Abstract
Despite the recent acceleration in the discovery of genetic risk factors for intellectual disability (ID), the genetic etiology of ID is unknown in approximately half of cases and remains a major frontier of genetics in medicine and psychiatry. The distinction between syndromal and non-syndromal forms of ID is of great clinical importance, but the boundary between these clinical entities is difficult to ascertain for many genes of interest. ID is more common in men than in women, but the genetic explanation of this gender asymmetry is incompletely understood . This Review systematically examines the reported cases of X-linked ID caused by de novo loss-of-function mutations in the gene IQSEC2. This gene is largely known as a cause of X-linked non-syndromal ID in male patients. However, depending on the severity of the mutation, the phenotypic spectrum of IQSEC2-related ID can range from the classic X-linked non-syndromal form of the disease to a severe syndrome that has been reported in the context of de novo mutations only, in both male and female patients. Bioinformatic analysis suggests that truncation of the longer of the two protein isoforms of the gene can be sufficient to lead to the syndrome, which may be caused by the disruption of cell signaling and signal transduction pathways. The clinical features of the syndrome converge on a pattern of global developmental delay, deficits in social communication, stereotypical hand movements and hypotonia. In addition, many if not all of these patients have seizures, microcephaly and language regression in addition to delay. We argue that it is clinically appropriate to test for IQSEC2 mutations in male and female patients with this symptom profile but without a known genetic mutation.
Keywords: Intellectual disability, Syndromal intellectual disability, Rett syndrome, Autism spectrum disorder, IQSEC2
Genetic causes of intellectual disability (ID) represent a major frontier of genetics in medicine, neurology and psychiatry. The prevalence of ID in developed countries is ~2%, with more than 100 currently known specific genetic etiologies (Kwok & Cheung 2007; Neul et al. 2010; Leonard & Wen 2002; Lubs et al. 2012). X-linked ID is responsible for 8-12% of ID in the male population and represents an important though less frequent cause of ID in the female population (Frints et al. 2002; Zhao et al. 2014; Lubs et al. 2012). Although technological advancements have yielded an acceleration in genetic discovery, it has been estimated that at least half of ID remains idiopathic with a presumed genetic basis (Suthers et al. 1988; Gécz et al. 2009). While cases of “non-syndromal” ID traditionally lack other distinguishing clinical signs/symptoms beyond cognitive dysfunction, cases of “syndromal” ID manifest distinctive patterns of physical, metabolic or neurologic features (Shoubridge et al. 2010; Leonard & Wen 2002; Mulley et al. 1992). Psychiatric symptoms are a less appreciated, but also significant, part of the disease burden (Shoubridge et al. 2010; Kwok & Cheung 2007). The diagnostic boundary between non-syndromal and syndromal ID is sometimes unclear, and historically genes associated with non-syndromal ID have been reclassified when characteristic syndromes have been identified (Frints et al. 2002).
The clinical genetics of IQSEC2
The path to the discovery of the clinical significance of dysfunction of the IQ motif and Sec7 domain 2 (IQSEC2) protein began with demonstration of linkage to chr Xp11-cen in a family with X-linked, non-syndromal ID (Suthers et al. 1988). Eventually, four families were identified in which moderate to severe ID followed a classic X-linked recessive inheritance pattern, due to missense mutations in the IQSEC2 gene at chrXp11.22 (Shoubridge et al. 2010). Although essentially fitting the expected phenotype for non-syndromal ID, some of the reported clinical features suggested a more complex clinical phenotype than cognitive dysfunction alone. Of the 32 male patients affected with ID across these families, there were 8 individuals with language delay, 2 with motor delay, 5 with seizures and 5 with deficits in social communication.
As exemplified by the illustrative clinical case below, recent evidence has indeed broadened the phenotype of IQSEC2 mutations to encompass a syndromal form of disease, which includes a convergent array of symptoms beyond ID (see Table 1) (Morleo et al. 2008; Tran Mau-Them et al. 2014; Rauch et al. 2012; Redin et al. 2014; Epi4K Consortium et al. 2013; Gandomi et al. 2013; Olson et al. 2015). Of the nine cases reported in seven published reports, all individuals had language delay and motor delay. In addition, social deficits, motor stereotypies and hypotonia were present in the seven cases that reported the presence or absence of these signs/symptoms. All but one of the reported individuals suffered visual impairment, either strabismus or cortical dysfunction. Distinctive physical characteristics (see Figure 1) and behavioral problems such as excessive tantrums, inappropriate screaming and intrusiveness have also been reported.
Table 1: Clinical details of reported IQSEC2 mutations.
