Abstract
Background:
This study aimed to evaluate the efficacy and safety of high-dose dual therapy for Helicobacter pylori (H. pylori) eradication compared to bismuth-containing quadruple therapy.
Methods:
The electronic database of PubMed, Embase, and Cochrane Library were searched from inception to March 18, 2021. Randomized, controlled trials that evaluated high-dose dual therapy versus bismuth-containing quadruple therapy for H. pylori infection were included.
Results:
We included 6 studies containing 1677 patients with H. pylori infection. This meta-analysis demonstrated that high-dose dual therapy achieved similar eradication rate compared with bismuth-containing quadruple therapy (intention-to-treat: 84.6% vs 83.7%, relative risk (RR) = 1.01, 95% CI: 0.97-1.06, P = .49; per-protocol = 88.4% vs 89.0%, RR = 1.00, 95% CI: 0.97-1.04, P = .99). However, high-dose dual therapy showed fewer side effects (13.1% vs 32.0%, RR = 0.51, 95% CI: 0.34-0.78, P = .002) and better compliance (96.1% vs 93.3%, RR = 1.03, 95% CI: 1.00-1.05, P = .03) compared to bismuth-containing quadruple therapy.
Conclusion:
This meta-analysis demonstrated that high-dose dual therapy is equally effective with bismuth-containing quadruple therapy in eradicating H. pylori, with fewer side effects and better compliance.
Keywords: Amoxicillin, Helicobacter pylori, high-dose dual therapy, meta-analysis, proton pump inhibitor
Main Points
Increasing antibiotic resistance is a major cause of treatment failure in Helicobacter pylori infection.
Despite bismuth-containing quadruple therapy (BQT) achieved excellent eradication rates, it still has some shortcomings.
High-dose dual therapy achieved a similar eradication rate compared to BQT but with fewer side effects and better compliance.
Introduction
Helicobacter pylori (H. pylori) is a gram-negative bacterium that colonizes the human stomach and infects more than half of the world’s population.1 H. pylori infection not only plays a causative role in chronic active gastritis, peptic ulcers, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma but also is closely associated with a number of extra-gastrointestinal disorders, such as idiopathic thrombocytopenic purpura and unexplained iron-deficiency anemia.2 In these conditions, this bacterial pathogen should be sought and eradicated.
With the increasing prevalence of metronidazole, clarithromycin, or levofloxacin resistance, the treatment for H. pylori remains a challenge.3 The eradication rate of standard triple therapy has globally fallen below 80%, which is an unacceptable level, and even less than 60% in many parts of the world.4-7 In order to address this dilemma, a variety of therapeutic strategies, such as the use of 4-drug regimens (bismuth-containing quadruple therapy [BQT], concomitant therapy, sequential therapy) and extending treatment duration to 14 days, have been proposed in clinical practice for increasing the eradication success rate. Bismuth-containing quadruple therapy, which consists of bismuth, a proton pump inhibitor (PPI), and 2 antibiotics, has been recommended by Maastricht V/Florence Consensus Report, the Toronto Consensus, and Fifth Chinese National Consensus Report as the first-line treatment regimen for H. pylori infection.2,8-9 However, BQT has some shortcomings as follows: bismuth salt is not available in some countries or regions; complex medication regimens and various adverse events may significantly lead to poor patient compliance, subsequently decreasing the eradication rate.
In the 1990s, Bayerdörffer et al.10 found that high-dose omeprazole (40 mg, 3 times daily) and amoxicillin (750 mg, 3 times daily) dual therapy achieved an eradication rate of over 91%. But later, there are not so many reports regarding its clinical studies. Thus, although the Maastricht V/Florence consensus and the American College of Gastroenterology have recommended that high-dose dual therapy (HDDT) can be used to treat H. pylori infection, the quality of evidence is low.2,11 Moreover, the results from previous studies on HDDT to eradicate H. pylori were controversial. Kwack et al.12 suggested that HDDT was ineffective to treat H. pylori as a first-line regimen in Korea. On the contrary, a study from China reported that HDDT was equally effective but safer and less costly compared with 14-day BQT.13 We therefore conducted a systematic review and meta-analysis to evaluate the efficacy and safety of HDDT for H. pylori eradication compared to BQT.
