Abstract
Background: Cardiac surgery in patients with infective endocarditis (IE) is still associated with high mortality and morbidity; an already present inflammation might further be aggravated due to a cardiopulmonary bypass-induced dysregulated immune response. Intraoperative hemoadsorption therapy may attenuate this septic response. Our objective was therefore to assess the efficacy of intraoperative hemoadsorption in active left-sided native- and prosthetic infective endocarditis. Methods: Consecutive high-risk patients with active left-sided infective endocarditis were enrolled between January 2015 and April 2021. Patients with intraoperative hemoadsorption (Cytosorbents, Princeton, NJ, USA) were compared to patients without hemoadsorption (control). Endpoints were the incidence of postoperative sepsis, sepsis-associated death and in-hospital mortality. Predictors for sepsis-associated mortality and in-hospital mortality were analysed by multivariable logistic regression. Results: A total of 202 patients were included, 135 with active left-sided native and 67 with prosthetic valve infective endocarditis. Ninety-nine patients received intraoperative hemoadsorption and 103 patients did not. Ninety-nine propensity-matched pairs were selected for final analyses. Postoperative sepsis and sepsis-related mortality was reduced in the hemoadsorption group (22.2% vs. 39.4%, p = 0.014 and 8.1% vs. 22.2%, p = 0.01, respectively). In-hospital mortality tended to be lower in the hemoadsorption group (14.1% vs. 26.3%, p = 0.052). Key predictors for sepsis-associated mortality and in-hospital mortality were preoperative inotropic support, lactate-levels 24 h after surgery, C-reactive protein levels on postoperative day 1, chest tube output, cumulative inotropes and white blood cell counts on postoperative day 2, and new onset of dialysis. Multivariate regression analysis revealed intraoperative hemoadsorption to be associated with lower sepsis-associated (OR 0.09, 95% CI 0.013–0.62, p = 0.014) as well as in-hospital mortality (OR 0.069, 95% CI 0.006–0.795, p = 0.032). Conclusions: Intraoperative hemoadsorption holds promise to reduce sepsis and sepsis-associated mortality after cardiac surgery for active left-sided native and prosthetic valve infective endocarditis.
Keywords: infective endocarditis, sepsis, hemoadsorption, CytoSorb, cytokine release syndrome
1. Introduction
Infective endocarditis (IE) is associated with significant cardiac and noncardiac morbidity. The growing number of cardiac interventions, valve implantations and staphylococcal infections constantly increases its prevalence [1,2]. Despite significant advancements, IE should not be underestimated, presenting with in-hospital mortality ranging from 20% to sometimes higher than 60% [1,2,3,4,5,6,7].
Surgical complexity, cardiopulmonary bypass (CPB)-induced hyperinflammation and postoperative sepsis have been identified as detrimental factors influencing outcomes [2,3,7]. A deranged immune response may activate a disseminated intravascular coagulation cascade [8,9,10,11]. Furthermore, an interplay of hyperinflammatory mechanisms culminates in end-organ deterioration and disproportionately decreased survival [4,12,13]. Multiorgan failure could be potentially reduced by removing pro-inflammatory circulating cytokines via blood purification, which has been introduced recently into modern cardiac surgery [11,14]. CytoSorb® is a CE-approved cytokine adsorption device with polymer beads that lowers circulating inflammatory mediators and bacterial enterotoxins in the range of up to 60 kDa [11,15]. Intraoperative adsorption demonstrated effective cytokine reduction during IE surgery [16,17], however with no detectable clinical benefit. Other recent studies evaluating intraoperative hemoadsorption during IE surgery reported ambiguous outcomes, from improved hemodynamic stabilization and sepsis attenuation and reduced bleeding with fewer transfusion requirements to increased bleeding diathesis postoperatively [4,5,18,19].
The aim of the present study was (1) to evaluate if intraoperative hemoadsorption could reduce sepsis occurrence or attenuate its severity in patients with active left-sided native valve (NVE) or prosthetic valve endocarditis (PVE) undergoing cardiac surgery, and (2) to evaluate predictors for sepsis, sepsis-associated mortality and in-hospital mortality.
2. Materials and Methods
2.1. Ethical Statement
The study was conducted in accordance with the Declaration of Helsinki, designed and reported in accordance with STROBE guidelines, [20] registered by the Institutional Study Centre (SZ_W_134.21-I-6) and approved by the Institutional Review Board (IRB-2021–031) on 10 December 2021. Informed consent was waived due to the study’s retrospective design, utilizing routinely obtained de-identified clinical and laboratory data.
2.2. Patients
Eligible candidates for this retrospective study were consecutive patients operated for active primarily left-sided NVE and PVE at the Department of Cardiac Surgery, Klinikum Nürnberg, Paracelsus Medical University, Nuremberg, Germany. Between January 2015 and April 2021, 204 patients fulfilling modified Duke criteria were identified [21]. According to guidelines, all patients received antibiotics and anti-infective therapy under supervision of an infectious disease specialist [1,2]. Intraoperative hemoadsorption during CPB was introduced in Nuremberg in 2018 and has been used ever since in most patients with IE. Exclusion criteria were isolated right-sided IE, fungal IE, age < 18 years and video-assisted right lateral approach to the mitral valve. Patients with recurrent IE within one year or identical pathogens before the second surgery were excluded from the analysis [22].
Patients received standardized anaesthesia. After implementing CPB via central cannulation, cardioplegic arrest was induced by cold crystalloid Bretschneider cardioplegia (Custodiol®, Dr. F.Koehler Chemie, Bensheim, Germany) or cold blood cardioplegia (Calafiore, custom preparation, Klinikum Nürnberg, Germany). The CytoSorb® (Cytosorbents, Monmouth Junction, NJ, USA) cartridge was installed into the venous system of the CPB between the oxygenator and venous reservoir for the entire duration of CPB. Operative strategies included excessive debridement, valve reconstruction or replacement with potential patch-plasty. Concomitant procedures such as myocardial revascularization, replacement of the ascending aorta, left atrial appendage amputation, or closure of persistent foramen ovale, atrial or ventricular septum defect, were performed whenever indicated.
2.3. Data Collection
Patient’s characteristics including demographical and comorbidity-related data, clinical and echocardiographic status including left ventricular ejection fraction, infective agent related informationintraoperative details, and outcomes were retrieved from archived patient files from SAP (Waldorf, Germany) and THG-QIMS (Terraconnect, Nottuln, Germany) quality management software. Laboratory parameters including Hemoglobin, blood lactate, C-reactive protein, white blood cells (WBC) and platelets were determined using the XE-5000 haematology analyser (Sysmex, Norderstedt, Germany), cobas® e602 and c702 module (Roche Diagnostics, Mannheim, Germany) or arterial blood gas analyzer ABL800 (Radiometer, Krefeld, Germany).
Primary outcome was the incidence of sepsis and sepsis-associated mortality. Sepsis was defined by the Third International Consensus Definitions [23]. In-hospital mortality was evaluated as a secondary outcome parameter [24,25]. The rationale of this evaluation was that intraoperative hemoadsorption could reduce sepsis occurrence or attenuate its severity [11,15,26].
2.4. Statistical Analysis
The hemoadsorption group was compared with the control group by using unadjusted and propensity scored-matched data. Propensity score matching was performed by first calculating the standardized mean differences (SMD) of the variables, and those variables with SMD > 0.1 were selected for propensity score matching.
