Abstract
An active sulfate-reducing consortium that degrades 2-methylnaphthalene (2-MNAP) at rates of up to 25 μM day−1 was established. Degradation was inhibited in the presence of molybdate and ceased in the absence of sulfate. As much as 87% of 2-[14C]MNAP was mineralized to 14CO2. 2-Naphthoic acid (2-NA) was detected as a metabolite, and incubation with either deuterated 2-MNAP or [13C]bicarbonate indicates that 2-NA is the result of oxidation of the methyl group. Also detected were carboxylated 2-MNAPs, suggesting the presence of an alternative pathway for 2-MNAP degradation.
Recently, many investigators have reported anaerobic biodegradation of polycyclic aromatic hydrocarbons (PAHs) (1–6, 8–10, 12, 14, 15, 17). Most of these studies focused on utilization of naphthalene (NAP) and other nonsubstituted PAHs. Nonetheless, substituted PAHs, such as alkyl PAHs, are major components of PAHs in the environment (7, 11). Among 19 PAHs detected in the sediments of the Passaic River and Newark Bay estuary in the New York-New Jersey harbor, 2-methylnaphthalene (2-MNAP) was the second most hazardous compound (7).
Early on, our laboratory described active sulfidogenic consortia from the Arthur Kill estuary in the New York-New Jersey harbor that are capable of mineralizing NAP and phenanthrene (PHE) (17). The proposed biochemical pathway for NAP degradation involved an initial carboxylation step to 2-naphthoic acid (2-NA) (17). 2-NA was then sequentially reduced starting at the unsubstituted ring through a series of five hydrogenation reactions (10, 18). Recently, Annweiler et al. (1) proposed a pathway for degradation of 2-MNAP. The upper portion of the pathway is analogous to toluene degradation, whereby fumarate is added to the methyl group and then subsequently oxidized to 2-NA (1). The proposed lower portion of the pathway proceeds from 2-NA through the same sequential reduction steps as shown for NAP degradation, although the origin of these metabolites was not confirmed. We describe here a stable consortium capable of mineralizing 2-MNAP under sulfidogenic conditions. Evidence is presented that confirms the origin of the lower pathway metabolites and verifies the oxidation of the methyl substituent using stable-isotope-labeled substrates. Detection of 2-NA and other, more reduced intermediates confirms that the proposed lower pathway is commonly used by bacteria for degradation of 2-MNAP. We also detected the previously unreported presence of other carboxylated 2-MNAPs during degradation of this substrate, which indicates the existence of an alternative pathway.
Initial degradation.
Sediment used as inoculum for this study was collected from the Arthur Kill, in the New York-New Jersey harbor estuary system. Enrichment cultures were established in basic mineral medium with 20 mM sulfate and 10% sediment using a strict anaerobic technique. Routine detection of 2-MNAP was done by gas chromatography (GC) with flame ionization detection as previously described (17). The enrichments initially showed no significant loss of substrate during the first 2 months of incubation, but in the third month 140 μM 2-MNAP was completely utilized. In contrast, other sulfate-reducing enrichments set up at the same time on NAP and PHE took 5 months for complete degradation (17). Upon refeeding, 2-MNAP (120 to 140 μM) was metabolized in 5 to 11 days without a lag, and no loss of substrate was observed in sterile controls. The metabolic activity has been successfully maintained for over 3 years by propagating the consortium as previously described (17).
Mineralization of 2-MNAP.
Complete substrate mineralization to CO2 was confirmed using 2-[14C]MNAP (labeled in the 8 position; Sigma Chemical Co., St. Louis, Mo.). Triplicate bottles of acclimated consortia (30 ml of culture) were amended with 0.12 μM 2-[14C]MNAP (dissolved in 4 μl of methanol; 62,263 total dpm) and 150 μM unlabeled 2-MNAP. Parallel cultures were set up with only unlabeled 2-MNAP and monitored by high-pressure liquid chromatography until the substrate decreased to less than 0.5 μM (24 days). After 24 days, 14CO2 was measured as previously described in detail (17). The 14CO2 produced (53,893 dpm) accounted for 86.6% of total radioactivity. The remaining radioactivity left in the slurry cultures was 6.8% (4,239 dpm), which yielded a total recovery of 93.4% with a standard deviation of less than 2%.
Dependence of 2-MNAP degradation on sulfate reduction.