Signs, symptoms and presumed genetic etiologies are listed for reported cases of IQSEC2 mutations.
| Source of Information |
Sex | ID | Language delay |
Social delay |
Motor delay |
Motor Stereotypies |
Developmental Regression |
Microcephaly | Seizures | Hypotonia | Vision Impairment |
Truncating mutation |
De novo Mutation |
Mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olson et al. 2015 | F | X | X | X | X | X | X | O | O | X | X | X | X | p.Asn91LysfsX112 (exon 1) |
| Morleo et al. 2008 | F | X | X | NR | X | NR | X | O | X | X | X | X | X | Translocation within intron 1 |
| Mau-Them et al. 2014, patient 1 | M | X | X | X | X | X | O | X | X | X | X | X | X | Duplication between exon 2 and the end of exon 4 |
| Rauch et al. 2012 / Mau-Them et al. 2014, patient 2 | M | X | X | X | X | X | O | X | O | X | X | X | X | p.Arg855X (exon 7) |
| Mau-Them et al. 2014, patient 3 | M | X | X | X | X | X | X | X | X | X | X | X | X | Duplication between intron 2 and the end of exon 8 |
| Redin et al. 2014 | M | X | X | X | X | X | NR | O | X | NR | X | X | X | p.Gln1033X (exon 11) |
| Epi4K consortium, 2013 | F | X | X | NR | X | NR | O | O | X | NR | NR | X | X | p.Gln1108X (exon 13) |
| Gandomi et al. 2013, patient 1 | M | X | X | X | X | X | O | O | X | X | X | X | X | p.Ser861Thr (exon 7) D |
| Gandomi et al. 2013, patient 2 | M | X | X | X | X | X | O | X | X | X | X | X | X | p.Cys684X (exon 5) |
| Shoubridge et al. 2010, probands | 32 M | 32/32 | 8/20A | 5/32 | 2/14 | NR | O A | O A | 5/32 | NR | 1/8 A | O B | O B | p.Arg359Cys (exon 4), p.Arg758Gln (exon 5), p.Gln801Pro (exon 6), p.Arg863Trp (exon 8) |
| Shoubridge et al. 2010, carriers | 25 F | 2 /25 C | NR | NR | NR | NR | NR | NR | 1/25 | NR | NR | O B | O B | p.Arg359Cys (exon 4), p.Arg758Gln (exon 5), p.Gln801Pro (exon 6), p.Arg863Trp (exon 8) |
| Totals (non-familial cases) | 6M, 3F | 9/9 | 9/9 | 7/7 A | 9/9 | 7/7 A | 3/8A | 4/9 | 7/9 | 7/7 A | 8/8 A | 9/9 | 9/9 |
Reported only for part of sample
Missense point mutations in 4 separate locations in 4 distinct families with X-linked inheritance
An additional 8 carrier female patients had borderline ID (IQ range 70-85)
Also causes splice site abolition. NR, not reported; X, present; O, absent.
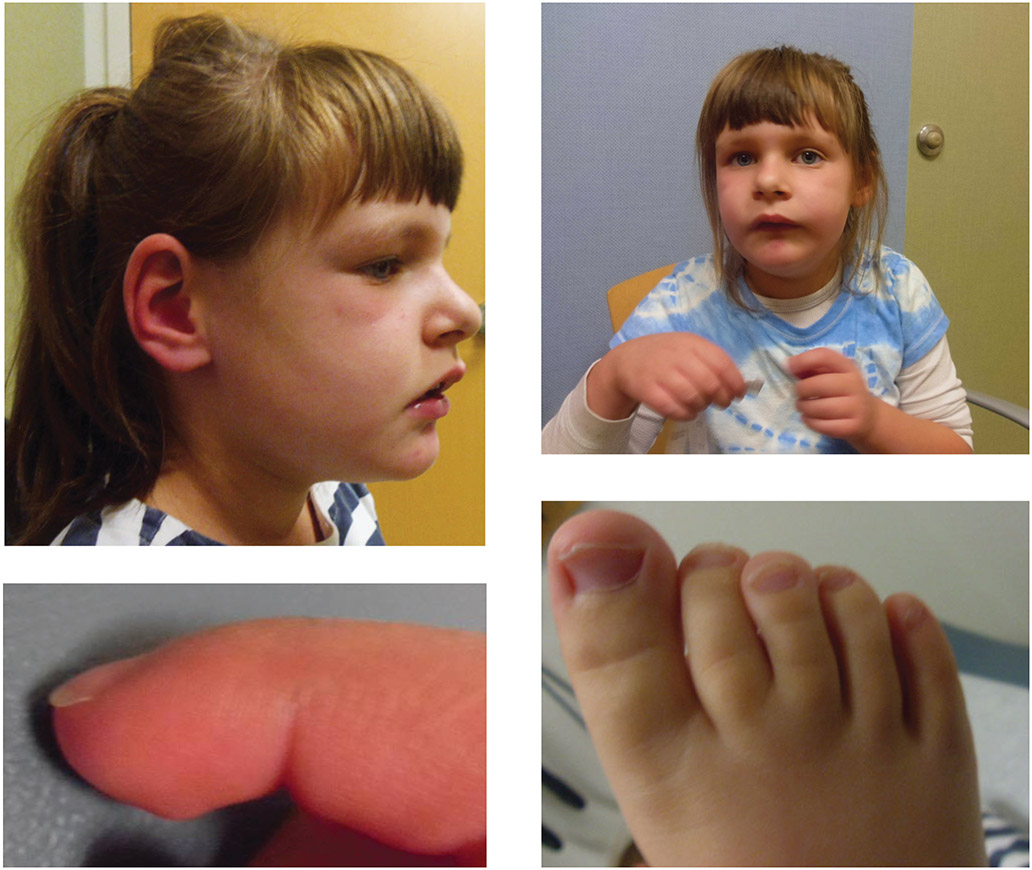
Figure 1: As described in the illustrative clinical case (originally reported in Olson et al. 2015), photographs of a 6-year-old female patient with IQSEC2-related illness.