Continuous variables were expressed as mean or median with standard deviation (SD) or interquartile range (IQR), respectively, and compared using Student’s t test or the Mann–Whitney test in the case of non-normally distributed data. Categorical data were expressed as the number of patients and frequencies and were compared using the chi-square test. Univariable and multivariable logistic regression analyses were performed to identify independent risk factors for sepsis-associated and in-hospital mortality. Only variables with a p value ≤ 0.1 in the univariable analysis were used for the forward stepwise multivariable logistic regression analysis. All statistical analyses were performed by using CRAN R (https://www.R-project.org/, version 3.6, The R Foundation for Statistical Computing, Vienna, Austria, accessed on 10 May 2022).
2.5. Definitions
Active IE was defined as an ongoing infection under antibiotic therapy. Coronary heart disease was defined as a history of percutaneous coronary intervention, coronary artery bypass or myocardial infarction. By EuroScore II, the updated system for calculation the risk of death in heart surgery was meant. Liver cirrhosis was defined as cirrhosis of any stage according to Child–Pugh classification. Chronic obstructive pulmonary disease was defined as chronic obstructive pulmonary disease of any stage after GOLD. Re-operation was any surgery due to endocarditis following previous heart surgery. Previous multiple valve surgery was defined as past surgery on two or more valves. Endocarditis of two or more valves was defined when 2 or more valves showed echocardiographic features of endocarditis that were confirmed during surgery. Concomitant right-sided endocarditis was defined as IE of the right heart that occurred secondarily to the endocarditis of the left heart. Concomitant tricuspid valve procedure was every tricuspid valve intervention in addition to the left-sided valve surgery due to endocarditis. Complex surgery was any additional procedure beyond valve repair/replacement, patch reconstruction and/or myocardial revascularization. Major adverse cardiac and cerebrovascular events were defined as per the Society of Thoracic Surgeons for Adult Cardiac Surgery (STS ACS) in-hospital mortality representing the greater of in-hospital, or 30-day mortality, sepsis-associated mortality, myocardial damage, or stroke. Central neurological complications included ischemic events, haemorrhages, cerebral embolisms and abscesses, encephalopathy and meningitis. Sepsis and septic shock were defined by the Third International Consensus Definitions, using quickSOFA criteria [23]. Cardiogenic shock was defined as systolic blood pressure <90 mmHg for >30 min or the need for inotropes to maintain systolic blood pressure >90 mmHg with clinical signs of impaired end-organ perfusion with at least one of the following: cool extremities, decreased urine output, altered mental status, laboratory-confirmed metabolic acidosis, elevated serum lactate and/or creatinine [27]. Postoperative atrial fibrillation (AF) was defined as any new onset of AF that occurred after the surgery during the time of hospitalization. CKD-EPI: the Chronic Kidney Disease Epidemiology Collaboration equation for QFR calculated:141 × min(SCr/κ, 1)α × max(SCr/κ, 1)−1.209 × 0.993 Age in years × 1.018 (if female) × 1.159 (if African American where SCr is standardized serum creatinine)(mg/dL),κ = 0.7 (females) or 0.9 (males),α = −0.329 (females) or −0.411 (males), min = indicates the minimum of SCr/κ or 1, max = indicates the maximum of SCr/κ or 1 [28]. Deep surgical wound infection was defined as sternal osteomyelitis or mediastinitis
3. Results
From the initial 204 patients, two were excluded (one patient because IE could not be confirmed and the other patient because he died during surgery). Of the remaining 202, 103 patients were part of the cohort without intraoperative hemoadsorption (operation between January 2015 and December 2017), and the remaining 99 formed the cohort in which intraoperative hemoadsorption was used (operation between January 2018 and April 2021). Fifteen patients from the control and twelve from the hemoadsorption group received an emergent operation. Demographics are summarized in Table 1. Preoperative characteristics were similar in both groups, except for a higher rate of prior cerebrovascular events in the hemoadsorption group (32.3% vs. 18.4%, p = 0.035, Table 1). Thus, propensity score matching was performed to mitigate the potential confounding effects yielding 99 pairs with similar baseline preoperative and operative characteristics as presented in the right columns of Table 1.
Table 1.
Preoperative patient characteristics.
| Preoperative Characteristics | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Propensity Score Match | |||||
| Control (n = 103) |
Hemoadsorption (n = 99) |
p Value | Control (n = 99) |
Hemoadsorption (n = 99) |
p Value | |
| Demographics | ||||||
| Age (years) | 69 [58;77] | 67 [58;75] | 0.612 | 68.0 [56.5;76.0] | 68.0 [56.5;76.0] | 0.745 |
| BMI (kg/m2) | 26.4 [23.8;30.8] | 26.8 [24.0;30.6] | 0.891 | 26.4 [23.9;31.0] | 26.8 [24.0;30.6] | 0.951 |
| Gender (% male) | 83 (80.6%) | 81 (81.8%) | 0.964 | 79 (79.8%) | 81 (81.8%) | 0.857 |
| EuroScore II (%) | 9 [3.6;22.2] | 9.9 [5.5;21.8] | 0.805 | 8.95 [3.58;21.1] | 9.89 [5.5;21.8] | 0.705 |
| Native valve IE | 76 (37.6%) | 49 (24.1%) | 0.106 | 73 (73.7%) | 49 (24.1%) | 0.110 |
| Prosthetic valve IE | 27 (13.3%) | 40 (19.9%) | 0.169 | 27 (27.3%) | 40 (19.9%) | 0.218 |
| CAD | 28 (27.5%) | 28 (28.3%) | 1.000 | 25 (25.5%) | 28 (28.3%) | 0.781 |
| Diabetes Mellitus (II) | 25 (24.3%) | 23 (23.2%) | 0.993 | 25 (25.3%) | 23 (23.2%) | 0.868 |
| pAOD | 6 (5.8%) | 3 (3%) | 0.499 | 4 (4.04%) | 3 (3.03%) | 1.000 |
| History of CVI | 19 (18.4%) | 32 (32.3%) | 0.035 | 19 (19.2%) | 32 (32.3%) | 0.051 |
| Septic embolisms (last 3 weeks) | 19 (18.4%) | 21 (21.2%) | 0.752 | 19 (19.2%) | 21 (21.2%) | 0.860 |
| COPD | 20 (19.4%) | 14 (14.1%) | 0.416 | 18 (18.2%) | 14 (14.1%) | 0.562 |
