Abstract
Background: Patients with advanced heart failure with reduced ejection fraction often cannot tolerate target doses of guideline-directed medical therapy due to symptomatic hypotension, renal dysfunction, and associated electrolyte abnormalities. While levosimendan can facilitate the titration of β-blockers in patients with advanced HFrEF, it is unclear whether ambulatory levosimendan infusions would offer the same benefit. In this prospective study, we investigate the effects of intermittent ambulatory levosimendan infusions on the uptitration of disease-modifying drugs. Methods: We enrolled 37 patients with advanced HFrEF who received repeated ambulatory infusions of levosimendan between January 2018 and January 2021. The demographic, clinical, and laboratory data were acquired 24 h before the first and the last ambulatory levosimendan infusion. Results: At the 1 year follow-up, the enrolled patients were on significantly higher doses of guideline-directed medical therapy, including bisoprolol (3.2 ± 2.8 mg vs. 5.9 ± 4.1 mg; p = 0.02), sacubitril/valsartan (41.67 ± 32.48 mg vs. 68.5 ± 35.72 mg; p = 0.01), and eplerenone (12.7 ± 8.5 mg vs. 22.8 ± 13.6 mg; p = 0.03). Furthermore, a substantial decrease in the furosemide dose was observed (123.2 ± 32.48 mg vs. 81.6 ± 19.47 mg; p < 0.0001). Conclusions: Levosimendan facilitates the optimization of disease-modifying heart failure medications in previously intolerant advanced HFrEF patients.
Keywords: levosimendan, disease modifier drugs, advanced heart failure, heart failure reduced ejection fraction
1. Introduction
Despite improvements in pharmacological and nonpharmacological treatments for patients with heart failure (HF) with reduced ejection fraction (HFrEF), approximately 10% of patients have a progressively worsening functional status culminating in advanced HF [1]. Furthermore, patients with advanced HFrEF develop distinct haemodynamic features that affect their natural history and disease-modifying drugs tolerance [2]. Symptomatic hypotension, renal dysfunction, and hyperkalaemia render the uptitration of β-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), and mineral receptor antagonists (MRAs) challenging [3]. Levosimendan is a calcium-sensitising medication [4] with two mechanisms of action, increased inotropy and vasodilation, and positive haemodynamic effects in acute HF [5]. Several studies of levosimendan in advanced HFrEF have been performed; however, they all included a bolus dose mimicking acute treatment [6,7]. More recently, the LIONHEART study showed that ambulatory intermittent levosimendan infusions reduced NT-proBNP plasma levels and hospitalisations [8]. Following this pivotal trial, subsequent studies demonstrated that intermittent ambulatory infusions of levosimendan improved haemodynamic parameters [9] and functional capacity [10], while reducing hospitalisation [11,12] in patients with advanced HFrEF. In addition, a 24-h infusion of levosimendan could facilitate the titration of β-blockers in previously intolerant advanced HFrEF patients [13]. However, the role of levosimendan ambulatory infusions in the optimization of guideline-directed medical therapy for HFrEF remains unknown. Therefore, the purpose of this prospective study was to investigate whether intermittent infusions of levosimendan could facilitate the titration of β-blockers, ARNIs, and MRAs in advanced patients with HFrEF and a documented intolerance to disease-modifying drugs uptitration.
2. Materials and Methods
2.1. Study Population
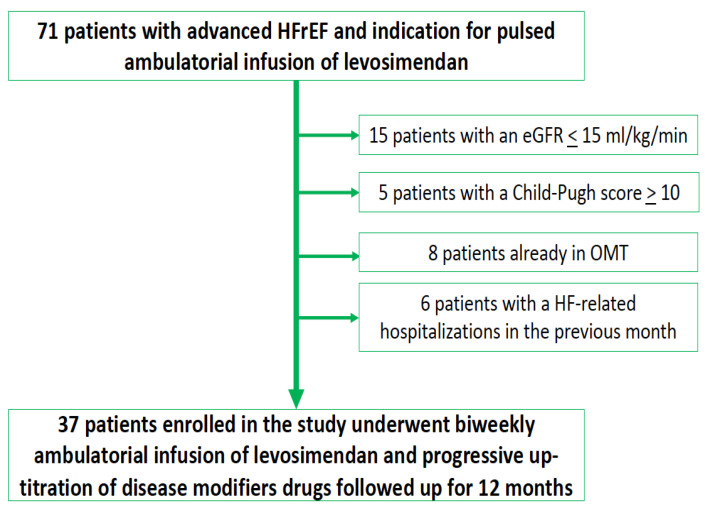
We enrolled the study population at the Heart Failure Unit of Monaldi Hospital between January 2018 and January 2021 (Figure 1).