Facial features are significant for deep-set eyes, prominent maxilla with overbite, downturned corners of mouth, broad central incisors with increased spacing, broad nasal root, full and everted lower lip and retrognathia. The patient has long, broad halluces and prominent fetal finger pads. She has a café-au-lait spot on the upper back and one on the left shin. Full written and informed consent was obtained for the use of the patient’s photographs and clinical data.
The clinical symptoms and signs of IQSEC2-related syndromal ID bear notable similarity to several other better-known syndromes. Common clinical features such as language regression, stereotypic hand movements, ataxia, hypotonia, microcephaly and seizures perhaps most closely resemble those of Rett syndrome. Although mutations in MECP2 account for close to 90% of cases of Rett syndrome, other genes including CDKL5, FOXG1 and MEF2C are thought to account for the reminder of cases of Rett and Rett-like syndrome (Zhao et al. 2014; Li et al. 2007; Rosas-Vargas et al. 2008; Bahi-Buisson et al. 2010; Jaillard et al. 2009; M. Zweier et al. 2010). It is tempting to conclude that IQSEC2 should be considered as another possible gene that when mutated can result clinically in Rett syndrome. However, it is also worth noting the clinical similarities with other syndromes, including the Pitt-Hopkins, Mowat-Wilson, Angelman and Angelman-like syndromes, which partially overlap in their clinical presentations as well as their genetic underpinnings (Hodge et al. 2014; Jaillard et al. 2009; de Pontual et al. 2009; C. Zweier et al. 2005). Because of their partial phenotypic overlap, evidence that they disrupt common functional genetic pathways and association with autism spectrum disorder, these disorders have been called “Rett Angelman spectrum disorders” (GeneDX, 2014; Mullegama et al., 2015; Tan et al., 2014; Jedele, 2007; Samaco et al., 2005). It is important to note that this concept of a Rett Angelman spectrum is largely favored in the research community, and its clinical use remains limited at this time.
The relationship between gender and IQSEC2-related disease requires further study. Case reports of IQSEC2-related ID in female patients remain relatively infrequent. Including both familial and de novo mutations, only five out of 43 reported cases of IQSEC2-associated ID occurred in female patients (excluding cases of borderline ID). Most of the female carriers were reported to be unaffected, although 2 out of the 25 had ID and an additional 8 had borderline ID (classified as IQ in the range of 70-85) (Shoubridge et al. 2010). However, limiting the focus to de novo syndromal cases, three out of nine reported cases have involved female patients. These data are not sufficient to reject the null hypothesis that the syndromal form of the disease is equally distributed between men and women (X-squared statistic= 1, df = 1, p-value = 0.32). Given a likely sampling bias towards male patients in studies of X-linked ID, it is plausible that female patients with IQSEC2-related ID are under-reported in the literature.
An illustrative clinical case
This patient (first reported in Olson et al. 2015) was referred to the Medical Genetics and Metabolism Division at MassGeneral Hospital for Children at age 6 years for possible exomic sequencing. She is the first-born child of healthy, non-consanguineous Caucasian parents. Her mother is of Ukranian and Ashkenazi Jewish descent, while her father is of Polish, German and English descent. At the time of the patient’s birth, her mother was age 30 years and her father was age 33 years. The pregnancy was complicated by fever and flu-like symptoms during the first trimester, treated with amoxicillin. The patient was born at 40 weeks gestation by normal spontaneous vaginal delivery after an 18-hour labor during which pitocin was utilized. At birth she had a weight of 7 pounds 4 ounces (56th percentile) and a length of 19 ¼ inches (44th percentile). Head circumference was within normal limits and remained within normal limits throughout development (mean percentile across 6 measurements in first 18 months of life, 44th percentile). After birth, she was noted to have mild latching and feeding difficulty secondary to low muscle tone. She went home with her parents within a few days. She had recurrent otitis media as an infant but was otherwise medically healthy.