| Liver cirrhosis | 4 (3.9%) | 6 (6.1%) | 0.533 | 4 (4.08%) | 6 (6.1%) | 0.747 |
| AF preop. | 20 (19.4%) | 27 (27.3%) | 0.248 | 20 (20.2%) | 27 (27.3%) | 0.316 |
| eGFRCKD-EPI (mL/min) | 62 [38;79] | 52 [40;74] | 0.380 | 62.0 [39.5;78.5] | 52.0 [40.0;74.0] | 0.340 |
| RRT preop. | 10 (9.7%) | 8 (8.1%) | 0.874 | 9 (9.09%) | 8 (8.1%) | 1.000 |
| Antiplatelet therapy | 32 (31.1%) | 22 (23.2%) | 0.276 | 30 (30.3%) | 22 (23.2%) | 0.337 |
| Oral anticoagulant therapy | 13 (12.7%) | 10 (10.4%) | 0.772 | 12 (12.2%) | 10 (10.4%) | 0.861 |
| Clinical status | ||||||
| NYHA (III-IV) | 73 (70.9%) | 78 (78.8%) | 0.257 | 72 (72.7%) | 78 (78.8%) | 0.407 |
| Inotropes preop. | 36 (35.0%) | 40 (40.8%) | 0.477 | 35 (35.4%) | 40 (40.8%) | 0.520 |
| Septic shock (within 48 h) | 17 (16.5%) | 19 (19.2%) | 0.753 | 16 (16.2%) | 19 (19.2%) | 0.709 |
| Ventilated preop. | 12 (11.7%) | 9 (9.2%) | 0.733 | 12 (12.1%) | 9 (9.2%) | 0.662 |
| Laboratory Parameters | ||||||
| CRP (mg/dL) preop. | 6 [3;10.9] | 5 [2;10.3] | 0.166 | 6.1 [3.00;11.0] | 5.0 [2.0;10.3] | 0.146 |
| Platelets (×103/µL) preop. | 236 [160;307] | 238 [171;300] | 0.920 | 237 [157;314] | 238 [171;300] | 0.983 |
| WBC (×103/µL) preop. | 9.7 [7.3;14.1] | 8.5 [6.2;12.5] | 0.077 | 9.30 [7.25;14.4] | 8.50 [6.2;12.5] | 0.086 |
| Hb (g/dL) preop. | 10.2 [9.1;11.4] | 10.1 [9.1;11.5] | 0.836 | 10.3 [9.15;11.5] | 10.1 [9.1;11.5] | 0.999 |
| Lactate (mmol/l) at start of surgery | 0.8 [0.6;1.1] | 0.7 [0.5;1] | 0.183 | 0.80 [0.60;1.10] | 0.70 [0.5;1.0] | 0.199 |
| Echocardiographic/Radiologic Characteristics | ||||||
| LV-EF lower than 50% | 29 (28.2%) | 23 (23.2%) | 0.523 | 25 (25.3%) | 23 (23.2%) | 0.868 |
| Vegetations | 98 (95.1%) | 94 (94.9%) | 1.000 | 94 (94.9%) | 94 (94.9%) | 1.000 |
| Paravalvular extension or abscess | 37 (35.9%) | 44 (44.4%) | 0.275 | 36 (36.4%) | 44 (44.4%) | 0.311 |
| Concomitant right-sided endocarditis | 5 (4.9%) | 2 (2.1%) | 0.446 | 5 (5.05%) | 2 (2.1%) | 0.445 |
| Causative infective agent | ||||||
| Staphylococcus spec. | 40 (38.8%) | 31 (32.0%) | 0.385 | 40 (40.4%) | 31 (32.0%) | 0.280 |
| Staphylococcus aureus | 26 (26%) | 19 (21.3%) | 0.563 | 26 (27.1%) | 19 (21.3%) | 0.461 |
| Streptococcus species | 29 (29%) | 18 (20.2%) | 0.221 | 26 (27.1%) | 18 (20.2%) | 0.357 |
| Enterococcus faecalis | 13 (13%) | 15 (16.9%) | 0.590 | 13 (13.5%) | 15 (16.9%) | 0.672 |
| Gram-bacteria | 5 (4.9%) | 5 (5.1%) | 1.000 | 5 (5.10%) | 5 (5.1%) | 1.000 |
| Antibiotic therapy (d) preop. | 6 [2;11] | 5 [3;10.8] | 0.702 | 6 [2;11] | 5 [3;11] | 0.850 |
Data are presented as median [interquartile range], mean (standard deviation), or n (%). AF: atrial fibrillation; BMI: body mass index; CAD: coronary artery disease; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration—for the equation see Section 2; CK-MB: Creatine kinase-MB; c-TnT: cardiac troponin; CRP: C-reactive protein; CVI: cerebrovascular insult; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; EuroScore II: updated system for calculation the risk of death in heart surgery; h: hour; Hb: haemoglobin; IE: infective endocarditis; Liver cirrhosis: cirrhosis of any stage according to Child-Pugh classification; LV-EF: left ventricular ejection fraction determined by echocardiography; NYHA: New York Heart Association Classification; oral anticoagulant therapy: Vitamin K antagonists or new oral anticoagulants; pAOD: peripheral arterial obstructive disease; preop.: preoperatively; RRT: renal replacement therapy; inotropes: (nor-) +epinephrine + dobutamine; WBC: white blood cells.
3.1. Operative Characteristics
As depicted in Table 2, the median interval between definitive diagnosis for surgical indication and surgery was 4 days (range 2–9 days) with no difference between both groups. In addition, both groups did not show any difference in the overall time of preoperative anti-infective treatment and were comparable with regard to operative characteristics both in non-adjusted and matched cohort. No device-related adverse events in the hemoadsorption group occurred, and none of the patients received postoperative hemoadsorption therapy.
Table 2.
Intraoperative data.
| Intraoperative Data | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Propensity Score Match | |||||
| Control (n = 103) |
Hemoadsorption (n = 99) | p Value | Control (n = 99) |
Hemoadsorption (n = 99) | p Value | |
| Time: diagnosis and surgery (d) | 4 [2;8.3] | 4 [2;9.8] | 0.697 | 4.00 [2.0;7.3] | 4.00 [2.0;9.8] | 0.546 |
| Re-operation | 43 (41.8%) | 46 (46.5%) | 0.769 | 43 (43.4%) | 46 (46.5%) | 0.891 |
| Cardiopulmonary Bypass (min) | 126 [93.0;168] | 134 [108;176] | 0.212 | 126 [93.0;168] | 134 [108;176] | 0.204 |
| Aortic cross-clamp (min) | 82 [59;116] | 91 [72.5;131] | 0.063 | 82.0 [58.0;114] | 91.0 [72.5;131] | 0.058 |
| Selective cerebral perfusion |
2 (1.9%) | 4 (4%) | 0.661 | 2 (2%) | 4 (4%) | 0.697 |
| Isolated aortic valve surgery |
56 (54.4%) | 46 (46.5%) | 0.601 | 56 (56.6%) | 46 (46.5%) | 0.833 |
| Aortic bioprosthesis | 56 (54.4%) | 50 (50.5%) | 0.852 | 50 (50.5%) | 46 (46.5%) | 0.833 |
| Aortic mechanical prosthesis |
8 (7.8%) | 9 (9.1%) | 0.953 | 8 (8.1%) | 9 (9.1%) | 1.000 |
| Complex aortic surgery | 14 (13.6%) | 23 (23.2%) | 0.196 | 10 (10.1%) | 23 (23.2%) | 0.056 |
| Aortic and mitral valve surgery |
9 (8.7%) | 12 (12.1%) | 0.631 | 14 (14.3%) | 8 (8.1%) | 0.248 |
| Isolated mitral valve surgery |
29 (28.2%) | 27 (27.3%) | 1.000 | 25 (25.3%) | 28 (28.3%) | 0.748 |
| Mitral bioprosthesis | 25 (24.3%) | 26 (26.3%) | 0.925 | 21 (21.2%) | 22 (22.2%) | 1.000 |
| Mitral mechanical prosthesis |
8 (7.8%) | 7 (7.1%) | 1.000 | 8 (8.1%) | 7 (7.1%) | 1.000 |
| Mitral valve reconstruction |
5 (4.9%) | 6 (6.1%) | 0.962 | 8 (8.1%) | 5 (5.1%) | 1.000 |
| Concomitant tricuspid valve surgery | 4 (3.9%) | 6 (6.1%) | 0.533 | 4 (4.08%) | 6 (6.1%) | 0.747 |
| Concomitant revascularization |
14 (14.0%) | 14 (14.6%) | 1.000 | 12 (12.1%) | 14 (14.1%) | 0.833 |
| Pericardial patch reconstruction |
36 (35.6%) | 36 (36.4%) | 1.000 | 36 (37.1%) | 36 (36.4%) | 1.000 |
Data are presented as median [interquartile range], mean (standard deviation) or n (%), d: day; min.: minute.