Materials and Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement.14 The study protocol was registered at International Prospective Register of Systematic Reviews (PROSPERO) (number: CRD42021244650). Ethics committee approval was not applicable for this meta-analysis.
Search Strategy
Two independent researchers (Z. Yin and J. Li) searched PubMed, Embase, and Cochrane Library from inception to March 18, 2021. The following search terms were used: (“Helicobacter pylori” OR “H. pylori” OR “Campylobacter pylori”) AND (“Proton Pump Inhibitors”) AND (“amoxicillin” OR “amoxycillin”) AND (“bismuth”).
Study Selection
Inclusion criteria for the studies were as follows: (a) only comparative studies that evaluated HDDT (PPI and amoxicillin were both administered 3 or 4 times a day and amoxicillin ≥ 2.0 g/day) versus BQT for H. pylori infection were included; (b) the study design was a randomized, controlled trial. Exclusion criteria were as follows: (a) duplicated publications; (b) overlapping data; (c) case reports, animal studies, editorials, comments, meta-analysis, and reviews; (d) age less than 18 years or greater than 70 years; (e) HDDT or BQT not treated with a 14-day therapeutic protocol; (f) use of traditional Chinese medicine or probiotics.
Data Extraction
Two investigators (Z. Yin and J. Li) independently extracted the data in duplicate according to the predesigned data collection form. Any discrepancies were resolved through group discussion or consulting a third independent reviewer (J. Zhang). The following data were collected: first author, year of publication, the country where the study was conducted, study design, participant characteristics, number of patients in each treatment group, details of the treatment regimens, diagnostic approach of H. pylori infection, and confirmative test for eradication, time to confirm H. pylori eradication. The primary outcome was the H. pylori eradication rates by intention-to-treat (ITT) analysis and per-protocol (PP) analysis, and the secondary outcomes were side effects and compliance.
Risk of Bias Assessment
Two independent reviewers (Z. Yin and J. Li) evaluated the risk of bias of each included study according to Cochrane Handbook for Systematic Reviews of Interventions. The risk of bias components was scored as follows: low risk, high risk, or uncertain risk of bias.15
Statistical Analysis
All data were aggregated and analyzed. The meta-analysis was performed using the software Review Manager (version 5.4.1 for mac). For each outcome, 95% CIs and 2-sided P-values were calculated. Heterogeneity among studies was evaluated by Q test and I² statistic. I² values of 25%, 50%, and 75% were considered as low, moderate, and high statistical heterogeneity, respectively. A fixed-effect model would be used if I 2 ≤ 50%, otherwise a random-effects model was applied.16 Funnel plots were used to visually estimate publication bias. Begg’s test was used to assess publication bias statistically.
Trial Sequential Analysis
The random errors and imprecision were assessed by trial sequential analysis (TSA).17 The TSA combines information size estimation for meta-analysis (cumulated sample size of included trials) with adjusted thresholds for statistical significance of meta-analysis and sequential monitoring boundaries for futility.18,19 If the cumulative Z-curve crosses the trial sequential monitoring boundary or futility boundary, a sufficient level of evidence may have been reached and further trials are unnecessary; otherwise, additional studies are required to confirm this idea. Trial sequential analysis was performed using TSA software (version 0.9.5.10 Beta).
Results
Study Selection
Figure 1 shows the flowchart of selection. A total of 1782 relevant records were retrieved with a systematic search. After removing duplicates, 1253 articles were screened for potential eligibility. After screening the titles and abstracts, 1227 irrelevant records were removed and 26 articles were found to be eligible for further analysis. After reviewing the full text, 20 articles were further excluded because of duplicate publications (n = 3), meta-analysis (n = 5), or not using 14-day BQT as the control group (n = 12). Finally, 6 studies with 1677 patients were included.13,20-24 The characteristics of the studies are presented in Table 1.
