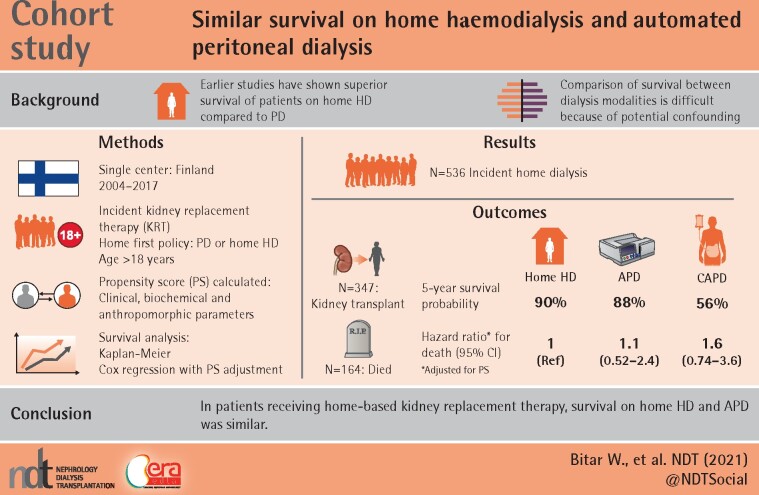
ABSTRACT
Background
Several studies have shown superior survival of patients on home haemodialysis (HD) compared with peritoneal dialysis (PD), but patients on automated PD (APD) and continuous ambulatory PD (CAPD) have not been considered separately. As APD allows larger fluid volumes and may be more efficient than CAPD, we primarily compared patient survival between APD and home HD.
Methods
All adult patients who started kidney replacement therapy (KRT) between 2004 and 2017 in the district of Helsinki-Uusimaa in Finland and who were on one of the home dialysis modalities at 90 days from starting KRT were included. We used intention-to-treat analysis. Survival of home HD, APD and CAPD patients was studied using Kaplan–Meier curves and Cox regression with adjustment for propensity scores that were based on extensive data on possible confounding factors.
Results
The probability of surviving 5 years was 90% for home HD, 88% for APD and 56% for CAPD patients. After adjustment for propensity scores, the hazard ratio of death was 1.1 [95% confidence interval (CI) 0.52–2.4] for APD and 1.6 (95% CI 0.74–3.6) for CAPD compared with home HD. Censoring at the time of kidney transplantation (KTx) or at transfer to in-centre HD did not change the results. Characteristics of home HD and APD patients at the start of dialysis were similar, whereas patients on CAPD had higher median age and more comorbidities and received KTx less frequently.
Conclusions
Home HD and APD patients had comparable characteristics and their survival appeared similar.
Keywords: home dialysis, home haemodialysis, kidney replacement therapy, peritoneal dialysis, survival
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS
What is already known about this subject?
Earlier studies have shown superior survival of patients on home haemodialysis (HD) compared with peritoneal dialysis (PD), but automated PD (APD) and continuous ambulatory PD have not been considered separately.
Comparison of survival between dialysis modalities is difficult because of potential confounding.
What this study adds?
Patients on APD and home HD have similar survival outcomes.
What impact this may have on practice or policy?
This study provides information on survival outcomes, which is important for healthcare professionals and patients when choosing the type of home dialysis modality.
INTRODUCTION
Since home dialysis has been associated with lower costs, higher participation in working life and better quality of life [1–3], many centres have a home-first policy. However, the proportion of patients on peritoneal dialysis (PD) and home haemodialysis (HD) varies markedly between countries, for instance, 3% in Japan, 7% in France, 12% in the USA, 26% in Finland, 47% in New Zealand and 74% in Hong Kong [4].
Several studies have compared survival of patients on PD and HD [5–8]. PD appears to be associated with better survival during the first 2 years of kidney replacement therapy (KRT), but the difference disappears thereafter. However, a reliable comparison is difficult because randomized trials of dialysis modalities have not been feasible. Outcomes of various types of dialysis are compared in observational studies, which require adequate adjustment for confounding, because patient characteristics typically differ considerably between dialysis modalities.
