Abstract
Cereals are of utmost importance for the nutrition of infants and children, as they provide important nutrients for their growth and development and, in addition, they are easily digestible, being the best choice for the transition from breast milk/infant formula to solid foods. It is well known that children are more susceptible than adults to toxic food contaminants, such as mycotoxins, common contaminants in cereals. Many mycotoxins are already regulated and controlled according to strict quality control standards in Europe and around the world. There are, however, some mycotoxins about which the level of knowledge is lower: the so-called emerging mycotoxins, which are not yet regulated. The current review summarizes the recent information (since 2014) published in the scientific literature on the amounts of mycotoxins in infants’ and children’s cereal-based food in Europe, as well as their behaviour during digestion (bioaccessibility). Additionally, analytical methods used for mycotoxin determination and in vitro methods used to evaluate bioaccessibility are also reported. Some studies demonstrated the co-occurrence of regulated and emerging mycotoxins in cereal products used in children’s food, which highlights the need to adopt guidelines on the simultaneous presence of more than one mycotoxin. Although very little research has been done on the bioaccessibility of mycotoxins in these food products, very interesting results correlating the fiber and lipid contents of such products with a higher or lower bioaccessibility of mycotoxins were reported. LC-MS/MS is the method of choice for the detection and quantification of mycotoxins due to its high sensibility and accuracy. In vitro static digestion models are the preferred ones for bioaccessibility evaluation due to their simplicity and accuracy.
Keywords: food toxins, mycotoxins, infancy/childhood nutrition, quality control, bioaccessibility, chromatography
1. Introduction
Nutrition in the first years of life is essential to provide optimal growth and development and to establish preferences and eating patterns [1,2,3]. At 6 months of age, breastfeeding should be gradually replaced by nutritionally adequate and safe complementary food, starting with small portions of food that gradually increase with child development [3,4]. From 6 to 23 months of age, children’s nutritional needs require that they eat foods from at least four of the subsequent groups per day: grains, roots and tubers, legumes and nuts, dairy products, meat and fish, eggs, vitamin A-rich fruits and vegetables, and other fruits and vegetables. If their diet lacks this diversity of food, there is a risk of potential micronutrient deficiency [3].
Infant cereals are, usually, among the first foods introduced as complementary foods during weaning because they can complement breastfeeding, guaranteeing the nutritional requirements of the infant, providing a large number of proteins, vitamins (B1, B2, B3, B6, B9, E, and K), minerals (iron, zinc, magnesium, sodium, potassium, and phosphorus), and bioactive compounds, and allowing iron fortification. Also, cereals provide non-digestible carbohydrates that are responsible for the development of microbiota (increasing the Bacteroides population). Due to their mild taste and semi-solid texture, cereals allow the transition from milk and the consequent acceptance of solid foods [5]. There are several different types of cereal-based products available for children, and they are composed of different base cereals, such as wheat, maize, barley, rice, and oats, which can be mixed with chocolate, honey, fruits, or nuts for the enhanced flavour and attractiveness of the product.
Cereals used in infant food are usually subjected to meticulous quality control processes before their release to the market; however, crops are susceptible to toxigenic fungi during both pre- and post-harvest steps, which can produce small toxic chemicals: mycotoxins. The main fungal producers of mycotoxins belong to the genera Aspergillus spp., Penicillium spp., and Fusarium spp. Generally, the identification of the fungi responsible for mycotoxin contamination is difficult since a single fungal species may be able to produce different mycotoxins, while a certain mycotoxin can be produced by more than one species. For instance, mycotoxins such as aflatoxins (AFs), ochratoxins (OTA), citrinin (CIT) [6], and sterigmatocystin (STE) [7], the last two being not regulated in this type of matrix, are produced by the Aspergillus species, while trichothecenes (TCs) (group A: HT-2, and T-2 toxins, and group B: deoxynivalenol (DON)), zearalenone (ZEA), fumonisins B1 (FB1) and B2 (FB2), and the emerging mycotoxins beauvericin (BEA) [8] and enniatins (ENs) [7] are often produced by the Fusarium species. Ergot alkaloids are produced by Claviceps [9].
Mycotoxins occur along all cereal food chains and can have a myriad of acute and/or chronic detrimental health effects, such as immunosuppressive, carcinogenic, estrogenic, gastrointestinal, and kidney events [10,11,12]. Therefore, several countries have adopted regulations to limit mycotoxin exposure through food and reduce their levels to as low as possible. In Europe, EC (European Community) set different maximum limits for mycotoxin exposure for adults and for children (Table 1)—Commission Regulation (EC) No 1881/2006, Commission Regulation (EC) No 165/2010, and Commission Recommendation (2013/165/EU). While the “classical mycotoxins” are already well-known and regulated, there are other mycotoxins, i.e., the so-called emerging mycotoxins, that are not routinely determined nor regulated, even though there is evidence of their rapidly increasing incidence [13].
Table 1.
Maximum levels of mycotoxins in cereals and cereal-derived products according to the European Commission.
| Mycotoxins | Processed Cereal-Based Foods and Baby Foods for Infants and Young Children (µg/kg) |
|---|---|
| Aflatoxin B1 | 0.1 |
| Ochratoxin A | 0.5 |
| Patulin * | 10 |
| Deoxynivalenol | 200 |
| Sum T-2 and HT-2 toxin | 15 |
| Zearalenone | 20 |
| Sum Fumonisin B1 and Fumonisin B2 | 200 |
* Baby foods other than processed cereal-based foods for infants and children.
This manuscript aims to present for the first time a holistic review of mycotoxins in infants’s and children’s cereal-based foods found in Europe since 2014. To accomplish the objective, the amounts of mycotoxins, their bioaccessibility as well as the advantages and drawbacks of the analytical methods used for their determination will be provided. The impact of mycotoxins in infants and children and the protective effect of the ingestion of cereals against mycotoxicosis will be briefly summarized. Additionally, different factors that determine mycotoxins’ bioaccessibility will be highlighted in order to understand whether the fraction of mycotoxins released from the food matrix into the gastrointestinal tract upon digestion could induce toxic health effects in the children. We provide an overview of the gaps in research on mycotoxins in cereal-based foods for infants and children, to stimulate and improve future research avenues.
2. Materials and Methods
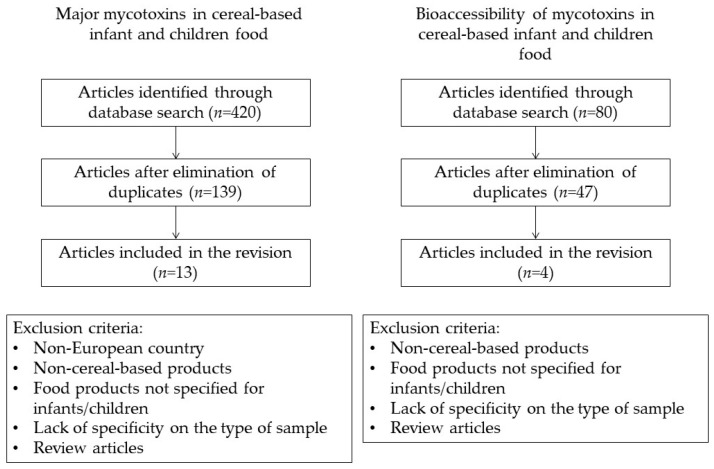
A review of the literature was performed by using the databases Scopus® (www.scopus.com), Web of Science®, PubMed®, and Google Scholar. Several keywords were included to identify published works for mycotoxin incidence in cereal and cereal-based products for infants and children. These included “occurrence”, “mycotoxin”, “aflatoxin”, “ochratoxin”, “deoxynivalenol”, “fumonisin”, “patulin” “T-2”, “HT-2”, “enniatin”, “beauvericin”, “cereal” or “cereal-based products”, “infant” or “children”, and “Europe”. The terms were searched across document titles, types, abstracts, and keywords lists across all years since 2014. Thirteen studies met the selected criteria and were included in the revision. The articles on bioavailability of mycotoxins in this matrix were selected using keywords such as “mycotoxin”, “bioaccessibility” or “bioavailability”, “cereal” or “cereal-based products”, “infant” or “children”; since only four studies were found, no timeframe was established. Data from the studies were divided into different sections: major mycotoxins in cereals, bioaccessibility of mycotoxins in cereal-based infants’ and children’s food and strategies for its reduction, methods for mycotoxin analysis in food and bioaccessibility studies, and gaps in the research of mycotoxins in infants’/children’s cereal-based food matrices (Figure 1).
Figure 1.
Methodology description diagram.
3. Mycotoxicosis in Infants and Children
Mycotoxin contamination is characterized by overtime exposure with the repeated consumption of the same kind of products. Children are more exposed to acute mycotoxicosis because they have stricter dietary patterns, consume some specific food products in larger amounts than adults (per kg of body weight), and their metabolism is not totally developed, so their system is more sensible [9,14,15,16]. However, as the levels of mycotoxins in food are usually low, the long-term effects are rarely seen in children [17,18].
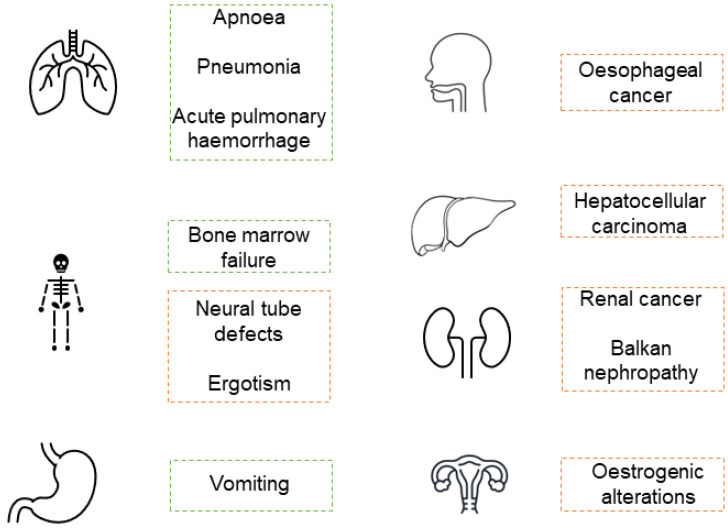
Among children and infants, the most characterized symptoms are recurrent vomiting, caused by exposure to AFs, fumonisins, T-2, patulin (PAT), and DON, bone marrow failure, a well-recognized effect of contamination by TCs [15,19], in addition to recurrent apnoea, pneumonia and/or acute pulmonary haemorrhages, when the exposure route is inhalation [19]. Although unusual in children, there are some long-term consequences due to exposure to these toxins, such as hepatocellular carcinoma by AFs [10], oesophageal cancer and neural tube defects by fumonisins [10,11,12], renal cancer and Balkan nephropathy by OTA [10,20], estrogenic effects by ZEA [21,22], and ergotism by EAs [10] (Figure 2).