Figure 1.
Study protocol. eGFR: estimated glomerular filtration rate. OMT: optimal medical therapy. HF: Heart failure. HFrEF: heart failure with reduced ejection fraction.
The following inclusion criteria were used:
-
(1)
HFrEF with a left ventricular ejection fraction <35%,
-
(2)
NYHA class III-IV,
-
(3)
NT-proBNP >2500 pg/mL,
-
(4)
walking distance at 6-min walking test <300 m,
-
(5)
indication for intermittent ambulatory levosimendan infusion due to episodes of pulmonary or systemic congestion requiring a high dose i.v. diuretics or episodes of low output requiring inotropes or causing >2 unplanned visits or hospitalisations in the last 12 months, and
-
(6)
guideline-directed medical therapy for HFrEF not at target dose [14,15,16], with documented intolerance to their uptitration in the six months prior to levosimendan infusion.
The following exclusion criteria were used:
-
(1)
End-stage renal disease (i.e., estimated glomerular filtration rate <15 mL/kg/min according to the CKD-EPI equation),
-
(2)
severe liver impairment (i.e., Child–Pugh score >10).
Signed informed consent was obtained, the Declaration of Helsinki was followed, and the institutional review board of AORN dei Colli–Ospedale Monaldi granted approval (deliberation n° 345 of November 2017). Demographic, clinical, and laboratory data were acquired from stable patients 24 h before the first and the last ambulatory levosimendan administration. The patients were followed up for 1 year during ambulatory infusions of levosimendan, and the follow-up was started at the first infusion of levosimendan.
2.2. Levosimendan Infusion
In all patients, levosimendan (Simdax®) was intravenously administered at 0.2 µg/kg/min for a total dosage of 6.25 mg every two weeks in an ambulatory setting. Levosimendan was administered in all patients for at least 1 year. No change in the dose of levosimendan occurred during the follow-up.
2.3. Evaluation of Disease Modifiers Drug Dose
During follow-up, the doses of disease-modifying drugs were uptitrated according to clinical judgment by two physicians with experience treating patients with advanced HFrEF (D.M., F.V.). The uptitration of the drugs was performed in an ambulatory setting on the same day as the levosimendan infusion. The doses of guideline-directed medical therapy were recorded 24 h before the first and the last ambulatory infusion of levosimendan; the latter doses were considered the maximum doses for each patient.
2.4. Statistical Analysis
All statistical analyses were performed using Prism 9 (GraphPad Software, San Diego, CA, USA). All demographic and clinical variables are expressed as the mean ± standard deviation. Categorical variables are expressed as numbers and percentages. Differences between the baseline and treatment values were compared using a Wilcoxon rank test for non-normal distribution and using a t-test for normal distribution. All p-values were two-sided; p < 0.05 indicated statistical significance.
3. Results
A total of 71 patients meeting the diagnostic criteria for advanced HFrEF with an indication for intermittent infusion of levosimendan were screened in our unit during the study period. Of these patients, fifteen (21%) did not receive an ambulatory infusion of levosimendan for end-stage renal disease, and five patients (7%) did not for severe liver failure. In addition, six patients (8%) had HF-related hospitalisations in the month before levosimendan administration, and eight patients (11%) had already achieved the target dose of disease-modifying drugs, so they were excluded from the study. The final population comprised 37 patients (mean age 55.8 ± 13.2 years, 84% male, mean ejection fraction 26.8 ± 9.4%). The demographic, clinical, and echocardiographic characteristics of the study population are presented in Table 1.
Table 1.
