Abstract
The filamentous fungus Cunninghamella elegans ATCC 36112 metabolized the triphenylmethane dye malachite green with a first-order rate constant of 0.029 μmol h−1 (mg of cells)−1. Malachite green was enzymatically reduced to leucomalachite green and also converted to N-demethylated and N-oxidized metabolites, including primary and secondary arylamines. Inhibition studies suggested that the cytochrome P450 system mediated both the reduction and the N-demethylation reactions.
Malachite green, an N-methylated diaminotriphenylmethane dye, has been widely used as the most efficacious antifungal agent in the fish farming industry (26). It is also used extensively in textile industries for dyeing nylon, wool, silk, leather, and cotton (10). Although malachite green is not approved by the U.S. Food and Drug Administration, its worldwide use in aquaculture will probably continue due to its relatively low cost, ready availability, and efficacy (26); therefore, potential human exposure to malachite green could result from the consumption of treated fish (2) and from working in the dye and aquaculture industries. Malachite green is highly toxic to mammalian cells; it promotes hepatic tumor formation in rodents and also causes reproductive abnormalities in rabbits and fish (13, 24). The structural similarity of malachite green to other carcinogenic triphenylmethane dyes also raises suspicion of carcinogenicity; gentian violet (crystal violet) is a thyroid and liver carcinogen in rodents (17), and pararosaniline is a bladder carcinogen in humans (7). Based on the potential for adverse human health effects, the U.S. Food and Drug Administration nominated malachite green as a priority chemical for carcinogenicity testing by the National Toxicology Program in 1993 (10). These studies are presently being conducted at the National Center for Toxicological Research, Jefferson, Ark.
From an environmental standpoint, there is concern about the fate of malachite green and its reduced form, leucomalachite green, in aquatic and terrestrial ecosystems, since they occur as contaminants (6, 21) and are potential human health hazards. Studies on the biodegradation of triphenylmethane dyes have focused primarily on the decolorization of dyes via reduction reactions (4, 19, 22, 23, 25). Intestinal microflora were shown to reduce crystal violet (18) and malachite green (16) to their respective leuco derivatives. The fungal metabolism of these compounds was first reported by Bumpus and Brock (5). The white rot fungus Phanerochaete chrysosporium, grown under ligninolytic conditions, was shown to metabolize crystal violet to three metabolites by sequential N demethylation of the parent compound, which was catalyzed by lignin peroxidase. They also reported (5) that nonligninolytic cultures of P. chrysosporium could also degrade crystal violet, although the N-demethylation products were not found under nonligninolytic conditions, suggesting that another mechanism for degrading crystal violet existed in this fungus. The present study was conducted to determine whether the filamentous fungus Cunninghamella elegans, which has been used as a microbial model for mammalian xenobiotic metabolism (1) as well as for the biodegradation of environmentally relevant chemicals (8), had a mechanism in triphenylmethane dye metabolism different from that of P. chrysosporium. C. elegans is capable of metabolizing a wide range of compounds, especially by N demethylation and N oxidation (14, 20, 27, 28). Little is known about the potential of nonligninolytic fungi to metabolize triphenylmethane dyes. This paper describes the metabolic fate of malachite green by cultures of C. elegans.
Biotransformation experiments were performed by the addition of malachite green (97% dye content; Aldrich Chemical Co., Milwaukee, Wis.) or leucomalachite green (Aldrich Chemical Co.) to 48-h-old cultures of C. elegans. Culture conditions were as described previously (20). Leucomalachite green was dissolved in dimethylformamide before addition. The data are averages based on three separate experiments performed with duplicates. After 5 days of incubation, fungal mycelia were removed by filtration and extracted with ethyl acetate (five times, each time with 100 ml). The supernatant was also extracted with ethyl acetate. The ethyl acetate extracts were then dried over anhydrous MgSO4 and evaporated in vacuo. The dried sample was dissolved in 10 ml of solution containing acetonitrile (60%) and 50 mM ammonium acetate (pH 4.5) (40%) for analysis by high-performance liquid chromatography (HPLC) and HPLC-mass spectrometry (MS).
