ABSTRACT
Background
Verinurad is a human uric acid (UA) transporter (URAT1) inhibitor known to decrease serum UA (sUA) levels and that may reduce albuminuria. In a Phase 2a study (NCT03118739), treatment with verinurad + febuxostat lowered urine albumin-to-creatinine ratio (UACR) at 12 weeks by 39% (90% confidence interval 4–62%) among patients with Type 2 diabetes mellitus, hyperuricaemia and albuminuria. The Phase 2b, randomized, placebo-controlled Study of verinurAd and alloPurinol in Patients with cHronic kIdney disease and hyperuRicaEmia (SAPPHIRE; NCT03990363) will examine the effect of verinurad + allopurinol on albuminuria and estimated glomerular filtration rate (eGFR) slope among patients with chronic kidney disease (CKD) and hyperuricaemia.
Methods
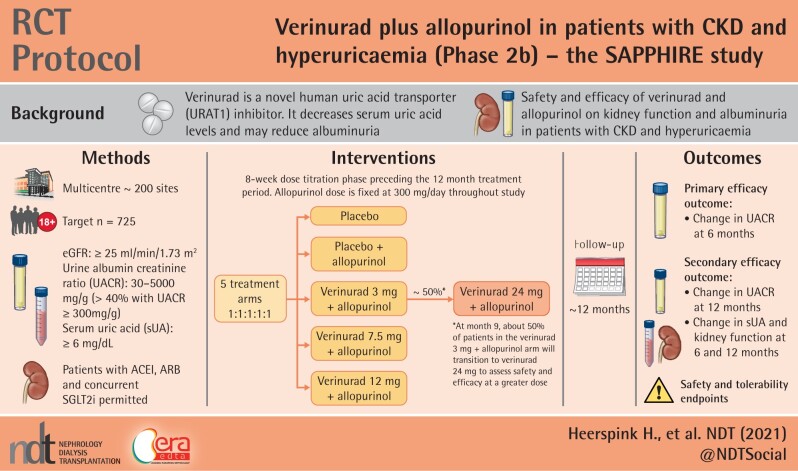
Adults (≥18 years of age) with CKD, eGFR ≥25 mL/min/1.73 m2, UACR 30–5000 mg/g and sUA ≥6.0 mg/dL will be enrolled. Approximately 725 patients will be randomized 1:1:1:1:1 to 12, 7.5 or 3 mg verinurad + allopurinol, allopurinol or placebo. An 8-week dose-titration period will precede a 12-month treatment period; verinurad dose will be increased to 24 mg at Month 9 in a subset of patients in the 3 mg verinurad + allopurinol arm. The primary efficacy endpoint the is change from baseline in UACR at 6 months. Secondary efficacy endpoints include changes in UACR, eGFR and sUA from baseline at 6 and 12 months.
Conclusions
This study will assess the combined clinical effect of verinurad + allopurinol on kidney function in patients with CKD, hyperuricaemia and albuminuria, and whether this combination confers renoprotection beyond standard-of-care.
Keywords: chronic kidney disease, hyperuricaemia, randomized controlled clinical trial, URAT1 inhibitor, verinurad
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS
What is already known about this subject?
Despite guideline-recommended therapies, patients with chronic kidney disease (CKD) are at high risk of end-stage kidney disease and cardiovascular complications.
Therapies that reduce albuminuria and slow estimated glomerular filtration rate decline have been associated with kidney protection during prolonged treatment.
Verinurad is a novel, potent and specific uric acid transporter (URAT1) inhibitor. In a Phase 2a study (NCT03118739) among patients with Type 2 diabetes mellitus and hyperuricaemia, verinurad + the xanthine oxidase inhibitor (XOI) febuxostat decreased the urine albumin-to-creatinine ratio by 39% (90% confidence interval 4–62%) compared with placebo at 12 weeks.
What this study adds?
The Phase 2b Study of verinurAd and alloPurinol in Patients with cHronic kIdney disease and hyperuRicaEmia (SAPPHIRE) will assess the clinical effect of verinurad + allopurinol on kidney function among patients with CKD, hyperuricaemia and albuminuria.
This study will establish whether an intensive uric acid lowering strategy with a URAT1 inhibitor plus XOI confers renoprotection beyond standard-of-care.
What impact may this have on practice or policy?
The findings from the SAPPHIRE study will confirm and expand our understanding of URAT1 inhibition with verinurad in combination with XO inhibition using allopurinol as a treatment for CKD.
INTRODUCTION
Chronic kidney disease (CKD) is associated with substantial cardiovascular morbidity and mortality as well as decreased quality of life [1–4]. Kidney failure and progression to end-stage kidney disease (ESKD) necessitating renal replacement therapy are predicted to increase in the next decade [5].
Control of intraglomerular hypertension, reduction in interstitial fibrosis and reduction in albuminuria are strategies to prevent CKD progression through use of renin–angiotensin–aldosterone system (RAAS) inhibitors [6, 7]. Recently, evidence has emerged that sodium–glucose cotransporter 2 (SGLT2) inhibitors reduce the risk of CKD progression and major kidney and cardiovascular outcomes, and they are now recommended for patients with Type 2 diabetes mellitus (T2DM) and CKD [8–10]. However, a risk remains of CKD progression in patients receiving SGLT2 inhibitors combined with RAAS inhibitors [11, 12]. Therefore, new treatment strategies are urgently needed.