3.2. Outcomes
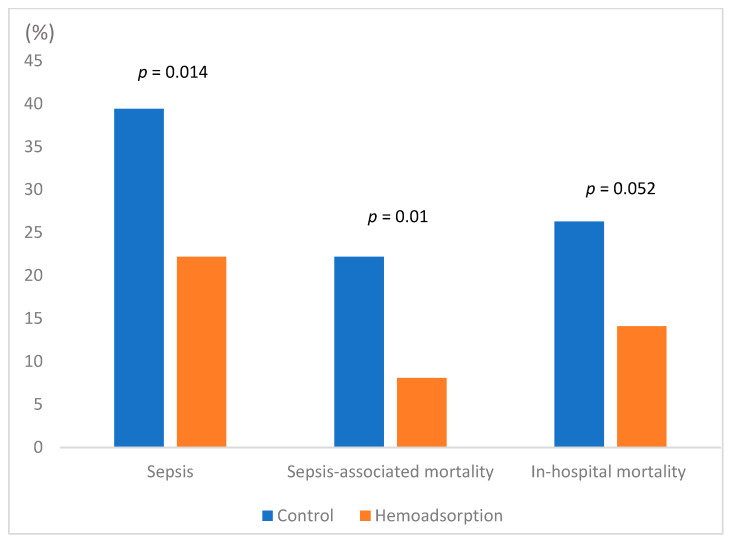
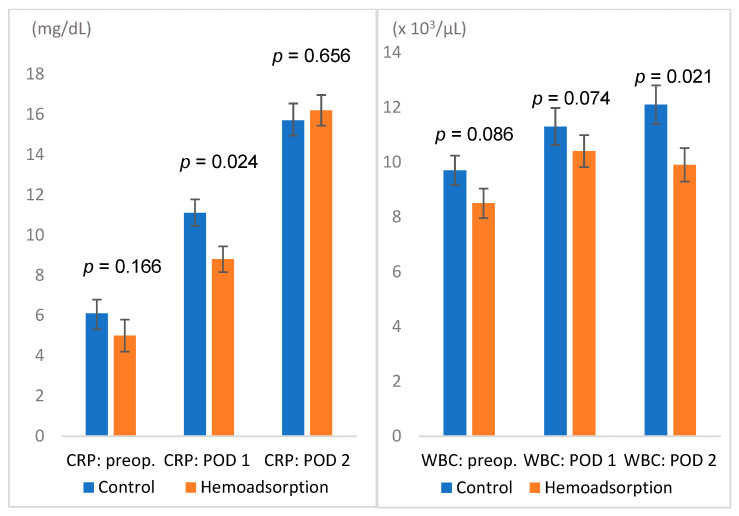
All outcome parameters are summarized in Table 3, demonstrating consistent results for unadjusted and propensity-matched cohorts. Therefore, only propensity-matched data are referred to further in the text. The incidence of postoperative sepsis was 22.2% in the hemoadsorption compared to 39.4% (p = 0.014) in the control group. Sepsis-associated mortality also differed between both groups (8.1% vs. 22.2%, p = 0.010) (Figure 1, Table 3). Overall, in-hospital mortality was notably but insignificantly lower in the hemoadsorption group (14.1% vs. 26.3, p = 0.052). Lower C-reactive protein (CRP) levels on the first postoperative day (8.8 vs. 11.1 mg/dL; p = 0.024) and lower leukocyte counts on the second postoperative day (9.9 vs. 12.1 × 103/µL; p = 0.021) were found in the hemoadsorption group (Figure 2).
Table 3.
Postoperative outcomes.
| Postoperative Outcomes | ||||||
|---|---|---|---|---|---|---|
| Unadjusted Data | Propensity Score Matched Data | |||||
| Control (n = 103) |
Hemoadsorption (n = 99) | p Value | Control (n = 99) |
Hemoadsorption (n = 99) | p Value | |
| Sepsis postop. | 39 (37.9%) | 22 (22.2%) | 0.023 | 39 (39.4%) | 22 (22.2%) | 0.014 |
| Sepsis-associated mortality | 22 (21.4%) | 8 (8.1%) | 0.014 | 22 (22.2%) | 8 (8.1%) | 0.010 |
| In-hospital mortality | 26 (25.2%) | 14 (14.1%) | 0.071 | 26 (26.3%) | 14 (14.1%) | 0.052 |
| Re-thoracotomy for bleeding | 17 (16.7%) | 10 (10.2%) | 0.258 | 17 (17.3%) | 10 (10.2%) | 0.214 |
| Mechanical ventilation (h) | 22 [8, 68.2] | 19.0 [10, 65.5] | 0.856 | 22.0 [7.25, 72.0] | 19.0 [10.0, 65.5] | 0.813 |
| Chest tube output (ml) 24 h postop. | 500 [300, 700] | 500 [250, 950] | 0.761 | 500 [300, 700] | 500 [250, 950] | 0.794 |
| New RRT postop. | 25 (24.3%) | 19 (19.2%) | 0.481 | 24 (24.2%) | 19 (19.2%) | 0.491 |
| Central neurological complications | 2 (1.9%) | 8 (8.1%) | 0.055 | 2 (2.02%) | 8 (8.1%) | 0.105 |
| Pneumonia | 10 (9.7%) | 6 (6.1%) | 0.484 | 10 (10.1%) | 6 (6.1%) | 0.434 |
| Deep Sternal Wound Infection | 1 (1.0%) | 0 | 1.000 | 1 (1.01%) | 0 (0.0%) | 1.000 |
| UTI | 2 (1.9%) | 1 (1.0%) | 1.000 | 2 (2.02%) | 1 (1.0%) | 1.000 |
| Cumulative inotropes (mg) POD1 | 12.6 [4.9, 40.1] | 17.8 [7.8, 37.2] | 0.204 | 12.6 [4.85, 40.1] | 17.8 [7.8, 37.2] | 0.227 |
| Cumulative inotropes (mg) POD2 | 5.8 [0.9, 15.2] | 4.9 [1.1, 16.9] | 0.927 | 5.82 [0.86, 16.2] | 4.93 [1.1, 16.9] | 0.877 |
| Lactate (mmol/L) at the end of Surgery | 1.3 [1, 1.7] | 1.2 [0.9, 1.8] | 0.656 | 1.30 [1.00, 1.70] | 1.20 [0., 1.80] | 0.652 |
| Lactate (mmol/L) POD1 | 1.20 [0.90, 1.60] | 1.30 [0.92, 1.75] | 0.191 | 1.20 [0.90, 1.60] | 1.30 [0.9, 1.8] | 0.148 |
| Lactate (mmol/L) POD2 | 1 [0.7, 1.2] | 1 [0.8, 1.3] | 0.142 | 1 [0.7, 1.2] | 1 [0.8, 1.3] | 0.191 |
| WBC (×103/µL) POD1 | 11.4 [9.10, 17.1] | 10.4 [8.10, 15.6] | 0.065 | 11.3 [9.05, 17.1] | 10.4 [8.1, 15.6] | 0.074 |
| WBC (×103/µL) POD2 | 12.1 [8.75, 15.1] | 9.90 [7.40, 14.2] | 0.025 | 12.1 [8.90, 15.3] | 9.90 [7.4, 14.2] | 0.021 |
| CRP (mg/dL) POD1 | 10.0 [7.2, 15.4] | 8.80 [5.0, 12.9] | 0.026 | 11.1 [7.0, 15.8] | 8.8 [5.0, 12.9] | 0.024 |
| CRP (mg/dL) POD2 | 15.4 [11.5, 21.8] | 16.2 [11, 20.6] | 0.824 | 15.7 [11.5, 22.3] | 16.2 [11, 20.6] | 0.656 |
| Hb (g/dL) POD1 | 9.20 [8.4, 9.9] | 9.70 [8.7, 10.3] | 0.011 | 9.2 [8.4, 9.9 | 9.70 [8.7, 10.3] | 0.012 |
| Hb (g/dL) POD2 | 8.75 [8.1, 9.3] | 8.90 [8.4, 9.6] | 0.166 | 8.8 [8.1, 9.3] | 8.90 [8.4, 9.6] | 0.200 |
| Platelets transfused (Units) | 0 [0, 2] | 0 [0, 1] | 0.719 | 0 [0, 1.5] | 0 [0, 1] | 0.801 |
| RBCs transfused | 3.0 [1.0, 6.0] | 1.0 [0.0, 4.0] | 0.016 | 3.0 [1.0, 6.0] | 1.0 [0.0, 4.0] | 0.016 |
| FFPs transfused | 0.0 [0.0, 4.00] | 0.0 [00, 6.00] | 0.014 | 0.0 [0.0, 4.0] | 0.0 [0.0, 6.0] | 0.023 |
| Intensive Care Unit stay (d) | 3 [2, 6] | 4 [2, 8.5] | 0.189 | 3 [2, 6] | 4 [2, 8.5] | 0.238 |
| Hospital stay (d) | 34.5 [24, 46] | 39 [22, 49.2] | 0.312 | 33.5 [24, 46] | 39 [22, 49] | 0.282 |
Data are presented as median [interquartile range], mean (standard deviation) or n (%), Central neurological complications: ischemic and haemorrhagic events, encephalopathy, meningitis and brain abscess-transient or permanent; cumulative inotropes: (nor--/ + epinephrine-/ + dobutamine; d: day; deep surgical wound infection: including osteomyelitis or mediastinitis; FFP: fresh frozen plasma; Hb: haemoglobin; h: hour; RBC: red blood cell; RRT: renal replacement therapy; POD: postoperative day; postop: postoperatively; inotropes: (nor-) + epinephrine + dobutamine; UTI: urinary tract infection: including urethritis, cystitis and pyelonephritis WBC: white blood cells.