Reverse-phase HPLC was performed with a Hewlett-Packard (Palo Alto, Calif.) 1050 series component system equipped with a photodiode array detector. Samples were resolved on a Spherisorb S5 nitrile column (4.6 by 250 mm; particle size, 5 μm) with a PbO2 post-column (4.6 by 10 mm) to detect nonchromatic leucomalachite green and its derivatives at 618 nm following oxidation to chromatic forms (3). The metabolites were eluted at a flow rate of 1.0 ml/min with a linear gradient running from 30% to 90% B (solvent A, 50 mM ammonium acetate, pH 4.5; solvent B, acetonitrile) for 30 min. An isocratic solvent system (solvent A/solvent B ratio = 40:60) was also used when the disappearance of malachite green was monitored. Conditions for liquid chromatography-atmospheric pressure chemical ionization-MS analysis were as described previously (12).
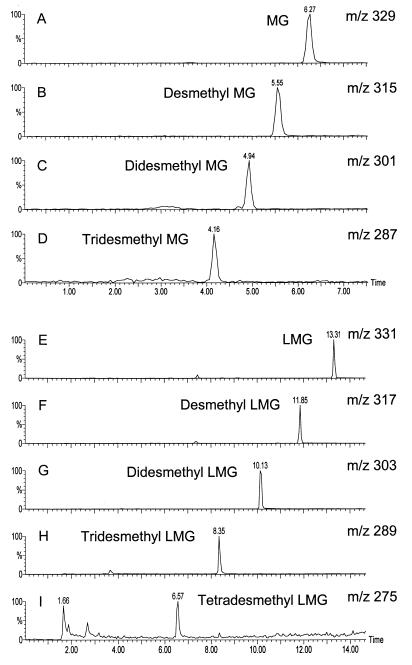
Cultures of C. elegans transformed malachite green, up to 54 μM, with a first-order rate constant of 0.029 μmol h−1 (mg of cells)−1. Apparently 85% of malachite green in culture flasks (81 μM) had disappeared after 24 h. A concentration of 108 μM malachite green inhibited fungal growth, and biotransformation did not occur. The absorption spectra of samples removed during biotransformation indicated that the wavelength (618 nm) at which malachite green exhibits its chromatic feature shifted to 608 nm after 8 h of incubation. These results suggested that malachite green might be undergoing N demethylation, since the N-demethylation products have absorption maxima at wavelengths lower than that of malachite green (B. P. Cho, personal communication). The loss of color was observed during incubation, suggesting that malachite green was reduced to its leuco- form (16). To confirm this observation, the metabolites from ethyl acetate extracts of C. elegans cultures incubated with malachite green and leucomalachite green were analyzed by HPLC in combination with atmospheric pressure chemical ionization-mass spectrometry. Figure 1 shows reconstructed molecular ion chromatograms from the samples extracted from the fungal cells after 5 days of incubation. Under these conditions, the mass spectra consisted primarily of molecular ions (protonated molecules for leucomalachite green and the demethylated derivatives and cationic molecules for malachite green and its derivatives). Based on previous reports (11, 12), these peaks correspond to malachite green (m/z 329) and its mono-, di-, and tri-desmethyl derivatives (m/z 315, 301, and 287, respectively) and leucomalachite green (m/z 331) and its mono-, di-, tri-, and tetra-desmethyl derivatives (m/z 317, 303, 289, and 275, respectively). The metabolites extracted from the culture supernatants were similar to those obtained from mycelium-extracted samples, except for malachite green N-oxide (m/z 345; retention time, 9.21 min), which was detected only in the mycelia. Control experiments with autoclaved cells did not produce a significant amount of metabolites. Only leuco- derivatives were observed as the final products of biotransformation after a prolonged incubation time (10 days), suggesting that the N-demethylated malachite green metabolites were also reduced to their corresponding leuco- derivatives. When leucomalachite green was used as the initial substrate, identical patterns of metabolites (mono-, di-, tri-, and tetra-desmethyl leucomalachite green) were observed.