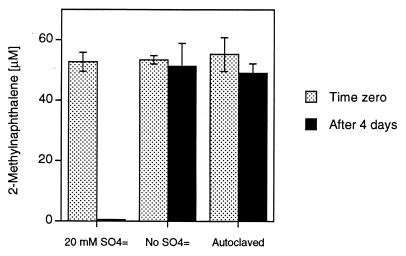
To confirm the dependence of 2-MNAP degradation on sulfate reduction, substrate loss was monitored in cultures grown in the presence or absence of sulfate (Fig. 1). To remove sulfate, cultures were anaerobically washed three times with sulfate-free media, and the sulfate concentration was confirmed in all bottles by ion chromatography as described previously (13). Complete loss of 2-MNAP occurred in cultures with 20 mM sulfate in 4 days but did not occur in cultures without sulfate or in sterile controls. To further confirm the dependence of 2-MNAP degradation on sulfate reduction, molybdate (20 mM), a specific inhibitor for dissimilatory sulfate reduction, was added to active cultures. 2-MNAP was not utilized in the cultures supplemented with molybdate after 16 days of incubation, whereas the substrate was utilized in the cultures without molybdate within 5 days (data not shown). These results indicate that 2-MNAP metabolism is coupled to dissimilatory sulfate reduction, though it remains unclear at this time whether the sulfate reducers are directly or indirectly responsible for the 2-MNAP utilization.
FIG. 1.
Effect of sulfate on utilization of 2-MNAP by the 2-MNAP-acclimated consortium. The autoclaved control is also shown. Data points are the means of duplicate cultures, and the standard deviations are shown.
Substrate specificity.
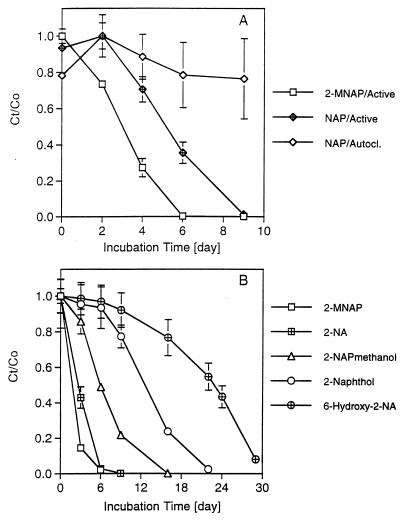
Utilization of other substrates (100 to 150 μM) by the acclimated 2-MNAP consortium was also tested. A series of cultures (30 ml each) was prepared from the active 2-MNAP-degrading enrichment, and loss of substrate was monitored by high-pressure liquid chromatography with UV detection at 280 nm as previously described (17). It is interesting to note that NAP can be readily utilized without a lag by the 2-MNAP-acclimated consortium (Fig. 2A), and conversely, the NAP consortium (17) is capable of readily degrading 2-MNAP as substrate (data not shown). 2-NA was utilized at a similar rate as was 2-MNAP, while 2-naphthalenemethanol (2-NAPmethanol) degradation was slower (Fig. 2B). Both 2-naphthol and 6-hydroxy-2-NA were utilized after a short lag period. Compounds which were not utilized by the 2-MNAP consortium after a 2-month incubation included 1-naphthol, 1-NA, benzene, toluene, biphenyl, PHE, and pyrene (data not shown).
FIG. 2.
Utilization of substrate by the enriched consortia. (A) Degradation of NAP by consortia that were originally enriched on either 2-MNAP (2-MNAP/Active) or NAP (NAP/Active). The autoclaved control is shown (NAP/Autocl.). (B) Degradation of other compounds by the 2-MNAP-degrading consortium. The y scale (Ct/Co) represents the normalized concentration of the tested compound and is calculated by dividing the final concentration of substrate by the initial concentration (∼100 μM). Data points are the means of duplicate cultures, and the standard deviations are shown. Note that the x scale (incubation times) differs between graphs A and B.
Metabolites detected during degradation of 2-MNAP.