Postnatal course was marked by global developmental delay, first noted at 6 months when she was not yet rolling over and could not sit without support. In terms of other aspects of motor development, she crawled at 15 months and walked at 18 months. She received physical therapy starting at 10 months. At age 2 years, she developed prominent motor stereotypies that have persisted to the present time including thrusting her tongue and mouthing, tapping and at times flapping her hands. At age 4 years, her gait was noted to be wide and ataxic. At age 6 years, she chewed primarily on the right side of her mouth and fed herself only with her right hand. She is unable to jump or to ride a tricycle, and she cannot dress herself. In terms of language, she initially had some development: by age 2 years, she had 10 clear words including hi, bye, mama, dada and cookie, and she was also able to repeat some of what others said to her. However, over a period of 6 months starting at age 2 years, she lost these words except for an occasional “hi”, and she also lost her ability to repeat, without subsequent regaining of these abilities. Receptive language is more advanced than expressive language. She understands commands related to her daily routine.
Socially, she enjoys being with other children and adults but does not know how to appropriately engage them in play. She displays motor hyperactivity that includes impulsive, intrusive and self-stimulatory behaviors. She is toilet trained based on a once-per-hour toileting schedule. She screams inappropriately at times. She sleeps well through the night. She does not have breath holding spells, though occasionally she had brief episodes of hyperventilation. She does not have a particular fascination with water. She lives with her parents and with her brother, who is 3 years younger and developmentally within normal limits. There is no family history of developmental or neurogenetic disorders. She has received adequate trials of aripiprazole, escitalopram, guanfacine, methylphenidate, ziprasidone and buspirone without improvement in her behavioral symptoms. A trial of risperidone resulted in improvement in hyperactivity but also weight gain and signs of precocious puberty before its discontinuation. A currently ongoing trial of quetiapine has resulted in improvement in some behavioral problems, but other behavioral problems persist including self-stimulatory behavior.
On exam, her height was at the 77%, weight 90%, and occipital-frontal head circumference 45%. Facial features were significant for deep-set eyes, prominent maxilla with overbite, downturned corners of mouth, broad central incisors with increased spacing, broad nasal root, full and everted lower lip and retrognathia. She had long and broad halluces, and prominent fetal finger pads (see Photographs). She had a café-au-lait spot on the upper back and one on the left shin. She was playful and smiling, constantly in motion, engaging in a variety of repetitive and intrusive behaviors. She attempted to take objects out of the examiner’s pocket and place them in her mouth. She showed pronounced right side dominance in her purposeful actions, and she chewed on the fingers of her left hand. She was hypotonic.
An MRI at age 2 years was notable for T2 prolongation at the lower limits of normal in frontal/periventricular white matter, consistent with delayed myelination, with some improvement on repeat scanning at age 4 years consistent with interval myelination. EKGs have been normal. Ophthalmological examinations suggested reduced visual acuity and cortical visual impairment. Although she has had no overt seizures, electrophysiological testing was consistent with Landau Kleffner Syndrome. Hearing testing was normal, but frequency modulated auditory evoked response testing was abnormal with a borderline 4hz response and a distorted click auditory evoked response, suggestive of deficits in early cortical and late peripheral auditory processing. Metabolic testing including urinary organic acids, urinary amino acids, plasma lactate, plasma amino acids and 7-dehydrocholesterol were within normal limits. Previous genetic testing was negative, including MECP-2, FOXG-1, UBE3A, TCF-4, STK9/CDKL5, MEKP2 and DHCR7. Karyotyping, Fragile X syndrome testing, myotonic dystrophy testing and 15q11-13 methylation, duplication and deletion testing for the Prader–Willi/Angelman syndrome region gave normal results. Whole exome sequencing revealed a de novo heterozygous truncating mutation within the gene IQSEC2 (see Table 1).
From genotype to clinical phenotype
Ongoing research suggests how syndromes like the illustrative clinical case could emerge from specific IQSEC2 mutations. Located on chromosome Xp11.22, the gene has been functionally implicated in processes including cellular homeostasis and synaptic plasticity via a critical role in regulatory processes (Mi et al. 2013; Shoubridge et al. 2010; Murphy et al. 2006; Sakagami et al. 2008). A guanine nucleotide exchange factor (GEF), IQSEC2 appears to regulate the activity of adenosine diphosphate (ADP)-ribosylation factor 6 (ARF6) by catalyzing the exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP, to which ARF6 is bound in its active state). When active, ARF6 plays a role in actin cytoskeleton reorganization and trafficking across biological membranes (Myers & Casanova 2008). It is not a coincidence that in three of the four original families described with X-linked inherited forms of IQSEC2 disease, missense mutations within the Sec7 functional domain of the IQSEC2 protein were experimentally demonstrated to diminish GTP binding by ARF6 when compared to wild-type protein (Shoubridge et al. 2010). Intriguingly, IQSEC2 mRNA and protein has been localized to post-synaptic dendrites of excitatory glutamatergic synapses in rodents, where activity-dependent expression may mediate a role in synaptic plasticity (Murphy et al. 2006; Myers et al. 2012).