To our knowledge, six studies have compared the survival prognosis between patients on home HD and PD [9–14]. In all of these studies, home HD was associated with lower mortality compared with PD. Notably, none of the studies analysed automated PD (APD) and continuous ambulatory PD (CAPD) separately. As APD allows more flexible adjustment of dialysis treatment, e.g. by easily increasing the number and volume of fluid exchanges, it may improve dialysis accuracy, and some studies have shown improved patient survival on APD compared with CAPD [15, 16], whereas others have not [17, 18]. APD requires more technical skills of the patient than CAPD, and in that regard APD and home HD patients can be expected to share similar characteristics. Therefore, when comparing outcomes of patients on home HD with those on PD, selection bias may be reduced if specifically comparing with patients on APD.
In this study performed in an academic hospital with a long commitment to home dialysis, we investigated patient survival in home therapies. We used comprehensive data to adjust for putative confounding factors and we primarily compared APD with home HD.
MATERIALS AND METHODS
Study design and population
Of 1640 patients ≥18 years of age who started KRT in 2004–2017 in the healthcare district of Helsinki-Uusimaa, Finland, 536 patients were on home dialysis 90 days after initiating KRT and were included in the analysis. This healthcare district serves 1.7 million inhabitants, which is 30% of Finland’s population. All home dialysis patients in the district are treated and followed up centrally at Helsinki University Hospital. The follow-up data were complete until moving outside the district (n =13), return of kidney function (n = 0), death (n = 164) or end of follow-up on 31 December 2019 (n = 359). During the follow-up, 347 patients received a kidney transplant.
During the study period in the healthcare district of Helsinki-Uusimaa, there was a systematic dialysis modality selection with a ‘home-first’ policy. This means that both PD and home HD were equally prioritized. Patients were informed about dialysis modalities by lectures and on-spot information before the modality choice was made. The medical team evaluated the patients for contraindications and suitability for various modalities and informed the patients accordingly. If there were no medical contraindications, the patient could choose the modality that was most suitable with regard to working life, social life, hobbies, etc. The decision was made in a multidisciplinary manner together with a dialysis nurse, nephrologist, the patient and family members.
Data collection
We established a structured research database and collected extensive data from the patient data system of the entire healthcare district. We collected data retrospectively on 34 various comorbidities, primary renal disease (PRD), laboratory results, blood pressure (BP), electrocardiogram (ECG) parameters, findings on heart ultrasound and social variables (Supplementary data, Table S1).
Statistical analysis
Survival probabilities were calculated using Kaplan–Meier curves according to the type of dialysis treatment (home HD, CAPD or APD) at 90 days from the start of KRT. Patients were followed up from the start of KRT until death (the event) or the end of follow-up, and if follow-up was >5 years, they were censored at 5 years from the start of KRT. Hazard ratios (HRs) of death associated with dialysis modality were assessed using Cox regression with adjustment for propensity scores [19, 20]. The proportional hazards assumption was assessed graphically.
Propensity scores were developed pairwise for APD versus home HD, CAPD versus home HD and PD (APD and CAPD as one group) versus home HD using binary logistic regression. Explanatory variables were selected from those listed in Supplementary data, Table S1 using a stepwise forward procedure. The variables human immunodeficiency virus and Parkinson’s disease were not included due to the small number of affected patients. The P-value for inclusion was <0.10. Missing values were statistically imputed using predictors as indicated in Supplementary data, Table S1. Additionally, the events waitlisting for kidney transplantation (KTx), KTx and death were used as predictors in the imputation. There were no missing data for comorbidities because each comorbidity was analysed as either found or not found in the patient files before the start of KRT. Table 1 displays the variables that were finally included for calculation of the propensity scores and Supplementary data, Table S1 further indicates which variables were used for calculating propensity scores for APD versus home HD, CAPD versus home HD and PD versus home HD.
Table 1.