Figure 2.
Most common mycotoxicosis health effects in children. Acute effects in green and chronic effects in orange.
Damage to the gastrointestinal system may also only be revealed in the long term. Mycotoxins affect the digestive system in two ways: first by altering the gut microbiota due to a direct toxic effect on the microorganisms and second by altering the structures of the intestine, e.g., TCs and PAT can affect the intestinal barrier, causing the weakening of the permeability and integrity of the intestinal epithelium, resulting in inflammation [17,23,24].
4. Protective Effects of Cereals against Mycotoxin Exposure
Cereals, especially whole-grain, provide several compounds with health-protective mechanisms that may diminish the effects caused by mycotoxins’ toxicity. Particularly, wheat bran has shown consistent cancer-protective properties in human and animal experimental models [25]. The anti-carcinogenic properties of whole-grain foods are mainly due to antioxidant and anti-inflammatory compounds, such as phenolic acids [26,27,28], flavonoids, carotenoids [29], vitamin E [30], n-3 fatty acids [31], phytic acid [32,33], and selenium [34]. Whole-grain cereals are also an indirect source of short-chain fatty acids such as acetate, butyrate, and propionate, which have cancer-preventing properties by lowering intestinal pH and reducing the ability of bile acids to act as carcinogens [35,36]. Moreover, the presence of insoluble fibres in whole-grain cereals increases colon transit time and faecal bulking, which leads to the dilution of carcinogens and reduces their interaction with epithelial cells, resulting in cancer prevention [35]. Dietary fibres can also adsorb xenobiotics, resulting in diminished exposure and absorption by the digestive tract [37]. Each of these compounds acts complementary to each other, which enhances their protective action [38].
5. Major Mycotoxins in Cereals
There are several studies on the prevalence of mycotoxins in cereal-based products for young infants and children, as reported in some previous review manuscripts [39,40,41]. In Table 2, there is a summary of the data from those reviews with special focus on the incidence of mycotoxins in cereal and cereal-based foods for infants and children commercialized in Europe.
Table 2.
Occurrence of mycotoxins in cereal-based infant and young children food in Europe (2014 –2021).
| Country | Sample | Mycotoxin | Total Samples |
Positive Samples | References | ||
|---|---|---|---|---|---|---|---|
| % | Mean (µg/kg) | Range (µg/kg) | |||||
| Italy | Infant formulas and baby food | OTA | 75 | 20 | 0.06 | 0.050–0.120 | Juan et al., 2014 [42] |
| DON | 25.3 | 102.60 | 1–268 | ||||
| NIV | 4 | 19.91 | 5.5–235 | ||||
| FUS-X | 24 | 146.51 | 5.5–604 | ||||
| HT-2 | 2.7 | 12.65 | 2–151 | ||||
| β-ZOL | 6.7 | 2.5 | 2–23.2 | ||||
| ENB | 13.3 | 101.30 | 5–832 | ||||
| ENB1 | 1.3 | 7.80 | 5–117 | ||||
| ENB4 | 5.3 | 38.08 | 5–311 | ||||
| ENA1 | 4 | 6.58 | 5–125 | ||||
| BEA | 1.3 | 1.18 | 5–21.3 | ||||
| Portugal | Cereal baby food (maize, wheat, rice, barley, rye, oat, sorghum, millet, spelt) | DON | 9 | 44 | 173.13 | 0.37–270.57 | Pereira et al., 2015 [43] |
| 15AcDON | 11 | 30.94 | 2.50–30.94 | ||||
| T2-Tetrol | 11 | 112.18 | 10.48–112.18 | ||||
| NEO | 11 | 87.21 | 1.28–87.21 | ||||
| Portugal | Breakfast cereals for children (maize, wheat, rice, and multi-grain) | AFB1 | 26 | 0.028 | 0.040–0.400 | Assunção et al., 2015 [44] | |
| AFB2 | 0.002 | 0.030–0.300 | |||||
| AFG1 | 0.006 | 0.045–0.450 | |||||
| AFM1 | 0.012 | 0.100–1.000 | |||||
| OTA | 0.026 | 0.200–2.000 | |||||
| DON | 59 | 15–360 | |||||
| NIV | 6 | 25–360 | |||||
| FB1 | 13 | 2.5–8.0 | |||||
| FB2 | 3 | 2.5–8.0 | |||||
| Turkey | Baby food (cereal based supplementary foods for infants and children) | OTA | 50 | 68 | 0.034–0.374 | 0.042–0.380 | Hampikyan et al., 2015 [45] |
| Portugal | Children cereal-based food | PAT | 20 | 75 50 40 |
2.33 0.061 |
3.2–40.0 0.2–2.0 |
Assunção et al., 2016 [46] |
| Portugal | Breakfast cereals | AFB1 | 26 | 69 | 0.013 | 0.003–0.130 | Martins et al., 2018 [47] |
| AFB2 | 27 | 0.004 | 0.001–0.011 | ||||
| AFG1 | 4 | 0.013 | 0.006–0.014 | ||||
| AFM1 | 12 | 0.017 | 0.011–0.240 | ||||
| OTA | 69 | 0.040 | 0.006–0.100 | ||||
| FB1 | 58 | 12.5 | 0.06–67.0 | ||||
| FB2 | 38 | 4.2 | 0.12–14.0 | ||||
| DON | 62 | 91.5 | 0.4–207.8 | ||||
| NIV | 4 | 27.1 | 5.6–27.1 | ||||
| ZEA | 19 | 0.7 | 0.12–5.6 | ||||
| Portugal | Cereal-based children food | AFB1 | 26 breakfast cereals | 73 | 0.036 | NM | Assunção et al., 2018 [48] |
| AFB2 | 46 | 0.07 | |||||
| AFG1 | 4 | NA | |||||
| AFM1 | 12 | 0.017 | |||||
| AFs | 73 | - | |||||
| OTA | 69 | 0.047 | |||||
| FB1 | 58 | 22.00 | |||||
| FB2 | 39 | 5.10 | |||||
| FMs | 58 | - | |||||
| ZEA | 73 | 1.20 | |||||
| DON | 62 | 95.9 | |||||
| NIV | 4 | NA | |||||
| AFB2 | 20 infant cereals (flours) | 5 | NA | NM | |||
| AFG1 | 10 | 0.014 | |||||
| AFM1 | 40 | 0.068 | |||||
| AFs | 45 | - | |||||
| OTA | 50 | 0.061 | |||||
| FB1 | 35 | 0.44 | |||||
| FMs | 35 | - | |||||
| ZEA | 30 | 0.48 | |||||
| DON | 20 | 41.8 | |||||
| OTAOTA | 6 biscuits | 100 | 0.086 | NM | |||
| DON | 50 | 43.8 | |||||
| Germany | Cereal-based baby food | AOH | 19 | 36.8 | 0.89 | 4.73–7.13 | Gotthardt et al., 2019 [49] |
| AME | 89.5 | 0.24 | 0.23–0.58 | ||||
| TEN | 94.7 | 1 | 0.18–7.53 | ||||
| ATX I | 15.8 | 0.17 | NA | ||||
| ATLP | 5.3 | 0.24 | NA | ||||
| TA | 50.2 | 5.66–221 | |||||
| Spain | Cereal-based baby food | AFB1 | 60 | 11 | 0.03 | 0.02–0.23 | Herrera et al., 2019 [50] |
| AFB2 | 1 | 0.01 | 0.02–0.20 | ||||
| AFG1 | 6 | 0.02 | 0.02–0.16 | ||||
| AFG2 | 1 | 0.01 | 0.02–0.11 | ||||
| DON | 12 | 37 | 33–245 | ||||
| Poland | Cereal-based infant and children food | DON | 302 | 17 | >LOD a <LOQ b | NM | Postupolski et al., 2019 [51] |
| NIV | 3 | ||||||
| ZEA | 14 | ||||||
| OTA | 4 | ||||||
| HT-2 | 0 | ||||||
| T-2 | 1 | ||||||
| FB1 | 3 | ||||||
| FB2 | 4 | ||||||
| Italy | Breakfast cereals Sweet cakes |
OTA | 84 | 2.38 | 1 | NM | Capei et al., 2019 [52] |
| 35.7 | 1.34 | ||||||
| Austria and Czech Republic | Processed cereal-based infant foods | AFL | 35 | 6 | - | <LOQ–1.1 | Braun et al., 2021 [53] * |
| AFB1 | - | - | |||||
| STG | 23 | - | <LOQ–0.5 | ||||
| ZEA | 3 | 0.24 | 1.2 | ||||
| DON | 6 | - | 25–62 | ||||
| NIV | 6 | 43 | <LOQ–20 | ||||
| T-2 | 26 | - | 0.8–3.0 | ||||
| BEA | 14 | 1.5 | <LOQ–3.1 | ||||
| ENA | 3 | −1.9 | <LOQ | ||||
| ENB | 11 | 0.7 | <LOQ–2.1 | ||||
| ENA1 | 60 | 5.9 | <LOQ–40 | ||||
| ENB1 | 26 | 3.9 | <LOQ–10 | ||||
| FB1 | 20 | 4.8 | <LOQ–8.3 | ||||
| AME | 20 | 0.6 | <LOQ–1.1 | ||||
| TA | 31 | 48 | <LOQ–124 | ||||
| TEN | 34 | 0.9 | <LOQ–1.5 | ||||
| ATPL | 23 | 11 | <LOQ–20 | ||||
| Poland | Cereal-based baby foods | DON | 110 | 9.09 | 107.8 | 62–148 | Mruczyk et al., 2021 [54] |
AFB1 (Aflatoxin B1), AFB2 (Aflatoxin B2), AFG1 (Aflatoxin G1), AFG2 (Aflatoxin G2), AFM1 (Aflatoxin M1), OTA (Ochratoxin A), DON (Deoxynivalenol), 15acDON (15-acetyldeoxynivelanol), NIV (Nivalenol), FUS-X (Fusarenon-x),T-2 (Mycotoxin T-2), HT-2 (Mycotoxin HT-2), T2-Tetrol (Mycotoxin T2-tetrol), β-ZOL (β-zearalenol), FB1 (Fumonisin B1), FB2 (Fumonisin B2), PAT (Patulin), ZEA (Zearalenone) ENB (Enniatin B), ENB1 (Enniatin B1), ENB2 (Enniatin B2), ENB4 (Enniatin B4), ENA (Enniatin A), ENA1 (Enniatin A1), ENA2 (Enniatin A2), BEA (Beauvericin), STG (Sterigmatocystin), NEO (Neosolaniol), AOH (Alternariol), AME (Alternariol monomethyl ether), TEN (Tentoxin), ATX I (Altertoxin 1), ATLP (Alterperylenol), TA (Tenuazonic acid) and AFL (Aflatoxicol). Maximum Limit (EC): AFB1—0.1 µg/kg; OTA—0.5 µg/kg; PAT—10.0 µg/kg; DON—200 µg/kg; ZEA—20 µg/kg; FB1 + FB2—200 µg/kg; T-2 + HT-2—15 µg/kg. Bold—above the maximum limit (EC). NA—not applicable; NM—not mentioned.a DON—2.0, NIV—18.6, ZEA—6.1, OTA—0.07, HT-2—1.1, T-2—0.1, FB1—1.4 and FB2—0.5 µg/kg. b DON—6.5, NIV—61.9, ZEA—20.5, OTA—0.24, HT-2—3.7, T-2—0.3, FB1—0.4 and FB2—1.5 µg/kg. * AFL—0.25; STG—0.1; NIV—16; BEA—0.4; ENA—0.4; ENA1—0.4; ENB—0.4; ENB1—0.1; FB1—7.0; AME—0.6; TA—24, TEN—1.0 and ATPL—10 µg/kg [53].