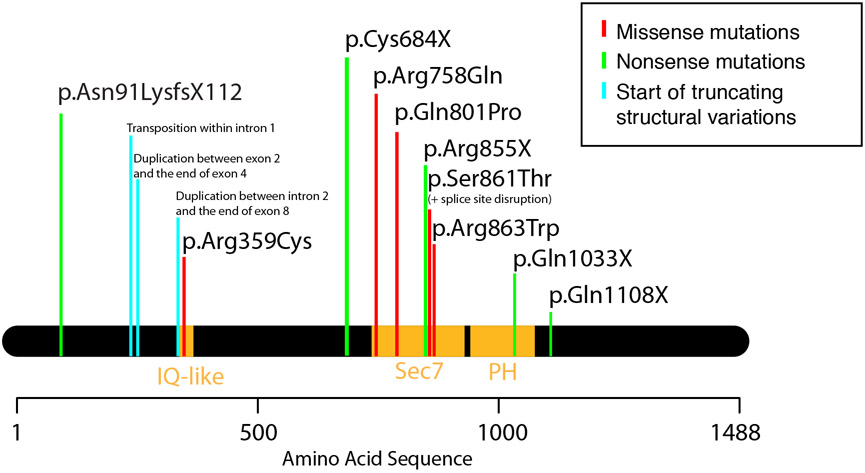
In contrast to the non-syndromal inherited missense mutations, the syndromal cases reported have all been the result of de novo, truncating mutations, stemming from a variety of specific mechanisms. In total there has been one report of a frameshift mutation that results in a premature truncation (Olson et al. 2015); four nonsense mutations (Gandomi et al. 2013; Rauch et al. 2012; Epi4K Consortium et al. 2013; Redin et al. 2014); two copy number variants with intra-genic duplications greater than 20-kb (Tran Mau-Them et al. 2014); and one balanced translocation with an intra-genic breakpoint (Morleo et al. 2008) (see Figure 2). One missense mutation abolished a canonical splice donor site (Gandomi et al. 2013), which is predicted to be a severely deleterious mutation. Epilepsy, microcephaly and ID were reported in a patient with a duplication of genetic material from TENM3 on Chromosome 4 inserted intra-genetically into IQSEC2, but other clinical features of this case were not reported (Gilissen et al. 2014). The phenotype of these mutations stands in notable contrast to the familial, “non-syndromal” ID that remains the best-known clinical phenotype of IQSEC2. Given the experimentally documented decreased ARF-associated GTP binding even in these missense-mutated proteins (Shoubridge et al. 2010), a profound disruption of function caused by truncation of the protein may result in the syndromal form of disease. It is likely that the truncated mRNAs are in fact not translated into proteins at all, as the process of nonsense-mediated mRNA decay may result in their degradation via mechanisms that generally contribute to cellular quality control (Lykke-Andersen and Jensen, 2015).
Figure 2: Illustration of reported genetic etiologies of IQSEC2-related ID.
Mutations from the literature are represented by their effect on the longer of the two protein isoforms. For clinical correlation, please see corresponding rows of Table 1.
The relatively greater proportion of female patients with syndromal as opposed to non-syndromal IQSEC2-related disease (although there remain fewer female cases than male cases) suggests that the nature of the genotype-phenotype relationship may differ in the syndromal and non-syndromal forms of the disease. One possible explanation is that although the missense mutation results in an X-linked recessive inheritance pattern, the more severe, truncating mutations represent a dominant form of the disease. Another possibility is that the affected female patients have X-inactivation profiles that result in disproportionate expression of the mutated gene. Indeed in one of the reported female cases, a completely skewed X-inactivation profile was demonstrated with preferential use of the affected chromosome (Morleo et al. 2008). Interestingly, there is evidence that while X-inactivation affects the mouse ortholog of IQSEC2, the human IQSEC2 gene escapes X-inactivation (Berletch et al. 2010; Yang et al. 2010; Carrel & Willard 2005; Shoubridge et al. 2010).
The illustrative case that we describe is unique in that only the longer of IQSEC2’s two protein isoforms would be IQSEC2 to be affected by the reported deletion in exon 1 (Olson et al. 2015). Both IQSEC2 protein isoforms have been reported experimentally in the rodent brain, the adult human brain and the fetal human brain (Murphy et al. 2006; Sakagami et al. 2008; Shoubridge et al. 2010). Moreover, exons 3 through 12 are identical in the longer isoform (NM_001111125) and the shorter isoform (NM_015075) of the protein. As exons 4-13 are involved in other reported point mutations, both isoforms should be affected (see Table 1). Reported CNV duplications also extend beyond exon 3 (Tran Mau-Them et al. 2014), and a reported translocation with an intragenic breakpoint would alter the pre-RNA in a manner that should affect both isoforms (Morleo et al. 2008). In contrast, the exon 1 mutation should be spliced out of the mRNA that is translated for the shorter isoform, suggesting that loss of function specific to the longer isoform is sufficient to cause the syndromal form of the disease. It is possible that disruption of the longer isoform is the mechanism of disease in all reported cases, or else disruption of either isoform may be sufficient.