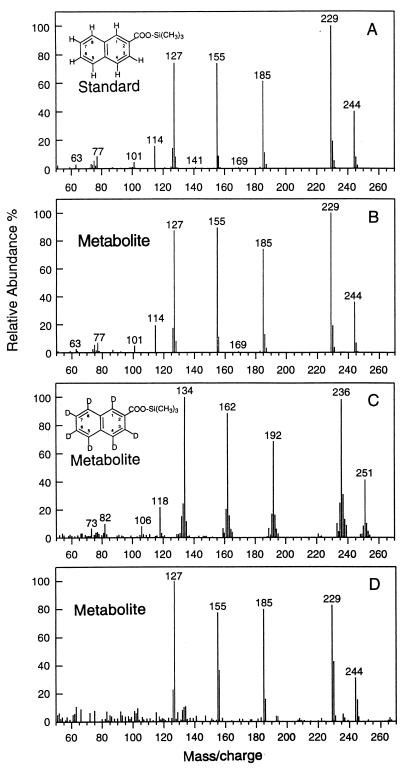
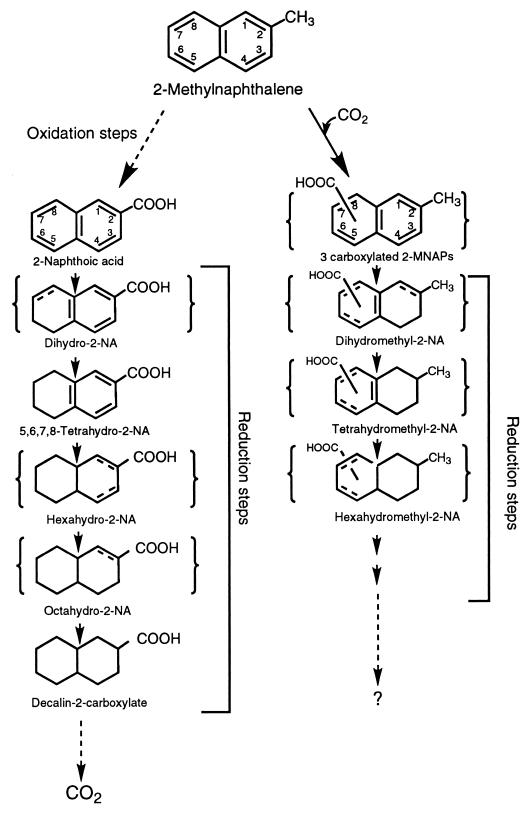
Metabolites produced during growth on 2-MNAP were extracted and detected using GC-mass spectrometry as previously described (17, 18). Identification of 2-NA as a metabolite was based on comparison of both its GC retention time and mass spectra to those of a 2-NA standard. The GC retention times for the standard and the 2-NA metabolite are the same (21.21 min) and are notably different from that of the 1-NA standard (20.62 min.). Furthermore, the mass spectra of trimethylsilyl-derivatized 2-NA standard (Fig. 3A) and 2-NA detected in the consortium (Fig. 3B) are identical, which confirms that 2-NA is the metabolite in the culture. Other, more reduced intermediates from later in the pathway were also detected, including dihydro-2-NA, 5,6,7,8-tetrahydro-2-NA, hexahydro-2-NA, and octahydro-2-NA (data not shown). The presence of these intermediates confirms that, once 2-MNAP is converted to 2-NA, it is further degraded by the same pathway proposed for NAP (10, 18).
FIG. 3.
The mass spectra of trimethylsilyl derivatives of standard 2-NA (A) and 2-NA metabolite detected in the 2-MNAP-degrading cultures supplemented with nondeuterated 2-MNAP (B), deuterated 2-MNAP (D10) (C), and nondeuterated 2-MNAP plus [13C]bicarbonate (D).
Deuterated 2-MNAP (D10) and [13C]bicarbonate (both from Cambridge Isotope Laboratories Inc., Andover, Mass.) were used to trace the flow of carbon from the substrate through intermediates. For the [13C]bicarbonate experiments, detailed experiment procedures were similar to those described previously (17) with the following modifications. The final concentration of either [13C]bicarbonate or [12C]bicarbonate was 25 mM, and 12.5 mM HCl was added to neutralize the alkalinity caused by the addition of bicarbonate. Entire cultures were extracted for GC-mass spectrometry analysis when 2-MNAP was reduced to 25% of the initial concentration in the active cultures. When deuterated 2-MNAP was used as the substrate, the detected intermediate had a retention time of 21.12 min., almost identical to that of the 2-NA standard. Up to a 6.8 μM level (6.8%) of the added deuterated 2-MNAP (100 μM) accumulated as deuterated 2-NA. The mass spectrum pattern (Fig. 3C) is quite similar to that of the 2-NA standard, except that five major ions (251, 236, 192, 162, and 134) were 7 mass units greater than the ions in the 2-NA standard (Fig. 3A). This mass unit increase confirms that the 2-NA metabolite originated from the labeled 2-MNAP substrate. The 7-mass-unit increase from the D10-labeled 2-MNAP can be explained by the loss of three deuterium atoms from the methyl group (-CD3 to -COOH) but no loss of the seven deuterium atoms on the ring. Hence, 2-NA is the result of the oxidation of the methyl group of 2-MNAP, and the methyl group carbon remains with the bicyclic moiety.