From 2014 to 2021, thirteen studies reported the presence of mycotoxins in cereal-based infants’/children’s food products in Europe. The majority of the studies verified that TCs, particularly DON, are the most prevalent mycotoxins [42,43,44,47,48,50,51,54]. In some of these studies, DON levels were above the maximum limit established by EC (200 µg/kg) [42,43,48,50]. HT-2 was detected in two studies [42,51], one of which found two samples above the established maximum limit (15 µg/kg) [42], and T-2 was also found in two studies at low levels in the first in four samples [51], and in the last in nine samples [53]. Nivalenol (NIV), a TCs unregulated mycotoxin, was found in the cereal samples in five studies, namely in four samples in the studies by Juan et al., 2014 [42], and Martins et al., 2018 [47], in three samples in the study by Postupolski et al., 2019 [51], and in two samples in the study by Braun et al., 2021 [53].
OTA was also a very prevalent mycotoxin in cereal-based food for young people, being reported in eight out of thirteen studies [42,44,45,46,47,48,51,52], usually in levels below the maximum limit established by the EC (0.5 µg/kg). In the study by Juan et al., 2014 [42], this mycotoxin was present in 15 of 75 (20%) samples of cereal-based baby food. Assunção and team [46] found that 40% of the samples presented co-occurrence of PAT and OTA, which underlines the necessity of establishing a maximum limit of PAT in processed cereal-based foods. Martins et al., 2018 [47] reported the presence of OTA in 18 samples. Assunção et al., 2018 [48] analysed different types of samples and reported the presence of OTA in 18 samples of breakfast cereals, 10 samples of infant flours, and 6 samples of biscuits. Lastly, Postupolski et al., 2019 [51], detected the presence of this mycotoxin in four samples.
Regarding AFs, despite being one of the most studied mycotoxins, only four studies reported their presence in cereal-based food samples marketed for children [44,47,48,50]. AFB1 was above the maximum limit established by the EC of 0.1 µg/kg in the works of Martins et al., 2018 [47] and Herrera et al., 2019 [50], both in six samples. In the last study, AFB2 (n = 1), AFG1 (n = 6), and AFG2 (n = 1) were also found.
Fumonisins and ZEA were found less in cereal-based baby foods, and when detected, the levels were below the maximum limits indicated by the EC, 200 µg/kg and 20 µg/kg, respectively [44,47,48,51]. In the study by Postupolski et al., 2019 [51], only one sample presented positive results for fumonisins, and 14 samples were contaminated with ZEA, while in the studies by Assunção et al., 2018 [48] and Martins et al., 2018 [47], both fumonisins and ZEA had a similar incidence of 19 and 15 positive samples, respectively.
ENs were quantified in cereal-based samples marketed for children in two works (Juan et al., 2014 and Braun et al., 2021), but their incidence was lower when compared to other regulated mycotoxins. ENB was the most detected enniatin, mostly in wheat cereal samples (n = 7) in the study by Juan et al., 2014 [42] and in 21 samples in the study by Braun et al., 2021 [53]. Even so, co-occurrence with these emerging mycotoxins demonstrates the importance of the establishment of new guidelines and the necessity of more studies in these matrices.
Other emerging mycotoxins such as STG [53], Alternaria toxins (AOH, AME, TEN, TA, ATX I or ATPL) [49,53], T2-tetrol [43], and AFL [53], were also found in the studies reported here, however, with a much lower frequency.
6. Bioaccessibility of Mycotoxins in Cereal-Based Infants’ and Children’s Food and Strategies for Its Reduction
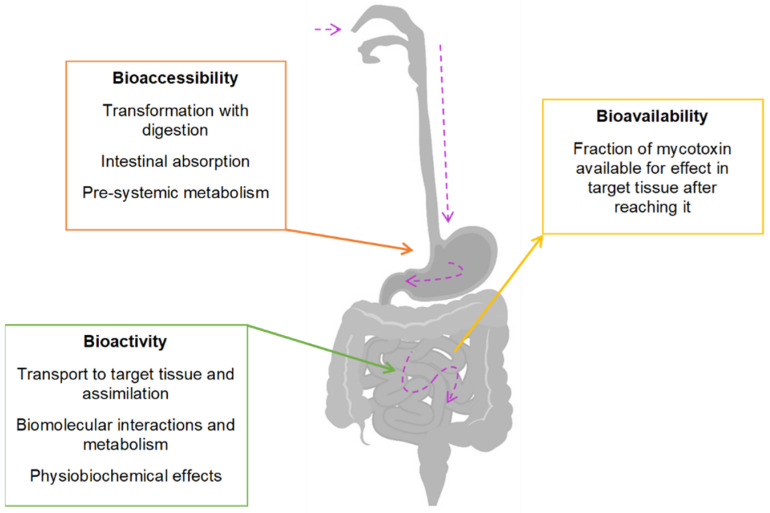
Despite the knowledge already acquired about the presence of mycotoxins in food products, the amounts that are effectively absorbed by the human body are not known, as little is known about their bioavailability and bioaccessibility once ingested [55,56,57] (Figure 3).
Figure 3.
Bioaccessibility, bioactivity, and bioavailability definitions. Purple arrow—mycotoxin path after ingestion.
Mycotoxin bioavailability is the measurement of the amount of mycotoxin that can reach circulation after absorption by the intestinal epithelium (Figure 2). Different food matrices can affect the bioaccessibility of a mycotoxin; however, the metabolism and absorption depend on the mycotoxin itself [55,57,58].
There are few studies on the bioaccessibility of mycotoxins in children (Table 3), but it is known that a child may be more susceptible to significant damage to the intestinal enterocytes caused by the presence of these toxins in the intestinal fluid when compared to adults [15,58,59].
Table 3.
Bioaccessibility of mycotoxins in cereal-based infants’ and young children’s food.
| Country | Sample | Mycotoxin | Total Samples | Bioaccessibility (%) | References |
|---|---|---|---|---|---|
| The Netherlands | Infant formula (spaghetti Bolognese) supplemented with 2 mL sunflower oil per 100 g of food | AFB1 OTA |
2 | 88 ± 16–94 ± 8 29 ± 6–32 ± 4 |
Kabak et al., 2009 [60] |
| Italy | Commercial pasta | DON | 6 | 2.12–41.5 | Raiola et al., 2012 [59] |
| Spain | Breakfast cereals Cookies Breads |
ENA ENA1 ENB ENB1 |
14 | 50 ± 3–80 ± 3 40 ± 2–64 ± 2 43 ± 3–70 ± 3 46 ± 3–74 ± 2 |
Prosperini et al., 2013 [61] |
| Portugal | Cereal-based food | PAT OTA PAT+OTA PAT+OTA |
6 | 30 ± 3–77 ± 2 95 ± 0–105 ± 2 33 ± 1–64 ± 2 (PAT) 103 ± 1–109 ± 0 (OTA) |
Assunção et al., 2016 [46] |
Kabak and colleagues evaluated the effects of probiotic bacteria on bioaccessibility of AFB1 and OTA alone and together in infant formula, with an in vitro static digestion model. The bioaccessibility levels of AFB1 and OTA differ from each other, 88–94% for AFB1 and 29–32% for OTA, which shows that the bioaccessibility depends on the mycotoxin [60]. The authors found that the effect on bioaccessibility depended on bacteria species and type of mycotoxin, with Lactobacillus acidophilus and L. casei Shirota being more effective in reducing the bioaccessibility of AFB1, while no effect was observed on the bioaccessibility of OTA.
In 2012, Raiola’s team evaluated the bioaccessibility of DON in six Italian commercial pastas marketed for young children using an in vitro static digestion model that simulated child physiology. The percentage of DON in the gastric fluid ranged from 2.12 ± 0.11–38.41 ± 2.95%, and the bioaccessibility of DON after the duodenal process ranged from 1.11 ± 0.01–17.91 ± 0.80%. The authors concluded that the bioaccessibility of DON is high enough to produce toxicity and cause higher damage to children, especially due to the small dimension of the intestinal epithelium [59].
Prosperini and colleagues [61] used an in vitro static digestion model to study the bioaccessibility of ENA, ENA1, ENB, and ENB1 in 14 samples of breakfast cereals, cookies, and bread. The samples were spiked with the mycotoxins at 1.5 and 3.0 µmol/g. Their results showed no significant differences between spiked levels in the same type of samples for all mycotoxins except ENB1, where there was higher bioaccessibility in the samples spiked with 1.5 µmol/g (67.3 ± 2.7%). Among the mycotoxins, ENA has the highest bioaccessibility, followed by ENB1, ENB, and ENA1. There are, however, differences in bioaccessibility between similar types of samples that may be related to the chemical structure of the mycotoxins, the food product composition, or matrix. When comparing different cereals, the ones with higher fibre content showed lower bioaccessibility, as the fibres combined with the mycotoxins, leaving less available to produce toxic effects. The inclusion of dietary fibres offers some protection against mycotoxicosis, resulting in a cheap and effective method for the detoxification of feed and food products. Likewise, when comparing different samples with different lipid content, the authors noticed that food products with a higher lipid content show lower bioaccessibility. When the mycotoxins interact with the fat content of the food product, they are not released completely.