FIG. 1.
LC-atmospheric pressure chemical ionization-mass spectrometry molecular ion chromatograms obtained at 20 V from an ethyl acetate extract of C. elegans incubated with 32 μM malachite green for 5 days. (A) m/z 329, malachite green (retention time, 6.27 min); (B) m/z 315, desmethyl malachite green (retention time, 5.55 min); (C) m/z 301, didesmethyl malachite green (retention time, 4.94 min); (D) m/z 287, tridesmethyl malachite green (retention time, 4.16 min); (E) m/z 331, leucomalachite green (retention time, 13.31 min); (F) m/z 317, desmethyl leucomalachite green (retention time, 11.85 min); (G) m/z 303, didesmethyl leucomalachite green (retention time, 10.13 min); (H) m/z 289, tridesmethyl leucomalachite green (retention time, 8.35 min); (I) m/z 275, tetradesmethyl leucomalachite green (retention time, 6.57 min)
The microsomal fraction from C. elegans, which was prepared as described previously (9), also appeared to mediate the transformation of malachite green. The incubation mixtures contained the following components in a total volume of 2 ml: 0.1 mg of malachite green, 1 mM NADPH, and 2.5 mg of microsomal protein in 50 mM sodium phosphate buffer, pH 7.0. Desmethyl and di-desmethyl malachite green and leucomalachite green were detected by HPLC. Boiled microsomal protein did not produce any demethylated metabolites. Leucomalachite green and its demethylated metabolites were not formed in the absence of NADPH, although demethylated metabolites of malachite green were still produced.
Cytochrome P450 inhibitors, such as 1-aminobenzotriazole (2 mM), metyrapone (2 mM), and SKF 525-A (1.5 mM), retarded biotransformation of malachite green. Metyrapone completely inhibited the reactions; 1-aminobenzotriazole inhibited the reactions by 67%, and SKF 525-A inhibited them by 70%. This suggested that the cytochrome P450 system of C. elegans mediated the N-demethylation reaction as well as the reduction of malachite green to leucomalachite green.
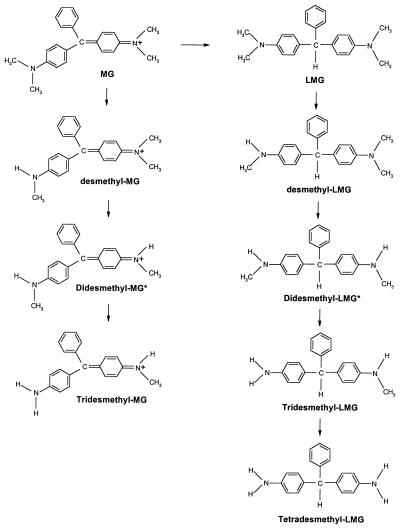
Previous studies (5, 22) demonstrated that the white rot fungus P. chrysosporium employed extracellular lignin peroxidases under ligninolytic conditions to decolorize crystal violet by sequential N demethylation. However, the present study shows that the nonligninolytic fungus C. elegans has multiple pathways to transform triphenylmethane dyes by intracellular cytochrome P450(s) which mediate(s) both the reduction and the N demethylation (Fig. 2). This study demonstrated that the decolorization of malachite green by C. elegans could be attributed mainly to its reduction to leucomalachite green since the demethylated metabolites of malachite green still exhibit absorption at 618 nm. The reduction of crystal violet by rat liver microsomes was shown to be catalyzed by a cytochrome P450 monooxygenase system via a one-electron reaction (15). The present study also suggested that C. elegans employs cytochrome P450 for the reduction of malachite green, because the cytochrome P450 inhibitors used in this study, especially metapyrone, clearly inhibited the reduction. Our study also demonstrated that this fungal system produced metabolite profiles similar to those observed in rat liver (11). Thus, C. elegans is a suitable microbial model for triphenylmethane dye metabolism and will be used to produce significant quantities of metabolites for toxicological evaluation.