Figure 1.
Flowchart of selection.
Table 1.
The Characteristics of the Included Studies
| Study | Initial Diagnosis/ Re-Diagnosis |
Treatment Experience | Subgroup (n) | Regimens |
Eradication Rate (ITT/PP, %) |
|---|---|---|---|---|---|
| Miehlke et al. 2003 | H, C/H, C, R, U | Experienced (84) | HDDT (41) BQT (43) |
O 40 mg qid, A 750 mg qid × 14 daysO 20 mg bid, M 500 mg qid, T 500 mg qid, B 107 mg qid × 14 days | 75.6/83.8 81.4/92.1 |
| Hu et al. 2017 | C, U, R/U | Naive (263) |
HDDT (174) BQT (89) |
R 10/20 mg qid, A 750 mg qid × 14 daysR 20 mg bid, A 1000 mg bid, C 500 mg bid, B 220 mg bid × 14 days | 79.9/81.3 84.3/86.2 |
| Sapmaz et al. 2017 | H/S, U | Naive (196) |
HDDT (98) BQT (98) |
R 20 mg tid, A 750 mg tid × 14 daysR 20 mg bid, M 500 mg tid, T 500 mg qid, B 120 mg qid × 14 days | 84.7/84.9 87.8/88.8 |
| Gao et al. 2018 | H, U/U | Naive (142) |
HDDT (70) BQT (72) |
E 20 mg qid, A 750 mg qid × 14 daysE 20 mg bid, A 1000 mg bid, C 500 mg bid, B 220 mg bid × 14 days | 82.9/89.2 86.1/93.9 |
| Yang et al. 2019 | U, R/U | Naive (232) |
HDDT (116) BQT (116) |
E 20 mg qid, A 750 mg qid × 14 daysE 20 mg bid, A 1000 mg bid, C 500 mg bid, B 220 mg bid × 14 days | 87.9/91.1 89.7/91.2 |
| Song et al. 2020 | H, R/U | Naive (760) |
HDDT (380) BQT (380) |
E 20 mg qid, A 750 mg qid × 14 daysE 20 mg bid, A 1000 mg bid, C 500 mg bid, B 220 mg bid × 14 days | 87.1/92.4 80.5/87.8 |
| H, histology; C, culture; R, rapid urease test; U, urea breath test; S, stool antigen test; HDDT, high-dose dual therapy; BQT, bismuth-containing quadruple therapy; O, omeprazole; A, amoxicillin; R, rabeprazole; C, clarithromycin; B, bismuth; M, metronidazole; T, tetracycline; E, esomeprazole; bid, twice a day; tid, 3 times a day; qid, 4 times a day; ITT, intent-to-treat; PP, per-protocol. | |||||
Risk of Bias in the Included Studies
Figures 2 and 3 show the risk of bias in included studies. One study did not describe the random sequence generation procedure and allocation concealment. One study mentioned the randomization sequence generation but did not describe the allocation concealment. By blinding participants and personnel, all included studies achieved a high risk of bias because none of them used the double-blinded method.
Figure 2.
Risk of bias graph.
Figure 3.
Risk of bias summary.
Meta-Analysis
Primary Outcome: Eradication Rate
Among the included studies, there were 879 patients in the HDDT group, and 798 patients in the BQT group. In the ITT analysis, no significant difference was observed in the eradication rate between HDDT and BQT (84.6% vs 83.7%; RR = 1.01, 95% CI: 0.97-1.06; P = .49), without significant heterogeneity among these studies (χ 2 = 7.72, I 2 = 35%, P = .17; Figure 4). In the PP analysis, there was also no significant difference in the eradication rate between HDDT and BQT (88.4% vs 89.0%; RR = 1.00, 95% CI: 0.97-1.04; P = .99), without significant heterogeneity among these studies (χ 2 = 8.60, I 2 = 42%, P = .13; Figure 5).