The most recent study on bioaccessibility of mycotoxins in cereal-based food for children is from Assunção et al. [46], who studied the bioaccessibility of PAT and OTA in processed cereal-based foods with an in vitro static digestion model. The authors analysed six samples (three with fruit and three without), which were spiked with 20 µg/kg of PAT and 1 µg/kg of OTA, and they verified that the bioaccessibility values of OTA were significantly higher than those of PAT, 106 ± 0.6%, and 56 ± 2.7%, respectively. When PAT was assayed alone or combined with OTA, there was no statistical difference in the values of bioaccessibility, with mean values of 52 ± 4.2% and 56 ± 2.7%, respectively. There was, however, a statistical difference when assaying OTA alone or combined with PAT, with mean values of 100 ± 1.1% and 106 ± 0.6%, which means that OTA demonstrated higher bioaccessibility when in a mixture with PAT; the values above 100% may be due to possible interactions between food matrix, mycotoxins, and digestive fluids.
In vitro biotransformation studies with cell lines offer deeper insight into the effect of the digestion process on mycotoxins and their bioavailability, and consequently, on the impact of these contaminants on the intestinal cells. The biotransformation process of mycotoxins is composed of two phases: in the first, mycotoxins may suffer oxidation, reduction, or hydrolyzation by cytochrome P (CYP) enzymes present in the endoplasmic reticulum and mitochondria, and in the second phase, the resulting metabolites may form conjugates with glutathione, glucuronic acid, and sulphate [62]. The Caco-2 (colorectal adenocarcinoma cell) cell line is the preferred in vitro model method to analyse the intestinal absorption of compounds because these cells form a continuous monolayer with tight junctions that mimic the intestinal wall [63]; also, the ability to express various phase I and II enzymes makes it a good option for biotransformation studies. Other models, such as IPEC-J2 (intestinal porcine epithelial cell), are also used [64,65]. Epithelial integrity and the organization of the tight junction in the cell monolayer are evaluated by monitoring the trans-epithelial electrical resistance (TEER) and the expression of tight junction proteins.
The biotransformation processes of the most studied mycotoxins are already known. AFB1, while not reactive by itself, after bioactivation by different CYP enzymes, forms different metabolites with different degrees of toxicity, such as AFB1-8,9-exposide (AFBO) [66], which is very toxic, with mutagenic and carcinogenic characteristics via the action of CYP450, aflatoxicol (AFL), which is mildly toxic via a ketoreduction, and AFM1, which is also mildly toxic via hydroxylation. The detoxification process of AFBO and AFM1 involves a phase II reaction, conjugation with glutathione-by-glutathione S-transferase (GST) [67,68]. Studies reported that AFB1 reduces TEER in a time-dependent manner in Caco-2 cell line culture [69,70,71] and significantly decreases mRNA expression of tight junction proteins, which are essential for maintenance of adhesive and selective permeability characteristics of the intestinal epithelium [70,71], while AFM1, which is less toxic than AFB1, damaged epithelial integrity to a lesser extent [71,72]. The metabolites resulting from OTA biotransformation are usually less toxic than the original compound [73]. The most common metabolites formed are OTα, formed by the action of carboxypeptidases, 4-hydroxy-ochratoxin A (4-OH-OTA), and 10-hydroxyochratoxin A (10-OH-OTA), formed by reaction with CYP450 enzymes [66,74,75]. A study by Huang et al., [72] showed that OTA has the capacity to reduce TEER in a dose-dependent manner, both individually and in conjugation with AFM1. A study by Alizadeh et al., 2019 [76] demonstrates that OTA reduced TEER in a dose- and time-dependent manner and also decreased the expression of tight junction proteins. The same effect was demonstrated in a different type of cellular model, porcine intestinal cell line IPEC-J2 [77]. DON metabolites are formed by phase II metabolism with glucuronide and sulphate conjugation. The most common metabolites are 3-acetyl-DON, 15-acetyl-DON, diepoxy-deoxynivalenol (DOM-1), DON-3-guccoside (D3G), DON-sulfonates and DON-3-glucuronides [78,79,80]. In studies with Caco-2 cell lines, there was no metabolization of DON, and TEER reduction was apparent only with high exposure times [81,82]. Kadota et al., 2013 compared DON and two metabolites, 3AcDON and 15AcDON, on their intestinal transport in Caco-2 cell line. The authors found that all compounds reduced TEER; however, 15AcDON induced a significantly higher loss of epithelial integrity than DON or 3AcDON [83]. In IPEC-J2 cell line, DON was capable of significantly reducing tight junction protein expression, reducing stability, and increasing the degradation of existing tight junction proteins, leading to increased epithelial permeability [65]. The effect of DON on proliferating and differentiated Caco-2 cell lines was evaluated by Luo et al., 2021. This mycotoxin induces a reduction of TEER in differentiated cells and delays the establishment of TEER in proliferating cells, which may lead to a reduction in the renewal and repair rates of the intestinal epithelium [84]. T-2 toxin can be metabolized by hydroxylation, hydrolysis, and deepoxidation reactions (phase I) and by conjugation (phase II). The most common metabolites formed are HT-2, neosolaniol (NEO), and T-2 triol. While in phase I, CYP450 enzymes are the major contributors, and in phase II, the conjugation is most common with glucuronide [79,85]. T-2 induces a reduction in TEER values in cytotoxic concentration in IPEC-J2 cells, while non-cytotoxic concentrations have little effect [64]. The study by Romero et al., 2016 demonstrated that T-2 was capable of decreasing TEER and the expression of tight junction proteins in a concentration-dependent manner [70]. FB1 is not metabolized by the action of CYP450 enzymes and can act as an inhibitor of specific CYP450 enzymes, such as CYP2C11 and CYP1A2. When FB1 is acetylated, the resulting N-acetylated metabolites are more toxic than the original FB1 [62,66,86,87]. This mycotoxin also affects epithelial integrity by downregulating tight junction protein expression and decreasing TEER. In a study evaluating four mycotoxins (AFB1, FB1, T-2, and OTA), FB1 caused the second-highest reduction of TEER, only surpassed by AFB1 [70]. In the case of ZEA, the most common metabolites are α-zearalenol (α-ZEA), which has a higher affinity for oestrogen receptors in humans, β-zearalenol (β-ZEA) via action of CYP450 enzymes, and zearalenone-14-glucoside (ZEA14Glc) via glycosylation [88,89]. The study by Videmann et al., 2008 demonstrated the metabolization of ZEA into mainly α-ZEA, followed by β-ZEA and ZEA glucuronide, leading to a higher susceptibility to the effects of this mycotoxin [90].
Besides good agricultural practices on crop cereals production, different types of food processing such as washing, roasting, grinding, cooking, radiation, and others [10,91] are strategies used to reduce the occurrence of mycotoxins in cereal food staples. For instance, Meca and co-workers studied the influence of heat treatment on the degradation of BEA, at 160, 180, and 200 °C and at different incubation times, 3, 6, 10, 15, and 20, in crispy breads with different flours. They found a 43% reduction of BEA bioaccessibility at 160 °C, 69% reduction at 180 °C, and 87% reduction at 200 °C, with increasing reduction with longer time of incubation [92]. There are, also, strategies to reduce the bioaccessibility of mycotoxins during digestion, such as the use of probiotic bacteria [58], chemical reduction, and food processing [93]. In 2009, Kabak and colleagues reported a reduction of 37% and 73% for AFB1 and OTA, respectively, in the presence of Lactobacillus and Bifidobacterium bacteria in pistachios, buckwheat, and infant food [60]. Another study on loaf bread samples showed a reduction of AFB1 (15–98%) and AFB2 (11–98%) bioaccessibility in gastric and duodenal digestion, with the presence of probiotic bacteria [94]. Isothiocyanates are compounds present in plants from the Brassicaceae family that have strong antimicrobial properties. Luciano and co-workers studied the reduction of BEA in cereals with BITC (benzyl isothiocyanate) and PITC (phenyl isothiocyanate) fumigation and found a significant reduction, with the highest rate at 92.5% in rice kernels [95].
7. Methods for Mycotoxin Analysis in Food and Bioaccessibility Studies
7.1. Analysis of Mycotoxin in Food
Identification and quantification of mycotoxins in food are critical steps to support production quality control and health risk assessment. Selective and robust methods are needed for mycotoxin analysis because they are very complex molecules with vastly different chemical structures that are present in a range of matrices, usually in low concentrations [96]. Co-occurrence of different mycotoxins is also an issue to consider when analyzing a product.
Mycotoxin analytical methods include several steps such as sampling, homogenization, extraction followed by a clean-up step to decrease and/or eliminate matrix compounds, sample concentration, separation, and detection. The detection step is performed either via a chromatographic technique with different detectors or via an immuno-chemical method [97,98]. More details about the sampling, sample preparation and detection method see Supplemental Material File S1.
7.2. Bioavailability and Bioaccessibility Analysis
Bioaccessibility assessment can be carried out by in vivo or in vitro assays. In vivo approaches are very complicated due to analytical and ethical questions, are very time-consuming and require specific planning, resources, and a high level of experimental control. Although less realistic, in vitro approaches have a wider experimental scope and are simpler to perform while still producing useful results [57].
In vivo studies can be divided in two groups: overall balance studies and determination of substances (or metabolites) in relevant tissues, while in vitro studies, can be divided into static and dynamic processes [57]. Static models mimic the digestive transit by the sequential exposure of the food product to the conditions of the stomach and small intestine, whereas dynamic models simulate the gradual transit of the food product through the different processes of the digestive tract, ensuring a more realistic simulation of the digestive process. These last models simulate the gastric emptying patterns, food transit times, pH value modifications, different concentrations of enzymes, bile salts and electrolytes, water absorption percentage, and in some models, the microbial activity in the different compartments of the gastrointestinal tract [58]. Some models simulate the digestive process in the stomach and small intestine of monogastric animals while other models mimic the large intestine and include microbiota derived from the intestine. As mycotoxin absorption occurs in the small intestine, large intestine models are not used in these studies [57,58].
In vitro digestion models have been developed for the study of several different contaminants, based on human and animal physiology. Some requirements need to be considered to achieve a good methodology, such as the following: (1) representative of the physiological processes of the organism in the study; (2) biochemical reactions, hydrodynamics, and mechanical forces used must match with digestion kinetics; (3) simulation of fasted and fed conditions including proper an-aerobic conditions for the presence of gastrointestinal microorganisms; and (4) easy, robust, reproducible, and applicable methodology [55,57].
The main physiological components of in vitro digestion models are (1) saliva, because the digestion process starts in the mouth with a mechanical action of chewing aided by salivary digestion. This fluid is an exocrine secretion consisting of 99% water, electrolytes, and proteins. In most models simulated saliva is a simplified version of this fluid containing electrolytes, urea, and α-amylase. (2) Gastric juices, which are secreted by the gastric glands in the stomach, are composed by mucus, enzymes, and aqueous component, and in models, they are simulated by a strong decrease of pH and addition of pepsin and electrolytes. (3) Intestinal juices, because after gastric digestion, the food is released into the duodenum, where enzymes from the pancreas and bile from the liver continue the digestion process, and the simulated fluid is composed of electrolytes, pancreatin, and bile salts. Other important components in the digestion models are temperature, peristalsis, incubation time, and microbial interactions, the last not usual component in static models for mycotoxins because these compounds are absorbed in the small intestine [55].