FIG. 2.
Proposed mechanism for the metabolism of malachite green (MG) and leucomalachite green (LMG) by C. elegans. The asterisk indicates that unsymmetrical didesmethyl MG and LMG are not shown.
Acknowledgments
We thank J. B. Sutherland, E. B. Hansen, and B. P. Cho for reading the manuscript and M. I. Churchwell for LC-MS analysis.
This work was supported in part by an appointment to the Postgraduate Research Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
REFERENCES
- 1.Abourashed E A, Clark A M, Hufford C D. Microbial models of mammalian metabolism of xenobiotics: an updated review. Curr Med Chem. 1999;6:359–374. [PubMed] [Google Scholar]
- 2.Alderman D J, Clifton-Hadley R S. Malachite green: a pharmacokinetic study in rainbow trout. Oncorhynchus mykiss (Walbaum) J Fish Dis. 1993;16:297–311. [Google Scholar]
- 3.Allen J L, Meinertz J R. Post-column reaction for simultaneous analysis of chromatic and leuco forms of malachite green and crystal violet by high-performance liquid chromatography with photometric detection. J Chromatogr. 1991;536:217–222. [Google Scholar]
- 4.Azmi W, Sani R K, Banerjee U C. Biodegradation of triphenylmethane dyes. Enzyme Microb Technol. 1998;22:185–191. doi: 10.1016/s0141-0229(97)00159-2. [DOI] [PubMed] [Google Scholar]
- 5.Bumpus J A, Brock B J. Biodegradation of crystal violet by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1988;54:1143–1150. doi: 10.1128/aem.54.5.1143-1150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burchmore S, Wilkinson M. Proposed environmental quality standards for malachite green in water (DWE 9026). United Kingdom Department of the Environment, Report no. 3167/2 (November 1993). Marlow, Buckinghamshire, United Kingdom: Water Research Center; 1993. [Google Scholar]
- 7.Case R A M, Pearson J T. Tumours of the urinary bladder in workmen engaged in the manufacture and the use of certain dyestuff intermediates in the British chemical industry. Br J Ind Med. 1954;11:213–221. doi: 10.1136/oem.11.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerniglia C E. Fungal metabolism of polycyclic aromatic hydrocarbons: past, present and future applications in bioremediation. J Ind Microbiol Biotechnol. 1997;19:324–333. doi: 10.1038/sj.jim.2900459. [DOI] [PubMed] [Google Scholar]
- 9.Cerniglia C E, Gibson D T. Metabolism of naphthalene by cell extracts of Cunninghamella elegans. Arch Biochem Biophys. 1978;186:121–127. doi: 10.1016/0003-9861(78)90471-x. [DOI] [PubMed] [Google Scholar]
- 10.Culp S J, Beland F A. Malachite green: a toxicological review. J Am Coll Toxicol. 1996;15:219–238. [Google Scholar]
- 11.Culp S J, Blankenship L R, Kusewitt D F, Doerge D R, Mulligan L T, Beland F A. Toxicity and metabolism of malachite green and leucomalachite green during short-term feeding to Fischer 344 rats and B6C3F(1) mice. Chem-Biol Interact. 1999;122:153–170. doi: 10.1016/s0009-2797(99)00119-2. [DOI] [PubMed] [Google Scholar]
- 12.Doerge D R, Churchwell M I, Gehring T A, Pu Y M, Plakas S M. Analysis of malachite green and metabolites in fish using liquid chromatography atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom. 1998;12:1625–1634. doi: 10.1002/(SICI)1097-0231(19981115)12:21<1625::AID-RCM373>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes C, Lalitha V S, Rao K V K. Enhancing effect of malachite green on the development of hepatic pre-neoplastic lesions induced by N-nitrosodiethylamine in rats. Carcinogenesis. 1991;12:839–845. doi: 10.1093/carcin/12.5.839. [DOI] [PubMed] [Google Scholar]