Figure 1.
Sepsis, sepsis-associated mortality, and in-hospital mortality (propensity-matched cohort).
Figure 2.
Basic laboratory values: C-reactive protein (mg/dL) and white blood cell counts (×103/µL); POD: postoperative day; WBC: white blood cell count.
3.3. Regression Analyses for Sepsis, Sepsis-Associated Mortality and In-Hospital Mortality
Several parameters predicted sepsis, sepsis-associated and in-hospital mortality, as summarized in Table 4, Table 5 and Table 6. Intraoperative hemoadsorption was an independent preventive factor both for sepsis-associated and in-hospital mortality (p = 0.014 and p = 0.032, respectively). Postoperative renal replacement therapy was independently associated with sepsis (p = 0.003), sepsis-associated (p = 0.015) and in-hospital mortality (p = 0.018). C-reactive protein after 24 h and white blood cell counts after 48 h were independent predictors of both sepsis (p = 0.021 and p < 0.001) and sepsis-associated mortality (p = 0.024 and p = 0.006, respectively). An additional independent predictor of sepsis was Staphylococcus species infection (p = 0.041).
Table 4.
Variables related to sepsis.
| Variables Related to Sepsis (Matched Cohort) | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Variable | OR (ClL, CLU) | p Value | OR (ClL, CLU) | p Value |
| BMI (m2/kg) | 1.098 (1.026, 1.174) | 0.007 | ||
| Hemoadsorption | 0.308 (0.130, 0.730) | 0.008 | 0.42 (0.16, 1.07) | 0.07 |
| Euroscore II | 1.022 (1.003, 1.041) | 0.024 | ||
| Cardiogenic shock in last 48 h | 6.125 (2.616, 14.339) | <0.001 | ||
| Ventilated preop. | 3.326 (1.214, 9.110) | 0.019 | ||
| Staphylococcus spec. | 2.506 (1.126, 5.577) | 0.024 | 2.65 (1.04, 6.75) | 0.041 |
| CRP preop. | 1.104 (1.054, 1.158) | <0.001 | ||
| WBC preop. | 1.18 (1.046, 1.196) | 0.001 | ||
| Lactate preop. | 1.878 (1.114, 3.165) | 0.018 | ||
| Inotropes preop. | 7.266 (2.933, 18.002) | <0.001 | ||
| CPB duration | 1.006 (1.001, 1.011) | 0.011 | ||
| Aortic cros-clamp | 1.007 (1.000, 1.014) | 0.046 | ||
| Lactate end of surgery | 2.207 (1.519, 3.209) | <0.001 | ||
| Inotropes cumulative POD 1 | 1.047 (1.031, 1.065) | <0.001 | ||
| Inotropes cumulative POD2 | 1.05 (1.030, 1.071) | <0.001 | ||
| WBC POD1 | 1.222 (1.131, 1.320) | <0.001 | ||
| WBC POD2 | 1.258 (1.155, 1.370) | <0.001 | 1.28 (1.15, 1.43) | <0.001 |
| Lactate 24 h postop. | 2.644 (1.650, 4.236) | <0.001 | ||
| c-TnT POD1 | 1.351 (1.162, 1.572) | <0.001 | ||
| CK-MB POD 1 | 1.01 (1.004, 1.016) | <0.001 | ||
| CRP POD 1 | 1.088 (1.025, 1.156) | 0.006 | ||
| CRP POD2 | 1.056 (0.998, 1.116) | 0.059 | 1.07 (1.01, 1.14) | 0.021 |
| New RRT postop. | 7.142 (3.101, 16.452) | <0.001 | 4.93 (1.70, 14.26) | 0.003 |
| IABP postop. | 6.308 (1.485, 26.792) | 0.013 | ||
| FFPs transfused | 1.088 (1.020, 1.161) | 0.011 | ||
Multivariate analysis was performed for p < 0.05; BMI: body mass index; CK-MB: creatin kinase MB; CPB: cardiopulmonary bypass; CRP: C-reactive protein; c-TnT: c-troponin; Inotropes: (nor)-/ + epinephrine-/ + dobutamine; FFP: fresh frozen plasma; IABP: intra-aortic balloon pump; min: minute; preop: preoperative; postop: postoperative; POD: postoperative day; RRT: renal replacement therapy, WBC: white blood cells.
Table 5.
Variables related to sepsis-associated mortality.