Patient characteristics that were used for propensity scores
| Characteristics | APD | CAPD | PD | Home HD |
|---|---|---|---|---|
|
Patients, n |
229 | 162 | 391 | 145 |
| Total deathsa, n | 46 | 90 | 136 | 28 |
| Deaths in 5 yearsb, n | 26 | 66 | 92 | 12 |
| Deaths per 1000 patient-yearsb, n | 108 | 252 | 146 | 87 |
| PRD, % | ||||
| Glomerulonephritis | 22 | 17 | 20 | 24 |
| Cystic kidney disease | 15 | 6 | 11 | 31 |
| Type 1 diabetes | 21 | 19 | 21 | 17 |
| Type 2 diabetes | 9 | 20 | 13 | 5 |
| Interstitial nephritis | 4 | 4 | 4 | 1 |
| Hypertension | 4 | 5 | 4 | 2 |
| Unknown | 13 | 20 | 16 | 10 |
| Others | 12 | 11 | 12 | 9 |
| Comorbidities, % | ||||
| Malignancy | 5 | 11 | 7 | 10 |
| Obesity | 17 | 22 | 19 | 34 |
| Atrial fibrillation | 5 | 15 | 9 | 5 |
| Visual problems | 26 | 29 | 27 | 14 |
| Type 2 diabetes | 9 | 21 | 14 | 10 |
| Hypertension | 84 | 82 | 83 | 89 |
| Compliance problem, % | 15 | 14 | 15 | 10 |
| Support in daily activities, % | 9 | 30 | 18 | 8 |
| Dialysis assistance, %b | ||||
| By professional | 1.7 | 4.9 | 3.1 | 0.7 |
| By family member | 4.4 | 18.5 | 10.2 | 4.1 |
| KTx-listed, %a,b | 85 | 51 | 71 | 88 |
| KTx, %a,b | 73 | 38 | 59 | 81 |
| Continuous variables, median (IQR) | ||||
| Age (years) | 50 (40–61) | 65 (52–74) | 55 (43–68) | 50 (42–60) |
| QT-interval on ECG (ms) | 412 (386–440) | 416 (390–440) | 413 (386–440) | 404 (381–436) |
| Systolic BP (mmHg) | 144 (132–159) | 148 (132–168) | 146 (132–161) | 148 (132–168) |
| Diastolic BP (mmHg) | 89 (79–95) | 85 (76–92) | 87 (78–94) | 84 (76–93) |
| Height (cm) | 173 (165–180) | 170 (164–178) | 173 (164–180) | 174 (168–180) |
| Weight (kg) | 77 (66–87) | 75 (63–85) | 77 (65–87) | 80 (70–93) |
| Body mass index (kg/m2) | 25 (23–28) | 26 (22–28) | 25 (23–28) | 26 (23–31) |
| Laboratory findings, median (IQR) | ||||
| P-creatinine (mmol/L) | 583 (484–708) | 558 (470–660) | 573 (480–689) | 621 (532–712) |
| P-albumin (g/L) | 36 (33–39) | 36 (32–38) | 36 (32–39) | 36 (33–38) |
| P-ionized calcium (mmol/L) | 1.2 (1.1–1.3) | 1.2 (1.2–1.3) | 1.2 (1.2–1.3) | 1.2 (1.1–1.2) |
| B-haemoglobin (g/L) | 111 (103–120) | 114 (105–122) | 113 (104–121) | 109 (100–118) |
| P-C-reactive protein (mg/L) | 3 (<3–5) | 5 (<3–7) | 4 (<3–7) | 4 (<3–5) |
| Total P-cholesterol (mmol/L) | 4.2 (3.5–5.0) | 4.0 (3.4–4.6) | 4.1 (3.4–4.8) | 3.9 (3.3–4.8) |
| P-triglycerides (mmol/L) | 1.4 (1.0–2.0) | 1.6 (1.1–2.0) | 1.5 (1.1–2.0) | 1.4 (0.97–1.9) |
IQR, interquartile range; CAD, coronary artery disease. aDuring the entire follow-up period. bNot included in propensity scores.
To ensure the robustness of the results, several sensitivity analyses were conducted. Cox regression was performed with censoring at either transplantation or transfer from home dialysis to in-centre HD. Additionally, models that included either KTx or waitlisting for transplantation as time-dependent variables were evaluated (Table 3). Models were also constructed without propensity score adjustment (data not shown).