Figure 4.
Forest plot of the efficacy of HDDT versus control regimens according to intention-to-treat analysis. HDDT, high-dose dual therapy.
Figure 5.
Forest plot of the efficacy of HDDT versus control regimens according to per-protocol analysis. HDDT, high-dose dual therapy.
Secondary Outcome: Side Effects and Compliance
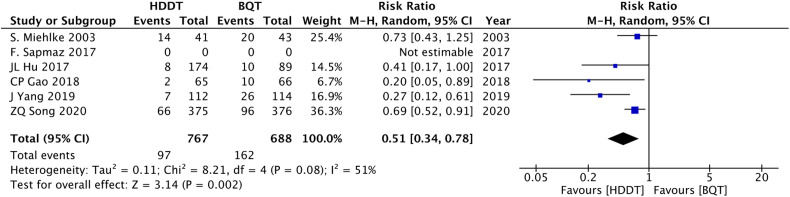
One study did not explicitly describe the number of people who occurred adverse events.22 The side effects in the HDDT group were significantly lower than that of the BQT group (13.1% vs 32.0%; RR = 0.51, 95% CI: 0.34-0.78; P = .002; I 2 = 51%, Figure 6). In terms of compliance, the HDDT group was statistically higher than that of the BQT group (96.1% vs 93.3%; RR = 1.03, 95% CI: 1.00-1.05; P = .03; I 2 = 23%, Figure 7).
Figure 6.
Forest plot of side effects of HDDT versus control regimens. HDDT, high-dose dual therapy.
Figure 7.
Forest plot of compliance of HDDT versus control regimens. HDDT, high-dose dual therapy.
Sensitivity Analysis
The sensitivity analyses revealed that the pooled RR with 95% CI was not obviously changed by removing 1 study at a time, indicating that our results were statistically robust (Table 2).
Table 2.
Sensitivity Analysis of Included Studies
| Study Removed | HDDT (%) | BQT (%) | Fixed-Effects Model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| RR (95% CI) | P | I ² (%) | P | |||
| Miehlke et al. 2003 | 85.1 | 83.8 | 1.02 (0.98, 1.06) | .38 | 44 | .13 |
| Hu et al. 2017 | 84.6 | 83.1 | 1.02 (0.98, 1.07) | .35 | 42 | .14 |
| Sapmaz et al. 2017 | 85.8 | 83.6 | 1.03 (0.98, 1.07) | .26 | 37 | .18 |
| Gao et al. 2018 | 84.8 | 83.8 | 1.02 (0.98, 1.06) | .38 | 44 | .13 |
| Yang et al. 2019 | 84.1 | 82.7 | 1.02 (0.97, 1.07) | .38 | 44 | .13 |
| Song et al. 2020 | 82.8 | 86.6 | 0.96 (0.91, 1.02) | .16 | 0 | .99 |
HDDT, high-dose dual therapy; BQT, bismuth-containing quadruple therapy.
Publication Bias
Visual examination of the funnel plot indicated that publication bias was possible (Figure 8). However, Begg’s test revealed no evidence of publication bias (P = .851). The reason for this difference might be attributed to the low number of included studies. and the sample sizes varied greatly among these studies.
Figure 8.
Publication bias.
Trial Sequential Analysis
The diversity-adjusted required information size (1331 participants) was calculated based on a relative risk reduction of 10%, an alpha of 5%, a beta of 20%, and a proportion of 83.7% in the control group. The cumulative Z-curve (blue line) did not cross the conventional boundary or trial sequential monitoring boundary but crossed the futility boundary and reached the required information size (vertical red line), suggesting that the efficacy of HDDT was equal to that of BQT (Figure 9).
Figure 9.