Verinurad is a novel, specific human uric acid (UA) transporter (URAT1) inhibitor in development for the treatment of CKD and heart failure [13]. In Phase 2 studies, verinurad significantly decreased serum UA (sUA) levels among patients with gout and/or asymptomatic hyperuricaemia [14]. When verinurad is combined with the xanthine oxidase inhibitors (XOIs) febuxostat or allopurinol, sUA levels are decreased by up to 80% post-dose [15, 16]. A recent Phase 2a study (NCT03118739) demonstrated that verinurad + febuxostat reduced sUA by 57% [95% confidence interval (CI) 48–64%] pre-dose and urine albumin-to-creatinine ratio (UACR) by 39% (90% CI 4–62%) after 12 weeks, compared with placebo, in patients with hyperuricaemia and T2DM [17]. These findings suggest that the UA-lowering effects of verinurad may also be accompanied by kidney protection. These promising findings have supported the design and conduct of a Phase 2b Study of verinurAd and alloPurinol in Patients with cHronic kIdney disease and hyperuRicaEmia (SAPPHIRE; NCT03990363), to assess the effects of verinurad on albuminuria and estimated glomerular filtration rate (eGFR) among patients with CKD.
MATERIALS AND METHODS
Study design
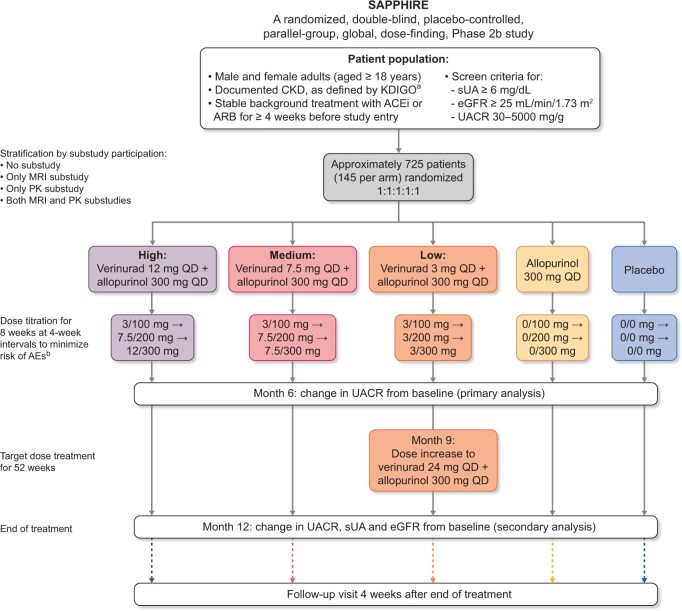
SAPPHIRE is a global, multicentre, randomized, double-blind, parallel-group, placebo- and active-controlled, dose-finding Phase 2b study (Figure 1), designed to assess the efficacy and safety of verinurad + allopurinol in patients with CKD and hyperuricaemia. Approximately 725 patients will be recruited at 200 sites across 13 countries worldwide (Figure 2).
FIGURE 1.
SAPPHIRE study design. aOccurrence ≥3 months before randomization of eGFR <60 mL/min/1.73 m2, UACR ≥30 mg/g and/or ≥1 other marker(s) of kidney damage. bPatients unable to tolerate the dosage may be down-titrated only by reversing the assigned steps within the treatment group.
FIGURE 2.
Countries participating in SAPPHIRE.
Participants
Key inclusion criteria are: age ≥18 years; eGFR of ≥25 mL/min/1.73 m2, UACR of 30–5000 mg/g and sUA concentration of ≥367 μmol/L (≥6 mg/dL); receiving stable background standard-of-care treatment for CKD and/or T2DM, including an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB), unless contraindicated, for ≥4 weeks; and a signed written informed consent (Table 1). Concurrent SGLT2 inhibitor therapy is permitted.
Table 1.
Key inclusion and exclusion criteria in SAPPHIRE
| Inclusion | Criteria |
|---|---|
| Pre-screening | sUA ≥6 mg/dL, and |
| eGFR ≥25 mL/min/1.73 m2, and | |
| UACR ≥30 mg/g and ≤5000 mg/g | |
| Patient characteristics | Male or female |
| ≥18 years of age | |
| Disease characteristics | Documented CKD, defined by KDIGO guidelines as abnormalities in kidney structure or function present for >3 months: occurrence ≥3 months before randomization of either eGFR <60 mL/min/1.73 m2, UACR ≥30 mg/g and/or ≥1 other markers of kidney damage (including abnormalities detected by histology or imaging, urine sediments, urine protein dipstick ≥1+, positive urine albumin dipstick or urinary protein to creatinine ratio ≥84 mg/g) |
| Medication | Background standard-of-care treatment for CKD and/or T2DM in accordance with locally recognized guidelines, as appropriate |
| Therapy should have been optimized and stable for ≥4 weeks before study entry and include an ACEi or an ARB, unless contraindicated, not tolerated or in the opinion of the investigator not practically available or suitable | |
| If treated with an SGLT2 inhibitor, the dose must have been stable for ≥4 weeks before randomization | |
| Pregnancy | Negative pregnancy test at investigation site (urine or serum) for female patients of childbearing potential |
| Exclusion | Criteria |
| Medical conditions | Autosomal dominant or autosomal recessive polycystic kidney disease, lupus nephritis, or anti-neutrophil cytoplasmic antibody-associated vasculitis |
| Carrier of the Human Leukocyte Antigen-B *58:01 allele | |
| History of kidney transplantation | |
| History of stroke, myocardial infarction, percutaneous coronary intervention or coronary artery bypass graft in the past 6 months | |
| Uncontrolled hypertension presenting with systolic blood pressure >180 mmHg and/or diastolic blood pressure >100 mmHg | |
| Evidence of significant liver diseasea | |
| Medication | Receiving cytotoxic therapy, immunosuppressive therapy or other immunotherapy for primary or secondary kidney disease within 6 months prior to enrolment |
| Treatment with any drug for hyperuricaemia in the past 6 months | |
| Treated with strong or moderate OATP inhibitors |
For example, aspartate transaminase or alanine transaminase >3× ULN; or total bilirubin >1.5× ULN.