- 14.Hansen E B, Jr, Cho B P, Korfmacher W A, Cerniglia C E. Fungal transformations of antihistamines: metabolism of brompheniramine, chlorpheniramine, and pheniramine to N-oxide and N-demethylated metabolites by the fungus Cunninghamella elegans. Xenobiotica. 1995;25:1081–1092. doi: 10.3109/00498259509061908. [DOI] [PubMed] [Google Scholar]
- 15.Harrelson W G, Jr, Mason R P. Microsomal reduction of gentian violet. Evidence for cytochrome P-450-catalyzed free radical formation. Mol Pharmacol. 1982;22:239–242. [PubMed] [Google Scholar]
- 16.Henderson A L, Schmitt T C, Heinze T M, Cerniglia C E. Reduction of malachite green to leucomalachite green by intestinal bacteria. Appl Environ Microbiol. 1997;63:4099–4101. doi: 10.1128/aem.63.10.4099-4101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Littlefield N A, Blackwell B-N, Hewitt C C, Gaylor D W. Chronic toxicity and carcinogenicity studies of gentian violet in mice. Fundam Appl Toxicol. 1985;5:902–912. doi: 10.1016/0272-0590(85)90172-1. [DOI] [PubMed] [Google Scholar]
- 18.McDonald J J, Cerniglia C E. Biotransformation of gentian violet to leucogentian violet by human, rat, and chicken intestinal microflora. Drug Metab Dispos. 1984;12:330–336. [PubMed] [Google Scholar]
- 19.Michaels G B, Lewis D L. Microbial transformation rates of azo and triphenylmethane dyes. Environ Toxicol Chem. 1986;5:161–166. [Google Scholar]
- 20.Moody J D, Zhang D L, Heinze T M, Cerniglia C E. Transformation of amoxapine by Cunninghamella elegans. Appl Environ Microbiol. 2000;66:3646–3649. doi: 10.1128/aem.66.8.3646-3649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson C R, Hites R A. Aromatic amines in and near the Buffalo River. Environ Sci Technol. 1980;14:1147–1149. [Google Scholar]
- 22.Ollikka P, Alhonmaki K, Leppanen V-M, Glumoff T, Raijola T, Suominen I. Decolorization of azo, triphenylmethane, heterocyclic, and polymeric dyes by lignin peroxidase isozymes from Phanerochaete chrysosporium. Appl Environ Microbiol. 1993;59:4010–4016. doi: 10.1128/aem.59.12.4010-4016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pointing S B, Vrijmoed L L P. Decolorization of azo and triphenylmethane dyes by Pycnoporus sanguineus producing laccase as the sole phenol oxidase. World J Microbiol Biotechnol. 2000;16:317–318. [Google Scholar]
- 24.Rao K V K. Inhibition of DNA synthesis in primary rat hepatocyte cultures by malachite green: a new liver tumor promoter. Toxicol Lett. 1995;81:107–113. doi: 10.1016/0378-4274(95)03413-7. [DOI] [PubMed] [Google Scholar]
- 25.Sani R K, Banerjee U C. Decolorization of triphenylmethane dyes and textile and dye-stuff effluent by Kurthia sp. Enzyme Microb Technol. 1999;24:433–437. [Google Scholar]
- 26.Schnick R A. The impetus to register new therapeutics for aquaculture. Prog Fish-Cult. 1988;50:190–196. [Google Scholar]
- 27.Zhang D, Evans F E, Freeman J P, Yang Y, Deck J, Cerniglia C E. Formation of mammalian metabolites of cyclobenzaprine by the fungus. Cunninghamella elegans. Chem-Biol Interact. 1996;102:79–92. doi: 10.1016/s0009-2797(96)03736-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, Freeman J P, Sutherland J B, Walker A E, Yang Y, Cerniglia C E. Biotransformation of chlorpromazine and methdilazine by Cunninghamella elegans. Appl Environ Microbiol. 1996;62:798–803. doi: 10.1128/aem.62.3.798-803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]