All studies on bioaccessibility reported here (Table 4) used the in vitro static digestive model, with three steps (mouth, stomach, and small intestine digestion) because it is an easier and cheaper model. Kabak et al., 2009 [60] used a method developed by Versantvoort et al., 2005 [99]. The process starts with adding simulated saliva, pH 6.8, to a food sample and incubation for 5 min at 37 °C. Then, artificial gastric juice, pH 1.3, was added, following 2 h incubation at 37 °C. Finally, artificial duodenal fluid at pH 8.1 is added and the mixture is incubated for another 2 h at 37 °C. After digestion, the mycotoxin levels were evaluated in the extract. Raiola et al., 2012 [59] and Prosperini et al., 2013 [61] used a similar method adapted from Gil-Izquierdo et al., 2002 [100], with small alterations to components of the artificial fluids and the pH in each phase of digestion. The first step combines oral and gastric digestion, where the pH starts at 6.5 and is then adjusted to 2, and in the duodenal digestion, the pH is 6.5. After each digestion, the extract is obtained for mycotoxin extraction and detection. For the child digestion model, in the study of Raiola and team [59], there are slight modifications, where the pH of the stomach is 3 and the amount of pepsin, pancreatin, and bile salts were reduced. Assunção and team [46] used a method adapted from Minekus et al., 2014 [101], a standardized method, very similar to the one used by Kabak’s team, with a pH of 7 in the oral digestion, 3 in the gastric digestion, 7 in the intestinal digestion, and some alterations to the components of the artificial digestion fluids, as reported in Table 4. After the intestinal digestion, the mycotoxin levels were quantified in the extract.
Table 4.
Methods for analysis of mycotoxins bioaccessibility in cereal-based food for infants and children.
| Matrix | Mycotoxin | Digestion Model | Extraction | Detection Method | References |
|---|---|---|---|---|---|
| Infant formula | AFB1 OTA |
Static in vitro digestion model: Oral phase (KCl/KSCN/NaH2PO4/NaSO4/NaCl/NaHCO3/urea/a-amylase/uric acid/mucin) Gastric phase (NaCl/NaH2PO4/KCl/CaCl2/NH4Cl/HCl/glucose/glucuronic acid/urea/glucosamine hydrochloride/BSA/pepsin/mucin) Intestinal phase (NaCl/NaHCO3/KCl/HCl/urea/CaCl2(2H2O)/BSA/bile) |
Phosphoric acid/chloroform + IAC AflaOchra HPLCTM | HPLC-FD | Kabak et al., 2009 [60] |
| Commercial pasta | DON | Static in vitro digestion model: Oral phase (KCl/KSCN/NaH2PO4/NaSO4/NaCl/NaHCO3/urea/a-amylase)Gastric phase (HCl/pepsin) Intestinal phase (NaHCO3/pancreatin/bile salts/H2O) |
ACN:water (84:16; v/v) | LC-MS/MS | Raiola et al., 2012 [59] |
| Breakfast cereals Cookies Breads |
ENA ENA1 ENB ENB1 |
Static in vitro digestion model: Oral phase (KCl/KSCN/NaH2PO4/NaSO4/NaCl/NaHCO3/urea/a-amylase) Gastric phase (HCl/pepsin) Intestinal phase (NaHCO3/pancreatin/bile salts/H2O) |
Ethyl acetate | LC-DAD | Prosperini et al., 2013 [61] |
| Cereal-based food | PAT | Static in vitro digestion model: Oral phase (KCl/KH2PO4/NaHCO3/MgCl2(H2O)6/(NH4)2CO3) Gastric phase (KCl/KH2PO4/NaHCO3/NaCl/MgCl2(H2O)6/(NH4)2CO3/pepsin) Intestinal phase (KCl/KH2PO4/NaHCO3/NaCl/MgCl2(H2O)6/pancreatin/bile) |
Ethyl acetate + sodium sulphate + sodium hydrogenocarbonate + SPE column | RP-HPLC-UV | Assunção et al., 2016 [46] |
| OTA | MeOH:water (80:20) + IAC AflaOchra | RP-HPLC-FD |
AFB1—Aflatoxin B1; DON—Deoxynivalenol; ENA—Enniatin A; ENA1—Enniatin A1; ENB—Enniatin B; ENB1—Enniatin B1; OTA—Ochratoxin; PAT—Patulin; ACN—Acetonitrile; HPLC-FD —High-performance liquid chromatography coupled to fluorescence detector; HPLC-UV—High-performance liquid chromatography coupled to ultraviolet detector; IAC—Immunoaffinity columns; LC-DAD—Liquid chromatography coupled to diode array detector; LC-MS/MS—Liquid chromatography coupled to tandem mass spectrometry; RP—Reverse-phase; SPE—Solid-phase extraction.
8. Gaps in the Research of Mycotoxins in Infant/Children Cereal-Based Food Matrices
This review of the available research on mycotoxin quantification and bioaccessibility in cereal-based children and infant food products makes it clear that this is a topic that requires much more attention.
The majority of the studies on mycotoxin determination and quantification in these types of food products reported the presence of regulated mycotoxins, in many cases presenting values above the EU legal limits, and the co-occurrence of several compounds. Although only three studies have evaluated the presence of emerging non-regulated mycotoxins, positive results were always found, which leads to the possibility of a higher mycotoxin presence in cereal-based food products than the one reported in the studies based on the assessment of only regulated mycotoxins.
The type of samples used was quite diversified over the years, with almost all studies differentiating the samples by cereal composition, such as wheat, rice, barley, or maize, or by the presence of fruits and/or cocoa and presenting results in relation to that differentiation. However, only two studies (Herrera and Braun) mentioned products with cereals from organic/biologic cultures [50,53], and only Herrera et al., 2019 [50] presented the results of these specific products. There is an ever-increasing search for organic and biological products for their assumed health benefits, and more families are starting to feed their infants and children with homemade products and formulas, leading to the necessity of more research on these types of products. Braun et al., 2021 [53], diversified their sample pool by also quantifying mycotoxins in raw flours samples intended for production of infant foods. Some studies present other types of cereal-based products for children such as sweet cakes [52] and biscuits [48], highlighting the necessity to add other cereal-based products for children such as cereal bars and cookies. It is also possible to see some distinctions between cereal-based food products for babies, for infants, and for children, as with different age groups there are different nutritional needs, different eating habits, and different susceptibilities. Some studies [48,50,54] clearly present their results with a separation of these types of products, where the first team divided their samples into infant cereals and breakfast cereals [48], the second team considered two types of samples, gluten-free samples for babies from 4 to 6 months of age and multi-cereals for infants aged 7 to 12 months [50], and the last team separated their samples by brand, type of cereal, and consumption age [54].
Bioaccessibility studies on cereal-based infant food are scarce (only four in total as far as we know) and cover a small number of mycotoxins. For instance, ZEA, T-2, HT-2, AME, and TA, which were found in several studies [42,44,47,48,49,53], were never accessed for their bioaccessibility. Despite these types of studies being time-consuming, they do provide valuable information on the potentially harmful effect of mycotoxins on infants and children. In the study by Prosperini and colleagues [61], they reported that in the same type of food, a different composition can change the bioaccessibility of a mycotoxin; for example, a higher percentage of fibre or fat can result in lower bioaccessibility. This highlights the importance of carefully choosing the type of matrix when analysing the effect of a mycotoxin.
The co-occurrence of different mycotoxins [42,46,47,50,53] and whether they act as antagonists or synergists of each other needs to be further researched. More bioavailability studies, with more and different matrices, and other mycotoxins, regulated and non-regulated, will be of extreme importance for the evaluation of the cumulative effect of the compounds in the organism. It is also important to note the lack of studies that correlate the health benefits of cereal ingestion and their protective action against mycotoxins.
9. Final Considerations
Child nutrition is of extreme importance to guarantee the nutritional and energy requirements for proper growth and development. Cereals are one of the best types of food for the weening period in children, as they offer the most important nutrients, are easy to digest, and have a tolerable texture to allow an easier transition to solid foods.
Mycotoxin occurrence in food and processed food products is a worldwide issue due to its high prevalence, particularly in cereals. Nowadays, there are several EU regulations on maximum limits allowed for the most recognized mycotoxins, such as AFs, OTA, ZEA, FMs, and PAT. These regulations are especially severe regarding the limits required for food and food products destined for children, as they are more susceptible to the effects of mycotoxin contamination.
This review about the presence of mycotoxins in cereal-based infant foods commercialized in Europe shows that besides the regular presence of “classical” mycotoxins, which in some cases are above the legal limits, the co-occurrence of the so-called emerging mycotoxins that are not yet regulated is quite common. This finding underlines the necessity of a careful re-evaluation of current guidelines, as these only take into consideration the individual effect of each mycotoxin. Moreover, regulated mycotoxins are the only ones regularly tested for quality control purposes, which may lead to a misguided belief that infant food products are within the safety limits.
Few studies were reported on the bioavailability and bioaccessibility of mycotoxins from cereal-based food matrices, which leads to a diminished understanding of the full effects of the presence of these compounds in infant foods. More studies are needed in this area to gather a consensus on the quality parameters required for the products being marketed for and consumed by children in Europe. The favored method for bioaccessibility analysis is the static in vitro digestion model, due to its cost-effectiveness ratio. It is predictable that in the future, there may be wider use of dynamic digestion models because they offer a more realistic approach to the digestion process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins14070488/s1, Table S1: Methods for analysis of mycotoxins in cereal-based food for infants and children, in Europe (2014–2021), File S1: Methods for Mycotoxin Analysis in Food. References [102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, S.C.C. and J.O.F.; Writing—Original Draft, C.P. Writing—Review and Editing, S.C.C. and J.O.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Summary of the latest scientific contributions on regulated and emerging mycotoxin incidence in cereal-based infant/children food with a review on their bioaccessibility.