Baseline clinical and echocardiographic characteristics of the study population.
| Variable | Total Population (n = 37) |
|---|---|
| Age (mean ± SD) | 55.8 ± 13.2 years |
| Female sex (n, %) | 6 (16%) |
| Ischaemic (n, %) | 20 (54%) |
| Hypertension (n, %) | 18 (48%) |
| Diabetes (n, %) | 17 (45%) |
| COPD (n, %) | 12 (32%) |
| NYHA class III (n, %) | 25 (67%) |
| NYHA class IV (n, %) | 12 (33%) |
| SBP (mean ± SD) | 97 ± 10 mmHg |
| DBP (mean ± SD) | 62 ± 8 mmHg |
| NT-pro BNP (mean ± SD) | 3448 ± 1187 pg/mL |
| Atrial fibrillation | 15 (40%) |
| Hb (mean ± SD) | 11.7 ± 1.8 g/dL |
| Creatinine (mean ± SD) | 1.4 ± 1.3 mg/dL |
| eGFR (mean ± SD) | 36.7 ± 18.1 mL/min/1.73 m2 |
| LVEDV (mean ± SD) | 2321.2 ± 85.9 mL |
| LVESV (mean ± SD) | 192.7 ± 80.2 mL |
| LVEF (mean ± SD) | 26.8 ± 9.4% |
| E wave (mean ± SD) | 128.1 ± 39.5 cm/s |
| e’ average (mean ± SD) | 6.9 ± 3.5 cm/s |
| E/e’ average (mean ± SD) | 21.2 ± 6.3 |
| DecT (mean ± SD) | 165.2 ± 28.3 m/s |
| LAVi (mean ± SD) | 52.5 ± 13.5 mL/m2 |
| PASP (mean ± SD) | 40.8 ± 12.6 mmHg |
| TAPSE (mean ± SD) | 14.1 ± 5.4 mm |
| Peak systolic s wave (mean ± SD) | 8.7 ± 3.2 cm/s |
| Loop diuretic (n, %) | 37 (100%) |
| Furosemide dose (mean ± SD) | 123.2 ± 32.48 mg |
| β-blocker (n, %) | 37 (100 %) |
| Bisoprolol dose (mean ± SD) | 3.2 ± 2.8 mg |
| ARNI (n, %) | 37 (100%) |
| ARNI dose (mean ± SD) | 41.67 ± 32.48 mg |
| MRA (n, %) | 37 (100%) |
| Eplerenone dose | 9.7 ± 8.8 mg |
COPD: chronic obstructive pulmonary disease; NYHA: New York Health Association; SBP: systolic blood pressure; DBP: diastolic blood pressure; NT-pro BNP: N terminal-pro brain natriuretic peptide; Hb: haemoglobin; eGFR: estimated glomerular filtration rate; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; LVEF: left ventricular ejection fraction; E wave: peak early mitral inflow velocity; e′ average: average of septal and lateral peak early diastolic mitral annular velocity; DecT: deceleration time; LAVi, left atrium volume index; PASP: pulmonary artery systolic pressure; TAPSE: tricuspid annular plane systolic excursion; ARNI: angiotensin receptor-neprilysin inhibitor; MRA: mineral receptor antagonist.
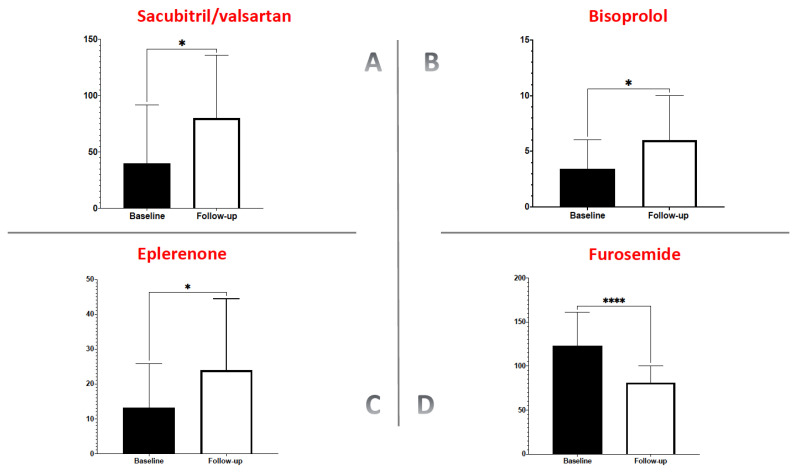
At the one-year follow-up, the ambulatory infusion of levosimendan had allowed a significant increase in the mean dose of sacubitril/valsartan compared with the dose before levosimendan treatment (41.67 ± 32.48 mg vs. 68.5 ± 35.72 mg; p = 0.01; Figure 2A).