When [13C]bicarbonate is added to the culture, no label is incorporated into the 2-NA metabolite (Fig. 3D compared to the standard in Fig. 3A). The [13C]bicarbonate data establishes that 2-NA is not derived from carboxylation of 2-MNAP, as was previously shown for NAP (10, 17, 18). In a number of experiments, we looked for the upper pathway of 2-MNAP degradation via the addition of fumarate as was previously reported (1), but no analogues with a molecular weight matching that of a fumarate addition product to 2-MNAP were found. Recognizing that absence of data is not proof, this does not exclude the possibility that the fumarate addition mechanism may occur in the initial steps.
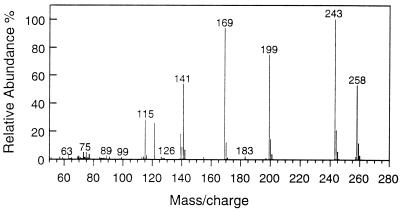
Besides 2-NA, three previously unreported carboxylated 2-MNAPs were also detected in the 2-MNAP-amended consortium. Their identification was based on their distinct GC retention times, molecular ion 258, and mass spectrum (Fig. 4). The position of the carboxyl group on the methylnaphthalene moiety is unknown because corresponding standards were not available. The concentrations of the carboxylated 2-MNAPs were at times as much as that detected for the 2-NA metabolite and were not detected in the sterile control. Also detected were the dihydromethyl-2-NA, tetrahydromethyl-2-NA, and hexahydromethyl-2-NA intermediates (data not shown), providing further support for the existence of this novel pathway based on the fact that multiple intermediates were detected. When deuterated 2-MNAP (D10) was added to the cultures, the deuterated form (with molecular ion 267) was also detected (data not shown). When [13C]bicarbonate was added, the carboxylated methylnaphthalene intermediates increased by 1 mass unit (molecular ion 259), indicating that the carboxyl group of the compounds was indeed derived from [13C]bicarbonate (data not shown).
FIG. 4.
Mass spectrum of trimethylsilyl derivatives of the carboxylated 2-MNAP detected in the 2-MNAP-degrading cultures supplemented with 2-MNAP. The molecular ion 258 equals the sum of 142 (2-MNAP), 44 (CO2), and 72 (trimethylsily group). Three GC peaks showed similar mass spectra, and one of them is shown here.
Conclusion.
As summarized in Fig. 5, we have enriched an active consortium that degrades 2-MNAP through 2-NA as a central intermediate and then proceeds via a series of sequential ring reductions before being mineralized to CO2. The presence of these intermediates confirms that degradation by this pathway is conserved in nature, as it has also been previously reported as part of the NAP degradation pathway (10, 18) and as the lower pathway of another 2-MNAP-degrading consortium (1). The use of stable-isotope-labeled substrates confirmed that the detected intermediates were derived from 2-MNAP and that the methyl group is oxidized to 2-NA. Also detected were carboxylated 2-MNAPs and their corresponding reduced metabolites, although the position of the carboxyl group and the double bonds remain unknown due to the lack of standards. The presence of these carboxylated 2-MNAPs and their reduced derivatives suggests that an alternative pathway exists for 2-MNAP degradation.
FIG. 5.
Proposed pathway for anaerobic biodegradation of 2-MNAP under sulfate-reducing conditions. (Left) Major pathway for 2-MNAP degradation. (Right) Degradation pathway for the carboxylated 2-MNAPs. Detected intermediates which are bracketed have no available standard and thus were deduced based on the mass spectra; the positions of both the double bonds and the carboxyl group are unknown.
Acknowledgments
We thank Beau Ranheim and the crew of the Osprey, NYC-DEP, for helping us collect Arthur Kill sediment and Carmela Palermo for technical support.
This research was funded in part by grants from DARPA (N0001492J1888), ONR (N00149311008), and NSF (9810248).