Table 3.
HR of death according to home dialysis modality (sensitivity analyses with propensity score adjustment)
| Explanatory variable | Hazard ratio | 95% CI for hazard ratio |
|---|---|---|
| Censoring at transfer to in-centre HD or at transplantationa | ||
| APD (compared with home HD) | 1.3 | 0.51–3.3 |
| CAPD (compared with home HD) | 1.1 | 0.40–2.9 |
| PD (compared with home HD) | 1.4 | 0.63–3.1 |
| KTx waitlisting as a time-dependent variablea | ||
| APD (compared with home HD) | 1.2 | 0.57–2.6 |
| CAPD (compared with home HD) | 1.5 | 0.68–3.2 |
| PD (compared with home HD) | 1.5 | 0.81–2.9 |
| KTx as a time-dependent variablea | ||
| APD (compared with home HD) | 0.96 | 0.44–2.1 |
| CAPD (compared with home HD) | 1.4 | 0.63–2.9 |
| PD (compared with home HD) | 1.3 | 0.66–2.4 |
| For the total study period, not censoring at 5 years | ||
| APD (compared with home HD) | 0.87 | 0.51–1.5 |
| CAPD (compared with home HD) | 1.3 | 0.74–2.3 |
| PD (compared with home HD) | 1.1 | 0.72–1.7 |
Censored at 5 years since the start of KRT.
RESULTS
Patients on home HD and APD showed similar characteristics. The median age in both patient groups was 50 years at the start of KRT and 51–54% had three or more comorbidities. In addition, the proportion of those waitlisted for KTx or of those receiving a transplant during the follow-up was similar (Supplementary data, Table S1). CAPD patients were older (65 years), 67% had three or more comorbidities and they more often needed support in daily activities and assistance in dialysis.
In unadjusted analyses, Kaplan–Meier curves showed similar survival probability for APD and home HD patients, whereas CAPD patients had lower survival probability (Figure 1). The 5-year survival was 90% for home HD, 88% for APD and 56% for CAPD patients. The 5-year survival for all PD patients was 75% (Figure 2).
FIGURE 1.
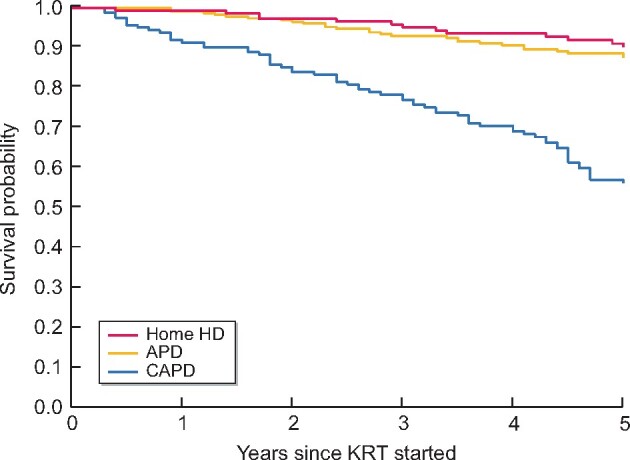
Survival of patients on home HD, APD or CAPD.
FIGURE 2.
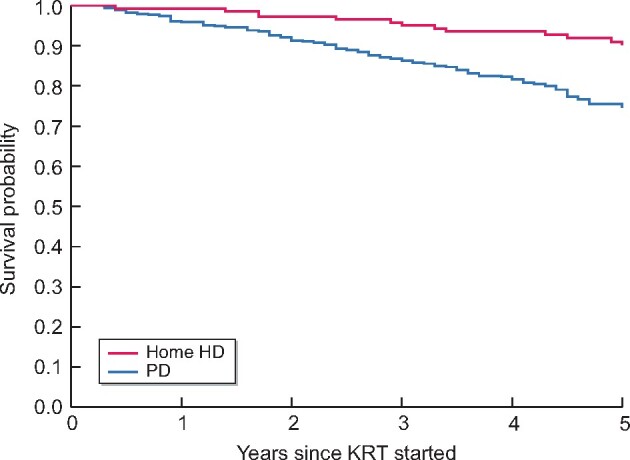
Survival of patients on home HD or PD.