Figure 2.
Change in the dose of disease modifier drugs (panel A–C) and diuretics at follow-up (panel D). *: p < 0.05; ****: p < 0.0001.
Likewise, we observed a significant increase in the mean dose of bisoprolol compared with the dose before levosimendan administration (3.2 ± 2.8 mg vs. 5.9 ± 4.1 mg; p = 0.02; Figure 2B), and the same change was seen with eplerenone (12.7 ± 8.5 mg vs. 22.8 ± 13.6 mg, p = 0.03; Figure 2C). Simultaneously with the increase in the dose of disease-modifying drugs, a substantial decrease in the dose of furosemide was observed compared with the dose before levosimendan treatment (123.2 ± 32.48 mg vs. 81.6 ± 19.47 mg; p < 0.0001; Figure 2D).
4. Discussion
One of the most complex clinical challenges in patients with advanced HFrEF is their intolerance to guideline-directed medical therapy or, if administered, inability to titrate to recommended doses due to hypotension, renal failure, and hyperkalaemia [17,18]. The poor tolerance of neurohormonal modulatory drugs in patients with advanced HFrEF could be related to the progression of the disease itself, leading to a critical reduction in the stroke volume resulting in hypotension and renal dysfunction. Alternatively, it could be associated with the direct effect of neurohormonal modulators or a combination of both [19]. Regardless of the cause, suboptimal doses of guideline-directed medical therapy in patients with advanced HFrEF are associated with poor prognoses. In this clinical scenario, levosimendan can assist in optimising therapy with β-blockers and drugs interfering with the renin-angiotensin-aldosterone system. Berger and colleagues demonstrated that levosimendan allowed the uptitration of β-blockers in previously intolerant HF patients. Levosimendan was periodically infused every four weeks, with a loading dose of 12 μg/kg for 10 min and an infusion rate of 0.1 μg/kg/min for 24 h. This protocol allowed for an increased dose of bisoprolol in patients in whom this had not been previously possible [13]. In our study, the use of levosimendan allowed for an increase in the dose of bisoprolol; this may have been due to the increase in cardiac output and consequent increase in blood pressure. The ability of levosimendan to increase cardiac output and cardiac performance, in addition to its positive effect on renal haemodynamics [20,21], may have allowed the uptitration of sacubitril/valsartan. Additionally, the positive impact on the renal performance, the reduction in the diuretic dose, and the reduction in potassium levels associated with levosimendan may have allowed the increase in the dose of MRAs [22]. Finally, in our study as well as in clinical trials [23] and in previous real-world experiences [24], the increasing dose of sacubitril/valsartan reduced the relative need for diuretics in patients with advanced HFrEF. This is potentially related to the natriuretic effects of sacubitril [25] or the presumed improvement in renal haemodynamics that may occur with sacubitril/valsartan [26].
5. Study Limitations
We recognise that the relatively small sample size, single-centre study design, the study’s observational nature, and the absence of a control arm could have affected our results. However, the data from our observational study should be taken into consideration when planning properly powered randomised clinical trials in this therapeutic setting.
6. Conclusions
Levosimendan facilitates the optimisation of guideline-directed medical therapy in patients with advanced HFrEF who were previously unable to achieve target doses. This therapeutic strategy may be used as a ‘bridge to optimisation’ and may justify, at least in part, the improvement in clinical outcomes that the intermittent infusion of levosimendan produces in patients with advanced HFrEF.
