5 (12), 1262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Kim ST, Kim SY, Klempner SJ, Yoon J, Kim N, Ahn S, Bang H, Kim KM, Park W, Park SH, Park JO, Park YS, Lim HY, Lee SH, Park K, Kang WK, and Lee J (2016) Rapamycin-insensitive companion of mTOR (RICTOR) amplification defines a subset of advanced gastric cancer and is sensitive to AZD2014-mediated mTORC1/2 inhibition. Ann. Oncol 28 (3), mdw669. [DOI] [PubMed] [Google Scholar]
- (59).Laugier F, Finet-Benyair A, Andre J, Rachakonda PS, Kumar R, Bensussan A, and Dumaz N (2015) RICTOR involvement in the PI3K/AKT pathway regulation in melanocytes and melanoma. Oncotarget 6 (29), 28120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Holmes B, Benavides-Serrato A, Freeman RS, Landon KA, Bashir T, Nishimura RN, and Gera J (2018) mTORC2/AKT/HSF1/HuR constitute a feed-forward loop regulating Rictor expression and tumor growth in glioblastoma. Oncogene 37 (6), 732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Morrison Joly M, Hicks DJ, Jones B, Sanchez V, Estrada MV, Young C, Williams M, Rexer BN, Sarbassov dos D, Muller WJ, Brantley-Sieders D, and Cook RS (2016) Rictor/mTORC2 Drives Progression and Therapeutic Resistance of HER2-Amplified Breast Cancers. Cancer Res. 76 (16), 4752–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Wang L, Qi J, Yu J, Chen H, Zou Z, Lin X, and Guo L (2017) Overexpression of Rictor protein in colorectal cancer is correlated with tumor progression and prognosis. Oncol. Lett. 14 (5), 6198–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Huang J, and Manning BD (2008) The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem. J. 412 (2), 179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Vadla R, and Haldar D (2018) Mammalian target of rapamycin complex 2 (mTORC2) controls glycolytic gene expression by regulating Histone H3 Lysine 56 acetylation. Cell Cycle 17 (1), 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, and Hall MN (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6 (11), 1122–1128. [DOI] [PubMed] [Google Scholar]
- (66).Zhang S, Qian G, Zhang QQ, Yao Y, Wang D, Chen ZG, Wang LJ, Chen M, and Sun SY (2019) mTORC2 Suppresses GSK3-Dependent Snail Degradation to Positively Regulate Cancer Cell Invasion and Metastasis. Cancer Res. 79 (14), 3725–3736. [DOI] [PubMed] [Google Scholar]
- (67).Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, and Su B (2006) SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127 (1), 125–37. [DOI] [PubMed] [Google Scholar]
- (68).Zhu X, Huang S, Zeng L, Ma J, Sun S, Zeng F, Kong F, and Cheng X (2017) HMOX-2 inhibits TGF-beta-induced epithelial-mesenchymal transition in the MCF-7 breast cancer cell line. Int. J. Mol. Med. 40, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, and Sabatini DM (2014) The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 9 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Parmigiani A, Nourbakhsh A, Ding B, Wang W, Kim YC, Akopiants K, Guan KL, Karin M, and Budanov AV (2014) Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep. 9 (4), 1281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Byun JK, Choi YK, Kim JH, Jeong JY, Jeon HJ, Kim MK, Hwang I, Lee SY, Lee YM, Lee IK, and Park KG (2017) A Positive Feedback Loop between Sestrin2 and mTORC2 Is Required for the Survival of Glutamine-Depleted Lung Cancer Cells. Cell Rep. 20 (3), 586–599. [DOI] [PubMed] [Google Scholar]
- (72).Wang M, Xu Y, Liu J, Ye J, Yuan W, Jiang H, Wang Z, Jiang H, and Wan J (2018) Recent Insights into the Biological Functions of Sestrins in Health and Disease. Cell. Physiol Biochem. 43, 1731–1741. [DOI] [PubMed] [Google Scholar]
- (73).Dibble CC, Asara JM, and Manning BD (2009) Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol. Cell. Biol. 29 (21), 5657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]