The IQSEC2 gene and other causative genes for disorders in the Rett Angelman spectrum appear to converge in their involvement with biological processes of cellular communication, signaling and signal transduction. This convergence is manifest in an “enrichment analysis” of Gene Ontology (GO) terms that are associated both with IQSEC2 and also with a subset of the 11 genes known to be involved in the Rett Angelman spectrum, including: CDKL5, FOXG1, MECP2, NRXN1, TCF4, ZEB2, CNTNAP2, MBD5, MEF2C, SLC9A6 and UBE3A (see Table 2). The GO database comprises a continuously updated set of annotated biological processes that are linked to specific genes by database resource groups (Ashburner et al. 2000), allowing for the statistical examination of whether a set of genes is enriched for particular biological annotation terms (Huang et al, 2012). Interestingly, in addition to cell signaling/transduction, higher-level biological processes such as social behavior, learning and memory are significantly associated with a subset of the other 11 genes, but not with IQSEC2, which may reflect the relative dearth of studies as opposed to a lack of involvement by IQSEC2 in these higher-level processes. It is important to note that such bioinformatics analysis is largely hypothesis-generating. While there are many examples of bioinformatics analysis including GO-term analysis providing insights into clinical and experimental data (eg Fromer et al. 2014; Heck et al. 2014; Parikshak et al. 2015), these hypotheses must be tested experimentally and proven in terms of their clinical utility. More broadly, it is worth pointing out that despite significant advances in the clinical science of whole-exome and whole-genome sequencing to discover and inform the interpretation of rare genetic mutations, the correlation between a clinical syndrome and a likely deleterious mutation is not a guarantee of causality. The utility of genome sequencing in neurodevelopmental disorders has yet to be fully established.
Table 2: Gene Ontology (GO) analysis.
GO terms for biological processes that are significantly associated both with IQSEC2 and with genes involved in the Rett Angelman spectrum including: CDKL5, FOXG1, MECP2, NRXN1, TCF4, ZEB2, CNTNAP2, MBD5, MEF2C, SLC9A6 and UBE3A.
| GO Term | ID | Background frequency |
Sample frequency |
Expected | +/− enriched |
P-value |
|---|---|---|---|---|---|---|
| Single-organism process | GO:0044699 | 12164 | 12 | 6.695 | + | 0.02636 |
| Regulation of biological process | GO:0050789 | 9875 | 11 | 5.435 | + | 0.03347 |
| Regulation of cellular process | GO:0050794 | 9486 | 11 | 5.221 | + | 0.02211 |
| Positive regulation of biological process | GO:0048518 | 4686 | 9 | 2.579 | + | 0.00328 |
| Localization | GO:0051179 | 4405 | 9 | 2.424 | + | 0.00196 |
| Positive regulation of cellular process | GO:0048522 | 4232 | 9 | 2.329 | + | 0.00140 |
| Regulation of cell communication | GO:0010646 | 2692 | 9 | 1.482 | + | 0.00003 |
| Regulation of signaling | GO:0023051 | 2680 | 9 | 1.475 | + | 0.00003 |
| Cellular component organization or biogenesis | GO:0071840 | 4569 | 8 | 2.515 | + | 0.02349 |
| Cellular component organization | GO:0016043 | 4449 | 8 | 2.449 | + | 0.01944 |
| Regulation of signal transduction | GO:0009966 | 2398 | 8 | 1.32 | + | 0.00020 |
| Regulation of response to stimulus | GO:0048583 | 3113 | 8 | 1.713 | + | 0.00144 |
| Regulation of nucleobase-containing compound metabolic process | GO:0019219 | 4196 | 8 | 2.309 | + | 0.01279 |
| Regulation of nitrogen compound metabolic process | GO:0051171 | 4292 | 8 | 2.362 | + | 0.01504 |
| Positive regulation of metabolic process | GO:0009893 | 3059 | 7 | 1.684 | + | 0.01281 |
| Positive regulation of cellular metabolic process | GO:0031325 | 2849 | 7 | 1.568 | + | 0.00816 |
| Positive regulation of nucleobase-containing compound metabolic process | GO:0045935 | 1915 | 7 | 1.054 | + | 0.00062 |
| Positive regulation of nitrogen compound metabolic process | GO:0051173 | 1943 | 7 | 1.069 | + | 0.00068 |
| Regulation of intracellular signal transduction | GO:1902531 | 1494 | 5 | 0.8222 | + | 0.02304 |
GO terms are organized hierarchically with more general processes at the top and more specific processes at the bottom of the table. Hypergeometric tests were performed of the null hypothesis that the frequencies of the term-by-gene associations were due to chance alone. All P-values were Bonferroni-corrected for multiple comparisons. All of the significant terms were associated with more of the genes than would be expected by chance (+) as opposed to fewer (−). The analysis utilized Panther 9.0 (Mi et al. 2013) and GO database release 2013-08-09 (Ashburner et al. 2000).