| Variables Related to Sepsis-Associated Mortality (Matched Cohort) | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Variable | OR (ClL, CLU) | p Value | OR (ClL, CLU) | p Value |
| BMI | 1.098 (1.026, 1.174) | 0.007 | ||
| Hemoadsorption | 0.308 (0.130, 0.730) | 0.008 | 0.09 (0.01, 0.62) | 0.014 |
| EuroScore II | 1.022 (1.003, 1.041) | 0.024 | ||
| Staphylococcus spec. | 2.506 (1.126, 5.577) | 0.024 | ||
| Inotropes preop. | 7.266 (2.933, 18.002) | <0.001 | ||
| CRP preop. | 1.104 (1.054, 1.158) | <0.001 | ||
| Cardiogenic shock in the last 48 h | 6.125 (2.616, 14.339) | <0.001 | ||
| Ventilated preop. | 3.326 (1.214, 9.110) | 0.019 | ||
| WBC preop. | 1.118 (1.046, 1.196) | 0.001 | ||
| Lactate: start of surgery | 1.878 (1.114, 3.165) | 0.018 | ||
| Concomitant tricuspid valve surgery | 4.32 (1.139, 16.390) | 0.031 | ||
| CPB duration (min.) | 1.006 (1.001, 1.011) | 0.011 | ||
| Aortic cross-clamp (min.) | 1.007 (1.000, 1.014) | 0.046 | ||
| Paravalvular extension or Abscess | 2.179 (0.992, 4.787) | 0.052 | 4.18 (0.73,23.99) | 0.109 |
| Lactate (mmol/L) end of surgery | 2.207 (1.519, 3.209) | <0.001 | ||
| WBC POD1 | 1.222 (1.131, 1.320) | <0.001 | ||
| WBC POD2 | 1.258 (1.155, 1.370) | <0.001 | 1.21 (1.06, 1.38) | 0.006 |
| Lactate at 24 h postop. | 2.644 (1.650, 4.236) | <0.001 | ||
| Inotropes cumulative POD1 | 1.047 (1.030, 1.064) | <0.001 | 1.03 (0.99, 1.06) | 0.068 |
| Inotropes cumulative POD2 | 1.005 (1.002, 1.007) | <0.001 | 1.01 (1.00, 1.01) | 0.015 |
| CRP POD 1 | 1.088 (1.025, 1.156) | 0.006 | 1.15 (1.02, 1.31) | 0.024 |
| CRP POD 2 | 1.056 (0.998, 1.116) | 0.059 | ||
| c-TnT POD 1 | 1.351 (1.162, 1.572) | <0.001 | ||
| CK-MB POD 1 | 1.01 (1.004, 1.016) | 0.001 | ||
| New RRT postop. | 7.142 (3.101, 16.452) | <0.001 | 8.16 (1.49, 44.37) | 0.015 |
| FFPs transfused | 1.088 (1.020, 1.161) | 0.011 | ||
| IABP postop. | 6.308 (1.485, 26.792) | 0.013 | ||
Multivariate analysis was performed for p < 0.05; BMI: body mass index; CK-MB: creatin kinase MB; CPB: Cardiopulmonary Bypass; CRP: C-reactive protein; c-TnT: c-troponin; Inotropes: (nor)-/ + epinephrine-/ + dobutamine; FFP: fresh frozen plasma; IABP: intra-aortic balloon pump; min: minute; preop: preoperative; postop: postoperative; POD: postoperative day; RRT: renal replacement therapy, WBC: White blood cells.
Table 6.
Variables related to in-hospital mortality.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | OR (ClL, CLU) | p Value | OR (ClL, CLU) | p Value |
| BMI | 1.068 (1.005, 1.135) | 0.034 | ||
| Hemoadsorption therapy | 0.462 (0.225, 0.951) | 0.036 | 0.07 (0.01, 0.79) | 0.032 |
| Staphylococcus spec. | 2.497 (1.223, 5.096) | 0.012 | ||
| Endocarditis of 2 or more valves | 2.654 (1.025, 6.873) | 0.044 | ||
| Cardiogenic chock in the last 48 h | 6.786 (3.047, 15.114) | <0.001 | ||
| Inotropes preop. | 6.362 (2.932, 13.801) | <0.001 | 17.36 (1.77, 170.13) | 0.014 |
| Preop Lactate | 2.147 (1.252, 3.679) | 0.005 | ||
| Ventilated preop. | 5.576 (2.168, 14.338) | <0.001 | ||
| WBC preop. | 1.129 (1.058, 1.204) | <0.001 | ||
| CRP preop. | 1.082 (1.036, 1.130) | <0.001 | ||
| CPB duration | 1.006 (1.002, 1.011) | 0.008 | ||
| Aortic cross-clamp | 1.007 (1.001, 1.014) | 0.026 | ||
| Lactate end of surgery | 2.637 (1.761, 3.948) | <0.001 | ||
| Lactate 24 h postop. | 2.754 (1.712, 4.432) | <0.001 | 4.14 (1.01, 17.01) | 0.049 |
| WBC POD1 | 1.173 (1.100,1.252) | <0.001 | ||
| Inotropes cumulative POD1 | 1.053 (1.035, 1.071) | <0.001 | ||
| Inotropes cumulative POD2 | 1.048 (1.028, 1.069) | <0.001 | 1.02 (1.00, 1.04) | 0.024 |
| WBC POD2 | 1.202 (1.120, 1.289) | <0.001 | ||
| c-TnT POD 1 | 1.365 (1.168, 1.595) | <0.001 | ||
| CK-MB POD 1 | 1.009 (1.003, 1.015) | 0.003 | ||
| eGFRCKD-EPI preop. | 0.984 (0.971, 0.998) | 0.022 | ||
| New RRT postop. | 6.833 (3.174, 14.709) | <0.001 | 14.10 (1.57, 126.67) | 0.018 |
| FFPs transfused | 1.151 (1.076, 1.230) | <0.001 | ||
| RBCs transfused | 1.085 (1.025, 1.149) | 0.005 | 0.9 (0.78, 1.03) | 0.123 |
| Drainage Output (ml) in 24 h | 1.001 (1.000, 1.001) | 0.004 | 1.004 (1.001, 1.006) | 0.002 |
| IABP postop. | 4.278 (1.021, 17.922) | 0.047 | ||
Multivariate analysis was performed for p < 0.05; BMI: body mass index; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration—for the equation see Definitions; CK-MB: creatin kinase MB; CRP: C-reactive protein; c-TnT: c-troponin; Inotropes: (nor)-/ + epinephrine-/ + dobutamine; eGFR: estimated glomerular filtration rate; FFP: fresh frozen plasma; IABP: intra-aortic balloon pump; min: minute; preop: preoperative; postop: postoperative; POD: postoperative day; RBC: red blood cell concentrate; RRT: renal replacement therapy; WBC: White blood cells.
Cumulative inotropes after 48 h were independent risk factors both for sepsis-associated and in-hospital mortality (p = 0.015 and p = 0.024, respectively). Preoperative levels of inotropes, lactate levels and chest tube output 24 h after surgery were identified as further independent risk factors for in-hospital mortality (p = 0.014, p = 0.049, and p = 0.002, respectively).
4. Discussion
The current study compared adjunctive intraoperative hemoadsorption therapy versus cardiac surgery without hemoadsorption in a cohort of consecutive high-risk patients presenting with active prosthetic or native valve left-sided IE. The following main observations can be inferred from the current study. First, the incidence of sepsis and sepsis-associated mortality was lower in the hemoadsorption group. The observed reduction corresponded to lower CRP levels 24 h after surgery and lower WBC counts on the second postoperative day. Second, a relevant however insignificant difference in overall in-hospital mortality was observed, with paralleled higher haemoglobin levels on the first postoperative day and lower RBC transfusion requirements in the hemoadsorption group. Third, multifactorial analyses revealed the association of intraoperative hemoadsorption with reduced sepsis-associated and in-hospital mortality. Finally, intraoperative hemoadsorption was safe and did not show any device-related events.
According to the European Infective Endocarditis Registry data, two thirds of in-hospital mortality could be attributed to non-cardiovascular or a combination of cardiovascular and non-cardiovascular causes, whereby sepsis accounted for 75% of non-cardiovascular causes (18). Therefore, a minority of in-hospital mortality is regarded as sepsis-independent. Especially in the surgical treatment of IE, outcomes were influenced mainly by surgical complexity, preoperative patient conditions and comorbidities, as well as the deleterious effects of prolonged CPB and aortic cross clamp (ACC) times [12,13,24]
Despite many advancements in modern anti-infective treatment strategies, IE remains a potentially fatal disorder, and currently, controversies about therapy, including medical and surgical approaches, persist. Anticipating an association between the infection-induced inflammatory response and IE outcomes, the profound understanding of underlying mechanisms including dysregulated immune response to IE-related triggers has become an important target of recent investigations [11,14,18,29,30,31].