OATP, organic anion transporting polypeptide; ULN, the upper limit of normal.
At pre-screening, included to minimize burden for patients and workload for study sites, potentially eligible patients meeting eGFR, UACR and sUA criteria are invited for a screening assessment. To account for day-to-day variability in laboratory parameters, a single repeat test is permitted if initial results were not within the eligible ranges, and patients can be pre-screened twice. At screening, patients who did not qualify initially based on inclusion or exclusion criteria can be re-screened once, following appropriate clinical management changes.
Randomization and stratification
Eligible patients are randomized on a 1:1:1:1:1 ratio to one of five treatment arms: placebo, placebo + allopurinol 300 mg/day, verinurad 3 mg + allopurinol 300 mg/day, verinurad 7.5 mg + allopurinol 300 mg/day or verinurad 12 mg + allopurinol 300 mg/day. Study medication is up-titrated according to the scheme in Table 2. These patients comprise the intention-to-treat population. Following Visit 9 (9 months after the end of the titration period), approximately half of patients in the verinurad 3 mg + allopurinol 300 mg/day arm will transition to verinurad 24 mg + allopurinol 300 mg/day to assess the safety and efficacy of verinurad at a greater dose. Patients not transitioning to the 24 mg verinurad dose will continue treatment according to their study arm. Randomization is performed centrally through an interactive web response system, based on a computer-generated randomization schedule prepared by AstraZeneca.
Table 2.
Study medication titration schedule
| Step 1 titration | Step 2 titration | Step 3 titration | |
|---|---|---|---|
| (verinurad/allopurinol) | (verinurad/allopurinol) | (verinurad/allopurinol) | |
| High dose, mg | 3/100 | 7.5/200 | 12/300 |
| Intermediate dose, mg | 3/100 | 7.5/200 | 7.5/300 |
| Low dose, mg | 3/100 | 3/200 | 3/300a |
| Allopurinol alone, mg | 0/100 | 0/200 | 0/300 |
| Placebo, mg | 0/0 | 0/0 | 0/0 |
Following Visit 9 (9 months after the end of the titration period), approximately half of patients in the verinurad 3 mg + allopurinol 300 mg/day arm will transition to verinurad 24 mg + allopurinol 300 mg/day to assess the safety and efficacy of verinurad at a higher dose.
Randomization is stratified by patient participation in two pharmacokinetic (PK) or magnetic resonance imaging (MRI) sub-studies, to ensure balance between treatment arms within each sub-study. Each patient is randomized into one of four levels within this factor: no sub-study, only MRI, only PKs, or both MRI and PKs. Recruitment is monitored to ensure ≥40% of patients have severely increased albuminuria (UACR ≥300 mg/g).
Patients and all study personnel (except the Independent Data Monitoring Committee) are blinded to treatment allocation. Study drugs are packaged in an identical manner, and with the same labelling and administration schedule. Laboratory assessment reports are masked regarding sUA and UACR readings.
Double-blind treatment and study procedures
Verinurad doses were selected based on earlier Phase 2 studies [14–17]. Following the first preplanned interim analysis, and the availability of data from a clinical study assessing the safety, tolerability and PKs of verinurad and allopurinol in healthy Asian subjects (NCT03836599), a protocol amendment introduced at Month 9 permitted a blinded dose increase to 24 mg verinurad in approximately half of patients randomized to 3 mg verinurad. Study treatments are administered orally once daily (QD) as a single capsule until Visit 9, with patients advised to take them with breakfast. Following Visit 9, study treatments are administered orally QD as two capsules to ensure blinding.
Following randomization, patients undergo an 8-week dose-titration phase (visits at Weeks 4 and 8) to minimize risk of gout flares and allopurinol hypersensitivity reactions (Figure 1), followed by treatment at target dose for 52 weeks. Study visits occur at Week 12 (after 4 weeks of treatment at target dose), followed by visits at Weeks 20, 34, 47 and 60. Efficacy endpoints will primarily be assessed at Week 34 (i.e. after 6 months of treatment at target dose) and Week 60 (i.e. 12 months after the end of the titration period). At Week 60, all patients will discontinue therapy, followed by a post-treatment follow-up visit 4 weeks later to assess off-study drug effects. Each study visit includes collection of blood and urine for laboratory measurements (including UACR and eGFR), adverse events (AEs), recording of vital signs, concomitant therapies and study drug adherence.
Efforts will be made to maintain a stable optimum dose of ACEi, ARB or SGLT2 inhibitors for each patient throughout the study in order to minimize UACR fluctuations. Management of blood pressure, lipids, glucose and use of other essential therapies is left to investigator discretion, in accordance with best clinical practice guidelines.
Reductions in eGFR and symptoms of kidney stones will be monitored, as these are known AEs of verinurad monotherapy [14]. Creatinine elevations ≥1.5× baseline in patients with baseline eGFR ≥40 mL/min/1.73 m2, or an eGFR decrease >25% in patients with baseline eGFR <40 mL/min/1.73 m2, will trigger further investigation, more intense monitoring, corrective actions (most notably hydration) and, depending on clinical situation, potentially treatment interruption or discontinuation. Study drug discontinuation is required for patients who become pregnant; develop kidney stones, confirmed acute kidney injury, skin reactions or hypersensitivity to allopurinol; or experience transaminase or bilirubin elevations. Patients who discontinue study drug prematurely (but do not withdraw consent) are encouraged to continue scheduled follow-up visits.