Both in unadjusted and in age- and sex-adjusted Cox regression analyses, the risks of death did not differ significantly between patients on APD and home HD (Table 2). Adjustment for propensity scores did not alter the result with an HR of death of 1.1 [95% confidence interval (CI) 0.52–2.4] for APD compared with home HD. The unadjusted HR of death associated with CAPD was almost 5.9-fold, while age- and sex-adjusted risk was 3.5-fold compared with that associated with home HD. However, after adjustment for propensity score, the HR of death diminished to 1.6 (95% CI 0.74–3.6), showing no statistically significant difference between CAPD and home HD. We also compared survival of all PD patients (both APD and CAPD) to that of home HD patients. With adjustment for propensity scores, the HR of death was 1.5 (95% CI 0.81–2.9) for PD compared with home HD.
Table 2.
HR of death in 5 years according to home dialysis modality
| Explanatory variable | Hazard ratio | 95% CI of hazard ratio |
|---|---|---|
| Unadjusted 5-year risk of death | ||
| Home HD (reference) | ||
| APD | 1.4 | 0.69–2.7 |
| CAPD | 5.9 | 3.2–10.9 |
| PD | 3.1 | 1.7–5.6 |
| Adjusted for age and sex 5-year risk of death | ||
| Home HD (reference) | ||
| APD | 1.4 | 0.69–2.7 |
| CAPD | 3.5 | 1.8–6.7 |
| PD | 2.2 | 1.2–4.1 |
| 5-year risk of death adjusted for propensity score | ||
| APD (compared with Home HD) | 1.1 | 0.52–2.4 |
| CAPD (compared with Home HD) | 1.6 | 0.74–3.6 |
| PD (compared with Home HD) | 1.5 | 0.81–2.9 |
We did several sensitivity analyses. When censoring at the time of transfer to in-centre HD or KTx, the HR of death for APD compared with home HD was 1.3 and the difference between the treatment modalities remained statistically insignificant (Table 3). Including waitlisting or KTx as a time-dependent variable in the model did not alter the results as compared with the propensity score–adjusted analysis in Table 2. We also performed an analysis with additional adjustment for the need for assistance in dialysis, but this did not alter the results. In addition, we included a sensitivity analysis without censoring at 5 years from the start of KRT, and this gave similar results.
DISCUSSION
In this study of 536 consecutive patients who entered home dialysis in 2004–2017, we observed similar survival among home HD and APD patients. After adjusting for potential confounders, this finding remained virtually unchanged, which was expected as these two patient groups showed many similar characteristics, e.g. age and number of comorbidities. Our study suggests that the choice between home HD and APD does not affect patients’ survival. CAPD patients showed worse survival, but the difference compared with home HD diminished and was not statistically significant after accounting for confounders. CAPD patients were significantly older and frailer and consequently the comparison of their survival with that of patients on other modalities was challenging, despite abundant data on confounders.
All earlier studies comparing home HD and PD have shown better patient survival on home HD. Studies from the UK, Australia, New Zealand and Sweden observed 30–40% lower risk of death associated with home HD compared with PD after adjustment for confounding factors [9–11, 14]. Our study also showed a survival advantage of home HD patients compared with PD patients in unadjusted analysis, but the difference disappeared after we accounted for confounding factors by propensity score adjustment. Two previous studies have also applied propensity scores when comparing survival on PD and home HD. In a study from Australia and New Zealand, home HD patients had 52% lower risk of premature death than PD patients in an analysis with propensity score matching [12]. In a study based on data from the US Renal Data System, Weinhandl et al. [13] showed a 20% lower mortality risk in 4201 daily home HD patients compared with a group of propensity score–matched PD patients. Our study took into account an exceptionally large number of confounders, among which there were 34 different comorbidities. Many of the potential confounders used in the analyses of our study, like patient compliance, need for support in daily activities, ECG findings and C-reactive protein, were assessed in earlier studies, and this might explain why adjustment for confounders made the survival difference disappear in our study, unlike in the earlier ones. There may be other explanations as well. When Weinhandl et al. [13] included only the patients who initiated home dialysis within 6 months from start of dialysis therapy, a situation more like that in our study, the survival difference between home HD and PD disappeared. Furthermore, a notable difference between our study and some of the earlier ones is the proportion of home HD patients out of all patients who entered chronic dialysis treatment. This proportion was 8% in our study and similar in the studies from Australia and New Zealand [10–12], but in all other studies [9, 13, 14] the proportion was <1%. A very small proportion of home HD probably indicates strict selection, meaning that only the fittest patients enter this treatment modality. A strong selection will favour home HD compared with PD and may be difficult to fully account for when adjusting for confounders.