Funding Statement
This work is co-financed by the European Regional Development Fund (ERDF) through the Intarreg VA Spain-Portugal (POCTEP) 2014-2020 Programme under Grant Agreement 0591_FOODSENS_1_E. This output reflects the views only of the authors, and the programme authorities cannot be held responsible for any use which may be made of the information contained therein. Sara C. Cunha acknowledges FCT for IF/01616/2015 contract. This research was partially supported by national funds through FCT within the scope of UIDB/04423/2020 and AgriFood XXI R&D&I project, operation No. NORTE-01-0145-FEDER-000041, co-financed by the European Regional Development Fund (ERDF) through NORTH 2020 (Northern Regional Operational Program 2014/2020).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cascant M.M., Garrigues S., de la Guardia M. Direct determination of major components in human diets and baby foods. Anal. Bioanal. Chem. 2015;407:1961–1972. doi: 10.1007/s00216-015-8461-4. [DOI] [PubMed] [Google Scholar]
- 2.Amezdroz E., Carpenter L., O’Callaghan E., Johnson S., Waters E. Transition from milks to the introduction of solid foods across the first 2 years of life: Findings from an Australian birth cohort study. J. Hum. Nutr. Diet. 2015;28:375–383. doi: 10.1111/jhn.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley M.E., Nulty A.K. When Does It All Begin: What, When, and How Young Children Are Fed. Nestle Nutr. Inst. Workshop Ser. 2020;93:15–24. doi: 10.1159/000503353. [DOI] [PubMed] [Google Scholar]
- 4.UNICEF . From the First Hour of Life. Data and Analytics, Division of Data, Research and Policy and Nutrition Section, Programe Division—UNICEF; New York, USA: 2016. [Google Scholar]
- 5.Klerks M., Bernal M.J., Roman S., Bodenstab S., Gil A., Sanchez-Siles L.M. Infant Cereals: Current Status, Challenges, and Future Opportunities for Whole Grains. Nutrients. 2019;11:473. doi: 10.3390/nu11020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doughari J.H. The Occurrence, Properties and Significance of Citrinin Mycotoxin. J. Plant Pathol. Microbiol. 2015;6:11. [Google Scholar]
- 7.Gruber-Dorninger C., Novak B., Nagl V., Berthiller F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017;65:7052–7070. doi: 10.1021/acs.jafc.6b03413. [DOI] [PubMed] [Google Scholar]
- 8.Mallebrera B., Prosperini A., Font G., Ruiz M.J. In vitro mechanisms of Beauvericin toxicity: A review. Food Chem. Toxicol. 2018;111:537–545. doi: 10.1016/j.fct.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Peraica M., Richter D., Rašić D. Mycotoxicoses in children. Arch. Ind. Hyg. Toxicol. 2014;65:347–363. doi: 10.2478/10004-1254-65-2014-2557. [DOI] [PubMed] [Google Scholar]
- 10.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelderblom W.C., Marasas W.F. Controversies in fumonisin mycotoxicology and risk assessment. Hum. Exp. Toxicol. 2012;31:215–235. doi: 10.1177/0960327110395338. [DOI] [PubMed] [Google Scholar]
- 12.Alizadeh A.M., Rohandel G., Roudbarmohammadi S., Roudbary M., Sohanaki H., Ghiasian S.A., Taherkhani A., Semnani S., Aghasi M. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pac. J. Cancer Prev. 2012;13:2625–2628. doi: 10.7314/APJCP.2012.13.6.2625. [DOI] [PubMed] [Google Scholar]
- 13.Vaclavikova M., Malachova A., Veprikova Z., Dzuman Z., Zachariasova M., Hajslova J. ‘Emerging’ mycotoxins in cereals processing chains: Changes of enniatins during beer and bread making. Food Chem. 2013;136:750–757. doi: 10.1016/j.foodchem.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Sherif S.O., Salama E.E., Abdel-Wahhab M.A. Mycotoxins and child health: The need for health risk assessment. Int. J. Hyg. Environ. Health. 2009;212:347–368. doi: 10.1016/j.ijheh.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Raiola A., Tenore G.C., Manyes L., Meca G., Ritieni A. Risk analysis of main mycotoxins occurring in food for children: An overview. Food Chem. Toxicol. 2015;84:169–180. doi: 10.1016/j.fct.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Hulin M., Bemrah N., Nougadère A., Volatier J.L., Sirot V., Leblanc J.C. Assessment of infant exposure to food chemicals: The French Total Diet Study design. Food Addit. Contam. Part A. 2014;31:1226–1239. doi: 10.1080/19440049.2014.921937. [DOI] [PubMed] [Google Scholar]
- 17.Arce-López B., Lizarraga E., López de Mesa R., González-Peñas E. Assessment of Exposure to Mycotoxins in Spanish Children through the Analysis of Their Levels in Plasma Samples. Toxins. 2021;13:150. doi: 10.3390/toxins13020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushnir-Sukhov N.M. A Novel Link between Early Life Allergen Exposure and Neuroimmune Development in Children. J. Clin. Exp. Immunol. 2020;5:188–195. doi: 10.33140/jcei.05.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etzel R.A. What the primary care pediatrician should know about syndromes associated with exposures to mycotoxins. Curr. Probl. Pediatr. Adolesc. Health Care. 2006;36:282–305. doi: 10.1016/j.cppeds.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Malir F., Ostry V., Pfohl-Leszkowicz A., Malir J., Toman J. Ochratoxin A: 50 Years of Research. Toxins. 2016;8:191. doi: 10.3390/toxins8070191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrigo D., Raiola A., Causin R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules. 2016;21:627. doi: 10.3390/molecules21050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee H.J., Ryu D. Advances in Mycotoxin Research: Public Health Perspectives. J. Food Sci. 2015;80:T2970–T2983. doi: 10.1111/1750-3841.13156. [DOI] [PubMed] [Google Scholar]
- 23.Guerre P. Mycotoxin and Gut Microbiota Interactions. Toxins. 2020;12:769. doi: 10.3390/toxins12120769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akbari P., Braber S., Varasteh S., Alizadeh A., Garssen J., Fink-Gremmels J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017;91:1007–1029. doi: 10.1007/s00204-016-1794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y., Sang S. Phytochemicals in whole grain wheat and their health-promoting effects. Mol. Nutr. Food Res. 2017;61:1600852. doi: 10.1002/mnfr.201600852. [DOI] [PubMed] [Google Scholar]
- 26.Schöneberg T., Kibler K., Sulyok M., Musa T., Bucheli T.D., Mascher F., Bertossa M., Voegele R.T., Vogelgsang S. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Addit. Contam. Part A. 2018;35:2455–2470. doi: 10.1080/19440049.2018.1538570. [DOI] [PubMed] [Google Scholar]
- 27.Stuper-Szablewska K., Kurasiak-Popowska D., Nawracała J., Perkowski J. Quantitative profile of phenolic acids and antioxidant activity of wheat grain exposed to stress. Eur. Food Res. Technol. 2019;245:1595–1603. doi: 10.1007/s00217-019-03262-8. [DOI] [Google Scholar]
- 28.Ferruz E., Loran S., Herrera M., Gimenez I., Bervis N., Barcena C., Carramiñana J.J., Juan T., Herrera A., Ariño A. Inhibition of Fusarium Growth and Mycotoxin Production in Culture Medium and in Maize Kernels by Natural Phenolic Acids. J. Food Prot. 2016;79:1753–1758. doi: 10.4315/0362-028X.JFP-15-563. [DOI] [PubMed] [Google Scholar]
- 29.Atanasova-Penichon V., Barreau C., Richard-Forget F. Antioxidant Secondary Metabolites in Cereals: Potential Involvement in Resistance to Fusarium and Mycotoxin Accumulation. Front. Microbiol. 2016;7:566. doi: 10.3389/fmicb.2016.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fardet A., Rock E., Rémésy C. Is the in vitro antioxidant potential of whole-grain cereals and cereal products well reflected in vivo? J. Cereal Sci. 2008;48:258–276. doi: 10.1016/j.jcs.2008.01.002. [DOI] [Google Scholar]
- 31.Slavin J. Why whole grains are protective: Biological mechanisms. Proc. Nutr. Soc. 2003;62:129–134. doi: 10.1079/PNS2002221. [DOI] [PubMed] [Google Scholar]
- 32.Graf E., Eaton J.W. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 1990;8:61–69. doi: 10.1016/0891-5849(90)90146-A. [DOI] [PubMed] [Google Scholar]
- 33.Feizollahi E., Mirmahdi R.S., Zoghi A., Zijlstra R.T., Roopesh M.S., Vasanthan T. Review of the beneficial and anti-nutritional qualities of phytic acid, and procedures for removing it from food products. Food Res. Int. 2021;143:110284. doi: 10.1016/j.foodres.2021.110284. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Dong R., Yang Y., Xie H., Huang Y., Chen X., Wang D., Zhang Z. Protective Effect of Organic Selenium on Oxidative Damage and Inflammatory Reaction of Rabbit Kidney Induced by T-2 Toxin. Biol. Trace Elem. Res. 2021;199:1833–1842. doi: 10.1007/s12011-020-02279-5. [DOI] [PubMed] [Google Scholar]
- 35.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010;23:65–134. doi: 10.1017/S0954422410000041. [DOI] [PubMed] [Google Scholar]
- 36.Slavin J., Jacobs D., Marquart L. Whole-grain consumption and chronic disease: Protective mechanisms. Nutr. Cancer. 1997;27:14–21. doi: 10.1080/01635589709514495. [DOI] [PubMed] [Google Scholar]
- 37.Aoudia N., Callu P., Grosjean F., Larondelle Y. Effectiveness of mycotoxin sequestration activity of micronized wheat fibres on distribution of ochratoxin A in plasma, liver and kidney of piglets fed a naturally contaminated diet. Food Chem. Toxicol. 2009;47:1485–1489. doi: 10.1016/j.fct.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Fardet A. 16—New Concepts and Paradigms for the Protective Effects of Plant-Based Food Components in Relation to Food Complexity. In: Mariotti F., editor. Vegetarian and Plant-Based Diets in Health and Disease Prevention. Academic Press; London, UK: 2017. pp. 293–312. [Google Scholar]
- 39.Hernández M., Juan-García A., Moltó J.C., Mañes J., Juan C. Evaluation of Mycotoxins in Infant Breast Milk and Infant Food, Reviewing the Literature Data. Toxins. 2021;13:535. doi: 10.3390/toxins13080535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppa C.F.S.C., Khaneghah A.M., Alvito P., Assunção R., Martins C., Eş I., Gonçalves B.L., Valganon de Neeff D., Sant’Ana A.S., Corassin C.H., et al. The occurrence of mycotoxins in breast milk, fruit products and cereal-based infant formula: A review. Trends Food Sci. Technol. 2019;92:81–93. doi: 10.1016/j.tifs.2019.08.014. [DOI] [Google Scholar]