.We calculated a diversity-adjusted required information size of 1331 participants using α = 0.05 (two-sided), β = 0.20 (power = 80%), a relative risk reduction of 10% and a proportion of 83.7% in the control group. The cumulative z-curve (blue line) was constructed using a fixed-effects model. The cumulative Z-curve (blue line) did not cross the conventional boundary or trial sequential monitoring boundary, but crossed the futility boundary and reached the required information size (vertical red line), suggesting that the efficacy of HDDT was equal to that of BQT. HDDT, high dose dual therapy. BQT, bismuth-containing quadruple therapy.
Discussion
It is well known that the main factor leading to treatment failure of H. pylori infections is considered to be antibiotic resistance. Unlike the high resistance rates to metronidazole, clarithromycin, or levofloxacin, the vast majority of studies have shown that amoxicillin resistance of H. pylori, both primary and acquired, is still generally uncommon.25 The failure of dual therapy (omeprazole 20 mg and amoxicillin 1 g, 2 times daily) used in the past few decades is largely due to the insufficiency in drug doses and dosing frequency. Amoxicillin is a unique antibiotic in that its bactericidal effects against H. pylori are time- and pH-dependent. On the one hand, amoxicillin can maximize the time above minimal inhibitory concentration if given 3 or 4 times daily.26 On the other hand, the bactericidal activity of amoxicillin was stable when the pH value in the stomach was higher than 6.27 The value of gastric pH is related to the PPI dose, dosing frequency, and CYP2C19 gene polymorphism. Increasing the dose or dosing frequency of PPI administration may maintain the gastric pH above 6. For example, the study performed by Sahara et al.28 showed that esomeprazole can achieve potent acid inhibition when given 20 mg qid (4 times a day). In this meta-analysis, we defined HDDT as PPI and amoxicillin 3 to 4 times a day and amoxicillin ≥ 2.0 g/day. The result of our study demonstrated that HDDT achieves comparable H. pylori eradication rate to BQT (ITT: 84.6% vs 83.7%, P = 0.49; PP = 88.4% vs 89.0%, P = 0.99). Although the number of included studies is not large, TSA indicated that this conclusion is reliable, and it is unlikely that further similar studies will alter the result substantially.
For H. pylori infection, as with other infectious diseases, susceptibility-guided therapy seems to be a reliable and excellent treatment strategy.29 It can avoid the misuse of antibiotics and improve the eradication rate. However, conventional culture has many limitations, such as it is invasive, time-consuming, and technically challenging. With the rapid development of molecular testing methods, both invasive and non-invasive, the detection of antibiotic resistance has become more convenient, rapid, and sensitive.30 Unfortunately, unlike other prevalent human pathogens, the researches in this area have just begun on H. pylori and they have not yet been widely applied in daily clinical practice.31 More comprehensive clinical trials are warranted to assess the therapeutic effectiveness and safety of susceptibility-guided therapy. In addition, as mentioned above, amoxicillin resistance of H. pylori is rare, so it is unnecessary to conduct a susceptibility test when using HDDT to eradicate H. pylori infection as the first-line or rescue therapeutic regimen.
Although the HDDT regimen increased the dose and the dosing frequency of PPI or amoxicillin, our meta-analysis showed HDDT was lower than BQT in terms of side effects (13.1% vs 32.0%; RR = 0.51, 95% CI: 0.34-0.78; P = .002). Most of the adverse events in the HDDT group were mild and well-tolerated, such as diarrhea, abdominal pain, and nausea. In addition, a high rate of adverse events and a relatively complicated dosing regimen with BQT may decrease patient compliance. Indeed, we found that HDDT was superior to BQT in compliance (96.1% vs 93.3%; RR = 1.03, 95% CI: 1.00-1.05; P = .03). This result is not in accordance with previous meta-analyses.32 This may be due to the small number of studies and sample size included in the latter study. Actually, besides antibiotic resistance, poor compliance with therapy is also an indispensable reason for eradication failure. A study from China has shown that enhancing the patient’s compliance can significantly improve the H. pylori eradication rate (compliance: 94.6% vs 54.1%, P < .05; eradication rate: 87.4% vs 763.1%, P < .001).33 Thus, compared to BQT, HDDT has advantages in terms of compliance.