Our study is unique in that it considered APD and CAPD separately. All earlier studies analysed PD patients as one group without distinction between CAPD and APD. Of the two types of PD, APD resembles home HD more in many aspects: both APD and home HD require the patient to manage a dialysis device independently at home with a certain degree of technical skills and sufficient physical and cognitive capacity. Thus it may be assumed that patients who select home HD have more characteristics in common with APD than with CAPD patients, which was also observed in our study. Consequently the similarity between home HD and APD patients may make the statistical comparison between these two groups more reliable.
When interpreting survival outcomes of home HD patients, it is important to have information about the frequency and duration of the dialysis sessions. These data were not directly available in our research database, but according to data collected by the Finnish Registry for Kidney Diseases about the home HD patients in our district, 76% had more than three weekly sessions, 53% had at least five sessions per week, 32% did >15 h of dialysis per week and 16% did nocturnal dialysis. Thus home HD patients in our cohort typically used more intense HD than just thrice-weekly 3- to 5-h sessions. In most of the earlier studies, the type of home HD was unspecified, except in one study that implied a similarity in survival between conventional and frequent/extended home HD [10]. The results may also be affected by the type of home HD device. In our study, all patients used conventional dialysis machines, whereas, for example, in the study of Weinhandl et al. [13], all home HD patients used one specific type of HD device with low dialysate volumes.
Our study has many strengths. The study is population-based, as we were able to include all patients in the healthcare district who were on home dialysis at 90 days from start of KRT. Comparing home HD patients separately with patients on APD and CAPD is unique for our study. Furthermore, the high proportion of home HD patients may reduce selection bias. We had complete data on the outcome and exceptionally extensive data on confounders. A lack of sufficient data on confounders was considered a concern by some of the earlier studies [10, 11, 13]. The observational nature of our study represents a limitation and leaves the possibility of residual confounding. Additionally, the relatively small number of patients makes analysis of subgroups difficult. Finally, patient selection differs between countries and centres, and our results may not be generalizable to other settings.
CONCLUSIONS
The results of our study may help healthcare professionals when counselling patients about selection of dialysis modalities. Our study suggests that patients’ survival may not be affected by the choice between home HD and APD. Consequently, factors other than survival should be considered when choosing dialysis modality. Further studies are needed to investigate outcomes other than survival, such as infections, cardiovascular events, hospitalizations and quality of life.
Supplementary Material
ACKNOWLEDGEMENTS
Study nurse Katja Henttunen is acknowledged for collecting data from patient files into the study database.
FUNDING
This study was supported by Finska läkaresällskapet, Liv och Hälsa and Helsinki University Hospital Governmental Research Funds. The funders had no role in the design and conduct of the study; collection, analysis or preparation of the data; or preparation, review or approval of the manuscript.
AUTHORS’ CONTRIBUTIONS
P.F. was responsible for the research idea. B.W., J.H. and P.F. were responsible for the study design and data analysis. B.W. and P.F. were responsible for data acquisition. B.W., J.H., E.H., V.R., M.H. and P.F. were responsible for data interpretation. J.H. and P.F. were responsible for supervision. Each author contributed important intellectual content during manuscript drafting and revision. All authors accept accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly because this was not allowed in the research permission provided by the Helsinki University Hospital.