Sub-studies
The first of the two stratifying sub-studies involves collection of kidney MRI data from ∼30 patients, at baseline and 34 weeks post-randomization, to examine structural changes in the kidney. The second sub-study among ≥95 patients will collect post-dose PKs and sUA blood samples within 3–4, 4–5, 5–6 and 8–9 h following study drug administration. A third study will include assessment of vascular reactivity.
Endpoints and event adjudication
Efficacy endpoint
The primary efficacy endpoint is the change from baseline in UACR at 6 months. Key secondary efficacy endpoints include change from baseline in UACR at 12 months and change in sUA, creatinine, cystatin-C and eGFR at 6 and 12 months, where eGFR is calculated using the CKD Epidemiology Collaboration (CKD-EPI) formulae (Table 3) [18, 19].
Table 3.
Key study endpoints in SAPPHIRE
| Endpoint | |
|---|---|
| Primary endpoint | Change from baseline in UACR at 6 months |
| Secondary endpoints | Change from baseline in UACR at 12 months |
| Change from baseline in UACR sUA at 6 and 12 months | |
| Change from baseline in UACR and sUA at 6 months for dose–response assessment up to 12 mg verinurad dose | |
| Change from baseline in eGFR at 6 and 12 months | |
| Change from baseline in creatinine at 6 and 12 months | |
| Change from baseline in cystatin-C at 6 and 12 months | |
| Exploratory endpoints | Plasma exposure of verinurad, allopurinol and oxypurinol (active metabolite of allopurinol) |
| Change from baseline at 6 months in kidney oxygenation, blood flow parameters, cortical and kidney volume measurements, MRI relaxation and diffusion measurements, pulse wave velocity | |
| Change from baseline at 6 and 12 months and EOT in blood pressure, NT-proBNP, high sensitivity CRP, high sensitivity troponin 1, flow-mediated dilation, emerging urine and serum biomarkers, AST, ALT | |
| Change from baseline at 6 and 12 months in UACR in pre-specified subsets of patients | |
| Change in renal function assessments at 6 and 12 months, and EOT | |
| Plasma concentrations of verinurad, allopurinol and oxypurinol, sUA, UACR and other PD variables | |
| Change in tophi from baseline at 6 months and at EOT | |
| Incidence of gout flare during the first titration period, the overall titration period and the whole study | |
| Safety endpoints | Rates of AEs and SAEs, including cardiovascular events |
| Change in vital signs, electrocardiograms and clinical laboratory parameters |
ALT, alanine aminotransferase; AST, aspartate transaminase; CRP, C-reactive protein; EOS, end-of-study; EOT, end-of-treatment; SAE, serious AE.
Key exploratory endpoints (Table 2) include plasma exposure of verinurad, allopurinol and oxypurinol; kidney structural and functional parameters; biomarkers; effects on UACR in pre-specified patient subsets; PK and pharmacodynamic (PD) analysis; gout parameters; and renal parameters (UACR, sUA, eGFR and creatinine, cystatin-C) at Week 60 for patients who received 24 mg verinurad.
Safety endpoints
Safety and tolerability endpoints include AEs, vital signs, physical examination, electrocardiograms and clinical laboratory parameters. An independent adjudication committee will assess potential cardiovascular events using pre-specified endpoint definitions. The independent adjudication committee is responsible for adjudication of all potential clinical events presented in Supplementary Appendix S1.
Statistical considerations
Sample size calculation
The estimated sample size of 145 patients randomized per group will yield 80% power to detect a 25% reduction in geometric mean UACR for the high dose of verinurad + allopurinol compared with placebo (treatment difference of approximately −0.29 on the natural log scale) at the two-sided alpha level of 0.1, assuming Type 1 error and a standard deviation (SD) of 1.0 on the natural log scale. The SD estimate is based on the results of a Phase 2a study [17]. A reduction in UACR of ≥25% has been shown to infer a high likelihood of clinical benefit on established kidney endpoints [20].
For the MRI sub-study, a sample size of 30 in each treatment arm will have at least 80% power to detect a difference (high dose verinurad + allopurinol versus placebo) in means of 0.05 in renal arterial resistive index assuming that the common SD is 0.05 with a two-sided significance level of 0.05. The SD of 0.05 is based on prior data [21].
Efficacy assessment
The primary efficacy analysis will be based on the intention-to-treat population. The analysis of change in UACR from baseline to Week 34 (i.e. 26 weeks of treatment at target dose) will be conducted on natural log-transformed UACR values using a mixed model for repeated measures, with fixed categorical effects of treatment, week, diabetes status, moderately or severely elevated albuminuria, N-terminal pro B-type natriuretic peptide (NT-proBNP) <360 or ≥360 pg/mL, baseline SGLT2 inhibitor use and treatment-by-week interaction. Continuous fixed covariates will be baseline log(UACR) and baseline log(UACR)-by-week interaction. We will provide estimates of the geometric mean percentage change from baseline in UACR for each treatment group under the mixed model (with 95% CIs), as well as the geometric mean treatment ratio between the active treatment groups and the placebo/comparator group (with 95% CI and P-value for a test of no treatment effect). As in previous dose-finding studies in patients with CKD, missing values will not be imputed, but all available longitudinal ACR values will be analysed under the assumption of missingness at random.
Secondary efficacy variables will be assessed in a similar manner to the primary efficacy variable. The dose–response relationship among the three doses of verinurad and allopurinol will also be assessed.
Safety assessment
All safety analyses will be performed on the safety population and summarized by treatment group, based on randomized treatment.
Interim analyses
Two interim analyses will be conducted by an independent team not involved in the study operations in order to support sponsor decision-making regarding the clinical programme. The first interim analysis, which already has occurred (see above), was to be performed no later than when 90% of patients have completed 12 weeks’ treatment after titration (Visit 7). The second analysis (for the primary endpoint) will be conducted when all patients have completed 26 weeks’ treatment after titration (Visit 8).