REFERENCES
- 1.Annweiler E, Materna A, Safinowski M, Kappler A, Richnow H H, Michaelis W, Meckenstock R U. Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture. Appl Environ Microbiol. 2000;66:5329–5333. doi: 10.1128/aem.66.12.5329-5333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedessem M E, Swoboda-Colberg N G, Colberg P J S. Naphthalene mineralization coupled to sulfate reduction in aquifer-derived enrichments. FEMS Microbiol Lett. 1997;152:213–218. [Google Scholar]
- 3.Coates J D, Woodward J, Allen J, Philp P, Lovley D R. Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Appl Environ Microbiol. 1997;63:3589–3593. doi: 10.1128/aem.63.9.3589-3593.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durant N D, Wilson L P, Bouwer E J. Microcosm studies of subsurface PAH-degrading bacteria from a former manufactured gas plant. J Contam Hydrol. 1995;17:213–237. [Google Scholar]
- 5.Galushko A, Minz D, Schink B, Widdel F. Anaerobic degradation of naphthalene by a pure culture of a novel type of marine sulphate-reducing bacterium. Environ Microbiol. 1999;1:415–420. doi: 10.1046/j.1462-2920.1999.00051.x. [DOI] [PubMed] [Google Scholar]
- 6.Hayes L A, Nevin K P, Lovley D R. Role of prior exposure on anaerobic degradation of naphthalene and phenanthrene in marine harbor sediments. Org Geochem. 1999;30:937–945. [Google Scholar]
- 7.Huntley S L, Bonnevie N L, Wenning R J. Polycyclic aromatic hydrocarbon and petroleum hydrocarbon contamination in sediment from the Newark Bay estuary, New Jersey. Arch Environ Contam Toxicol. 1995;28:93–107. [Google Scholar]
- 8.Langenhoff A A M, Zehnder A J B, Schraa G. Behavior of toluene, benzene and naphthalene under anaerobic conditions in sediment columns. Biodegradation. 1996;7:267–274. [Google Scholar]
- 9.McNally D L, Mihelcic J R, Lueking D R. Biodegradation of three- and four-ring polycyclic aromatic hydrocarbons under aerobic and denitrifying conditions. Environ Sci Technol. 1998;32:2633–2639. [Google Scholar]
- 10.Meckenstock R U, Annweiler E, Michaelis W, Richnow H H, Schink B. Anaerobic naphthalene degradation by a sulfate-reducing enrichment culture. Appl Environ Microbiol. 2000;66:2743–2747. doi: 10.1128/aem.66.7.2743-2747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller J G, Lantz S E, Blattmann B O, Chapman P J. Bench-scale evaluation of alternative biological treatment processes for the remediation of pentachlorophenol- and creosote-contaminated materials: solid-phase bioremediation. Environ Sci Technol. 1991;25:1045–1055. [Google Scholar]
- 12.Parker W J, Monteith H D. Fate of polynuclear aromatic hydrocarbons during anaerobic digestion of municipal wastewater sludges. Water Environ Res. 1995;67:1052–1059. [Google Scholar]
- 13.Phelps C D, Young L Y. Anaerobic biodegradation of BTEX and gasoline in various aquatic sediments. Biodegradation. 1999;10:15–25. doi: 10.1023/a:1008303729431. [DOI] [PubMed] [Google Scholar]
- 14.Rockne K J, Chee-Sanford J C, Sanford R A, Hedlund B P, Staley J T, Strand S E. Anaerobic naphthalene degradation by microbial pure cultures under nitrate-reducing conditions. Appl Environ Microbiol. 2000;66:1595–1601. doi: 10.1128/aem.66.4.1595-1601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharak Genthner B R, Townsend G T, Lantz S E, Mueller J G. Persistence of polycyclic aromatic hydrocarbon components of creosote under anaerobic enrichment conditions. Arch Environ Contamin Toxicol. 1997;32:99–105. doi: 10.1007/s002449900160. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland J B, Rafii F, Khan A A, Cerniglia C E. Mechanisms of polycyclic aromatic hydrocarbon degradation. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss, Inc.; 1995. pp. 77–126. [Google Scholar]
- 17.Zhang X, Young L Y. Carboxylation as an initial reaction of anaerobic biodegradation of naphthalene and phenanthrene by sulfidogenic consortia. Appl Environ Microbiol. 1997;63:4759–4764. doi: 10.1128/aem.63.12.4759-4764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Sullivan E R, Young L Y. Evidence for aromatic ring reduction in the biodegradation pathway of carboxylated naphthalene by a sulfate reducing consortium. Biodegradation. 2000;11:117–124. doi: 10.1023/a:1011128109670. [DOI] [PubMed] [Google Scholar]