Specifically, the recognition of biofilm formation coming with increased antibiotic resistance and hyperinflammatory host response amenable to blood purification therapies are subjects of great interest [11,15,16,30,31]. In the treatment armamentarium of patients presenting with sepsis, various blood purification techniques have been investigated thus far, and most recently, these new adjunctive therapies are also being investigated in infective endocarditis patients.
A first study reporting reduced inotropic support, sepsis and sepsis-associated mortality in patients with native mitral valve IE coupled with hemoadsorption therapy was published by Haidari et al. [18]
Holmén confirmed these results in a small-randomized control trial in patients requiring urgent surgery for IE where hemoadsorption therapy was used intraoperatively. Not only was the accumulated dose of inotropes double in the control group, there was a significantly lower need for blood products in the treatment group [19]. However, another study published by Santer et al. showed different results without beneficial effects of hemoadsorption therapy in IE patients compared to Haidari et al. [5]. More recently, the REMOVE (Revealing Mechanisms and Investigating Efficacy of Hemoadsorption for Prevention of Vasodilatory Shock in Cardiac Surgery Patients with Infective Endocarditis) randomized controlled trial was published, showing no difference in the primary endpoint of the delta (Δ) Sequential Organ Failure Assessment (SOFA) score [17]. In contrast to REMOVE, in the present analysis, we focused on sepsis and sepsis-related mortality, rather than on the ΔSOFA score, as the SOFA score has not been validated for cardio-surgical patients. Moreover, in the present study, over one third of patients from the overall cohort were on preoperative inotropic support; 16.2% in the control and 19.2% in the hemoadsorption group (p = 0.709) presented with septic shock (≈80% in NYHA class III/IV). Specifically, this means that in the present analysis, all-comer and consecutive patients were enrolled to reflect “real-world” data, compared to REMOVE, where only 288 out of 740 screened patients were recruited [17].
As the major finding, we observed significantly reduced sepsis-associated mortality and milder postoperative hyperinflammation. Whether this finding could translate into better overall survival is, however, debatable, but the present analysis demonstrated a relevant difference in overall in-hospital mortality without reaching statistical significance (26.3% vs. 14.1%, p = 0.052). Among other factors, we could show for the first time that intraoperative hemoadsorption is associated with lower sepsis-associated (OR 0.091, 95% CI 0.013–0.620, p = 0.014) and in-hospital mortality (OR 0.069, 95% CI 0.0006–0.795, p = 0.032) in IE patients. If it is postulated that intraoperative hemoadsorption could result in less sepsis and sepsis-associated mortality, the treatment time may be considered a limitation, and a longer duration of hemoadsorption may exert additional effects as also mentioned by the REMOVE investigators. In all published trials conducted thus far regarding evaluating the effect of hemoadsorption in IE, the device was only used during the index procedure. We are only at the beginning of a better understanding of the adjunctive potential of hemoadsorption therapy in IE patients. The REMOVE trial showed a significant reduction in all plasma-circulating harmful cytokines including cell-free DNA in the hemoadsorption group. Moreover, within the REMOVE trial, the direct correlation between CPB-time and elevation of interleukin (IL)-6 was demonstrated. Innovative markers such as pro-adrenomedullin, as mentioned in REMOVE, might serve for better patient selection in the future [17].
Limitations
The present study comes with the following limitations: The study was not a randomized controlled trial, but both groups were comparable. Nonetheless, bias cannot be completely excluded. Moreover, although the current trial represents the largest single-centre cohort of IE patients treated by hemoadsorption thus far, the sample size is still too small to draw definitive conclusions. Due to the overall recruitment period of 6 years, surgical techniques and antimicrobial therapy have changed slightly, opening the study to treatment biases. Second, this was a single-centre, retrospective database analysis, whereby special advanced clinical and inflammatory parameters (e.g., systemic vascular resistance, IL-6, Procalcitonin, N-terminal prohormone of brain natriuretic peptide) were not available for meaningful analyses due to missing values. Further, the retrospective design precluded routine determination of coagulation and endogenous vasoconstricting factors, such as protein C, antithrombin III, coagulation factors VII and X, cortisol, thromboxane, endothelin-1 and albumin.
5. Conclusions
As the main finding, the present analysis demonstrates a reduction in sepsis and sepsis-associated mortality by the intraoperative use of hemoadsorption with CytoSorb® in high-risk IE patients with affected left-sided native or prosthetic valves. Intraoperative hemoadsorption was safe and easy to use without any adjustment to the intraoperative heparin regime. Excessive postoperative inflammatory response with the increased need of postoperative inotropic support and kidney failure requiring dialysis were independent risk factors for sepsis-associated mortality. The intraoperative use of CytoSorb® was the only preventive factor in this regard. Preoperative inotropes, elevated lactate levels at 24 h after the surgery, kidney failure requiring dialysis and excessive postoperative bleeding were independently associated with in-hospital mortality. Again, the intraoperative use of CytoSorb® was a preventive factor. More evidence is needed to better define the value of hemoadsorption in cardiac surgery, especially in the setting of IE relating to appropriate patient selection, timing and dosing.
Author Contributions
Conceptualization, J.M.K., M.F. and T.F.; methodology, J.Z. and T.B.; software, J.Z.; validation, J.M.K. and J.Z.; formal analysis, F.A.V., T.B. and H.M.; investigation, J.M.K. and S.L.; resources, T.F..; data curation, S.L., H.M. and J.M.K.; writing—original draft preparation, J.M.K., J.Z. and F.A.V.; writing—review and editing, J.M.K., E.B. and T.F.; visualization, S.L. and H.M.; supervision, T.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, registered by the Institutional Study Centre (SZ_W_134.21-I-6), and approved by the Institutional Review Board (IRB-2021-031). Informed consent was waived due to the study’s retrospective design, utilizing routinely obtained de-identified clinical and laboratory data.