- 41.De Sá S.V.M., Monteiro C., Fernandes J.O., Pinto E., Faria M.A., Cunha S.C. Emerging mycotoxins in infant and children foods: A review. Crit. Rev. Food Sci. Nutr. 2021:1–15. doi: 10.1080/10408398.2021.1967282. [DOI] [PubMed] [Google Scholar]
- 42.Juan C., Raiola A., Mañes J., Ritieni A. Presence of mycotoxin in commercial infant formulas and baby foods from Italian market. Food Control. 2014;39:227–236. doi: 10.1016/j.foodcont.2013.10.036. [DOI] [Google Scholar]
- 43.Pereira V.L., Fernandes J.O., Cunha S.C. Comparative assessment of three cleanup procedures after QuEChERS extraction for determination of trichothecenes (type A and type B) in processed cereal-based baby foods by GC–MS. Food Chem. 2015;182:143–149. doi: 10.1016/j.foodchem.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 44.Assunção R., Vasco E., Nunes B., Loureiro S., Martins C., Alvito P. Single-compound and cumulative risk assessment of mycotoxins present in breakfast cereals consumed by children from Lisbon region, Portugal. Food Chem. Toxicol. 2015;86:274–281. doi: 10.1016/j.fct.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Hampikyan H., Bingol E.B., Colak H., Cetin O., Bingol B. Determination of ochratoxin a in baby foods by ELISA and HPLC. Acta Aliment. 2015;44:578–584. doi: 10.1556/066.2015.44.0030. [DOI] [Google Scholar]
- 46.Assunção R., Martins C., Dupont D., Alvito P. Patulin and ochratoxin A co-occurrence and their bioaccessibility in processed cereal-based foods: A contribution for Portuguese children risk assessment. Food Chem. Toxicol. 2016;96:205–214. doi: 10.1016/j.fct.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Martins C., Assunção R., Cunha S.C., Fernandes J.O., Jager A., Petta T., Oliveira C.A., Alvito P. Assessment of multiple mycotoxins in breakfast cereals available in the Portuguese market. Food Chem. 2018;239:132–140. doi: 10.1016/j.foodchem.2017.06.088. [DOI] [PubMed] [Google Scholar]
- 48.Assunção R., Martins C., Vasco E., Jager A., Oliveira C., Cunha S.C., José O.F., Nunes B., Loureiro S., Alvito P. Portuguese children dietary exposure to multiple mycotoxins—An overview of risk assessment under MYCOMIX project. Food Chem. Toxicol. 2018;118:399–408. doi: 10.1016/j.fct.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 49.Gotthardt M., Asam S., Gunkel K., Moghaddam A.F., Baumann E., Kietz R., Rychlik M. Quantitation of Six Alternaria Toxins in Infant Foods Applying Stable Isotope Labeled Standards. Front. Microbiol. 2019;10:109. doi: 10.3389/fmicb.2019.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrera M., Bervis N., Carraminana J.J., Juan T., Herrera A., Arino A., Loran S. Occurrence and Exposure Assessment of Aflatoxins and Deoxynivalenol in Cereal-Based Baby Foods for Infants. Toxins. 2019;11:150. doi: 10.3390/toxins11030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Postupolski J., Starski A., Ledzion E., Kurpinska-Jaworska J., Szczesna M. Exposure assessment of infants and young children on selected Fusarium toxins. Rocz. Panstw. Zakl. Hig. 2019;70:5–14. doi: 10.32394/rpzh.2019.0050. [DOI] [PubMed] [Google Scholar]
- 52.Capei R., Pettini L., Mando Tacconi F. Occurrence of Ochratoxin A in breakfast cereals and sweet snacks in Italy: Dietary exposure assessment. Ann Ig. 2019;31:130–139. doi: 10.7416/ai.2019.2265. [DOI] [PubMed] [Google Scholar]
- 53.Braun D., Eiser M., Puntscher H., Marko D., Warth B. Natural contaminants in infant food: The case of regulated and emerging mycotoxins. Food Control. 2021;123:107676. doi: 10.1016/j.foodcont.2020.107676. [DOI] [Google Scholar]
- 54.Mruczyk K., Cisek-Woźniak A., Mizgier M., Wójciak R.W. Natural Occurrence of Deoxynivalenol in Cereal-Based Baby Foods for Infants from Western Poland. Toxins. 2021;13:777. doi: 10.3390/toxins13110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.González-Arias C.A., Marín S., Sanchis V., Ramos A.J. Mycotoxin bioaccessibility/absorption assessment using in vitro digestion models: A review. World Mycotoxin J. 2013;6:167–184. doi: 10.3920/WMJ2012.1521. [DOI] [Google Scholar]
- 56.Arce-López B., Lizarraga E., Vettorazzi A., González-Peñas E. Human Biomonitoring of Mycotoxins in Blood, Plasma and Serum in Recent Years: A Review. Toxins. 2020;12:147. doi: 10.3390/toxins12030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardoso C., Afonso C., Lourenço H., Costa S., Nunes M.L. Bioaccessibility assessment methodologies and their consequences for the risk–benefit evaluation of food. Trends Food Sci. Technol. 2015;41:5–23. doi: 10.1016/j.tifs.2014.08.008. [DOI] [Google Scholar]
- 58.Rebellato A.P., dos Santos Caramês E.T., Pallone J.A.L., de Oliveira Rocha L. Mycotoxin bioaccessibility in baby food through in vitro digestion: An overview focusing on risk assessment. Curr. Opin. Food Sci. 2021;41:107–115. doi: 10.1016/j.cofs.2021.03.010. [DOI] [Google Scholar]
- 59.Raiola A., Meca G., Mañes J., Ritieni A. Bioaccessibility of Deoxynivalenol and its natural co-occurrence with Ochratoxin A and Aflatoxin B1 in Italian commercial pasta. Food Chem. Toxicol. 2012;50:280–287. doi: 10.1016/j.fct.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 60.Kabak B., Brandon E.F.A., Var I., Blokland M., Sips A.J.A.M. Effects of probiotic bacteria on the bioaccessibility of aflatoxin B1 and ochratoxin A using an in vitro digestion model under fed conditions. J. Environ. Sci. Health Part B. 2009;44:472–480. doi: 10.1080/03601230902935154. [DOI] [PubMed] [Google Scholar]
- 61.Prosperini A., Meca G., Font G., Ruiz M.-J. Bioaccessibility of Enniatins A, A1, B, and B1 in Different Commercial Breakfast Cereals, Cookies, and Breads of Spain. J. Agric. Food Chem. 2013;61:456–461. doi: 10.1021/jf3044023. [DOI] [PubMed] [Google Scholar]
- 62.Tran V.N., Viktorová J., Ruml T. Mycotoxins: Biotransformation and Bioavailability Assessment Using Caco-2 Cell Monolayer. Toxins. 2020;12:628. doi: 10.3390/toxins12100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Assunção R., Ferreira M., Martins C., Diaz I., Padilla B., Dupont D., Bragança M., Alvito P. Applicability of In Vitro Methods to Study Patulin Bioaccessibility and Its Effects on Intestinal Membrane Integrity. J. Toxicol. Environ. Health Part A. 2014;77:983–992. doi: 10.1080/15287394.2014.911138. [DOI] [PubMed] [Google Scholar]
- 64.Goossens J., Pasmans F., Verbrugghe E., Vandenbroucke V., De Baere S., Meyer E., Haesebrouck F., De Backer P., Croubels S. Porcine intestinal epithelial barrier disruption by the Fusariummycotoxins deoxynivalenol and T-2 toxin promotes transepithelial passage of doxycycline and paromomycin. BMC Vet. Res. 2012;8:245. doi: 10.1186/1746-6148-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li E., Horn N., Ajuwon K.M. Mechanisms of deoxynivalenol-induced endocytosis and degradation of tight junction proteins in jejunal IPEC-J2 cells involve selective activation of the MAPK pathways. Arch. Toxicol. 2021;95:2065–2079. doi: 10.1007/s00204-021-03044-w. [DOI] [PubMed] [Google Scholar]
- 66.Wen J., Mu P., Deng Y. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Toxicol. Res. 2016;5:377–387. doi: 10.1039/c5tx00293a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gross-Steinmeyer K., Eaton D.L. Dietary modulation of the biotransformation and genotoxicity of aflatoxin B1. Toxicology. 2012;299:69–79. doi: 10.1016/j.tox.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 68.Deng J., Zhao L., Zhang N.-Y., Karrow N.A., Krumm C.S., Qi D.-S., Sun L.-H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res./Rev. Mutat. Res. 2018;778:79–89. doi: 10.1016/j.mrrev.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Gratz S., Wu Q.K., El-Nezami H., Juvonen R.O., Mykkänen H., Turner P.C. Lactobacillus rhamnosus strain GG reduces aflatoxin B1 transport, metabolism, and toxicity in Caco-2 Cells. Appl. Environ. Microbiol. 2007;73:3958–3964. doi: 10.1128/AEM.02944-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romero A., Ares I., Ramos E., Castellano V., Martínez M., Martínez-Larrañaga M.-R., Anadón A., Martínez M.-A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology. 2016;353–354:21–33. doi: 10.1016/j.tox.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Gao Y., Bao X., Meng L., Liu H., Wang J., Zheng N. Aflatoxin B1 and Aflatoxin M1 Induce Compromised Intestinal Integrity through Clathrin-Mediated Endocytosis. Toxins. 2021;13:184. doi: 10.3390/toxins13030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang X., Gao Y., Li S., Wu C., Wang J., Zheng N. Modulation of Mucin (MUC2, MUC5AC and MUC5B) mRNA Expression and Protein Production and Secretion in Caco-2/HT29-MTX Co-Cultures. Following Exposure to Individual and Combined Aflatoxin M1 and Ochratoxin A. Toxins. 2019;11:132. doi: 10.3390/toxins11020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kőszegi T., Poór M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins. 2016;8:111. doi: 10.3390/toxins8040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ringot D., Chango A., Schneider Y.-J., Larondelle Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem.-Biol. Interact. 2006;159:18–46. doi: 10.1016/j.cbi.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 75.Tao Y., Xie S., Xu F., Liu A., Wang Y., Chen D., Pan Y., Huang L., Peng D., Wang X., et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018;112:320–331. doi: 10.1016/j.fct.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Alizadeh A., Akbari P., Varasteh S., Braber S., Malekinejad H., Fink-Gremmels J. Ochratoxin A challenges the intestinal epithelial cell integrity: Results obtained in model experiments with Caco-2 cells. World Mycotoxin J. 2019;12:399–407. doi: 10.3920/WMJ2019.2451. [DOI] [Google Scholar]