Study oversight
The trial was designed in collaboration with a scientific advisory committee, consisting of academic members and sponsor representatives. The scientific advisory committee will oversee the study and supervise the analysis of data. The study is being conducted by the contract research organization Covance, who will maintain responsibility for the collection and analysis of data in conjunction with the sponsor. Independent safety and data monitoring committees will review, interpret and adjudicate safety data and overall study conduct, respectively, throughout the trial.
Current status
The study is currently ongoing and has enrolled 860 patients from 12 countries. Demographics and baseline characteristics for the enrolled patients, extracted from the study database on 16 March, are presented in Table 4. Of the 860 patients enrolled, mean age was 65.3 years and 67.0% were male. Patients had a mean (SD) eGFR of 47.8 (18) mL/min/1.73 m2. Patients had a wide range of eGFR levels: 100 (11.6%) patients had an eGFR <30 mL/min/1.73 m2 and 179 (20.8%) had an eGFR ≥60 mL/min/1.73 m2. Median UACR was 246 mg/g and median serum urate was 7.7 mg/dL. The majority (66.0%) of patients had a diagnosis of diabetic nephropathy as reported by the treating physician. The second most common aetiology of CKD was ischaemic/hypertensive nephropathy, diagnosed in 156 (18.1%) patients.
Table 4.
Baseline demographic and disease characteristics
| Characteristic | Total (n = 860) |
|---|---|
| Age, years | |
| Mean (SD) | 65.3 (10.8) |
| Sex, n (%) | |
| Male | 576 (67.0) |
| Female | 284 (33.0) |
| Race, n (%) | |
| White | 621 (72.2) |
| Black or African American | 116 (13.5) |
| American Indian or Alaska Native | 29 (3.4) |
| Asian | 20 (2.3) |
| Other | 70 (8.1) |
| Missing | 2 (0.2) |
| Region,an (%) | |
| North America | 373 (43.4) |
| Europe | 376 (43.7) |
| South Africa | 111 (12.9) |
| Body mass index, kg/m2 | |
| Mean (SD) | 32.0 (6.3) |
| Blood pressure, mmHg | |
| Systolic | 136.4 (16.6) |
| Diastolic | 75.4 (10.3) |
| eGFR, mL/min/1.73 m2 | |
| Mean (SD) | 47.8 (17.9) |
| eGFR categories, mL/min/1.73 m2, n (%) | |
| <30 | 100 (11.6) |
| 30 to <60 | 581 (67.6) |
| 60 to <90 | 149 (17.3) |
| ≥90 | 30 (3.5) |
| NT-proBNP, pg/mL | |
| Median (range) | 188 (25–46025) |
| Serum urate, mg/dL | |
| Median (range) | 7.7 (5.5–13.5) |
| UACR, mg/g | |
| Median (range) | 246 (10–5579) |
| HbA1c, % | |
| Mean (SD) | 7.5 (1.8) |
| Comorbidity, n (%) | |
| Diabetes mellitus | 706 (82.1) |
| Kidney disease aetiology, n (%) | |
| Diabetic nephropathy | 568 (66.0) |
| Ischaemic/hypertensive nephropathy | 156 (18.1) |
| Chronic glomerulonephritis | 25 (2.9) |
| Other/unknown | 111 (12.9) |
| SGLT2 inhibitor, n (%) | 84 (9.8) |
Europe: Czech Republic, France, Hungary, Israel, Italy, Poland, Romania, Slovakia and Spain; North America: USA and Mexico; South Africa: South Africa. Baseline data presented in the table are extracted from the study database on 16 March 2021 while the trial was ongoing and before database lock. HbA1c, glycated haemoglobin; n, number of patients.
DISCUSSION
Despite advancements in pharmacotherapies and clinical care programmes, the mortality and morbidity associated with CKD are substantial, resulting in significant financial and societal burden [1, 2, 22, 23]. Targeted therapies that reduce albuminuria and slow eGFR decline have been associated with kidney protection following prolonged treatment [6, 8, 9, 24].
The Phase 2b SAPPHIRE study will assess the effect of URAT1 inhibition with verinurad combined with the XOI allopurinol on albuminuria and eGFR among patients with CKD and hyperuricaemia. The primary pharmacological action of the verinurad and allopurinol combination is lowering of sUA by increasing renal UA excretion and lowering UA production. The result is a consistent lowering of sUA by >70% [15, 16]. In SAPPHIRE, the inclusion of allopurinol and placebo treatment arms, as well as three treatment arms comprising different verinurad doses (which are expected to lower sUA by 50–75%), will allow careful analysis of the relationship between the sUA-lowering effect and the effect on albuminuria. This analysis may allow conclusions to be drawn regarding the relative contribution of inhibition of XO and URAT1 on albuminuria.
The SAPPHIRE study was preceded by the Phase 2a CITRINE study that enrolled 60 patients with T2DM and albuminuria to verinurad and febuxostat versus placebo for 24 weeks [17]. The primary endpoint was UACR at 12 weeks, although treatment was continued for 24 weeks. Verinurad + febuxostat, compared with placebo, decreased UACR by 39% (90% CI 4–62%) at 12 weeks and by 49% (90% CI 19–68%) at 24 weeks (Figure 3). These data supported further development of verinurad, as improvement in UACR of >25% after 6 months of treatment has been linked to clinically important improvements in kidney outcomes in subsequent outcomes studies [20]. However, owing to the broad CIs reported in the CITRINE study and uncertainty in the dose–response relationship between verinurad and UACR, it would be premature to initiate a Phase 3 programme based on this study alone. Therefore, the Phase 2b SAPPHIRE study is the next logical step in the development of verinurad.