Helicobacter pylori has no resistance to bismuth compounds. Many previous studies have demonstrated that the addition of bismuth can partially overcome the H. pylori resistance to clarithromycin or levofloxacin, increasing the overall cure rate by 20% to 30%. However, research has also found that the high gastric pH caused by high PPI dosing frequency may reduce the effectiveness of bismuth.34 Lou et al.35 found that adding bismuth to HDDT could not enhance the overall eradicate rates, it only improved treatment effectiveness among smokers.35 Therefore, whether the addition of bismuth to HDDT can improve the eradication of H. pylori remains to be further inves tigated.
Vonoprazan (VPZ), a novel potassium-competitive acid blocker, was marketed in February 2015 and approved for application in the treatment of H. pylori infection in October 2019 by Food and Drug Administration. Compared to PPI, VPZ has the following advantages: (1) it does not need acid activation and is stable in the acidic environment; (2) it has a longer half-life, so it can achieve rapid, strong, and long-lasting gastric acid inhibition; (3) it is not affected by diet and CYP2C19 polymorphism.36-39 A Japanese study demonstrated that a 7-day VPZ–amoxicillin dual therapy was not inferior to VPZ-based triple therapy (ITT = 92.9% vs 91.9%, P = .728).40 Similarly, another Japanese study showed that a 7-day VPZ–amoxicillin dual therapy provided acceptable H. pylori eradication rate and a similar efficacy to VPZ-based triple therapy (ITT = 84.5% vs 89.2%, P = .203; PP = 87.1% vs 90.2%, P = .372) in regions with high clarithromycin resistance.41 Obviously, VPZ–amoxicillin dual therapy provides sufficient first-line efficacy, without the need for other antibiotics, such as metronidazole, clarithromycin, or levofloxacin. It is worth noting that these VPZ–amoxicillin dual therapy regimens have only been carried out in Japan, and further large-scale, prospective, randomized, well-designed studies are needed to validate these findings in other countries or regions.
There are some limitations of this study. First, all included studies did not examine the patients’ intragastric pH, thus we were unable to assess whether PPI could successfully inhibit gastric acid and maintain pH values higher than 6. Second, the majority of the studies originated from Asia, which could be potentially affected by the CYP2C19 genotype polymorphism. Third, none of these trials used the double-blinding method, and the missing blinding might have influenced patient-reported outcome measures. Finally, despite we performed rigorous statistics, the number of included studies was relatively small.
Conclusion
In summary, our study demonstrated that HDDT is equally effective with BQT in eradicating H. pylori, with fewer side effects and better compliance. The result of this meta-analysis supports that HDDT is an effective, safe, and well-tolerated regimen for the eradication of H. pylori infection as first-line or rescue therapy.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Z.K.Y, J.Y.Z; Design – J.Y.Z.; Supervision – J.Y.Z.; Resource – Z.K.Y., H.L.; Materials – Z.K.Y.; Data collection and/or processing – Z.K.Y., J.L.; Analysis and/or interpretation – W.F.H., X.Y.L., D.X., G.H.X.; Literature search – Z.K.Y., J.L.; Writing – Z.K.Y.; Critical reviews – J.Y.Z.
Declaration of Interest: The authors have no conflict of interest to declare.
Funding: This study received no funding.