Author Contributions
Conceptualization D.M., M.M.K., M.L.M. and G.P.; investigation F.V., R.G., M.V., E.A., A.C. and A.P.; writing—original draft preparation D.M., C.C. and V.D.P.; writing—review and editing, G.P., M.M.K. and P.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of AORN dei Colli-Ospedale Monaldi (deliberation n° 345 of November 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
D.M., M.M.K., M.L.M., F.V., R.G., M.V., E.A., C.C., V.D.P., A.C., A.P. and G.P. report no conflicts of interest in this work. P.P. is a full-time employee of Orion Pharma, where levosimendan was discovered and developed.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Truby L.K., Rogers J.G. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC Heart Fail. 2020;8:523–536. doi: 10.1016/j.jchf.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Masarone D., Kittleson M., Petraio A., Pacileo G. Advanced heart failure: State of the art and future directions. Rev. Cardiovasc. Med. 2022;23:48. doi: 10.31083/j.rcm2302048. [DOI] [PubMed] [Google Scholar]
- 3.Iacoviello M., Vitale E., Corbo M.D., Correale M., Brunetti N.D. Disease-modifier Drugs in Patients with Advanced Heart Failure: How to Optimize Their Use? Heart Fail. Clin. 2021;17:561–573. doi: 10.1016/j.hfc.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Masarone D., Melillo E., Gravino R., Errigo V., Martucci M.L., Caiazzo A., Petraio A., Pölzl G., Pacileo G. Inotropes in Patients with Advanced Heart Failure: Not Only Palliative Care. Heart Fail. Clin. 2021;17:587–598. doi: 10.1016/j.hfc.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Long Y.X., Cui D.Y., Kuang X., Hu S., Hu Y., Liu Z.Z. Effect of Levosimendan on Ventricular Systolic and Diastolic Functions in Heart Failure Patients: A Meta-Analysis of Randomized Controlled Trials. J. Cardiovasc. Pharmacol. 2021;77:805–813. doi: 10.1097/FJC.0000000000001010. [DOI] [PubMed] [Google Scholar]
- 6.Mavrogeni S., Giamouzis G., Papadopoulou E., Thomopoulou S., Dritsas A., Athanasopoulos G., Adreanides E., Vassiliadis I., Spargias K., Panagiotakos D., et al. A 6-month follow-up of intermittent levosimendan administration effect on systolic function, specific activity questionnaire, and arrhythmia in advanced heart failure. J. Card. Fail. 2007;13:556–559. doi: 10.1016/j.cardfail.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Parissis J.T., Adamopoulos S., Farmakis D., Filippatos G., Paraskevaidis I., Panou F., Iliodromitis E., Kremastinos D.T. Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure. Heart. 2006;92:1768–1772. doi: 10.1136/hrt.2005.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comín-Colet J., Manito N., Segovia-Cubero J., Delgado J., Pinilla J.M.G., Almenar L., Crespo-Leiro M.G., Sionis A., Blasco T., Pascual-Figal D., et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: The LION-HEART multicentre randomised trial. Eur. J. Heart Fail. 2018;20:1128–1136. doi: 10.1002/ejhf.1145. [DOI] [PubMed] [Google Scholar]
- 9.Masarone D., Melillo E., Errigo V., Martucci M.L., Pacileo R., Pollesello P., Petraio A., Pacileo G. Hemodynamic Effects of Levosimendan in Outpatients With Advanced Heart Failure: An Echocardiographic Pilot Study. J. Cardiovasc. Pharmacol. 2021;79:e36–e40. doi: 10.1097/FJC.0000000000001163. [DOI] [PubMed] [Google Scholar]
- 10.Apostolo A., Vignati C., Della Rocca M., De Martino F., Berna G., Campodonico J., Contini M., Muratori M., Palermo P., Mapelli M., et al. Why Levosimendan Improves the Clinical Condition of Patients With Advanced Heart Failure: A Holistic Approach. J. Card. Fail. 2021;28:509–514. doi: 10.1016/j.cardfail.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Masarone D., Valente F., Verrengia M., Ammendola E., Gravino R., D’Alterio G., Petraio A., Pacileo G. Efficacy and safety of repeated infusion of levosimendan in outpatients with advanced heart failure: A real-world experience. J. Cardiovasc. Med. 2020;21:919–921. doi: 10.2459/JCM.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 12.Oliva F., Perna E., Marini M., Nassiacos D., Cirò A., Malfatto G., Morandi F., Caico I., Perna G., Meloni S., et al. Scheduled intermittent inotropes for Ambulatory Advanced Heart Failure. The RELEVANT-HF multicentre collaboration. Int. J. Cardiol. 2018;272:255–259. doi: 10.1016/j.ijcard.2018.08.048. [DOI] [PubMed] [Google Scholar]