FIGURE 3.
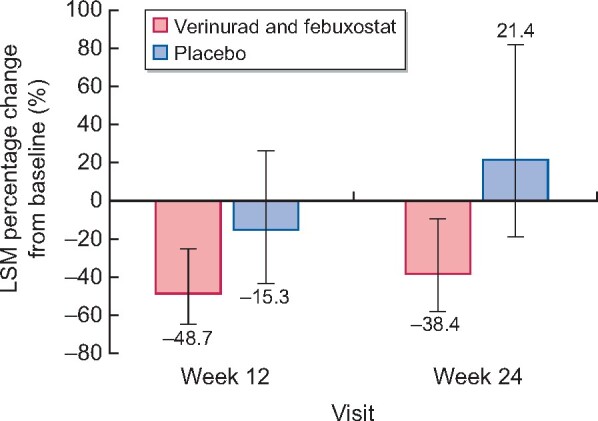
LSM percentage change from baseline in UACR at Weeks 12 and 24 in the CITRINE study. Error bars are 95% CIs. Results are based on a mixed-effects model for repeated measures. Baseline mean (SD) UACR was 459.1 (824.7) mg/g for verinurad plus febuxostat and 411.6 (547.8) mg/g for placebo. LSM, least-squares mean.
The rationale for combining verinurad with allopurinol is derived from observations that have linked transient creatinine elevations with potent URAT1 inhibitor monotherapy [25]. Observed elevations in serum creatinine levels are possibly attributable to UA crystalluria but the precise mechanism is not yet confirmed. The risk of UA crystalluria can be mitigated substantially by reducing UA production through combination of a URAT1 inhibitor with an XOI. Previous reports support the notion that the combination of verinurad and an XOI is not associated with an increased rate of creatinine elevations [15, 16]. Previously, verinurad was investigated in combination with febuxostat [15, 17], based on the lower risk of hypersensitivity reactions compared with other XOIs [26]. However, the CARES study suggested that febuxostat may be associated with an increased risk of all-cause and cardiovascular mortality compared with allopurinol [27]. Accordingly, SAPPHIRE is conducted using allopurinol instead of febuxostat, in combination with verinurad, and an event adjudication committee will ensure a robust assessment of cardiovascular events during the trial. Following completion of recruitment for the SAPPHIRE trial, results from the FAST study confirmed that febuxostat is non-inferior to allopurinol [28].
Whether lowering sUA alone is sufficient to reduce the rate of CKD progression is a topic of intense debate [29–31]. Key issues in this debate include the mechanism of action and extent of sUA lowering required to achieve clinical benefit. Four recent clinical trials have evaluated the efficacy of sUA-lowering strategies for the treatment of hyperuricaemia. In PERL, 530 patients with Type 1 diabetes with or without hyperuricaemia, who were at high risk of CKD progression, were randomized to allopurinol 200–400 mg or placebo for 3 years plus a 2-month washout period [32]. The trial showed no benefit of sUA reduction with allopurinol on iohexol-measured glomerular filtration rate or albuminuria [32]. Similarly, in the CKD-FIX trial, which enrolled 369 patients with Stage 3/4 CKD to 24 months’ treatment with allopurinol 100–300 mg, allopurinol did not slow the rate of eGFR decline compared with placebo [33]. In the FEATHER trial, 467 patients with Stage 3 CKD and asymptomatic hyperuricaemia were randomized to febuxostat or placebo for 108 weeks [34]. The study showed no difference in the rate of eGFR after 108 weeks of treatment with febuxostat compared with placebo [34]. Finally, FREED, a randomized, open-label, blinded endpoint trial, compared the effect of 40 mg febuxostat with physician’s choice of allopurinol 100 mg or no therapy on the risk of major cardiovascular and renal events among 1070 elderly patients with hyperuricaemia (7–9 mg/dL) with adverse cardiovascular profiles [35]. Febuxostat reduced both the primary composite outcome and the renal endpoint (i.e. development of microalbuminuria/mild proteinuria, progression to overt albuminuria/severe proteinuria or worsening of overt albuminuria, doubling of serum creatinine level, progression to ESKD) by 25% versus the non-febuxostat group, driven primarily by a lower rate of development of microalbuminuria, development of overt albuminuria and worsening of overt albuminuria [35]. However, there was no significant difference between treatment groups for doubling of serum creatinine [35]. The findings from these recent studies point to residual uncertainty as to the benefit of urate-lowering therapy as a treatment to prevent CKD progression. Limitations in the design and conduct of these studies, such as the relatively small sample sizes and heterogeneity in the study populations, have been reviewed elsewhere [36]. It remains to be seen whether the conflicting results were a consequence of differences in study populations, specific interventions tested, extent of sUA-lowering, insufficient power due to small sample sizes, or the appropriateness of study designs. The SAPPHIRE study will contribute to our current understanding of sUA in kidney disease and the potential kidney protective effects of urate-lowering therapies. First, it will explore the benefit of a new urate-lowering drug with a novel mechanism of action (i.e. URAT1 inhibition) on albuminuria and eGFR decline. Secondly, it will assess the impact of an intensive urate-lowering strategy (ranging from 35% to 80% reduction in sUA) on these outcomes, in order to elucidate a potential dose–response effect.