In summary, there have been at least nine reported cases of syndromal ID secondary to loss of function mutations in the IQSEC2 gene. The X-linked recessive, non-syndromal form of the disease appears to be associated with non-truncating missense mutations. In contrast, all reported truncating mutations are de novo and appear to result in a syndromal form of disease. Clinically, the cluster of features converges on a pattern of global developmental delay, deficits in social communication, stereotypical hand movements, and hypotonia. In addition, many if not all of these patients have seizures, microcephaly and language regression in addition to delay. These clinical features resemble those of what has been called the “Rett Angelman spectrum.” At the present time, we believe the data support either IQSEC2 testing in patients with a suspicious phenotype not attributable to a known genetic mutation or else the inclusion of IQSEC2 on Rett Angelman gene panels. Both the natural history and the mechanism of the IQSEC2 syndrome should be clarified as more data become available through genetic sequencing of a greater number of individuals with unexplained ID.
Footnotes
Conflicts of interest: none.
REFERENCES
- Ashburner M et al. , 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature Genetics, 25(1), pp.25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi-Buisson N et al. , 2010. Revisiting the phenotype associated with FOXG1 mutations: two novel cases of congenital Rett variant. Neurogenetics, 11(2), pp.241–249. [DOI] [PubMed] [Google Scholar]
- Berletch JB, Yang F & Disteche CM, 2010. Escape from X inactivation in mice and humans. Genome Biology, 11(213). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel L & Willard HF, 2005. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature Neuroscience, 434(7031), pp.400–404. [DOI] [PubMed] [Google Scholar]
- de Pontual L et al. , 2009. Mutational, functional, and expression studies of the TCF4 gene in Pitt-Hopkins syndrome. Human Mutation, 30(4), pp.669–676. [DOI] [PubMed] [Google Scholar]
- Epi4K Consortium et al. , 2013. De novo mutations in epileptic encephalopathies. Nature Neuroscience, 501(7466), pp.217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frints SGM et al. , 2002. X-linked mental retardation: vanishing boundaries between non-specific (MRX) and syndromic (MRXS) forms. Clinical Genetics, 62(6), pp.423–432. [DOI] [PubMed] [Google Scholar]
- Fromer M et al. , 2014. De novo mutations in schizophrenia implicate synaptic networks. Nature Neuroscience, 506(7487), pp.179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandomi SK et al. , 2013. Diagnostic exome sequencing identifies two novel IQSEC2 mutations associated with X-Linked intellectual disability with seizures: implications for genetic counseling and clinical diagnosis. Journal of Genetic Counseling, 23(3), pp.289–298. [DOI] [PubMed] [Google Scholar]
- GeneDX, 2014. Rett/Angelman syndrome and related disorders genetic testing panel. www.genedx.com. Available at: https://www.genedx.com/test-catalog/available-tests/rettangelman-syndrome-panel/ [Accessed November 8, 2014].
- Gécz J, Shoubridge C & Corbett M, 2009. The genetic landscape of intellectual disability arising from chromosome X. Trends in Genetics, 25(7), pp.308–316. [DOI] [PubMed] [Google Scholar]
- Gilissen C et al. , 2014. Genome sequencing identifies major causes of severe intellectual disability. Nature Neuroscience, 511(7509), pp.344–347. [DOI] [PubMed] [Google Scholar]
- Heck A et al. , 2014. Converging genetic and functional brain imaging evidence links neuronal excitability to working memory, psychiatric disease, and brain activity. Neuron, 81(5), pp.1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge JC et al. , 2014. Disruption of MBD5 contributes to a spectrum of psychopathology and neurodevelopmental abnormalities. Molecular Psychiatry, 19(3), pp.368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT & Lempicki RA, 2009. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Research, 37(1), pp.1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillard S et al. , 2009. 2q23.1 microdeletion identified by array comparative genomic hybridisation: an emerging phenotype with Angelman-like features? Journal of Medical Genetics, 46(12), pp.847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedele KB, 2007. The overlapping spectrum of rett and angelman syndromes: a clinical review. Seminars in pediatric neurology, 14(3), pp.108–117. [DOI] [PubMed] [Google Scholar]
- Kwok H & Cheung PWH, 2007. Co-morbidity of psychiatric disorder and medical illness in people with intellectual disabilities. Current Opinion in Psychiatry, 20(5), pp.443–449. [DOI] [PubMed] [Google Scholar]
- Leonard H & Wen X, 2002. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Mental Retardation and Developmental Disabilities Research Reviews, 8(3), pp.117–134. [DOI] [PubMed] [Google Scholar]
- Li M-R et al. , 2007. MECP2 and CDKL5 gene mutation analysis in Chinese patients with Rett syndrome. Journal of Human Genetics, 52(1), pp.38–47. [DOI] [PubMed] [Google Scholar]