References
- 1. .Zamani M, Ebrahimtabar F, Zamani V.et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–876.. 10.1111/apt.14561) [DOI] [PubMed] [Google Scholar]
- 2. .Malfertheiner P, Megraud F, O'Morain CA.et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30.. 10.1136/gutjnl-2016-312288) [DOI] [PubMed] [Google Scholar]
- 3. .Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155(5):1372-1382.e17. 10.1053/j.gastro.2018.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. .Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12(4):275–278.. 10.1111/j.1523-5378.2007.00518.x) [DOI] [PubMed] [Google Scholar]
- 5. .Mégraud F.H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53(9):1374–1384.. 10.1136/gut.2003.022111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. .Kuo YT, Liou JM, El-Omar EM.et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(10):707–715.. 10.1016/S2468-1253(17)30219-4) [DOI] [PubMed] [Google Scholar]
- 7. .Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: past, present and future. World J Gastrointest Pathophysiol. 2014;5(4):392–399.. 10.4291/wjgp.v5.i4.392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. .Fallone CA, Chiba N, van Zanten SV.et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151(1):e14. 10.1053/j.gastro.2016.04.006) [DOI] [PubMed] [Google Scholar]
- 9. .Liu WZ, Xie Y, Lu H.et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23(2):e12475. 10.1111/hel.12475) [DOI] [PubMed] [Google Scholar]
- 10. .Bayerdörffer E, Miehlke S, Mannes GA.et al. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology. 1995;108(5):1412–1417.. 10.1016/0016-5085(95)90689-4) [DOI] [PubMed] [Google Scholar]
- 11. .Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–239.. 10.1038/ajg.2016.563) [DOI] [PubMed] [Google Scholar]
- 12. .Kwack W, Lim Y, Lim C, Graham DY, Ilaprazole HDose. High Dose Ilaprazole/Amoxicillin as first-line regimen for Helicobacter pylori Infection in Korea. Gastroenterol Res Pract. 2016;2016:1648047. 10.1155/2016/1648047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. .Yang J, Zhang Y, Fan L.et al. Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am J Gastroenterol. 2019;114(3):437–445.. 10.14309/ajg.0000000000000132) [DOI] [PubMed] [Google Scholar]
- 14. .Moher D, Liberati A, Tetzlaff J, Altman DGPRISMA Group. Reprint--preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880.. 10.1093/ptj/89.9.873) [DOI] [PubMed] [Google Scholar]
- 15. .Higgins JP, Altman DG, Gøtzsche PC.et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. .DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(A):139–145.. 10.1016/j.cct.2015.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. .Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):39. 10.1186/s12874-017-0315-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. .Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75.. 10.1016/j.jclinepi.2007.03.013) [DOI] [PubMed] [Google Scholar]
- 19. .Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive--trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009;38(1):287–298.. 10.1093/ije/dyn188) [DOI] [PubMed] [Google Scholar]
- 20. .Miehlke S, Kirsch C, Schneider-Brachert W.et al. A prospective, randomized study of quadruple therapy and high-dose dual therapy for treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter. 2003;8(4):310–319.. 10.1046/j.1523-5378.2003.00158.x) [DOI] [PubMed] [Google Scholar]
- 21. .Hu JL, Yang J, Zhou YB, Li P, Han R, Fang DC. Optimized high-dose amoxicillin-proton-pump inhibitor dual therapies fail to achieve high cure rates in China. Saudi J Gastroenterol. 2017;23(5):275–280.. 10.4103/sjg.SJG_91_17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. .Sapmaz F, Kalkan IH, Atasoy P, Basyigit S, Guliter S. A non-inferiority study: modified dual therapy consisting higher doses of rabeprazole is as successful as standard quadruple therapy in eradication of Helicobacter pylori. Am J Ther. 2017;24(4):e393–e398.. 10.1097/MJT.0000000000000316) [DOI] [PubMed] [Google Scholar]