The conclusions from the analyses conducted for a joint National Kidney Foundation/US Food and Drug Administration/European Medicines Agency workshop in 2018 suggest that UACR at 6 months is a likely valid surrogate endpoint, and that eGFR slope over 2 or 3 years is a valid surrogate endpoint, for kidney failure in Phase 3 randomized controlled trials [37]. The SAPPHIRE study focuses on UACR as the primary endpoint, owing to the smaller sample size and shorter treatment duration required compared with assessment of eGFR slope. The SAPPHIRE study design has several novel features to maximize assessment of the treatment effects on eGFR (Table 5). Namely, the 60-week treatment duration allows preliminary assessment of effects on eGFR, and analysing the effects of verinurad on UACR and eGFR may provide an improved estimate of the long-term treatment effects on clinical endpoints. Moreover, implementation of interim analyses conducted independently of the study team allowed the study to be adapted, enabling the addition of a 24 mg verinurad dose to ensure adequate exploration of the full dose range.
Table 5.
Novelty of the SAPPHIRE study design
| (i) Treatment extended beyond 6 months to collect longer-term safety and eGFR data compared with traditional Phase 2 studies focused on UACR in CKD |
| (ii) In the SAPPHIRE study, both UACR and eGFR are endpoints; having results from both in the same study will improve the planning and design of a subsequent renal outcomes study |
| (iii) The adaption of SAPPHIRE to include a 24 mg verinurad treatment period from Month 9 in a subset of patients following an interim analysis represents a step-wise approach to collect safety and efficacy data for the full dose range of verinurad in response to emerging PK, PD and safety results from SAPPHIRE and other trials, without affecting the scientific integrity of SAPPHIRE |
The SAPPHIRE trial enrolled patients with a variety of eGFR levels, which allows exploration of the efficacy and safety of verinurad in various stages of CKD. The majority of participants had diabetic nephropathy, followed by ischaemic/hypertensive nephropathy and chronic glomerulonephritis. Compared with the DAPA-CKD trial, which also enrolled patients with CKD with and without T2DM, participants in the SAPPHIRE trial were more likely to have a diagnosis of diabetic nephropathy and less likely to be diagnosed with chronic glomerulonephritis [38]. The CKD-FIX and PERL trials previously assessed the effects of allopurinol on kidney function in patients with CKD [33, 39]. In contrast to the CKD-FIX trial, the SAPPHIRE trial enrolled a somewhat older patient cohort with a higher prevalence of T2DM, a higher eGFR and lower albuminuria and serum urate levels.[33] Comparing the SAPPHIRE cohort with the PERL trial, our cohort had a lower eGFR and higher albuminuria and serum urate levels [39].
Clinical practice guidelines recommend SGLT2 inhibitors for the treatment of CKD in patients with Type 2 diabetes. Recruitment of the SAPPHIRE trial started well before these recommendations were issued. As a result, a relatively small proportion of 10% of patients participating in the SAPPHIRE trial was using an SGLT2 inhibitor at baseline. However, this subgroup allows us to explore the efficacy of verinurad and allopurinol as adjunct to SGLT2 inhibitors and ACEi or ARB. Establishing the safety of verinurad and allopurinol in this subgroup is also important since there have been initial concerns that the combination of verinurad with SGLT2 inhibitors may increase UA excretion, thereby theoretically increasing the risk of UA nephrolithiasis. However, a dedicated drug–drug interaction study with dapagliflozin and verinurad demonstrated that adding dapagliflozin to a combination of verinurad plus febuxostat did not increase urinary UA excretion rates but led to a marked and significant lowering of sUA compared with verinurad plus febuxostat alone [40]. These data indicate that combining verinurad with dapagliflozin further reduces sUA without adversely impacting UA excretion.
In conclusion, CKD remains an unmet medical need despite recent advances in therapeutic interventions. Verinurad in combination with an XOI may represent a novel therapy to slow progression of CKD based on early clinical data. As such, the Phase 2b SAPPHIRE study was designed to confirm the clinical benefits of verinurad and expand our understanding urate-lowering strategies in CKD.
Supplementary Material
ACKNOWLEDGEMENTS
Medical writing support, including assisting authors with the development of the initial draft and incorporation of comments, was provided by Lucy Ambrose, DPhil and Jessica Gorrill, MSc; editorial support, including referencing, figure preparation, formatting, proofreading and submission, was provided by Rachael Cazaly, BSc, all of Core Medica, London, UK, supported by AstraZeneca according to Good Publication Practice guidelines. The Members of the SAPPHIRE Scientific Advisory Committee designing the study: H.J.L. Heerspink, Vlado Perkovic, Austin G. Stack and Robert Terkeltaub. Members of the Independent Data Monitoring Committee: Marc C. Hochberg (Chair, University of Maryland), David Charytan (New York University), Michelle O’Donoghue (Brigham and Women’s Hospital) and Robert Makuch (Yale University). Members of the Event Adjudication Committee: William B. White (Chair; Calhoun Cardiology Center), Peter R. Kowey (Main Line Health System), Donna Simonds (Main Line Health System) and P.B. Gorelick (Thorek Memorial Hospital).
FUNDING
The SAPPHIRE study is sponsored by AstraZeneca. The sponsor was involved in the study design, writing of the article and the decision to submit the article for publication.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.