- 77.Wang H., Zhai N., Chen Y., Fu C., Huang K. OTA induces intestinal epithelial barrier dysfunction and tight junction disruption in IPEC-J2 cells through ROS/Ca2+-mediated MLCK activation. Environ. Pollut. 2018;242:106–112. doi: 10.1016/j.envpol.2018.06.062. [DOI] [PubMed] [Google Scholar]
- 78.Maresca M. From the Gut to the Brain: Journey and Pathophysiological Effects of the Food-Associated Trichothecene Mycotoxin Deoxynivalenol. Toxins. 2013;5:784–820. doi: 10.3390/toxins5040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu Q.-H., Wang X., Yang W., Nüssler A.K., Xiong L.-Y., Kuča K., Dohnal V., Zhang X.-J., Yuan Z.-H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014;88:1309–1326. doi: 10.1007/s00204-014-1280-0. [DOI] [PubMed] [Google Scholar]
- 80.Payros D., Alassane-Kpembi I., Pierron A., Loiseau N., Pinton P., Oswald I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016;90:2931–2957. doi: 10.1007/s00204-016-1826-4. [DOI] [PubMed] [Google Scholar]
- 81.Sergent T., Parys M., Garsou S., Pussemier L., Schneider Y.-J., Larondelle Y. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol. Lett. 2006;164:167–176. doi: 10.1016/j.toxlet.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 82.Akbari P., Braber S., Gremmels H., Koelink P.J., Verheijden K.A.T., Garssen J., Fink-Gremmels J. Deoxynivalenol: A trigger for intestinal integrity breakdown. FASEB J. 2014;28:2414–2429. doi: 10.1096/fj.13-238717. [DOI] [PubMed] [Google Scholar]
- 83.Kadota T., Furusawa H., Hirano S., Tajima O., Kamata Y., Sugita-Konishi Y. Comparative study of deoxynivalenol, 3-acetyldeoxynivalenol, and 15-acetyldeoxynivalenol on intestinal transport and IL-8 secretion in the human cell line Caco-2. Toxicol. In Vitro. 2013;27:1888–1895. doi: 10.1016/j.tiv.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 84.Luo S., Terciolo C., Neves M., Puel S., Naylies C., Lippi Y., Pinton P., Oswald I.P. Comparative sensitivity of proliferative and differentiated intestinal epithelial cells to the food contaminant, deoxynivalenol. Environ. Pollut. 2021;277:116818. doi: 10.1016/j.envpol.2021.116818. [DOI] [PubMed] [Google Scholar]
- 85.Li Y., Wang Z., Beier R.C., Shen J., De Smet D., De Saeger S., Zhang S. T-2 toxin, a trichothecene mycotoxin: Review of toxicity, metabolism, and analytical methods. J. Agric. Food Chem. 2011;59:3441–3453. doi: 10.1021/jf200767q. [DOI] [PubMed] [Google Scholar]
- 86.Voss K.A., Smith G.W., Haschek W.M. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007;137:299–325. doi: 10.1016/j.anifeedsci.2007.06.007. [DOI] [Google Scholar]
- 87.EFSA Panel on Contaminants in the Food Chain (CONTAM) Knutsen H.-K., Alexander J., Barregård L., Bignami M., Brüschweiler B., Ceccatelli S., Cottrill B., Dinovi M., Edler L., et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018;16:e05242. doi: 10.2903/j.efsa.2018.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malekinejad H., Maas-Bakker R.F., Fink-Gremmels J. Bioactivation of zearalenone by porcine hepatic biotransformation. Vet. Res. 2005;36:799–810. doi: 10.1051/vetres:2005034. [DOI] [PubMed] [Google Scholar]
- 89.Malekinejad H., Maas-Bakker R., Fink-Gremmels J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006;172:96–102. doi: 10.1016/j.tvjl.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 90.Videmann B., Mazallon M., Tep J., Lecoeur S. Metabolism and transfer of the mycotoxin zearalenone in human intestinal Caco-2 cells. Food Chem. Toxicol. 2008;46:3279–3286. doi: 10.1016/j.fct.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 91.Sforza S., Dall’asta C., Marchelli R. Recent advances in mycotoxin determination in food and feed by hyphenated chromatographic techniques/mass spectrometry. Mass Spectrom. Rev. 2006;25:54–76. doi: 10.1002/mas.20052. [DOI] [PubMed] [Google Scholar]
- 92.Meca G., Ritieni A., Mañes J. Influence of the heat treatment on the degradation of the minor Fusarium mycotoxin beauvericin. Food Control. 2012;28:13–18. doi: 10.1016/j.foodcont.2012.04.016. [DOI] [Google Scholar]
- 93.Luz C., Saladino F., Luciano F.B., Mañes J., Meca G. Occurrence, toxicity, bioaccessibility and mitigation strategies of beauvericin, a minor Fusarium mycotoxin. Food Chem. Toxicol. 2017;107:430–439. doi: 10.1016/j.fct.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 94.Saladino F., Posarelli E., Luz C., Luciano F.B., Rodriguez-Estrada M.T., Mañes J., Meca G. Influence of probiotic microorganisms on aflatoxins B1 and B2 bioaccessibility evaluated with a simulated gastrointestinal digestion. J. Food Compos. Anal. 2018;68:128–132. doi: 10.1016/j.jfca.2017.01.010. [DOI] [Google Scholar]
- 95.Luciano F.B., Meca G., Manyes L., Mañes J. A chemical approach for the reduction of beauvericin in a solution model and in food systems. Food Chem. Toxicol. 2014;64:270–274. doi: 10.1016/j.fct.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 96.Zheng M.Z., Richard J.L., Binder J. A Review of Rapid Methods for the Analysis of Mycotoxins. Mycopathologia. 2006;161:261–273. doi: 10.1007/s11046-006-0215-6. [DOI] [PubMed] [Google Scholar]
- 97.Pereira V.L., Fernandes J.O., Cunha S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014;36:96–136. doi: 10.1016/j.tifs.2014.01.005. [DOI] [Google Scholar]
- 98.Alshannaq A., Yu J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health. 2017;14:632. doi: 10.3390/ijerph14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Versantvoort C.H.M., Oomen A.G., Van de Kamp E., Rompelberg C.J.M., Sips A.J.A.M. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005;43:31–40. doi: 10.1016/j.fct.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 100.Gil-Izquierdo A., Zafrilla P., Tomás-Barberán F.A. An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur. Food Res. Technol. 2002;214:155–159. doi: 10.1007/s00217-001-0428-3. [DOI] [Google Scholar]
- 101.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Carrière F., Boutrou R., Corredig M., Dupont D., et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014;5:1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- 102.Rahmani A., Jinap S., Soleimany F. Qualitative and Quantitative Analysis of Mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009;8:202–251. doi: 10.1111/j.1541-4337.2009.00079.x. [DOI] [PubMed] [Google Scholar]
- 103.Shanakhat H., Sorrentino A., Raiola A., Romano A., Masi P., Cavella S. Current methods for mycotoxins analysis and innovative strategies for their reduction in cereals: An overview. J. Sci. Food Agric. 2018;98:4003–4013. doi: 10.1002/jsfa.8933. [DOI] [PubMed] [Google Scholar]
- 104.Xie L., Chen M., Ying Y. Development of Methods for Determination of Aflatoxins. Crit. Rev. Food Sci. Nutr. 2016;56:2642–2664. doi: 10.1080/10408398.2014.907234. [DOI] [PubMed] [Google Scholar]
- 105.Turner N.W., Bramhmbhatt H., Szabo-Vezse M., Poma A., Coker R., Piletsky S.A. Analytical methods for determination of mycotoxins: An update (2009–2014) Anal. Chim. Acta. 2015;901:12–33. doi: 10.1016/j.aca.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 106.Bueno D., Istamboulie G., Muñoz R., Marty J. Determination of Mycotoxins in Food: A Review of Bioanalytical to Analytical Methods. Appl. Spectrosc. Rev. 2015;50:728–774. doi: 10.1080/05704928.2015.1072092. [DOI] [Google Scholar]
- 107.Turner N.W., Subrahmanyam S., Piletsky S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta. 2009;632:168–180. doi: 10.1016/j.aca.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 108.Krska R., Schubert-Ullrich P., Molinelli A., Sulyok M., MacDonald S., Crews C. Mycotoxin analysis: An update. Food Addit. Contam. Part A. 2008;25:152–163. doi: 10.1080/02652030701765723. [DOI] [PubMed] [Google Scholar]
- 109.Wilson T.J., Romer T.R. Use of the Mycosep Multifunctional Cleanup Column for Liquid Chromatographic Determination of Aflatoxins in Agricultural Products. J. Assoc. Off. Anal. Chem. 1991;74:951–956. doi: 10.1093/jaoac/74.6.951. [DOI] [PubMed] [Google Scholar]
- 110.Cunha S.C., Fernandes J.O. Development and validation of a method based on a QuEChERS procedure and heart-cutting GC-MS for determination of five mycotoxins in cereal products. J. Sep. Sci. 2010;33:600–609. doi: 10.1002/jssc.200900695. [DOI] [PubMed] [Google Scholar]
- 111.Cunha S.C., Fernandes J.O. Chapter 21—Application in Food Analysis. In: Poole C.F., editor. Liquid-Phase Extraction. Elsevier; Amsterdam, The Netherlands: 2020. pp. 643–665. [Google Scholar]
- 112.Tamura M., Uyama A., Mochizuki N. Development of a Multi-mycotoxin Analysis in Beer-based Drinks by a Modified QuEChERS Method and Ultra-High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry. Anal. Sci. 2011;27:629. doi: 10.2116/analsci.27.629. [DOI] [PubMed] [Google Scholar]
- 113.Shephard G.S. Current Status of Mycotoxin Analysis: A Critical Review. J. AOAC Int. 2016;99:842–848. doi: 10.5740/jaoacint.16-0111. [DOI] [PubMed] [Google Scholar]
- 114.Boyer R.F. Modern Expeimental Biochemestry. 3rd ed. Benjamin Cummings; San Francisco, CA, USA: 2000. [Google Scholar]
- 115.Bessaire T., Mujahid C., Mottier P., Desmarchelier A. Multiple Mycotoxins Determination in Food by LC-MS/MS: An International Collaborative Study. Toxins. 2019;11:658. doi: 10.3390/toxins11110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Korfmacher W.A. Foundation review: Principles and applications of LC-MS in new drug discovery. Drug Discov. Today. 2005;10:1357–1367. doi: 10.1016/S1359-6446(05)03620-2. [DOI] [PubMed] [Google Scholar]
- 117.Wang X., Wang S., Cai Z. The latest developments and applications of mass spectrometry in food-safety and quality analysis. TrAC Trends Anal. Chem. 2013;52:170–185. doi: 10.1016/j.trac.2013.08.005. [DOI] [Google Scholar]
- 118.Santos Pereira C., Cunha S.C., Fernandes J.O. Prevalent Mycotoxins in Animal Feed: Occurrence and Analytical Methods. Toxins. 2019;11:290. doi: 10.3390/toxins11050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.