- 23. .Gao CP, Xiao X, Liu PX, Zhou Z, Li LP, Han SX. High-dose amoxicillin/esomeprazole dual therapy as a first-line therapy for Helicobacter pylori eradication. World Chin J Digestology. 2018;26(6):353–359.. 10.11569/wcjd.v26.i6.353) [DOI] [Google Scholar]
- 24. .Song Z, Zhou L, Xue Y, Suo B, Tian X, Niu Z. A comparative study of 14-day dual therapy (esomeprazole and amoxicillin four times daily) and triple plus bismuth therapy for first-line Helicobacter pylori infection eradication: a randomized trial. Helicobacter. 2020;25(6):e12762. 10.1111/hel.12762) [DOI] [PubMed] [Google Scholar]
- 25. .Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155(5):1372.e17-1382.e17. 10.1053/j.gastro.2018.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. .Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori infection. Gastroenterol Clin North Am. 2010;39(3):465–480.. 10.1016/j.gtc.2010.08.007) [DOI] [PubMed] [Google Scholar]
- 27. .Berry V, Jennings K, Woodnutt G. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrob Agents Chemother. 1995;39(8):1859–1861.. 10.1128/AAC.39.8.1859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. .Sahara S, Sugimoto M, Uotani T.et al. Potent Gastric Acid Inhibition over 24 hours by 4-times daily dosing of esomeprazole 20 mg. Digestion. 2015;91(4):277–285.. 10.1159/000381419) [DOI] [PubMed] [Google Scholar]
- 29. .Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12(2):177:e3. 10.1016/j.cgh.2013.05.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. .Arslan N, Yılmaz Ö, Demiray-Gürbüz E. Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World J Gastroenterol. 2017;23(16):2854–2869.. 10.3748/wjg.v23.i16.2854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. .Ierardi E, Giorgio F, Iannone A.et al. Noninvasive molecular analysis of Helicobacter pylori: is it time for tailored first-line therapy? World J Gastroenterol. 2017;23(14):2453–2458.. 10.3748/wjg.v23.i14.2453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. .Yang X, Wang JX, Han SX, Gao CP. High dose dual therapy versus bismuth quadruple therapy for Helicobacter pylori eradication treatment: a systematic review and meta-analysis. Med (Baltim). 2019;98(7):e14396. 10.1097/MD.0000000000014396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. .Luo M, Hao Y, Tang M.et al. Application of a social media platform as a patient reminder in the treatment of Helicobacter pylori. Helicobacter. 2020;25(2):e12682. 10.1111/hel.12682) [DOI] [PubMed] [Google Scholar]
- 34. .Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65(5):870–878.. 10.1136/gutjnl-2015-311019) [DOI] [PubMed] [Google Scholar]
- 35. .Yu L, Luo L, Long X.et al. High-dose PPI-amoxicillin dual therapy with or without bismuth for first-line Helicobacter pylori therapy: a randomized trial. Helicobacter. 2019;24(4):e12596. 10.1111/hel.12596) [DOI] [PubMed] [Google Scholar]
- 36. .Sugimoto M, Yamaoka Y. Role of Vonoprazan in Helicobacter pylori eradication therapy in Japan. Front Pharmacol. 2018;9:1560. 10.3389/fphar.2018.01560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. .Otake K, Sakurai Y, Nishida H.et al. Characteristics of the novel potassium-competitive acid blocker Vonoprazan fumarate (TAK-438). Adv Ther. 2016;33(7):1140–1157.. 10.1007/s12325-016-0345-2) [DOI] [PubMed] [Google Scholar]
- 38. .Inatomi N, Matsukawa J, Sakurai Y, Otake K. Potassium-competitive acid blockers: advanced therapeutic option for acid-related diseases. Pharmacol Ther. 2016;168:12–22.. 10.1016/j.pharmthera.2016.08.001) [DOI] [PubMed] [Google Scholar]
- 39. .Kagami T, Sahara S, Ichikawa H.et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther. 2016;43(10):1048–1059.. 10.1111/apt.13588) [DOI] [PubMed] [Google Scholar]
- 40. .Furuta T, Yamade M, Kagami T.et al. Dual therapy with Vonoprazan and amoxicillin is as effective as triple therapy with Vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori. Digestion. 2020;101(6):743–751.. 10.1159/000502287) [DOI] [PubMed] [Google Scholar]
- 41. .Suzuki S, Gotoda T, Kusano C.et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69(6):1019–1026.. 10.1136/gutjnl-2019-319954) [DOI] [PMC free article] [PubMed] [Google Scholar]