REFERENCES
- 1. Wang H, Naghavi M, Allen C. et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1459–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D. et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 3. Mujais SK, Story K, Brouillette J. et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol 2009; 4: 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Webster AC, Nagler EV, Morton RL. et al. Chronic kidney disease. Lancet 2017; 389: 1238–1252 [DOI] [PubMed] [Google Scholar]
- 5. Liyanage T, Ninomiya T, Jha V. et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015; 385: 1975–1982 [DOI] [PubMed] [Google Scholar]
- 6. KDIGO. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf (14 April 2020, date last accessed). [DOI] [PubMed]
- 7. Remuzzi G, Perico N, Macia M. et al. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl 2005; 99: S57–S65 [DOI] [PubMed] [Google Scholar]
- 8. KDIGO. KDIGO Clinical Practice Guideline on Diabetes Management in Chronic Kidney Disease (Public Review Version). https://kdigo.org/wp-content/uploads/2018/03/KDIGO-Diabetes-Management-in-CKD_Public-Review.pdf (June 9, 2020. date last accessed)
- 9. Li J, Neal B, Perkovic V. et al. Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int 2020; 98: 769–777 [DOI] [PubMed] [Google Scholar]
- 10. Li J, Woodward M, Perkovic V. et al. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail 2020; 8: 57–66 [DOI] [PubMed] [Google Scholar]
- 11. Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 12. Heerspink HJL, Stefansson BV, Correa-Rotter R. et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446 [DOI] [PubMed] [Google Scholar]
- 13. Tan PK, Liu S, Gunic E. et al. Discovery and characterization of verinurad, a potent and specific inhibitor of URAT1 for the treatment of hyperuricemia and gout. Sci Rep 2017; 7: 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fitz-Patrick D, Roberson K, Niwa K. et al. Safety and efficacy of verinurad, a selective URAT1 inhibitor, for the treatment of patients with gout and/or asymptomatic hyperuricemia in the United States and Japan: findings from two phase II trials. Mod Rheumatol 2019; 29: 1042–1052 [DOI] [PubMed] [Google Scholar]
- 15. Fleischmann R, Winkle P, Hall J. et al. Pharmacodynamic and pharmacokinetic effects and safety of verinurad in combination with febuxostat in adults with gout: a phase IIa, open-label study. RMD Open 2018; 4: e000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleischmann R, Winkle P, Miner JN. et al. Pharmacodynamic and pharmacokinetic effects and safety of verinurad in combination with allopurinol in adults with gout: a phase IIa, open-label study. RMD Open 2018; 4: e000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stack AG, Dronamraju N, Parkinson J. et al. Effect of intensive urate lowering with combined verinurad and febuxostat on albuminuria in patients with type 2 diabetes: a randomized trial. Am J Kidney Dis 2021; 77: 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH. et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michels WM, Grootendorst DC, Verduijn M. et al. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 2010; 5: 1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heerspink HJL, Greene T, Tighiouart H. et al. ; Chronic Kidney Disease Epidemiology Collaboration. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 2019; 7: 128–139 [DOI] [PubMed] [Google Scholar]
- 21. Leoncini G, Martinoli C, Viazzi F. et al. Changes in renal resisitve index and urinary albumin excretion in hypertensive pateints under long-term treatment with lisinopril or nifedipine GITS. Nephron 2002; 90: 169–173 [DOI] [PubMed] [Google Scholar]
- 22. Hill NR, Fatoba ST, Oke JL. et al. Global prevalence of chronic kidney disease - A systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Golestaneh L, Alvarez PJ, Reaven NL. et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care 2017; 23: S163–S172 [PubMed] [Google Scholar]
- 24. Xie X, Liu Y, Perkovic V. et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis 2016; 67: 728–741 [DOI] [PubMed] [Google Scholar]
- 25. Sanchez-Niño MD, Zheng-Lin B, Valiño-Rivas L. et al. Lesinurad: what the nephrologist should know. Clin Kidney J 2017; 10: 679–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jordan A, Gresser U.. Side effects and interactions of the xanthine oxidase inhibitor febuxostat. Pharmaceuticals (Basel) 2018; 11: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. White WB, Saag KG, Becker MA. et al. ; CARES Investigators. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018; 378: 1200–1210 [DOI] [PubMed] [Google Scholar]
- 28. Mackenzie IS, Ford I, Nuki G. et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 2020; 396: 1745–1757 [DOI] [PubMed] [Google Scholar]
- 29. Miyaoka T, Mochizuki T, Takei T. et al. Serum uric acid levels and long-term outcomes in chronic kidney disease. Heart Vessels 2014; 29: 504–512 [DOI] [PubMed] [Google Scholar]
- 30. Kumagai T, Ota T, Tamura Y. et al. Time to target uric acid to retard CKD progression. Clin Exp Nephrol 2017; 21: 182–192 [DOI] [PubMed] [Google Scholar]
- 31. Badve SV, Brown F, Hawley CM. et al. Challenges of conducting a trial of uric-acid-lowering therapy in CKD. Nat Rev Nephrol 2011; 7: 295–300 [DOI] [PubMed] [Google Scholar]
- 32. Doria A, Galecki AT, Spino C. et al. ; PERL Study Group. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med 2020; 382: 2493–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Badve SV, Pascoe EM, Tiku A. et al. ; CKD-FIX Study Investigators. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med 2020; 382: 2504–2513 [DOI] [PubMed] [Google Scholar]
- 34. Kimura K, Hosoya T, Uchida S. et al. ; FEATHER Study Investigators. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis 2018; 72: 798–810 [DOI] [PubMed] [Google Scholar]
- 35. Kojima S, Matsui K, Hiramitsu S. et al. Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy. Eur Heart J 2019; 40: 1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russo E, Verzola D, Leoncini G. et al. Treating hyperuricemia: the last word hasn’t been said yet. J Clin Med 2021; 10: 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levey AS, Gansevoort RT, Coresh J. et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 2020; 75: 84–104 [DOI] [PubMed] [Google Scholar]
- 38. Wheeler DC, Stefansson BV, Batiushin M. et al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 2020; 35: 1700–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Afkarian M, Polsky S, Parsa A. et al. ; PERL Study Group. Preventing early renal loss in diabetes (PERL) study: a randomized double-blinded trial of allopurinol-rationale, design, and baseline data. Diabetes Care 2019; 42: 1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stack AG, Han D, Goldwater R. et al. Dapagliflozin added to verinurad plus febuxostat further reduces serum uric acid in hyperuricemia: the QUARTZ study. J Clin Endocrinol Metab 2021; 106: e2347–e2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.