- 13.Berger R., Moertl D., Huelsmann M., Bojic A., Ahmadi R., Heissenberger I., Pacher R. Levosimendan and prostaglandin E1 for uptitration of beta-blockade in patients with refractory, advanced chronic heart failure. Eur. J. Heart Fail. 2007;9:202–208. doi: 10.1016/j.ejheart.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 14.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 15.Rosano G.M., Moura B., Metra M., Böhm M., Bauersachs J., Ben Gal T., Adamopoulos S., Abdelhamid M., Bistola V., Čelutkienė J., et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021;23:872–881. doi: 10.1002/ejhf.2206. [DOI] [PubMed] [Google Scholar]
- 16.Heidenreich P.A., Bozkurt B., Aguilar D., Allen L.A., Byun J.J., Colvin M.M., Deswal A., Drazner M.H., Dunlay S.M., Evers L.R., et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 17.Ameri P., Bertero E., Maack C., Teerlink J.R., Rosano G., Metra M. Medical treatment of heart failure with reduced ejection fraction: The dawn of a new era of personalized treatment? Eur. Heart J. Cardiovasc. Pharmacother. 2021;7:539–546. doi: 10.1093/ehjcvp/pvab033. [DOI] [PubMed] [Google Scholar]
- 18.Komajda M., Follath F., Swedberg K., Cleland J., Aguilar J., Cohen-Solal A., Dietz R., Gavazzi A., van Gilst W., Hobbs R., et al. The EuroHeart Failure Survey programme--a survey on the quality of care among patients with heart failure in Europe. Part 2: Treatment. Eur. Heart J. 2003;24:464–474. doi: 10.1016/S0195-668X(02)00700-5. [DOI] [PubMed] [Google Scholar]
- 19.Crespo-Leiro M.G., Barge-Caballero E. Advanced Heart Failure: Definition, Epidemiology, and Clinical Course. Heart Fail. Clin. 2021;17:533–545. doi: 10.1016/j.hfc.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Caira C., Brasolin B., Maestrini V., Scardala R., Orvieto G., Mancone M., Fedele F. 475 Effects of Levosimendan on renal function and renal hemodynamic parameters in patients with acute heart failure. Eur. J. Heart Fail. Suppl. 2007;6:103. doi: 10.1016/S1567-4215(07)60284-7. [DOI] [Google Scholar]
- 21.Zemljic G., Bunc M., Yazdanbakhsh A.P., Vrtovec B. Levosimendan Improves Renal Function in Patients With Advanced Chronic Heart Failure Awaiting Cardiac Transplantation. J. Card. Fail. 2007;13:417–421. doi: 10.1016/j.cardfail.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Nieminen M.S., Akkila J., Hasenfuss G., Kleber F.X., Lehtonen L.A., Mitrovic V., Nyquist O., Remme W.J. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J. Am. Coll. Cardiol. 2000;36:1903–1912. doi: 10.1016/S0735-1097(00)00961-X. [DOI] [PubMed] [Google Scholar]
- 23.Mcmurray J.J.V., Packer M., Desai A.S., Gong J., Lefkowitz M.P., Rizkala A.R., Rouleau J.L., Shi V.C., Solomon S.D., Swedberg K., et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. PARADIGM-HF Investigators and Committees. N. Engl. J. Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 24.Masarone D., Melillo E., Errigo V., Valente F., Pacileo G. Clinical relevance of transient worsening renal function after initiation of sacubitril/valsartan. Curr. Med. Res. Opin. 2020;37:9–12. doi: 10.1080/03007995.2020.1853509. [DOI] [PubMed] [Google Scholar]
- 25.Wang T.-D., Tan R.S., Lee H.-Y., Ihm S.-H., Rhee M.-Y., Tomlinson B., Pal P., Yang F., Hirschhorn E., Prescott M.F., et al. Effects of Sacubitril/Valsartan (LCZ696) on Natriuresis, Diuresis, Blood Pressures, and NT-proBNP in Salt-Sensitive Hypertension. Hypertension. 2017;69:32–41. doi: 10.1161/HYPERTENSIONAHA.116.08484. [DOI] [PubMed] [Google Scholar]
- 26.Pontremoli R., Borghi C., Filardi P.P. Renal protection in chronic heart failure: Focus on sacubitril/valsartan. Eur. Heart J. Cardiovasc. Pharmacother. 2021;7:445–452. doi: 10.1093/ehjcvp/pvab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.