- Lubs HA, Stevenson RE & Schwartz CE, 2012. Fragile X and X-linked intellectual disability: four decades of discovery. American Journal of Human Genetics, 90(4), pp.579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A & Thomas PD, 2013. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Research, 41(Database issue), pp.D377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morleo M et al. , 2008. Disruption of the IQSEC2 transcript in a female with X;autosome translocation t(X;20)(p11.2;q11.2) and a phenotype resembling X-linked infantile spasms (ISSX) syndrome. Molecular Medicine Reports, 1(1), pp.33–39. [PubMed] [Google Scholar]
- Mullegama SV et al. , 2015. Phenotypic and molecular convergence of 2q23.1 deletion syndrome with other neurodevelopmental syndromes associated with autism spectrum disorder. International journal of molecular sciences, 16(4), pp.7627–7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulley JC et al. , 1992. Nomenclature guidelines for X-linked mental retardation. American Journal of Medical Genetics, 43(1-2), pp.383–391. [DOI] [PubMed] [Google Scholar]
- Murphy JA, Jensen ON & Walikonis RS, 2006. BRAG1, a Sec7 domain-containing protein, is a component of the postsynaptic density of excitatory synapses. Brain Research, 1120(1), pp.35–45. [DOI] [PubMed] [Google Scholar]
- Myers KR & Casanova JE, 2008. Regulation of actin cytoskeleton dynamics by Arf-family GTPases. Trends in Cell Biology, 18(4), pp.184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KR et al. , 2012. Arf6-GEF BRAG1 regulates JNK-mediated synaptic removal of GluA1-containing AMPA receptors: a new mechanism for nonsyndromic X-linked mental disorder. The Journal of Neuroscience, 32(34), pp.11716–11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL et al. , 2010. Rett syndrome: revised diagnostic criteria and nomenclature. Annals of Neurology, 68(6), pp.944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson HE et al. , 2015. Mutations in epilepsy and intellectual disability genes in patients with features of Rett Syndrome. American Journal of Medical Genetics, 9999A, pp.1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikshak NN, Gandal MJ & Geschwind DH, 2015. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nature Reviews Genetics, 16(8), pp.441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A et al. , 2012. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet, 380(9854), pp.1674–1682. [DOI] [PubMed] [Google Scholar]
- Redin C et al. , 2014. Efficient strategy for the molecular diagnosis of intellectual disability using targeted high-throughput sequencing. Journal of Medical Genetics, 51(11), pp.724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Vargas H et al. , 2008. Impairment of CDKL5 nuclear localisation as a cause for severe infantile encephalopathy. Journal of Medical Genetics, 45(3), pp.172–178. [DOI] [PubMed] [Google Scholar]
- Sakagami H et al. , 2008. IQ-ArfGEF/BRAG1 is a guanine nucleotide exchange factor for Arf6 that interacts with PSD-95 at postsynaptic density of excitatory synapses. Neuroscience Research, 60(2), pp.199–212. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Hogart A & LaSalle JM, 2005. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Human Molecular Genetics, 14(4), pp.483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge C et al. , 2010. Mutations in the guanine nucleotide exchange factor gene IQSEC2 cause nonsyndromic intellectual disability. Nature Genetics, 42(6), pp.486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers GK, Turner G & Mulley JC, 1988. A non-syndromal form of X-linked mental retardation (XLMR) is linked to DXS14. American Journal of Medical Genetics, 30(1-2), pp.485–491. [DOI] [PubMed] [Google Scholar]
- Tan W-H et al. , 2014. If not Angelman, what is it? a review of Angelman-like syndromes. American Journal of Medical Genetics, 164(4), pp.975–992. [DOI] [PubMed] [Google Scholar]
- Tran Mau-Them F et al. , 2014. Expanding the phenotype of IQSEC2 mutations: truncating mutations in severe intellectual disability. European Journal of Medical Genetics, 22(2), pp.289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F et al. , 2010. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Research, 20(5), pp.614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y et al. , 2014. Clinical features and gene mutational spectrum of CDKL5-related diseases in a cohort of Chinese patients. BMC Medical Genetics, 15, p.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier C et al. , 2005. Clinical and mutational spectrum of Mowat-Wilson syndrome. European Journal of Medical Genetics, 48(2), pp.97–111. [DOI] [PubMed] [Google Scholar]
- Zweier M et al. , 2010. Mutations in MEF2C from the 5q14.3q15 microdeletion syndrome region are a frequent cause of severe mental retardation and diminish MECP2 and CDKL5 expression. Human Mutation, 31(6), pp.722–733. [DOI] [PubMed] [Google Scholar]