Informed Consent Statement
Informed consent was waived due to the study’s retrospective design, utilizing routinely obtained de-identified clinical and laboratory data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are primarily not publicly available due to the data protection policy of the institution.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pettersson G.B., Hussain S.T. Current AATS guidelines on surgical treatment of infective endocarditis. Ann. Cardiothorac. Surg. 2019;8:630–644. doi: 10.21037/acs.2019.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.P., Del Zotti F., Dulgheru R., El Khoury G., Erba P.A., Iunget B., et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 3.Farag M., Borst T., Sabashnikov A., Zeriouh M., Schmack B., Arif R., Beller C.J., Popov A.-F., Kallenbach K., Ruhparwar A., et al. Surgery for Infective Endocarditis: Outcomes and Predictors of Mortality in 360 Consecutive Patients. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017;23:3617–3626. doi: 10.12659/MSM.902340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boss K., Jahn M., Wendt D., Haidari Z., Demircioglu E., Thielmann M., Ruhparwar A., Kribben A., Tyczynski B. Extracorporeal cytokine adsorption: Significant reduction of catecholamine requirement in patients with AKI and septic shock after cardiac surgery. PLoS ONE. 2021;16:e0246299. doi: 10.1371/journal.pone.0246299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santer D., Miazza J., Koechlin L., Gahl B., Rrahmani B., Hollinger A., Eckstein F., Siegemund M., Reuthebuch O. Hemoadsorption during Cardiopulmonary Bypass in Patients with Endocarditis Undergoing Valve Surgery: A Retrospective Single-Center Study. J. Clin. Med. 2021;10:564. doi: 10.3390/jcm10040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Della Corte A., Di Mauro M., Dato G.A., Barili F., Cugola D., Gelsomino S., Santè P., Carozza A., della Ratta E., Galletti L. Surgery for prosthetic valve endocarditis a retrospective study of a national registry. Eur. J. Cardiothorac. Surg. 2017;52:105–111. doi: 10.1093/ejcts/ezx045. [DOI] [PubMed] [Google Scholar]
- 7.Pang P.Y., Sin Y.K., Lim C.H., Tan T.E., Lim S.L., Chao V.T., Chua Y.L. Surgical management of infective endocarditis: An analysis of early and late outcomes. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2015;47:826–832. doi: 10.1093/ejcts/ezu281. [DOI] [PubMed] [Google Scholar]
- 8.Iba T., Levy J.H., Warkentin T.E., Thachil J., van der Poll T., Levi M. the Scientific and Standardization Committee on DIC; the Scientific and Standardization Committee on Perioperative and Critical Care of the International Society on Thrombosis and Haemostasis. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 2019;17:1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 9.Holub M., Džupová O., Růžková M., Stráníková A., Bartáková E., Máca J., Beneš J., Herwald H., Beran O. Selected Biomarkers Correlate with the Origin and Severity of Sepsis. Mediat. Inflamm. 2018;2018:e7028267. doi: 10.1155/2018/7028267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buyukasýk N.S., Ileri M., Alper A., Senen K., Atak R., Hisar I., Yetkin E., Turhan H., Demirkan D. Increased blood coagulation and platelet activation in patients with infective endocarditis and embolic events. Clin. Cardiol. 2004;27:154–158. doi: 10.1002/clc.4960270312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honore P.M., Hoste E., Molnár Z., Jacobs R., Joannes-Boyau O., Malbrain M.L., Forni L.G. Cytokine removal in human septic shock: Where are we and where are we going? Ann. Intensive Care. 2019;9:56. doi: 10.1186/s13613-019-0530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olmos C., Vilacosta I., Habib G., Maroto L., Fernández C., López J., Sarriá C., Salaun E., Di Stefano S., Carnero M., et al. Risk score for cardiac surgery in active left-sided infective endocarditis. Heart Br. Card Soc. 2017;103:1435–1442. doi: 10.1136/heartjnl-2016-311093. [DOI] [PubMed] [Google Scholar]
- 13.Tattevin P., Fillâtre P., Tchamgoué S., Lesouhaitier M., Nesseler N., Tadié J.M. Should we include microorganisms in scores to predict outcome in candidates for cardiac surgery during the acute phase of endocarditis? J. Thorac. Dis. 2019;11:E158–E162. doi: 10.21037/jtd.2019.09.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Träger K., Fritzler D., Fischer G., Schröder J., Skrabal C., Liebold A., Reinelt H. Treatment of post-cardiopulmonary bypass SIRS by hemoadsorption: A case series. Int. J. Artif. Organs. 2016;39:141–146. doi: 10.5301/ijao.5000492. [DOI] [PubMed] [Google Scholar]
- 15.Gruda M.C., Ruggeberg K.G., O’Sullivan P., Guliashvili T., Scheirer A.R., Golobish T.D., Capponi V.J., Chan P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS ONE. 2018;13:e0191676. doi: 10.1371/journal.pone.0191676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diab M., Tasar R., Sponholz C., Lehmann T., Pletz M.W., Bauer M., Brunkhorst F.M., Doenst T. Changes in inflammatory and vasoactive mediator profiles during valvular surgery with or without infective endocarditis: A case control pilot study. PLoS ONE. 2020;15:e0228286. doi: 10.1371/journal.pone.0228286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diab M., Lehmann T., Bothe W., Akhyari P., Platzer S., Wendt D., Deppe A.-C., Strauch J., Hagel S., Günther A., et al. Cytokine Hemoadsorption during Cardiac Surgery Versus Standard Surgical Care for Infective Endocarditis (REMOVE): Results from a Multicenter Randomized Controlled Trial. Circulation. 2022;145:959–968. doi: 10.1161/CIRCULATIONAHA.121.056940. [DOI] [PubMed] [Google Scholar]
- 18.Haidari Z., Wendt D., Thielmann M., Mackowiak M., Neuhäuser M., Jakob H., Ruhparwar A., El-Gabry M. Intraoperative Hemoadsorption in Patients with Native Mitral Valve Infective Endocarditis. Ann. Thorac. Surg. 2020;110:890–896. doi: 10.1016/j.athoracsur.2019.12.067. [DOI] [PubMed] [Google Scholar]
- 19.Holmén A., Corderfeldt A., Lannemyr L., Dellgren G., Hansson E.C. Whole Blood Adsorber during CPB and Need for Vasoactive Treatment after Valve Surgery in Acute Endocarditis: A Randomized Controlled Study. J. Cardiothorac. Vasc. Anesth. 2022;36:3015–3020. doi: 10.1053/j.jvca.2022.02.028. [DOI] [PubMed] [Google Scholar]
- 20.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007;335:806–888. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J.S., Sexton D.J., Mick N., Nettles R., Fowler V.G., Jr., Ryan T., Bashore T., Corey G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 22.Chu V.H., Sexton D.J., Cabell C.H., Barth R.L., Pappas P.A., Singh R.K., Fowler V.G., Ralph C.G., Aksoy O., Woods C.W. Repeat Infective Endocarditis: Differentiating Relapse from Reinfection. Clin. Infect. Dis. 2005;41:406–409. doi: 10.1086/431590. [DOI] [PubMed] [Google Scholar]
- 23.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habib G., Erba P.A., Iung B., Donal E., Cosyns B., Laroche C., Popescu B.A., Prendergast B., Tornos P., Sadeghpour A., et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019;40:3222–3232. doi: 10.1093/eurheartj/ehz620. [DOI] [PubMed] [Google Scholar]
- 25.Howitt S.H., Herring M., Malagon I., McCollum C.N., Grant S.W. Incidence and outcomes of sepsis after cardiac surgery as defined by the Sepsis-3 guidelines. Br. J. Anaesth. 2018;120:509–516. doi: 10.1016/j.bja.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kogelmann K., Jarczak D., Scheller M., Drüner M. Hemoadsorption by CytoSorb in septic patients: A case series. Crit Care. 2017;21:74. doi: 10.1186/s13054-017-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vahdatpour C., Collins D., Goldberg S. Cardiogenic Shock. J. Am. Heart Assoc. 2019;8:e011991. doi: 10.1161/JAHA.119.011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., III, Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rugg C., Klose R., Hornung R., Innerhofer N., Bachler M., Schmid S., Fries D., Ströhle M. Hemoadsorption with CytoSorb in Septic Shock Reduces Catecholamine Requirements and In-Hospital Mortality: A Single-Center Retrospective “Genetic” Matched Analysis. Biomedicines. 2020;8:539. doi: 10.3390/biomedicines8120539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerche C.J., Schwartz F., Theut M., Fosbøl E.L., Iversen K., Bundgaard H., Høiby N., Moser C. Anti-biofilm Approach in Infective Endocarditis Exposes New Treatment Strategies for Improved Outcome. Front. Cell Dev. Biol. 2021;9:643335. doi: 10.3389/fcell.2021.643335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe S.E., Wagner N.J., Li L., Beam J.E., Wilkinson A.D., Radlinski L.C., Zhang Q., Miao E.A., Conlon B.P. Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection. Nat. Microbiol. 2020;5:282–290. doi: 10.1038/s41564-019-0627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are primarily not publicly available due to the data protection policy of the institution.