REFERENCES
- 1. Couchoud C, Couillerot AL, Dantony E. et al. Economic impact of a modification of the treatment trajectories of patients with end-stage renal disease. Nephrol Dial Transplant 2015; 30: 2054–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kraus MA, Fluck RJ, Weinhandl ED. et al. Intensive hemodialysis and health-related quality of life. Am J Kidney Dis 2016; 68(5 Suppl 1): S33–S42 [DOI] [PubMed] [Google Scholar]
- 3. Helanterä I, Haapio M, Koskinen P. et al. Employment of patients receiving maintenance dialysis and after kidney transplant: a cross-sectional study from Finland. Am J Kidney Dis 2012; 59: 700–706 [DOI] [PubMed] [Google Scholar]
- 4. United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 5. Heaf JG, Løkkegaard H, Madsen M.. Initial survival advantage of peritoneal dialysis relative to hemodialysis. Nephrol Dial Transplant 2002; 17: 112–117 [DOI] [PubMed] [Google Scholar]
- 6. McDonald SP, Marshall MR, Johnson DW. et al. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009; 20: 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haapio M, Helve J, Kyllönen L. et al. Modality of chronic renal replacement therapy and survival—a complete cohort from Finland, 2000–2009. Nephrol Dial Transplant 2013; 28: 3072–3081 [DOI] [PubMed] [Google Scholar]
- 8. Korevaar JC, Feith GW, Dekker FW. et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int 2003; 64: 2222–2228 [DOI] [PubMed] [Google Scholar]
- 9. Nitsch D, Steenkamp R, Tomson CR. et al. Outcomes in patients on home hemodialysis in England and Wales, 1997–2005: a comparative cohort analysis. Nephrol Dial Transplant 2011; 26: 1670–1677 [DOI] [PubMed] [Google Scholar]
- 10. Marshall MR, Hawley CM, Kerr PG. et al. Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis 2011; 58: 782–793 [DOI] [PubMed] [Google Scholar]
- 11. Marshall MR, Walker RC, Polkinghorne KR. et al. Survival on home dialysis in New Zealand. PLoS One 2014; 9: e96847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nadeau-Fredette AC, Hawley CM, Pascoe EM. et al. An incident cohort study comparing survival on home hemodialysis and peritoneal dialysis (Australia and New Zealand Dialysis and Transplantation Registry). Clin J Am Soc Nephrol 2015; 10: 1397–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinhandl ED, Gilbertson DT, Collins AJ.. Mortality, hospitalization, and technique failure in daily home hemodialysis and matched peritoneal dialysis patients: a matched cohort study. Am J Kidney Dis 2016; 67: 98–110 [DOI] [PubMed] [Google Scholar]
- 14. Rydell H, Ivarsson K, Almquist M. et al. Improved long-term survival with home hemodialysis compared with institutional hemodialysis and peritoneal dialysis: a matched cohort study. BMC Nephrol 2019; 20: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li X, Xu H, Chen N. et al. The effect of automated versus continuous ambulatory peritoneal dialysis on mortality risk in China. Perit Dial Int 2018; 38(Suppl 2): S25–S35 [DOI] [PubMed] [Google Scholar]
- 16. Beduschi G. D C, Figueiredo AE, Olandoski M. et al. Automated peritoneal dialysis is associated with better survival rates compared to continuous ambulatory peritoneal dialysis: a propensity score matching analysis. PLoS One 2015; 1010: e0134047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Michels WM, Verduijn M, Boeschoten EW. et al. Similar survival on automated peritoneal dialysis and continuous ambulatory peritoneal dialysis in a large prospective cohort. Clin J Am Soc Nephrol 2009; 4: 943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang IK, Yu TM, Yen TH. et al. Comparison of patient survival and technique survival between continuous ambulatory peritoneal dialysis and automated peritoneal dialysis. Perit Dial Int 2020; 40: 563–572 [DOI] [PubMed] [Google Scholar]
- 19. Cox D. Regression models and life-tables. J R Stat Soc Ser B Stat Methodol 1972; 34: 187–220 [Google Scholar]
- 20. Edouard LF, Groenwold RHH, Zoccali C. et al. Merits and caveats of propensity scores to adjust for confounding. Nephrol Dial Transplant 2019; 34: 1629–1635 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly because this was not allowed in the research permission provided by the Helsinki University Hospital.